Abstract
The high-pressure behavior of jamesonite (FePb4Sb6S14, a = 4.08(3) Å, b = 19.08(3) Å, c = 15.67(3) Å, β= 91.89°, space group P21/c) has been investigated using in situ HP synchrotron X-ray single-crystal diffraction up to ~17 GPa with a diamond anvil cell under hydrostatic conditions. Results of the volume isothermal equation of state (EoS), determined by fitting the P-V data with a third-order Birch–Murnaghan (BM) EoS, are V0 = 1207.1(4) Å3, K0 = 36(1) GPa and K’ = 5.7(7). At high pressure, jamesonite undergoes a phase transition to an orthorhombic structure with a Pmcb space group (β-jamesonite). The analysis of β-jamesonite’s compressibility up to 16.6 GPa, studied by fitting the data with a second-order BM-EoS, gives V0 = 1027(2) Å3, K0 = 74(2) GPa. The comparison of the structural refinements at different pressures indicates that Fe, Pb and Sb do not change their coordination number over the whole investigated P range, respectively, 6 for Fe, 7 and 8 for Pb and 5 + 2 for Sb. However, a significant change occurs on the orientation of Sb lone electron pairs upon the phase transition in accordance with the change in symmetry. Furthermore, a discontinuity in the Fe chain evolution at the transition pressure is observed.
1. Introduction
The term “sulfosalts” was proposed for complex salts in which sulfur plays the role of the anion instead of oxygen, with a general chemical formula of AxXySz, where A stands for metallic cations (e.g., Pb2+, Ag1+; Cu1+); X for semimetal elements As3+, Sb3+, Bi3+ and Te4+; and S for sulfur [1]. Chalcogenides, because of their distinctive characteristics, are interesting as next-generation smart materials, such as thermoelectrics [2], photoelectrics [3], photovoltaics [4], materials for optical data storage [5] and solid-state electrolytes [6]. The wide application range of sulfosalts in these fields is due not only to their chemical and structural construction units but also to their physical properties, such as the band gap, which can be adjusted from almost 0 eV to more than 2.0 eV [7]. This allows sulfosalts to be used in a wide range of semiconductor materials.
Jamesonite (FePb4Sb6S14) belongs to the group of rod-based sulfosalt structures made of infinite rods of M-S polyhedral clusters [8]. Jamesonite crystals are commonly found with needlelike morphology associated with sulfides such as pyrite (FeS2), stibnite (Sb2S3), galena (PbS) and sphalerite (ZnS) in hydrothermal veins [9]. The crystals are lead-gray in color, up to a centimeter long and filling the quartz veins [10]. Due to its morphology and properties, jamesonite attracted scientific interest for applications in selective absorption and catalysis [11] and microelectronic devices [7]. In addition, jamesonite is one of the most important antimony resources [12]. With benavidesite (Pb4MnSb6S14), jamesonite forms a complete solid-solution series in the PbS-FeS-MnS-Sb2S3 system with a melting point from 583 ± 3 °C (Fe) to 592 ± 3 °C (Mn) [13].
Jamesonite’s crystal structure was first described and determined by Niizeki and Buerger [14] with space group P21/a and unit-cell dimensions equal to the following: a = 15.07 Å, b = 18.98 Å, c = 4.03 Å and β = 91.48°. Recently, Léone et al. [15] refined the crystal structure of jamesonite in the space group P21/c and unit-cell dimensions equal to the following: a = 4.02 Å, b = 19.07 Å, c = 15.73 Å and β = 91.89°. The (Pb6Sb6S14)∞ rods extending along a are connected by the Fe coordination octahedra into layers parallel to (010). Each rod shares its Pb2 atoms with four rods, two from each (010) neighboring layer, leaving for each rod four Pb on average. The Fe coordination octahedra share two opposite edges with the neighboring two Fe coordination octahedra to form the chains (FeS4)∞, parallel to the a axis. All orientations refer to the P21/c setting (Figure 1).
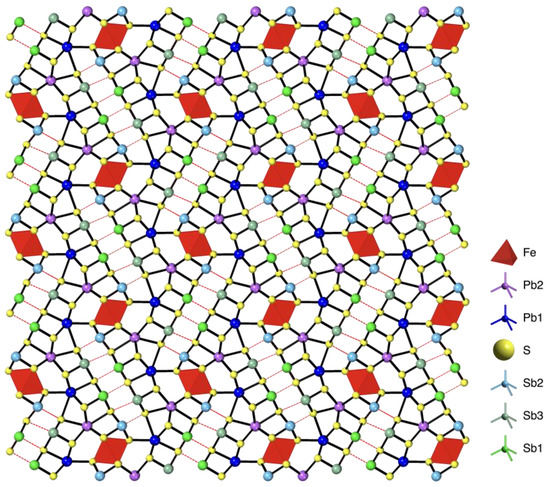
Figure 1.
Crystal structure of jamesonite along [100]. The color legend for the atoms is on the right. The red dashed lines represent the longest S-Sb distances (see text).
The rods are four atomic planes thick and three coordination pyramids long in the direction perpendicular to the rod extension. They are based on the SnS archetype [16] with a mutual 2 Å shift of their two halves along the rod’s extension, when compared to a simple PbS-like arrangement. The middle of a rod comprises a lone electron pair’s (LEP) micelle, where Sb atoms orient their LEP. The full coordination of Sb atoms can thus be described as a monocapped trigonal prism (CN 7) in which tight Sb-S bonds, forming a square pyramid, are supplemented by two S atoms across the micelle at significantly longer distances, forming the body of the prism together with the pyramid’s base. The coordination is, strictly speaking, a 5 + 2 one, or even a 3 + 2 + 2 one, because of a bond-length gap between the closest three and the next-closest two in the distorted square pyramid. The Sb configuration is described in Comodi et al. [17] and, as shown by Balic-Zunic and Makovicky [18], the centroid of the coordination, calculated considering the seven coordination, expresses the maximum of the electron density of the lone electron pairs. The additional two long distances between Sb and S, measured in all three Sb polyhedra, indicate that at room pressure, there are just weak interactions between the Sb and S across the LEP micelles (in Figure 1, these bonds are represented with red dashed lines).
Pb polyhedra are significantly distorted. Pb1 has a 6 + 1 coordination with six distances lower than 3.1 Å, and Pb2 has a 7 + 1 coordination with seven bond distances lower than 3.2 Å. The longest distances are at 3.3 and 3.5 Å, respectively.
Figure 2 shows the jamesonite structure projected along the a axis, where the octahedral iron chains, running along the a axis, are well evident. That projection shows the layered character of the jamesonite structure, i.e., layers with Fe octahedra are alternated with Sb and Pb polyhedra along the a axis.
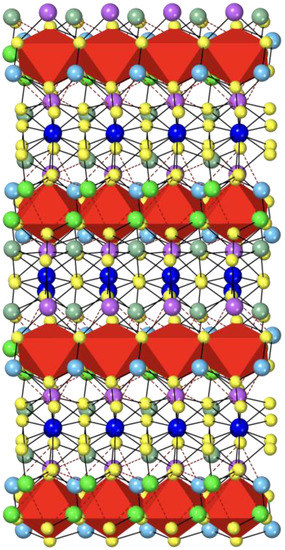
Figure 2.
Jamesonite structure viewed along [001]. The colors of the atoms are the same as in Figure 1. It shows the red iron polyhedra running along the a axis and the Pb and Sb polyhedra in the interchain region (see text).
The distance between the iron and the sulfur shared with other iron is significantly longer (over 2.6 Å) than the distance between the iron and sulfur not shared with other iron (2.4 Å), producing a distorted Fe octahedron. The same behavior is observed at room pressure on the edges of iron polyhedra: S3-S3 (shared edges) = 3.445 Å and S3-S3 (unshared edges) = 4.023 Å.
In jamesonite, the strength of covalent bonds Sb-S and Pb-S can drastically modify the octahedral coordination and thus also influence the nature and degree of the two-spin superposition interference on the contiguous centers of the transition metal [19,20]. A possible polymorph of jamesonite, the orthorhombic parajamesonite, has been debated in the literature record [19,21,22]. To date, there is only one report containing mineralogical information on the parajamesonite phase [22]. However, in recent studies, no evidence of the potential presence of parajamesonite in nature has been obtained [21]. It must be noted, however, that the structure of the monoclinic jamesonite is conspicuously close to an orthorhombic one.
Although the interest in the flotation, smelting, selective leaching and extraction of antimony prompted serious investigations [10,23], analyses on the crystal structure of jamesonite at nonambient conditions are extremely limited [19].
Jamesonite has remarkably interesting electric and magnetic properties. Having low electric conductivity, this compound is an intrinsic semiconductor with an optical band gap equal to 0.48 eV. In magnetic measurements, Matsushita and Ueda [18] found a 1d-HAF behavior and a change in the magnetic susceptibility at 120 K, together with other anomalies at lower temperatures. Furthermore, Leone et al. [15] showed an unusual magnetic anisotropy: the susceptibility parallel to the a axis is superior that the susceptibility perpendicular to that axis. The a axis is an easy axis of magnetization.
High-pressure studies of selected sulfosalts such as heyrovskyite Pb6Bi2S9 [24], lillianite Pb3Bi2S6 [25] and chalcostibite CuSbS2 [17] evidenced the occurrence of pressure-induced phase transitions, and these physical parameters could play a key role in the structural properties of these thermoelectric compounds.
In this work, the high-pressure (HP) behavior of a natural jamesonite sample was investigated, for the first time, by means of in situ synchrotron single-crystal X-ray diffraction (SC-XRD) to study the effect of pressure on the jamesonite crystal structure, to evaluate the possible occurrence of pressure-induced phase transitions and to measure the lattice parameters’ compressibility as well as the main components of the elastic tensors. Moreover, the effect of pressure on the lone pair activity of Sb and Pb, and the change in Fe coordination, which might have a profound influence on the magnetic properties, were studied.
2. Materials and Methods
2.1. Experimental Procedure
A natural jamesonite sample was provided by Yves Moëlo. The quality of the mineral sample was first evaluated by means of SC-XRD at the Department of Physics and Geology of the University of Perugia (Italy), confirming its typical diffraction pattern. A crystal with the dimensions 80 × 20 × 30 μm was selected for the HP SC-XRD analyses (ESRF synchrotron in Grenoble, France). The HP SC-XRD experiments were performed on the ESRF synchrotron in Grenoble (France) at the ID-15B beamline. The measurements were made using a membrane-type diamond anvil cell with an opening angle of 64°, equipped with 600 μm culet diamonds. The pressure-transmitting medium was helium, and a ruby sphere was loaded with the sample as the P-calibrant. The pre-indented stainless-steel gasket had a 300 μm diameter hole and 80 μm thickness. A monochromatized beam (λ = 0.41125 Å) was used, focused down to a 10 × 10 μm2 area. Data were registered with an angular step of 0.5° using an MAR555 flat-panel detector with a 430 × 350 mm2 (555 mm diagonal) active area. The wavelength and the sample-to-detector distance (279.88 mm) were calibrated using Si standard and Fit2D software [26].
The investigated pressure range varied from 1.26 to 16.6 GPa. Measurements were made in 7 steps in increasing pressure. Pressure was measured before and after each data collection, and the gasket relaxation was ensured by waiting ~15 min after changing the load. CrysAlisPro software (Agilent Technologies U.K. Ltd., Yarnton, UK) was used for data extraction, reflection intensity correction, reflection merging and refinement of the lattice parameters. Diffraction peak indexing and integration of the peak intensities were performed using the CrysAlisPro package (Rigaku Oxford Diffraction 2018, Agilent Technologies U.K. Ltd., Yarnton, UK). X-ray absorption effects corrections were made by applying the semiempirical ABSPECK routine present in CrysAlisPro.
2.2. Refinement Protocol and Elasticity Analysis
The structural refinements were performed using ShelXle software [27]. The crystal structure of jamesonite was refined up to 6.76 GPa, starting from the crystal structure published in Léone et al. [15], in the space group P21/c. The unit cell and crystal structure refinements of data collected from 9.70 to 16.60 GPa showed the beta angle of jamesonite approaching 90° and the x atomic positions of all the atoms in the unit cell approaching special positions. Thus, the occurrence of a phase transition from monoclinic to orthorhombic symmetry was checked, and SHELXT [28] software was used to solve the crystal structure of jamesonite after phase transition. Satisfactory structure refinements were performed in the Pmcb space group for the last four data collections. The crystal structures before and after phase transition are hereafter named α-jamesonite and β-jamesonite, respectively, which can be explained by a displacive character of the phase transition. The good crystallinity of the jamesonite sample was maintained during the whole experiment.
The unit-cell parameters and volumes are reported in Table 1 together with data collection and refinement details. Figure 3 shows the evolution of lattice parameters with pressure, normalized to the room-pressure value and to 9.7 GPa for α- and β-jamesonite, respectively. Atomic coordinates at different pressures (Table S1) and crystal information files (CIFs) are deposited in the Supplementary Materials section.

Table 1.
Unit-cell parameters of α- and β-jamesonite and details of data collection and refinement at different pressures. P precision ± 0.05 GPa.
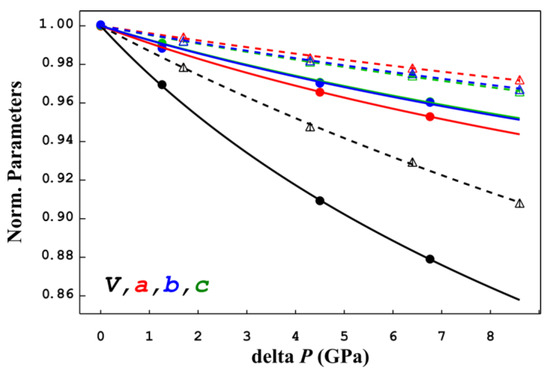
Figure 3.
High-pressure evolution of the lattice parameters of α-jamesonite (continuous lines and full symbols) with respect to β-jamesonite (dashed lines and empty symbols). All values are normalized to room pressure for α-jamesonite and to 9.7 GPa for β-jamesonite. Lines are the results of the Birch–Murnaghan equation-of-state fitting (details are given in the text).
The compressibility of jamesonite before and after phase transition was analyzed using EosFit GUI [29] (Figure 3). The f–F plots in Figure 4 [30] show that data for α-jamesonite lie on an inclined straight line (Figure 4a), that is, they can be adequately described by a 3rd-order Birch–Murnaghan (BM) equation of state (EoS), whereas, data for β-jamesonite lie on a horizontal line of constant F (Figure 4b), meaning that a 2nd-order BM-EoS (K’ fixed to 4) is the best choice to describe its volumetric elastic behavior [30]. Results of the BM-EoS fittings are listed in Table 2 and shown in Figure 3.
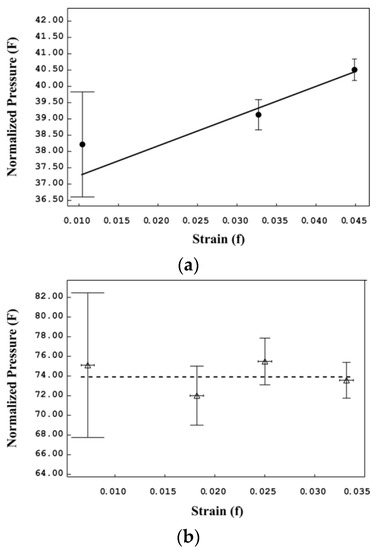
Figure 4.
F–f plot. Eulerian finite strain (f) versus normalized stress (F) with the weighted linear regression through the volume cell data points for α-jamesonite (a,b) β-jamesonite.

Table 2.
Results of the EoS fittings for α- and β-jamesonite using a 3rd-order BM-EoS and a 2nd-order BM-EoS, respectively.
3. Results
3.1. Compressibility
Results of the BM-EoS fittings show that α-jamesonite has an anisotropic compressional behavior with the highest compressibility along [100] and the lowest compressibility along [001], whereas the [010] direction has an intermediate value close to [001] (Table 2). The monoclinic beta angle decreases with P approaching the 90° at the transition pressure (>9 GPa) (Table 1). The β-jamesonite (dashed lines in Figure 3) shows a more isotropic rigid behavior with respect to the α polymorph. Moreover, while in α-jamesonite, the most compressible lattice parameter is a, in the β polymorph, it becomes the stiffest direction.
This behavior is well shown, considering the ratio between the lattice parameters moduli, which indicates a quite anisotropic compressional behavior in both α and β polymorphs, with 1/M0a:1/M0b:1/M0c = 1.24:1.00:1.02 in the former and 1/M0a:1/M0b:1/M0c = 1.00:1.24:1.1 in the latter.
3.2. High-Pressure Structural Evolution from α-Jamesonite to β-Jamesonite
The geometry and distortion of the coordination polyhedra were analyzed by means of the IVTON program [31]. Table 3 reports the bond distances for all Fe, Sb and Pb polyhedra, and Figure 5 reports the compressibility of all polyhedra normalized to the room-pressure values.

Table 3.
Selected bond distances (Å), average bond length (Å) and polyhedral volume (Å3) with estimated standard deviations in parentheses for α- and β-jamesonite. The apexes mean different atoms related by symmetry operations. P values are given in Table 1.
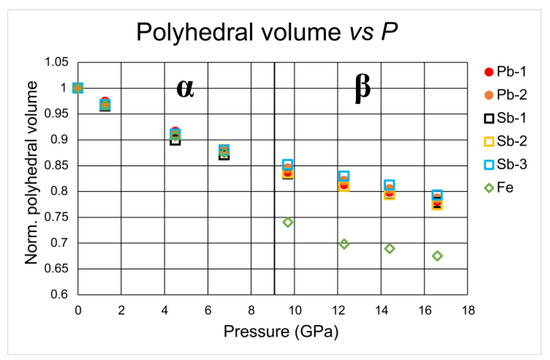
Figure 5.
Polyhedral evolution in α- and β-jamesonite. All values are normalized to room-pressure value.
The stereochemical activity of the lone electron pair (LEP) of Sb and Pb, quantified by measuring the eccentricity, namely, the distance of the cation from the centroid of the coordination polyhedra (centroid = the point with the smallest standard deviation of distances from all the coordinated ligands), as well as their evolution with P, are given in Table 4. In addition, in Table 4, sphericity values, defined as the inverse standard deviation of ligands from the sphere fitted to the positions of ligands, are also given.

Table 4.
Polyhedral eccentricity, sphericity, total polyhedral volume (Å3) and volume distortion at different pressures in α- and β-jamesonite. P values are given in Table 1.
The comparison of the structural data collected at different P allows us to describe the evolution of Sb, Pb and Fe polyhedra.
3.2.1. Sb polyhedra
- (a)
- All Sb polyhedra turn out to have quite similar compressibilities: the polyhedral bulk moduli, calculated as the reciprocal value of the average polyedral compressibilities in the investigated P range, are 53, 53 and 31 GPa for Sb1, Sb2 and Sb3, respectively, in α-jamesonite; in contrast, they become 100, 90 and 96 GPa for Sb1, Sb2 and Sb3, respectively, in β-jamesonite. A similar value was measured for Sb polyhedra in chalcostibite by Comodi et al. [17]. The pyramid apexes are directed perpendicular to the rods, and the lone electron pairs of Sb are located in the LEP micelle. However, a significant change occurs in the orientation of the LEP upon the phase transition in accordance with the change in symmetry (Figure 6). Note that the three shortest Sb-S bonds from which the LEP is pointing include the apical atom of the square pyramid and the two atoms in its base; in α-jamesonite, these are the two below or above Sb (looking along the rod extension), whereas in the β polymorph, they are both on the side of the Sb atom. In other words, the LEP becomes perpendicular to the rods, which is a consequence of their mutual repulsion from Sb atoms to those Sb on the opposite side of a micelle (Figure 7). With increasing P, the distances between Sb and S on the opposite side of the micelle drastically decrease: at 1.6 GPa and 16.6 GPa, Sb1-S1 changes from 4.125(5) to 3.234(5) Å, Sb2-S6 from 3.992(5) to 3.108(5) Å and Sb3-S5 from 4.169(5) to 3.467(6) Å.
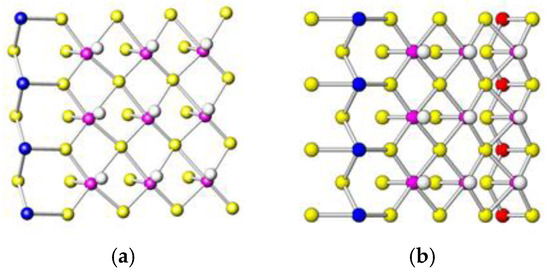 Figure 6. The orientation of the LEP in α-jamesonite at 1.26 GPa (a) and in the β-jamesonite at 14.4 GPa (b). The LEPs are projected as white spheres centered on the centroids of coordination. Only one wall of a micelle is depicted, and the projection is slightly inclined from perpendicular to give a better view.
Figure 6. The orientation of the LEP in α-jamesonite at 1.26 GPa (a) and in the β-jamesonite at 14.4 GPa (b). The LEPs are projected as white spheres centered on the centroids of coordination. Only one wall of a micelle is depicted, and the projection is slightly inclined from perpendicular to give a better view.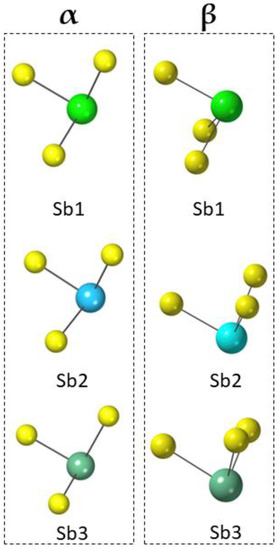 Figure 7. Distribution of the 3 Sb-S shortest distances in the Sb1, Sb2 and Sb3 polyhedra. On the left, distances in α-jamesonite are shown, and on the right, the distances in β-jamesonite. All the projections are on the plane [100]. From α- to β-jamesonite, a significant change in the orientation of the LEP is observed, considering the LEP point from the three shortest Sb-S distances.
Figure 7. Distribution of the 3 Sb-S shortest distances in the Sb1, Sb2 and Sb3 polyhedra. On the left, distances in α-jamesonite are shown, and on the right, the distances in β-jamesonite. All the projections are on the plane [100]. From α- to β-jamesonite, a significant change in the orientation of the LEP is observed, considering the LEP point from the three shortest Sb-S distances. - (b)
- The stereochemical activity of Sb LEPs at room pressure is quite high: the eccentricities are 0.32, 0.29 and 0.33 for Sb1, Sb2 and Sb3, respectively, very similar to the value measured in chalcostibite by Comodi et al. [17] that is equal to 0.31 (Table 4). With increasing P, the stereochemical activity of the three Sb decreases (Table 4) in a similar way as can be observed with the reduction in the eccentricity (Figure 8a) and the increase in the sphericity (Figure 8b) of the Sb polyhedra. At the same time, the distorted quadratic bases of Sb polyhedra become more regular as P increases, as indicated by the Sb-S distance in Table 4, with a usually equalizing trend observed in other sulfosalts, where the longest Sb-S distances decrease more strongly than the shortest ones. This behavior can be also evaluated by the reduction in distortion (Figure 9) and eccentricity evolution (Table 4) with P. The regularization of polyhedra is a common behavior in high-pressure structures [32], as regular polyhedra have lower volume with respect to distorted polyhedra with the same superficial extension.
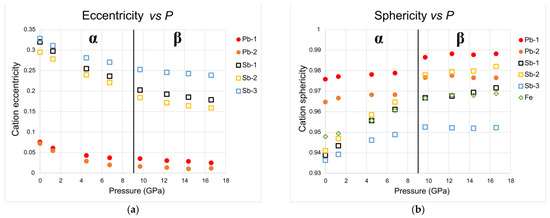 Figure 8. Evolution with P of eccentricity (a) and sphericity (b) of the different polyhedra in α- and β-jamesonite.
Figure 8. Evolution with P of eccentricity (a) and sphericity (b) of the different polyhedra in α- and β-jamesonite.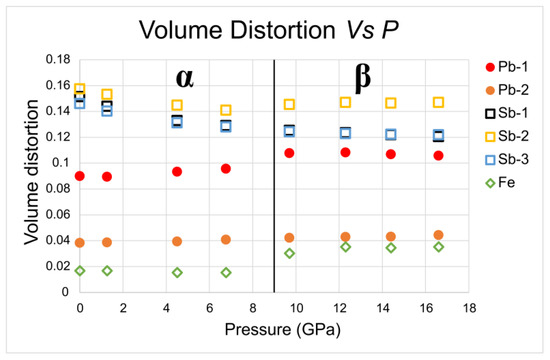 Figure 9. Evolution with pressure of the volume distortion [33] of the different polyhedra in α-jamesonite and β-jamesonite.
Figure 9. Evolution with pressure of the volume distortion [33] of the different polyhedra in α-jamesonite and β-jamesonite.
3.2.2. Pb polyhedra
Lead atoms are located along the rod edges, and Pb-S bonds connect two adjacent rod layers. At ambient P, the coordination of Pb1 is 6 + 1 and that of Pb2 is 7 + 1, considering the 6 and 7 distances less than 3.2 Å in Pb1 and Pb2 coordination polyhedra, respectively. With increasing pressure, both Pb atoms increase their coordination as the Pb1-S6 distance decreases from 3.272 to 2.8 Å and the Pb2-S4 distance decreases from 3.37 to 2.94 Å. The bulk moduli of Pb1 and Pb2 (by considering 7 and 8 for Pb1 and Pb2 coordination, respectively) are 54 and 54 GPa, respectively, for the α-jamesonite and 98 and 95 GPa, respectively, for the β-jamesonite. They represent the most rigid polyhedra in the jamesonite structure.
Again, this is the well-known high-pressure behavior in which atoms, both cations and anions, tend to increase their coordination number with increasing pressure to increase packing efficiency. In jamesonite, the increase in the lead coordination number promotes better bonding between the rods in the 3D directions.
Usually, the LEP activity of lead is less expressed than that of Sb or Bi, and in some cases, as in galena, it is completely suppressed [34]. In α-jamesonite at room pressure, the LEP activity of Pb atoms is lower than in Sb; the eccentricity and sphericity values of Pb1 and Pb2 are 0.076 and 0.976 for Pb1 and 0.072 and 0.965 for Pb2, respectively. The same behavior is observed in in β-jamesonite, where eccentricity and sphericity are 0.025 and 0.988 for Pb1 and 0.012 and 0.977 for Pb2 at 16.6 GPa (Table 4), respectively.
3.2.3. Fe polyhedra
FeS6 octahedra are connected by two opposite edges and form chains parallel to [100]. The iron polyhedral bulk moduli results are equal to 50 and 72 GPa in α- and β-jamesonite, respectively.
The evolution of the Fe chain can be described considering that the S-S shared edges are shorter with respect to the S-S unshared edges to increase the distance between the two iron atoms.
With pressure, the shared and unshared edges have a different compressibility. In Figure 10, the FeS6 chain is shown and both the shared and unshared edges (S3-S3 distances) are indicated. The evolution with P of the shared and unshared S3-S3 distances are in Table 5. The plot in Figure 11 shows how the compressibility of shared edges is higher than that of the unshared ones, that is, the distortion of Fe polyhedra increases with P (Figure 9).
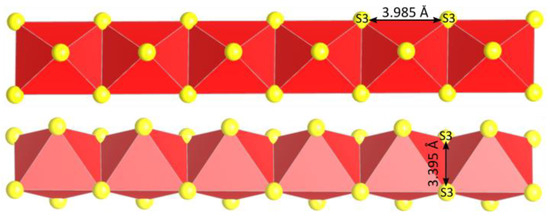
Figure 10.
View along y (above) and z (below) axes of the Fe chain. The shared and unshared edge dimensions at 1.26 GPa are reported.

Table 5.
Evolution with pressure of significant interatomic distance along the Fe chain and in the interchain region (See Figure 1) in α- and β-jamesonite.
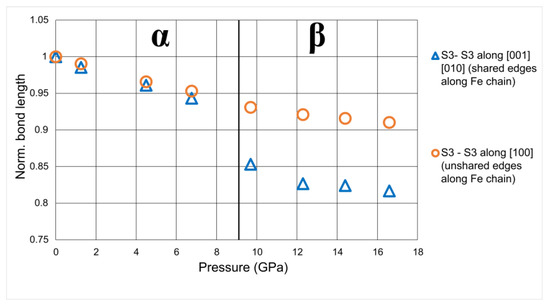
Figure 11.
Evolution of the octahedral edge, normalized to the room-pressure values, with P. At the transition pressure, a strong drop in the evolution of the shared S-S edge is well evident.
This apparently anomalous behavior, i.e., the increase in polyhedral distortion with P (exactly the opposite of what is observed in Sb polyhedra) may be related to the balancing effect whereby the reduction in volume must avoid the strong approach of the Fe atoms, which would produce a strong increase in repulsive power.
Looking in more detail at the evolution of iron edges along the chains, at the transition from the α to the β polymorph, a sharp drop in the evolution of the shared edges of the Fe polyhedra is clear (Figure 11). Polyhedral iron evolution could be associated with the high–low spin transition of iron, as observed in other sulfide structures, which occurs at pressures around 10 GPa, very low compared to similar oxygenated compounds [35]. Indeed, Huang et al. [35] observed in greigite Fe3S4, a mineral isostructural with magnetite Fe3O4, the high-spin–low-spin transition of the Fe2+ and Fe3+ at approximately 9 GPa and at 17 GP, respectively.
However, new high-pressure spectroscopic measurements, currently in progress, are needed to determine whether the electron–iron transition in jamesonite occurs around the same pressure (~9 GPa) as in the other S-structures.
4. Discussions and Conclusions
In conclusion, this in situ HP-synchrotron SC-XRD study of natural jamesonite allowed the carefully study of the high-pressure phase transition from α-jamesonite (P21/c space group) to β-jamesonite (Pmcb space group) and the compressibility of the two polymorphs. Both crystal structures showed an anisotropic compressional behavior, with the a lattice parameter being the softest and the stiffest one in α-jamesonite and β-jamesonite, respectively. At 9.7 GPa, a change in the structural evolution and a phase transition to β-jamesonite occurs, which has a much more rigid behavior [K9.7 = 74(2) GPa, K’ = 4] with respect to the α polymorph [K0 = 36(1) GPa, K’ = 5.7(7)], as also reflected in the increased bulk moduli of Sb, Pb and Fe polyhedra in the β polymorph. A similar behavior was observed in lillianite [25], for which the authors measured K0 = 44(2) GPa and K’ = 7(1) for lillianite, and K4.9 = 67(3) GPa and K’ = 5.1(4) for its high-pressure polymorph β-Pb3Bi2S6.
Sulfosalts have a rather compressible behavior with pressure, with bulk moduli ranging from 27 to 72 GPa and a value of the first derivative of the bulk modulus ranging from 4 to 8, as measured in stibnite by Lundegaard et al. [36]. The classic inverse behavior between the bulk modulus and its first derivative observed in several mineral groups is also shown in sulfosalts. The bulk modulus of α-jamesonite has an intermediate value among those measured in other sulfosalts, as can be seen in Figure 12, being close to other Sb sulfosalts, such as berthierite and chalcostibite, but with a lower first derivative. β-jamesonite shows the highest bulk modulus and the lowest first derivative (akin to heyrovskiite).
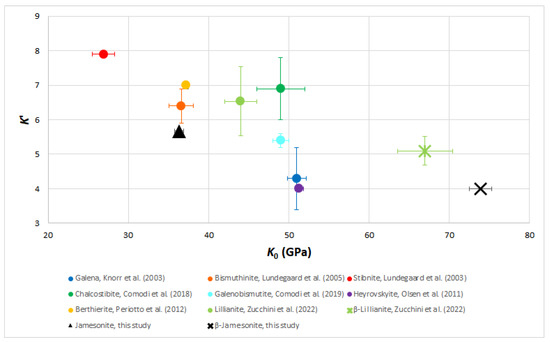
Figure 12.
Bulk moduli (K0) vs. their pressure derivative (K’) for different sulfosalts taken from [17,24,25,34,36,37,38,39].
Satisfactory crystal structure refinements of α- and β-jamesonite were performed, and the results pointed out, on the one hand, a significant change in the orientation of Sb lone electron pairs upon the phase transition, in accordance with the change in symmetry, and, on the other hand, a discontinuity in the Fe chain evolution at the transition pressure. A complex cooperative mechanism between the structural modules should be involved, as well as the different LEP activities, which impacts on polyhedral distortion and in turn on structural compressibility.
Jamesonite shows an interesting physical characteristic, in particular the strong anisotropy in magnetic susceptibility. In fact, as described by Leone et al. [15], the magnetic susceptibility of jamesonite along the a axis is significantly higher than the susceptibility values measured along b and c. As P increases, the proximity of the Fe atoms increases this effect even more, and the magnetic anisotropy is expected to increase with pressure; however, if suspicion of the high- to low-spin transition is correct, the magnetism vanishes after the phase transition to β-jamesonite.
The 1D magnetic property as well as the band-gap properties should change in quite interesting ways in the new β-jamesonite polymorph, following the different anisotropic compressibility of the observed lattice parameters. That is, the next measurements of band-gap and magnetic susceptibility at high-pressure conditions are welcome.
Finally, the possibility of a high- to low-spin iron transition is under investigation through the elaboration of additional diffractometric and spectroscopic data. The new results might explain the role of the electron spin transition in the phase transition here reported and the relationship with stereochemical activity of Pb and Sb.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13081258/s1, CIF files: JAM_c1_p1_s_23Feb23; JAM_c1_p2_s_22Mar23; JAM_c1_p3_s_22Mar23; JAM_c1_p4_s_ortho; JAM_c1_p5_s_ortho; JAM_c1_p6_s_ortho; JAM_c1_p7_s_ortho and Table S1: Atomic coordinates and Ueq for jamesonite α and β at different pressures. P values are given in Table 1. P0 values come from the refinement of jamesonite reported in [15].
Author Contributions
Conceptualization, P.C. and T.B.-Ž.; data curation, P.C., M.F., M.H., I.C. and A.Z.; formal analysis, P.C. and A.Z.; methodology, M.H., I.C. and A.Z.; software, M.F., M.H., I.C. and A.Z.; supervision, P.C.; validation, T.B.-Ž.; writing—original draft, P.C. and T.B.-Ž.; writing—review and editing, P.C., T.B.-Ž., M.F. and A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data available under request.
Acknowledgments
Thanks to Yves Moëlo for kindly providing the natural jamesonite sample. The European Synchrotron Facility is acknowledged for allocating beam-time for the experiment ES-723 (main proposer Paola Comodi).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kharbish, S.; Jeleň, S. Raman Spectroscopy of the Pb-Sb Sulfosalts Minerals: Boulangerite, Jamesonite, Robinsonite and Zinkenite. Vib. Spectrosc. 2016, 85, 157–166. [Google Scholar] [CrossRef]
- Hsu, K.F.; Loo, S.; Guo, F.; Chen, W.; Dyck, J.S.; Uher, C.; Hogan, T.; Polychroniadis, E.K.; Kanatzidis, M.G. Cubic AgPb m SbTe2+ m: Bulk Thermoelectric Materials with High Figure of Merit. Science 2004, 303, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Shuklov, I.A.; Razumov, V.F. Lead Chalcogenide Quantum Dots for Photoelectric Devices. Russ. Chem. Rev. 2020, 89, 379. [Google Scholar] [CrossRef]
- Dittrich, H.; Bieniok, A.; Brendel, U.; Grodzicki, M.; Topa, D. Sulfosalts—a New Class of Compound Semiconductors for Photovoltaic Applications. Thin Solid Film. 2007, 515, 5745–5750. [Google Scholar] [CrossRef]
- Abdelkader, D.; Rabeh, M.B.; Khemiri, N.; Kanzari, M. Investigation on Optical Properties of SnxSbySz Sulfosalts Thin Films. Mater. Sci. Semicond. Process. 2014, 21, 14–19. [Google Scholar] [CrossRef]
- Peng, H.-J.; Huang, J.-Q.; Zhang, Q. A Review of Flexible Lithium–Sulfur and Analogous Alkali Metal–Chalcogen Rechargeable Batteries. Chem. Soc. Rev. 2017, 46, 5237–5288. [Google Scholar] [CrossRef]
- Dittrich, H.; Stadler, A.; Topa, D.; Schimper, H.-J.; Basch, A. Progress in Sulfosalt Research. Phys. Status Solidi 2009, 206, 1034–1041. [Google Scholar] [CrossRef]
- Makovicky, E. Rod-Based Sulphosalt Structures Derived from the SnS and PbS Archetypes. Eur. J. Mineral. 1993, 545–592. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy; Mineral Data Publishing: Tucson, AZ, USA, 2001; Volume 1. [Google Scholar]
- Zhou, X.; Zhao, C.; Li, Y.; Chen, J.; Chen, Y. The Flotation Process, Smelting Process and Extraction Products on Jamesonite: A Review. Miner. Eng. 2021, 172, 107146. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Minerals as Advanced Materials I; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 3-540-77123-9. [Google Scholar]
- Nishad, P.A.; Bhaskarapillai, A. Antimony, a Pollutant of Emerging Concern: A Review on Industrial Sources and Remediation Technologies. Chemosphere 2021, 277, 130252. [Google Scholar] [CrossRef]
- Chang, L.L.; Li, X.; Zheng, C. The Jamesonite-Benavidesite Series. Can. Mineral. 1987, 25, 667–672. [Google Scholar]
- Niizeki, Ν.; Buerger, M.J. The Crystal Structure of Jamesonite, FePb4Sb6S14. Z. Für Krist.-Cryst. Mater. 1957, 109, 161–183. [Google Scholar] [CrossRef]
- Léone, P.; Le Leuch, L.-M.; Palvadeau, P.; Molinié, P.; Moëlo, Y. Single Crystal Structures and Magnetic Properties of Two Iron or Manganese-Lead-Antimony Sulfides: MPb4Sb6S14 (M: Fe, Mn). Solid State Sci. 2003, 5, 771–776. [Google Scholar] [CrossRef]
- Makovicky, E. The Building Principles and Classification of Sulphosalts Based on the SnS Archetype. Fortschritte Der Mineral. 1985, 63, 45–89. [Google Scholar]
- Comodi, P.; Guidoni, F.; Nazzareni, S.; Balić-Žunić, T.; Zucchini, A.; Makovicky, E.; Prakapenka, V. A High-Pressure Phase Transition in Chalcostibite, CuSbS2. Eur. J. Mineral. 2018, 30, 491–505. [Google Scholar] [CrossRef]
- Balić Žunić, T.; Makovicky, E. Determination of the Centroid Orthe Best Centre’of a Coordination Polyhedron. Acta Crystallogr. Sect. B Struct. Sci. 1996, 52, 78–81. [Google Scholar] [CrossRef]
- Matsushita, Y.; Ueda, Y. Structure and Physical Properties of 1D Magnetic Chalcogenide, Jamesonite (FePb4Sb6S14). Inorg. Chem. 2003, 42, 7830–7838. [Google Scholar] [CrossRef]
- Poudeu, P.F.P.; Takas, N.; Anglin, C.; Eastwood, J.; Rivera, A. FexPb4−xSb4Se10: A New Class of Ferromagnetic Semiconductors with Quasi 1D {Fe2Se10} Ladders. J. Am. Chem. Soc. 2010, 132, 5751–5760. [Google Scholar] [CrossRef]
- Papp, G.; Criddle, A.J.; Stanley, C.J.; Kriston, L.; Nagy, G. Parajamesonite Revisited: Background of the Discreditation of an Enigmatic Mineral Species. Swiss J. Geosci. 2007, 100, 495–502. [Google Scholar] [CrossRef][Green Version]
- Zsivny, V.; Náray-Szabó, I. Parajamesonit, Ein Neues Mineral von Kisbánya. Schweiz. Mineral. Petrogr. Mitt 1947, 27, 183–189. [Google Scholar]
- Baláž, P.; Achimovičová, M. Selective Leaching of Antimony and Arsenic from Mechanically Activated Tetrahedrite, Jamesonite and Enargite. Int. J. Miner. Process. 2006, 81, 44–50. [Google Scholar] [CrossRef]
- Olsen, L.A.; Friese, K.; Makovicky, E.; Balić-Žunić, T.; Morgenroth, W.; Grzechnik, A. Pressure Induced Phase Transition in Pb 6 Bi 2 S 9. Phys. Chem. Miner. 2011, 38, 1–10. [Google Scholar] [CrossRef]
- Zucchini, A.; Balić-Žunić, T.; Collings, I.E.; Hanfland, M.; Comodi, P. High-Pressure Single-Crystal Synchrotron X-Ray Diffraction Study of Lillianite. Am. Mineral. 2022, 107, 1752–1759. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-Dimensional Detector Software: From Real Detector to Idealised Image or Two-Theta Scan. Int. J. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Angel, R.; Gonzalez-Platas, J. ABSORB-7 and ABSORB-GUI for Single-Crystal Absorption Corrections. J. Appl. Crystallogr. 2013, 46, 252–254. [Google Scholar] [CrossRef]
- Angel, R.J. Equations of State. Rev. Mineral. Geochem. 2000, 41, 35–59. [Google Scholar] [CrossRef]
- Balić Žunić, T.; Vicković, I. IVTON–a Program for the Calculation of Geometrical Aspects of Crystal Structures and Some Crystal Chemical Applications. J. Appl. Crystallogr. 1996, 29, 305–306. [Google Scholar] [CrossRef]
- Comodi, P.; Ballaran, T.B.; Zanazzi, P.F.; Capalbo, C.; Zanetti, A.; Nazzareni, S. The Effect of Oxo-Component on the High-Pressure Behavior of Amphiboles. Am. Mineral. 2010, 95, 1042–1051. [Google Scholar] [CrossRef]
- Makovicky, E.; Balić-Žunić, T. New Measure of Distortion for Coordination Polyhedra. Acta Crystallogr. Sect. B Struct. Sci. 1998, 54, 766–773. [Google Scholar] [CrossRef]
- Comodi, P.; Zucchini, A.; Balić-Žunić, T.; Hanfland, M.; Collings, I. The High Pressure Behavior of Galenobismutite, PbBi2S4: A Synchrotron Single Crystal X-Ray Diffraction Study. Crystals 2019, 9, 210. [Google Scholar] [CrossRef]
- Huang, S.; Kang, D.; Wu, X.; Niu, J.; Qin, S. Pressure-Induced Structural and Spin Transitions of Fe3S4. Sci. Rep. 2017, 7, 46334. [Google Scholar] [CrossRef] [PubMed]
- Lundegaard, L.F.; Miletich, R.; Balic-Zunic, T.; Makovicky, E. Equation of State and Crystal Structure of Sb2S3 between 0 and 10 GPa. Phys. Chem. Miner. 2003, 30, 463–468. [Google Scholar] [CrossRef]
- Knorr, K.; Ehm, L.; Hytha, M.; Winkler, B.; Depmeier, W. The High-Pressure α/β Phase Transition in Lead Sulphide (PbS) X-Ray Powder Diffraction and Quantum Mechanical Calculations. Eur. Phys. J. B-Condens. Matter Complex Syst. 2003, 31, 297–303. [Google Scholar] [CrossRef]
- Periotto, B.; Balić-žunić, T.; Nestola, F. The Role of the Sb3+ Lone-Electron Pairs and Fe2+ Coordination in the High-Pressure Behavior of Berthierite. Can. Mineral. 2012, 50, 201–218. [Google Scholar] [CrossRef]
- Lundegaard, L.F.; Makovicky, E.; Boffa-Ballaran, T.; Balic-Zunic, T. Crystal Structure and Cation Lone Electron Pair Activity of Bi2S3 between 0 and 10 GPa. Phys. Chem. Miner. 2005, 32, 578–584. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).