Abstract
Thermoelectric materials enable the direct conversion of heat into electricity, but progress is often limited by challenges in reproducibility and stability. Bi2O2Se has recently attracted attention as a promising candidate; however, reported transport properties of undoped polycrystalline samples vary by several orders of magnitude, complicating its use as a baseline for doping studies. In this work, we investigate the sources of variability and identify key factors including precursor contamination, reactions with quartz ampoules and graphite dies, grain size effects, and surface oxidation. To mitigate these issues, we employed calcination of Bi2O3 precursors, synthesis with controlled temperature gradients, coarse-fraction powders, and hot pressing in Si3N4 dies. The resulting polycrystalline Bi2O2Se exhibits improved reproducibility, reduced sensitivity to thermal cycling, and characteristic transport values around σRT ≈ 500 S·m−1 and S ≈ −300 μV·K−1 at room temperature. This is a good starting point for further doping studies and a prerequisite of thermoelectric efficiency studies in the future, which can reveal the true thermoelectric potential of this material.
1. Introduction
The search for novel low-cost and nontoxic thermoelectric materials with sufficient conversion efficiency is the major focus of thermoelectric materials research. Bi2O2Se has become a widely studied quasi-2D semiconductor with promising transport properties of single crystals, such as exceptionally high electron mobility, and for the ease of formation of Bi2SeO5 insulating layers, which is an important attribute in the fabrication and integration of electronic devices [1,2]. According to the literature, Bi2O2Se in single-crystal form is a well-characterized compound [3]. As a single-crystalline 2D material, it has been established as a photochemical detector [4], field-effect transistor [5] or oxygen detector [6]. In its polycrystalline form, Bi2O2Se has been explored as a potential cost-effective, stable and easy-to-prepare alternative to tellurium-based materials in flexible and wearable thermoelectric electronics [7,8,9]. However, the poor reproducibility of reported properties in Bi2O2Se—particularly in the polycrystalline form—remains a significant obstacle. Inconsistencies in the reported properties of supposedly identical pure materials have been the motivation for this work.
Over the past decade, most of the research has focused on the optimization of thermoelectric parameters through doping, namely electrical conductivity, the Seebeck coefficient, and thermal conductivity [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. The primary motivation for doping was to improve the low electrical conductivity of pure Bi2O2Se. However, doping frequently induces the formation of foreign phases (FPs), which complicates the material’s stability and performance. This problem led to an alternative approach, which involves doping by the intentional addition of FPs to the pure Bi2O2Se phase. In this context, several composite designs have been proposed [15,16,28,29]. Additionally, studies have explored the correlation between the nonstoichiometry of individual elements and the material’s transport and thermoelectric properties [30,31,32,33]. The purpose of stoichiometric variation was to induce the formation of corresponding native defects. However, since the resulting concentration of native defects and thus the nonstoichiometry are much lower than expected, these materials often contain compositionally related phases such as Bi2O3, Bi2Se3, and Bi2SeO5.
As a result, many reported polycrystalline samples lack true phase purity, complicating the investigation of the intrinsic properties of polycrystalline Bi2O2Se. While X-ray diffraction is typically used to detect FPs, the identification of very thin 2D inclusions within the 2D matrix of Bi2O2Se is challenging because of their low concentration, small size, and limited coherence length, further shown in Supplementary Materials, Section S6. This leads to the potential misinterpretation of the results as if they were for a phase pure material.
Indeed, our review of the literature highlights two major inconsistencies: (1) electrical conductivity of undoped polycrystals varies by four orders of magnitude, with some studies reporting metallic behavior [10,12,15,16,17,18,19,21,23,24] while others find semiconducting characteristics [17,28,34,35]; and (2) there is almost no data on thermal stability under repeated heating cycles, despite its clear relevance for high-temperature applications.
The aim of this work is therefore to systematically analyze the changes in transport properties of undoped Bi2O2Se in relation to synthesis and compaction conditions. We identify key factors—including precursor contamination, ampoule reactions, surface oxidation, grain size, and pressing-induced reduction—that contribute to the poor reproducibility observed in the literature. We propose a modified processing strategy to mitigate these issues and improve reproducibility and stability under thermal cycling. This paves the way for further doping studies that will not be affected by impurities caused by precursor contamination, ampoule reactions, surface oxidation or reduction induced by the pressing die. Using this new method of sample preparation, we have successfully produced Mn-doped samples exhibiting p-type conductivity for the first time [36].
2. Materials and Methods
Bi2O2Se samples were prepared via both high- and low-temperature synthesis. Bismuth chunks (Sigma Aldrich, Burlington, MA, USA, 5 N) were ground in an MM500 nano oscillating mill (Retsch, Haan, Germany) at 30 Hz for 20 min, selenium shards (Sigma Aldrich, Burlington, MA, USA, 5 N) were ground in an agate mortar, and Bi2O3 powder (Alfa Aesar, Haverhill, MA, USA, 5 N) was calcined (decarbonized) at 450 °C for 30 min in an argon flow and subsequently rapidly cooled to remove traces of Bi2O2CO3. These powders were mixed in a stoichiometric ratio in an agate mortar and sealed in a quartz ampoule under a pressure of <10−3 Pa.
The first set of samples was synthesized via a low-temperature method, with a ramp of 1 °C/min to 400 °C, maintained for 10 days, followed by free cooling. To suppress the reaction between Bi2O3 and SiO2, the surface of the ampoules was crystallized at 1000 °C for 48 h to improve chemical resistance and then cleaned with aqua regia, followed by deionized water, before use. To prevent agglomeration and ensure a homogeneous solid-state reaction, the ampoules were rotated in the tube furnace at approximately 1 rpm.
For comparison with synthesis methods from the literature, a second set of samples was synthesized via a common high-temperature method, with a ramp of 1 °C/min to 300 °C, maintained for 1 day, followed by a ramp of 1 °C/min to 700 °C, and maintained for 10 days, followed by free cooling. As in the low-temperature method, the ampoules were rotated in the furnace (Clasic, Řevnice, Czech Republic).
To further investigate the effect of particle size on the properties of polycrystalline Bi2O2Se, a coarse-grained powder was prepared. This was achieved by synthesizing Bi2O2Se via the low-temperature method, followed by crystal growth in a temperature gradient of 860 °C to 800 °C maintained for 14 days. This process led to the formation of visible crystals, some of which reached sizes up to 1 cm. The crystals were sieved to obtain particle-size fractions for subsequent pressing.
The phase purity of the samples was checked by powder X-ray diffraction (PXRD) (Cu Kα, λ = 1.5418 Å) on a D8 ADVANCE DAVINCI diffractometer (Bruker AXS, Karlsruhe, Germany) with a Bragg–Brentano Θ-Θ goniometer (radius 280 mm) equipped with a LynxEye XE-T detector. Scanning was performed at room temperature from 5° to 90° (2Θ) in 0.01° steps with a counting time of 1.5 s (total step time 288 s).
The thermal properties of powdered Bi2O2Se were studied with an STA 449 F5 Jupiter apparatus (Netzsch, Selb, Germany) operating in the DTA mode. Measurements were performed at a heating rate of 5 °C/min on powder samples (250 mg) placed in a platinum crucible with a silica insert and heated under a flowing N2 atmosphere.
To compact the powders, both spark plasma sintering (SPS) and hot pressing (HP) were used for comparison. Hot press samples were prepared using an HP apparatus (HP 20-4560, Thermal Technology, Minden, NV, USA). Exactly 2 g of powder was poured into either a graphite die or a silicon nitride (Si3N4) die (MTI Corp., Richmond, CA, USA) with an inner diameter of 12 mm under an Ar atmosphere. The temperature and pressure gradually increased for 45 min until reaching the target temperatures of 580 °C, 620 °C, or 730 °C, and pressure of 70 MPa. The temperature and pressure were maintained for 1 h for normal powders and 3 h for coarse fraction powders, followed by rapid pressure release. The samples were allowed to cool freely inside the press for approximately 3 h. The density of all the samples was calculated via volumetric method. The density of the pressed tablets ranged from 95 to 100% of the theoretical density, with a median 97%.
Spark plasma sintered samples were prepared using SPS apparatus (HP D 10-SD, FCT Systeme, Effelder-Rauenstein, Germany). Exactly 5 g of powder was poured into a graphite die with an inner diameter of 20 mm lined with graphite paper. The temperature was increased to 580, 650 or 730 °C at 100 °C/min, followed by a dwell time of 5 min. Cooling was maintained at 50 °C/min. A pressure of 70 MPa was applied continually from 300 °C to the maximum temperature, and released within 1 min during cooling. All the experiments were performed in vacuum. The temperature was measured by an optical pyrometer in a non-through hole in the top piston (5 mm from the sample surface). The density of the pressed tablets ranged from 87% to 95% of the theoretical density.
The circular tablets obtained were cut into rectangular shapes (approximately 10 × 3 × 2 mm3) for the measurement of transport properties using a custom-built, solvent-free diamond wire cutter. Final polishing was performed on a diamond grinding wheel to achieve parallel-sided rectangular samples.
The electrical conductivity σ was measured via the four-terminal method using an LSR-3 instrument (Linseis, Selb, Germany) over a temperature range from 300 K to 780 K on round or rectangular hot-pressed samples. The Seebeck coefficient S was measured using the static DC method on the LSR-3, with electrical conductivity measured simultaneously. The measurements were performed in a He atmosphere at an overpressure of 0.1 bar. The Seebeck coefficient was measured with an accuracy of ±7% and repeatability of ±3.5%. Electrical conductivity was measured with an accuracy of ±10% and a repeatability of ±5%. All the transport properties were measured perpendicular to the sintering pressing direction.
The microstructure analysis was performed using a LYRA 3 GMH scanning electron microscope (SEM) (Tescan, Brno, Czech Republic). Prior to analysis, one set of samples was finely polished to a 5000-grit finish, etched in 20% nitric acid for approximately 10 s and neutralized in a diluted ammonia solution. The second set of samples was left unpolished and unetched. Finally, the surfaces of all the samples were cleaned by brief sonication (≈10 s) in an ethanol and ammonia solution (80/20 by volume), rinsed in deionized water and air-dried. The sample composition was analyzed with energy-dispersive spectroscopy (EDS) Aztec X-Max 20 system at 5 kV (Oxford Instruments, Abingdon, UK).
3. Results and Discussion
A critical review of the literature reveals significant inconsistencies in the reported properties of Bi2O2Se.
Reported electrical conductivity values for undoped polycrystalline Bi2O2Se span more than four orders of magnitude. Most studies describe metallic behavior [10,12,15,16,17,18,19,21,23,24], whereas others report semiconducting characteristics [17,28,34,35]. This spread can be attributed to variations in stoichiometry and the presence of foreign phases (FPs) (Figure 1). The pronounced effect of nonstoichiometry on both conductivity and Seebeck coefficient is illustrated in Supplementary Materials Figure S2. Since the number of FPs often increases with doping (Table S6), separating the effects of dopants from those caused by stoichiometry and secondary phases is difficult. The issue of FPs complicating transport analysis was first noted in 2018 [24]. Also, systematic data on the thermal stability of Bi2O2Se under cycling are lacking for both undoped and doped materials, despite its relevance for high-temperature applications. In our experiments, polycrystalline samples displayed marked changes in transport properties after repeated thermal cycling (Figures 5 and 6). These changes likely arise from the combined effects of grain boundaries, foreign phases, and the intrinsic quality of individual grains, which is governed by native point defects.
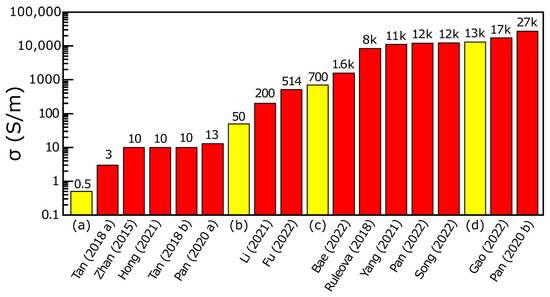
Figure 1.
Comparison of the room temperature electrical conductivity σ of undoped polycrystalline materials in various doping studies. The red columns represent literature data of undoped materials prepared by solid-state reactions at high temperature. The yellow columns represent our measured data from a batch of synthesized Bi2O2Se with the presented low-temperature method hot-pressed at 730 °C and 70 MPa ((a) <35 μm fraction, Si3N4 die; (b) all fractions, Si3N4 die; (c) 35–340 μm fraction, Si3N4 die; (d) all fractions, graphite die). The numbers above the columns are approximate values of room temperature electrical conductivity (S·m−1), and the numbers below the columns are citation numbers. The preparation methods used in the cited studies are listed in Table S7. The ‘k’ in the labels denotes ×103. The electrical conductivities of the prepared SPS samples are given in the Supplementary Materials Figures S8 and S9 [10,11,12,13,14,16,17,18,19,24,25,27,29,35].
In our study we focused on and identified several factors influencing reproducibility, including precursor contamination, reactions with ampoule material, surface-driven phase formation, grain size effects, and pressing-induced reduction (Figure 2).
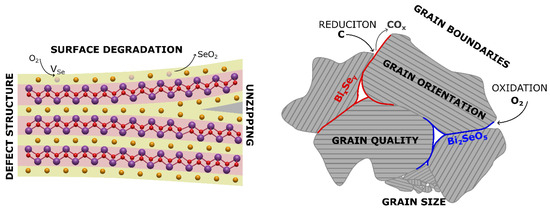
Figure 2.
Schematics of the Bi2O2Se crystal structure (left) and grains in the polycrystalline sample (right) showing various processes influencing the properties of the prepared polycrystalline samples. Yellow atoms represent selenium, red atoms oxygen and purple atoms bismuth.
3.1. Difficulties Common to Both Undoped and Doped Bi2O2Se
The commonly used high-temperature synthesis procedures involve several critical challenges that are likely major contributors to the difficulty in reproducibility of transport properties reported in the literature. The challenges outlined apply to both doped and undoped Bi2O2Se-based materials.
- Purity of precursors and their reactivity with SiO2 during synthesis [24,37,38].
- Air stability of the as-synthesized Bi2O2Se powder [6].
- Reaction with die during compacting.
- Equilibrium reactions between the matrix and foreign phases—grain boundaries—stoichiometry.
Other related problems arise in the context of doping. These are beyond the scope of this article but are addressed to some extent in Section 3.2.
3.1.1. Purity of the Precursors and Their Reactivity with SiO2 During Synthesis
Commercial high-purity (99.999%) Bi2O3 stored under ambient conditions often contains significant amounts of impurities, reducing the stoichiometric Bi content. The most significant impurity, Bi2O2CO3, forms readily in air at room temperature [37], leading to a shift in the stoichiometry of precursors if not accounted for. Calcination (decarbonation) at elevated temperatures to eliminate carbonate content is commonly noted in Bi2O3 studies but is mostly omitted in Bi2O2Se studies. The reported decomposition temperature of Bi2O2CO3 to β- and α- Bi2O3 varies significantly, but a temperature of 450 °C is generally sufficient [38]. To minimize the amount of impurities, Bi2O3 calcination is recommended immediately prior to the synthesis of Bi2O2Se [39].
Polycrystalline Bi2O2Se is commonly synthesized from elemental or binary precursors via solid-phase reactions at high temperatures ranging from 600 °C to 800 °C. At these temperatures, finding a chemically inert material that can fully withstand this specific combination of compounds is challenging. Bi2O3 strongly corrodes most ceramic and glass materials, whereas selenium and bismuth readily react with most metals that can be used as reaction vessels instead of quartz. To investigate the reactions between precursors (Se, Bi, and Bi2O3) and the quartz glass ampoule wall, a series of polycrystalline batches were synthesized at various temperatures (400 °C, 730 °C, and 860 °C). The observed reaction can be summarized as
3x SiO2 + 2/3·Bi2O3 + 2/3·Bi +·Se → x Bi4(SiO4)3 + Bi2−4xO2−6xSe
As shown in Figure 3, Bi4(SiO4)3 covers the inner wall of the quartz ampoule. This reaction shifts the stoichiometry toward Se-rich, O- and Bi-poor composition. This conclusion is also supported by the presence of both Bi4(SiO4)3 and Bi2Se3 detected by XRD on the inner wall of the ampoule. Interestingly, most of the synthesized powders show a single-phase diffraction pattern.
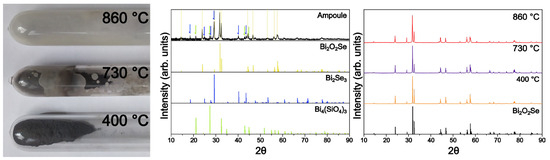
Figure 3.
Reaction of Bi2O2Se with SiO2 ampoules. The photo on the left shows the temperature dependence of the reaction rate of Bi2O3 with SiO2 at 400 °C, 730 °C, and 860 °C. In the middle, the XRD pattern of the inner wall of the ampoules shows Bi2SeO5 formed on the ampoule surface. The right XRD pattern shows how products prepared at various temperatures can appear to be XRD pure independent of the reaction with the ampoules and adequately shifted stoichiometry.
The reaction between Bi2O3 and quartz glass can be reduced by the surface crystallization of quartz through high-temperature annealing (1000 °C for 48 h). A thin layer of crystalline quartz is more resistant to reactions with Bi2O3, leading to the slower formation of Bi4(SiO4)3. Furthermore, the reaction of precursors with quartz glass is much faster when these precursors are in the molten state, which limits the optimal synthesis to a solid-phase reaction [40,41]. Due to these reactions, a temperature of approximately 400 °C with a prolonged synthesis time and well-mixed powders (to avoid the presence of unreacted Bi2O3 and Bi2Se3 in the product) is the preferred synthesis method.
3.1.2. Air Stability of the As-Synthesized Bi2O2Se Powder
During our experiments, as-synthesized pure polycrystalline Bi2O2Se powder handled and stored exclusively in a vacuum or inert argon atmosphere prior to hot pressing in a Si3N4 die could not be effectively densified, even at elevated temperatures (730 °C, 3 h, 70 MPa). The resulting pellets often fail to densify entirely or have a lower density (<95%) than those sintered from identical batches stored in ambient air. This difference is probably caused by the adsorption of atmospheric oxygen on the particle surfaces, resulting in the formation of an amorphous surface phase driven by electrostatic dipoles on the mosaic surface [6]. Notably, the estimated average adsorption energy (≈3.7 eV per O2 molecule) is very high and comparable to the reaction enthalpies of common chemical reactions, such as carbon combustion, rather than the adsorption energy. This issue was not observed during pressing in the graphite die (see Section 3.1.3 below).
As the surface Se layer vacancies fill with oxygen, the properties of the bulk material beneath it change, leading to a measurable decrease in electrical conductivity. This extreme sensitivity of Bi2O2Se nanoplatelets to the ppm level of O2 has been used for Bi2O2Se ppm oxygen detectors [6]. Oxidation has been reported for very thin (10–100 nm) and small (≈20 × 20 μm2) single crystals but is difficult to study in bulk polycrystalline materials. In these materials, the amount of surface-adsorbed oxygen varies depending on the duration of exposure, temperature, and particle morphology and size, which are factors that are difficult to control simultaneously.
The data and findings in ref. [6] are inconsistent with the general perception of Bi2O2Se stability in the single-crystal form [5,41]. For a very thin sample (≈10 nm), the resistivity increased by two orders of magnitude after 10 min of exposure to air. While the cationic layer of Bi2O2 is extremely stable, the anionic part (S, Se, Te) is easily replaced by other structures, such as Bi2O2(OH)(NO3) [42], Bi2O2NCN [43] or similar structures such as Bi2O2CO3 [44]. These surface reactions cause the formation of foreign phases at the grain boundaries during compaction. This modifies the transport properties of polycrystalline Bi2O2Se in several ways, such as through changes in the concentration of point defects, modulation doping, or simply through intergrain carrier scattering, all of which depend on the nature of the foreign phase [45]. The use of coarse particle fractions improves reproducibility by reducing the surface-to-volume ratio and helping optimal grain orientation, as discussed in Section 3.1.4 below.
3.1.3. Reaction with Die During Compacting
Compacting synthesized powders in a graphite die is possible at lower temperatures (e.g., 580 °C) compared with dies made of other materials (e.g., ZrO2 and Si3N4). This is because Bi2O2Se is reduced by carbon at temperatures above ≈400 °C, as shown in Figures S3 and S4, forming a BixSey phase that melts at a lower temperature and binds Bi2O2Se powder particles together. The reaction between the graphite die and compacted powder can be summarized as [46]
3 Bi2O2Se + 3 C → Bi4Se3 + 2 Bi + 3 CO2 (ΔH = +110 kJ (+1.14 eV)/reaction).
This chemical reduction is endothermic and takes place only at higher temperatures. However, the diffusion of CO2 out of the press chamber shifts the reaction equilibrium toward the products.
In Figure 4, the DTA (TG) measurement shows a comparison of the reactions of pure sample and a sample mixed with carbon at a stoichiometric ratio. In the pure sample, the reaction with the quartz crucible starts gradually at approximately 500 °C, with the reaction rate increasing at higher temperatures. The slight mass loss observed can be attributed to the surface evaporation of selenium. The sample mixed with carbon begins to experience a mass loss above 300 °C. This is caused by the surface reduction of the Bi2O2Se particles and the formation of BixSey on the surface. This BixSey layer then acts as a protective barrier, shielding the interior of the particles from further reduction. Above 600 °C, the BixSey surface phase melts, allowing further reduction of the particle. With a stoichiometric ratio of Bi2O2Se:C of 1:1, a complete reduction to BixSey phases is observed. The total mass loss of 6% at 800 °C corresponds to the total oxygen content in Bi2O2Se. Notably, for the full reduction of 2 g of Bi2O2Se (typical weight of compacted powder) to BixSey, only 0.045 g of carbon is needed. After hot-pressing the powder at 730 °C in a graphite die, small metallic Bi-Se spheres squeezed out of the die were observed, with the resulting pressed pellet reaching close to 100% theoretical density of Bi2O2Se (Figure S4b and Table S2). This suggests that the presence of foreign phases facilitates the compaction process of the Bi2O2Se particles by acting as a lubricant/wetting agent at the grain boundaries. BixSey phases were also observed on the unpolished surfaces of the SPS samples across all tested temperatures, with their content increasing with temperature.
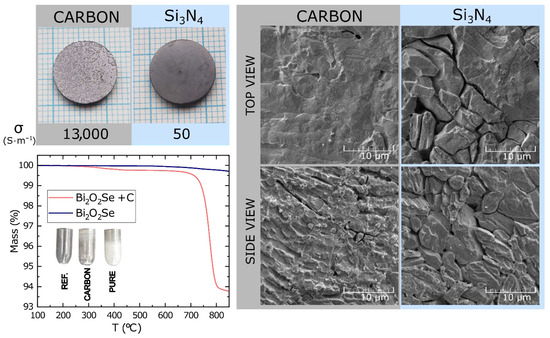
Figure 4.
Comparison of optical images, SEM micrographs, thermogravimetry results, and electrical conductivities of pellets hot-pressed at 730 °C in C (graphite) and Si3N4 dies. The SEM images of polished and etched samples pressed in a graphite die show increased BixSey content on the grain surfaces, which is responsible for an increase in electrical conductivity. In contrast, no chemical reduction of Bi2O2Se occurs in a Si3N4 die, resulting in mere particle compaction with no BixSey phase wetting the grain surface. This results in low electrical conductivity even in high-density samples. (A more detailed of the temperature dependence of C reduction is presented in Figure S4a). The onset temperatures of the ongoing reactions are evident in the thermogravimetric measurements.
For good grain contact, the mentioned BixSey phases appear to enhance intergranular charge transport [13,21,29,33]. However, this enhancement comes at the cost of altering the chemical identity of the sample and is also likely related to the instability of the transport properties during thermal cycling as suggested in Section 3.1.4. The presence of these secondary phases may also result in misleading conclusions regarding the effects of doping.
3.1.4. Equilibrium Reactions Between the Matrix and Foreign Phases—Grain Boundaries—Stoichiometry
The issues described in Section 3.1.1, Section 3.1.2 and Section 3.1.3 likely result in a shift in stoichiometry and the formation of foreign phases, which may further react with the matrix during thermal cycling. The eventual change in composition depends on the temperature and its rate of change in terms of reaction kinetics and appears to be the key contributor to the phase instability of the bulk polycrystalline material under thermal cycling. While most of these obstacles are directly solvable, the reaction with ambient oxygen is difficult to address. During compaction at elevated temperatures, the oxidized surface of the powder under ambient conditions can also react with the particle core [46]
3 Bi2O2Se + 2 SeO2 ⇌ 2 Bi2SeO5 + Bi2Se3 (ΔH = −138 kJ (−1.43 eV)/reaction),
2 Bi2O2Se + 3 SeO2 ⇌ 2 Bi2SeO5 + 3 Se (ΔH = −133 kJ (−1.38 eV)/reaction).
If CO2 and SeO2 are present, the second reaction will apply, shifting equilibria in favor of the products [46]
3 Bi2O2Se + SeO2 + CO2 ⇌ Bi2SeO5 + Bi2Se3 + Bi2O2CO3 (ΔH = −118 kJ (−1.22 eV)/reaction).
Other possible reactions are listed in the Supplementary Materials, Section S5. These reactions are associated with relatively low reaction enthalpies < 0.4 eV per Bi2O2Se formula unit, suggesting that the equilibrium of these reactions can be significantly shifted with small changes in temperature or composition [47]. This tendency for surface driven reaction/decomposition appears to be a property of Bi2O2Se, inevitably leading to the formation of foreign phases at the grain boundaries. Grain boundaries in conjunction with foreign phases play a critical role in the properties of polycrystalline Bi2O2Se and its stability during thermal cycling (see Figure 5 and Figure 6). One reasonable way to mitigate the effect of the particle surface/grain boundary is to reduce the total surface area of the particles used for compaction, thereby reducing the amount of oxygen adsorbed. This can be achieved by using coarse-grained, well-crystallized, low-morphology material for compaction, that is, as-grown, uncrushed material.
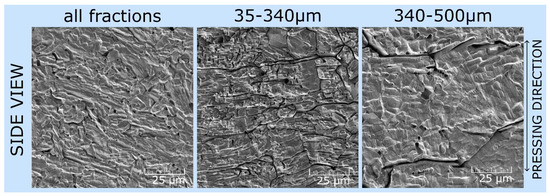
Figure 5.
SEM images (100 × 100 μm2) of samples pressed in a Si3N4 die with various particle size fractions. The images show that using a larger particle size fraction allows for better grain alignment within the sample, potentially improving the structural orientation of the material. In contrast, smaller particle sizes result in a more compact sample, which helps prevent the formation of intergranular voids. We also observed a correlation between particle size and electrical conductivity (Figure 1). The samples pressed from larger particles had a higher in-plane conductivity (perpendicular to the pressing direction), whereas those pressed from smaller particles had a lower conductivity. This reduction is likely due to the increased number of grain boundaries and suboptimal grain orientations.
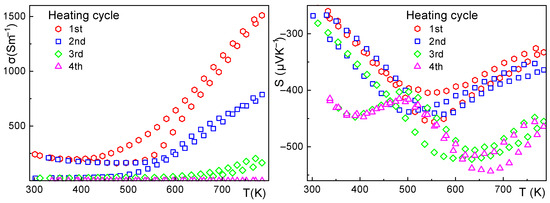
Figure 6.
Sample 1—electrical conductivity (left), Seebeck coefficient (right), and the effect of thermal cycling up to 500 °C. The electrical conductivity shows significant hysteresis during cycling, lowering the electrical conductivity by two orders of magnitude with subsequent cycling. The Seebeck coefficient also shows hysteresis with a complex curve for evaluation.
The growth step in single-crystal (SC) synthesis requires a considerable amount of time. In this regard, the surface crystallization of quartz is quite beneficial as it improves the ampoule chemical resistance. Since the reaction of Bi2O2Se with SiO2 is slower than that with Bi2O3, performing a low-temperature synthesis step before the high-temperature growth of SC is recommended. At high temperatures of approximately 860 °C, the presence of a temperature gradient around 50 °C ensures the separation of the Se, Bi2Se3 and Bi2SeO5 phases on the cooler side of the ampoule, serving as a self-purification process. This results in the formation of pure well-crystallized Bi2O2Se material on the warmer side of the gradient. The main problem is the formation of a fine powder of Bi4(SiO4)3 on the ampoule walls (see Figure S1). Owing to the low vapor pressure, Bi4(SiO4)3 is not transported to the colder side of the ampoule and remains with Bi2O2Se. However, it is a fine powder that can be largely removed by sieving (Table S1).
This high-temperature step produces both a few large single crystals and a significant amount of smaller crystals or crystal clusters, which can be effectively sieved into defined particle fractions suitable for compaction. The use of these well-crystallized coarse particle fractions minimizes the surface area for adsorption, leading to a lower amount of foreign phases and promoting grain orientation during compaction (Figure 5). The samples prepared from these fractions exhibit greater reproducibility of transport properties and improved resistance to thermal cycling (Figure 7 and Figure 8). Notably, for 2D materials, particle size selection by sieving is inherently suboptimal due to anisotropic shape and the frequent occurrence of intergrown crystallite clusters.
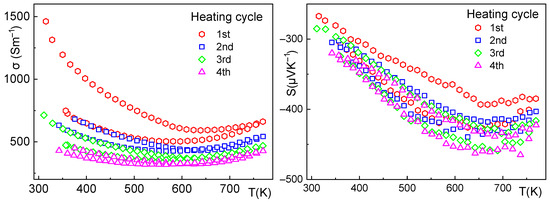
Figure 7.
Sample 2—electrical conductivity (left), Seebeck coefficient (right), and the effect of thermal cycling up to 500 °C. The electrical conductivity shows hysteresis during the first cycle, stabilizing after the third cycle at approximately 500 S·m−1 at 300 K. The Seebeck coefficient also shows slight hysteresis during the first cycle.
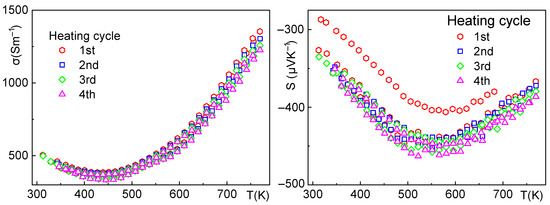
Figure 8.
Sample 3—electrical conductivity (left), Seebeck coefficient (right), and the effect of thermal cycling up to 500 °C. The electrical conductivity does not show any hysteresis with increasing cycle count. Similarly to sample 2, the Seebeck coefficient shows hysteresis during the first cycle, but then stabilizes.
3.2. Summary and Results of the Improved Synthesis Method
To mitigate some of the most persistent synthesis-related problems, we propose a synthesis procedure that improves the reproducibility of transport properties and enhances the thermal cycling stability of polycrystalline Bi2O2Se. While these steps reduce certain sources of variability, they do not fully eliminate specific challenges associated with pure Bi2O2Se.
3.2.1. Low-Temperature Synthesis of Pure Bi2O2Se
Decarbonation of Bi2O3 at 450 °C under Ar flow for 30 min prior to synthesis to minimize the content of Bi2O2CO3 and thus maintain the correct stoichiometry. Low-temperature synthesis of homogenized fine powder precursors was performed at 400 °C in a crystallized SiO2 ampoule to minimize the reaction of Bi2O3 with SiO2 to Bi4(SiO4)3, which also leads to a stoichiometric change. Rotation of the ampoule during synthesis likely accelerates the reaction. We assume that this is due to gas phase reaction kinetics (this assumption is supported by how large single crystals grow).
3.2.2. High-Temperature Gradient Growth for the Optimization of Grain Boundaries
Preparation of coarse particle fractions is performed from presynthesized material in a crystallized SiO2 ampoule at 860 °C in a temperature gradient. Formed Bi2SeO5 and SeO2 segregate in a cooler region of the ampoule and can be discarded, whereas BixSey phases remain in the hottest part of the ampoule walls (Figure S1).
The use of a defined coarse particle fraction for pressing to reduce surface-to-volume ratio of the powder, minimizing the effect of ambient conditions and improving grain orientation in a pressed pellet. Traces of Bi4(SiO4)3 formed during the high-temperature step have a small particle size and can be largely removed by sieving.
3.2.3. Pressing
The use of a Si3N4 die for pressing is essential to prevent the reduction of Bi2O2Se to BixSey phases. Pressing at temperatures close to the melting point (730 °C) is necessary to achieve a high density of samples.
While spark plasma sintering with a tungsten carbide (WC) die might serve as a suitable alternative for graphite dies, such setups are expensive and relatively uncommon. In our experiments with graphite dies, we attempted to prevent direct contact between the Bi2O2Se powder and graphite by using a separating layer of ZrO2 powder, but this approach was only partially successful. The use of BN powder resulted in the formation of a significant amount of the BiSe phase and several unidentified peaks in PXRD.
By following the presented synthesis steps, we achieved improved reproducibility and stability in thermal cycling, as documented in Figure 7 and Figure 8.
To evaluate the effects of individual synthesis parameters, we present 4 samples following some or all of the suggested steps. All the samples were pressed in a Si3N4 die to prevent the formation of the BixSey phase due to rection with carbon.
Sample 1 was prepared by a common synthesis method presented in the literature, with a synthesis temperature of 700 °C for 10 days, without prior Bi2O3 calcination. No high-temperature growth in gradient or sieving was performed.
Sample 2 followed low-temperature synthesis, but without high-temperature growth, temperature gradient purification or sieving.
Samples 3 and 4 were subjected to both low-temperature synthesis and subsequent high-temperature gradient growth. Only the 35–340 μm particle fraction was used for pressing. For Sample 4, the powder was stored under ambient conditions for 1 month before being pressed to assess the influence of air exposure and surface oxidation.
All four samples exhibited a single-phase PXRD pattern of Bi2O2Se, although the presence of undetectable nanoscale or amorphous foreign phases cannot be excluded. The density of all the samples was >98% of the theoretical density.
For samples 3 and 4 (Figure 8 and Figure 9), the electrical conductivity shows exponential growth at temperatures above 500 K. The activation energy calculated from the temperature dependence with Arrhenius analysis is EA ≅ 0.20 eV, which would translate to an energy band gap of Eg ≅ 0.40 eV for fundamental excitation. This value is approximately half of the theoretical value of the band gap Eg ≅ 0.85 eV reported in the literature, e.g., [48], indicating that the polycrystalline material may still deviate from ideal stoichiometry and likely contains native defects and/or foreign phases at grain boundaries. This is consistent with the slight decrease in electrical conductivity with cycling in sample 4. While the stability of the samples under thermal cycling has improved, we believe that the quality of the grain boundaries continues to influence the transport behavior.
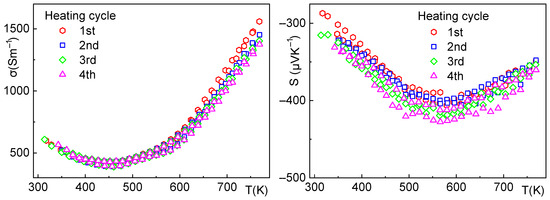
Figure 9.
Sample 4—electrical conductivity (left), Seebeck coefficient (right), and the effect of thermal cycling up to 500 °C. Both the electrical conductivity and Seebeck coefficient remain stable. The small decrease in electrical conductivity can be attributed to the slow surface oxidation and formation of an insulating layer of Bi2SeO5 due to the ppm concentration of O2 in the Ar used as an inert gas for measurement.
Additional cycling stability results, including comparative analysis of hot-pressed samples with various surface treatments and spark plasma sintered (SPS) samples, are presented in the Supplementary Materials, Figures S6–S9 and Tables S2–S5. These data confirm that even samples exhibiting phase purity according to PXRD may undergo significant changes in transport properties during cycling, depending on factors such as porosity, particle surface conditions, or inhomogeneities introduced during pressing.
For further comparison, the properties of single crystals from this batch are shown in Figure S16. The band gap could not be measured from the temperature dependence of electrical conductivity because of contact instability at temperatures above 150 °C. This highlights additional challenges related to the fabrication of long-term stable contacts for potential high-temperature thermoelectric applications.
3.3. Difficulties Associated with the Doping of Bi2O2Se
Published data and our own experiments indicate that the solubility of the dopants in the Bi2O2Se matrix is often very limited [24], Table S6. Ti-doped material [16] can serve as an example. In Bi2−xTixO2Se, TiO2 segregates as a foreign phase instead of substituting in the Bi2O2 layer. This results in an O-poor and Se-rich composition of the matrix
Bi2−xTixO2Se → Bi2−xO2−2xSe + xTiO2.
This stoichiometric change has implications on the character and concentration of native defects and foreign phases (at grain boundaries). Since the Bi-O layer in Bi2O2Se is extremely stable, doped materials that focus on Bi substitution often contain foreign phases. As a result, doping often leads to the formation of compositionally related foreign phases, e.g., BiSe, Bi4Se3, Bi2Se3, and dopant oxides. Among lanthanoids, Ce [17] and La [35] show reasonable solubilities in the Bi2O2Se matrix, as evidenced by the validity of Vegard’s law. The effects of doping and connected stoichiometric changes are summarized in Figure S2a,b, Supplementary Materials, Sections S4–S6, and Table S6.
A better doping approach is the direct use of a specific foreign phase [15,16,28,29]. This phase can serve both as a facilitator of charge transport between grains and as a phase for modulation doping. The doping phase should ideally be stable in contact with Bi2O2Se and should not form any potential barrier. This is still a challenge. In this direction, it is worth noting that the relative permittivity of Bi2O2Se is high, close to 200 [49], which is very favorable for modulation doping [45] and is unique for a non-ferroelectric material.
4. Conclusions
Bi2O2Se is a unique material with ultra-high mobility, but achieving consistent charge and heat transport properties, especially in polycrystalline form, is challenging. Our results confirm that polycrystalline samples exhibit limited reproducibility of the transport parameters, while these parameters also change due to temperature cycling. These problems are closely related to stoichiometry, leading to changes in the concentration of native point defects, the concentration of foreign phases, and the nature of grain boundaries, all of which remain difficult to control, even with careful synthesis procedures.
We present several synthesis improvements that mitigate key sources of variability in polycrystalline Bi2O2Se, resulting in improved reproducibility and stability of the sample properties under temperature cycling. The presented methods include the calcination of the Bi2O3 precursor to maintain the correct stoichiometry, low-temperature synthesis of Bi2O2Se in a crystallized silica ampoule, high-temperature crystal growth of the presynthesized Bi2O2Se and purification of the material in a temperature gradient, the use of a defined particle fraction for pressing to aid in particle orientation and minimize the number of foreign phases and grain boundaries, and finally, the pressing of the powder in a Si3N4 die, rather than the conventional graphite die to prevent the reduction of Bi2O2Se to BixSey phases.
The implementation of these steps improves reproducibility and partially mitigates degradation effects during thermal cycling, although full property stabilization remains unresolved. At room temperature, the as-prepared pure sample pressed from the 35–340 μm particle fraction has an electrical conductivity of σRT ≈ 500 S·m−1 and a Seebeck coefficient of S ≈ −300 μV·K−1 with a corresponding figure of merit ZTRT of 0.003 (Figure S17). This synthesis approach may serve as a useful reference for further systematic studies, particularly in the context of doping and microstructure–property correlations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst15110951/s1. References [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, Č.D. and J.Z.; Methodology, J.Z. and Č.D.; Validation, T.P., K.K. and J.H.; Formal analysis, T.P., K.K. and J.H.; Investigation, J.Z., T.P., A.S., P.L., P.R., S.Š. and V.N.; Resources, Č.D. and K.K.; Data curation, J.Z. and T.P.; Writing—original draft preparation, J.Z.; Writing—review and editing, J.N., K.K., J.H. and Č.D.; Visualization, A.S. and J.Z.; Supervision, Č.D.; Project administration, Č.D.; Funding acquisition, Č.D. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Czech Science Foundation for financial support (Project No. 22-05919S), financial support from the grant of the Ministry of Education, Youth and Sports of the Czech Republic (grant LM2023037).
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank P. Mošner and T. Hostinský from the University Pardubice for the DTA measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BM | Ball Milling |
| BSE | Backscattered Electron imaging |
| CP | Cold pressing |
| DTA | Differential thermal analysis |
| EDS | Energy-Dispersive Spectroscopy |
| FP | Foreign Phases |
| HP | Hot Pressing |
| (P)XRD | (Powder) X Ray Diffraction |
| SEM | Scanning Electron Microscopy |
| STA | Simultaneous Thermal Analysis |
| TE | Thermoelectric |
| XRF | X Ray Fluorescence |
References
- Zhang, C.; Tu, T.; Wang, J.; Zhu, Y.; Tan, C.; Chen, L.; Wu, M.; Zhu, R.; Liu, Y.; Fu, H.; et al. Single-crystalline van der Waals layered dielectric with high dielectric constant. Nat. Mater. 2023, 22, 832–837. [Google Scholar] [CrossRef]
- Wu, J.; Qiu, C.; Fu, H.; Chen, S.; Zhang, C.; Dou, Z.; Tan, C.; Tu, T.; Li, T.; Zhang, Y.; et al. Low Residual Carrier Concentration and High Mobility in 2D Semiconducting Bi2O2Se. Nano Lett. 2019, 19, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhou, X.; Li, W.; Peng, H. Preparation of two-dimensional [Bi2O2]-based layered materials: Progress and prospects. APL Mater. 2021, 9, 060905. [Google Scholar] [CrossRef]
- Liu, J.; Han, Z.; Ding, J.; Guo, K.; Yang, X.; Hu, P.; Jiao, Y.; Teng, F. Preparation and Performance Study of Photoconductive Detector Based on Bi2O2Se Film. Photonics 2023, 10, 1187. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, H.; Meng, M.; Chen, C.; Sun, Y.; Chen, Z.; Dang, W.; Tan, C.; Liu, Y.; Yin, J.; et al. High electron mobility and quantum oscillations in non-encapsulated ultrathin semiconducting Bi2O2Se. Nat. Nanotechnol. 2017, 12, 530–534. [Google Scholar] [CrossRef]
- Xu, S.; Fu, H.; Tian, Y.; Deng, T.; Cai, J.; Wu, J.; Tu, T.; Li, T.; Tan, C.; Liang, Y.; et al. Exploiting Two-Dimensional Bi2O2Se for Trace Oxygen Detection. Angew. Chem. Int. Ed. 2020, 59, 17938–17943. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Shi, X.-L.; Ao, D.-W.; Liu, W.-D.; Li, M.; Kou, L.-Z.; Chen, Y.-X.; Li, F.; Wei, M.; Liang, G.-X.; et al. Harvesting waste heat with flexible Bi2Te3 thermoelectric thin film. Nat. Sustain. 2023, 6, 180–191. [Google Scholar] [CrossRef]
- Yang, D.; Shi, X.-L.; Li, M.; Nisar, M.; Mansoor, A.; Chen, S.; Chen, Y.; Li, F.; Ma, H.; Liang, G.X.; et al. Flexible power generators by Ag2Se thin films with record-high thermoelectric performance. Nat. Commun. 2024, 15, 923. [Google Scholar] [CrossRef]
- Ao, D.; Liu, W.; Chen, Y.; Wei, M.; Jabar, B.; Li, F.; Shi, X.; Zheng, Z.; Liang, G.; Zhang, X.; et al. Novel Thermal Diffusion Temperature Engineering Leading to High Thermoelectric Performance in Bi2Te3 -Based Flexible Thin-Films. Adv. Sci. 2022, 9, 2103547. [Google Scholar] [CrossRef]
- Yang, N.; Pan, L.; Chen, C.; Wang, Y. Effects of Sb-doping on the electron-phonon transport properties of Bi2O2Se. J. Alloys Compd. 2021, 858, 157748. [Google Scholar] [CrossRef]
- Bae, S.Y.; Kim, H.-S.; Lee, S.W.; Park, O.; Park, H.; Kim, S. Enhanced thermoelectric properties of I-doped polycrystalline Bi2O2Se oxyselenide. J. Mater. Res. Technol. 2022, 19, 2831–2836. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, J.-L.; Dong, S.-T.; Yu, M.-C.; Zhao, L.; Wang, L.; Yao, S.-H. Effects of Zr substitution on structure and thermoelectric properties of Bi2O2Se. J. Mater. Res. Technol. 2022, 21, 640–647. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, Z.; Yang, N.; Xing, W.; Zhang, J.; Liu, Y.; Chen, C.; Li, D.; Wang, Y. Effects of sulfur substitution for oxygen on the thermoelectric properties of Bi2O2Se. J. Eur. Ceram. Soc. 2020, 40, 5543–5548. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Hu, K.; Ren, G.; Li, Y.; Liu, R.; Lin, Y.; Lan, J.; Nan, C. Synergistically optimizing electrical and thermal transport properties of Bi2O2Se ceramics by Te-substitution. J. Am. Ceram. Soc. 2018, 101, 326–333. [Google Scholar] [CrossRef]
- Niu, X.; Gao, Y.; Pan, L.; Chen, C.; Wang, Y. Thermoelectric properties of Bi2O2Se-x%AgSnSe2 composites via liquid assisted shear exfoliation- restacking process. J. Alloys Compd. 2022, 921, 166087. [Google Scholar] [CrossRef]
- Song, C.; Song, Y.; Pan, L.; Chen, C.; Zong, P.; Wang, Y. Thermoelectric properties of Bi2-xTixO2Se with the shear exfoliation-restacking process. J. Alloys Compd. 2022, 892, 162147. [Google Scholar] [CrossRef]
- Hong, H.Y.; Kim, D.H.; Won, S.O.; Park, K. Enhancement of the thermoelectric performance of n−type Bi2O2Se by Ce4+ doping. J. Mater. Res. Technol. 2021, 15, 4161–4172. [Google Scholar] [CrossRef]
- Li, Y.; Huo, H.; Huang, H.; Guo, K.; Yang, X.; Xing, J.; Luo, J.; Rao, G.-H.; Zhao, J.-T. Optimization of electrical and thermal transport properties of layered Bi2O2Se via Nb doping. J. Mater. Sci. 2021, 56, 12732–12739. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Pan, L.; Chen, C.; Zong, P.; Wang, Y. Enhancing thermoelectric performance of Bi2O2Se by W-doping with the shear exfoliation- restacking process. Mater. Lett. 2022, 308, 131291. [Google Scholar] [CrossRef]
- Tan, X.; Lan, J.; Ren, G.; Liu, Y.; Lin, Y.-H.; Nan, C.-W. Enhanced thermoelectric performance of n-type Bi2O2Se by Cl-doping at Se site. J. Am. Ceram. Soc. 2017, 100, 1494–1501. [Google Scholar] [CrossRef]
- Song, C.; Zhou, H.; Gu, Y.; Pan, L.; Chen, C.; Wang, Y. Enhanced thermoelectric properties of Bi2O2Se by Bi2Te2.7Se0.3 addition. J. Alloys Compd. 2023, 930, 167439. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Liu, R.; Zhou, Z.; Liu, C.; Lan, J.; Zhang, Q.; Lin, Y.; Nan, C. Synergistical Enhancement of Thermoelectric Properties in n-Type Bi2O2Se by Carrier Engineering and Hierarchical Microstructure. Adv. Energy Mater. 2019, 9, 1900354. [Google Scholar] [CrossRef]
- Kim, M.; Park, D.; Kim, J. Enhancement of Bi2O2Se thermoelectric power factor via Nb doping. J. Alloys Compd. 2021, 851, 156905. [Google Scholar] [CrossRef]
- Ruleova, P.; Plechacek, T.; Kasparova, J.; Vlcek, M.; Benes, L.; Lostak, P.; Drasar, C. Enhanced Thermoelectric Performance of n-type Bi2O2Se Ceramics Induced by Ge Doping. J. Electron. Mater. 2018, 47, 1459–1466. [Google Scholar] [CrossRef]
- Zhan, B.; Butt, S.; Liu, Y.; Lan, J.-L.; Nan, C.-W.; Lin, Y.-H. High-temperature thermoelectric behaviors of Sn-doped n-type Bi2O2Se ceramics. J. Electroceramics 2015, 34, 175–179. [Google Scholar] [CrossRef]
- Zhan, B.; Liu, Y.; Lan, J.; Zeng, C.; Lin, Y.-H.; Nan, C.-W. Enhanced Thermoelectric Performance of Bi2O2Se with Ag Addition. Materials 2015, 8, 1568–1576. [Google Scholar] [CrossRef]
- Pan, L.; Shi, X.; Song, C.; Liu, W.; Sun, Q.; Lu, C.; Liu, Q.; Wang, Y.; Chen, Z. Graphite Nanosheets as Multifunctional Nanoinclusions to Boost the Thermoelectric Performance of the Shear-Exfoliated Bi2O2Se. Adv. Funct. Mater. 2022, 32, 2202927. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, L.; Zhang, X.; Chen, C.; Yao, P.; Jiang, C.; Shen, X.; Lyu, Y.; Lu, C.; Zhao, L.-D.; et al. Significant Optimization of Electron–Phonon Transport of n-Type Bi2O2Se by Mechanical Manipulation of Se Vacancies via Shear Exfoliation. ACS Appl. Mater. Interfaces 2019, 11, 21603–21609. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, W.-D.; Zhang, J.-Y.; Shi, X.-L.; Gao, H.; Liu, Q.; Shen, X.; Lu, C.; Wang, Y.-F.; Chen, Z.-G. Synergistic effect approaching record-high figure of merit in the shear exfoliated n-type Bi2O2−2xTe2xSe. Nano Energy 2020, 69, 104394. [Google Scholar] [CrossRef]
- Zhan, B.; Liu, Y.; Tan, X.; Lan, J.; Lin, Y.; Nan, C. Enhanced Thermoelectric Properties of Bi2O2Se Ceramics by Bi Deficiencies. J. Am. Ceram. Soc. 2015, 98, 2465–2469. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, T.; Jabar, B.; Ao, D.; Li, F.; Chen, Y.; Liang, G.; Luo, J.; Fan, P. Enhanced Thermoelectric Performance in n -Type Bi2O2Se by an Exquisite Grain Boundary Engineering Approach. ACS Appl. Energy Mater. 2021, 4, 10290–10297. [Google Scholar] [CrossRef]
- Wei, T.; Xu, B.; Ji, X. Enhanced thermoelectric properties of Bi2O2Se ceramics by Bi deficiencies. Eur. Phys. J. B 2019, 92, 17. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, J.; Chen, C.; Wang, Y. Enhanced thermoelectric properties of highly textured Bi2O2-xSe1+x with liquid-phase mechanical exfoliation. Scr. Mater. 2020, 178, 376–381. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Liu, G.; Yang, X.; Wei, T.; Zhang, H.; Zhou, J.; Zhu, J. Tuning power factors of two-dimensional Bi2O2Se nanoplates through vacancy engineering. Mater. Today Energy 2021, 21, 100810. [Google Scholar] [CrossRef]
- Tan, X.; Lan, J.; Hu, K.; Xu, B.; Liu, Y.; Zhang, P.; Cao, X.; Zhu, Y.; Xu, W.; Lin, Y.; et al. Boosting the thermoelectric performance of Bi2O2Se by isovalent doping. J. Am. Ceram. Soc. 2018, 101, 4634–4644. [Google Scholar] [CrossRef]
- Sojka, A.; Zich, J.; Plecháček, T.; Levinský, P.; Navrátil, J.; Ruleová, P.; Šlang, S.; Beneš, L.; Knížek, K.; Holý, V.; et al. Mn-doping reveals a thermal gap and natural p-type conductivity in Bi2O2Se. Mater. Adv. 2025, 6, 7526–7534. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.-L.; Zumeta-Dubé, I.; Díaz, D.; Nava-Etzana, N.; Cruz-Zaragoza, E.; Santiago-Jacinto, P. Bismuth Oxide Nanoparticles Partially Substituted with EuIII, MnIV, and SiIV: Structural, Spectroscopic, and Optical Findings. Inorg. Chem. 2017, 56, 3394–3403. [Google Scholar] [CrossRef]
- Moré, R.; Olah, M.; Balaghi, S.E.; Jäker, P.; Siol, S.; Zhou, Y.; Patzke, G.R. Bi2O2CO3 Growth at Room Temperature: In Situ X-ray Diffraction Monitoring and Thermal Behavior. ACS Omega 2017, 2, 8213–8221. [Google Scholar] [CrossRef]
- Ding, X.; Li, M.; Chen, P.; Zhao, Y.; Zhao, M.; Leng, H.; Wang, Y.; Ali, S.; Raziq, F.; Wu, X.; et al. Bi2O2Se: A rising star for sem-iconductor devices. Matter 2022, 5, 4274–4314. [Google Scholar] [CrossRef]
- Tong, T.; Zhang, M.; Chen, Y.; Li, Y.; Chen, L.; Zhang, J.; Song, F.; Wang, X.; Zou, W.; Xu, Y.; et al. Ultrahigh Hall mobility and suppressed backward scattering in layered semiconductor Bi2O2Se. Appl. Phys. Lett. 2018, 113, 072106. [Google Scholar] [CrossRef]
- Xu, L.; Luo, Y.-C.; Lv, Y.-Y.; Zhang, Y.-Y.; Wu, Y.-Z.; Yao, S.-H.; Zhou, J.; Chen, Y.B.; Chen, Y.-F. Electrical scattering mechanism evolution in un-doped and halogen-doped Bi2O2Se single crystals. J. Phys. Condens. Matter 2020, 32, 365705. [Google Scholar] [CrossRef]
- Henry, N.; Evain, M.; Deniard, P.; Jobic, S.; Abraham, F.; Mentre, O. [Bi2O2]2+ Layers in Bi2O2(OH)(NO3): Synthesis and Structure Determination. Z. Für Naturforschung 2005, 60b, 322–327. [Google Scholar] [CrossRef]
- Corkett, A.J.; Chen, Z.; Bogdanovski, D.; Slabon, A.; Dronskowski, R. Band Gap Tuning in Bismuth Oxide Carbodiimide Bi2O2NCN. Inorg. Chem. 2019, 58, 6467–6473. [Google Scholar] [CrossRef]
- Huang, H.; Tian, N.; Jin, S.; Zhang, Y.; Wang, S. Syntheses, characterization and nonlinear optical properties of a bismuth subcarbonate Bi2O2CO3. Solid State Sci. 2014, 30, 1–5. [Google Scholar] [CrossRef]
- Sojka, A.; Janíček, P.; Zich, J.; Navrátil, J.; Ruleová, P.; Plecháček, T.; Kucek, V.; Knížek, K.; Drašar, Č. Extraneous doping and its necessary preconditions. Comput. Mater. Sci. 2024, 243, 113138. [Google Scholar] [CrossRef]
- Jain, A.; Hautier, G.; Ong, S.P.; Moore, C.J.; Fischer, C.C.; Persson, K.A.; Ceder, G. Formation enthalpies by mixing GGA and GGA + U calculations. Phys. Rev. B 2011, 84, 045115. [Google Scholar] [CrossRef]
- Yu, W.-X.; Liu, B.; Huang, W.-Q.; Zhou, H.; Xie, S.-Y. Phase evolution for oxidizing bismuth selenide. J. Phys. Condens. Matter 2023, 35, 075401. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Yun, W.S.; Seong, S.; Tahir, Z.; Kim, Y.S.; Ko, M.; Ryu, S.; Bae, J.-S.; Ahn, C.W.; Kang, J. Hidden Direct Bandgap of Bi2O2Se by Se Vacancy and Enhanced Direct Bandgap of Bismuth Oxide Overlayer. J. Phys. Chem. Lett. 2024, 15, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, J.; Wang, T.; Hu, W.; Yang, X.; Lin, X. Huge permittivity and premature metallicity in Bi2O2Se single crystals. Sci. China Phys. Mech. Astron. 2021, 64, 267312. [Google Scholar] [CrossRef]
- Fu, H.; Wu, J.; Peng, H.; Yan, B. Self-modulation doping effect in the high-mobility layered semiconductor Bi2O2Se. Phys. Rev. B 2018, 97, 241203. [Google Scholar] [CrossRef]
- Onderka, B.; Fitzner, K.; Kopyto, M.; Przybyło, W. Thermodynamics of Bi2O3-SiO2 system. J. Min. Met. Sect. B: Met. 2017, 53, 223–231. [Google Scholar] [CrossRef]
- Liu, R.; Lan, J.-L.; Tan, X.; Liu, Y.-C.; Ren, G.-K.; Liu, C.; Zhou, Z.-F.; Nan, C.-W.; Lin, Y.-H. Carrier concentration optimization for thermoelectric performance enhancement in n-type Bi2O2Se. J. Eur. Ceram. Soc. 2018, 38, 2742–2746. [Google Scholar] [CrossRef]
- Li, T.; Peng, H. 2D Bi2O2Se: An Emerging Material Platform for the Next-Generation Electronic Industry. Accounts Mater. Res. 2021, 2, 842–853. [Google Scholar] [CrossRef]
- Xu, R.; Wang, S.; Li, Y.; Chen, H.; Tong, T.; Cai, Y.; Meng, Y.; Zhang, Z.; Wang, X.; Wang, F. Layered Semiconductor Bi2O2Se for Broadband Pulse Generation in the Near-Infrared. IEEE Photon-Technol. Lett. 2019, 31, 1056–1059. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Wang, T.; Xu, Z.; Wu, J.; Hu, W.; Ren, Z.; Liu, S.; Behnia, K.; Lin, X. T-square resistivity without Umklapp scattering in dilute metallic Bi2O2Se. Nat. Commun. 2020, 11, 3846. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Geng, X.; Yang, J.; Zhang, J.; Zhu, S.; Yu, Q.; Wang, Y.; Li, H.; Li, R.; Hao, H. Synthesis and electrical transport properties of Bi2O2Se single crystals. J. Cryst. Growth 2018, 498, 244–247. [Google Scholar] [CrossRef]
- Lv, Y.-Y.; Xu, L.; Dong, S.-T.; Luo, Y.-C.; Zhang, Y.-Y.; Chen, Y.B.; Yao, S.-H.; Zhou, J.; Cui, Y.; Zhang, S.-T.; et al. Electron-electron scattering dominated electrical and magnetotransport properties in the quasi-two-dimensional Fermi liquid single-crystal Bi2O2Se. Phys. Rev. B 2019, 99, 195143. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.; Wu, J.; Fu, H.; Yang, H.; Tian, Z.; Tu, T.; Peng, H.; Sun, Y.; Xu, X.; et al. Electronic structures and unusually robust bandgap in an ultrahigh-mobility layered oxide semiconductor, Bi2O2Se. Sci. Adv. 2018, 4, eaat8355. [Google Scholar] [CrossRef] [PubMed]
- Drasar, C.; Ruleova, P.; Benes, L.; Lostak, P. Preparation and Transport Properties of Bi2O2Se Single Crystals. J. Electron. Mater. 2012, 41, 2317–2321. [Google Scholar] [CrossRef]
- Li, P.; Han, A.; Zhang, C.; He, X.; Zhang, J.; Zheng, D.; Cheng, L.; Li, L.-J.; Miao, G.-X.; Zhang, X.-X. Mobility-Fluctuation-Controlled Linear Positive Magnetoresistance in 2D Semiconductor Bi2O2Se Nanoplates. ACS Nano 2020, 14, 11319–11326. [Google Scholar] [CrossRef]
- Zhu, Z.; Yao, X.; Zhao, S.; Lin, X.; Li, W. Giant Modulation of the Electron Mobility in Semiconductor Bi2O2Se via Incipient Ferroelectric Phase Transition. J. Am. Chem. Soc. 2022, 144, 4541–4549. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).