Abstract
Two ferrocene derivatives, namely, 1,2-(tetramethylene)-ferrocene and 1,2,1′,2′-bis(tetramethylene)-ferrocene, were synthesized in a four-step reaction sequence starting from ferrocene. Friedel–Crafts acylation of ferrocene using succinic anhydride gave mono- or bis(3-carboxypropinoyl)-ferrocene depending on the stoichiometry of succinic anhydride. The reduction of the keto groups to methylene followed by ring-closing using trifluoroacetic anhydride gave 1,2-(α-ketotetramethylene)-ferrocene or 1,2,1′,2′-bis(α-ketotetramethylene)-ferrocene. The diastereomeric mixture of the latter diketones was separated using column chromatography, characterized via single-crystal X-ray analysis, and assigned its stereochemistry. Reduction of the keto groups to methylene under Clemmensen conditions gave homoannular mono- or bis(tetramethylene)-ferrocene derivatives. The molecular structure of 1,2-(tetramethylene)-ferrocene revealed that the ipso carbon atoms of the cyclopentadienyl group are 0.023(3) Å farther away from Fe(II) compared to the remaining three carbon atoms. Both complexes exhibit lower half-wave oxidation potentials than ferrocene, possibly due to the electron-releasing effects of the tetramethylene bridges.
1. Introduction
Ferrocene and its derivatives play important roles in modern-day material chemistry owing to their air stability, aromatic reactivity, reversible electrochemistry, and low toxicity [1]. Ferrocene, since its discovery in 1951 [2], has been considered an analog of benzene for its aromatic properties. Benzene has been homologated up to 13 linearly fused rings [3], and the higher polycyclic aromatic hydrocarbons are promising candidates for organic optoelectronic applications [4]. However, the benzannulation of ferrocene to extend the π-conjugation of its cyclopentadienide (Cp−) ring is limited to indenide (Ind−) [5,6,7,8]. The indenide complexes of iron are commonly synthesized via direct metalation of the conjugate base of indene using Fe(II) synthons [9,10]. This method has limitations associated with the inherent synthetic difficulties of ligands for higher benzannulated ferrocene derivatives.
With our continuous interests in extending the π-conjugation of metallocenes [11], we synthesized 1′,2′,3′,4′,5′-pentamethylruthenocene-fused quinones from the double Friedel–Crafts acylation between metallocene-1,2-diacylchloride and organic aromatics [12]. Our attempts to reductively aromatize quinones were not successful. Later, we realized that switching the functionality of two reaction partners can give such complexes via a much simpler method. Ferrocene is an excellent nucleophile for Friedel–Crafts reactions; its reaction with succinic anhydride can eventually lead us to the conjugation of tetramethylene groups to one or both of its cyclopentadienyl rings [13]. These compounds can serve as the simplest possible precursors for extending the π-conjugation in ferrocene via dehydrogenation. In this contribution, we report the synthesis of 1,2-(tetramethylene)-ferrocene and 1,2,1′,2′-bis(tetramethylene)-ferrocene starting from ferrocene, molecular structures of key synthetic products, and the half-wave oxidation potentials of the final products.
2. Materials and Methods
2.1. General Procedures
Reactions were carried out using standard Schlenk line techniques under nitrogen unless otherwise described. Reagent-grade solvents and chemicals were purchased and used as received. Ferrocene, succinic anhydride, aluminum chloride, zinc powder, mercuric chloride (Alfa Aesar, Haverhill, MA, USA), and trifluoroacetic anhydride (Oakwood Chemicals, Estill, SC, USA) were used as purchased.
1H and 13C NMR spectra were recorded on a JEOL—400 ESZ spectrometer at room temperature and were referenced to residual solvent peaks. Infrared spectra were recorded on a Bruker Alpha-E FT-IR spectrometer using a diamond crystal ATR accessory. Melting points were taken on a Thomas–Hoover capillary melting point apparatus and were uncorrected. Half-wave oxidation potentials were determined using a BASi Epsilon—Electrochemical Workstation purchased from BASI® Corporation, West Lafayette, IN, USA.
2.2. X-ray Crystallography
X-ray diffraction data were measured at T = 90 K on a Bruker Kappa Apex-II diffractometer (Bruker, USA) equipped with a sealed-tube MoKα source (λ = 0.71073 Å), a Triumph focusing monochromator, and a CCD detector. Structures were solved using SHELXT [14] and refined using SHELXL [15]. Hydrogen atoms were visible in difference maps and were placed in idealized positions during refinement and treated as riding. The structure of 3b′ had a disorder involving partially occupied carbonyl groups at CH2 sites. The crystal structures and refinement data are presented in Table 1.

Table 1.
Crystal data and refinement parameters.
2.3. Experimental Procedures
1-(3-Carboxypropionyl)-ferrocene (1a). To a stirred solution of ferrocene (3.02 g, 16.3 mmol) in dichloroethane (40 mL), a suspension of succinic anhydride (1.83 g, 18.1 mmol) and aluminum chloride (4.20 g, 32.1 mmol) in dichloroethane (80 mL) was slowly added over a 45 min period. The reaction mixture was stirred for an additional 2 h at room temperature and then poured into ice-cold water (50 mL). The organic phase was extracted in dichloromethane (3 × 50 mL). The product was again extracted in 1.0 M NaOH (3 × 20 mL), and the aqueous layer was neutralized using concentrated HCl. The precipitate was collected via filtration, washed with water, and dried to give 1a (1.78 g, 38%) as an orange powder. Melting point: 165–166 °C (Lit. [16] 164–165). 1H NMR (400 MHz, CDCl3): δ 2.72–2.76 (t, 2 H, 3J = 6.8 Hz), 3.06–3.09 (t, 2 H, 3J = 6.4 Hz), 4.23 (s, 5 H, Cp), 4.51–4.52 (t, 2 H, 3J = 2.4 Hz), and 4.80–4.81 (t, 2 H, 3J = 2.0 Hz). IR: 1705 cm−1 (carboxylic C=O), 1657 cm−1 (ketonic C=O).
1,1′-Bis(3-carboxypropionyl)-ferrocene (1b). To a suspension of succinic anhydride (10.8 g, 108 mmol) and anhydrous aluminum chloride (28.8 g, 216 mmol) in dichloroethane (80 mL), a solution of ferrocene (10.0 g, 53.8 mmol) in dichloroethane (80 mL) was added slowly. The reaction was stirred for 48 h at room temperature and poured into ice-cold water (100 mL). The organic phase was collected and the aqueous phase was extracted using dichloromethane (2 × 100 mL). The product was extracted from the organic phase using 2 M sodium hydroxide (3 × 100 mL). The aqueous phase was neutralized using conc. HCl. The precipitate was collected via filtration. The crude product was suspended in boiling water (100 mL) and filtered to give 1b (14.9 g, 72%) as a dark red powder. Melting Point: 178–179 °C (Lit. [17] 179 °C). 1H NMR (400 MHz, acetone-d6, ppm): δ 2.60–2.64 (t, 4 H, 3J = 6.4 Hz), 3.02–3.05 (t, 4H, 3J = 6 Hz), 4.62–4.63 (t, 4H, 3J = 3.6 Hz), and 4.89–4.90 (t, 4 H, 3J = 2.4 Hz).
1-(3-carboxypropyl)-ferrocene (2a). This compound was synthesized using procedures reported in the literature [18]. Yield: 54%. Melting Point: 115–116 °C (Lit. [16] 115–116 °C). 1H NMR (400 MHz, CDCl3, ppm): δ 1.80–1.87 (m, 4 H), 2.35–2.40 (m, 8 H), and 3.98–4.09 (m, 8 H).
1,1′-bis(3-carboxypropyl)-ferrocene (2b). This compound was synthesized following procedures reported in the literature [17]. Yield: 90%. Melting Point: 72–73 °C (Lit. [17] 73 °C). 1H NMR (400 MHz, CDCl3, ppm): δ 1.80–1.87 (m, 4H), 2.35–2.40 (m, 8H), and 43.98–4.09 (m, 8H). 13C{1H} NMR (100 MHz, CDCl3, ppm): δ 25.95, 28.58, 33.64, 67.89, 68.88, 77.32, 88.08, and 179.97. IR: 1703 cm−1 (C=O), 3000–3200 cm−1 (OH).
1,2-(α-ketotetramethylene)-ferrocene (3a). This compound was synthesized by following procedures in the literature [19]. Yield: 81%. Melting Point: 82–83 °C (Lit. [20] 83–85 °C) 1H NMR (400 MHz, CDCl3, ppm): δ 2.02–2.22 (m, 2H), 2.25–2.45 (m, 2H), 2.59–2.67 (m, 2H), 4.17 (s, 5H, Cp), 4.45–4.47 (m, 2H, Cp), and 4.81–4.82 (m, 1H, Cp). 13C{1H} NMR (100 MHz, acetone-d6, ppm): δ 23.4, 23.9, 38.8, 64.6, 70.0, 75.8, 92.5, and 202.4. IR: 1664 cm−1 (C=O). The product was analyzed via single crystal X-ray analysis.
Isomeric 1,2,1′2′-bis(α-ketotetramethylene)-ferrocenes (3b and 3b′). To a stirred solution of 2b (0.501 g, 1.40 mmol) in dichloromethane (15 mL), a solution of trifluoroacetic anhydride (0.778 mL, 5.59 mmol) in dichloromethane (15 mL) was added dropwise. The reaction was stirred for 20 h at room temperature. A saturated solution of sodium bicarbonate (100 mL) was added to the mixture. The organic phase was collected, and the aqueous phase was extracted using dichloromethane (50 mL). The organic phase was washed with water (2 × 20 mL), dried with magnesium sulfate, and filtered. The volatiles were removed in vacuo to give 3b and 3b′ (0.190 g, 42%) as a bright red powder. The two diastereomers (3b and 3b′) were separated via silica column chromatography using a mixture of ethyl acetate and dichloromethane (2:1) as eluent. The racemic mixture (dark red) 3b′ was followed by the meso (dark orange) 3b in the column.
3b (Meso): Melting Point: 168–189 °C (Lit. [21] 161–167 °C). 1H NMR (400 mHz, CDCl3): δ 2.00–2.11 (m, 4H, CH2), 2.15–2.40 (m, 6H, CH2), 2.56–2.66 (m 4H CH2), 4.42–4.45 (m, 2H, Cp), and 4.73 (b, 2H, Cp). 13C{1H} NMR (100 MHz, CDCl3, ppm): δ 22.92, 23.66, 39.27, 67.38, 72.37, 73.19, 76.58, 93.42, and 204.33. IR: 1661 cm−1 (C=O). The product was characterized via single crystal X-ray analysis.
3b′ (Racemic): Melting Point: 156–157 °C (Lit. [21] 153–161 °C). 1H NMR (400 MHz, CDCl3): δ 2.01–2.15 (m, 2 H), 2.21–2.47 (m, 6H), 2.61 (dt, 2J = 15.6 Hz, 3J = 4.4 Hz, 4 H), 4.39 (d, 3J = 1.2 Hz, 2H), 4.42–4.43 (m, 4 H), and 4.65 (t, 3J = 1.2 Hz, 2 H). 13C{1H} NMR (100 MHz, CDCl3, ppm): δ 23.32, 23.52, 39.20, 68.03, 70.84, 71.85, 77.32, 94.00, and 203.40. IR: 1663 cm−1 (C=O). The product was analyzed via single crystal X-ray analysis.
1,2-(tetramethylene)-ferrocene (4a) and 1,2,1′,2′-bis(tetramethylene)-ferrocene (4b). The experimental setup and procedure were the same as that for 2a.
4a. Yield: 87%. Melting point: 39 °C (Lit. [22] 39–41 °C) 1H NMR (400 MHz, Acetone-d6, ppm): δ 1.53–1.64 (m, 2H), 1.85–1.92 (m, 2H), 2.17–2.26 (m, 2H), 2.60–2.67 (m, 2H), 3.85 (t, 3J = 2.4 Hz, 1H, CHCHCH), 3.94 (d, 3J = 2.4 Hz, 2H, CHCHCH), and 3.95 (s, 5H, Cp). 13C{1H} NMR (100 MHz, acetone-d6, ppm): δ 23.5, 24.6, 64.8, 65.1, 69.4, and 84.9.
4b. Yield: 59%. 1H NMR (400 MHz, acetone-d6): δ 1.53–1.57 (m, 4H), 1.85–1.88 (m, 4H), 2.16–2.23 (m, 4H), 2.57–2.64 (m, 4H), 3.65 (t, 3J = 2.4 Hz, 2H, CHCHCH), and 3.76 (d, 3J = 2.4 Hz, 4H, CHCHCH). 13C{1H} NMR (100 MHz, acetone-d6, ppm): δ 23.6, 23.9, 67.5, 68.3, and 84.2.
3. Results and Discussion
3.1. Synthesis and Structural Elucidation of Compounds 1a, 1b–4a, 4b
Synthesis of the target compounds (4a and 4b) was performed by following the reaction sequence as shown in Scheme 1. Our attempts to reproduce the procedures reported in the literature [23,24] resulted in the formation of an approximately equimolar mixture of mono-and bis(3-carboxypropenoyl)-ferrocene. To avoid the tedious separation of these two keto-carboxylic acids, we synthesized (3-carboxypropenoyl)-ferrocene, 1a (38%), by slowly adding a suspension of succinic anhydride and aluminum chloride in dichloroethane to the solution of ferrocene in the same solvent. The procedure left some unreacted ferrocene, but the product was purified via solvent extraction using NaOH solution. Synthesis of bis(3-carboxypropenoyl)-ferrocene, 1b (72%), was performed by adding ferrocene into the suspension of succinic anhydride and aluminum chloride and stirring the solution at room temperature for 48 h. After the base extraction, the product was found to contain 1a as an impurity, which was removed via washing with boiling water. The Clemmensen reduction of both keto carboxylic acids, 1a and 1b, was performed by following the literature procedures [17,18] to give 1-(3-carboxypropyl)-ferrocene, 2a (54%), and 1,1′-bis(3-carboxypropyl)-ferrocene, 2b (90%), respectively.
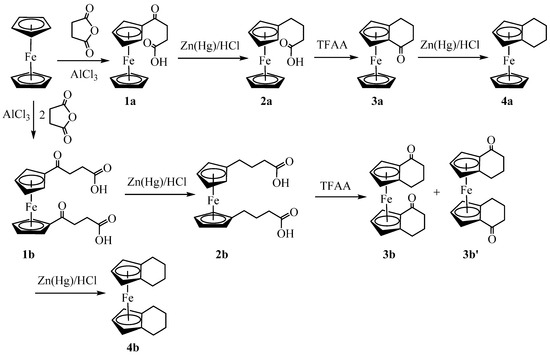
Scheme 1.
Synthesis of homoannular ferrocene derivatives.
The ring closing of 2a and 2b was performed in the presence of trifluoroacetic anhydride [19,21] to give homoannularly cyclized products 1,2(α-ketotetramethylene)-ferrocene, 3a (81%), and 1,2,1′,2′-bis(α-ketotetramethylene)-ferrocene, 3b (42%), respectively. The 1H NMR analysis of the crude product 3b revealed the formation of two geometric isomers (3b and 3b’) in an approximately 1:1 ratio. The diastereomers were separated using column chromatography. Distinctive ABC patterns of substituted cyclopentadienyl rings in 1H NMR for each compound indicated the formation of desired products. Similarly, the diastereotopic nature of exo- and endo-protons of the methylene groups exhibited a complex coupling pattern giving a set of multiplets (Figures S1, S4 and S7 see Supplementary Materials). Assignment of the stereochemistry of these geometrical isomers (meso—3b; racemic—3b′) was achieved via single crystal X-ray analysis (vide infra). Although Nesmeyanov [25] and Rinehart [13,21] independently reported their synthesis, their full characterization data of 3a and 3b were not available. The authors were unable to assign the configurations of the geometric isomers of 3b [21].
Reductions of the carbonyl groups of 3a and 3b to methylene were performed under Clemmensen conditions to give 1,2-(tetramethylene)-ferrocene, 4a (87%), and 1,2,1′,2′-bis(tetramethylene)-ferrocene, 4b (59%), respectively. The appearance of triplets (1H) and doublets (2H) on the substituted cyclopentadienyl rings in 1H NMR indicated the formation of desired products. Like ketones (3a, 3b, and 3b′), the diastereotopic nature of the exo- and endo-hydrogens of the methylene group gave a complicated coupling pattern in 1H NMR. Both signals of the cyclopentadienyl rings are shifted upfield for both 4a and 4b, but the shift is more prominent for 4b (triplet at 3.65 and doublet at 3.75 ppm) (Figures S10 and S12). Synthesis of compounds 4a [22] and 4b [26,27,28] was previously reported with limited characterization data. Our attempts to dehydrogenate 4a and 4b using 2,3-dichloro-5,6-dicyanoquinone (DDQ) resulted in the formation of intractable black products with limited solubility in organic solvents, possibly due to the formation of charge-transfer complexes [29].
3.2. X-ray Crystal Structures
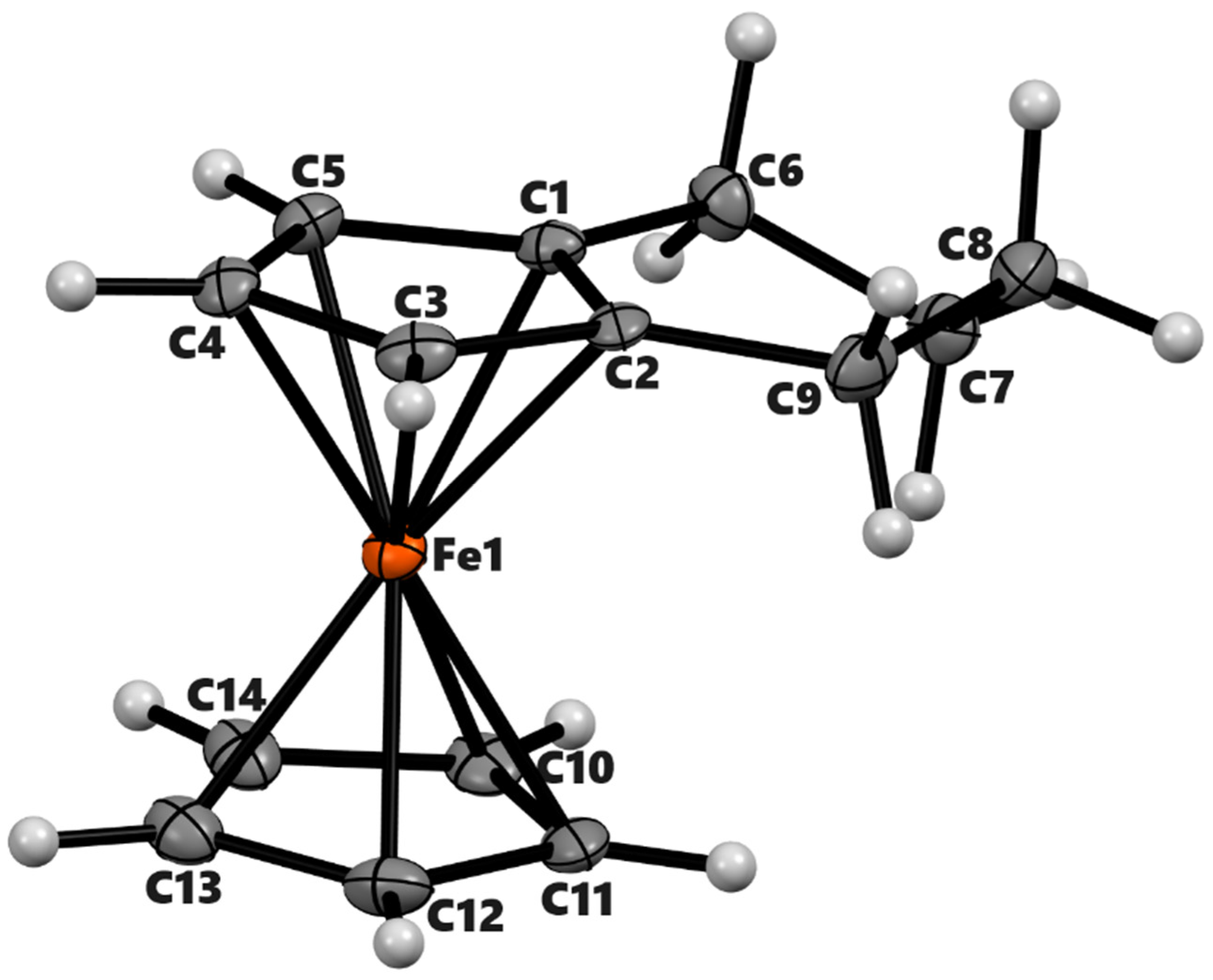
The structures of ferrocene derivatives 3a, 3b, 3b’, and 4a were determined using X-ray crystallography. All crystals were grown via slow evaporation of concentrated ethyl ether solutions in air at room temperature. Thermal ellipsoid plots with numbering schemes are shown in Figure 1, Figure 2, Figure 3 and Figure 4. The crystal and refinement data for these compounds are given in Table 1. The crystal structure of 3a has been reported by Fleischer et al. at room temperature [30]. We have redetermined its crystal structure at 90 K with much higher precision. The compound 3a exhibits planar chirality since two different substituents are connected to the cyclopentadienyl ring [31]. As the compound crystallizes in the centrosymmetric space group (P − 1) the two enantiomers Rp/Sp are present equally in the crystals. The iron atom in compound 3b lies on a crystallographic inversion center. Compound 3b′ crystallizes with Z’ = 2 in which one of the molecules has a disorder of the O atom in two positions. The 3b′ crystallizes as a racemic mixture in a centrosymmetric space group P21/n. The two molecules have roughly perpendicular orientations. Each molecule possesses an approximate two-fold rotation axis (Figure 3). Compound 4a crystallizes in the monoclinic space group P21/c with one molecule per asymmetric unit.
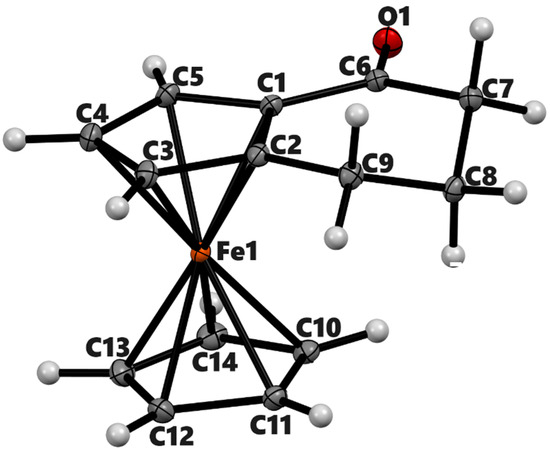
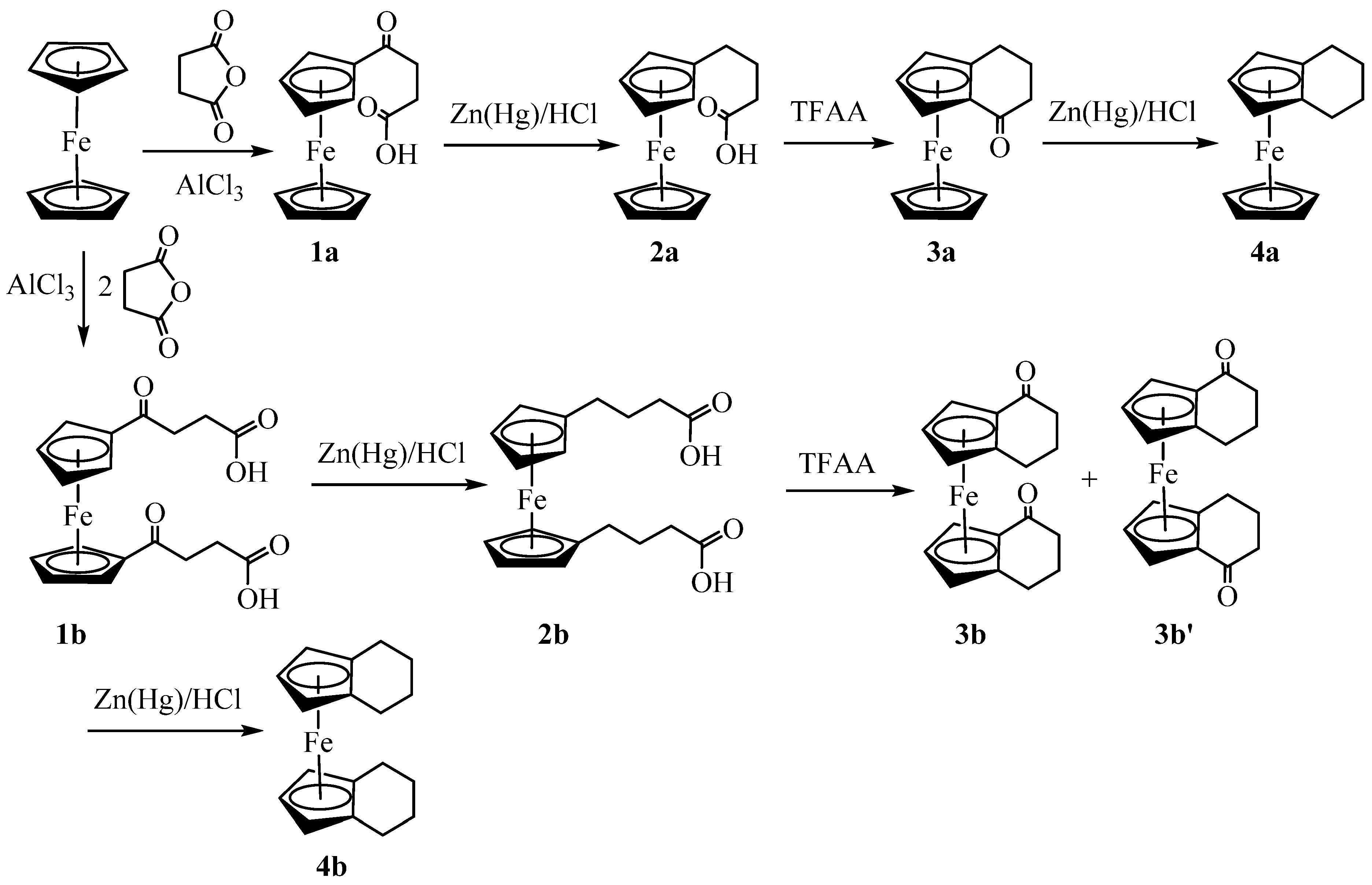
Figure 1.
ORTEP diagram of solid-state structure showing the atom-numbering scheme of compound 3a. Thermal ellipsoids are drawn at the 50% probability level. Selected bond lengths (Å) for the complex: Fe1–C1 2.0397(9), Fe1–C2 2.0508(8), Fe1–C3 2.0526(6), Fe1–C4 2.0527(7), Fe1–C5 2.0456(9), Fe1–C10 2.0553(7), Fe1–C11 2.0530(8), Fe1–C12 2.045(1), Fe1–C13 2.0548(9), Fe1–C14 2.0554(6), C1–C6 1.465(1), C6–O1 1.2265(9), Cp (centroid, substituted)–Fe 1.647, and Cp (centroid, unsubstituted) 1.655.
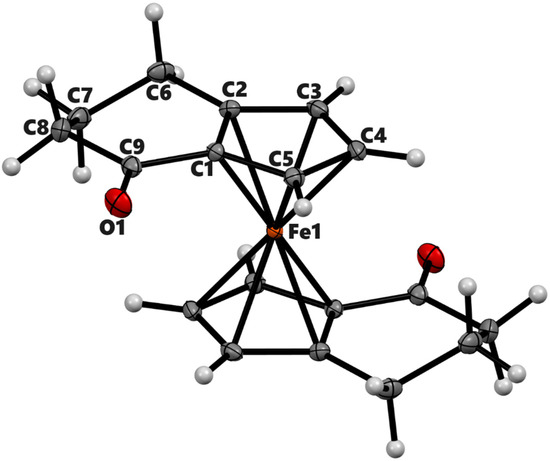
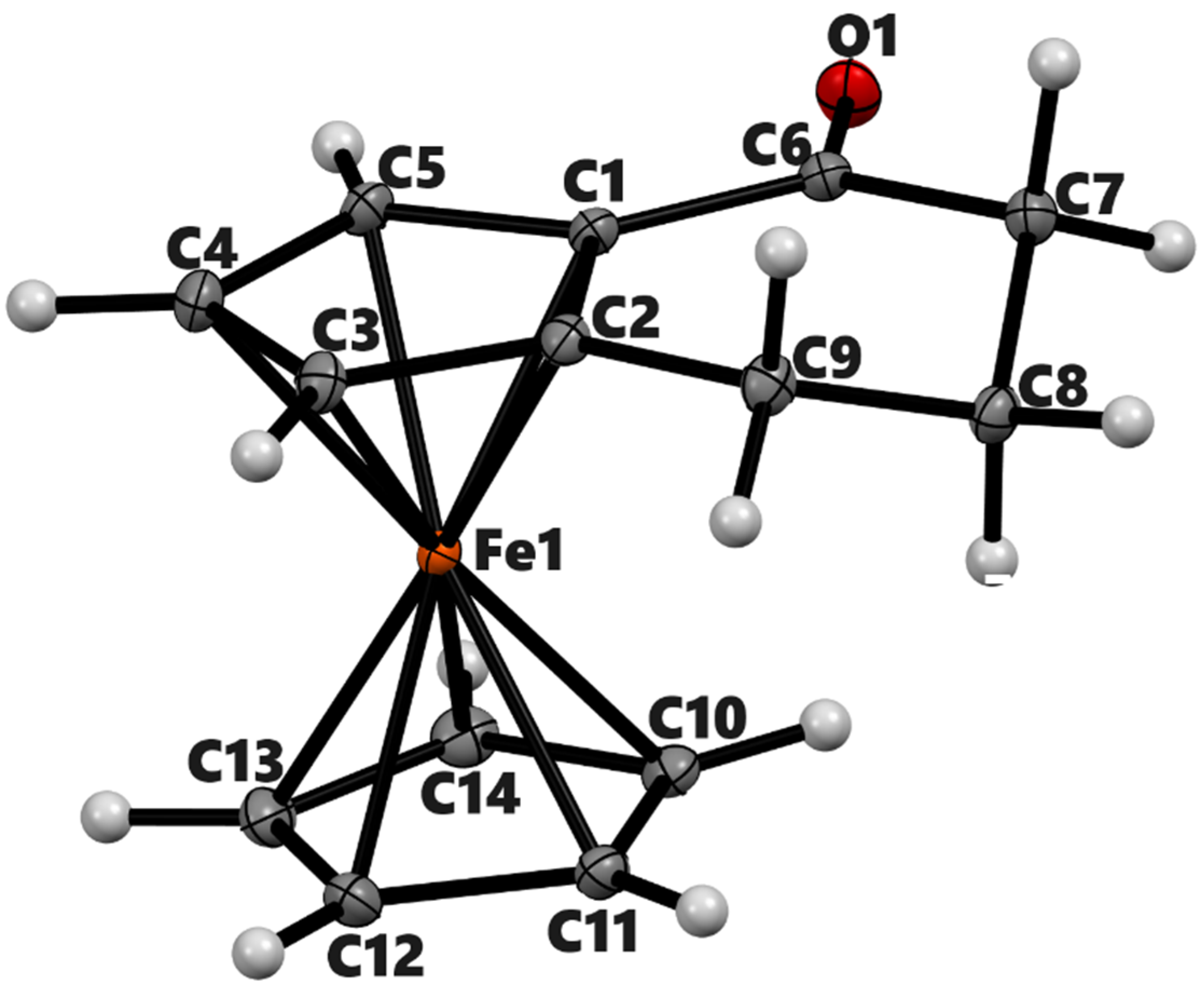
Figure 2.
ORTEP diagram of the solid-state structure showing the atom-numbering scheme of compound 3b (meso). Thermal ellipsoids are drawn at the 50% probability level. Selected bond lengths (Å) for the complex: Fe1–C1 2.0397(8), Fe1–C2 2.0627(7), Fe1–C3 2.0605(8), Fe1–C4 2.0664(8), Fe1–C5 2.0511(7), O1–C9 1.2230(9), and Cp (centroid)–Fe 1.660.
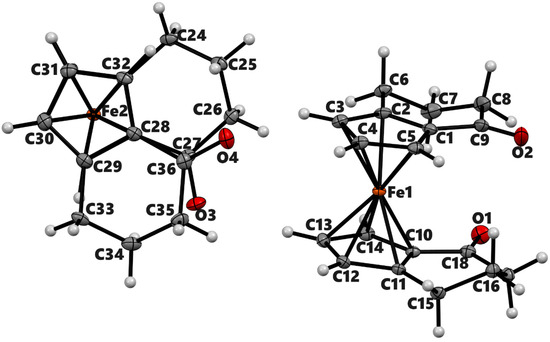
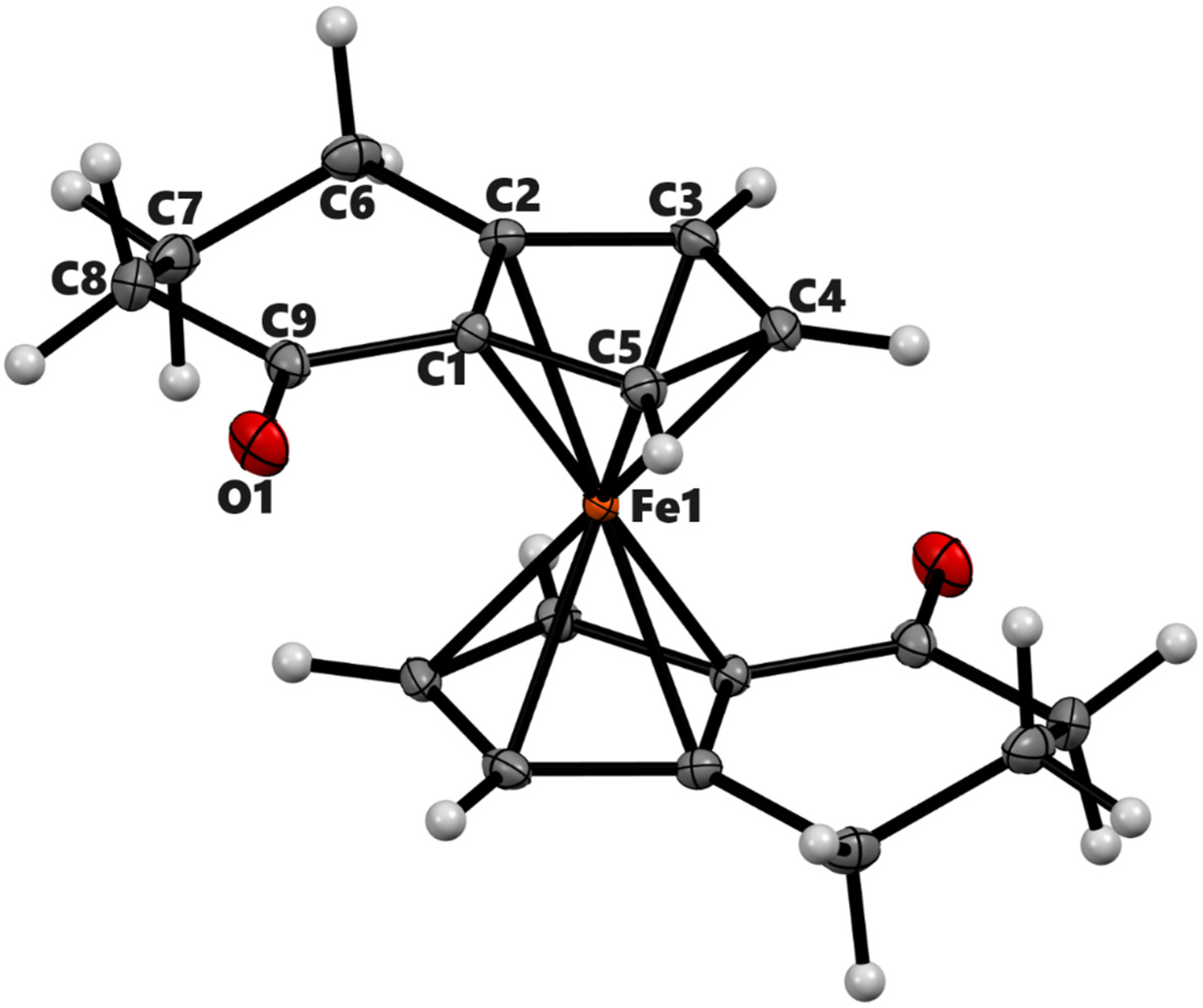
Figure 3.
ORTEP diagram of the solid-state structure showing the atom-numbering scheme of compound 3b′. Thermal ellipsoids are drawn at the 50% probability level. The minor component of the disordered O atom is not shown. Selected bond lengths (Å) for the complexes: Fe1–C1 2.0409(7), Fe1–C2 2.0575(7), Fe1–C3 2.0681(7), Fe1–C4 2.0534(7), Fe1–C5 2.0442(7), Fe1–C10 2.0469(6), Fe1–C11 2.0688(6), Fe1–C12 2.0615(7), Fe1–C13 2.0566(7), Fe1–C4 2.0534(7), Fe1–C4 2.0534(7), O2–C9 1.2212(17), and average Cp(Centroid)–Fe 1.652.
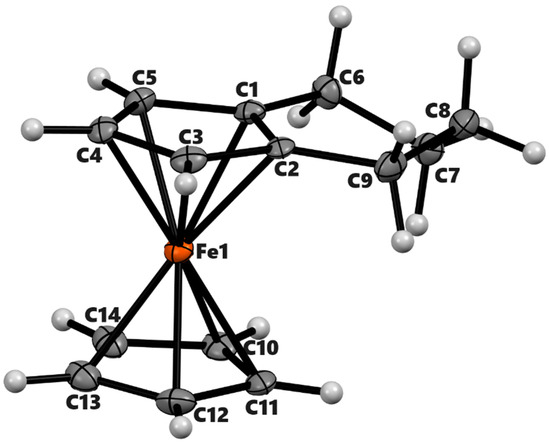
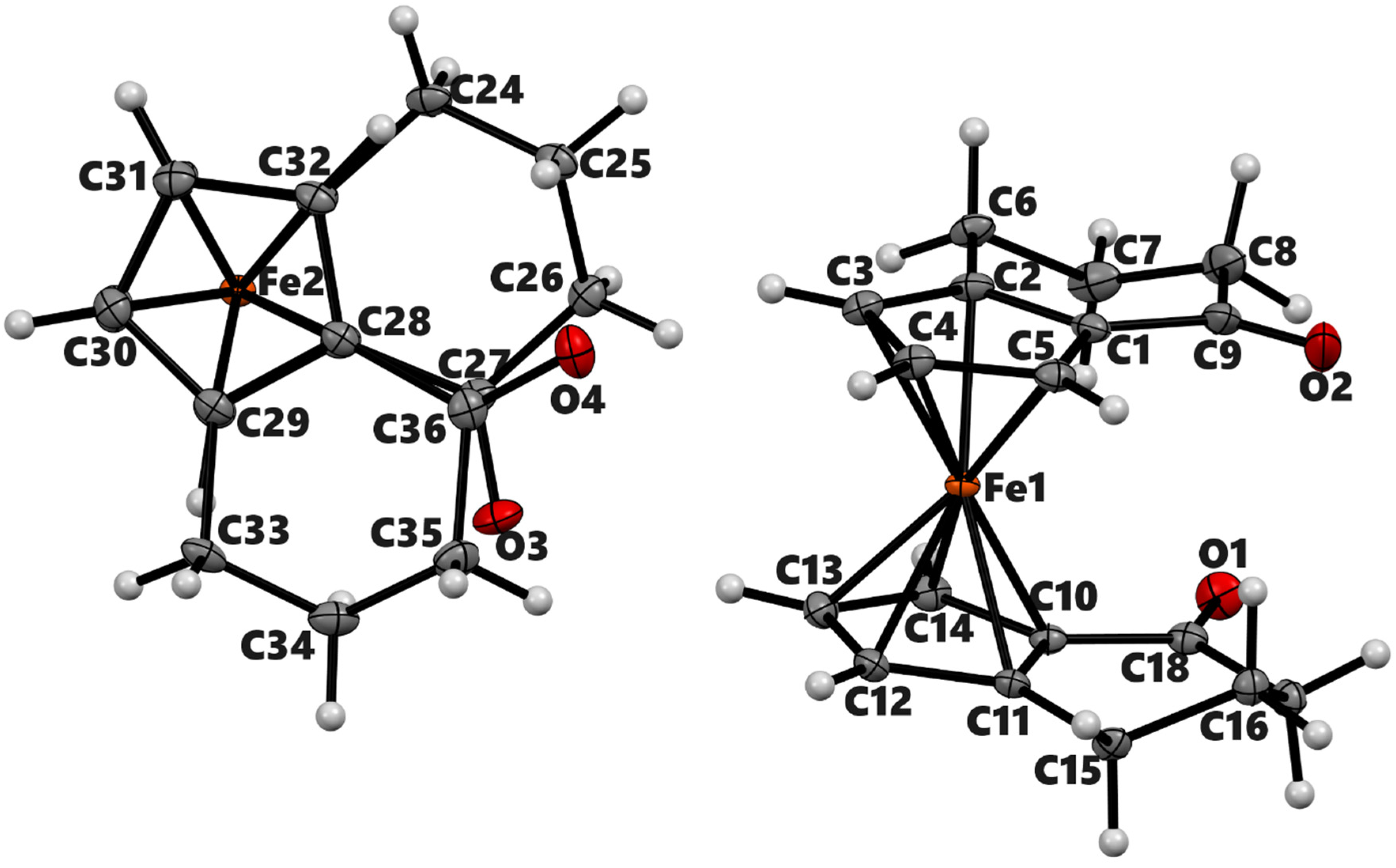
Figure 4.
ORTEP diagram of the solid-state structure showing the atom-numbering scheme of compound 4a. Thermal ellipsoids are drawn at the 50% probability level. Selected bond lengths (Å) for the complexes: Fe1–C1 2.049(3), Fe1–C2 2.064(3), Fe1–C3 2.040(3), Fe1–C4 2.034(2), Fe1–C5 2.031(3), Fe1–C10 2.043(3), Fe1–C11 2.036(3), Fe1–C12 2.045(3), Fe1–C13 2.051(3), Fe1–C14 2.042(3), Cp (centroid, substituted)–Fe 1.647, and Cp (centroid, unsubstituted) 1.648.
In the molecules 3a, 3b, and 3b′ the ferrocenyl moiety is fused with α-keto tetramethylene rings on one or both cyclopentadienyl rings, while in the molecule 4a the ferrocenyl moiety is fused with a tetramethylene ring. In all cases, the angle between Fe and two Cp ring centroids is nearly 180° with a maximum deviation of 4.85° from linear geometry in 3b′. The Cp rings in the ferrocene system are almost parallel, as the dihedral angles between the planes of the two Cp rings in 3a, 3b′, and 4a are 1.84°, 3.46° (average of two), and 1.92°, respectively. The Cp rings display nearly eclipsed conformation in 3a, 3b′, and 4a, as demonstrated by C–Cg1–Cg2–C average torsion angles of 9.12°, 0.26°, and 6.12°, respectively. The Cp rings in 3b are perfectly staggered (torsional angles 36.00°) as required by the inversion center located at Fe.
Within the ferrocenyl moieties, the mean Fe–C bond distances for unsubstituted Cp are unremarkable. However, the Fe–C distance for the ipso carbon-bearing keto group next to it is slightly shorter than the rest of the distances. For instance, the Fe1–C1 bond in compound 3a is 2.0397(9) Å while the average distance of Fe1 with C2 to C5 is 2.0505(7) Å. The slippage of the Fe center towards C1 has been observed in other α-keto ferrocenyl derivatives [32,33]. It presumably occurs to maximize the interaction of the Fe center with the exocyclic double bond in the resonance of such ketones [34]. The attached carbonyl group lies almost co-planar with the substituted cyclopentadienyl ring in 3a, 3b, and 3b’ as given by the torsional angle (C5–C1–C6–O1 = 1.52(11)°) in 3a. The C=O bond lengths in 3a (1.2265(9) Å), 3b (1.2230(9) Å), and 3b′ (1.225 (17) Å) are in normal range of similar α–ferrocenyl ketones [35,36]. In 4a, the average distance of Fe1–C1 and Fe1–C2 is higher by 0.023(3) Å than the rest of the Fe–C bonds of the substituted cyclopentadienyl ring. The distance from the Fe center to the centroid of cyclopentadienyl rings ranges from 1.647 Å to 1.660 Å.
The six-membered rings in all structures adopt half-chair conformations. The Cremer & People puckering parameters [37] of the six-membered rings, namely, 3a, 3b, 3b′, and 4a, are QT = 0.4340(8) Å, θ = 131.43(11)°, and φ = 2.47(14)°; QT = 0.4227(8) Å, θ = 129.68(10)° and φ = 358.42(13)°; QT = 0.4356(12) Å, θ = 53.54(14)°, and φ = 178.20(18)°; and QT = 0.521(3) Å, θ = 51.7(3)° and φ = 219.0(4)°, respectively. In 3a, atoms C6/C7/C9 lie on the same plane as the substituted cyclopentadienyl ring with a dihedral angle of 1.80°. The C8 projects inward from the main plane of Cp by 0.582 Å. Similar folding of six-membered rings can be seen in 3b and 3b′ as well. The two α-ketotetramethylene groups are oriented with dihedral angles of 180° and 72° in 3b and 3b′, respectively. The six-membered ring of 4a is more twisted than in 3a or 3b. In this molecule, C6 and C9 are almost coplanar with the substituted Cp (deviation: C6 = 0.018 Å and C9 = 0.033 Å). The C7 is projected down by 0.498 Å and C8 is projected up by 0.305 Å from the Cp plane.
In their crystal structures, compounds 3a, 3b, and 3b′ display similar intermolecular interactions. The most prominent contacts are weak intermolecular C–H⋯O hydrogen bonds and C-H⋯π interactions. For example, molecules of 3b exhibit intermolecular C–H4⋯O1, C–H5⋯O1 interactions along the crystallographic b-axis. Similarly, atom H8A is positioned almost perpendicularly above the cyclopentadienyl ring centroid of the adjacent molecule (Figure S14). In the crystal structure of 4a, there are C–H⋯π interactions between methylene hydrogen and the cyclopentadienyl rings (Figure S15).
3.3. Electrochemical Studies
To investigate the effects of tetramethylene substituents on the oxidation potential of the ferrocene moiety, we measured the half-wave redox potentials (E1/2) of 4a and 4b via cyclic voltammetry using 0.1 M tetrabutylammonium hexafluorophosphate in dichloromethane as a supporting electrolyte at a scan rate of 50 mV s−1 in 10−3 M concentration. All measurements were carried out at room temperature under a dry nitrogen atmosphere by the use of a three-electrode system: glassy carbon as the working electrode, Ag/AgCl as the reference electrode, and platinum wire as a counter electrode.
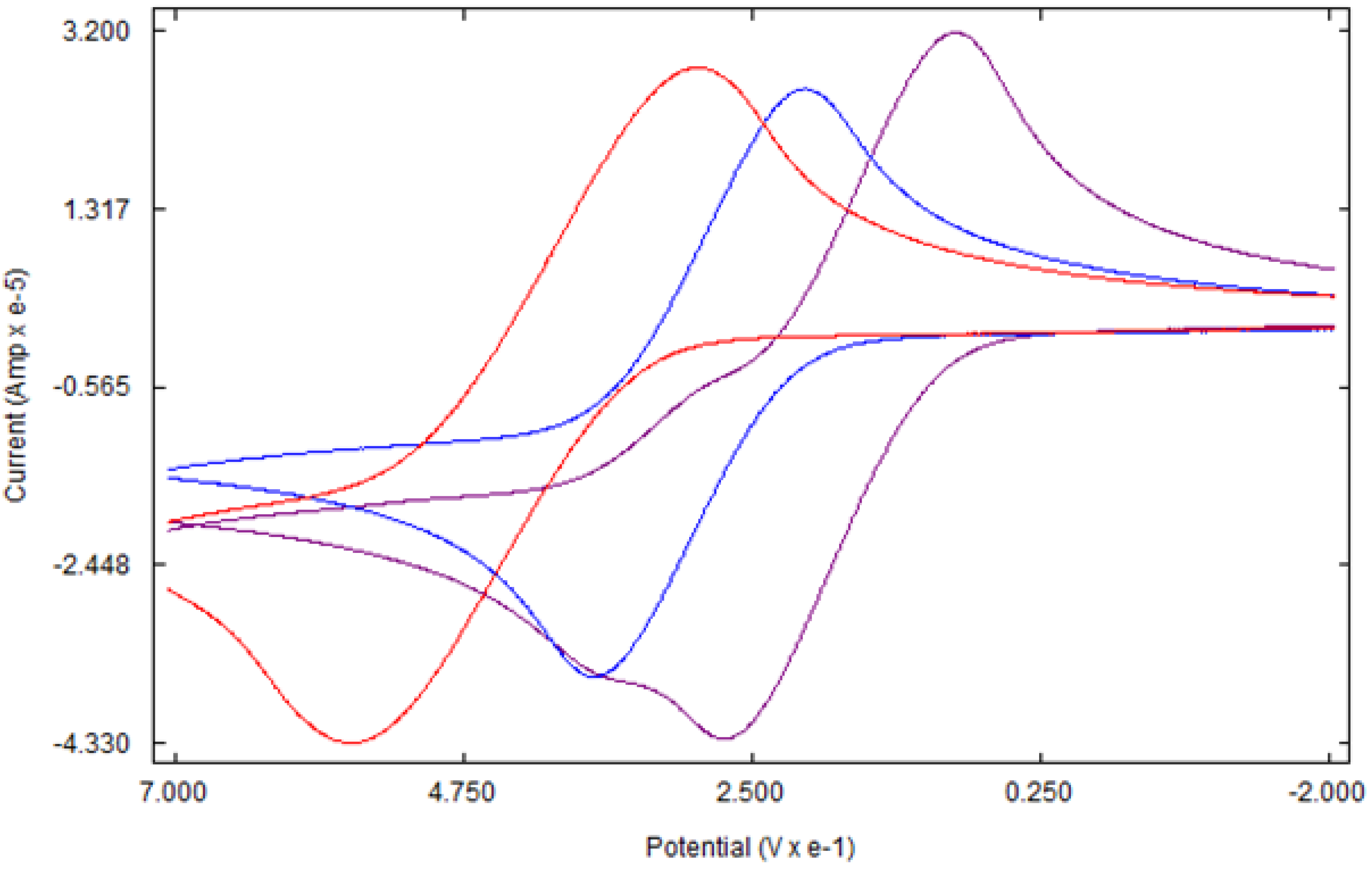
Like ferrocene, the cyclic voltammetry of 4a and 4b shows a single-electron reversible redox process (Figure 5). As expected, the redox potentials of both complexes are lower than that of ferrocene due to the electron-donating ability of tetramethylene rings. The higher electron density at the iron center due to the tetramethylene ring causes the iron center to lose an electron more easily as compared to ferrocene [38]. Under the experimental conditions, the E1/2 of ferrocene, 4a, and 4b are 499 mV, 303 mV, and 183 mV, respectively, versus Ag/Ag+. Thus, the oxidation potential of 4a is 196 mV lower than that of ferrocene. The addition of the second tetramethylene group, as in 4b, lowers the potential by 316 mV, nearly twice the value found between 4a and ferrocene. A slight deviation of the values from linearity might be due to experimental errors. Similar additivity effects of substituents on the oxidation potential of ferrocene have been reported in ferrocene derivatives with an increasing number of similar substituents [39,40].
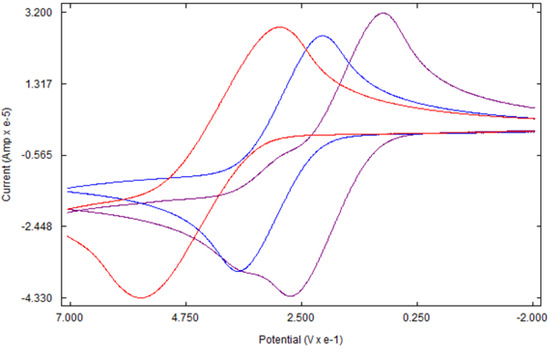
Figure 5.
Cyclic voltammogram of Ferrocene (red), 4a (blue), and 4b (purple).
4. Conclusions
We have synthesized and characterized mono- and bis-(tetramethylene)-ferrocenes as viable precursors for π-extended ferrocene derivatives. Four compounds of the reaction sequence have been studied using single-crystal X-ray analysis. The effects of tetramethylene groups on the half-wave oxidation potential were studied via cyclic voltammetry. Our attempts to dehydrogenate the final products of with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) were unsuccessful. Currently, we are working on alternative methods to aromatize the ferrocene-bound aliphatic rings.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst14020141/s1, Figure S1: 1H NMR of 3a; Figure S2: 13C NMR of 3a; Figure S3: IR spectrum of 3a; Figure S4: 1H NMR of 3b; Figure S5: 13C NMR of 3b; Figure S6: IR spectrum of 3b; Figure S7: 1H NMR of 3b′; Figure S8: 13C NMR of 3b′; Figure S9: IR spectrum of 3b′; Figure S10: 1H NMR of 4a; Figure S11: 13C NMR of 4a; Figure S12: 1H NMR of 4b; Figure S13: 13C NMR of 4b; Figure S14: Packing diagram of 3a; Figure S15: Packing diagram of 4a.
Author Contributions
Conceptualization, U.R.P.; synthesis and spectroscopic characterization, D.P.D., S.D.N. and G.S.E.; X-ray crystallography, F.R.F.; electrochemistry, M.A.L. and U.R.P.; writing—original draft preparation, U.R.P.; writing—review and editing, U.R.P. and F.R.F.; visualization, U.R.P.; supervision, U.R.P.; project administration, U.R.P.; funding acquisition, U.R.P. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the Louisiana Board of Regents. Contract number: LEQSF (2017–18)-RD-A-28.
Data Availability Statement
The original contributions presented in the study are included in the article and supplementary material, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors extend their appreciation to the Department of Chemistry and Physical Sciences, Nicholls State University for providing funds for purchasing chemicals and the Department of Chemistry, Louisiana State University for providing X-ray crystallography services free of charge.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Astruc, D. Why is ferrocene so exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Kealy, T.J.; Pauson, P.L. A New Type of Organo-Iron Compound. Nature 1951, 168, 1039–1040. [Google Scholar] [CrossRef]
- Yang, X.; Rominger, F.; Mastalerz, M. Benzo-Fused Perylene Oligomers with up to 13 Linearly Annulated Rings. Angew. Chem. Int. Ed. 2021, 60, 7941–7946. [Google Scholar] [CrossRef]
- Anthony, J.E. The larger acenes: Versatile organic semiconductors. Angew. Chem. Int. Ed. 2008, 47, 452–483. [Google Scholar] [CrossRef]
- Hewison, L.; Crook, S.H.; Johnson, T.R.; Mann, B.E.; Adams, H.; Plant, S.E.; Sawle, P.; Motterlini, R. Iron indenyl carbonyl compounds: CO-releasing molecules. Dalton Trans. 2010, 39, 8967–8975. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, H.K.; Lee, S.S.; Chung, Y.K. Convenient Synthesis of Mixed Ferrocenes. Organometallics 1997, 16, 304–306. [Google Scholar] [CrossRef]
- Perekalin, D.S.; Babak, M.V.; Karslyan, E.E.; Nelyubina, Y.V.; Kudinov, A.R. Synthesis of iron complexes [(η5-indenyl)FeL3]+ from the readily available [(η5-indenyl)Fe(η6-indene)]+. Inorganica Chem. Acta 2012, 392, 73–76. [Google Scholar] [CrossRef]
- Shirokii, V.L.; Knizhnikov, V.A.; Konovalov, T.P.; Zubreichuk, Z.P.; Erdman, A.A.; Nefedov, S.E.; Eremenko, I.L.; Yanovskii, A.I.; Struchkov, Y.T.; Maier, N.A. Electrochemical synthesis of π-indenyl-π-(3)-1,2-dicarbollyliron(III). Izv. Akad. Nauk Seriya Khimicheskaya 1993, 42, 764–765. [Google Scholar] [CrossRef]
- Belmont, J.A.; Wrighton, M.S. Photochemical conversion of (η5-C5H5)Fe(CO)2(η1-C5H5) and related complexes to ferrocene and related derivatives: Reactivity of the monocarbonyl intermediate. Organometallics 1986, 5, 1421. [Google Scholar] [CrossRef]
- Curnow, O.J.; Fern, G.M. Synthesis, structures and spectroelectrochemistry of methyl-substituted bis(η5-indenyl)iron(II) complexes. J. Organomet. Chem. 2005, 690, 3018–3026. [Google Scholar] [CrossRef]
- Pokharel, U.R. Organometallic Heterocycles and Acene-Quinone Complexes of Ruthenium, Iron and Manganese. Ph.D. Dissertation, University of Kentucky, Lexington, KY, USA, 2012. [Google Scholar]
- Pokharel, U.R.; Selegue, J.P.; Parkin, S. Ruthenocene 1,2-dicarboxylic acid, carboxylic anhydride, and acid chloride: A facile route to metallocene-fused acenequinones. Organometallics 2011, 30, 3254–3256. [Google Scholar] [CrossRef]
- Rinehart, K.L., Jr.; Curby, R.J., Jr. Ferrocene bridging and homoannular cyclizations. J. Am. Chem. Soc. 1957, 79, 3290–3291. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Patwa, A.N.; Gupta, S.; Gonnade, R.G.; Kumar, V.A.; Bhadbhade, M.M.; Ganesh, K.N. Ferrocene-linked thymine/uracil conjugates: Base pairing directed self-assembly and supramolecular packing. J. Org. Chem. 2008, 73, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Apreutesei, D.; Lisa, G.; Hurduc, N.; Scutaru, D. Synthesis and un-isotherm kinetic study of some ferrocene acids. Cent. Eur. J. Chem. 2004, 2, 553–562. [Google Scholar] [CrossRef]
- Overbaugh, S.C.; Allen, C.F.H.; Martin, E.L.; Fieser, L.F. γ-Phenylbutyric acid. Org. Synth. 1935, XV, 64. [Google Scholar] [CrossRef]
- Weißenbacher, M.; Sturm, T.; Kalchhauser, H.; Kratky, C.; Weissensteiner, W. Synthesis and characterization of novel aminophosphine ligands based on ferrocenodecaline backbones. Monatshefte Für Chem. 2002, 133, 991–1009. [Google Scholar] [CrossRef]
- Huffman, J.; Rabb, D. Notes- The Preparation of 1,2-(α-Ketotetramethylene)ferrocene. J. Org. Chem. 1961, 26, 3588–3589. [Google Scholar] [CrossRef]
- Rinehart, K.L., Jr.; Curby, R.J., Jr.; Gustafson, D.H.; Harrison, K.G.; Bozak, R.E.; Bublitz, D.E. Organic chemistry of ferrocene. V. Cyclization of ω-ferrocenylaliphatic acids. J. Am. Chem. Soc. 1962, 84, 3263. [Google Scholar] [CrossRef]
- King, R.B.; Bisnette, M.B. π-Cyclopentadienyl-π-indenyliron. Angew. Chem. Int. 1963, 75, 642. [Google Scholar] [CrossRef]
- Bernhard, Y.; Gilbert, J.; Bousquet, T.; Favrelle-Huret, A.; Zinck, P.; Pellegrini, S.; Pelinski, L. One-Pot Synthesis of 2,5-Disubstituted Furans through In Situ Formation of Allenes and Enolization Cascade. Eur. J. Org. Chem. 2019, 2019, 7870–7873. [Google Scholar] [CrossRef]
- Liu, G.; He, H.; Wang, J. Ferrocene redox controlled reversible immobilization of ruthenium carbene in ionic liquid: A versatile catalyst for ring-closing metathesis. Adv. Synth. Catal. 2009, 351, 1610–1620. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Vol’kenau, N.A.; Vil’chevskaya, V.D. Intramolecular acylation in the ferrocene series. Cyclization of γ-ferrocenyl substituted acids and oxo acids. Dokl. Akad. Nauk SSSR 1958, 118, 512. [Google Scholar]
- Osiecki, J.H.; Hoffman, C.J.; Hollis, D.P. Organometallic compounds. Ruthenium and iron derivatives of indene. J. Organomet. Chem. 1965, 3, 107. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Vol’kenau, N.A.; Vil’chevskaya, V.D. Intramolecular acylation in the ferrocene series. Dokl. Akad. Nauk SSSR 1956, 111, 362–364. [Google Scholar]
- Hallam, B.F.; Pauson, P.L. Ferrocene derivatives. IV. Indenyl- and tetrahydroindenyliron carbonyls. J. Chem. Soc. 1958, 646. [Google Scholar] [CrossRef]
- Brandon, R.L.; Osiecki, J.H.; Ottenberg, A. Reactions of metallocenes with electron acceptors. J. Org. Chem. 1966, 31, 1214. [Google Scholar] [CrossRef]
- Fleischer, E.B.; Hawkinson, S.W. The structure of α-keto-1,5-tetramethyleneferrocene. Acta Crystallogr. 1967, 22, 376–381. [Google Scholar] [CrossRef]
- Schlögl, K. Stereochemistry of Metallocenes. In Topics in Stereochemistry; John Wiley & Sons: Hoboken, NJ, USA, 1967; pp. 39–91. [Google Scholar]
- Paramasivam, S.; Purushothaman, S.; Seshadri, P.R.; Raghunathan, R. (E)-1-Ferrocenyl-3-[2-(2-hydroxyethoxy)phenyl]prop-2-en-1-one. Acta Crystallogr. Sect. E 2013, 69, m144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, S.; He, Z.; Xu, L.; Huang, S. 2-Amino-4-(4-chlorophenyl)-6-ferrocenylpyridine-3-carbonitrile. Acta Crystallogr. Sect. E 2008, 64, m730. [Google Scholar] [CrossRef]
- Bratych, N.; Hassall, K.; White, J. Redetermination of the structure of diferrocenyl ketone at low temperature. Acta Crystallogr. Sect. E 2003, 59, m33–m35. [Google Scholar] [CrossRef]
- Erben, M.; Ruzicka, A.; Vinklarek, J.; Stava, V.; Handlir, K. 1′-Acetylferrocene-1-carbonitrile. Acta Crystallogr. Sect. E 2007, 63, m2145–m2146. [Google Scholar] [CrossRef]
- Erben, M.; Vinklarek, J.; Ruzicka, A. Acetylferrocene-2-chloro-1-ferrocenylethanone (1/1). Acta Crystallogr. Sect. E 2011, 67, m1447–m1448. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.T.; Pople, J. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Okuda, J.; Albach, R.W.; Herdtweck, E.; Wagner, F.E. Substituent effects in multiply trimethylsilyl-substituted ferrocenes. Molecular structure of 1,1′,2,2′,4,4′-hexakis(trimethylsilyl)ferrocenium tetrafluoroborate. Polyhedron 1991, 10, 1741–1748. [Google Scholar] [CrossRef]
- Tateaki, O.; Kazuo, O.; Tadashi, F.; Shunsuke, M.; Taeko, I.; Akira, K.; Nobuyuki, T. Electrochemical properties of ferrocenophanes. I. Voltammetric studies on the oxidation of mono-, di-, and tri-bridged ferrocenophanes in acetonitrile. Bull. Chem. Soc. Jpn. 1981, 54, 3723–3726. [Google Scholar] [CrossRef]
- Kuwana, T.; Bublitz, D.E.; Hoh, G. Chronopotentiometric Studies on the Oxidation of Ferrocene, Ruthenocene, Osmocene and Some of their Derivatives. J. Am. Chem. Soc. 1960, 82, 5811–5817. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).