Abstract
Hexagonal ferrites with the formula SrxBa(1−x)Fe12O19 (x = 0; 0.3; 0.5; 0.7; and 1) were prepared using the citrate method. The main feature of this synthesis is a relatively low calcination temperature of 700 °C. An X-ray diffraction study revealed a single-phase material. According to SEM, the particles were 50−70 nm in diameter. The Curie temperature of the samples that were determined using the DSC method varied in a very narrow range of 455−459 °C. Analysis of the magnetic hysteresis loops obtained at 300 K and 50 K indicated all samples as magnetically hard materials in a single-domain state. The maximal magnetic characteristics encompass strontium hexaferrite. The terahertz spectra of complex dielectric permittivity and the spectra of infrared reflectivity were measured at room temperature in the range of 6–7000 cm−1. The obtained broad-band spectra of the real and imaginary parts of permittivity reveal significant changes associated with structural distortions of the (Sr,Ba)O12 anti-cuboctahedron caused by the substitution of Ba2+ with Sr2+ in the same crystallographic positions.
1. Introduction
Hexagonal ferrites are the complex oxide systems structurally related to the magnetoplumbite mineral, which has the formula PbFe7.5Mn3.5Al0.5Ti0.5O19. There are several known types of hexagonal ferrites, M, Z, W, X, Y, U, and V. Among them, M-type ferrites are the most widely used due to their specific properties. M-type ferrites possess a range of useful properties: high chemical resistance, a wide range of magnetic resonance frequencies, high values of electrical resistance along with a high Curie temperature, and magnetic anisotropy constants, while having an easy magnetization axis along the c axis. [1,2,3].
Currently, several methods are commonly employed for the synthesis of ferrites. The most widely used among them is the ceramic method. It involves mixing the powders of the initial compounds and the calcination of the mixture at high temperatures. Powder mixing is frequently accompanied by mechanochemical activation. The duration and temperature of the heat treatment, the time of mechanochemical activation, and the nature of the starting materials play important roles [4,5,6,7].
The citrate method is the most universal and involves the preparation of a gel containing evenly distributed metal ions, which is then dried and combusted [8,9,10,11]. Another well-known method is hydrothermal synthesis, which is carried out in autoclaves at high pressures, wherein an aqueous solution of metal salts is hydrolyzed. This process is controlled by the temperature, time, and concentration of surfactants and alkali compounds [12,13,14].
The co-precipitation method could also be used to obtain hexaferrites. This method commences with a solution containing soluble forms of metals followed by their co-precipitation. After drying, the co-precipitated solid is calcined, resulting in the formation of the target phase. For such a process, it is essential to select reagents with similar solubility and precipitation rates [15,16,17].
The flux method is employed to obtain hexaferrites in large crystal structures. The mixture of metal oxides or salts with an addition of flux is heated up in a platinum crucible to form a melt. When the molten solution is slowly cooled, the crystals of the target product begin to grow. The purity of the starting materials and the cooling rate affect the quality and size of the crystals [18,19,20].
Other methods for complex oxide production include self-propagating high-temperature synthesis [21], combustion in the presence of carbon [22], and microwave combustion [23]. These methods are not often used to obtain hexagonal ferrites, since they are more specific than the methods described above.
Materials based on M-type ferrites are suitable for the production of permanent magnets, in data-recording and storage devices, ferrite cores, pigments, sensors, and catalysts [2,3,24]. M-type ferrites could also be applied as a component for the hyperthermal method of tumor treating [25,26,27].
The doping of barium hexaferrite is commonly performed to change its properties. Thus, substitution with Al3+ ions increases the coercive force of hexaferrite, but at the same time reduces the residual magnetization, which makes such a material suitable for further use as a microwave scanner [1]. The use of two substituent ions, Y3+ and Co2+, is beneficial in obtaining materials for microwave devices [24]. The substitution of Ba2+ with Pb2+ makes it possible to obtain a material suitable for application in transformers and in ferrite filters operating at high frequencies [28]. The material obtained through doping with La3+–Ca2+–Co3+ ions is used under high frequency and microwave engineering [29]. By including Tb3+ at the positions where Ba2+ resides, it is possible to obtain a material suitable for high-frequency devices and magnetic data-recording devices [30].
If Co2+ and Ti4+ are simultaneously used for the substitution of Fe3+, the resulting hexaferrite is optimally suitable for recording devices, like computer hard drives (SSDs), due to having a small grain size, low coercive force, and high saturation magnetization [1]. For the same purposes, the simultaneous substitution of Fe3+ with Ni2+, Co2+, and Ba2+ for Sr2+ ions has been shown to be suitable [31].
Barium hexaferrite substituted with Ga3+, Zn2+, and Sr2+ ions can be used to create microwave absorbers [32]. Another promising material for a microwave absorber is Nd3+–Cd2+-substituted hexaferrite [33]. The combined use of Sr2+, Ca2+, and Al3+ ions makes it possible to achieve a higher level of energy in a permanent magnet [34,35]. In addition to doping, another relevant field of research is the preparation of hexaferrite-based composite materials, where spinel ferrite could be used as a secondary compound to enhance magnetic properties [36] or to create polymer composites for microwave absorption [37,38,39]. These are two primary applications for the M-type ferrites. However, systems like SrFe12O19 still attract attention from the synthesis process-tuning point of view [40,41].
The purpose of this study was to conduct the sol-gel synthesis of nanosized hexaferrites with the formula SrxBa(1−x)Fe12O19, where x = 0; 0.3; 0.5; 0.7; and 1, and the investigation of the structural parameters, morphology, and magnetic properties of the products.
2. Materials and Methods
The sol-gel synthesis of SrxBa(1−x)Fe12O19 (x = 0; 0.3; 0.5; 0.7; 1) was carried out according to the following scheme: the initial compounds Sr(NO3)2, Ba(NO3)2, and Fe(NO3)3·9H2O, in stoichiometric amounts, were taken along with the citric acid monohydrate, which was added in a 2-fold excess relative to the metal ions and dissolved in distilled water. For example, 4.2598 g of Fe(NO3)3·9H2O, 0.0579 g of Sr(NO3)2, 0.1668 g of Ba(NO3)2, and 7.5 g of C6H8O7·H2O was dissolved in 20 mL of distilled water for the synthesis of Sr0.3Ba0.7Fe12O19. Following the dissolution of all components, the solution was kept at 100 °C in an oven until a dry dark brown gel was formed. The resulting gel was placed in a muffle furnace and heated up to 500 °C at 10 °C/min, held for 3 h at 500 °C, and then cooled. The obtained light brown powder was ground in a mortar and calcinated at 700 °C for 3 h.
In order to compare the influence of the preparation method used on the magnetic properties of a product, solid solutions of strontium-substituted barium hexaferrite were synthesized using a convenient ceramic method. For this method, SrCO3, BaCO3, and Fe2O3 were mixed with a mortar, pressed in a pellet, and calcined in the air at 1400 °C for 5 h. The product of this ceramic technique was a dark brown powder.
Both the sol-gel and ceramic materials were investigated using the following methods: powder X-ray diffraction (XRD) scanning electron microscopy (SEM), energy-dispersive X-ray fluorescence spectroscopy (EDX), differential scanning calorimetry (DSC), vibrating magnetometry, and terahertz (THz) and infrared (IR) spectroscopy. The following equipment were used for these methods: the powder diffractometer Rigaku Ultima IV (Chelyabinsk, Russia) (CuKα radiation), the scanning electron microscope JEOL JSM-7001F with an energy-dispersive X-ray fluorescence spectrometer Oxford INCA X-max 80 (Chelyabinsk, Russia), the simultaneous thermal analyzer Netzsch STA 449F1 “Jupiter” (Chelyabinsk, Russia), the vibrating magnetometer Quantum Design PPMS VersaLab (Chelyabinsk, Russia), the terahertz time-domain spectrometer Menlo Tera K15 (Moscow, Russia), and the vacuum infrared Fourier transform spectrometer Bruker Vertex 80v (Moscow, Russia). Structural parameters were calculated using Jana 2006, FullProf 2021, and Rigaku PDXL software.
3. Results and Discussion
3.1. XRD Analysis
The diffraction patterns of the obtained samples confirmed the purity of the materials obtained using the citrate method (Figure 1) and the ceramic method (Figure 2).
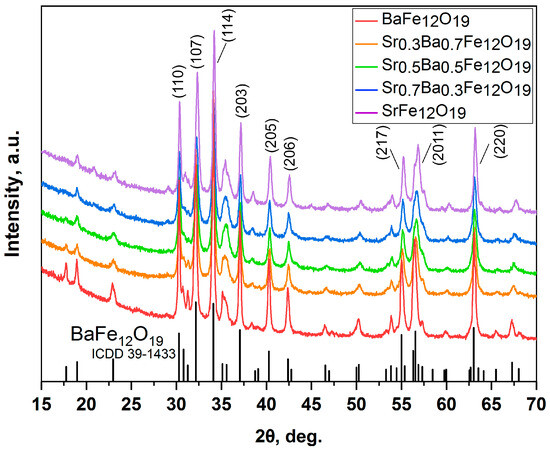
Figure 1.
XRD patterns of SrxBa(1−x)Fe12O19 obtained using the citrate method (x = 0; 0.3; 0.5; 0.7; and 1).
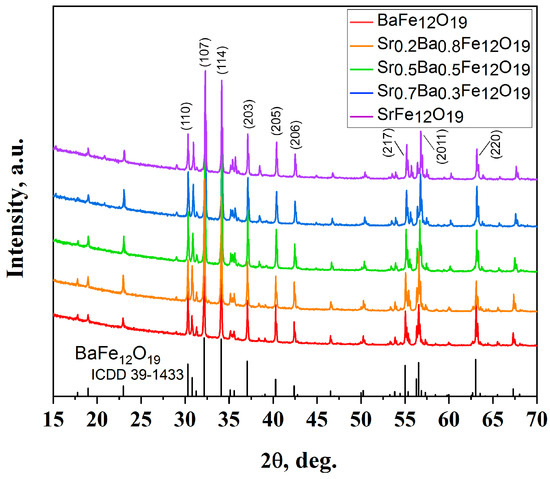
Figure 2.
XRD patterns of SrxBa(1−x)Fe12O19 obtained using the ceramic method (x = 0; 0.2; 0.5; 0.7; and 1).
Rietveld refinement (Figures S1–S5) of the obtained XRD patterns for the citrate samples was carried out under the following conditions: for x = 0 and 0.3, BaFe12O19 was used as a starting model for refinement, for the x = 0.5, 0.7, and 1, it was SrFe12O19. The parameters Rp (profile fitting R-value), Rwp (weighted profile R-value), and χ2 (goodness-of-fit quality factor) obtained after refinement are presented in Table 1. The Rp and Rwp were smaller than 4% and χ2 was lower than 3, which indicates a good agreement between our simulated structures and the actual structures. Following this refinement, there were obtained values for the unit cell parameters a and c and the CSR sizes (DR) (Table 1).

Table 1.
Curie temperatures, unit cell parameters, and CSR size for the citrate method-obtained samples.
Using the formula detailed in the Wiliamson–Hall method (1) (Figure 3), the coherent scattering region (CSR) size, as well as the lattice strain, were calculated for each sample (Table 1). As outlined in Equation (1) D—size of the CSR, ε—lattice strain, k—shape factor equal to 0.94, λ = 1.54 Å—wavelength of copper radiation, and β—full width at half maximum of the reflection:
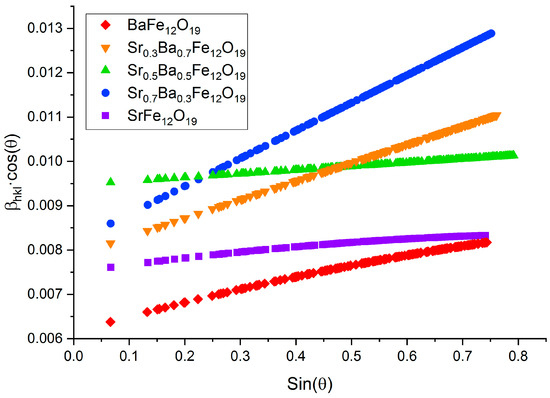
Figure 3.
Wiliamson–Hall patterns of SrxBa(1−x)Fe12O19 obtained using the citrate method (x = 0; 0.3; 0.5; 0.7; and 1).
From this method, the average CSR size for the synthesized hexaferrites was 19.7 nm, which indicates that they are present in their nanodispersed state. Values of lattice strain reaching the order of 10−3 may refer to the initial stages of crystal formation.
Indexing the obtained XRD patterns made it possible to determine the unit cell parameters for each obtained solid solution (Table 1). Since the ionic radius of Sr2+ (1.44 Å) is smaller than that of Ba2+ (1.61 Å), the unit cell and its parameters decreased with x (Figure 4). The linear dependence indicates the Vegard’s law, which confirms the formation of the substitutional solid solutions in both sets of powders. Small deviations in the a and c parameters from Vegard’s law at x = 0.5 and 0.7 may indicate their tendency to separate into two solid solutions.
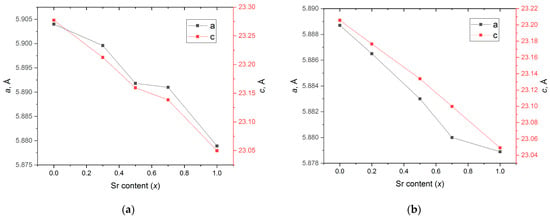
Figure 4.
Dependence of the unit cell parameters a and c on x in SrxBa(1−x)Fe12O19 prepared using the (a) citrate method and the (b) ceramic method.
3.2. Morphological Analysis
SEM images of the citrate samples (Figure 5) indicate an average particle size of the samples of about 50 nm. The obtained maps (as shown in Figure 6) display a homogeneous distribution of elements within the samples. SEM images of the ceramic samples (Figure 7) present a grain size of about 1 μm; such an increase in size occurred as a result of recrystallisation effects.
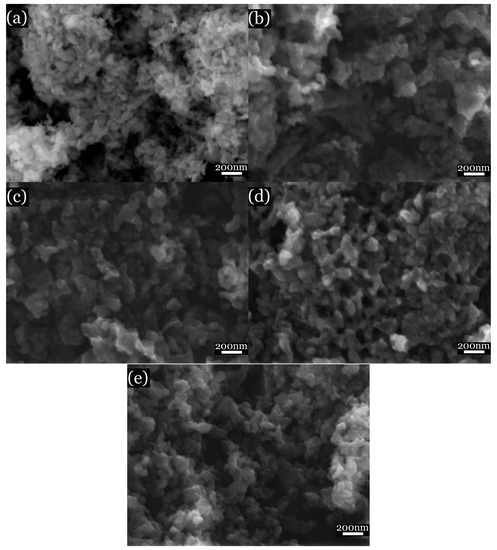
Figure 5.
SEM images of SrxBa(1−x)Fe12O19 citrate samples. (a) x = 0; (b) x = 0.3; (c) x = 0.5; (d) x = 0.7; and (e) x = 1.

Figure 6.
Maps of oxygen, iron, and strontium over a SrFe12O19 citrate sample.
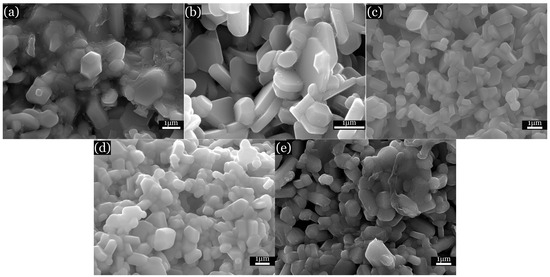
Figure 7.
SEM images of SrxBa(1−x)Fe12O19 ceramic samples. (a) x = 0; (b) x = 0.2; (c) x = 0.5; (d) x = 0.7; and (e) x = 1.
3.3. DSC Analysis
Differentiation of the DSC curves provided the DDSC curves (Figure 8), which permit a more accurate determination of the Curie temperature (Tc) values (Table 1). Our values of Tc do not differ strongly from the values obtained with bulk materials [1]. Tc was found to increase with x, which was probably related to shorter interatomic distances, which is known to increase superexchange interactions between Fe sites. Decreasing interatomic distances formed as a result of the substitution of the larger Ba2+ ions with the smaller Sr2+ ions have already been investigated in previous studies [42,43].
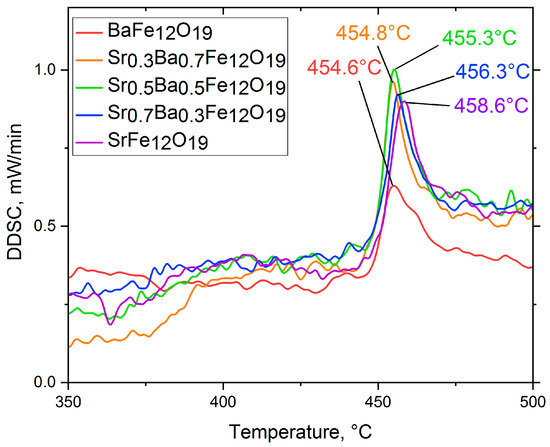
Figure 8.
DDSC data of the SrxBa(1−x)Fe12O19 citrate samples.
3.4. Magnetic Measurements
Magnetic measurements were carried out with a magnetic flux density of up to 3T at the temperature range from 50 K to 300 K. The resulting wide hysteresis loops (Figure 9) indicate the magnetically hard material with a decrease in the remnant magnetization (Mr) and saturation magnetization (Ms) with the temperature. For each sample, Ms is almost twice as large as Mr, which indicates a random orientation of the single-domain particles and the existence of uniaxial anisotropy.
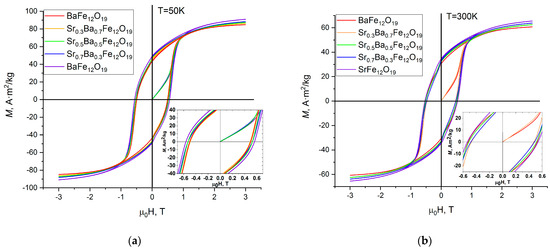
Figure 9.
Hysteresis loops at 50 K (a) and 300 K (b) for the SrxBa(1−x)Fe12O19 citrate samples.
According to the Stoner–Wohlfarth model, Ms and effective magnetic anisotropy (Keff) can be determined using Equations (2) and (3) [32], respectively, from the hysteresis data at a high-field region of the plot. By plotting magnetization (M) against 1/H2 and extrapolating the linear plot to a zero value of 1/H2 (Figure 10), we can thereby obtain values of Ms, while the slope value provides the parameter B multiplied by Ms.
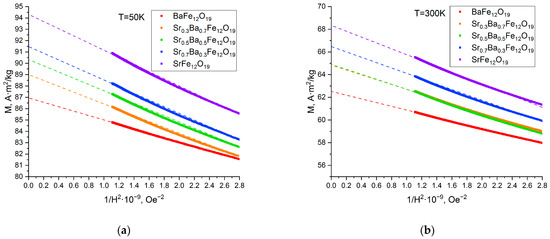
Figure 10.
M against 1/H2 plot at 50 K (a) and 300 K (b) for the SrxBa(1−x)Fe12O19 citrate samples. Dashed line—linear extrapolation of the M function.
Using these data, can be evaluated using Equation (3):
The obtained values of Ms and (Table 2) were slightly higher than the values for Ba0.5Sr0.5Fe12O19 known from the literature: 0.497 × 105 J/m3 at 300 K [32]. Obviously, annealing at 1200 °C resulted in the growth of the grain size that exceeded the single domain. Much higher values of for SrFe12O19 was presented by the authors of [44], 4.47–5.45 × 105 J/m3 (at 300 K), with sintering temperatures of 850–950 °C. These may have resulted from the grain size being closer to a critical size of a single domain, but not exceeding it.

Table 2.
Magnetic characteristics at 50 K and 300 K for the SrxBa(1−x)Fe12O19 citrate samples.
From the magnetization plots for samples obtained using the citrate method (Figure 9b) and samples obtained using the ceramic method (Figure 11), a strong difference in the Hc parameter was observed. The differences in Hc, Ms, and Mr can be explained by the single-domain state of the particles that were obtained using citrate method. The particle size for samples calcinated at 1400 °C (Table 3) is an order of magnitude larger than the citrate samples calcined at 700 °C. In the single-domain state, no motion of the domain border is possible in the particle itself, which has a positive effect on the value of the coercive force.
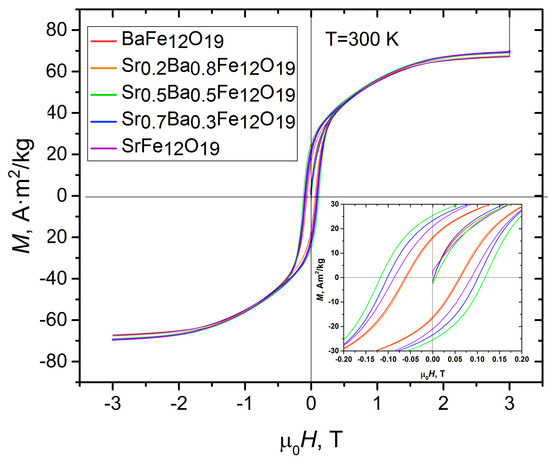
Figure 11.
Hysteresis loop at 300 K for the SrxBa(1−x)Fe12O19 ceramic samples.

Table 3.
Magnetic characteristics at 300 K of the samples obtained at 1400 °C and their grain sizes using SEM.
Figure 12 shows the dependence of the magnetization on the temperature at a magnetic field of 0.05 T for the SrxBa(1−x)Fe12O19 samples that were obtained using the citrate method. Compounds with x equal to 0 and 0.3 are depicted by a dashed line and have large values of magnetization, which were determined to have been formed as a result of their prior saturation with a magnetic field. For each sample, their magnetization increased linearly when cooled from 300 K to 100 K, following which the rate of increase slowed down.
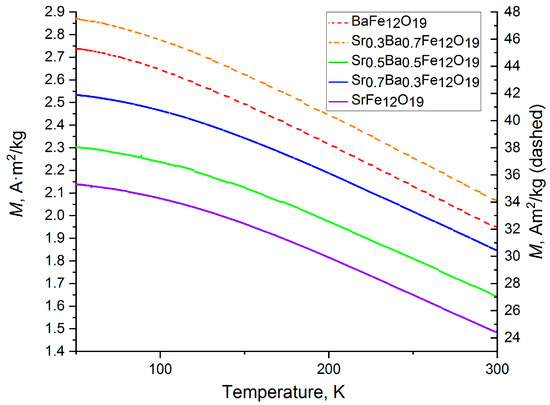
Figure 12.
Field-cooled magnetization curves of the citrate SrxBa(1− x)Fe12O19 samples at 0.05 T.
Fitting the M(T) plot according to the modified Bloch’s law for nanoparticles (Equation (4)) [45] resulted in β and B parameters (Table 4). The obtained β exponent was much larger than standard value of 1.5, which indicates that the material is not bulk, but rather nanodispersed.

Table 4.
Results of Bloch’s law fitting for the SrxBa(1−x)Fe12O19 citrate samples.
3.5. Terahertz–Infrared Spectroscopy
For terahertz and infrared measurements, plane-parallel pellets 5 mm in diameter and 0.7–0.8 mm thick were carefully prepared through pressing. At terahertz frequencies ( = 6–60 cm−1), room-temperature spectra of the complex transmission coefficient (amplitude and phase) were measured that allowed for direct determination of the spectra of the real and imaginary parts of complex dielectric permittivity, With the standard Fresnel equations, the THz spectra of the reflection coefficients of the bulk and layered samples were calculated from the spectra of the real and imaginary parts of permittivity, and merged with the infrared spectra of the reflection coefficient measured up to 7000 cm−1. Broad-band terahertz–infrared spectra of real and imaginary permittivity were obtained through a least square analysis of the combined THz–IR reflectivity together with the directly measured THz spectra of and These spectra allowed for the detailed analysis of the nature of the observed absorption resonances. An example of such data processing is shown in Figure 13. Figure 14 displays the so-obtained broad-band spectra of imaginary permittivity of all studied samples Ba1−xSrxFe12O19, with x = 0, …, and 1. According to the factor-group analysis of the M-type hexaferrite’s structure with the P63/mmc space group, 17 E1u and 13 A2u phonon modes were expected for the a‖E and c‖E geometries of the single-crystal experiments, respectively [46] (E is the electric field vector of the probing terahertz/infrared radiation). Since the samples are powders, the spectra contain a superposition of all these phonon modes due to the orientation averaging of grains/particles with different directions of the crystallographic axes. For the same reason, not all symmetry-resolved lines were able to be separated and recognized in the experimental spectrum (Figure 13). That is why we only considered 22 phonon modes to fit the THz–IR reflectivity/permittivity spectra, each modeled through Lorentzian expression for the complex dielectric permittivity:
where is the high-frequency permittivity that is weakly dependent on the temperature; is the oscillator strength, Δεj denotes the dielectric contribution, νj represents the frequency, and denotes the damping of the j-th mode.
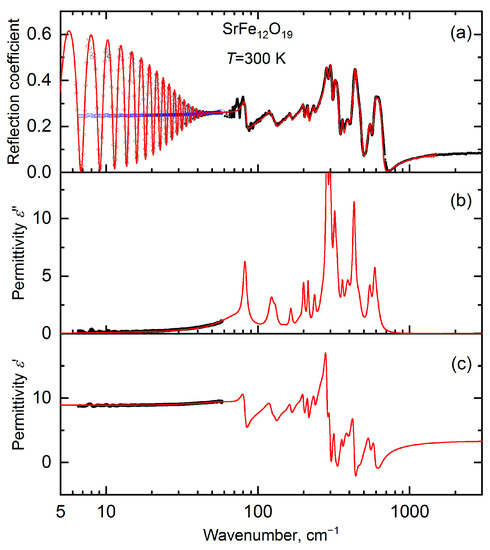
Figure 13.
(a) Room-temperature spectra of the reflection coefficient of a 0.737 mm-thick pellet pressed of SrFe12O19 powder: black dots—far-infrared and mid-infrared data; open blue and black dots—terahertz reflection coefficient spectra of a bulk sample and of a 0.737 mm layer sample, respectively, calculated using the terahertz spectra of imaginary and real permittivity that are shown by dots in panels (b) and (c), respectively. Periodic oscillations in the spectrum seen below ≈40 cm−1 are due to multiple reflections of the radiation within the plane-parallel sample (Fabry–Perot effect). The line in panel (a) corresponds to the results of a least squares fit of the infrared reflection coefficient spectrum together with the terahertz spectra of and (dots in panels (b,c)). Regular Lorentzian expression was used to model the absorption lines. The obtained broad-band spectra of imaginary and real permittivity are shown by lines (and dots for the THz data) in panels (b) and (c), respectively.
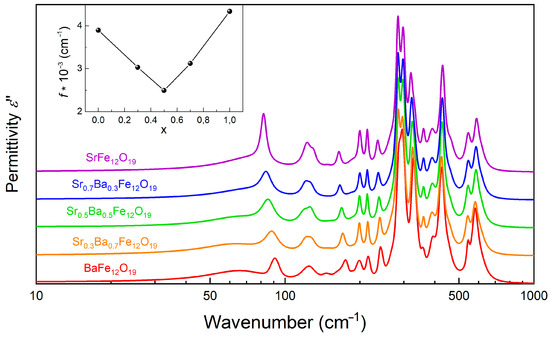
Figure 14.
Room-temperature broad-band terahertz-infrared spectra of the imaginary permittivity of pellets pressed of Ba1−xSrxFe12O19 powders (0 < x < 1) obtained by fitting the infrared spectra of the reflection coefficient together with the terahertz spectra of real and imaginary permittivity, as described in the text and demonstrated in Figure 12. Spectra are vertically shifted for clarity. Inset shows dependence of the oscillator strength of the phonon with the lowest frequency on the strontium concentration.
General analysis of the lattice dynamics in hexaferrites reveals that the main type of oscillations leads to the appearance of the corresponding phonon modes in vibrational spectra [47]. The oscillations of different type of FeO6 octahedrons contribute to peak formations in the range of ~300–620 cm−1, while the peaks with the highest oscillator strength are caused by the strontium oscillations located below 200 cm−1. In this regard, the IR spectra of both pure SrFe12O19 and BaFe12O19 (Figure 13), as well as of their solid solutions (Figure 14), demonstrate characteristic absorption lines, which correspond to known phonon modes of the hexaferrite structure [46,47]. This observation is in agreement with the X-ray diffraction data on the relatively single-phase crystal structure of these samples.
Figure 14 shows the composition-induced evolution of the phonon mode parameters. While the frequencies of the low-frequency modes associated with the oscillations of the (Sr,Ba) cations increase and their dielectric contribution decreases with the growth of x(Sr2+), the high-frequency modes (oscillations of different types of FeO6 octahedrons) remain less sensitive to the compositional changes. This confirms the formation of a solid solution of the substitutional type, which is consistent with the fulfillment of Vegard’s rules (Figure 4). Certain splitting of the phonon modes in the range ~280–600 cm−1 was still observed, indicating the inter-sublattice coupling inherent to the hexaferrite structure due to the corner-shared connection between different types of the oxygen polyhedra [1]. It is important to note that the substitution of Ba2+ with Sr2+ in the 2d Wyckoff position of the hexaferrite’s structure results in spectral changes that are similar to the temperature-induced behavior of the ferroelectric soft mode, signs of which were found in pure BaFe12O19 [46]. The fundamental difference is that isovalent substitutions in the SrxBa(1−x)Fe12O19 samples lead to a simultaneous shift in several phonon modes associated with vibrations of the A-cations, in contrast to the BaFe12O19 single crystal, for which only the A2u ferroelectric mode (for E‖c geometry) is softened with decreasing temperature [46]. These composition-induced changes of the lattice dynamics indicate an increase in the structural distortions of the (Sr,Ba)O12 anti-cuboctahedron due to significant differences in the ionic radii of Sr2+ and Ba2+, but not in an increase in the ferroelectric instability, as expected when the Ba2+ ions were replaced with stoichiometrically active Pb2+ ions with a lone pair of electrons in PbxBa1−xFe12O19 [48].
Since Sr2+ and Ba2+ ions randomly occupy one crystallographic position without any signs of ordering (Table 1), this indicates an increase in structural disorder, primarily in the system of the (Sr,Ba)O12 anti-cuboctahedron. A clear manifestation of the effect of structural disorder is the pronounced broadening of the lines corresponding to low-frequency oscillations ( < 280 cm−1) and the enhancement of mode damping observed in the THz and IR spectra of the samples with intermediate x(Sr2+) concentrations (0 < x < 1), especially with equal contents of barium and strontium (x = 0.5). Note that the oscillator strength of the phonon with the lowest frequency (and highest dielectric contribution) changes from f = 3894 cm−2 (ε = 0.82) in BaFe12O19 to f = 4331 cm−2 (ε = 0.78) in SrFe12O19. However, the f (x) dependence (Figure 14, inset) as well as the change, are both nonmonotonic and demonstrate a clear minimum at x = 0.5 (f = 2493 cm−2, ε = 0.57), which may indicate the competition between two processes inherent to pure BaFe12O19 and to the pure SrFe12O19 compounds. While the dominant type of dielectric contribution for BaFe12O19 originates from the A2u ferroelectric mode softening [46], the origin of the unstable phonon dynamics for SrFe12O19 is still unknown and needs further study of the pure single crystals.
4. Conclusions
Solid solutions of SrxBa(1−x)Fe12O19 (x = 0; 0.3; 0.5; 0.7; and 1) were obtained using the low-temperature sol-gel method. The average particle size that was observed using the SEM method is 50 nm; the average-calculated CSR size according to the Wiliamson–Hall method is 19.7 nm. Analysis of XRD patterns confirms the single-phase state of the hexaferrite samples. Linear dependence of the unit cell parameters a and c, obtained through Rietveld refinement, as well as the dependencies of the infrared phonon modes frequencies on x confirms the formation of substitutional solid solutions according to Vegard’s law. Small deviations in the a and c parameters from Vegard’s law at x = 0.5 and 0.7 may indicate their tendency to separate into two solid solutions. Decreases in the unit cell parameters with x is associated with the substitution of Ba2+ (ionic radius = 1.61 Å) with Sr2+ (ionic radius = 1.44 Å). There is a systematic increase in the Curie temperature with an increase in the degree of substitution, x. The terahertz and infrared spectra demonstrate clear signs of structural disorder originated from the random-type distortion of the (Sr,Ba)O12 anti-cuboctahedron via substitution of Ba2+ with Sr2+ in the same crystallographic position. The resulting solid solutions are single-domain, which is confirmed with large values of the coercive force, a high degree of magnetization, and comparison with large particles of samples of the same composition. A wide hysteresis loop indicates a hard magnetic material; Ms, Mr, and Hc displayed the maximum values for a SrFe12O19 composition. The obtained hexaferrite samples SrxBa(1−x)Fe12O19 (x = 0; 0.3; 0.5; 0.7; 1) were suitable for exploitation under the magnetic field of 0.49–0.56 T and at temperatures under 448–451 °C without demagnetization. High values of Hc, as well as Ms and Mr, made the obtained samples suitable for permanent magnet application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13091354/s1, Figure S1: Rietveld refinement of the XRD patterns of sample BaFe12O19, obtained using the citrate method; Figure S2: Rietveld refinement of the XRD patterns of sample S0.3Ba0.7Fe12O19, obtained using the citrate method; Figure S3: Rietveld refinement of the XRD patterns of sample S0.5Ba0.5Fe12O19, obtained using the citrate method; Figure S4: Rietveld refinement of the XRD patterns of sample S0.7Ba0.3Fe12O19, obtained using the citrate method; Figure S5: Rietveld refinement of the XRD patterns of sample SrFe12O19, obtained using the citrate method.
Author Contributions
Data curation, A.K.; formal analysis, A.K., D.Z., S.T., E.Z. and M.T.; funding acquisition, D.V. and S.G.; investigation, A.K., D.Z., V.Z., S.T., E.Z., A.A. and P.A.; methodology, D.Z.; project administration, D.V.; resources, D.V., S.G., V.Z. and S.T.; supervision, D.V.; writing—original draft preparation, A.K.; writing—review and editing, D.Z.; visualization, A.K.; validation, A.K., E.Z., A.A., P.A. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (Agreement No. 21-79-10115). Additionally in part of spectroscopy, the research was supported by the Russian Science Foundation Grant No. 22-72-10022 (THz-IR spectroscopy: measurements and spectrum analysis). The research was partially funded by the Ministry of Science and Higher Education of the Russian Federation (No. FSMG-2021-0005).
Data Availability Statement
The data presented in this study are openly available in MDPI.
Acknowledgments
The authors would like to thank our colleagues from the chemical department of Chelyabinsk State University for their support and providing materials for this research. We thank A. Gurchenko for their assistance with the IR experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pullar, R.C. Hexagonal Ferrites: A Review of the Synthesis, Properties and Applications of Hexaferrite Ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Nikmanesh, H.; Hoghoghifard, S.; Hadi-Sichani, B. Study of the Structural, Magnetic, and Microwave Absorption Properties of the Simultaneous Substitution of Several Cations in the Barium Hexaferrite Structure. J. Alloys Compd. 2019, 775, 1101–1108. [Google Scholar] [CrossRef]
- Hashhash, A.; Hassen, A.; Baleidy, W.S.; Refai, H.S. Impact of Rare-Earth Ions on the Physical Properties of Hexaferrites Ba0.5Sr0.5RE0.6Fe11.4O19, (RE = La, Yb, Sm, Gd, Er, Eu, and Dy). J. Alloys Compd. 2021, 873, 159812. [Google Scholar] [CrossRef]
- Zhuravlev, V.A.; Nevmyvaka, A.A.; Itin, V.I.; Svetlichnyi, V.A.; Lapin, I.N.; Wagner, D.V. Influence of the Reagent Types on the Characteristics of Barium Hexaferrites Prepared by Mechanochemical Method. Mater. Today Commun. 2019, 21, 100614. [Google Scholar] [CrossRef]
- Gunanto, Y.E.; Izaak, M.P.; Sitompul, H.; Adi, W.A. Composite Paint Based on Barium-Strontium-Hexaferrite as an Absorber of Microwaves at X-Band Frequency. Mater. Today Proc. 2019, 13, 1–4. [Google Scholar] [CrossRef]
- Brightlin, B.C.; Balamurugan, S. Magnetic, Micro-Structural, and Optical Properties of Hexaferrite, BaFe12O19 Materials Synthesized by Salt Flux-Assisted Method. J. Supercond. Nov. Magn. 2017, 30, 215–225. [Google Scholar] [CrossRef]
- Verma, S.; Chawla, A.; Pushkarna, I.; Singh, A.; Godara, S.K.; Pathak, D.K.; Kumar, R.; Singh, M. Understanding the Phase Evolution with Temperature in Pure (BaFe12O19) and Zinc-Zirconium Co-Doped Barium Hexaferrite (BaZnZrFe10O19) Samples Using Pawley and Rietveld Analysis. Mater. Today Commun. 2021, 27, 102291. [Google Scholar] [CrossRef]
- Joseph, N.; Kaipamangalath, A.; Varma, M.R.; Thomas, S. Size Controlled Growth of Barium Hexaferrite Platelets Using Salt Assisted Sol-Gel Technique. Mater. Chem. Phys. 2021, 272, 124971. [Google Scholar] [CrossRef]
- Godara, S.K.; Kaur, V.; Chuchra, K.; Narang, S.B.; Singh, G.; Singh, M.; Chawla, A.; Verma, S.; Bhadu, G.R.; Chaudhari, J.C.; et al. Impact of Zn2+-Zr4+ Substitution on M-Type Barium Strontium Hexaferrite’s Structural, Surface Morphology, Dielectric and Magnetic Properties. Results Phys. 2021, 22, 103892. [Google Scholar] [CrossRef]
- Patel, C.D.; Dhruv, P.N.; Meena, S.S.; Singh, C.; Kavita, S.; Ellouze, M.; Jotania, R.B. Influence of Co4+-Ca2+ Substitution on Structural, Microstructure, Magnetic, Electrical and Impedance Characteristics of M-Type Barium–Strontium Hexagonal Ferrites. Ceram. Int. 2020, 46, 24816–24830. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Güngüneş, H.; Baykal, A.; Alhamed, N.A.; Trukhanov, A.V.; Trukhanov, S.V. Structure, Mössbauer and AC Susceptibility of Strontium Nanohexaferrites: Effect of Vanadium Ions Doping. Ceram. Int. 2019, 45, 11615–11624. [Google Scholar] [CrossRef]
- Kaman, O.; Kubániová, D.; Knížek, K.; Kubíčková, L.; Klementová, M.; Kohout, J.; Jirák, Z. Structure and Magnetic State of Hydrothermally Prepared Mn-Zn Ferrite Nanoparticles. J. Alloys Compd. 2021, 888, 161471. [Google Scholar] [CrossRef]
- Cao, L.; Zeng, Y.; Ding, C.; Li, R.; Li, C.; Zhang, C. One-Step Synthesis of Single Phase Micro-Sized BaFe12O19 Hexaplates via a Modified Hydrothermal Approach. Mater. Chem. Phys. 2016, 184, 241–249. [Google Scholar] [CrossRef]
- Soria, G.D.; Jenus, P.; Marco, J.F.; Mandziak, A.; Sanchez-Arenillas, M.; Moutinho, F.; Prieto, J.E.; Prieto, P.; Cerdá, J.; Tejera-Centeno, C.; et al. Strontium Hexaferrite Platelets: A Comprehensive Soft X-Ray Absorption and Mössbauer Spectroscopy Study. Sci. Rep. 2019, 9, 11777. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G.A.; Zhang, L.; Abbas, W.; Murtaza, G. Magnetic and Optical Properties of Gd-Tl Substituted M-Type Barium Hexaferrites Synthesized by Co-Precipitation Technique. Curr. Appl. Phys. 2019, 19, 506–515. [Google Scholar] [CrossRef]
- Gordani, G.R.; Ghasemi, A.; Saidi, A. Enhanced Magnetic Properties of Substituted Sr-Hexaferrite Nanoparticles Synthesized by Co-Precipitation Method. Ceram. Int. 2014, 40, 4945–4952. [Google Scholar] [CrossRef]
- Basma, H.; Rahal, H.T.; Awad, R. Enhancement of the Magnetic Properties of Ba1-XBixFe12O19 Nanoparticles. J. Magn. Magn. Mater. 2021, 539, 168413. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zherebtsov, D.A.; Mashkovtseva, L.S.; Nemrava, S.; Semisalova, A.S.; Galimov, D.M.; Gudkova, S.A.; Chumanov, I.V.; Isaenko, L.I.; Niewa, R. Growth, Structural and Magnetic Characterization of Co- and Ni-Substituted Barium Hexaferrite Single Crystals. J. Alloys Compd. 2015, 628, 480–484. [Google Scholar] [CrossRef]
- Liu, R.; Wang, L.; Yu, X.; Xu, Z.; Gong, H.; Zhao, T.; Hu, F.; Shen, B. Magnetocrystalline Anisotropy Study of Co-Substituted M-Type Strontium Hexaferrite Single Crystals. Ceram. Int. 2023, 49, 1888–1895. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Gudkova, S.A.; Zherebtsov, D.A.; Trofimov, E.A.; Mashkovtseva, L.S.; Trukhanov, A.V.; Trukhanov, S.V.; Nemrava, S.; Blaschkowski, B.; Niewa, R. Flux Single Crystal Growth of M-Type Strontium Hexaferrite SrFe12O19 by Spontaneous Crystallization. J. Magn. Magn. Mater. 2019, 470, 97–100. [Google Scholar] [CrossRef]
- You, L.; Qiao, L.; Zheng, J.; Jiang, M.; Jiang, L.; Sheng, J. Magnetic Properties of La-Zn Substituted Sr-Hexaferrites by Self-Propagation High-Temperature Synthesis. J. Rare Earths 2008, 26, 81–84. [Google Scholar] [CrossRef]
- Martirosyan, K.S.; Galstyan, E.; Hossain, S.M.; Wang, Y.-J.; Litvinov, D. Barium Hexaferrite Nanoparticles: Synthesis and Magnetic Properties. Mater. Sci. Eng. B 2011, 176, 8–13. [Google Scholar] [CrossRef]
- Mahmoud, M.H.; Hassan, A.M.; Said, A.E.-A.A.; Taha, T.A. Structural, Magnetic, and Catalytic Studies of Microwave-Combustion/Ball-Mill Synthesized Zinc Ferrite Nanoparticles. Inorg. Chem. Commun. 2022, 144, 109932. [Google Scholar] [CrossRef]
- Li, L.-Z.; Sokolov, A.; Yu, C.-J.; Li, Q.-F.; Li, Q.-F.; Qian, K.; Harris, V.G. Effects of Y–Co Co-Substitution on the Structural and Magnetic Properties of M-Type Strontium Hexaferrites. Ceram. Int. 2021, 47, 25514–25519. [Google Scholar] [CrossRef]
- Tkachenko, M.V.; Ol’khovik, L.P.; Kamzin, A.S. Polyfunctional Bioceramics Modified by M-Type Hexagonal Ferrite Particles for Medical Applications. Tech. Phys. Lett. 2011, 37, 494–496. [Google Scholar] [CrossRef]
- Tkachenko, M.V.; Ol’khovik, L.P.; Kamzin, A.S.; Keshri, S. Polyfunctional Bioceramics Based on Calcium Phosphate and M-Type Hexagonal Ferrite for Medical Applications. Tech. Phys. Lett. 2014, 40, 4–6. [Google Scholar] [CrossRef]
- Danewalia, S.S.; Singh, K. Bioactive Glasses and Glass–Ceramics for Hyperthermia Treatment of Cancer: State-of-Art, Challenges, and Future Perspectives. Mater. Today Bio 2021, 10, 100100. [Google Scholar] [CrossRef] [PubMed]
- Prathap, S.; Madhuri, W.; Meena, S.S. Multiferroic Properties and Mössbauer Study of M-Type Hexaferrite PbFe12O19 Synthesized by the High Energy Ball Milling. Mater. Charact. 2021, 177, 111168. [Google Scholar] [CrossRef]
- Dai, Y.; Lan, Z.; Yu, Z.; Sun, K.; Guo, R.; Wu, G.; Jiang, X.; Wu, C.; Liu, Y.; Liu, H.; et al. Effects of La Substitution on Micromorphology, Static Magnetic Properties and Low Ferromagnetic Resonance Linewidth of Self-Biased M-Type Sr Hexaferrites for High Frequency Application. Ceram. Int. 2021, 47, 8980–8986. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Gungunes, H.; Manikandan, A.; Baykal, A. Investigation of the Effects of Tm3+ on the Structural, Microstructural, Optical, and Magnetic Properties of Sr Hexaferrites. Results Phys. 2019, 13, 102166. [Google Scholar] [CrossRef]
- Alna’washi, G.A.; Alsmadi, A.M.; Bsoul, I.; Salameh, B.; Alzoubi, G.M.; Shatnawi, M.; Hamasha, S.M.; Mahmood, S.H. Investigation on X-ray Photoelectron Spectroscopy, Structural and Low Temperature Magnetic Properties of Ni-Ti Co-Substituted M-Type Strontium Hexaferrites Prepared by Ball Milling Technique. Results Phys. 2021, 28, 104574. [Google Scholar] [CrossRef]
- Huang, K.; Yu, J.; Zhang, L.; Xu, J.; Yang, Z.; Liu, C.; Wang, W.; Kan, X. Structural and Magnetic Properties of Gd–Zn Substituted M-Type Ba–Sr Hexaferrites by Sol-Gel Auto-Combustion Method. J. Alloys Compd. 2019, 803, 971–980. [Google Scholar] [CrossRef]
- Singh, V.P.; Batoo, K.M.; Singh, M.; Kumar, S.; Kumar, G. Giant Magnetization and Ultra-Low Loss in Non-Magnetic Ion-Substituted Barium Nanohexaferrite Matrix. J. Mater. Sci. Mater. Electron. 2020, 31, 3951–3959. [Google Scholar] [CrossRef]
- Elkhouad, S.; Yamkane, Z.; Louafi, J.; Moutataouia, M.; Omari, L.H.; Elouafi, A.; Moubah, R.; Lassri, H.; El Moussaoui, H. Structural, Morphological and Magnetic Properties of Sr0,54Ca0,46Fe6,5Al5,5O19 M-Type Hexaferrites: Effects of Annealing Temperature. Solid State Commun. 2021, 337, 114453. [Google Scholar] [CrossRef]
- Han, G.; Sui, R.; Yu, Y.; Wang, L.; Li, M.; Li, J.; Liu, H.; Yang, W. Structure and Magnetic Properties of the Porous Al-Substituted Barium Hexaferrites. J. Magn. Magn. Mater. 2021, 528, 167824. [Google Scholar] [CrossRef]
- Abarna, S.T.; Ezhil Vizhi, R.; Harikrishnan, V. Examining the Structural, Magnetic and Dielectric Properties of Exchange Spring Nanocomposite Magnets Comprising Ba0.5Sr0.5Fe12O19 and Zn0.5Co0.5Fe2O4. Results Phys. 2023, 48, 106419. [Google Scholar] [CrossRef]
- Abhilash, G.P.; Sharma, D.; Bose, S.; Shivakumara, C. PANI-Wrapped BaFe12O19 and SrFe12O19 with RGO Composite Materials for Electromagnetic Interference Shielding Applications. Heliyon 2023, 9, e13648. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Pratap, V.; Chaurasia, A.K.; Soni, A.K.; Dubey, A.; Dixit, A.K. Combined Effect of Exfoliated Graphite/Ferrite Filled Epoxy Composites on Microwave Absorbing and Mechanical Properties. Phys. Open 2023, 14, 100138. [Google Scholar] [CrossRef]
- Zulkimi, M.M.M.; Azis, R.S.; Ismail, I.; Mokhtar, N.; Ertugrul, M.; Hamidon, M.N.; Hasan, I.H.; Yesilbag, Y.O.; Tuzluca, F.N.; Ozturk, G.; et al. Enhancing Radar Absorption Performance of Sr-Hexaferrite by Hybridization with Coiled Carbon Nanotubes via Chemical Vapour Deposition Method. Diam. Relat. Mater. 2023, 137, 110118. [Google Scholar] [CrossRef]
- Rahman, M.L.; Rahman, S.; Biswas, B.; Ahmed, M.F.; Rahman, M.; Sharmin, N. Investigation of Structural, Morphological and Magnetic Properties of Nanostructured Strontium Hexaferrite through Co-Precipitation Technique: Impacts of Annealing Temperature and Fe/Sr Ratio. Heliyon 2023, 9, e14532. [Google Scholar] [CrossRef]
- Marjeghal, M.A.; Sedghi, A.; Baghshahi, S. The Effect of the Citric Acid to Metal Nitrates Molar Ratio on the Structural and Magnetic Properties of Strontium Hexaferrite Nanoparticles Synthesized by the Sol-Gel Combustion Method. J. Alloys Compd. 2023, 968, 171765. [Google Scholar] [CrossRef]
- Obradors, X.; Collomb, A.; Pernet, M.; Samaras, D.; Joubert, J.C. X-Ray Analysis of the Structural and Dynamic Properties of BaFe12O19 Hexagonal Ferrite at Room Temperature. J. Solid State Chem. 1985, 56, 171–181. [Google Scholar] [CrossRef]
- Fang, C.M.; Kools, F.; Metselaar, R.; de With, G.; Groot, R.A. de Magnetic and Electronic Properties of Strontium Hexaferrite SrFe12O19 from First-Principles Calculations. J. Phys. Condens. Matter 2003, 15, 6229–6237. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.K.; Satyapal, H.K.; Monalisa; Kumar, A.; Sharma, S. Lattice Strain Mediated Structural and Magnetic Properties Enhancement of Strontium Hexaferrite Nanomaterials through Controlled Annealing. Phys. B Condens. Matter 2021, 600, 412592. [Google Scholar] [CrossRef]
- Nadeem, K.; Krenn, H. Exchange Bias, Memory and Freezing Effects in NiFe2O4 Nanoparticles. J. Supercond. Nov. Magn. 2011, 24, 717–720. [Google Scholar] [CrossRef]
- Mikheykin, A.S.; Zhukova, E.S.; Torgashev, V.I.; Razumnaya, A.G.; Yuzyuk, Y.I.; Gorshunov, B.P.; Prokhorov, A.S.; Sashin, A.E.; Bush, A.A.; Dressel, M. Lattice anharmonicity and polar soft mode in ferrimagnetic M-type hexaferrite BaFe12O19 single crystal. Eur. Phys. J. B 2014, 87, 232. [Google Scholar] [CrossRef]
- Kreisel, J.; Lucazeau, G.; Vincent, H. Raman Spectra and Vibrational Analysis of BaFe12O19 Hexagonal Ferrite. J. Solid State Chem. 1998, 137, 127–137. [Google Scholar] [CrossRef]
- Alyabyeva, L.; Zhukova, E.; Zhukov, S.; Ahmed, A.; Vinnik, D.; Gorshunov, B. Tuning the terahertz electrodynamics in Ba-Pb hexaferrite single crystals. Mater. Res. Bul. 2023, 161, 112155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).