Abstract
A universal equation of state of solids is one of the far goals of condensed matter science. Here, it is shown that within pressures of 2–100 GPa, the compression of oxides and oxide-based networks follows a linear relation between the molar volume and the combined ionic volume that is based on the pressure-dependent crystal radii at any pressure. This relation holds for simple and complex oxides and modified networks such as alumosilicates, beryllosilicates, borates, and empty zeolites. Available compression data for halides and metal-organic frameworks are also consistent with this relation. Thus, the observed relation also serves as a measure for pore-space filling in cage structures.
1. Introduction
Advanced methods of computing the band structures of solids also provide good assessments of their elastic properties. With suitable corrections of electron–electron interactions, ab initio computation reproduces experimentally determined volumes over extended ranges of pressure with small systematic uncertainties [1,2,3].
The present study looks for general concepts of compression across different structure types and compositions. This purpose requires an approach somewhat different from that of ab initio calculations: finding a general pattern for a large number of compounds of a different structure, composition, and stoichiometry requires abstracting from the specific direction-dependent bonding of the individual crystalline species, which vastly differs between many of these materials. It is the purpose of this paper to examine if there is any such general pattern of material compression beyond vague correlations with large variances.
The compression of solids over intervals of pressure in the range of tens to hundreds of GPa is commonly described in terms of Eulerian finite strain [4,5,6], and there are various non-analytical correlations between volume, pressure, the bulk modulus, and its pressure derivative that successfully describe solid-state compression over an extended range of pressures, for instance, the Birch–Murnaghan [5,6] and the Vinet equation [7], or other equations that are based on empirical potentials [6].
In a recent study [8], it was shown that pressure-dependent crystal radii can be defined such that interatomic distances in more than 100 different observed crystal structures between ambient and 160 GPa are reproduced within small uncertainties. It was found that cations generally compress linearly over the examined range of pressures, whereas the anions O2−, Cl−, and Br− follow an inverse power law. For a given valence, the cation and anion compression vary systematically with the nuclear charge number, the screening of the valence shell from the nuclei, and the azimuthal and principal quantum numbers [8]. Furthermore, a power–law correlation between crystal radii and electronegativity gives an approximate quantitative measure of the change from localized ionic to more covalent bonding along with pressure [9] that previously had been calculated [10] and inferred from empirical data [11,12].
These findings raise the question how the compression of solids relates to the compression of the constituting atoms in the limiting case of ionic bonding—and if there is any meaningful correlation. Although the ionic bond model falls short in describing the properties of most solids, it is shown here that the molar and ionic volume (as defined below) are linearly correlated for a larger number of solids of various structures and compositions. In other words, the volume compression of solids, as far as they are examined here, is rather independent of directional variations in electron bond states. The linear coefficient in the relation between the molar and ionic volume for each solid obeys a general systematic trend.
2. Materials and Methods
The pressure dependencies of crystal radii that were recently obtained [8] are compared to the observed compression of crystalline oxides, zeolites a metal–organic framework (MOF), and a few halogenides. Compression of glasses and liquids shall be discussed in a separate paper. The set of data includes polymorphs of same composition and isotypic structures of different composition. All compression studies whose results have been used here were conducted in diamond anvil cells at 300 K and compression was evaluated based on X-ray diffraction and structure analysis. If available, single-crystal diffraction studies were given preference over power diffraction data. Wherever available, data obtained under hydrostatic or nearly hydrostatic compression in solid He or Ne media were given preference over non-hydrostatic compression studies. However, compression studies of empty zeolites and MOFs are conducted with silicon oil pressure media in order to shift the filling of the framework to higher pressure. Silicon oils do not provide hydrostatic stress to as high pressures as neon or helium at 300 K.
The following classes of materials were examined (Table 1): (a) simple oxides and halogenides of the NaCl- and CsCl-type (henceforth, B1- and B2-type); (b) polymorphic AO2, A2O3-oxides of the corundum-type; (c) the polymorphic silicates MgSiO3, CaSiO3, and Mg2SiO4 and their isotypic equivalents MgGeO3 and Al2BeO4; (d) alumo-, boro-, and beryllosilicates including framework structures; and (e) one MOF.

Table 1.
Fitted values A and V′ for Relation (2). Mineral species are given with the mineral name. Synthetic phases by their chemical sum formula or, for framework structures, by the commonly used compound name.
Pressure-dependent ionic volumes of solids are defined here as the sum of the cubes of the pressure-dependent crystal radii of the constituting atoms. For a compound such as AiBjCk…, where i, j, k… gives the stoichiometry of the chemical species A, B, C…, the ‘ionic volume’ is
4π/3 (i·rA3 + j·rB3 + k·rC3 …),
Thence, the total ionic volume at pressure P is calculated from (1) using the crystal radii rA (P), rB (P), and rC (P) at pressure P. At each pressure P, the ionic volumes are compared to the observed molar volumes of the material AiBjCk… The pressure-dependent radii that are used in this study are taken from [8] (that is, either from the Tables 1 and 2 that are given in reference [8] or, if not measured, are calculated by Formula (1) in reference [8]). Bond coordinations are given as Roman numbers. The assessment of bond coordination has been discussed in [8]. The issue of applicability of the crystal radii concept to MOFs is discussed below.
3. Results
Figure 1 shows the correlation of the ionic and molar volume for several materials at different pressures at 300 K. The pressures that correspond to these volumes differ for different materials and range from a few GPa for the zeolite RHO-A to more than 100 GPa for MgO and B2-type KCl. All materials shown in Figure 1 exhibit a linear correlation between ionic and molar volumes. The same strong linear correlation was found for all materials listed in Table 1 over most of the range of their isothermal compression.
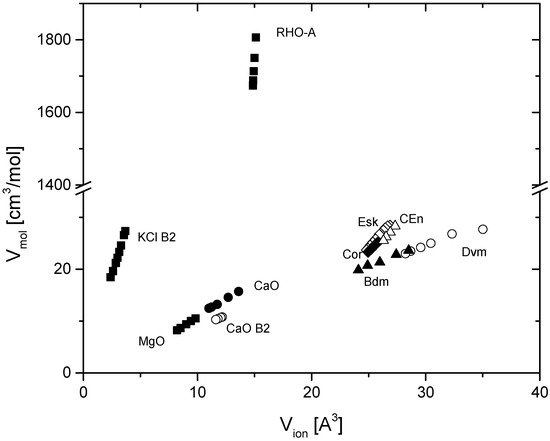
Figure 1.
Correlation of molar and ionic volumes of various materials. The relation is linear for all materials listed in Table 1. Ionic volumes are calculated based on Equation (1). Abbreviations of mineral names are: Bdm = bridgmanite, Dvm = davemaoite, Esk = eskolaite, Cor = corundum, and CEn = (high-pressure) clinoenstatite.
The observed linear correlation of the ionic and molar volume over large ranges of pressures for many materials of different compositions and structures (Table 1) is not a trivial finding because the ionic bond concept is a limiting case of bonding; it neglects actual electron density gradients along and perpendicular to the bond vectors and directional variations in the bond strength in favor of a spherical average. Thus, Figure 1 and Table 1 show that volume compression is rather independent of these directional variations.
Figure 1 unites examples of materials of different structure types and compositions. For instance, corundum (γ-Al2O3) and eskolaite (Cr2O3) are isotypic and the linear relation between the ionic and molar volume
is very similar with A = 2.25(4)·1024/mol = 3.74(6)NA and 2.53(4) × 1024/mol = 4.20(7)NA and V′ = 32.9(9) and 39.0(10) × 10−6 m3/mol for corundum and eskolaite, respectively (NA = Avogadro number). Bridgmanite and davemaoite are both ABO3-type perovskites with B = SiVI. Within uncertainties, they exhibit equal linear coefficient A (Table 1) which, furthermore, is nearly equal to that of CaO (B2), whereas CaO (B1) and MgO (B1) have nearly an equal factor A, which, however, differs markedly from that of CaO (B2) (Figure 1). Hence, materials of similar basic structure type, such as CsCl-and NaCl-type structures, exhibit similar linear coefficients A in Relation (2). It is noted that Relation (2) deviates from linearity if, intentionally, ionic volumes with incorrect coordinations of ions are used. However, the worsening of the R2 value of the fits are generally small and the main difference is a shift of A and V’. More noticeable deviations from linearity are observed if the ionic volumes for an incorrect stoichiometry are used. This is not surprising, but the two observations combined emphasize that the compression of oxides is controlled by that of the anion, which dominates the ionic volume by its large radius, its strong compression, and by stoichiometry. This suggests that Relation (2) does not provide a good discrimination between correct and incorrect coordinations, but it is shown below that the shift of A and V′ in Relation (2) allows for this distinction.
Vmol(P) = A·Vion(P) − V′,
4. Discussion
High-pressure clinoenstatite (HCEn) MgSiO3 has the same composition as bridgmanite, but assumes a linear chain structure of silica tetrahedra with interstitial Mg on two distorted octahedral sites. The correlation between the ionic and molar volume of HCEn is linear, but different from that of bridgmanite (Figure 1). In fact, it is close to that of corundum and eskolaite in agreement with the fact that HCEn compression is controlled by the contraction of the MgO6 octahedra [19], which form edge-sharing chains in the pyroxene structure. In sum, isotypic phases and phases of similar structures exhibit the same or a similar relation between molar and ionic volumes at any pressure, but isochemical compounds of different structures give different linear coefficients A in Relation (2). This rule avails for all studied oxides, but it does not extend to isotypic solids with other anions. KCl (B2) follows a much steeper linear correlation than CaO (B2), probably because the monovalent chloride anion ismore compressibile than the divalent oxide anion. Based on the few available data, the cause of this difference cannot be evaluated, but it is suggested that it is related to the anion valence.
Returning to oxides, it is noteworthy that even structures as large as zeolites [32,54] obey the linear Relation (2) between the ionic and molar volume (Figure 1 and Figure 2). The relation appears to extend to metal–organic frameworks; at least, ZIF-4 [55] follows a linear relationship between a reference ‘ionic volume’ 4π/3 · [r(ZnIV,2+)3 + 6·r(CIV,4+)3 + 4·r(NIII,2+)3] and its molar volume between 1 and 6 GPa (and the contribution of H has been neglected in the reference ionic volume). The relationships of the molar and the reference ionic volume of some large framework materials like Na-X, sodalite, cristobalite, and ZiF-4 are shown in Figure 2. It is understood that crystal radii are not good representations of the chemical bonding in MOFs, besides that H is not even considered in the used reference ionic volume. Yet, ZIF-4 exhibits a linear relation between the experimental molar volume [55] and the reference ionic volume, as defined above. While the ionic volumes of such framework materials may not be considered as fully quantitative, Relation (2) provides a measure for pressure-induced void filling in such structures and for the irreversible collapse of compressed frameworks through deviations of the measured volumes from Relation (2).
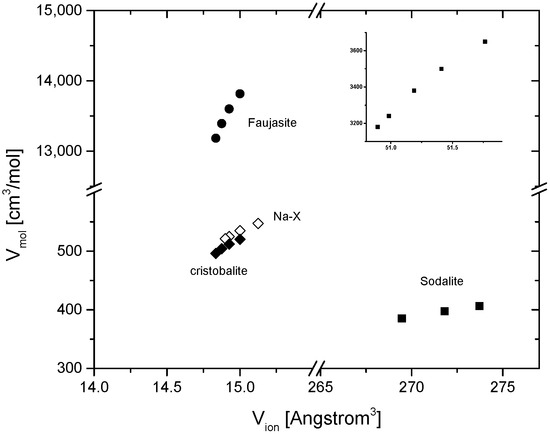
Figure 2.
Relation of molar and ionic volume of larger framework structures. Insert: the same relation for ZIF-4 with the equivalent.
Most materials that are listed in Table 1 show minor deviations from the linear regime of the ionic–molar volume relationship at pressures below 1–2 GPa. This can be seen in Figure 1 for the largest volume of eskolaite and davemaoite, respectively, with each corresponding to about 1 GPa of pressure. This low-pressure offset signifies a slight contraction of the molar relative to the ionic volume and is interpreted as reflecting directional components in elastic bond compression that are beyond the spherically symmetric model of pressure-dependent crystal radii and which have been assessed through the concept of bonded radii [61]. While the 0–2 GPa regime is important in many respects, the present study focuses on the linear regime that avails for the elastic compression of all examined materials above this low-pressure regime and up to the highest pressures examined.
It may be objected that many of the examined materials do not exhibit a strongly ionic bond character or include bonds of different degrees of valence electron transfer between cations and anions. However, the observed linear correlation between ionic and molar volume (Figure 1 and Figure 2, Table 1) shows that the pressure-dependent crystal radii reproduce the compression of solids of vastly different compositions, structures, and bond topologies over a large range of pressures. While this novel correlation is not a substitute for the more precise first-principles-based computation of the elasticity of specific solids, it allows for the direct comparison of the compression behavior across structure types and compositional spaces as well as the prediction of bulk moduli for very large structures or multicomponent solid solutions where computation is costly.
In a second step, the nature of the constant factor A and the constant term V′ has to be examined. V′ has the dimension of a volume and does not vanish when Vion becomes zero. Thus, on a first glance, V′ may be taken as a geometric measure of the interstitial voids in a given structure. However, V′ remains invariant over the examined ranges of pressure since Relation (2) remains linear within narrow bounds (Figure 1 and Figure 2, Table 1). In fact, for a given composition, V′ changes significantly only upon phase transitions, such as from the B1 to the B2 phase of CaO, or from enstatite to HCEn and further to akimotoite and bridgmanite, all four of which are polymorphs of MgSiO3 (see Figure 1). In addition, V′ is quite different for isotypic halogenides and oxides such as KCl and CaO in the B2-type structure. Thus, V′ is not purely geometric. Rather, V′ represents a rigid minimal void space of a given structure, but not the actual interstitial space that is reduced along with the reduction of interatomic distances in a solid. For oxides, A/NA is related to V′/V0 (V0 being the molar volume at standard conditions) as (V′/V0)3/2 = 0.61(2)A/NA-1.06(9) with an R2 of 0.975. This is shown in Figure 3. The fit has been conducted over all data points with A/NA and (V′/V0)3/2 < 15.
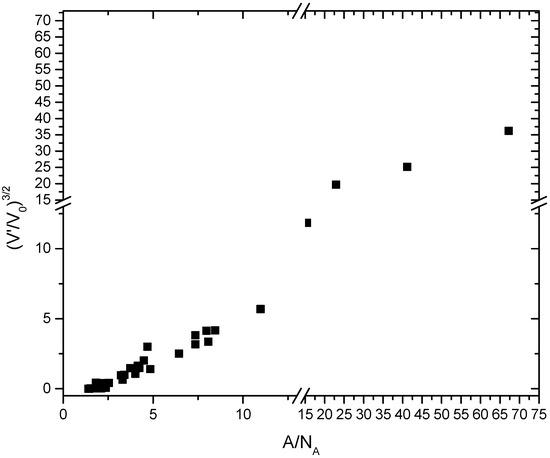
Figure 3.
Relationship between the normalized reference volume V′/V0 and the factor A in Relation (2). V0 is the molar volume at standard conditions and V′ is obtained through Relation (2) (see Table 1). A (here divided by the Avogadro number NA) relates to V′/V0 to the power 3/2 for all materials listed in Table 1. However, very large framework structures appear to have a constant offset.
Consequently, this range of data (2) can be reformulated as
where NA is the Avogadro Number, V0 the molar volume at standard conditions, and V(P) and Vion(P) are the molar and ionic volumes at pressures P and V′, as defined in Equations (1) and (2), respectively.
Relation (3) holds for simple and complex oxides including garnets, pyrochlore (Tb-Ti), sodalite, inyoite, and tourmaline. At the end of the Section 3, it was mentioned that changes of the ionic volume to the wrong composition or wrong coordination cause only minor deviations from linearity in Relation (2), but cause shifts of the constant factor A and the constant term V′. For instance, if the molar volumes of HCEn are (arbitrarily) correlated with the ionic volumes of grossular, the coefficient A changes from 5.06 (11) to 0.47 (2), and V′ changes from 44.8 (2.8) to 23.7 (2.8).,These values are distinctly shifted off the main trend of data in Figure 3 and Relation (3). The ionic volume for MgX, SiVI, and OVI are used instead of MgVI, SiIV, and OIII for HCEn shifts A and V′ to 0.69 (3) and 9.3 (9), respectively, which is still noticeably off the trend defined by Equation (3). Thus, Relation (3) provides a distinction for correct and incorrect bond coordinations and stoichiometry, though it is limited by the fact that the oxide anion compression dominates over cation compression and by the variance in a correlation such as (3) that compares materials of vastly different structures and compositions (see Figure 3).
However, very large framework structures such as the zeolites RHO-A, Na-X, and ZIF-4 deviate markedly from (3) while they obey Relation (2). ZIF-4 is not an oxide and compliance to (3) is not expected since halogenides also do not follow Relation (3). For the zeolites, it is noted that a geometric rescaling of the chemical formula unit Z based on the reduced chemical formula brings the molar–ionic volume relation close to match Relation (3). These are the data points shown in Figure 3 for A NA and (V′/V0)3/2 > 15. The relation appears to be the same, but with a constant offset different from that for A/NA and (V′/V0)3/2 < 15. The reduced chemical formulas have been calculated as follows: For Na-X, a faujasite-type zeolite, the chemical formula Na4.38H1.62Al6Si6O24 with Z = 16 has been rewritten as (Na,H)3/4Al3/4Si3/4O3, where the three highest symmetric sites in the structure, 32e (partially occupied by Na and H), are mapped onto one site, and the reduced unit cell gives a volume at standard conditions of 70.1 rather than 560.8 cm3/mol. It requires more compression data for empty zeolites beyond 2 GPa to assess if the proposed rescaling is justified. At the present state, the validity of Relation (3) (Figure 3) is only confirmed for A/NA and (V′/V0)3/2 < 15, while Relation (2) holds for all examined materials, including zeolites (see Figure 1 and Figure 2). It should be noted that deviations from hydrostatic pressure in the experiments on empty framework materials may affect these data more than those collected in helium, neon, or liquid methanol–ethanol mixtures (see Section 2).
In sum, the molar volume at any pressure and of any of the examined phases is represented as a sum of the cubes of the crystal radii of the constituting chemical species and a rigid reference volume V′ that relates the ionic to the molar volume. Thus, for compounds with O2− as a constituting anion and with a known composition and molar volume at standard conditions, their compression at 300 K is easily computed based on Relations (2) and (3).
5. Conclusions
An extensive set of compression data for simple and complex oxides is used to show a strong linear correlation between the molar and ionic volume at any examined pressure above 2 GPa. Ionic volumes are computed as a sum of the cubes of the pressure-dependent crystal radii multiplied by their stoichiometric factors (Section 2, Equation (1)). The linearity of the relation between the molar and ionic volume, thus defined, extends to large framework structures such as zeolites and MOFs. The relation also applies to halogenides.
For oxides, the linear relationship between the molar and ionic volume is further reduced by defining a single volumetric parameter that relates the molar and ionic volume and that can be obtained at standard conditions. This extended Relation (Section 4, Equation (3)) holds for simple oxides and for silicates including garnets, tourmaline, and sodalite. Based on this relation, known pressure-dependent crystal radii and the molar volume at standard conditions volume compression is easily computed. This approach is of interest for assessing the compression of multicomponent solid solutions and of very large structures such as zeolites where ab initio predictions are costly. Moreover, Equation (1) through (3) allow for assessing empty framework compression through deviations from the linear relation between ionic and molar volumes as function of pressure, and, thus, for discriminating the effect of compression-induced void filling and irreversible framework collapse.
In the same way, the observed relation can be used to monitor volume misfits from deviatoric stresses in high-pressure experiments. Furthermore, the relationship between the molar and ionic volume is useful in assessing the pressure-derivative of the bulk modulus, k0′, which is the same for the ionic and the molar volumes, but is commonly hard to assess directly through fits of pressure–volume data by equations of state. In addition, the relation can be used to assess isotherms at temperatures different from 300 K, which are experimentally more challenging and are subject to combined uncertainties of pressure and temperature assessments.
Overall, the present work shows that above 2 GPa, the volume compression of oxides and halides is largely independent of directional differences in bond strength and electron distribution.
Finally, it should be noted that the general linear relationship between empirical molar volumes and the ionic volumes that are derived from the pressure-dependent crystal radii confirms that the latter are meaningful physical parameters because they properly represent the volume compression of a large number of compounds and structures.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article. All data are given in the paper and the references for Table 1. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thanks the two reviewers for their helpful comments.
Conflicts of Interest
The author declares no conflict of interest.
References
- Karki, B.B.; Stixrude, L.; Wentzcovitch, R.M. High-pressure elastic properties of major materials of Earth’s mantle from first principles. Rev. Geophys. 2001, 39, 507–534. [Google Scholar] [CrossRef]
- Dewaele, A.; Torrent, M.; Loubeyre, P.; Mezouar, M. Compression curves of transition metals in the Mbar range: Experiments and projector augmented-wave calculations. Phys. Rev. B 2008, 78, 104102. [Google Scholar] [CrossRef]
- Lejaeghere, K.; Bihlmayer, G.; Björkman, T.; Blaha, P.; Blügel, S.; Blum, V.; Caliste, D.; Castelli, I.E.; Clark, S.J.; Corso, A.D.; et al. Reproducibility in density functional theory calculations of solids. Science 2016, 351, aad3000. [Google Scholar] [CrossRef]
- Bridgman, P.W. The compression of 46 substances to 50,000 kg/cm2. Proc. Am. Acad. Art Sci. 1940, 74, 21–51. [Google Scholar] [CrossRef]
- Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Holzapfel, W.B. Effects of intrinsic anharmonicity in the Mie-Gruneisen equation of state and higher order corrections. High Press. Res. 2005, 25, 187–203. [Google Scholar] [CrossRef]
- Vinet, P.; Ferrante, J.; Rose, J.H.; Smith, J.R. Compressibility of Solids. J. Geophys. Res.-Sol Earth Planet 1987, 92, 9319–9325. [Google Scholar] [CrossRef]
- Tschauner, O. Pressure-Dependent Crystal Radii. Solids 2023, 4, 235–253. [Google Scholar] [CrossRef]
- Grochala, W.; Hoffmann, R.; Feng, J.; Ashcroft, N.W. The Chemical Imagination at Work in Very Tight Places. Angew. Chem. Int. Ed. 2007, 46, 3620–3642. [Google Scholar] [CrossRef]
- Rahm, M.; Cammi, R.; Ashcroft, N.W.; Hoffmann, R. Squeezing All Elements in the Periodic Table: Electron Configuration and Electronegativity of the Atoms under Compression. J. Am. Chem. Soc. 2019, 141, 10253–10271. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D.; Prewitt, C.T. Coordination and volume changes accompanying high-pressure phase transformations of oxides. Mat. Res. Bul. 1969, 4, 57–59. [Google Scholar] [CrossRef]
- Prewitt, C.T.; Downs, R.T. High-pressure crystal chemistry. In Ultrahigh-Pressure Mineralogy: Physics and Chemistry of the Earth’s Deep Interior; Hemley, R.J., Ed.; Mineralogical Society of America: Washington, DC, USA, 1998; Volume 37, pp. 283–317. [Google Scholar]
- Yamanaka, T.; Komatsu, Y.; Sugahara, M.; Nagai, T. Structure change of MgSiO3, MgGeO3, and MgTiO3 ilmenites under compression. Am. Min. 2005, 90, 1301–1307. [Google Scholar] [CrossRef]
- Ross, N.L.; Hazen, R.M. High-pressure crystal chemistry of MgSiO3 perovskite. Phys. Chem. Min. 1990, 17, 228–237. [Google Scholar] [CrossRef]
- Kudoh, Y.; Ito, E.; Takeda, H. Effect of pressure on the crystal structure of perovskite type MgSiO3. Phys. Chem. Min. 1987, 14, 350–354. [Google Scholar] [CrossRef]
- Sugahara, M.; Yoshiasa, A.; Komatsu, Y.; Yamanaka, T.; Bolfan Casanova, N.; Nakatsuka, A.; Sasaki, S.; Tanaka, M. Reinvestigation of the MgSiO3 perovskite structure at high pressure. Am. Min. 2006, 91, 533–536. [Google Scholar] [CrossRef]
- Murakami, M.; Hirose, K.; Kawamura, K.; Sata, N.; Ohishi, Y. Post-perovskite phase transition in MgSiO3. Science 2004, 304, 855–858. [Google Scholar] [CrossRef]
- Ono, S.; Kikegawa, T.; Ohishi, Y. Equation of state of CaIrO3-type MgSiO3 up to 144 GPa. Am. Min. 2006, 91, 475–478. [Google Scholar] [CrossRef]
- Lazarz, J.D.; Dera, P.; Hu, Y.; Meng, Y.; Bina, C.R.; Jacobsen, S.D. High-pressure phase transitions of clinoenstatite. Am. Min. 2019, 104, 897–904. [Google Scholar] [CrossRef]
- Periotto, B.; Balic-Zunic, T.; Nestola, F.; Katerinopoulou, A.; Angel, R.J. Re-investigation of the crystal structure of enstatite under high-pressure conditions. Am. Min. 2012, 97, 1741–1748. [Google Scholar] [CrossRef]
- Hazen, R.M. Comparative Compressibilities of Silicate Spinels: Anomalous Behavior of (Mg,Fe)2SiO4. Science 1993, 259, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.D.; Holl, C.M.; Adams, K.A.; Fischer, R.A.; Martin, E.S.; Bina, C.R.; Lin, J.-F.; Prakapenka, V.B.; Kubo, A.; Dera, P. Compression of single-crystal magnesium oxide to 118 GPa and a ruby pressure gauge for helium pressure media. Am. Miner. 2008, 93, 1823–1828. [Google Scholar] [CrossRef]
- Milani, S.; Comboni, D.; Lotti, P.; Fumagalli, P.; Ziberna, L.; Maurice, J.; Hanfland, M.; Merlini, M. Crystal structure evolution of CaSiO3 polymorphs at earth’s mantle pressures. Minerals 2021, 11, 652. [Google Scholar] [CrossRef]
- Kubo, A.; Kiefer, B.; Shim, S.-H.; Shen, G.; Prakapenka, V.B.; Duffy, T.S. Rietveld structure refinement of MgGeO3 post-perovskite phase to 1 Mbar. Am. Miner. 2008, 93, 965–976. [Google Scholar] [CrossRef]
- Richet, P.; Mao, H.-K.; Bell, P.M. Static compression and equation of state of CaO to 1.35 Mbar. J. Geophys. Res. 1988, 93, 15279–15288. [Google Scholar] [CrossRef]
- Zhao, J.; Ross, N.L.; Angel, R.J. Polyhedral control of the rhombohedral to cubic phase transition in LaAlO3 perovskite. J. Phys. Cond. Matt. 2004, 16, 8763–8773. [Google Scholar] [CrossRef]
- Andrault, D.; Angel, R.J.; Mosenfelder, J.L.; Le Bihan, T. Equation of state of stishovite to lower mantle pressures. Am. Min. 2003, 88, 301–307. [Google Scholar] [CrossRef]
- Ross, N.L.; Shu, J.-F.; Hazen, R.M.; Gasparik, T. High-pressure crystal chemistry of stishovite. Am. Min. 1990, 75, 739–747. [Google Scholar]
- Yamanaka, T.; Fukuda, T.; Tsuchiya, J. Bonding character of SiO2 stishovite under high pressures up to 30 GPa. Phys. Chem. Min. 2002, 29, 633–641. [Google Scholar] [CrossRef]
- Sugiyama, M.; Endo, S.; Koto, K. The crystal structure of stishovite under pressure up to 6 GPa. Miner. J. 1987, 13, 455–466. [Google Scholar] [CrossRef][Green Version]
- Kudoh, Y.; Takeda, H. Single crystal X-ray diffraction study on the bond compressibility of fayalite, Fe2SiO4 and rutile, TiO2 under high pressure. Phys. B+C Phys. Cond. Matt. 1986, 139, 333–336. [Google Scholar] [CrossRef]
- Colligan, M.; Forster, P.M.; Cheetham, A.K.; Lee, Y.; Vogt, T.; Hriljac, J.A. Synchrotron X-ray Powder Diffraction and Computational Investigation of Purely Siliceous Zeolite Y under Pressure. J. Am. Chem. Soc. 2004, 126, 12015–12022. [Google Scholar] [CrossRef]
- Dera, P.; Lazarz, J.D.; Prakapenka, V.B.; Barkley, M.; Downs, R.T. New insights into the high-pressure polymorphism of SiO2. Phys. Chem. Min. 2011, 38, 517–529. [Google Scholar] [CrossRef]
- Hazen, R.M.; Finger, L.W.; Hemley, R.J.; Mao, H.K. High-pressure crystal chemistry and amorphization of alpha quartz. Solid State Commun. 1989, 72, 507–511. [Google Scholar] [CrossRef]
- Levien, L.; Prewitt, C.T.; Weidner, D.J. Structure and elastic properties of quartz at pressure. Am. Min. 1980, 65, 920–930. [Google Scholar]
- Kantor, A.; Kantor, I.; Merlini, M.; Glazyrin, K.; Prescher, C.; Hanfland, M.; Dubrovinsky, L. High-pressure structural studies of eskolaite by means of single-crystal X-ray diffraction. Am. Min. 2012, 97, 1764–1770. [Google Scholar] [CrossRef]
- Finger, L.W.; Hazen, R.M. Crystal structure and compression of ruby to 46 kbar. J. Appl. Phys. 1978, 49, 5823–5826. [Google Scholar] [CrossRef]
- Kim-Zajonz, J.; Werner, S.; Schulz, H. High pressure single crystal X-ray diffraction study on ruby up to 31 GPa. Z. Krist. 1999, 214, 331–336. [Google Scholar] [CrossRef]
- Lin, J.; Degtyareva, O.; Prewitt, C.T.; Dera, P.; Sata, N.; Gregoryanz, E.; Mao, H.-K.; Hemley, R.J. Crystal structure of a high-pressure/high-temperature phase of alumina by in situ X-ray diffraction. Nat. Mat. 2004, 3, 389–393. [Google Scholar] [CrossRef]
- Au, Y.; Hazen, R.M. Polyhedral modeling of the elastic properites of corundum and chrysoberyl. Geophys. Res. Lett. 1985, 12, 725–728. [Google Scholar] [CrossRef]
- Finkelstein, G.J.; Dera, P.K.; Jahn, S.; Oganov, A.R.; Holl, C.M.; Meng, Y.; Duffy, T.S. Phase transitions and equation of state of forsterite to 90 GPa from single-crystal X-ray diffraction and molecular modeling. Am. Min. 2014, 99, 35–43. [Google Scholar] [CrossRef]
- Chen, H.; Shim, S.-H.; Leinenweber, K.; Prakapenka, V.; Meng, Y.; Prescher, C. Crystal structure of CaSiO3 perovskite at 28–62 GPa and 300 K under quasi-hydrostatic stress conditions. Am. Min. 2021, 103, 462–468. [Google Scholar] [CrossRef]
- Martin, C.D.; Smith, R.I.; Marshall, W.G.; Parise, J.B. High-pressure structure and bonding in CaIrO3: The structure model of Mg Si O3 post-perovskite investigated with time-of-flight neutron powder diffraction. Am. Min. 2007, 92, 1912–1918. [Google Scholar] [CrossRef]
- Levy, D.; Pavese, A.; Hanfland, M. Synthetic MgAl2O4 (spinel) at high-pressure conditions (0.0001–30 GPa): A synchrotron X-ray powder diffraction study. Am. Min. 2003, 88, 93–98. [Google Scholar] [CrossRef]
- Lotti, P.; Gatta, G.D.; Rotiroti, N.; Camara, F. High-pressure study of a natural cancrinite. Am. Min. 2012, 97, 872–882. [Google Scholar] [CrossRef]
- Gatta, G.D.; Angel, R.J.; Zhao, J.; Alvaro, M.; Rotiroti, N.; Carpenter, M.A. Phase stability, elastic behavior, and pressure-induced structural evolution of kalsilite: A ceramic material and high-T/high-P mineral. Am. Min. 2011, 96, 1363–1372. [Google Scholar] [CrossRef]
- Gatta, G.D.; Angel, R.J. Elastic behavior and pressure-induced structural evolution of nepheline: Implications for the nature of the modulated superstructure. Am. Min. 2007, 92, 1446–1455. [Google Scholar] [CrossRef]
- Zhang, L.; Ahsbahs, H.; Kutoglu, A. Hydrostatic compression and crystal structure of pyrope to 33 GPa. Phys. Chem. Min. 1998, 25, 301–307. [Google Scholar] [CrossRef]
- Hazen, R.M.; Finger, L.W. Crystal structure and compressibilities of pyrope and grossular to 60 kbar. Am. Min. 1978, 63, 297–303. [Google Scholar]
- O’Bannon, E., III; Beavers, C.M.; Kunz, M.; Williams, Q. High-pressure study of dravite tourmaline: Insights into the accommodating nature of the tourmaline structure. Am. Min. 2018, 103, 1622–1633. [Google Scholar] [CrossRef]
- Burt, J.B.; Ross, N.L.; Angel, R.J.; Koch, M. Equations of state and structures of andalusite to 9.8 GPa and sillimanite to 8.5 GPa. Am. Min. 2006, 91, 319–326. [Google Scholar] [CrossRef]
- Comboni, D.; Battiston, T.; Pagliaro, F.; Lotti, P.; Gatta, G.D.; Hanfland, M. High-pressure behaviour and atomic-scale deformation mechanisms in inyoite, CaB3O3(OH)5·4H2O. Phys. Chem. Min. 2022, 49, 4. [Google Scholar] [CrossRef]
- Apetrei, A.; Mirebeau, I.; Goncharenko, I.; Crichton, W.A. Crystal structure under pressure of geometrically frustrated pyrochlores. J. Phys. Cond. Mat. 2007, 19, 376208. [Google Scholar] [CrossRef]
- Nearchou, A.; Cornelius, M.L.U.; Jones, Z.L.; Collings, I.E.; Wells, S.A.; Raithby, P.R.; Sartbaeva, A. Pressure-induced symmetry changes in body-centred cubic zeolites. R. Soc. Open Sci. 2019, 6, 182158. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Simoncic, P.; Moggach, S.A.; Gozzo, F.; Macchi, P.; Keen, D.A.; Tana, J.-C.; Cheetham, A.K. Reversible pressure-induced amorphization of a zeolitic imidazolate framework (ZIF-4). Chem. Commun. 2011, 47, 7983–7985. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, A. Equations of State of Simple Solids (Including Pb, NaCl and LiF) Compressed in Helium or Neon in the Mbar Range. Minerals 2019, 9, 684. [Google Scholar] [CrossRef]
- Dewaele, A.; Belonoshko, A.B.; Garbarino, G.; Occelli, F.; Bouvier, P.; Hanfland, M.; Mezouar, M. High-pressure high-temperature equation of state of KCl and KBr. Phys. Rev. B 2012, 85, 214105. [Google Scholar] [CrossRef]
- Yang, H.-X.; Hazen, R.M.; Prewitt, C.T.; Finger, L.W.; Lu, R.; Hemley, R.J. High-pressure single-crystal X-ray diffraction and infrared spectroscopic studies of the C2/m-P21/m phase transition in cummingtonite. Am. Min. 1998, 83, 288–299. [Google Scholar] [CrossRef]
- Angel, R.J.; Shaw, C.S.J.; Gibbs, G.V. Compression mechanisms of Coesite. Phys. Chem. Min. 2003, 30, 167–176. [Google Scholar] [CrossRef]
- Werner, S.; Barth, S.; Jordan, R.; Schulz, H. Single crystal study of sodalite at high pressure. Z. Krist. 1996, 211, 158–162. [Google Scholar] [CrossRef]
- Gibbs, G.V.; Ross, N.L.; Cox, D.F.; Rosso, K.M.; Iversen, B.B.; Spackman, M.A. Bonded radii and the contraction of the electron density of the oxygen atom by bonded interactions. J. Phys. Chem. A 2013, 117, 1632–1640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).