An Overview of Strengths and Directionalities of Noncovalent Interactions: σ-Holes and π-Holes
Abstract
1. σ-Holes and π-Holes
2. Molecular Electrostatic Potentials
3. Coulombic Nature of Interactions
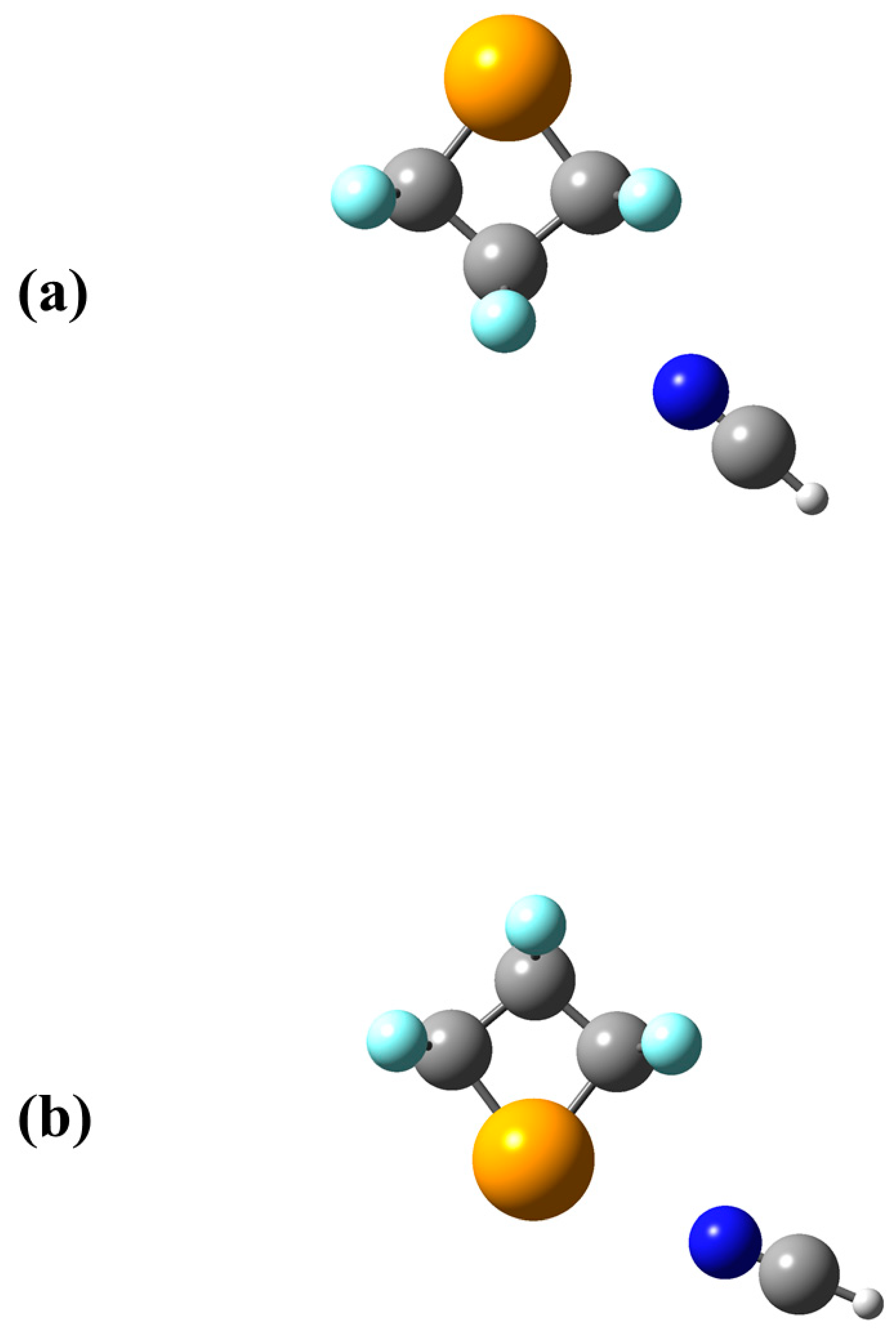
4. Significance of VS,max
5. Overlapping of Positive Potentials: Implications for Interpretation of Close Contacts


6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Schneider, H.-J. Binding Mechanisms in Supramolecular Complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. σ-Holes, π-Holes and Electrostatically-Driven Interactions. J. Mol. Model. 2012, 18, 541–548. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen Bonding and Other σ-Hole Interactions: A Perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The Bright Future of Unconventional σ/π-Hole Interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The σ-Hole Revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.D. Experimental Electron Density Distributions of Molecular Chlorine. Mol. Phys. 1979, 37, 27–45. [Google Scholar] [CrossRef]
- Row, T.N.G.; Parthasarathy, R. Directional Preferences of Nonbonded Atomic Contacts with Divalent Sulfur in Terms of Its Orbital Orientations. 2. S---S Interactions and Nonspherical Shape of Sulfur in Crystals. J. Am. Chem. Soc. 1981, 103, 477–479. [Google Scholar] [CrossRef]
- Nyburg, S.C.; Faerman, C.H. A Revision of Van der Waals Atomic Radii for Molecular Crystals: N, O, F, S, Cl, Se, Br and I Bonded to Carbon. Acta Cryst. 1985, B41, 274–279. [Google Scholar] [CrossRef]
- Ikuta, S. Anisotropy of Electron Density Distribution Around Atoms in Molecules: N, P, O and S Atoms. J. Mol. Struct. (Theochem) 1990, 205, 191–201. [Google Scholar] [CrossRef]
- Tsirelson, V.C.; Zou, P.F.; Tang, T.-H.; Bader, R.F.W. Topological Definition of Crystal Structure Determination of the Bonded Interactions in Solid Molecular Chlorine. Acta Cryst. 1995, A51, 143–153. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen Bonding: An Interim Discussion. ChemPhysChem 2013, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Benesi, H.A.; Hildebrsand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Hassel, O.; Rømming, C. Direct Structural Evidence for Weak Charge Transfer Bonds in Solids Containing Chemically Saturated Molecules. Quart. Rev. Chem. Soc. 1962, 16, 1–18. [Google Scholar] [CrossRef]
- Bent, H.A. Structural Chemistry of Donor-Acceptor Interactions. Chem. Rev. 1968, 68, 587–648. [Google Scholar] [CrossRef]
- Blackstock, S.C.; Lorand, J.P.; Kochi, J.K. Charge-Transfer Interactions of Amines with Tetrahalomethanes. X-Ray Crystal Structures of the Donor-Acceptor Complexes of Quinuclidine and Diazabicyclo[2.2.2]octane with Carbon Tetrabromide. J. Org. Chem. 1987, 52, 1451–1460. [Google Scholar] [CrossRef]
- Ramasubbu, N.; Parthasarathy, R.; Murray-Rust, P. Angular Preferences of Intermolecular Forces Around Halogen Centers: Preferred Directions of Approach of Electrophiles and Nuclophiles Around Carbon-Halogen Bond. J. Am. Chem. Soc. 1986, 108, 4308–4314. [Google Scholar] [CrossRef]
- Rosenfeld, R.E., Jr.; Parthasarathy, R.; Dunitz, J.D. Directional Preferences of Nonbonded Atomic Contacts with Divalent Sulfur. 1. Electrophiles and Nucleophiles. J. Am. Chem. Soc. 1977, 99, 4860–4862. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Janjič, G.V.; Zarič, S.D. σ-Hole Interactions of Covalently-Bonded Nitrogen, Phosphorus and Arsenic: A Survey of Crystal Structures. Crystals 2014, 4, 12–31. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef]
- Scilabra, P.; Terraneo, G.; Resnati, G. The Chalcogen Bond in Crystalline Solids: A World Parallel to Halogen Bond. Acc. Chem. Res. 2019, in press. [Google Scholar]
- Shields, Z.; Murray, J.S.; Politzer, P. Directional Tendencies of Halogen and Hydrogen Bonding. Int. J. Quantum Chem. 2010, 110, 2823–2832. [Google Scholar] [CrossRef]
- Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Halogen Bonding Based Recognition Processes: A World Parallel to Hydrogen Bonding. Acc. Chem. Res. 2005, 38, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Naming Interactions from the Electrophilic Site. Cryst. Growth Des. 2014, 14, 2697–2702. [Google Scholar] [CrossRef]
- Stewart, R.F. On the Mapping of Electrostatic Properties from Bragg Diffraction Data. Chem. Phys. Lett. 1979, 65, 335–342. [Google Scholar] [CrossRef]
- Politzer, P.; Truhlar, D.G. Chemical Applications of Atomic and Molecular Electrostatic Potentials; Plenum Press: New York, NY, USA, 1981. [Google Scholar]
- Klein, C.L.; Stevens, E.D. Charge Density Studies of Drug Molecules. In Structure and Reactivity; Liebman, J.F., Goldberg, A., Eds.; VCH Publishers: New York, NY, USA, 1988; pp. 26–64. [Google Scholar]
- Price, S.L. Applications of Realistic Modelling to Molecules in Complexes, Solids and Proteins. J. Chem. Soc. Faraday Trans. 1996, 92, 2997–3008. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. The Electrostatic Potential: An Overview. WIREs Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Carroll, M.T.; Cheeseman, J.R.; Chang, C. Properties of Atoms in Molecules. Atomic Volumes. J. Am. Chem. Soc. 1987, 109, 7968–7979. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Molecular Electrostatic Potentials and Chemical Reactivity. In Reviews in Computational Chemistry; Lipkowitz, K.B., Boyd, D.B., Eds.; VCH Publishers: New York, NY, USA, 1991; Volume 2, pp. 273–312. [Google Scholar]
- Wheeler, S.E.; Houk, K.N. Through-Space Effects of Substituents Dominate Molecular Electrostatic Potentials of Substituted Arenes. J. Chem. Theory Comput. 2009, 5, 2301–2312. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Molecular Electrostatic Potentials and Noncovalent Interactions. WIREs Comput. Mol. Sci. 2017, 7, e1326. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. σ-Holes and π-Holes: Similarities and Differences. J. Comput. Chem. 2017, 39, 464–471. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. σ-Hole Interactions: Perspectives and Misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Henneker, W.H.; Cade, P.E. Molecular Charge Distributions and Chemical Binding. J. Chem. Phys. 1967, 46, 3341–3363. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bulat, F.A.; Toro-Labbé, A.; Brinck, T.; Murray, J.S.; Politzer, P. Quantitative Analysis of Molecular Surfaces: Volumes, Electrostatic Potentials and Average Local Ionization Energies. J. Mol. Model. 2010, 16, 1679–1691. [Google Scholar] [CrossRef]
- Hellmann, H. Einführung in die Quantenchemie; Deuticke: Leipzig, Germany, 1937. [Google Scholar]
- Feynman, R.P. Forces in Molecules. Phys. Rev. 1939, 56, 340–343. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Bader, R.F.W. Pauli Repulsions Exist Only in the Eye of the Beholder. Chem. Eur. J. 2006, 12, 2896–2901. [Google Scholar] [CrossRef]
- Lennard-Jones, J.E.; Pople, J.A. The Molecular Orbital Theory of Chemical Valency: IX. The Interaction of Paired Electrons in Chemical Bonds. Proc. R. Soc. Lond. Ser. A 1951, 210, 190–206. [Google Scholar]
- Politzer, P.; Riley, K.E.; Bulat, F.A.; Murray, J.S. Perspectives on Halogen Bonding and Other σ-Hole Interactions: Lex parsimoniae (Occam’s Razor). Comput. Theoret. Chem. 2012, 998, 2–8. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. σ-Hole Bonding: A Physical Interpretation. Top. Curr. Chem. 2015, 358, 19–42. [Google Scholar]
- Politzer, P.; Murray, J.S.; Clark, T. Mathematical Modeling and Physical Reality in Noncovalent Interactions. J. Mol. Model. 2015, 21, 52. [Google Scholar] [CrossRef]
- Levine, I.N. Quantum Chemistry, 5th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- Solimannejad, M.; Malekani, M.; Alkorta, I. Cooperative and Diminutive Unusual Weak Bonding in F3C-X---HMgH---Y and F3C-X---Y---HMgH Trimers (X = Cl, Br; Y = HCN and HNC). J. Phys. Chem. A 2010, 114, 12106–12111. [Google Scholar] [CrossRef]
- Hennemann, M.; Murray, J.S.; Politzer, P.; Riley, K.E.; Clark, T. Polarization-Induced σ-Holes and Hydrogen Bonding. J. Mol. Model. 2012, 18, 2461–2469. [Google Scholar] [CrossRef]
- Clark, T.; Politzer, P.; Murray, J.S. Correct Electrostatic Treatment of Non-Covalent Interactions: The Importance of Polarisation. WIREs Comput. Mol. Sci. 2015, 5, 169–177. [Google Scholar] [CrossRef]
- Riley, K.E.; Hobza, P. Investigations into the Nature of Halogen Bonding Including Symmetry Adapted Perturbation Theory Analyses. J. Chem. Theory Comput. 2008, 4, 232–242. [Google Scholar] [CrossRef]
- Mulliken, R.S. Molecular Compounds and Their Spectra. II. J. Am. Chem. Soc. 1952, 74, 811–824. [Google Scholar] [CrossRef]
- Flurry, R.L., Jr. Molecular Orbital Theory of Electron Donor-Acceptor Complexes. I. A Simple Semiempirical Treatment. J. Phys. Chem. 1969, 69, 1927–1933. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Thompson, C.C. A Critique for Charge Transfer and Stability Constants for Some TCNE-Hydrocarbon Complexes. Tetrahedron 1966, 7, 97–114. [Google Scholar] [CrossRef]
- Slifkin, M.A. Charge Transfer Interactions of Biomolecules; Academic Press: London, UK, 1971. [Google Scholar]
- Stone, A.J.; Price, S.L. Some New Ideas in the Theory of Intermolecular Forces: Anisotropic Atom-Atom Potentials. J. Phys. Chem. 1988, 92, 3325–3335. [Google Scholar] [CrossRef]
- Sokalski, W.A.; Roszak, S.M. Efficient Techniques for the Decomposition of Intermolecular Interaction Energy at SCF Level and Beyond. J. Mol. Struct. (Theochem) 1991, 234, 387–400. [Google Scholar] [CrossRef]
- Stone, A.J.; Misquitta, A.J. Charge-Transfer in Symmetry-Adapted Perturbation Theory. Chem. Phys. Lett. 2009, 473, 201–205. [Google Scholar] [CrossRef]
- Stone, A.J. Natural Bond Orbitals and the Nature of the Hydrogen Bond. J. Phys. Chem. A 2017, 121, 1531–1534. [Google Scholar] [CrossRef]
- Clark, T.; Murray, J.S.; Politzer, P. A Perspective on Quantum Mechanics and Chemical Concepts in Describing Noncovalent Interactions. Phys. Chem. Chem. Phys. 2018, 20, 30076–30082. [Google Scholar] [CrossRef] [PubMed]
- Brinck, T.; Borrfors, A.N. Electrostatics and Polarization Determine the Strength of the Halogen Bond: A Red Card for Charge Transfer. J. Mol. Model. 2019, in press. [Google Scholar]
- Wang, W.; Wang, N.B.; Zheng, W.; Tian, A. Theoretical Study on the Blueshifting Halogen Bond. J. Phys. Chem. A 2004, 108, 1799–1805. [Google Scholar] [CrossRef]
- Murray, J.S.; Concha, M.C.; Lane, P.; Hobza, P.; Politzer, P. Blue Shifts vs. Red Shifts in σ-Hole Bonding. J. Mol. Model. 2008, 14, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Ramasubbu, N.; Parthsarathy, R. Stereochemistry of Incipient Electrophilic and Nucleophililc Reactions at Divalent Selenium Center: Electrophilic-Nucleophilic Pairing and Anisotropic Shape of Se in Se…Se Interactions. Phosphorus Sulfur 1987, 31, 221–229. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Concha, M.C. Halogen Bonding and the Design of New Materials: Organic Bromides, Chlorides and Perhaps Even Fluorides as Donors. J. Mol. Model. 2007, 13, 643–650. [Google Scholar] [CrossRef]
- Chopra, D.; Guru Row, T.N. Role of Organic Fluorine in Crystal Engineering. CrystEngComm 2011, 13, 2175–2186. [Google Scholar] [CrossRef]
- Metrangolo, P.; Murray, J.S.; Pilati, T.; Politzer, P.; Resnati, G.; Terraneo, G. Fluorine-Centered Halogen Bonding: A Factor in Recognition Phenomena and Reactivity. Cryst. Growth Des. 2011, 11, 4238–4246. [Google Scholar] [CrossRef]
- Pathak, R.K.; Gadre, S.R. Maximal and Minimal Characteristics of Molecular Electrostatic Potentials. J. Chem. Phys. 1990, 93, 1770–1773. [Google Scholar] [CrossRef]
- Riley, K.E.; Murray, J.S.; Politzer, P.; Concha, M.C.; Hobza, P. Br---O Complexes as Probes of Factors Affecting Halogen Bonding: Interactions of Bromobenzenes and Bromopyrimidines with Acetona. J. Chem. Theory Comput. 2009, 5, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Murray, J.S.; Fanfrlίk, J.; Řezáč, J.; Solá, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen Bond Tunability I: The Effects of Aromatic Fluorine Substitution on the Strengths of Halogen-Bonding Interactions Involving Chlorine, Bromine and Iodine. J. Mol. Model. 2011, 17, 3309–3318. [Google Scholar] [CrossRef]
- Bundhun, A.; Ramasami, P.; Murray, J.S.; Politzer, P. Trends in σ-Hole Strengths and Interactions of F3MX Molecules (M = C, Si, Ge and X = F, Cl, Br, I). J. Mol. Model. 2013, 19, 2739–2746. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. A Unified View of Halogen Bonding, Hydrogen Bonding and Other σ-Hole Interactions. In Noncovalent Forces; Scheiner, S., Ed.; Springer: Heidelberg, Germany, 2015; Chapter 10; pp. 291–321. [Google Scholar]
- Politzer, P.; Murray, J.S. Halogen Bonding and Beyond: Factors Influencing the Nature of CN-R and SiN-R Complexes with FCl and Cl2. Theoret. Chem. Acc. 2012, 131, 1114. [Google Scholar] [CrossRef]
- Murray, J.S.; Shields, Z.P.-I.; Seybold, P.G.; Politzer, P. Intuitive and Counterintuitive Noncovalent Interactions of Aromatic π-Regions with the Hydrogen and Nitrogen of HCN. J. Comput. Sci. 2015, 10, 209–216. [Google Scholar] [CrossRef]
- Clark, T.; Hesselmann, A. The Coulombic σ-Hole Model Describes Bonding in CX3I···Y− Complexes Completely. Phys. Chem. Chem. Phys. 2018, 20, 22849–22855. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Murray, J.S.; Politzer, P. The σ-Hole Coulombic Interpretation of Trihalide Anion Formation. ChemPhysChem 2018, 19, 3044–3049. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Dance, I. Distance Criteria for Crystal Packing Analysis of Supramolecular Motifs. New J. Chem. 2003, 27, 22–27. [Google Scholar] [CrossRef]
- Alvarez, S. A Cartography of the van der Waals Territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Nayak, S.K.; Kumar, V.; Murray, J.S.; Politzer, P.; Terraneo, G.; Pilati, T.; Metrangolo, P.; Renati, G. Fluorination Promotes Chalcogen Bonding in Crystalline Solids. CrystEngComm 2017, 19, 4955–4959. [Google Scholar] [CrossRef]
- Politzer, P.; Resnati, G.; Murray, J.S. Close Contacts and Noncovalent Interactions in Crystals. Faraday Disc. 2017, 203, 113–130. [Google Scholar]
- Davies, D.R.; Blum, J.J. The Crystal Structure of Parabanic Acid. Acta Cryst. 1955, 8, 129–136. [Google Scholar] [CrossRef]
- Bolton, W. The Crystal Structure of Alloxan. Acta Cryst. 1964, 17, 147–152. [Google Scholar] [CrossRef]
- Swaminathan, S.; Craven, B.M.; McMullan, R.K. Alloxan—Electrostatic Properties of an Unusual Structure from X-ray and Neutron Diffraction. Acta Cryst. 1985, B41, 113–122. [Google Scholar] [CrossRef]
- Gavezzotti, A. Molecular Packing and Other Structural Properties of Crystalline Oxohydrocarbons. J. Phys. Chem. 1991, 95, 8948–8955. [Google Scholar] [CrossRef]
- Spackman, M.A.; Weber, H.P.; Craven, B.M. Energies of Molecular Interactions from Bragg Diffraction Data. J. Am. Chem. Soc. 1988, 110, 775–782. [Google Scholar] [CrossRef]
- Coombes, D.S.; Nagi, G.K.; Price, S.L. On the Lack of Hydrogen Bonds in the Crystal Structure of Alloxan. Chem. Phys. Lett. 1997, 265, 532–537. [Google Scholar] [CrossRef]
- Paulini, R.; Müller, K.; Diederich, F. Orthogonal Multipolar Interactions in Structural Chemistry and Biology. Angew. Chem. Int. Ed. 2005, 44, 1788–1805. [Google Scholar] [CrossRef]
- Hunter, C.A. Quantifying Intermolecular Interactions: Guidelines for the Molecular Recognition Toolbox. Angew. Chem. Int. Ed. 2004, 43, 5310–5324. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Wijethunga, T.K.; Desper, J. Molecular Electrostatic Potential Dependent Selectivity of Hydrogen Bonding. New J. Chem. 2015, 39, 822–828. [Google Scholar] [CrossRef]
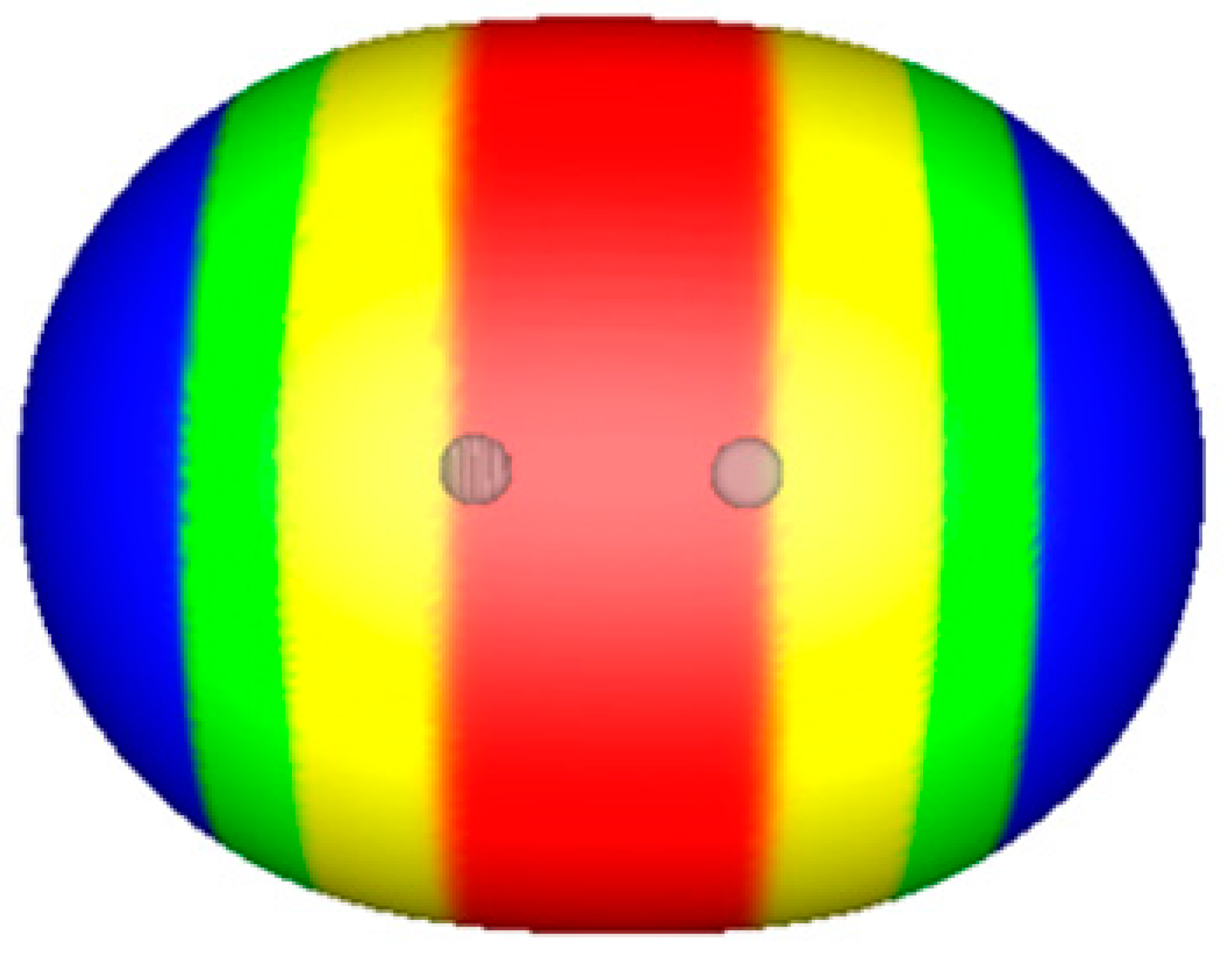
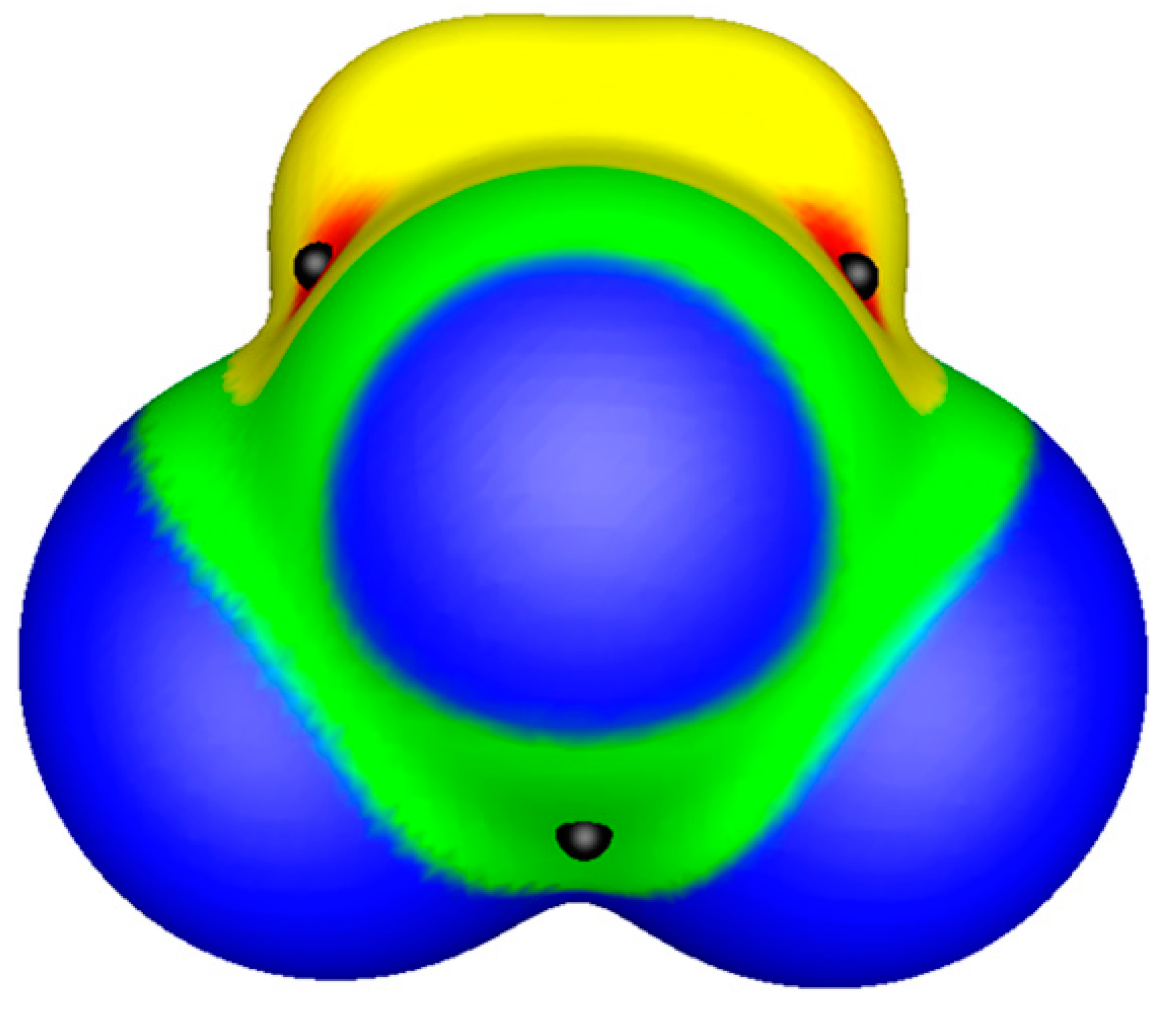
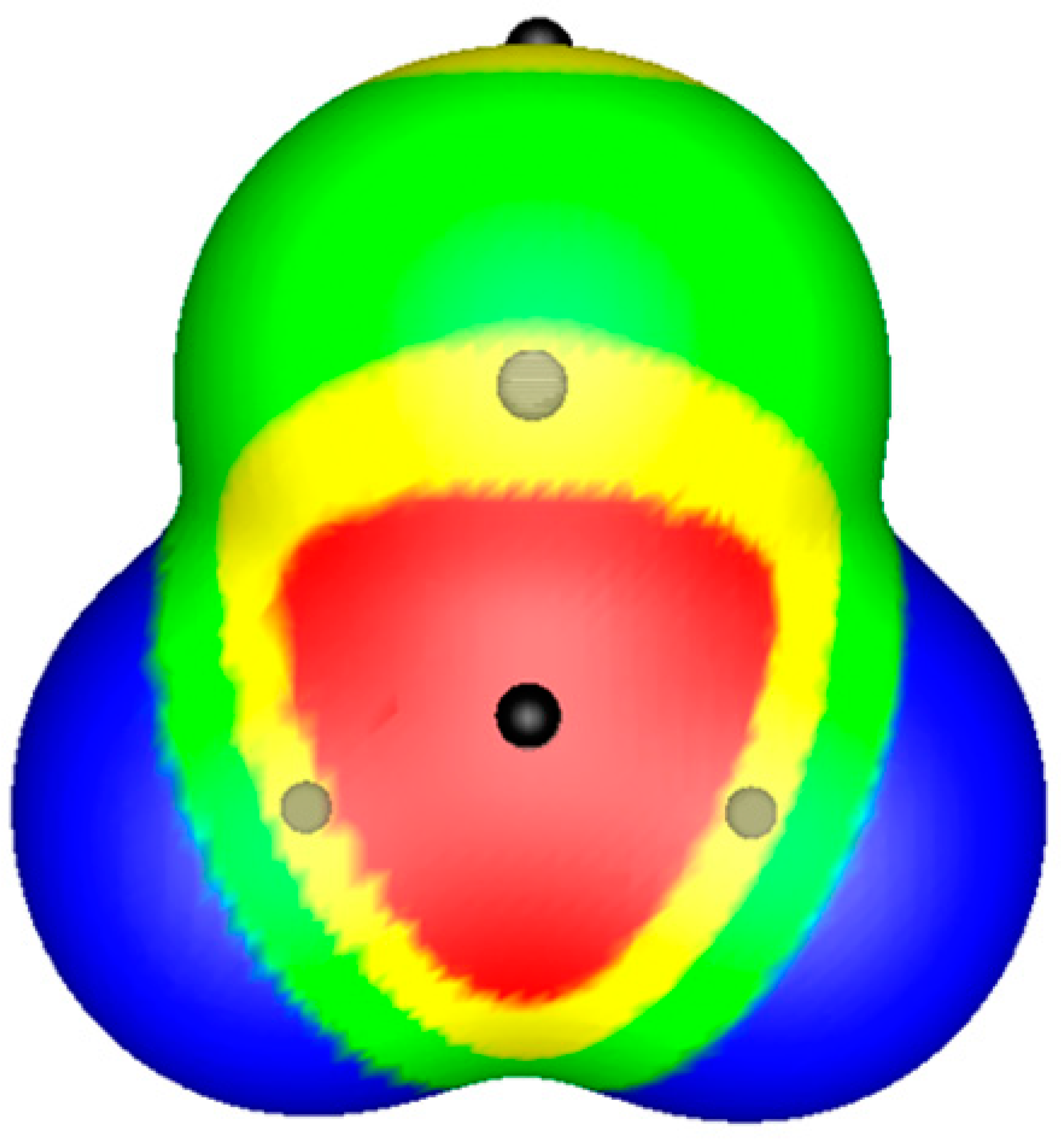
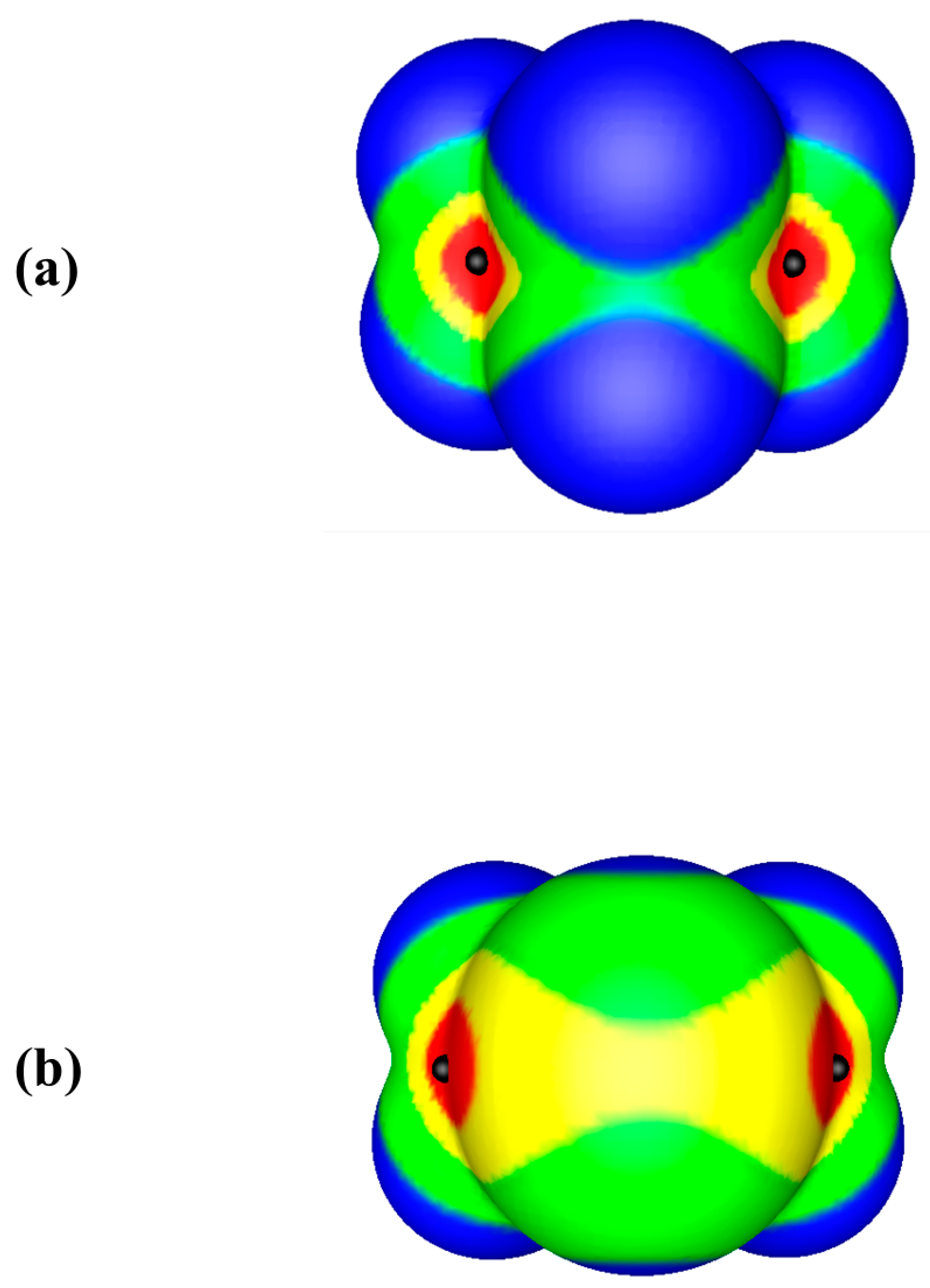


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politzer, P.; Murray, J.S. An Overview of Strengths and Directionalities of Noncovalent Interactions: σ-Holes and π-Holes. Crystals 2019, 9, 165. https://doi.org/10.3390/cryst9030165
Politzer P, Murray JS. An Overview of Strengths and Directionalities of Noncovalent Interactions: σ-Holes and π-Holes. Crystals. 2019; 9(3):165. https://doi.org/10.3390/cryst9030165
Chicago/Turabian StylePolitzer, Peter, and Jane S. Murray. 2019. "An Overview of Strengths and Directionalities of Noncovalent Interactions: σ-Holes and π-Holes" Crystals 9, no. 3: 165. https://doi.org/10.3390/cryst9030165
APA StylePolitzer, P., & Murray, J. S. (2019). An Overview of Strengths and Directionalities of Noncovalent Interactions: σ-Holes and π-Holes. Crystals, 9(3), 165. https://doi.org/10.3390/cryst9030165




