Single Crystal X-Ray Structure Determination and Vibrational Spectroscopy of 2-Aminopyrimidinium Hydrogen Trioxofluorophosphate and bis(2-Aminopyrimidinium) Trioxofluorophosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Crystallization
2.2. Structure Determination and Refinement
2.3. Vibrational Spectroscopy Instrument
3. Results and Discussion
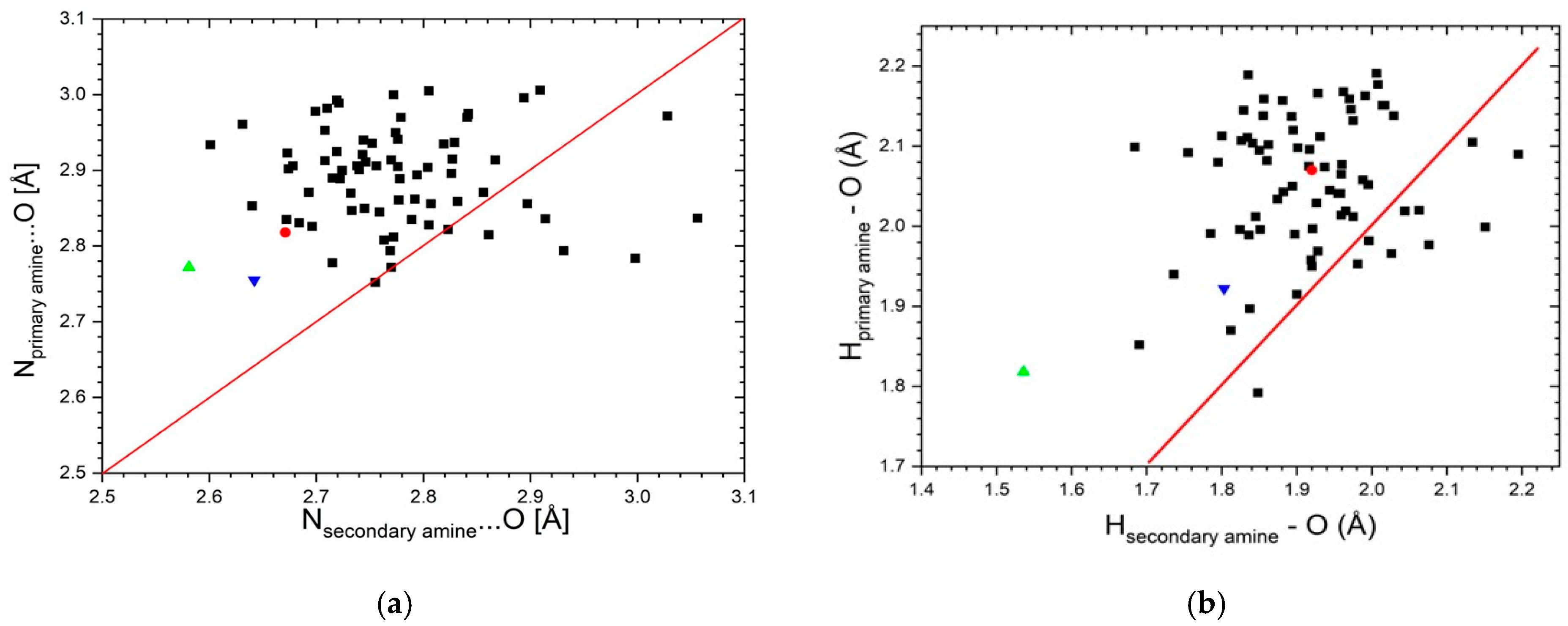
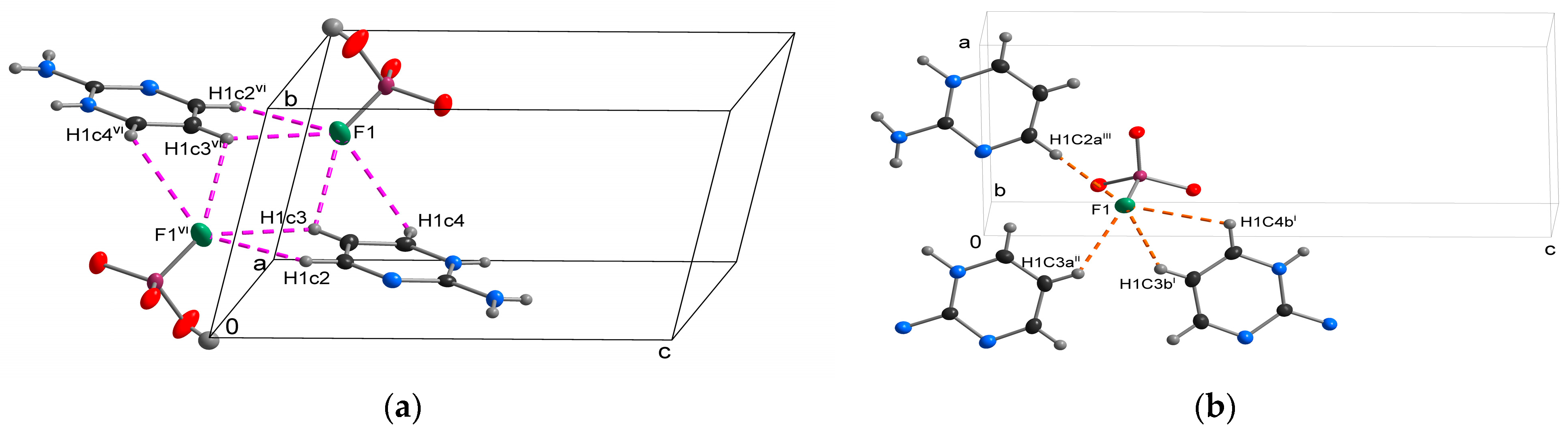
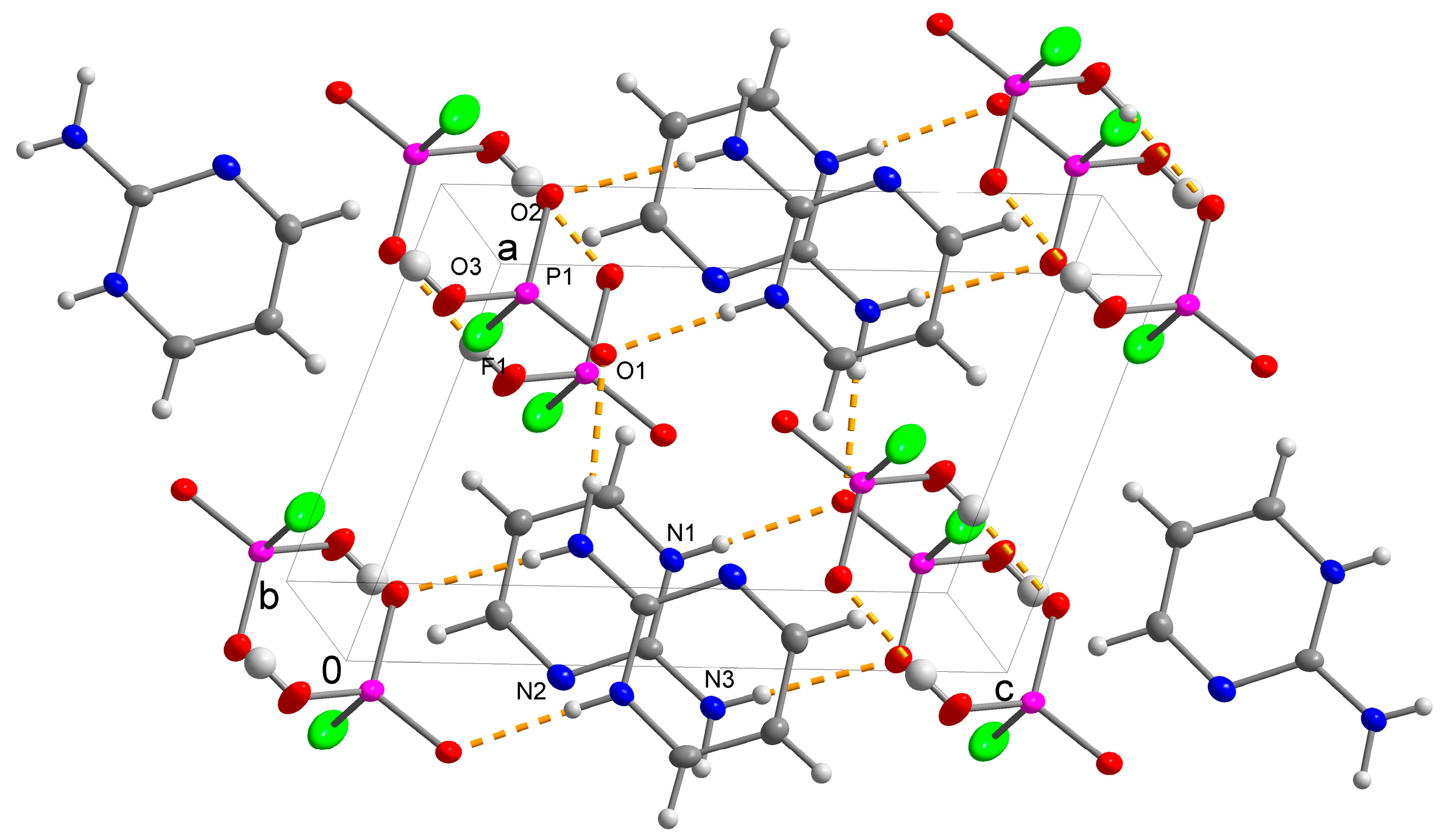
3.1. Crystal Structure Determination
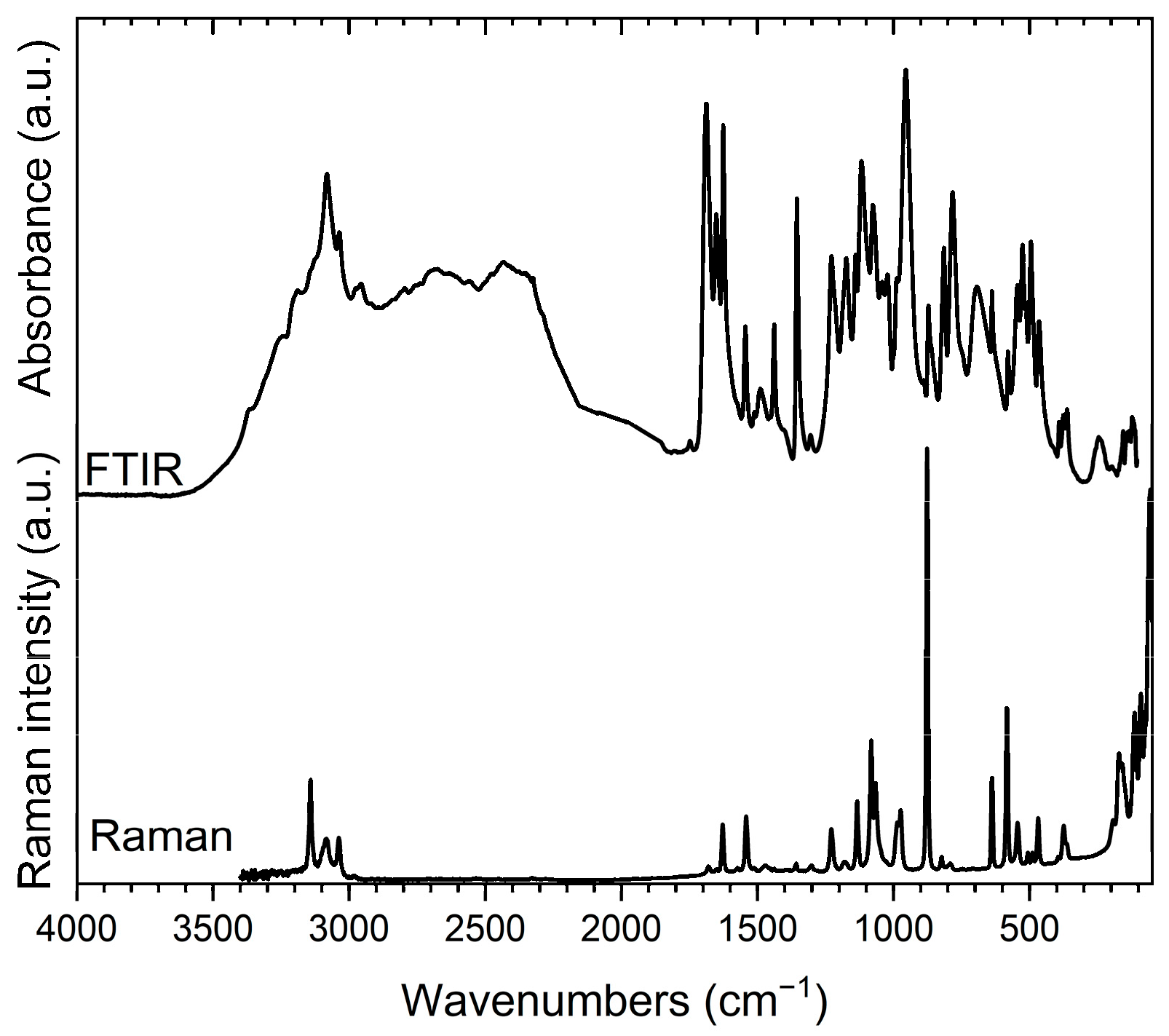
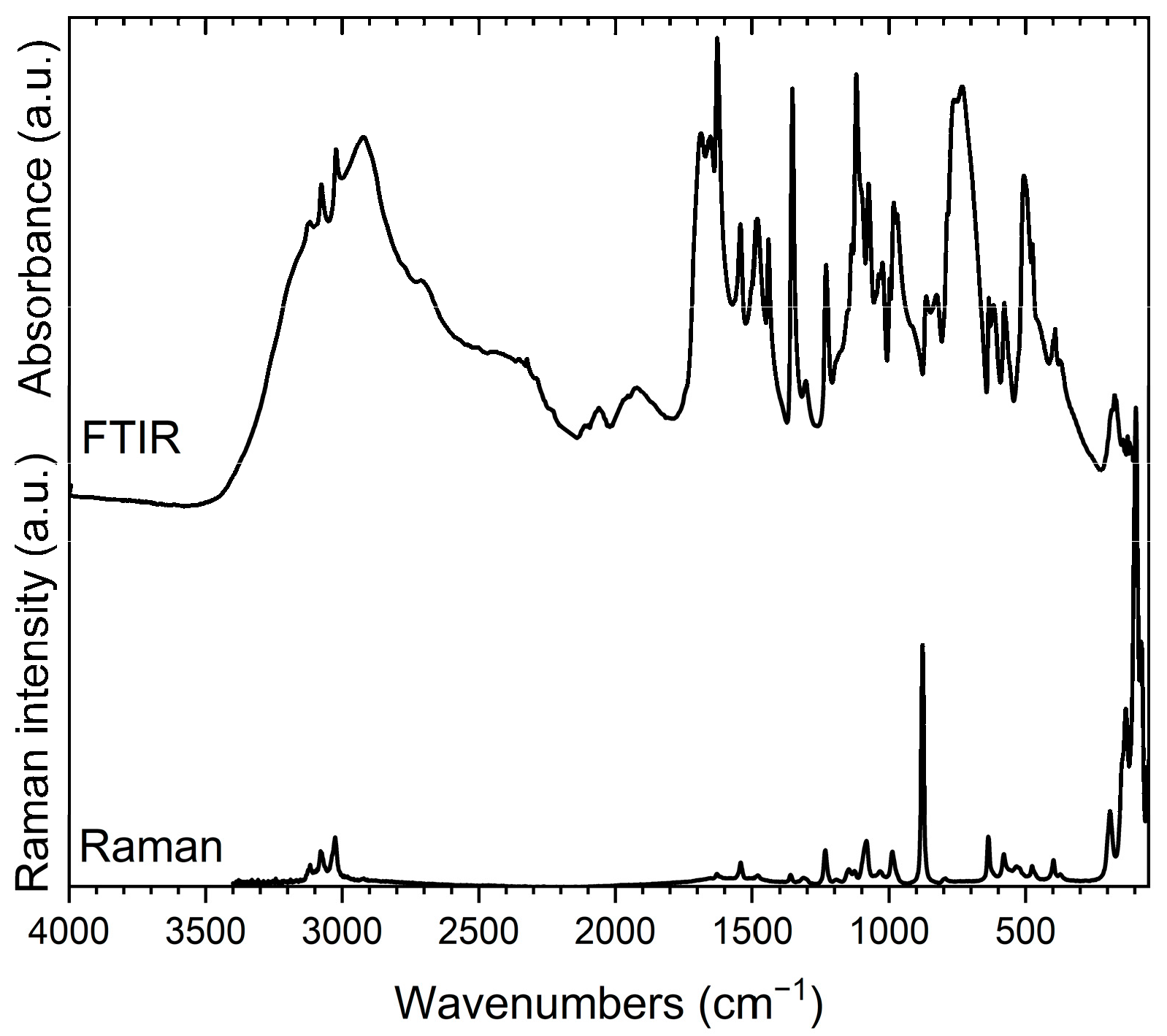
3.2. Vibrational Spectroscopy
3.2.1. Vibrational Bands Associated with 2-Aminopyrimidinium Hydrogen Trioxofluorophosphate, (I)
| FTIR cm−1 | Raman (780 nm) cm−1 | Assignment | FTIR cm−1 | Raman (780 nm) cm−1 | Assignment |
|---|---|---|---|---|---|
| 56 vs | External modes | 1076 s | 1082 s | ρNH2, νrg, δrg | |
| 75 sh | 1118 s | νasPO2 | |||
| 91 s | 1139 m | 1133 m | δCH, νrg | ||
| 114 s | 1173 mb | 1178 w | νasPO2 | ||
| 161 s | 1228 mb | 1228 m | δPOH, δCH, νrg | ||
| 172 s | 1305 wb | 1301 w | δCH, νrg, δNHx | ||
| 198 wb | 193 m | γrg | 1355 s | 1358 w | δCH, νrg, δNH |
| 245 wb | 1407 wb | νC-NH2, νrg, δNHx, δCH | |||
| 362 w | 366 sh | ρPO2 | 1438 m | 1437 vw | |
| 376 w | 375 m | 1455 w | 1457 sh | δCH, νrg, δNHx | |
| 392 w | 395 vw | γrg, γCH, γNHx | 1488 mb | 1470 w | |
| 465 m | 469 m | δCNC | 1511 w | 1512 vw | |
| 495 m | 489 w | δFPO2 | 1544 m | 1541 m | νrg, δCH, δNHx, δNCN |
| 514 m | 506 w | 1575 sh | 1574 vw | ||
| 526 m | δPO2 | 1626 s | 1628 m | νrg, δNHx, δCH | |
| 545 m | 544 m | 1651 mb | 1650 vw | νC-NH2, νrg, δNHx, δrg | |
| 580 m | 583 s | δrg | 1688 sb | 1680 w | |
| 638 m | 638 m | 1748 vw | |||
| 694 mb | γNH, τNH2 | 2430 mb | νO-H(···O), νN-H(···O) | ||
| 755 mb | γN-H(···O) | 2560 mb | |||
| 783 m | 791 wb | γCH, γrg, δCN3 | 2675 mb | ||
| 815 m | 823 w | νPF | 2795 sb | ||
| 860 mb | δNH | 2957 s | |||
| 872 m | 876 vs | νsrg, δsrg, δNH | 2975 s | ||
| 889 sh | 3036 s | 3038 m | νCH, νN-H(···O) | ||
| 955 sb | νsPO2 | 3081 s | 3083 m | ||
| 974 m | νsPO2 | 3121 sh | |||
| 988 m | 985 m | δrg, νrg, δNH | 3140 sh | 3142 s | νN-H(···O) |
| 1000 sh | 3190 s | ||||
| 1022 m | γCH, γrg | 3240 sh | |||
| 1042 m | 3366 sh | ||||
| 1066 m | ρNH2, νrg, δrg |
3.2.2. Vibrational Bands Associated with bis(2-Aminopyrimidinium) Trioxofluorophosphate, (II)
| FTIR cm−1 | Raman (780 nm) cm−1 | Assignment | FTIR cm−1 | Raman (780 nm) cm−1 | Assignment |
|---|---|---|---|---|---|
| 76 s | External modes | 1037 m | γCH, γrg | ||
| 96 vs | 1075 s | 1083 mb | νasPO3, ρNH2, νrg, δrg | ||
| 133 s | 1105 sh | δCH, δNHx | |||
| 145 sh | 1120 s | 1126 m | νasPO3 | ||
| 174 wb | 191 m | γrg | 1137 m | δCH, νrg | |
| 374 w | 373 w | ρPO3 | 1152 sh | 1147 m | νasPO3 |
| 392 sh | 398 m | γrg, γCH, γNHx | 1180 mb | ||
| 400 m | 1231 m | 1234 m | δCH, νrg | ||
| 475 m | 476 m | δFPO3, δCNC | 1305 m | 1314 wb | δCH, νrg, δNHx |
| 507 mb | 528 sh | δPO3 | 1354 s | 1361 w | δCH, νrg, δNH |
| 533 mb | 1442 s | νC-NH2, νrg, δNHx, δCH | |||
| 579 mb | 580 m | δPO3, δrg | 1482 s | 1481 w | δCH, νrg, δNHx |
| 618 mb | δPO3 | 1542 m | 1541 m | νrg, δCH, δNHx, δNCN | |
| 635 m | 637 m | δrg | 1627 s | 1629 w | νrg, δNHx, δCH |
| 732 mb | δFPO3 | 1651 s | νC-NH2, νrg, δNHx, δrg | ||
| 761 mb | γCH, γrg, δCN3 | 1687 m | |||
| 786 sh | 794 w | νPF, γCH | 1925 wb | νN-H(···O) | |
| 826 mb | νPF | 2060 mb | |||
| 864 m | 878 s | νsrg, δsrg, δNH | 2700 mb | ||
| 925 mb | γCH | 2922 sb | |||
| 971 m | δrg, νrg, δNH | 3023 sb | 3026 m | νCH, νN-H(···O) | |
| 983 m | 988 mb | νsPO3 | 3076 s | 3079 m | |
| 998 m | 3118 m | 3118 m | |||
| 1024 sh | 1033 m | νsPO3, γrg, γCH | 3160 sh | νN-H(···O) |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, D.-X.; Lv, Y.-L.; Guo, J.-D.; Gao, W.-Y.; Liu, W.; Tang, R.-L. From (C6H5N2)2CdCl4 to (C6H5N2)2ZnCl4, Chirality Transformation to Realize a Nonlinear Optical Organic-Inorganic Hybrid Halide with Balanced Comprehensive Performance. Inorg. Chem. 2025, 64, 3643–3648. [Google Scholar] [CrossRef]
- Tang, R.L.; Yang, D.X.; Ma, L.; Lv, Y.L.; Liu, W.L.; Guo, S.P. (C6H5N2)HgCl3: Discovery of a Polar Hg-Based Hybrid Halide as Preeminent Nonlinear Optical and Birefringent Material Activated by π-Conjugated Organic Cation Substitution. Adv. Opt. Mater. 2025, 13, 2403044. [Google Scholar] [CrossRef]
- Etter, M.C.; Adsmond, D.A.; Britton, D. 2-Aminopyrimidine Succinic Acid (1/1) Cocrystal. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1990, 46, 933–934. [Google Scholar] [CrossRef]
- Byriel, K.A.; Kennard, C.H.L.; Lynch, D.E.; Smith, G.; Thompson, J.G. Molecular Cocrystals of Carboxylic-Acids. 9. Carboxylic-Acid Interactions with Organic Heterocyclic Bases-the Crystal-Structures of the Adducts of (2,4-Dichlorophenoxy)Acetic Acid with 3-Hydroxypyridine, 2,4,6-Trinitrobenzoic Acid with 2-Aminopyrimidine, and 4-Nitrobenzoic Acid with 3-Amino-1,2,4-Triazole. Aust. J. Chem. 1992, 45, 969–981. [Google Scholar] [CrossRef]
- Lynch, D.E.; Smith, G.; Freney, D.; Byriel, K.A.; Kennard, C.H.L. Molecular Cocrystals of Carboxylic-Acids. 15. Preparation and Characterization of Heterocyclic Base Adducts with a Series of Carboxylic-Acids, and the Crystal-Structures of the Adducts of 2-Aminopyrimidine with 2,6-Dihydroxybenzoic Acid, 4-Aminobenzoic Acid, Phenoxyacetic Acid, (2,4-Dichlorophenoxy)Acetic Acid, (3,4-Dichlorophenoxy)-Acetic Acid and Salicylic-Acid, and 2-Aminopyridine with 2,6-Dihydroxybenzoic Acid. Aust. J. Chem. 1994, 47, 1097–1115. [Google Scholar] [CrossRef]
- Jin, S.; Wang, D. Hydrogen Bonded 3D Supramolecular Architectures of Two Organic Salts Assembled from 2-Aminoheterocyclic Compounds and Phosphoric Acid. J. Chem. Crystallogr. 2012, 42, 276–282. [Google Scholar] [CrossRef]
- Shan, N.; Bond, A.D.; Jones, W. Supramolecular architectures of cyclohexane-1, 3, 5-tricarboxylic acid in acid:base complexes. N. J. Chem. 2003, 27, 365–371. [Google Scholar] [CrossRef]
- Goswami, S.; Mahapatra, A.K. Molecular recognition induced supramolecular array of 2-aminopyrimidine with terephthalic acid, 1,4-phenylenediacetic acid and fumaric acid in solid state H-bonding and π-stacking interactions. Supramol. Chem. 1999, 11, 25–33. [Google Scholar] [CrossRef]
- Mirzaei, M.; Sadeghi, F.; Molcanov, K.; Zareba, J.K.; Gomila, R.M.; Frontera, A. Recurrent Supramolecular Motifs in a Series of Acid Base Adducts Based on Pyridine-2,5-Dicarboxylic Acid-Oxide and Organic Bases: Inter- and Intramolecular Hydrogen Bonding. Cryst. Growth Des. 2020, 20, 1738–1751. [Google Scholar] [CrossRef]
- Thangarasu, S.; Kumar, S.S.; Athimoolam, S.; Sridhar, B.; Bahadur, A.A.; Shanmugam, R.; Thamaraichelvan, A. Synthesis, structure, spectral, thermal analyses and DFT calculation of a hydrogen bonded crystal: 2-Aminopyrimidinium dihydrogenphosphate monohydrate. J. Mol. Struct. 2014, 1074, 107–117. [Google Scholar] [CrossRef]
- Kloda, M.; Matulková, I.; Císařová, I.; Becker, P.; Bohatý, L.; Němec, P.; Gyepes, R.; Němec, I. Cocrystals of 2-Aminopyrimidine with Boric Acid—Crystal Engineering of a Novel Nonlinear Optically (NLO) Active Crystal. Crystals 2019, 9, 403. [Google Scholar] [CrossRef]
- Zhang, Z.-P.; Liu, X.; Liu, X.; Lu, Z.-W.; Sui, X.; Zhen, B.-Y.; Lin, Z.; Chen, L.; Wu, L.-M. Driving nonlinear optical activity with dipolar 2-aminopyrimidinium cation in (C4H6N3)+(H2PO3)−. Chem. Mater. 2022, 34, 1976–1984. [Google Scholar] [CrossRef]
- Matulková, I.; Bohatý, L.; Becker, P.; Císařová, I.; Gyepes, R.; Fridrichová, M.; Kroupa, J.; Němec, P.; Němec, I. Extended study of crystal structures, optical properties and vibrational spectra of polar 2-aminopyrimidinium hydrogen phosphite and three centrosymmetric salts-bis(2-aminopyrimidinium) sulfate monohydrate and two 2-aminopyrimidinium hydrogen sulfate polymorphs. Opt. Mater. 2025, 164, 117033. [Google Scholar] [CrossRef]
- Fábry, J.; Fridrichova, M.; Dušek, M.; Fejfarová, K.; Krupková, R. Mixed crystals of 2-carbamoylguanidinium with hydrogen fluorophosphonate and hydrogen phosphite in the ratios 1:0, 0.76 (2):0.24 (2) and 0.115 (7):0.885 (7). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2012, 68, o76–o83. [Google Scholar] [CrossRef]
- Matulková, I.; Andreoni, R.; Císařová, I.; Němec, I.; Fábry, J. Crystallographic aspects of hydrated salts of 4,6-diaminopyrimidine with the first five dicarboxylic acids. Z. Krist. Cryst. Mater. 2017, 232, 471–484. [Google Scholar] [CrossRef]
- Matulková, I.; Fábry, J.; Eigner, V.; Dušek, M.; Kroupa, J.; Němec, I. Isostructural crystals of bis(guanidinium) trioxofluoro- phosphate/phosphite in the ratio 1/0, 0.716/0.284, 0.501/0.499, 0.268/0.732, 0/1–crystal structures, vibrational spectra and second harmonic generation. Crystals 2022, 12, 1694. [Google Scholar] [CrossRef]
- Lima-de-Faria, J.; Hellner, E.; Liebau, F.; Makovicky, E.; Parthe, E. Nomenclature of Inorganic Structure Types—Report of the International-Union-of-Crystallography Commission-on-Crystallographic-Nomenclature Subcommittee on the Nomenclature of Inorganic Structure Types. Acta Crystallogr. A 1990, 46, 1–11. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, J.; Lu, J.; Pan, C.-Y.; Wu, L.-M. Monofluorophosphates: A New Source of Deep-Ultraviolet Nonlinear Optical Materials. Chem. Mater. 2018, 30, 7823–7830. [Google Scholar] [CrossRef]
- Schülke, U.; Kayser, R. Herstellung von Fluorophosphaten, Difluorophosphaten, Fluorophsophonaten und Fluorophosphiten in fluoridhaltigen Harnstoffschmelzen. Z. Anorg. Allg. Chem. 1991, 600, 221–226. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sevvana, M.; Ruf, M.; Usón, I.; Shedrick, G.M.; Herbst-Irmer, R. Non-merohedral twinning: From minerals to proteins. Acta Crystallogr. D 2019, 75, 1040–1050. [Google Scholar] [CrossRef]
- Bruker. SADABS, Bruker/Siemens Area Detector Absorption Program, version 2014/2; Bruker AXS: Madison, WI, USA, 2005.
- Becker, P.J.; Coppens, P. Extinction within the Limit of Validity of the Darwin Transfer Equations. I. General Formalisms for Primary and Secondary Extinction and Their Application to Spherical Crystals. Acta Crystallogr. A 1974, 30, 129–147. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Bruker. SAINT. Data Reduction and Correction Program, version 8.40B; Bruker AXS Inc.: Madison, WI, USA, 2016.
- Brandenburg, K. Diamond, version 3.2q; Crystal Impact GbR: Bonn, Germany, 1997.
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Struct. Biol. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- OriginPro, version v6.1052; OriginLab Corporation: Northampton, MA, USA, 2015.
- Omnic for Dispersive Raman 8. Omnic for Dispersive Raman 8.2.121, version 8.2; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2010.
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond, Outline of a Comprehensive Hydrogen Bond Theory; Oxford University Press: Oxford, UK; New York, NY, USA, 2009. [Google Scholar]
- Bertolasi, V.; Pretto, L.; Gilli, P.; Ferretti, V.; Gilli, G. Hydrogen-bonded supramolecular structures in co-crystals of β- or ζ-diketone enols with 2,6-diaminopyridine or 2,4-diaminopyrimidine. N. J. Chem. 2002, 26, 1559–1566. [Google Scholar] [CrossRef]
- Matulková, I.; Fábry, J.; Němec, I.; Císařová, I.; Vaněk, P. Migrating hydrogen in 2,4,6-triaminopyrimidinium(1+)x hydrogen trioxofluorophosphate(-)x monohydrate/2,4,6-triaminopyrimidinium(2+)1–x trioxofluorophosphate(2–)1–x monohydrate (0.0 < x < 0.73) with changing temperature. Acta Crystallogr. Sect. B Struct. Sci 2017, 73, 1114–1124. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Fábry, J. A resonance-assisted intra molecular hydrogen bond in compounds containing 2-hydroxy-3,5-di nitro benzoic acid and its various deprotonated forms: Redetermination of several related structures. Acta Crystallogr. E 2018, 74, 1344–1357. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Taylor, R. Organic Fluorine Hardly Ever Accepts Hydrogen Bonds. Chem. Eur. J. 1997, 3, 89–98. [Google Scholar] [CrossRef]
- Shimoni, L.; Glusker, J.P. The Geometry of Intermolecular Interactions in Some Crystalline Fluorine-Containing Organic-Compounds. Struct. Chem. 1994, 5, 383–397. [Google Scholar] [CrossRef]
- Krupková, R.; Fábry, J.; Císarová, I.; Vanek, P. Bis(ammonium) fluorophosphate at room temperature. Acta Crystallogr. Sect. C Struct. Chem. 2002, 58, i66–i68. [Google Scholar] [CrossRef]
- Thakuria, R.; Nath, N.K.; Saha, B.K. The Nature and Applications of π-π Interactions: A Perspective. Cryst. Growth Des. 2019, 19, 523–528. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Weil, M.; Baran, E.J.; Kremer, R.K.; Libowitzky, E. Synthesis, Crystal Structure, and Properties of Mn(PO3F)(H2O)2. Z. Anorg. Allg. Chem. 2015, 641, 184–191. [Google Scholar] [CrossRef]
- Bühler, K.; Bues, W. Schwingungsspektren von Fluorophosphatschmelzen und -kristallen. Z. Anorg. Allg. Chem. 1961, 308, 62–71. [Google Scholar] [CrossRef]
- Weil, M.; Puchberger, M.; Baran, E.J. Preparation and Characterization of Dimercury(I) Monofluorophosphate(V), Hg2PO3F: Crystal Structure, Thermal Behavior, Vibrational Spectra, and Solid-State 31P and 19F NMR Spectra. Inorg. Chem. 2004, 43, 8330–8335. [Google Scholar] [CrossRef]
- Nelson, D.G.A.; Williamson, B.E. Raman Spectra of Phosphate and Monofluorophosphate Ions in Several Dentally-Relevant Materials. Caries Res. 1985, 19, 113–121. [Google Scholar] [CrossRef]
- Rousseau, D.L.; Bauman, R.P.; Porto, S.P.S. Normal Mode Determination in Crystals. J. Raman Spectrosc. 1981, 10, 253–290. [Google Scholar] [CrossRef]
- Novak, A. Hydrogen Bonding in solids Correlation of Spectroscopic and Crystallographic Data. In Large Molecules. Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 1974; Volume 18, pp. 177–216. [Google Scholar] [CrossRef]
- Lautié, A.; Froment, F.; Novak, A. Relationship between NH Stretching Frequencies and N···O Distances of Crystals Containing NH···O Hydrogen-Bonds. Spectrosc. Lett. 1976, 9, 289–299. [Google Scholar] [CrossRef]
- Votrubcová, I. Novel Prospective Materials for Nonlinear Optics. Bachelor’s Thesis, Charles University, Prague, Czech Republic, 2010. [Google Scholar]
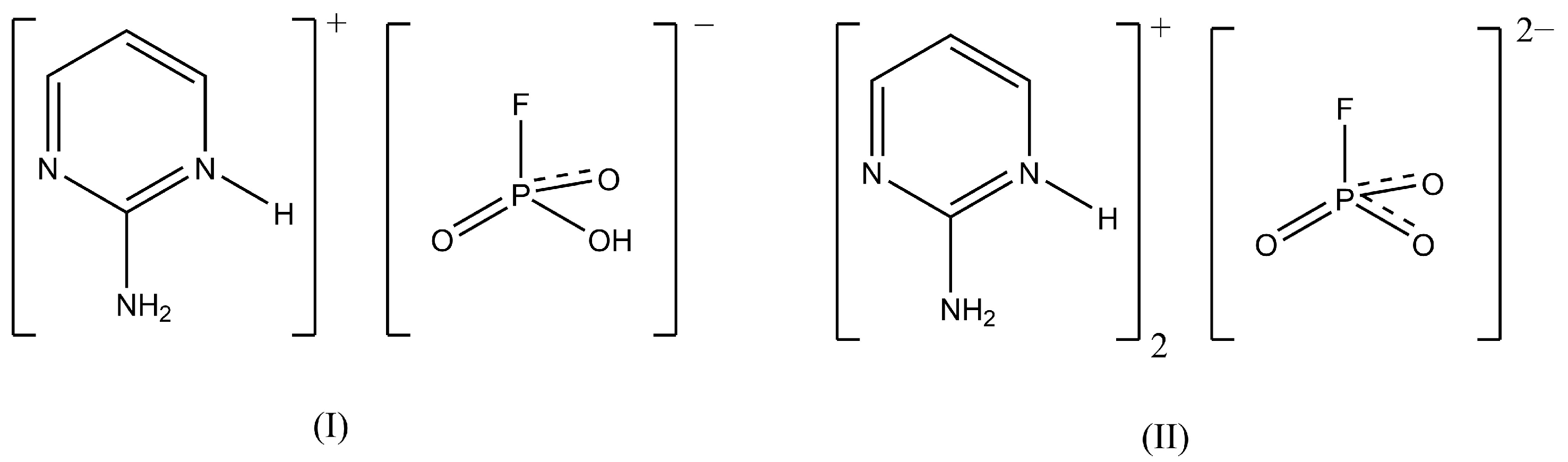
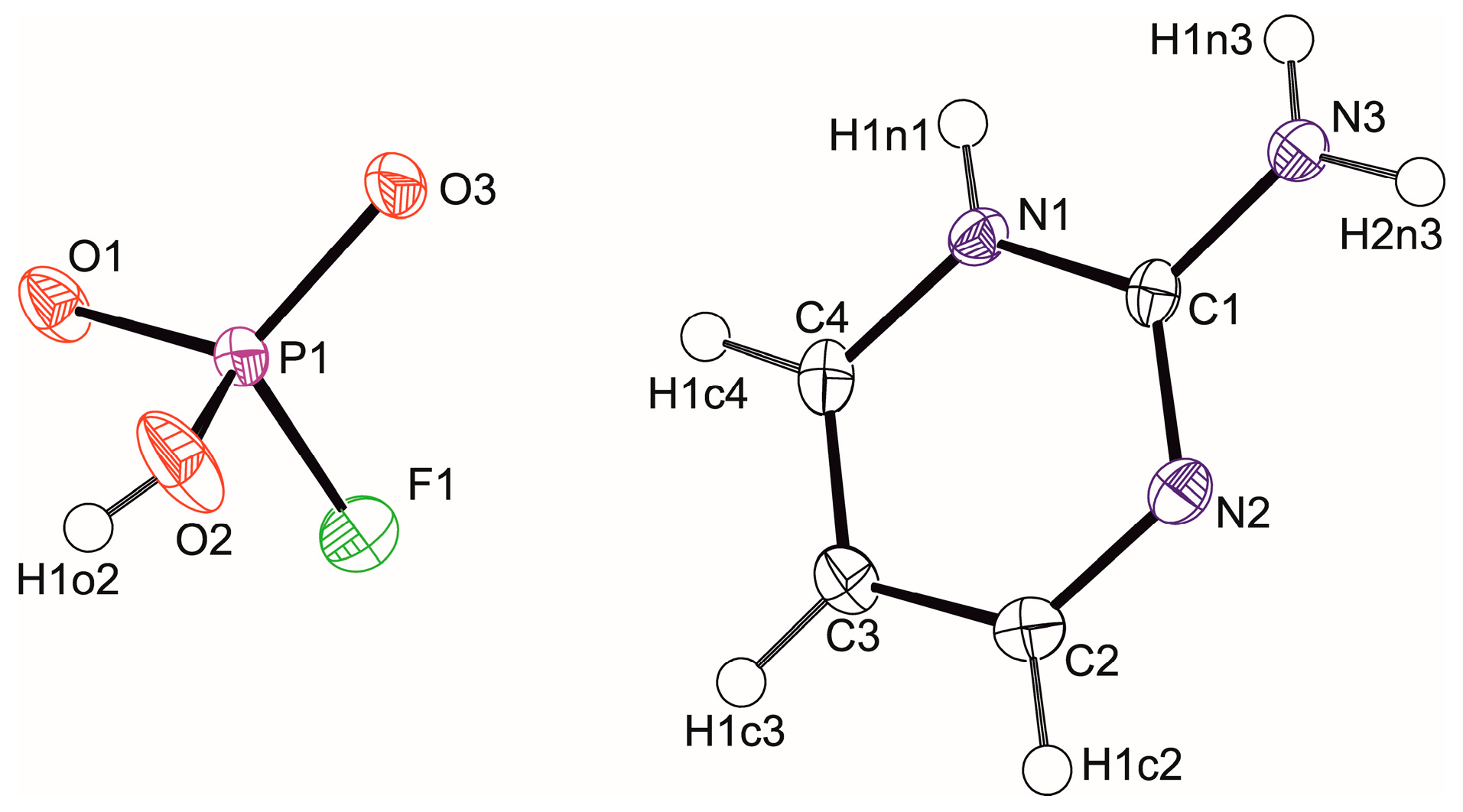
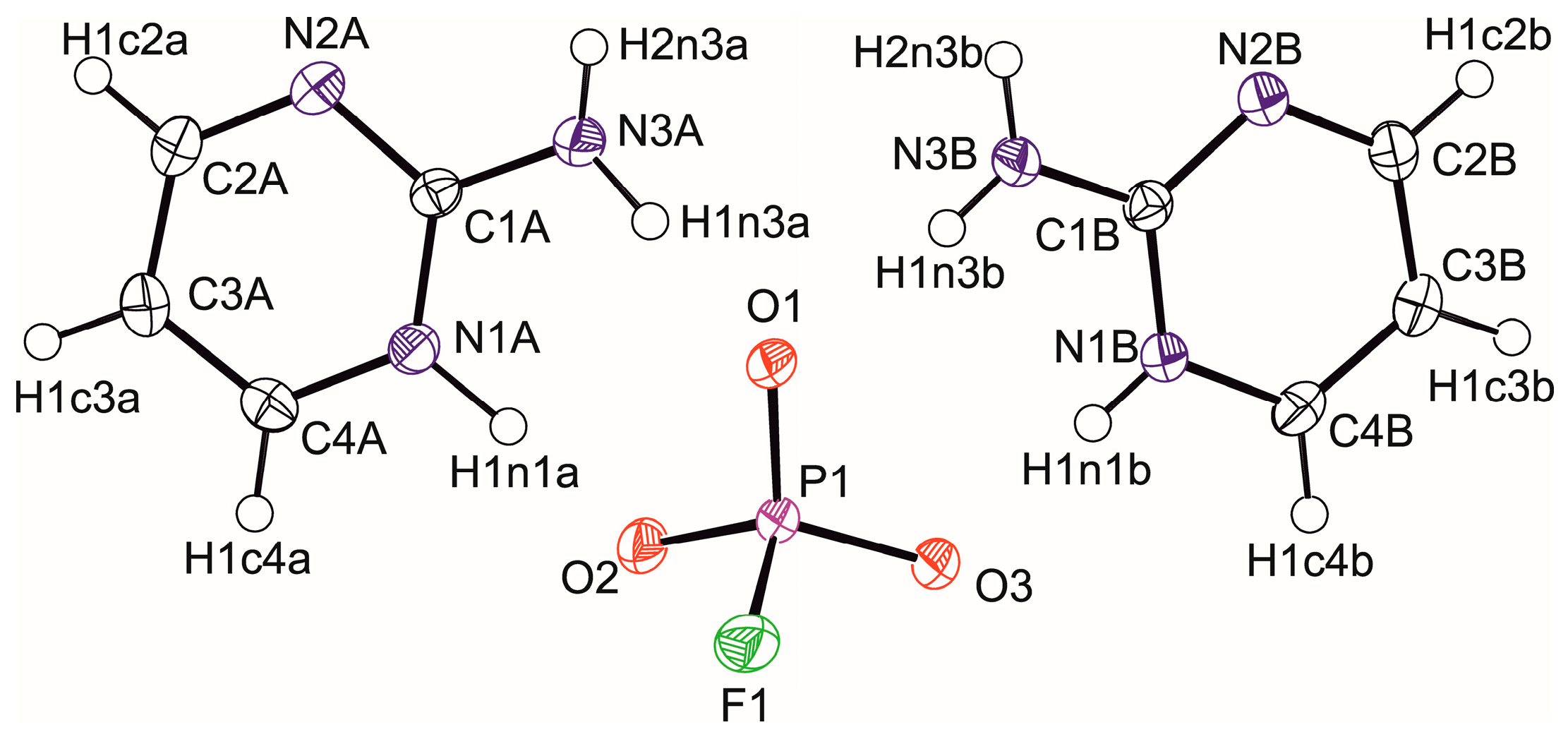

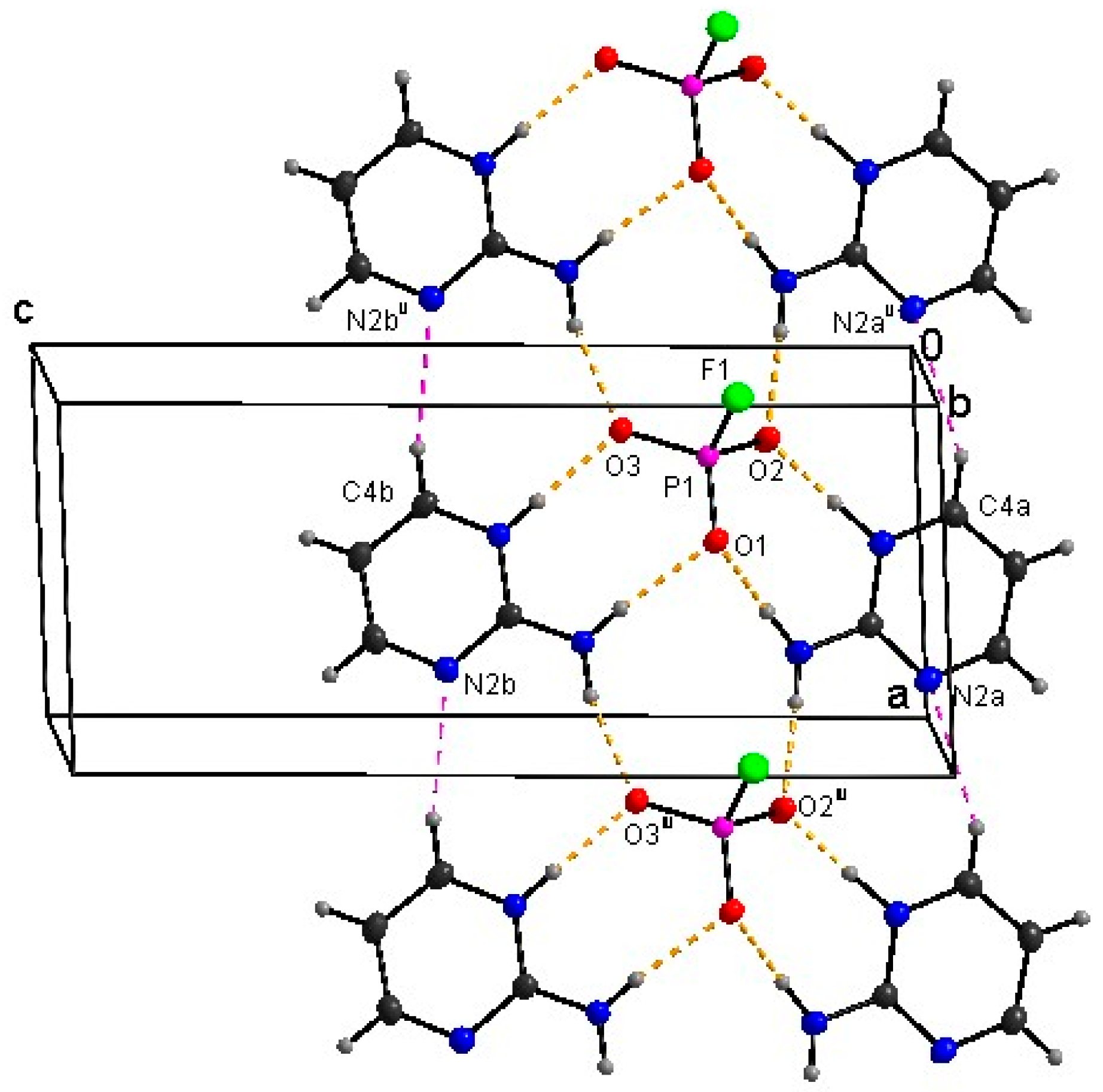
| Chemical Formula | (C4H6N3)+.(HFO3P)− | (C4H6N3)+2·(FO3P)2− |
| Chemical name | 2-aminopyrimidinium hydrogen trioxofluorophosphate, (I) | bis(2-aminopyrimidinium) trioxofluorophosphate, (II) |
| Mr | 195.1 | 290.2 |
| Crystal system, space group | Triclinic, P-1 | Triclinic, P-1 |
| a, b, c (Å) | 6.2794(4), 6.3363(5), 10.1553(7) | 6.2020(4), 7.0417(4), 14.8458(9) |
| α, β, γ (°) | 80.195(3), 73.215(2), 89.695(2) | 79.859(1), 89.627(1), 70.463(1) |
| V (Å3) | 380.78(5) | 600.72(6) |
| Temperature (K) | 120 | 150 |
| Cooling device | Cryostream 800 Long Leg configuration (Oxford Cryosystems, Oxford, UK) | Cryostream 800 Long Leg configuration (Oxford Cryosystems, Oxford, UK) |
| Z | 2 | 2 |
| Radiation type | Mo Kα | Cu Kα |
| sinθfull/λ/sinθmax/λ (Å−1) | 0.600006/0.667476 | 0.617483/0.617483 |
| μ (mm−1) | 3.51 | 2.349 |
| Crystal size (mm) | 0.30 × 0.17 × 0.14 | 0.70 × 0.29 × 0.08 |
| F(000) | 200 | 300 |
| Data collection | ||
| Diffractometer | Bruker D8 Venture | Bruker D8 VENTURE KappaDuo PHOTON 100 CMOS |
| Absorption correction | Multi-Scan [22] | Numerical [23] |
| Tmin, Tmax | 0.9030, 0.9530 | 0.40, 0.83 |
| No. of measured, independent and observed [I > 3σ (I)] reflections | 4242, 2996, 2790 | 11729, 2346, 2154 |
| Rint | 0 | 0.035 |
| Refinement | ||
| R [I > 3σ (I)], wR (I), S | 0.0488, 0.1227, 3.33 | 0.0311, 0.0888, 2.32 |
| No. of reflections | 2996 | 2346 |
| No. of parameters/constraints/restraints | 122/16/0 | 191/30/0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.60, −0.57 | 0.22, −0.30 |
| Extinction Twinning matrix/proportion of the second domain | none [h2 k2 l2] = [h1 k1 l1] · [−1.0005 0.0010 0.0010/ −0.0033 1.0034 −0.0134/ −0.0033 0.5118 −1.0030]/0.363(1) | 4400(500) [B-C type 1 Lorentzian isotropic [24] |
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | D-H···A (°) |
|---|---|---|---|---|
| O2-H1o2···O1i | 0.71(2) | 1.85(2) | 2.5596(16) | 172(3) |
| N1-H1n1···O3ii | 0.76(2) | 1.92(2) | 2.671(2) | 175(2) |
| N3-H1n3···O1ii | 0.75(2) | 2.07(2) | 2.818(2) | 179(2) |
| N3-H2n3···O3iii | 0.91(2) | 1.94(2) | 2.8429(18) | 174.4(19) |
| C4-H1c4···N2iv | 0.95 | 2.63 | 3.558(2) | 164.36 |
| C2-H1c2···F1v | 0.95 | 2.92 | 3.567(2) | 126.40 |
| C2-H1c2···F1vi | 0.95 | 2.81 | 3.388(2) | 119.75 |
| C3-H1c3···F1vi | 0.95 | 2.57 | 3.246(2) | 128.36 |
| C3-H1c3···F1 | 0.95 | 2.71 | 3.163(2) | 110.23 |
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | D-H···A (°) |
|---|---|---|---|---|
| N1a-H1n1a···O2 | 1.026(17) | 1.556(18) | 2.5810(15) | 176.3(18) |
| N3a-H1n3a···O1 | 0.941(17) | 1.847(16) | 2.7779(14) | 169.6(14) |
| N3a-H2n3a···O2iv | 0.88(2) | 1.97(2) | 2.834(2) | 167.2(15) |
| N1b-H1n1b···O3 | 0.906(16) | 1.738(17) | 2.6415(15) | 175.1(16) |
| N3b-H1n3b···O1 | 0.847(17) | 1.910(17) | 2.7536(14) | 174.1(18) |
| N3b-H2n3b···O3iv | 0.90(2) | 1.93(2) | 2.8152(19) | 166.9(14) |
| C4a-H1c4a···N2av | 0.95 | 2.71 | 3.567(2) | 150.22 |
| C4b-H1C4b···N2bv | 0.95 | 2.53 | 3.459(2) | 167.37 |
| C2a-H1c2a···F1vii | 0.95 | 2.67 | 3.4661(17) | 141.96 |
| C3a-H1c3a···F1viii | 0.95 | 2.49 | 3.2519(17) | 136.68 |
| C3b-H1c3b···F1ix | 0.95 | 2.77 | 3.2265(16) | 110.43 |
| C4b-H1c4b···F1ix | 0.95 | 3.05 | 3.3796(15) | 102.17 |
| Code | Symmetry Operation |
|---|---|
| i | 2 − x, 2 − y, −z |
| ii | 1−x, 1−y, 1−z |
| iii | −x, 1−y, 1−z |
| iv | x+1, y, z |
| v | x−1, y, z |
| vi | −x + 1, −y + 1, −z |
| vii | 1−x, 2−y, −z |
| viii | −x, 2−y, −z |
| ix | −x, 2−y, 1−z |
| x | −x, −y, −z+1 |
| xi | x, y−1, z |
| xii xiii | 1 + x, y, z −x+1, −y+2, −z+1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulková, I.; Fábry, J.; Císařová, I. Single Crystal X-Ray Structure Determination and Vibrational Spectroscopy of 2-Aminopyrimidinium Hydrogen Trioxofluorophosphate and bis(2-Aminopyrimidinium) Trioxofluorophosphate. Crystals 2025, 15, 952. https://doi.org/10.3390/cryst15110952
Matulková I, Fábry J, Císařová I. Single Crystal X-Ray Structure Determination and Vibrational Spectroscopy of 2-Aminopyrimidinium Hydrogen Trioxofluorophosphate and bis(2-Aminopyrimidinium) Trioxofluorophosphate. Crystals. 2025; 15(11):952. https://doi.org/10.3390/cryst15110952
Chicago/Turabian StyleMatulková, Irena, Jan Fábry, and Ivana Císařová. 2025. "Single Crystal X-Ray Structure Determination and Vibrational Spectroscopy of 2-Aminopyrimidinium Hydrogen Trioxofluorophosphate and bis(2-Aminopyrimidinium) Trioxofluorophosphate" Crystals 15, no. 11: 952. https://doi.org/10.3390/cryst15110952
APA StyleMatulková, I., Fábry, J., & Císařová, I. (2025). Single Crystal X-Ray Structure Determination and Vibrational Spectroscopy of 2-Aminopyrimidinium Hydrogen Trioxofluorophosphate and bis(2-Aminopyrimidinium) Trioxofluorophosphate. Crystals, 15(11), 952. https://doi.org/10.3390/cryst15110952







