Abstract
Antibiotics are pervasive contaminants in aqueous systems that pose an environmental threat to aquatic life and humans. Typically, antibiotics are developed to counteract bacterial infections; however, their prolonged and excessive use has provoked unintended consequences. The presence of excessive amounts of antibiotics and anti-inflammatory, anti-depressive, and contraceptive drugs in hospital and industrial wastewater poses a significant threat to the ecosystem, with groundwater containing drug concentrations of <1 mg/L to hundreds of µg/L. According to the literature, 33,000 people die directly from drug-resistant bacterial infections in Europe annually, which costs EUR 1.5 billion in health care and productivity loss. Consequently, the continuous spread of antibiotics in the ecosystem has led to greater interest in developing a sustainable method for effective antibiotic removal from wastewater. This critical review aims to present and discuss recent advances in the photocatalytic degradation of widely used drugs by ZnO-based nanostructures, namely (i) antibiotics; (ii) antidepressants; (iii) contraceptives; and (iv) anti-inflammatories. This study endows a comprehensive understanding of the degradation of antibiotics using ZnO-based nanomaterials (bare, doped, and composites) for effective treatment of wastewater containing antibiotics. In addition, the operational conditions and mechanisms involved during the photocatalytic degradation process are systematically discussed. Finally, particular emphasis is devoted to future challenges and the corresponding outlook with respect to toxic effects following the utilization of ZnO-based nanomaterials.
1. Introduction
Antibiotic use among human beings and animals has massively increased with the swift evolution of the pharma and medical industries. Antibiotics can prevent the onset of many illnesses by curing infections quickly [1]. The widespread use of antibiotics averts bacterial infections in humans and animals, hence saving numerous lives. Apart from the extreme water pollution, the persistence and difficult-to-degrade characteristics of antibiotics leads to important environmental issues, such as the development of drug-resistant bacteria [2]. Pharmaceutical drugs are released further and further into the environment, which is a serious threat to the environment. The presence of antibiotics in the water for a prolonged time span will make it easier for bacteria to develop antibiotic resistance, posing a more serious danger to human health and the efficacy of antibiotic medications [3]. The current review aims to portray the response to the critical environmental challenges caused by antibiotics, antidepressants, contraceptives, and anti-inflammatory drugs. In this context, diverse approaches, including adsorption, photocatalysis, biodegradation, electrochemical treatment, and others, have been effectively employed to address the troubles brought on by antibiotic contamination [4]. As a unique and appealing catalytic technique with several advantages, such as being green, eco-friendly, and economically viable, photocatalysis was developed to address issues with earlier catalytic technologies. Owing to the desire to exploit abundant solar energy as a sustainable energy source, photocatalysis has emerged as a hot topic in recent years [5,6,7,8,9,10,11,12]. Photocatalysis is a field of chemistry that studies chemical reactions triggered by light and a photocatalyst (a semiconductor that enhances reaction kinetics). Photocatalysts change the reaction rate by absorbing light and acting as a catalyst in chemical reactions [13].
The semiconductor ZnO has drawn great attention from scientists mainly because of its energy band gap, which allows light to be absorbed for photocatalytic reactions to take place [14]. In addition to photocatalysis, ZnO is a potential candidate in transparent thin-film transistors, transducers, transparent ohmic contacts, light absorption amplification structures for GaN-based light-emitting diodes (LEDs), and other possible optical devices [15]. Numerous review and research papers addressing the synthesis and validation of ZnO nanomaterial synthesis have previously been published. Therefore, the applicability of ZnO nanomaterials to the degradation of antibiotics, antidepressants, contraceptives, and anti-inflammatory drugs is significantly emphasized. The following review primarily addresses the structural characteristics of ZnO and the photocatalytic degradation performance of pristine, doped, and composite ZnO towards the aforementioned drugs under diverse conditions, including temperature, pH, and irradiation contact time.
2. Principal Approaches Driving Photocatalysis
Photocatalysts are essentially semiconductors that catalyze the reaction upon exposure to light. In recent years, photocatalysts evolved as benchmark green catalysts owing to their hazardless nature, unlike other energy sources. Upon exposure to light, an electron-hole pair is generated within the semiconductor material. The energy band gap is a major determinant of the physical characteristics of semiconductors. The energy band gap (Eg) represents the difference in energy between the valence band (HOMO) and conduction band (LUMO) [16]. Figure 1 illustrates the energy band gaps of different materials.
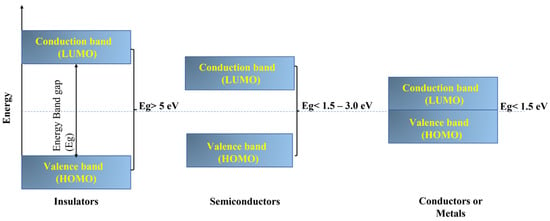
Figure 1.
Material-based energy band gap (Eg).
Since it aids in resolving the issue associated with quick charge recombination, semiconductor-mediated photocatalysis has attracted much interest and metal oxides possess the required characteristics, such as the necessary electronic structure, light-absorbing capabilities, and charge transport characteristics. Figure 2 depicts a schematic illustration of photocatalytic degradation by ZnO.
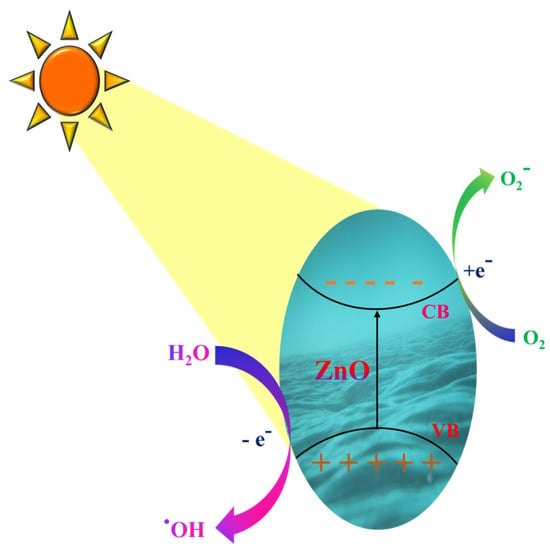
Figure 2.
Schematic representation of photocatalytic degradation by ZnO.
2.1. Degradation Mechanism of ZnO
Hermann et al. [17] demonstrated a detailed mechanism for the photocatalytic oxidation steps involved in the use of ZnO: (i) initially, the pollutants disseminate from the liquid phase to the outer surface of ZnO nanoparticles so adsorption takes place on ZnO. (ii) During the adsorption process, redox reactions take place and desorption of the products occurs. (iii) Finally, the polluted products are removed from the interface region. Typically, when a ZnO photocatalyst is photo-induced by UV/solar light with a photonic energy (hv) higher than the excitation energy (Eg), the electrons present in the filled valence band (VB) are transferred to the empty conduction band (CB). During the photo-induced process, electron-hole (e−/h+) pairs are produced. These electron-hole pairs travel to the ZnO surface to undergo redox reactions where H+ combines with water and hydroxide ions to generate hydroxyl radicals, while electrons combine with oxygen to generate superoxide radical anions and then produce hydrogen peroxide. The generated hydrogen peroxide reacts with superoxide radicals to produce hydroxyl radicals. Subsequently, the powerful hydroxyl radicals, as oxidizing agents, attack the adsorbed contaminants present at the surface of ZnO to rapidly generate intermediate compounds [18,19]. These compounds are converted to produce H2O, CO2, and mineral acids. Figure 3 illustrates the detailed mechanism of ZnO photocatalysis [20].
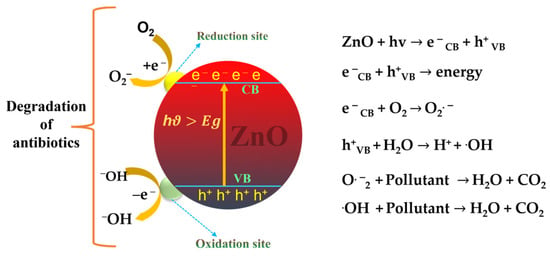
Figure 3.
Photocatalysis mechanism of ZnO.
Therefore, the ZnO photocatalyst is controlled by its ability to generate photogenerated electron-hole pairs. However, the major limitation of ZnO photocatalysts is the high rate of photogenerated electron-hole recombination, which complicates the photodegradation mechanism. The wide energy band gap of ZnO only adsorbs UV light, which limits photocatalytic activity to the UV region. Hence, improving the efficiency of ZnO photocatalysts is a top research topic in recent decades.
2.2. Enhancing Semiconductor Performance: An Overview of Techniques and Approaches
There are four main approaches to improve a semiconductor’s performance, including: employing a semiconductor with a low Eg; creating a localized state just above the valence band or creating a localized state just beneath the conduction band; forming a color center in the band gap; and surface modification. Consequently, the following techniques are used to achieve the required modifications: (i) metal and non-metal doping, (ii) co-doping, (iii) composites, (iv) substitution, (v) sensitization, and (vi) various other methods.
2.3. Metal and Non-Metal Doping
Metal and non-metal doping is regarded as a component of energy band gap engineering, which entails introducing an electron or hole into the semiconductor used in photocatalysis. By introducing new energy levels (also referred to as the impurity state) between the valence and conduction bands, metal and non-metal doping boosts the photocatalyst’s photoresponsiveness to the visible region. Metal dopants (impurities) such as Cu, Zn [21], Mn [22], Co, Cr [23], Fe, Ni, Mo [24], etc., give rise to a new band below the conduction band, whereas non-metals such as N [25], P [26], F, Si, S, Cl, Se, Br, I, etc., [27] give rise to a new band above the valence band. The subsequent explanations highlight why the addition of dopants to photocatalysts improves their performance. Doping prevents electron-hole recombination, promotes surface area, increases particle size in porous structure, enhances crystallinity, and increases sensitivity across a diverse range [28]. Studies have shown that non-metals, such as S, N, F, and C, can alter the energy band gap of ZnO via doping, resulting in increased oxygen vacancy defects and enhanced photocatalytic activity under visible light [29]. S-doping specifically modifies electrical, optical, and photocatalytic properties due to the difference in electronegativity and size between S and O. Doping with sulfur is an effective method to narrow ZnO’s energy band gap and shift its threshold wavelength towards visible light [30].
2.4. Co-doping
Co-doping raises the valence band edge while dropping the conduction band edge to minimize the band gap. This approach also enables the resolution of challenges such as the host material’s lack of responsiveness to visible light, the carrier recombination solubility limit, and poor carrier mobility. In contrast to pure TiO2, the integration of ZnO and Fe2O3 into TiO2 improved the photodegradation of phenol. This TiO2 co-doping led to a coordinated rise in activity, which was attributed to the synergistic interaction between the co-dopants and the energy bands of TiO2 [31].
2.5. Composites
The fabrication of composites is an alternate approach to boost the photoresponsiveness of semiconductors in the visible range of the whole spectrum. The semiconductors used to develop new composites must have varying band gaps. A semiconductor with a small energy band gap and greater negative conduction band level is typically coupled with a semiconductor with a large energy band gap. As a result, the conduction band electrons are transferred from the semiconductor with a small energy band gap to the semiconductor with a large band gap. The ZnO/-Fe2O3 nanocomposite catalyst was found to be easily removed from water after photocatalytic treatment due to the presence of paramagnetic -Fe2O3 nanoparticles. This made it simple to recover the catalyst and reuse it in future degradation cycles with the application of a weak external magnetic field [32].
2.6. Substitution
An additional approach to modify the activity of a photocatalyst is to replace one metal with another, called substitution. For example, when W is replaced in WO3 by another metal of the same valency, such as Cr or Mo, the Eg decreases due to the movement of the conduction band edge to the lower state. In contrast, the introduction of lower valency metals, such as Ti, Zr, and Hf, causes an elevation in Eg owing to the upward movement of the conduction band edge [33]. The results of a photocatalytic degradation experiment showed that 5% Pb-substituted ZnO nanoparticles were an effective photocatalyst for degradation [34]. When exposed to visible light irradiation, the photocatalytic degradation of organic pollutants was significantly enhanced by substituting Al3+ with Zn2+ at Zn2+ sites within the ZnO host lattice [35]
2.7. Sensitization
Organic and inorganic compounds that chemisorb or physisorb on the surface of semiconductors are known as sensitizers. Sensitization is another intriguing concept for photocatalyst surface modification. Because of their redox capabilities and sensitivity to visible light, dyes and complexes can be employed in photocatalytic devices, including solar cells, to enhance the photocatalytic activity. Some chemical compounds containing chromophores, such as dyes or natural pigments, might be employed to improve the photosensitivity of semiconductors. When photosensitizers are exposed to visible light, absorbed light injects electrons into the semiconductor’s conduction band, triggering a catalytic process. Even when exposed to indoor vis-LED lighting, C-sensitized and N-doped TiO2 had the ability to purify and mineralize water by effectively removing any harmful chemicals [36].
2.8. Other Methods
Undoped TiO2 nanoparticles possess an energy band gap of 3.1 eV, which can be narrowed significantly up to 2.2 eV by the formation of a midgap state-induced energy gap during synthesis. Thus, undoped TiO2 synthesized using the mixed phase solution method eventually possessed greater surface area. The as-synthesized material possessed enhanced photocatalytic activity [37]. The utilization of synthetic techniques for surface functionalization is an efficient approach to address challenges such as chemical reactivity in solution and inherent flaws that impede the incorporation of catalysts into practical applications. These techniques encompass everything from complete coverings to the utilization of low-dimensional elements such as nanoparticles, photosensitive dyes, quantum dots, and organic compounds. This straightforward and reliable method may be used to modify the surface charge of different kinds of nano photocatalysts and enhance their photocatalytic activities. The photodegradation rate and adsorption effectiveness of a dye were altered by employing TiO2 nanoparticles with various surface charges [38].
3. Structural and Electronic Aspects of ZnO
ZnO is a discrete semiconductor with a suitable energy band gap (≈3.30 eV) and an unusually large exciton binding energy of 60 meV [39,40,41,42]. The greatest ionization energy of any element in the sixth group of the periodic table is oxygen, which leads to the strongest bonding between Zn (3d) and O (2p) orbitals [43]. Substantial electromechanical incorporating effects in piezoelectric and pyroelectric properties can be applied in piezoelectric sensors and mechanical actuators owing to the deficiency of a center of symmetry in ZnO’s wurtzite structure [44,45,46,47,48]. Due to its unique characteristics, ZnO has attracted much attention, particularly because of its hexagonal wurtzite-type structure. Cubic rock-salt and blende forms are two further structural variations ZnO, but the wurtzite form is thermodynamically stable at moderate temperature and pressure [49]. High-temperature and high-power operation, reduced noise production, larger breakdown voltages, and the capacity to withstand strong electric fields are positive characteristics related to the large bandgap of ZnO. For both low and high electric fields, electron transport in semiconductors can be taken into account (i) in a suitably weak electric field that has no impact on the energy distribution of electrons and (ii) in a suitably high electric field where electron distribution function differs considerably from its equilibrium [15]. SEM at a magnification of 10,000× was utilized to identify the surface morphological characteristics of the materials prior to and after calcination (Figure 4) [50]. The uncalcined material’s morphology (Figure 4a) showed the aggregation of particles. However, the morphology of the calcined materials showed nanoprisms and nanorods at various temperatures. The TEM images (Figure 4b) confirmed that the uncalcined material was agglomerated and great homogeneity and crystallinity were observed when the temperature rose.
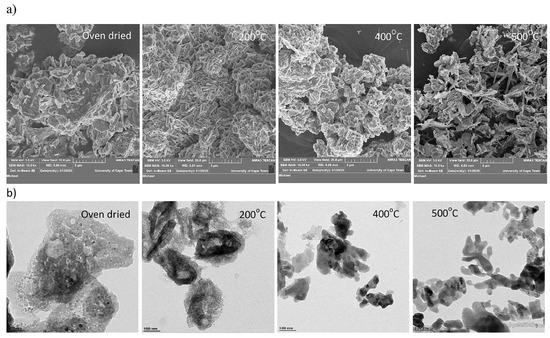
Figure 4.
SEM (a) and TEM (b) images of ZnO at different annealing temperatures.
The Raman spectra of spherical and commercial ZnO particles in the range of 250–680 cm−1 are shown in Figure 5 [51]. No apparent Raman peaks of flower-like ZnO were observed. Better optical quality was achieved in as-prepared ZnO crystals than in commercial ZnO, which had a hexagonal wurtzite structure with four atoms per unit cell and corresponded to the space group C46m (P63mc).
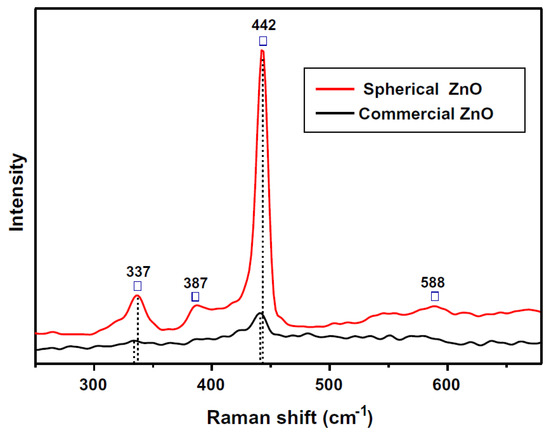
Figure 5.
Raman spectra of spherical and commercial ZnO (☐—Prominent peaks).
Nanoparticles are favored due to their increased surface area to volume ratio. This property allows nanoparticles to absorb more energy and, as an outcome, generate more hydroxyl groups, which aids in the oxidation of organic contaminants [52]. The fundamental drawback of ZnO, like all other semiconductor materials, is that the recombination of h+/VB–e−/CB has an adverse influence on photocatalytic performance. Using simple control techniques, ZnO/Ag/CdO, FexZn1−xO, ZnO/CeO2, and certain nanocomposites were synthesized. The photocatalytic efficiency undoubtedly increased since the surface of ZnO was altered. ZnO has been synthesized through a variety of routes as a matrix material with varied structure and forms. The catalytic performance of nanoparticles is significantly influenced by their shape. ZnO’s enhanced photocatalytic performance is made possible by its crystallinity and spherical form [53]. The photodegradation of tetracycline under visible light irradiation showed maximum efficiency, which was related to the synergistic action of tetracycline desorption because of its high surface area. Employing ZnO nanoparticles will greatly enhance the economic future of photocatalysis by massively decreasing the required dosage due to its greater surface area and more active sites. For example, 50% of ciprofloxacin was eliminated with just 20 mg/L of nano-ZnO. Moreover, utilizing 20 mg/L of nano-ZnO achieved higher efficiency, i.e., 90% and 59% elimination of tetracycline and ibuprofen, respectively [54].
3.1. The Influence of pH and Zeta Potential on the Photocatalytic Degradation of Antibiotics in Aqueous Solutions
The pH of a solution plays a crucial role in photodegradation processes as it affects the size and charge of the photocatalyst [55]. For example, ZnO nanowires showed improved photodegradation of cephalexin under alkaline conditions (pH 7.2–9.2). However, the ZnO nanowires’ surface charge changed from positive to negative at pH 6.8, thereby affecting the species involved in the oxidation process. The stability of colloidal solutions depends on the zeta potential value, which is in turn influenced by the surface charge. The calcination temperature can also affect the activity of photocatalysts, with the highest efficiency (82%) achieved at 4000 °C. However, higher calcination temperatures can reduce photocatalytic activity due to a decrease in surface area and increase in crystal size [56].
3.2. Structural Stability and Reusability of ZnO as Photocatalysts
ZnO has a broad UV absorption spectrum and excellent photostability, biocompatibility, and degradability. Recyclability is a crucial factor to consider when examining the structural stability and reuse of a photocatalyst. The ZnO photocatalyst synthesized using the solvothermal method to eliminate the antibiotic ofloxacin exhibited good cycling stability and retained 95% efficiency even after three cycles. This showed the ZnO photocatalyst’s benefits of outstanding structural stability and enticing reusability [57]. Maximum photocatalytic activity was shown by ZnO photocatalysts prepared using the hydrothermal method in the removal of the antibiotic ofloxacin even after undergoing a third cycle. The ZnO photocatalyst continued to perform at a high level, demonstrating its superior cycling capacity [58]. N,S-doped carbon quantum dot-embedded ZnO nanoflowers had a high degree of stability and could be utilized repeatedly [59]. In five successive cycles, the Cr2O3@ZnO photocatalyst tested for the degradation of ciprofloxacin showed 100% destruction and high reusability [60].
3.3. Corrosion Effects of ZnO
ZnO’s efficiency and stability as a photocatalyst are reduced when exposed to UV radiation due to its susceptibility to corrosion. Thus, ZnO is being combined with carbon-based compounds, noble metals, or other metal oxides in an effort to increase its performance [61]. For instance, the durability and photocatalytic efficacy of ZnO nanoparticles were enhanced by coating them with reduced graphene oxide. ZnO can have improved visible light responsiveness and greater photo corrosion resistance when doped with Ag, Au, or Pt, as demonstrated by the Ag-ZnO photocatalyst’s degradation of the antibiotic ofloxacin [62].
4. Pristine ZnO Photocatalysts
Zinc oxide is a well-known n-type semiconductor that has garnered widespread use as a photocatalyst. This is due to its high absorption efficiency and large exciton binding energy, which result in its exceptional mechanical, electrical, and optical properties. The use of ZnO-based materials is widespread across a variety of applications, including biological and catalytic processes. Among these materials, pristine ZnO nanoparticles are particularly favored for their exceptional photocatalytic activity [63]. Furthermore, the production cost of ZnO nanoparticles is much lower than that of other materials, as reported by Liang et al. [64]. However, the incorporation of dopants in ZnO nanoparticles and the fabrication of composite-based materials can achieve improved efficiency.
The combination of ZnO with other materials to produce a hybrid photocatalyst system subsequently improves the separation of photogenerated electron-hole pairs [65,66]. Above all, nanostructured ZnO is divided into zero-, one-, two-, and three-dimensions and each of these are subdivided into planar, dots, and quantum arrays. One-dimensional ZnO arrays include nanofibers, nanotubes, nanorods, nanoneedles, and nanowires, while two- and three-dimensional ZnO arrays include nanoflowers. Luo et al. [67] reported that ZnO has a higher specific surface area, which is advantageous in the photocatalytic degradation process as more pollutants can be easily adsorbed. Additionally, the lower crystallinity of ZnO promotes the trapping of photoinduced electron-hole pairs, which enhances easy separation.
There are several approaches for the synthesis of ZnO nanostructures (Figure 6), but vapor phase and solution-based approaches are primarily employed [68]. The solution-based approach is the easiest and least energy-consuming technique to improve the morphology and control the size of the nanostructure [69]. The techniques used to produce ZnO in a solution-based approach include hydrothermal, precipitation, solvothermal, sol-gel, microwave, electrospinning, and wet chemical methods. Among them, sol-gel is the most commonly used and effective technique due to its advantages [70]. Ciciliati et al. used Fe-based ZnO nanoparticles for photocatalysis, which were synthesized using the sol-gel method [71]. The vapor phase approach includes physical vapor deposition, plasma enhanced chemical vapor deposition, and metal-organic chemical vapor deposition methods [72].
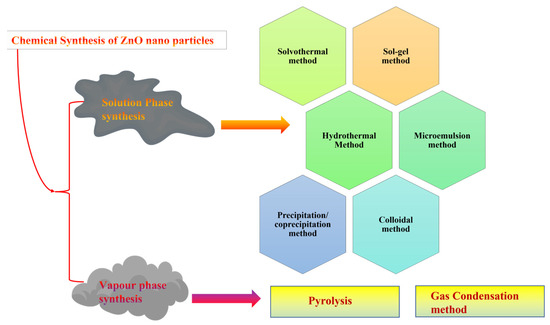
Figure 6.
Methods used for the synthesis of ZnO nanostructures.
5. Metal/Non-Metal-Doped ZnO Photocatalysts
The improvement of ZnO as a photocatalyst can be achieved via metal doping and fabrication of composites. Generally, ZnO contains a tetrahedral bonding configuration with a largely ionic nature that is classified between a covalent and ionic semiconductor. The ZnO photocatalyst forms three crystalline structures, including hexagonal wurtzite (P63mmc), cubic rock-salt (Fm3m), and cubic zinc-blende (F43m). ZnO hexagonal wurtzite is the most stable at ambient conditions [73]. Metal doping has been adopted to modify the physicochemical properties of ZnO such as to alter the valence band energy upward and minimize the energy band gap to the ultraviolet-visible region. On the other hand, non-metal doping is also used to shift the bandgap of ZnO. Specifically, fluorine, oxygen, carbon, and nitrogen interfere with lattice interstices and bind to atoms via the oxidation process. Among all, carbon is an effective candidate as a non-metal dopant due to its explicit properties, such as good mechanical strength and chemical resistance, and electronic properties. Moreover, the incorporation of metal/non-metals produces greater OH. radicals, thereby leading to enhanced photocatalytic degradation of pollutants [74]. This is owing to the fact that the metal/non-metal dopants act as scavengers, control the recombination of electron-hole pairs, and produce H+ for the formation of OH. radicals. Transition metal incorporation into the ZnO crystal lattice is highly regarded due to its tunability of the energy band gap of ZnO to produce an effective visible light photocatalyst. The transition metal can significantly modify the particle size and shape of ZnO and controls ZnO growth, which leads to the generation of ultrafine nanostructures with higher surface area. Transition metal oxides such as Fe and Cu have oxidation states that improve the photocatalytic activity of the doped ZnO catalyst [75]. The Ag-doped ZnO catalyst is a commonly used photocatalyst. Li et al. reported that the properties of Ag helped to improve the doped ZnO photocatalyst in which Ag acted as an electron acceptor [76]. Mn is another efficient material that enhances the photocatalytic activity of the doped ZnO photocatalyst due to changes in the optical properties. The charge transfer between the valence band and Mn-induced levels and d-d transition in the crystal field induces visible-light irradiation. The charge carrier defect eliminates the photo-induced electron/hole pair, thereby increasing the lifetime of the photocatalyst [77]. Copper is a cost-effective element that can be easily incorporated in substitutional sites of ZnO, which helps to modify the emission and absorption spectra into the visible-light region [78]. Polat et al. synthesized a Cu-doped ZnO photocatalyst for effective dye degradation [79]. Fu et al. utilized a Cu-doped ZnO photocatalyst to examine degradation under UV light. However, the addition of high concentrations of copper acted as a recombination center, which decreased the photocatalytic activity [80]. Likewise, Mohan et al. demonstrated that Cu-doped ZnO produced more holes, which were a surface defect that enhanced the photocatalytic activity. The cobalt-doped ZnO catalyst has received enormous attention due to its great response as photocatalyst [81]. The incorporation of Co in the ZnO lattice shifts the optical absorption edge toward the visible-light region and narrows the energy band gap. The addition of Co hinders the growth of ZnO nanoparticles, thus producing a smaller particle size and favoring photocatalytic activity. Fe doping is similarly used for ZnO modification [82]. Fe doping of ZnO causes a narrow energy band gap and increases the electron excitation rate. The introduction of Fe reduces the crystallite size and increases the oxygen vacancies, which enhances the electron/hole separation efficiency [83]. Yu et al. demonstrated that Al-doped ZnO adsorbed large amounts of organic pollutants compared to pure ZnO [84]. The incorporation of Al in ZnO generates more oxygen defects, which increase the charge density of ZnO and therefore enhance its performance. Alam et al. reported that magnesium-doped ZnO produced a higher response than pure ZnO due to the unpaired electrons [85]. Mrindha et al. showed that Al-doped ZnO exhibited a better photocatalytic response than the pristine ZnO [86]. Further, Dai et al. produced Cu-doped ZnO nanoparticles with improved photocatalytic activity over five consecutive cycles [87]. However, crystal distortion and high levels of metal concentrations reduced the photocatalytic activity.
6. ZnO Composite Photocatalysts
The commercialization of ZnO as a photocatalyst is constrained by the effects of photocorrosion, which can be prevented by preparing composites of ZnO with other metal oxides [88]. The composites have the tendency to modify the energy band gap and efficiently allow visible light. Wang et al. synthesized the MnO-ZnO nanocomposite with a reduced energy band gap that moved the absorption from UV to visible light and enhanced the photocatalytic activity [89]. Sigh et al. demonstrated the preparation of the CuO-ZnO nanocomposite and produced lower energy band gap [90]. Carbon-based nanocomposites are attractive materials owing to their improved activities in many applications. Wang et al. developed the ZnO-rGO nanocomposite as a visible light photocatalyst [91]. The photogenerated electrons in the conduction band of ZnO migrated to rGO, thereby suppressing their recombination with the hole in ZnO. Similarly, Tran et al. synthesized the ZnO-rGO nanocomposite with a reduced energy band gap for the effective photocatalytic degradation of dye under visible light. Spinel ferrite-based ZnO photocatalysts are commonly used as effective photocatalysts [92]. Azar et al. developed the ZnAl2O4–ZnO nanocomposite with improved photocatalytic activity [93]. Similarly, Dlugosz et al. used the combination of magnetic and catalytic activities to improve the degradation activity of dye [94]. Zhang et al. synthesized the SnO2/ZnO composite with a reduction of the energy band gap for improved photocatalytic activity of dye [95]. Trakulmututa et al. developed the CuO/ZnO composite with modified optical properties for the degradation of antibiotics [96]. A study by Hemnil et al. produced a novel Fe2O3/ZnO nanocomposite with high absorbance in the ultraviolet and visible regions and proposed the synthesized photocatalyst with a suitable energy band gap for effective photodegradation [97]. Krishnan et al. delivered a suitable MOS2/ZnO nanocomposite for biological photocatalytic degradation under visible light [98]. Zhang et al. proposed a tertiary ZnO/ZnFe2O4/TiO2 nanocomposite with enhanced photocatalytic activity under visible light degradation [99]. The ZnO/SiO2 nanocomposite developed by Fatimah et al. was found to be an effective reusable photocatalyst with a reduced energy band gap for improved photocatalytic degradation of dye pollutants [100]. Sabri et al. fabricated a novel ZnO/CuBi2O4 nanocomposite for the photocatalytic degradation of dye contaminants under visible light. The proposed photocatalyst had improved photocatalytic activity due to its larger surface area, formation of a heterojunction at ZnO and CuBi2O4 and better absorption of visible light. The small energy band gap significantly absorbed visible light, and the nanocomposite was effectively utilized as an efficient composite-based photocatalyst [101].
7. Impact of Antibiotic and Pharmaceutical Pollution on the Environment and Human Health
Antibiotics are pharmaceutically active compounds (PACs) that alleviate bacterial ailments and are necessary to protect overall survival [102]. Antibiotics are spilling into the ecosystem to a greater extent and are becoming a prominent contributor to water contamination. The menace that antibiotic waste poses to the purity of aquatic systems and human health is one of the greatest ecological concerns of the twenty-first century [103]. Tetracyclines (TC) are a category of expansive antibiotics prevalently prescribed to combat numerous infectious diseases in both people and animals [104]. More than 70% of TC deployed for pharmacologic therapy is eliminated through renal excretion in metropolitan wastewater and animal liquid effluents due to inefficient adhesion and metabolic activity by animals and people. Tetracyclines have a plethora of repercussions on soil microbes and photosynthetic marine creatures, which correlate with the surge in substantial human health complications and antibiotic-resistant pathogens [105,106,107]. The World Health Organization (WHO) cited antibiotic resistance as a global health concern in 2019 [108] and estimated that veterinary utilization accounted for over 80% of all antibiotic consumption. The United Nations General Assembly has highlighted livestock consumption of antibiotics as one of the major factors contributing to the escalation in antibiotic resistance, with an anticipated total usage of 200,000 tons annually [109,110]. Antibiotic-resistant genes can be acquired by bacterium when antibiotic dosages are very low, barring them from affecting the bacterium. In turn, other bacterial spores can accumulate these genes. If the prevailing trend continues, 10,000,000 people may perish annually by 2050 from contagious diseases spurred by bacterium with diverse impedances [111,112,113,114]. Macrolides, primarily spiramycin (SPY), serve as the most ubiquitous antibiotics in industrial effluents. As a result of their putative assimilation by crops fertilized with product from sewage treatment plants, antibiotics may impede recycling of waste in agriculture [115,116].
A prevalent antibiotic, tetracycline hydrochloride (TC-HCl), is produced in enormous quantities and holds second place in usage worldwide. Through the metabolic pathways of individuals and livestock, and pharmaceutical corporation waste, TC-HCl is transferred into the ambient aquatic system and then reaches the food system of humans. Drug-resistant infectious diseases have evolved as a consequence of the deposition of TC-HCl, as well as other pharmaceuticals, resulting in a serious influence on both public welfare and marine life [117,118]. The World Health Organization (WHO) stated in 2014 that the inclusion of gemifloxacin (GMF), a quinolone drug, in numerous ecosystems could result in significant health and ecological risks, such as cytotoxic effects and bacterium development of resistance [119]. Investigations conducted in Spain, Germany, Australia, Italy, Brazil, Canada, Greece, and the United States have revealed that more than 80 distinct types of pharma drugs and metabolites produced as a consequence of consuming multiple medications have contaminated marine ecosystems. Substantial concentrations of pharma drugs per liter were observed in materials collected from surface fluids downstream of urban sewage treatment facilities, inlet effluent, and contaminated water [120,121].
Naproxen (NAP) and ibuprofen (IBU) are non-steroidal, anti-inflammatory pharmaceuticals implicated in health risks to people, such as renal dysfunction and intestinal problems, as per toxicity testing [122]. One of the most pervasive hazardous pollutants is diclofenac (DCF), a non-steroidal, anti-inflammatory medicine frequently employed to relieve arthritis or rheumatism. Despite having a low toxic effect, diclofenac can incentivize the emergence of drug-resistant pathogens that pose a risk to human wellbeing and aquatic creatures and are thus destructive to the ecosystem and mankind [123].
The tricyclic antidepressant that receives the most usage is amitriptyline hydrochloride (AMI). Moreover, it is also employed in the treatment of sleeplessness, acute headaches, and migraines [124,125]. In France, amitriptyline hydrochloride has been found in drinking water at a concentration of 1.4 ng/L, whereas ground waterways in the UK have been found to contain amitriptyline hydrochloride at a concentration of 0.5 to 21 ng/L. Furthermore, AMI was detected in solid sewage treatment effluent in Canada at a concentration of 448 ng/g [126,127,128].
Humans, aquatic life, and other species in the environment are gravely endangered by water pollution by endocrine-disrupting pollutants such as estrone, estradiol, ethinylestradiol, and estriol steroid hormones [129]. Endocrine-disrupting compounds (EDCs) are agents that interfere with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development, and behavior [130,131]. Waterways in the Czech Republic have been reported to possess steroid hormones at 34–41 ng/L [132]. Most frequently encountered in oral contraceptive tablets, artificial hormones such as 17α-ethinylestradiol (EE2) are also utilized to cure prostatic disease and irregular menstrual cycles. They remain active as an outcome of interactions between hormone residues in the environment and the ensuing deconjugation of EE2 during incomplete degradation at wastewater plants, and their leftovers are also a substantial pollutant in the environment [27,133]. The impacts of EE2 encompass testicular weight reduction in Japanese quail, rapid development in female fish, and reproductive issues [134]. Comparable effects, including lowering of sperm counts and elevated risks of ovarian and breast cancer, are reported in humans [135].
8. Photocatalytic Degradation of Antibiotics
Various methods, including carbon filtration, ozonation [136], catalytic membranes [137], Fenton-like catalysis [138], sorption, and biodegradation [139] have been employed to eliminate such enduring antibiotic substances. As a result, wastewater accumulates effluents from corporations, clinics, and farmland. Sewage treatment is typically regarded as the preferred method for treating these antibiotics. However, investigations have demonstrated that the established methods do not eliminate these pollutants, which are primarily water-soluble but neither volatile nor compostable [140,141].
Photocatalysis has gained a lot of interest as an effective method for eliminating antibiotic contaminants since it is inexpensive, effective, and environmentally benign as it eliminates antibiotics in sunlight and under ambient conditions [142,143]. The reactions endured by a semiconductor and the potential to absorb photons with energies higher than its energy band gap is called “photocatalytic degradation” [144]. The degradation of antibiotics by photocatalysis can be categorized into five major steps: (1) the passage of antibiotics from the fluid to the surface, (2) antibiotic sorption, (3) change during the adsorption phase, (4) desorption of the product, (5) and product separation from the interface area [145,146]. Due to their superior light absorption under UV, visible light, or both, together with their biocompatibility, safety, and stability when subjected to various circumstances, metal oxide-based photocatalysts have recently attracted much attention [147].
8.1. Antibiotic Degradation Utilizing Pristine ZnO Photocatalyst
ZnO has multiple benefits because of its antigen-free, antimicrobial, antitumor, and wound-healing characteristics [148]. ZnO is an affordable material with minimal technical aspects, rendering it the more beneficial choice compared to TiO2 as a photocatalyst. ZnO has a significant exciton binding energy of 60 meV and good capacity to absorb UV irradiation. ZnO exhibited better photocatalytic performance than TiO2 due to its photogenerated electron-hole pairs, mobility, and isolation [149].
Effective photodegradation of ciprofloxacin (CIP) was exhibited by ZnO nanoparticles synthesized using an ethanolic root extract of Japanese knotweed with specific operational conditions, as shown in Table 1. A UV-vis spectrophotometer at 271 nm was used to analyze the level of CIP while the degradation process was being carried out under ultraviolet light (λmax = 365 nm). It is necessary to highlight that the λmax of CIP changed from 272 to 265 nm from the start to completion of degradation, emphasizing the disturbance of the chromophoric conjugation network. The perfect nanoparticle size of 14 nm had a perceived rate constant (kapp) of 0.038 min−1 and completely degraded CIP during exposure for 100 min [150]. The degradation of CIP in water solutions with varied pH values under exposure to UV light was examined with ZnO nanoparticles synthesized using a chemical precipitation method. After 60 min of exposure, the maximal recorded CI antibiotic degradation efficacy was around 18% at pH 4, 42% at pH 7, and 50% at pH 10. Pseudo-first order kinetics govern the photocatalytic degradation of ciprofloxacin. The free hydroxyl ions interact with holes (h+) and generate hydroxyl radicals (OH.) that possess strong oxidizing potential, thereby enhancing the photocatalytic degradation of ciprofloxacin. At pH 10, ZnO nanoparticles notably demonstrated enhanced degradation of ciprofloxacin (Table 1).

Table 1.
Photocatalytic degradation of antibiotics by pristine ZnO as photocatalyst.
From the above findings, it can be inferred that the pH might modify the photocatalyst’s surface charge features and presumably the chemical morphology of the molecule; as a corollary, the photocatalysis process is pH-dependent. It has been revealed that thin ZnO films possessed photocatalytic performance in the photocatalytic decomposition of the drugs chloramphenicol (levomycetin) and paracetamol with conditions (Table 1). When the catalyst is irradiated by ultraviolet light with either an energy equal to or higher than that of the energy band gap, an electron–hole pair is formed. Water adsorption over the ZnO layer or hydroxyl groups occurs in connection with photo-induced holes at the valence band. The outcome of such activity is the powerful OH. radical. Conduction band electrons react with electrophiles, such as O2, deposited on the surface or dispersed in the water, leading to the production of super oxide radicals. By reacting with paracetamol and chloramphenicol, the ensuing strongly reactive radicals produced intermediates that degraded to give ionic species. The degradation efficiency for paracetamol and chloramphenicol under UV light was 14% and 40% with conditions (Table 1) [153]. Table 1 also summarizes numerous ZnO photocatalysts that effectively photodegraded various drug compounds, such as spiramycin (95–99%), ofloxacin (95%), metronidazole (100%), and tetracycline hydrochloride (69.8%), respectively.
8.2. Antibiotic Degradation Utilizing Metal-Integrated ZnO Photocatalysts
ZnO nanoparticles have several beneficial usages in piezoelectric devices, semiconductors, photovoltaic cells, polymers, skincare, and pharmaceuticals. However, they have specified drawbacks, such as: (i) potential to degrade in water solutions as a consequence of the photocorrosive effect; (ii) scattering of light, which can be minimized by modifying the catalyst dosage rate; (iii) a high energy band gap facilitates performance in ultraviolet light, which may not be realistic for massive sewage treatment; (iv) due to the recombination pathway being more rapid than the surface redox reaction, impulsive e−/h+ recombination in the photocatalyst; (v) minimal reuse. In an attempt to tackle these issues, ZnO nanomaterials have been doped with metal nanoparticles. By adding defective modes and tunning the active energy band gap, doping helps to improve the surface region of ZnO nanoparticles and also boosts their ability to absorb photons. Platinum (Pt), gold (Au), and silver (Ag) are the noble metals utilized as dopants. Noble metals easily absorb in the visible part of the electromagnetic spectrum through the surface plasmon resonance (SPR) mechanism. ZnO becomes a visible light-sensitive photocatalyst by incorporating noble metals into its crystal lattice or on its interface [156]. Transition metals, alkali metals, alkaline earth metals, as well as heavy metals such as lanthanides have also been utilized to dope ZnO photocatalysts in addition to noble metals. For instance, the minor misalignment between magnesium (Mg) and ZnO crystal structures leads to the dopant raising the energy band gap of ZnO. The basic pathway of antibiotic photodegradation by metal-doped ZnO is as follows: (i) the antibiotic drug adheres to the photocatalyst’s surface; (ii) production of electron-hole pairs; and (iii) reactive oxygen species (ROS) are caused by redox processes to decompose the antibiotic [157].
Comparing the photocatalytic activity of the ZnO photocatalyst under UV light irradiation (duration: 120 min) and the Ag-ZnO photocatalyst under solar light irradiation (duration: 80 min) against the antibiotic ofloxacin revealed that 95% degradation efficiency was achieved by the ZnO photocatalyst and 100% degradation efficiency was achieved by Ag-ZnO photocatalyst (5 wt% silver) under similar operating conditions. Due to its higher electron-hole separating efficacy than pure ZnO, the Ag-doped ZnO photocatalyst exhibited complete degradation of ofloxacin. The Schottky barrier arises at the Ag/ZnO interface as metal silver is coated onto the ZnO photocatalyst, which leads to better photocatalytic activity and greater quantum efficacy [150,158]. Under ideal conditions, the Ag-ZnO photocatalyst exhibited significant degradation rates for medicinal residues, i.e., 70.2% for atenolol (ATL) and 90.8% for acetaminophen (ACT) (Table 2).

Table 2.
Photocatalytic degradation of antibiotics by metal-integrated ZnO as photocatalyst.
ATL and ACT degradation is governed by pseudo-first-order kinetics. Increased surface area, improved charge transport with both ZnO and Ag, and their synergistic influence were the main factors that contributed to the increase in efficiency. An additional finding about the photocatalyst mechanism revealed that the main method for removing ATL and ACT was the OH. pathway [151]. The Ag/ZnO synthesized using the rapid, one-pot, surfactant-free, microwave-assisted, aqueous solution method showed complete degradation of the antibiotic tetracycline (TC) in 30 min under visible light [154]. Silver nanoparticles act as an electron sink in Ag-doped ZnO nanoparticles, improving the charge separation. This enhances the generation of hydroxyl radicals in the reaction media and consequently improves the photocatalytic performance of ZnO nanoparticles [146]. Employing a conventional solid-state process, Mg-doped ZnO nanocrystallites were synthesized and exhibited an 87% alprazolam degradation rate. Mg-doped ZnO annealed at 700 °C showed excellent photocatalytic properties, reaching 100% degradation within 20 min. Meanwhile, under similar conditions, bare ZnO showed 78% removal of alprazolam. Due to excellent electron-hole separation and good textural characteristics, doping ZnO nanoparticles with Mg enhanced the photocatalytic performance triggered by sunlight and the results indicated that doping ZnO with Mg promoted alprazolam elimination by 10% [153]. Under UV light, a hydrothermally prepared Ce-doped ZnO photocatalyst demonstrated 96% degradation rates for nizatidine and levofloxacin and a 65% degradation rate for acetaminophen [159]. Research revealed that the metal doping of ZnO was necessary for controlling the ZnO nanoparticle surface and optical features. It must be highlighted that the photodegradation of metal-doped ZnO depends on the light source. This is due to ZnO’s ability to absorb UV light whereas metal-doped ZnO absorbs light primarily in the visible spectrum. Therefore, due to the fact that sunlight carries UV along with visible light, the excitonic formation under solar irradiation may arise by either ZnO or the doped metal [146].
8.3. Antibiotic Degradation Utilizing ZnO Composites Photocatalysts
The major concern with ZnO in photocatalysis is mainly its high rate of electron-hole recombination. With the aim of enhancing the performance of photocatalysis, hetero-junction semiconductors have been developed and used to prevent electron-hole recombination [147], consequently enhancing the degradation efficiency with better regeneration and recycling.
The photodegradation of tetracycline by the ZnO/γ-Fe2O3 composite showed 88.52% degradation efficiency under UV-visible light over 150 min. Both the catalyst’s surface and pore volume were increased by the inclusion of iron oxide in its structure, thereby promoting the analyte’s adsorption on the nanostructure’s surface, which is a crucial step in improving photocatalytic degradation. Additionally, it was observed that ZnO served a vital function in the photocatalytic degradation supported by γ-Fe2O3, raising the rate of TC degradation to 20% [163]. The photodegradation activity of the ZnO globular-gC3N4 nanocomposite showed that 78.4% of tetracycline degraded in 50 min and 63.5% of oxytetracycline degraded in 50 min. Reactive species were regarded as the primary cause in the photodegradation mechanism [160]. As Ag loading rose during the photocatalytic degradation of the antibiotic tetracycline hydrochloride, the catalytic performance of the Ag@ZnO/BiOCl composite first rose and then fell. ZnO has minimal photocatalytic performance and achieved a degradation rate of38.5% over 80 min. The degradation rate achieved by ZnO/BiOCl was moderately greater, attaining 42.7%. It is noteworthy that the degradation rates of Ag-loaded samples were better than that of ZnO/BiOCl, and the Ag nanocomposite achieved the maximum degradation rate with 80.4% removal of tetracycline hydrochloride (Table 3).

Table 3.
Photocatalytic degradation of antibiotics by ZnO nanocomposites as photocatalysts.
This was achieved as a result of enhanced absorption of visible light, better charge separation as a function of surface plasmon resonance, the potential of Ag to capture electrons, and enhanced surface catalysis. Hence, adding noble metals to a semiconductor with a wide energy band gap is a practical approach to improving its photocatalytic performance [164]. A simple hydrothermal method was utilized to synthesize the SnO2/ZnO nanocomposite investigated for the photocatalytic degradation of quinolone antibiotics (ciprofloxacin, ofloxacin, and norfloxacin). Over 60 min, ciprofloxacin, ofloxacin, and norfloxacin were all degraded at rates of 91.23%, 91.26%, and 88.39%, respectively. Strong oxidation potential is present in SnO2, whereas high reduction ability is found in ZnO. This can substantially increase the separation efficacy of photo-induced e−/h+. Therefore, a simple method for improving the photocatalytic properties is to combine SnO2 with ZnO to produce a composite photocatalyst [165]. The photodegradation of the most used antibiotics by several ZnO nanocomposites is summarized in Table 3. Ag–ZnO/GP composite, SnO2/ZnO nanocomposite, GO@Fe3O4/ZnO/SnO2 composite, and ZnO/γ-Fe2O3 composite are recognized for exhibiting the maximum degradation for the frequently prescribed antibiotics metronidazole, ciprofloxacin and ofloxacin, azithromycin, and tetracycline, respectively. Therefore, ZnO nanocomposites are potential and effective preferences for the photocatalytic degradation of antibiotics.
9. Photocatalytic Degradation of Anti-Depressives, Anti-Inflammatories, and Contraceptives
Pharmaceuticals and endocrine-disrupting compounds (EDCs) constitute a substantial category of emerging contaminants (ECs) that are ingested in enormous quantities around the world. These pollutants are pervasive in the environment, primarily as a result of their limited degradability and high resistance. Therefore, it is critically necessary to find effective strategies for removing or lowering the concentrations of such developing pollutants. Because of its nontoxicity, accessibility, lack of mass transfer restriction, chemical stability, and potential functioning at room temperature, photocatalytic degradation has been proven to be a viable approach.
ZnO is probably the most common catalyst for the photocatalytic removal of organic contaminants [171]. The advantages and mechanisms of ZnO photocatalysts have already been mentioned above. The most popular tricyclic antidepressant is amitriptyline hydrochloride (AMI), which is used to treat migraines, sleepiness, and severe headaches. The ZnO photocatalyst in the degradation of AMI showed 94.3% degradation efficiency under solar irradiation with variable operating parameters (Table 4).

Table 4.
Photocatalytic degradation of antidepressants, anti-inflammatories, and contraceptives by ZnO/ZnO based nanomaterials as photocatalysts.
The increased mobility, production, and separation of e−/h+ pairs might be the cause of ZnO’s enhanced photocatalytic activity [169]. Under ultraviolet (UV) light irradiation, the ZnO photocatalyst degraded ibuprofen (IBU) and naproxen (NAP) with high rates of 94.5% and 98.7% after 120 min, respectively [167]. Aquatic life, mankind, and other species in the ecosystem are greatly threatened by water contamination with endocrine-disrupting compounds such as estrone (E1), estradiol (E2), 17-ethinylestradiol (EE2), and estriol (E3), which are steroid hormones. ZnO nanoparticles are extremely potent at eliminating estrogenic hormones through photodegradation. The degradation rate of endocrine-disrupting compounds by ZnO nanoparticles was 84–93% altogether, indicating a propensity for fast photocatalytic degradation [168]. Endocrine-disrupting compounds (EDCs), namely estrone (E1), 17-estradiol (E2), 17-ethinylestradiol (EE2), and bisphenol A (BPA) were photodegraded using ZnO nanorod arrays. The simplest EDC to eliminate over six hours was estrone (E1), followed by 17-ethinylestradiol (EE2) and 17-estradiol (E2) with a similar trend. Bisphenol A (BPA) degraded the least quickly of all EDC substances. Electrons will shift from the valence band to the conduction band as ZnO nanorods are exposed to UV light with a light energy greater than or equivalent to their energy band gap, thus creating electron-hole pairs. In subsequent reaction with water, the electrons and holes will produce hydroxyl radicals (OH.). The reactive oxidative species, called OH. radicals, will target EDCs and break them down into CO2 and water. Additionally, the EDCs may be effectively oxidized by the holes.
10. Conclusions and Future Prospects
This review highlighted the repercussions of hazardous, non-biodegradable medications (antibiotics, antidepressants, anti-inflammatories, and contraceptives). A comprehensive overview of the photocatalytic degradation of such widely used drugs using bare ZnO, metal- and non-metal-doped ZnO, as well as ZnO-based nanocomposites is provided. ZnO demonstrated superior photocatalytic performance because of its photogenerated electron-hole pairs, mobility, and isolation. In practice, heteroatom doping is utilized to improve the catalyst’s photocatalytic performance, especially when metal atoms are used as dopants. Moreover, it must be mentioned that metal dopants may act as recombination centers at higher concentrations, which can decrease the effectiveness of a photocatalyst. Consideration should be given to operating parameters such as pH, temperature, catalyst dosage, and pollutant concentration on the complete photodegradation rate. There are limited studies on the photocatalytic activity of ZnO-based catalysts in the degradation of developing pollutants, such as non-biodegradable medicines. Extensive research on multi-component photocatalytic degradation is required to assess probable competition among a variety of pollutants for the photocatalyst, light absorption, and interactions with oxidizing agents. Decreased rapid recombination and photocorrosion should be addressed because they influence the photocatalyst’s potential to effectively degrade pollutants, regenerate, and its lifecycle.
Economic Evaluation of Degradation Techniques
The average retail price of electricity in the United States is approximately USD 0.13 per kilowatt-hour (kWh) and it may vary based on the provider and local regions [179]. A lamp that operates at 500 watts will consume 0.5 kilowatt-hours (kWh) of electricity per hour, and this usage will cost USD 0.07. The cost of a UV lamp can vary greatly depending on the type of lamp, its size, the manufacturer, and the intended application. On average, a simple handheld UV lamp can cost between USD 20 to 100. On average, ZnO nanoparticles cost USD 4.6 per gram, which may vary based on the purity of the chemicals and supplier. The cost of photocatalytic degradation studies can vary depending on several factors, such as the location, size of the study, type of equipment used, and duration of the study.
Author Contributions
K.M.M.: validation, investigation, resources, writing—original draft, writing—review & editing, visualization. J.J.B.: investigation, resources, writing—original draft, writing—review & editing, visualization. J.J.V.: conception, methodology, resources, writing—original draft, writing—review & editing, visualization, supervision.; M.B.: validation, writing—original draft, writing—review & editing, visualization, co-supervision. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data and material are available from the corresponding author upon reasonable request.
Conflicts of Interest
All authors declare that they have no conflict of interest.
References
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A Review of the Influence of Treatment Strategies on Antibiotic Resistant Bacteria and Antibiotic Resistance Genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, H.; Wang, T.; Wang, B.; Wei, W.; Li, H.; Yuan, S.; An, T.; Zhao, H.; Yu, J.; et al. Nature-Based Catalyst for Visible-Light-Driven Photocatalytic CO2 Reduction. Energy Environ. Sci. 2018, 11, 2382–2389. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the Biological and Chemical Treatment Technologies for Emerging Contaminant Removal from Wastewater: A Critical Review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Liu, J.; Fang, W.; Wei, Z.; Qin, Z.; Jiang, Z.; Shangguan, W. Efficient Photocatalytic Hydrogen Evolution on N-Deficient g-C3N4 Achieved by a Molten Salt Post-Treatment Approach. Appl. Catal. B 2018, 238, 465–470. [Google Scholar] [CrossRef]
- Fang, W.; Qin, Z.; Liu, J.; Wei, Z.; Jiang, Z.; Shangguan, W. Photo-Switchable Pure Water Splitting under Visible Light over Nano-Pt@P25 by Recycling Scattered Photons. Appl. Catal. B 2018, 236, 140–146. [Google Scholar] [CrossRef]
- Liu, J.; Fang, W.; Wei, Z.; Qin, Z.; Jiang, Z.; Shangguan, W. Metallic 1T-Li x MoS 2 Co-Catalyst Enhanced Photocatalytic Hydrogen Evolution over ZnIn2S4 Floriated Microspheres under Visible Light Irradiation. Catal. Sci. Technol. 2018, 8, 1375–1382. [Google Scholar] [CrossRef]
- Qin, Z.; Fang, W.; Liu, J.; Wei, Z.; Jiang, Z.; Shangguan, W. Zinc-Doped g-C3N4/BiVO4 as a Z-Scheme Photocatalyst System for Water Splitting under Visible Light. Chin. J. Catal. 2018, 39, 472–478. [Google Scholar] [CrossRef]
- Fang, W.; Liu, J.; Yu, L.; Jiang, Z.; Shangguan, W. Novel (Na, O) Co-Doped g-C3 N4 with Simultaneously Enhanced Absorption and Narrowed Bandgap for Highly Efficient Hydrogen Evolution. Appl. Catal. B 2017, 209, 631–636. [Google Scholar] [CrossRef]
- Wei, Z.; Li, R.; Wang, R. Enhanced Visible Light Photocatalytic Activity of BiOBr by in SituReactable Ionic Liquid Modification for Pollutant Degradation. RSC Adv. 2018, 8, 7956–7962. [Google Scholar] [CrossRef]
- Wei, Z.-D.; Wang, R. Hierarchical BiOBr Microspheres with Oxygen Vacancies Synthesized via Reactable Ionic Liquids for Dyes Removal. Chin. Chem. Lett. 2016, 27, 769–772. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Waste Water Treatment; Academic Press: London, UK, 2018; pp. 135–175. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. band gap Engineered Zinc Oxide Nanostructures via a Sol–Gel Synthesis of Solvent Driven Shape-Controlled Crystal Growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Ameta, R.; Ameta, S.C. Photocatalysis; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor Heterojunction Photocatalysts: Design, Construction, and Photocatalytic Performances. Chem. Soc. Rev. 2014, 43, 5234. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous Photocatalysis: Fundamentals and Applications to the Removal of Various Types of Aqueous Pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Shanthi, M. Photocatalytic Degradation of an Organic Pollutant by Zinc Oxide—Solar Process. Arab. J. Chem. 2016, 9, S1858–S1868. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental Principles and Application of Heterogeneous Photocatalytic Degradation of Dyes in Solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Li, L.; Wang, M. Advanced Nanomatericals for Solar Photocatalysis. In Advanced Catalytic Materials—Photocatalysis and Other Current Trends; InTechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Khairy, M.; Zakaria, W. Effect of Metal-Doping of TiO2 Nanoparticles on Their Photocatalytic Activities toward Removal of Organic Dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef]
- Li, N.; Teng, H.; Zhang, L.; Zhou, J.; Liu, M. Synthesis of Mo-Doped WO 3 Nanosheets with Enhanced Visible-Light-Driven Photocatalytic Properties. RSC Adv. 2015, 5, 95394–95400. [Google Scholar] [CrossRef]
- Bae, S.W.; Borse, P.H.; Hong, S.J.; Jang, J.S.; Lee, J.S.; Jeong, E.D.; Hong, T.E.; Yoon, J.H.; Jin, J.S.; Kim, H.G. Photophysical Properties of Nanosized Metal-Doped TiO2 Photocatalyst Working under Visible Light. J. Korean Phys. Soc. 2007, 51, 22. [Google Scholar] [CrossRef]
- Feng, Y.; Ji, W.-X.; Huang, B.-J.; Chen, X.; Li, F.; Li, P.; Zhang, C.; Wang, P.-J. The Magnetic and Optical Properties of 3d Transition Metal Doped SnO2 Nanosheets. RSC Adv. 2015, 5, 24306–24312. [Google Scholar] [CrossRef]
- Zhang, D.; Gong, J.; Ma, J.; Han, G.; Tong, Z. A Facile Method for Synthesis of N-Doped ZnO Mesoporous Nanospheres and Enhanced Photocatalytic Activity. Dalton Trans. 2013, 42, 16556. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Wu, C.-H.; Wu, J.-T.; Chen, Y.-R. Synthesis and Characterization of a Phosphorus-Doped TiO2 Immobilized Bed for the Photodegradation of Bisphenol A under UV and Sunlight Irradiation. React. Kinet. Mech. Catal. 2015, 114, 753–766. [Google Scholar] [CrossRef]
- Guo, W.; Guo, Y.; Dong, H.; Zhou, X. Tailoring the Electronic Structure of β-Ga2O3 by Non-Metal Doping from Hybrid Density Functional Theory Calculations. Phys. Chem. Chem. Phys. 2015, 17, 5817–5825. [Google Scholar] [CrossRef]
- Raza, W.; Faisal, S.M.; Owais, M.; Bahnemann, D.; Muneer, M. Facile Fabrication of Highly Efficient Modified ZnO Photocatalyst with Enhanced Photocatalytic, Antibacterial and Anticancer Activity. RSC Adv. 2016, 6, 78335–78350. [Google Scholar] [CrossRef]
- Patil, A.B.; Patil, K.R.; Pardeshi, S.K. Ecofriendly Synthesis and Solar Photocatalytic Activity of S-Doped ZnO. J. Hazard. Mater. 2010, 183, 315–323. [Google Scholar] [CrossRef]
- Yuan, Z.; Jia, J.; Zhang, L. Influence of Co-Doping of Zn(II)+Fe(III) on the Photocatalytic Activity of TiO2 for Phenol Degradation. Mater. Chem. Phys. 2002, 73, 323–326. [Google Scholar] [CrossRef]
- Saad, A.M.; Abukhadra, M.R.; Abdel-Kader Ahmed, S.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.-J.; Rabie, A.M. Photocatalytic Degradation of Malachite Green Dye Using Chitosan Supported ZnO and Ce–ZnO Nano-Flowers under Visible Light. J. Environ. Manag. 2020, 258, 110043. [Google Scholar] [CrossRef]
- Ran, L.; Zhao, D.; Gao, X.; Yin, L. Highly Crystalline Ti-Doped SnO2 Hollow Structured Photocatalyst with Enhanced Photocatalytic Activity for Degradation of Organic Dyes. CrystEngComm 2015, 17, 4225–4237. [Google Scholar] [CrossRef]
- Gnanamozhi, P.; Rajivgandhi, G.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Pandiyan, V.; Li, W.-J. Enhanced Antibacterial and Photocatalytic Degradation of Reactive Red 120 Using Lead Substituted ZnO Nanoparticles Prepared by Ultrasonic-Assisted Co-Precipitation Method. Ceram. Int. 2020, 46, 19593–19599. [Google Scholar] [CrossRef]
- Sharma, H.K.; Archana, R.; Sankar ganesh, R.; Singh, B.P.; Ponnusamy, S.; Hayakawa, Y.; Muthamizhchelvan, C.; Raji, P.; Kim, D.Y.; Sharma, S.K. Substitution of Al3+ to Zn2+ Sites of ZnO Enhanced the Photocatalytic Degradation of Methylene Blue under Irradiation of Visible Light. Solid State Sci. 2019, 94, 45–53. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, T.; Wang, R.; Lim, T.-T. Carbon-Sensitized and Nitrogen-Doped TiO2 for Photocatalytic Degradation of Sulfanilamide under Visible-Light Irradiation. Water Res. 2011, 45, 5015–5026. [Google Scholar] [CrossRef]
- Yadav, S.; Jaiswar, G. Review on Undoped/Doped TiO2 Nanomaterial; Synthesis and Photocatalytic and Antimicrobial Activity. J. Chin. Chem. Soc. 2017, 64, 103–116. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Z.; Zapien, J.A.; Lee, C.-S.; Zhang, W. Surface Engineering of ZnO Nanostructures for Semiconductor-Sensitized Solar Cells. Adv. Mater. 2014, 26, 5337–5367. [Google Scholar] [CrossRef]
- Yaghoubi, H.; Li, Z.; Chen, Y.; Ngo, H.T.; Bhethanabotla, V.R.; Joseph, B.; Ma, S.; Schlaf, R.; Takshi, A. Toward a Visible Light-Driven Photocatalyst: The Effect of Midgap-States-Induced Energy Gap of Undoped TiO2 Nanoparticles. ACS Catal. 2015, 5, 327–335. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: Material, Physics and Applications. ChemPhysChem 2007, 8, 782–803. [Google Scholar] [CrossRef]
- Klingshirn, C.; Hauschild, R.; Priller, H.; Decker, M.; Zeller, J.; Kalt, H. ZnO Rediscovered—Once Again!? Superlattices Microstruct. 2005, 38, 209–222. [Google Scholar] [CrossRef]
- Thangavel, R.; Singh Moirangthem, R.; Lee, W.-S.; Chang, Y.-C.; Wei, P.-K.; Kumar, J. Cesium Doped and Undoped ZnO Nanocrystalline Thin Films: A Comparative Study of Structural and Micro-Raman Investigation of Optical Phonons. J. Raman Spectrosc. 2010, 41, 1594–1600. [Google Scholar] [CrossRef]
- Ellmer, K.; Klein, A. ZnO and Its Applications. In Transparent Conductive Zinc Oxide; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–33. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Gao, P.X.; Song, J.; Liu, J.; Wang, Z.L. Nanowire Piezoelectric Nanogenerators on Plastic Substrates as Flexible Power Sources for Nanodevices. Adv. Mater. 2007, 19, 67–72. [Google Scholar] [CrossRef]
- Wang, Z.L. Nanopiezotronics. Adv. Mater. 2007, 19, 889–892. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Liu, J.; Liu, H.; Li, Y.; Wang, Z.L. Piezoelectric Potential Output from ZnO Nanowire Functionalized with P-Type Oligomer. Nano Lett. 2008, 8, 203–207. [Google Scholar] [CrossRef]
- Lu, M.-P.; Song, J.; Lu, M.-Y.; Chen, M.-T.; Gao, Y.; Chen, L.-J.; Wang, Z.L. Piezoelectric Nanogenerator Using P-Type ZnO Nanowire Arrays. Nano Lett. 2009, 9, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A Review on ZnO: Fundamental Properties and Applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Aremu, O.H.; Akintayo, C.O.; Sodeinde, K.O.; Igboama, W.N.; Oseghe, E.O.; Nelana, S.M. Sonocatalytic Degradation of Amoxicillin from Aquaculture Effluent by Zinc Oxide Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100513. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The Effect of Surface Charge on Photocatalytic Degradation of Methylene Blue Dye Using Chargeable Titania Nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef]
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal Oxides and Metal Organic Frameworks for the Photocatalytic Degradation: A Review. J. Environ. Chem. Eng. 2020, 8, 103726. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, J.; Jiang, T.; Tang, Q.; Cheng, Y. Photocatalytic Degradation of Sulfamethazine in Aqueous Solution Using ZnO with Different Morphologies. R. Soc. Open Sci. 2018, 5, 171457. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of Pharmaceuticals and Endocrine Disrupting Compounds from Water by Zinc Oxide-Based Photocatalytic Degradation: A Review. Sustain. Cities Soc. 2016, 27, 407–418. [Google Scholar] [CrossRef]
- Qamar, M.; Muneer, M. A Comparative Photocatalytic Activity of Titanium Dioxide and Zinc Oxide by Investigating the Degradation of Vanillin. Desalination 2009, 249, 535–540. [Google Scholar] [CrossRef]
- Gupta, D.; Chauhan, R.; Kumar, N.; Singh, V.; Srivastava, V.C.; Mohanty, P.; Mandal, T.K. Enhancing Photocatalytic Degradation of Quinoline by ZnO:TiO2 Mixed Oxide: Optimization of Operating Parameters and Mechanistic Study. J. Environ. Manag. 2020, 258, 110032. [Google Scholar] [CrossRef]
- He, S.; Hou, P.; Petropoulos, E.; Feng, Y.; Yu, Y.; Xue, L.; Yang, L. High Efficient Visible-Light Photocatalytic Performance of Cu/ZnO/RGO Nanocomposite for Decomposing of Aqueous Ammonia and Treatment of Domestic Wastewater. Front. Chem. 2018, 6, 219. [Google Scholar] [CrossRef]
- Sansenya, T.; Masri, N.; Chankhanittha, T.; Senasu, T.; Piriyanon, J.; Mukdasai, S.; Nanan, S. Hydrothermal Synthesis of ZnO Photocatalyst for Detoxification of Anionic Azo Dyes and Antibiotic. J. Phys. Chem. Solids 2022, 160, 110353. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, X.; Huang, R.; Qi, W.; Su, R.; He, Z. Enhanced Photocatalytic Degradation of Antibiotics in Water over Functionalized N,S-Doped Carbon Quantum Dots Embedded ZnO Nanoflowers under Sunlight Irradiation. Chem. Eng. J. 2020, 382, 123016. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ismail, A.A.; Alhaddad, M. A Novel Design of Porous Cr2O3@ZnO Nanocomposites as Highly Efficient Photocatalyst toward Degradation of Antibiotics: A Case Study of Ciprofloxacin. Sep. Purif. Technol. 2021, 266, 118588. [Google Scholar] [CrossRef]
- Dworschak, D.; Brunnhofer, C.; Valtiner, M. Photocorrosion of ZnO Single Crystals during Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 51530–51536. [Google Scholar] [CrossRef]
- Fang, Y.; Li, Z.; Xu, S.; Han, D.; Lu, D. Optical Properties and Photocatalytic Activities of Spherical ZnO and Flower-like ZnO Structures Synthesized by Facile Hydrothermal Method. J. Alloy. Compd. 2013, 575, 359–363. [Google Scholar] [CrossRef]
- Pal, M.; Bera, S.; Sarkar, S.; Jana, S. Influence of Al Doping on Microstructural, Optical and Photocatalytic Properties of Sol–Gel Based Nanostructured Zinc Oxide Films on Glass. RSC Adv. 2014, 4, 11552–11563. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, K.; Mo, Y.; Huang, X. A Novel ZnO Nanoparticle Blended Polyvinylidene Fluoride Membrane for Anti-Irreversible Fouling. J. Memb. Sci. 2012, 394–395, 184–192. [Google Scholar] [CrossRef]
- Meng, Y.; Lin, Y.; Yang, J. Synthesis of Rod-Cluster ZnO Nanostructures and Their Application to Dye-Sensitized Solar Cells. Appl. Surf. Sci. 2013, 268, 561–565. [Google Scholar] [CrossRef]
- Jimenez-Cadena, G.; Comini, E.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Synthesis of Different ZnO Nanostructures by Modified PVD Process and Potential Use for Dye-Sensitized Solar Cells. Mater. Chem. Phys. 2010, 124, 694–698. [Google Scholar] [CrossRef]
- Luo, J.; Ma, S.Y.; Sun, A.M.; Cheng, L.; Yang, G.J.; Wang, T.; Li, W.Q.; Li, X.B.; Mao, Y.Z.; GZ, D.J. Ethanol Sensing Enhancement by Optimizing ZnO Nanostructure: From 1D Nanorods to 3D Nanoflower. Mater. Lett. 2014, 137, 17–20. [Google Scholar] [CrossRef]
- Srujana, S.; Bhagat, D. Chemical-Based Synthesis of ZnO Nanoparticles and Their Applications in Agriculture. Nanotechnol. Environ. Eng. 2022, 7, 269–275. [Google Scholar] [CrossRef]
- Banerjee, P.; Chakrabarti, S.; Maitra, S.; Dutta, B.K. Zinc Oxide Nano-Particles—Sonochemical Synthesis, Characterization and Application for Photo-Remediation of Heavy Metal. Ultrason. Sonochem. 2012, 19, 85–93. [Google Scholar] [CrossRef]
- Vafaee, M.; Ghamsari, M.S. Preparation and Characterization of ZnO Nanoparticles by a Novel Sol–Gel Route. Mater. Lett. 2007, 61, 3265–3268. [Google Scholar] [CrossRef]
- Ciciliati, M.A.; Silva, M.F.; Fernandes, D.M.; de Melo, M.A.C.; Hechenleitner, A.A.W.; Pineda, E.A.G. Fe-Doped ZnO Nanoparticles: Synthesis by a Modified Sol–Gel Method and Characterization. Mater. Lett. 2015, 159, 84–86. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Introduction. In Mechanical Alloying; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–12. [Google Scholar] [CrossRef]
- Özgür, Ü.; Avrutin, V.; Morkoç, H. Zinc Oxide Materials and Devices Grown by MBE. In Molecular Beam Epitaxy; Elsevier: Amsterdam, The Netherlands, 2013; pp. 369–416. [Google Scholar] [CrossRef]
- Shen, J.-H.; Chiang, T.-H.; Tsai, C.-K.; Jiang, Z.-W.; Horng, J.-J. Mechanistic Insights into Hydroxyl Radical Formation of Cu-Doped ZnO/g-C3N4 Composite Photocatalysis for Enhanced Degradation of Ciprofloxacin under Visible Light: Efficiency, Kinetics, Products Identification and Toxicity Evaluation. J. Environ. Chem. Eng. 2022, 10, 107352. [Google Scholar] [CrossRef]
- Bechambi, O.; Sayadi, S.; Najjar, W. Photocatalytic Degradation of Bisphenol A in the Presence of C-Doped ZnO: Effect of Operational Parameters and Photodegradation Mechanism. J. Ind. Eng. Chem. 2015, 32, 201–210. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Fan, W. Structural, Electronic, and Optical Properties of Ag-Doped ZnO Nanowires: First Principles Study. J. Phys. Chem. C 2011, 115, 3552–3557. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Ahmed, W.; Elhissi, A.; Hong, Z.L.; Khalid, N.R. Enhancing Visible Light Responsive Photocatalytic Activity by Decorating Mn-Doped ZnO Nanoparticles on Graphene. Ceram. Int. 2014, 40, 10085–10097. [Google Scholar] [CrossRef]
- Kaur, J.; Singhal, S. Facile Synthesis of ZnO and Transition Metal Doped ZnO Nanoparticles for the Photocatalytic Degradation of Methyl Orange. Ceram. Int. 2014, 40, 7417–7424. [Google Scholar] [CrossRef]
- Polat, İ.; Yılmaz, S.; Altın, İ.; Bacaksız, E.; Sökmen, M. The Influence of Cu-Doping on Structural, Optical and Photocatalytic Properties of ZnO Nanorods. Mater. Chem. Phys. 2014, 148, 528–532. [Google Scholar] [CrossRef]
- Fu, M.; Li, Y.; Wu, S.; Lu, P.; Liu, J.; Dong, F. Sol–Gel Preparation and Enhanced Photocatalytic Performance of Cu-Doped ZnO Nanoparticles. Appl. Surf. Sci. 2011, 258, 1587–1591. [Google Scholar] [CrossRef]
- Mohan, R.; Krishnamoorthy, K.; Kim, S.-J. Enhanced Photocatalytic Activity of Cu-Doped ZnO Nanorods. Solid State Commun. 2012, 152, 375–380. [Google Scholar] [CrossRef]
- Le, T.H.; Bui, A.T.; Le, T.K. The Effect of Fe Doping on the Suppression of Photocatalytic Activity of ZnONanopowder for the Application in Sunscreens. Powder Technol. 2014, 268, 173–176. [Google Scholar] [CrossRef]
- Mardani, H.R.; Forouzani, M.; Ziari, M.; Biparva, P. Visible Light Photo-Degradation of Methylene Blue over Fe or Cu Promoted ZnO Nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 27–33. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, L.-C.; Xie, Y.; Liu, G.; Ma, X.; Cheng, H.-M. Crystallinity-Dependent Substitutional Nitrogen Doping in ZnO and Its Improved Visible Light Photocatalytic Activity. J. Colloid Interface Sci. 2013, 400, 18–23. [Google Scholar] [CrossRef]
- Alam, M.S.; Manzoor, U.; Mujahid, M.; Bhatti, A.S. Highly Responsive UV Light Sensors Using Mg-Doped ZnO Nanoparticles. J. Sens. 2016, 2016, 8296936. [Google Scholar] [CrossRef]
- Mridha, S.; Basak, D. Aluminium Doped ZnO Films: Electrical, Optical and Photoresponse Studies. J. Phys. D Appl. Phys. 2007, 40, 6902–6907. [Google Scholar] [CrossRef]
- Dai, W.; Pan, X.; Chen, C.; Chen, S.; Chen, W.; Zhang, H.; Ye, Z. Enhanced UV Detection Performance Using a Cu-Doped ZnO Nanorod Array Film. RSC Adv. 2014, 4, 31969. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; Hessien, M.; Alresheedi, F.; Al-Baradi, A.M.; Alrowaili, Z.A.; Kebaili, I.; Olarinoye, I.O. ZnO– Bi2O3 Nanopowders: Fabrication, Structural, Optical, and Radiation Shielding Properties. Ceram. Int. 2022, 48, 3464–3472. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.; Wang, Z.; Dong, M.; Cui, L. Facile Fabrication of Mn2+ -Doped ZnO Photocatalysts by Electrospinning. R. Soc. Open Sci. 2020, 7, 191050. [Google Scholar] [CrossRef]
- Singh, J.; Soni, R.K. Controlled Synthesis of CuO Decorated Defect Enriched ZnO Nanoflakes for Improved Sunlight-Induced Photocatalytic Degradation of Organic Pollutants. Appl. Surf. Sci. 2020, 521, 146420. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Jiang, J.; Wan, Z.; Su, Y.; Tang, H. Insight into Charge Carrier Separation and Solar-Light Utilization: RGO Decorated 3D ZnO Hollow Microspheres for Enhanced Photocatalytic Hydrogen Evolution. J. Colloid Interface Sci. 2020, 564, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Van Tuan, P.; Phuong, T.T.; Tan, V.T.; Nguyen, S.X.; Khiem, T.N. In-Situ Hydrothermal Fabrication and Photocatalytic Behavior of ZnO/Reduced Graphene Oxide Nanocomposites with Varying Graphene Oxide Concentrations. Mater. Sci. Semicond. Process 2020, 115, 105114. [Google Scholar] [CrossRef]
- Eskandari Azar, B.; Ramazani, A.; TaghaviFardood, S.; Morsali, A. Green Synthesis and Characterization of ZnAl2O4@ZnO Nanocomposite and Its Environmental Applications in Rapid Dye Degradation. Optik 2020, 208, 164129. [Google Scholar] [CrossRef]
- Długosz, O.; Szostak, K.; Krupiński, M.; Banach, M. Synthesis of Fe3O4/ZnO Nanoparticles and Their Application for the Photodegradation of Anionic and Cationic Dyes. Int. J. Environ. Sci. Technol. 2021, 18, 561–574. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Chen, N.; Du, Y.; Ding, T.; Li, Y.; Chang, W. Synthesis of SnO2/ZnO Flowerlike Composites Photocatalyst for Enhanced Photocatalytic Degradation of Malachite Green. Opt. Mater. 2022, 133, 112978. [Google Scholar] [CrossRef]
- Trakulmututa, J.; Chuaicham, C.; Shenoy, S.; Srikhaow, A.; Sasaki, K.; Smith, S.M. Effect of Transformation Temperature toward Optical Properties of Derived CuO/ZnO Composite from Cu–Zn Hydroxide Nitrate for Photocatalytic Ciprofloxacin Degradation. Opt. Mater. 2022, 133, 112941. [Google Scholar] [CrossRef]
- Hemnil, P.; Prapawasit, Y.; Karthikeyan, V.; Wongwuttanasatian, T.; Seithtanabutara, V. Novel Cubic Heterojunction Fe2O3/ZnO Composite for the Photocatalyst Application. Mater. Today Proc. 2022; in press. [Google Scholar] [CrossRef]
- Krishnan, U.; Kaur, M.; Kaur, G.; Singh, K.; Dogra, A.R.; Kumar, M.; Kumar, A. MoS2/ZnO Nanocomposites for Efficient Photocatalytic Degradation of Industrial Pollutants. Mater. Res. Bull. 2019, 111, 212–221. [Google Scholar] [CrossRef]
- Zhang, J.; Kuang, M.; Cao, Y.; Ji, Z. Environment-Friendly Ternary ZnO/ZnFe2O4/TiO2 Composite Photocatalyst with Synergistic Enhanced Photocatalytic Activity under Visible-Light Irradiation. Solid State Sci. 2022, 129, 106913. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, G.; Sahroni, I.; Kamari, A.; Sagadevan, S.; Doong, R.-A. Nanoflower-like Composites of ZnO/SiO2 Synthesized Using Bamboo Leaves Ash as Reusable Photocatalyst. Arab. J. Chem. 2021, 14, 102973. [Google Scholar] [CrossRef]
- Sabri, M.; Habibi-Yangjeh, A.; Ghosh, S. Novel ZnO/CuBi2O4 Heterostructures for Persulfate-Assisted Photocatalytic Degradation of Dye Contaminants under Visible Light. J. Photochem. Photobiol. A Chem. 2020, 391, 112397. [Google Scholar] [CrossRef]
- Abdurahman, M.H.; Abdullah, A.Z.; Shoparwe, N.F. A Comprehensive Review on Sonocatalytic, Photocatalytic, and Sonophotocatalytic Processes for the Degradation of Antibiotics in Water: Synergistic Mechanism and Degradation Pathway. Chem. Eng. J. 2021, 413, 127412. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, J.; Shangguan, W. A Review on Photocatalysis in Antibiotic Wastewater: Pollutant Degradation and Hydrogen Production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Rizzi, V.; Lacalamita, D.; Gubitosa, J.; Fini, P.; Petrella, A.; Romita, R.; Agostiano, A.; Gabaldón, J.A.; ForteaGorbe, M.I.; Gómez-Morte, T.; et al. Removal of Tetracycline from Polluted Water by Chitosan-Olive Pomace Adsorbing Films. Sci. Total Environ. 2019, 693, 133620. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, C.; Li, Y.; Ke, H.; Cheng, G. Efficient Removal of Tetracycline Hydrochloride from Aqueous Solution by Mesoporous Cage MOF-818. SN Appl. Sci. 2020, 2, 669. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, X.; Li, S.; Han, R.; Wang, G. Insights into Tetracycline Adsorption onto Kaolinite and Montmorillonite: Experiments and Modeling. Environ. Sci. Pollut. Res. 2015, 22, 17031–17040. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, L.; Qi, C.; Zhang, M. Highly Effective Removal of Tetracycline from Water by Hierarchical Porous Carbon: Batch and Column Adsorption. Ind. Eng. Chem. Res. 2019, 58, 20036–20046. [Google Scholar] [CrossRef]
- World Health Organization. Ten Threats to Global Health in 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Rodi, P.; Obermeyer, W.; Pablos-Mendez, A.; Gori, A.; Raviglione, M.C. Political Rationale, Aims, and Outcomes of Health-Related High-Level Meetings and Special Sessions at the UN General Assembly: A Policy Research Observational Study. PLoS Med. 2022, 19, e1003873. [Google Scholar] [CrossRef] [PubMed]
- Lofrano, G.; Libralato, G.; Brown, J. (Eds.) Nanotechnologies for Environmental Remediation; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Rizzo, L.; Agovino, T.; Nahim-Granados, S.; Castro-Alférez, M.; Fernández-Ibáñez, P.; Polo-López, M.I. Tertiary Treatment of Urban Wastewater by Solar and UV-C Driven Advanced Oxidation with Peracetic Acid: Effect on Contaminants of Emerging Concern and Antibiotic Resistance. Water Res. 2019, 149, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Zammit, I.; Vaiano, V.; Ribeiro, A.; Silva, A.; Manaia, C.; Rizzo, L. Immobilised Cerium-Doped Zinc Oxide as a Photocatalyst for the Degradation of Antibiotics and the Inactivation of Antibiotic-Resistant Bacteria. Catalysts 2019, 9, 222. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- Brisson-Noël, A.; Trieu-Cuot, P.; Courvalin, P. Mechanism of Action of Spiramycin and Other Macrolides. J. Antimicrob. Chemother. 1988, 22 (Suppl. B), 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bellino, A.; Lofrano, G.; Carotenuto, M.; Libralato, G.; Baldantoni, D. Antibiotic Effects on Seed Germination and Root Development of Tomato (Solanum lycopersicum L.). Ecotoxicol. Environ. Saf. 2018, 148, 135–141. [Google Scholar] [CrossRef]
- Fan, X.; Gao, J.; Li, W.; Huang, J.; Yu, G. Determination of 27 Pharmaceuticals and Personal Care Products (PPCPs) in Water: The Benefit of Isotope Dilution. Front. Environ. Sci. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Dehghan, A.; Zarei, A.; Jaafari, J.; Shams, M.; Mousavi Khaneghah, A. Tetracycline Removal from Aqueous Solutions Using Zeolitic Imidazolate Frameworks with Different Morphologies: A Mathematical Modeling. Chemosphere 2019, 217, 250–260. [Google Scholar] [CrossRef]
- Ibrahim, F.A.; Al-Ghobashy, M.A.; Abd El-Rahman, M.K.; Abo-Elmagd, I.F. Optimization and in Line Potentiometric Monitoring of Enhanced Photocatalytic Degradation Kinetics of Gemifloxacin Using TiO2 Nanoparticles/H2O2. Environ. Sci. Pollut. Res. 2017, 24, 23880–23892. [Google Scholar] [CrossRef]
- Lindberg, R.; Jarnheimer, P.-Å.; Olsen, B.; Johansson, M.; Tysklind, M. Determination of Antibiotic Substances in Hospital Sewage Water Using Solid Phase Extraction and Liquid Chromatography/Mass Spectrometry and Group Analogue Internal Standards. Chemosphere 2004, 57, 1479–1488. [Google Scholar] [CrossRef]
- Shokoohi, R.; Leili, M.; Dargahi, A.; Vaziri, Y.; Khamutian, R. Common Antibiotics in Wastewater of Sina and Besat Hospitals, Hamadan, Iran. Arch. Hyg. Sci. 2017, 6, 152–159. [Google Scholar] [CrossRef]
- Hu, Y.; Song, C.; Liao, J.; Huang, Z.; Li, G. Water Stable Metal-Organic Framework Packed Microcolumn for Online Sorptive Extraction and Direct Analysis of Naproxen and Its Metabolite from Urine Sample. J. Chromatogr. A 2013, 1294, 17–24. [Google Scholar] [CrossRef]
- Vitiello, G.; Iervolino, G.; Imparato, C.; Rea, I.; Borbone, F.; de Stefano, L.; Aronne, A.; Vaiano, V. F-Doped ZnO Nano- and Meso-Crystals with Enhanced Photocatalytic Activity in Diclofenac Degradation. Sci. Total Environ. 2021, 762, 143066. [Google Scholar] [CrossRef]
- Bendtsen, L.; Jensen, R.; Olesen, J. A Non-Selective (Amitriptyline), but Not a Selective (Citalopram), Serotonin Reuptake Inhibitor Is Effective in the Prophylactic Treatment of Chronic Tension-Type Headache. J. Neurol. Neurosurg. Psychiatry 1996, 61, 285–290. [Google Scholar] [CrossRef]
- Li, H.; Sumarah, M.W.; Topp, E. Persistence of the Tricyclic Antidepressant Drugs Amitriptyline and Nortriptyline in Agriculture Soils. Environ. Toxicol. Chem. 2013, 32, 509–516. [Google Scholar] [CrossRef]
- Togola, A.; Budzinski, H. Multi-Residue Analysis of Pharmaceutical Compounds in Aqueous Samples. J. Chromatogr. A 2008, 1177, 150–158. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The Occurrence of Pharmaceuticals, Personal Care Products, Endocrine Disruptors and Illicit Drugs in Surface Water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef]
- Sabourin, L.; Duenk, P.; Bonte-Gelok, S.; Payne, M.; Lapen, D.R.; Topp, E. Uptake of Pharmaceuticals, Hormones and Parabens into Vegetables Grown in Soil Fertilized with Municipal Biosolids. Sci. Total Environ. 2012, 431, 233–236. [Google Scholar] [CrossRef]
- Snyder, S.A. Endocrine Disruptors as Water Contaminants: Toxicological Implications for Humans and Wildlife. Southwest Hydrol. 2003, 2, 14–15. [Google Scholar]
- Vymazal, J.; Březinová, T.; Koželuh, M. Occurrence and Removal of Estrogens, Progesterone and Testosterone in Three Constructed Wetlands Treating Municipal Sewage in the Czech Republic. Sci. Total Environ. 2015, 536, 625–631. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Cao, Z.; Hu, J.; Gao, W. Adsorption of Ethinylestradiol (EE2) on Polyamide 612: Molecular Modeling and Effects of Water Chemistry. Water Res. 2013, 47, 2273–2284. [Google Scholar] [CrossRef]
- Limpiyakorn, T.; Homklin, S.; Ong, S.K. Fate of Estrogens and Estrogenic Potentials in Sewerage Systems. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1231–1270. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I. Adsorption and Transport of Trace Contaminant Estrone in NF/RO Membranes. Environ. Eng. Sci. 2002, 19, 441–451. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, W. Photodegradation of Endocrine Disrupting Chemicals by ZnO Nanorod Arrays. Mol. Cryst. Liq. Cryst. 2014, 603, 194–201. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, X.; Bi, Y.; Li, C.; Feng, Y.; Han, X. High-Concentration Organic Dye Removal Using Fe2O3 ·3.9MoO3 Nanowires as Fenton-like Catalysts. Environ. Sci. Nano 2018, 5, 2069–2076. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Xia, X.; Han, J.-L.; Ding, Y.-C.; Haider, M.R.; Wang, A.-J. Graphene Modified Electro-Fenton Catalytic Membrane for in Situ Degradation of Antibiotic Florfenicol. Environ. Sci. Technol. 2018, 52, 9972–9982. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, Z.; Zhang, H.; Shen, X.; Qiu, Y.; Yin, D. Enhanced Removal of Veterinary Antibiotic Florfenicol by a Cu-Based Fenton-like Catalyst with Wide PH Adaptability and High Efficiency. ACS Omega 2019, 4, 1982–1994. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef]
- Shehu Imam, S.; Adnan, R.; MohdKaus, N.H. Photocatalytic Degradation of Ciprofloxacin in Aqueous Media: A Short Review. Toxicol. Environ. Chem. 2018, 100, 518–539. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2019, 12, 102. [Google Scholar] [CrossRef]
- Boxi, S.S.; Paria, S. Visible Light Induced Enhanced Photocatalytic Degradation of Organic Pollutants in Aqueous Media Using Ag Doped Hollow TiO2 Nanospheres. RSC Adv. 2015, 5, 37657–37668. [Google Scholar] [CrossRef]
- Kushwaha, H.S.; Halder, A.; Jain, D.; Vaish, R. Visible Light-Induced Photocatalytic and Antibacterial Activity of Li-Doped Bi0.5Na0.45K0.5TiO3–BaTiO3 Ferroelectric Ceramics. J. Electron. Mater. 2015, 44, 4334–4342. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Ching Ng, A.M. Strategies for Improving the Efficiency of Semiconductor Metal Oxide Photocatalysis. Mater. Horiz. 2014, 1, 400. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Han, C.; Sannino, D.; Dionysiou, D.D. Photocatalytic Removal of Atrazine Using N-Doped TiO2 Supported on Phosphors. Appl. Catal. B 2015, 164, 462–474. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, J.; Zhou, F.; Chen, X.; Wei, L.; Gao, Q.; Wang, K.; Zhao, Q. Construction of a Visible-Light-Driven Magnetic Dual Z-Scheme BiVO4/g-C3N4/NiFe2O4 Photocatalyst for Effective Removal of Ofloxacin: Mechanisms and Degradation Pathway. Chem. Eng. J. 2021, 405, 126704. [Google Scholar] [CrossRef]
- Bai, X.; Chen, W.; Wang, B.; Sun, T.; Wu, B.; Wang, Y. Photocatalytic Degradation of Some Typical Antibiotics: Recent Advances and Future Outlooks. Int. J. Mol. Sci. 2022, 23, 8130. [Google Scholar] [CrossRef]
- Yashni, G.; Al-Gheethi, A.; Mohamed, R.; Hossain, M.S.; Kamil, A.F.; AbiramaShanmugan, V. Photocatalysis of Xenobiotic Organic Compounds in Greywater Using Zinc Oxide Nanoparticles: A Critical Review. Water Environ. J. 2021, 35, 190–217. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Yenjai, C.; Nanan, S. Utilization of Formononetin and Pinocembrin from Stem Extract of Dalbergia Parviflora as Capping Agents for Preparation of ZnO Photocatalysts for Degradation of RR141 Azo Dye and Ofloxacin Antibiotic. Catal. Today 2022, 384–386, 279–293. [Google Scholar] [CrossRef]
- Ravbar, M.; Kunčič, A.; Matoh, L.; SmoleMožina, S.; Šala, M.; Šuligoj, A. Controlled Growth of ZnO Nanoparticles Using Ethanolic Root Extract of Japanese Knotweed: Photocatalytic and Antimicrobial Properties. RSC Adv. 2022, 12, 31235–31245. [Google Scholar] [CrossRef]
- Vignati, D.; Lofrano, G.; Libralato, G.; Guida, M.; Siciliano, A.; Carraturo, F.; Carotenuto, M. Photocatalytic ZnO-Assisted Degradation of Spiramycin in Urban Wastewater: Degradation Kinetics and Toxicity. Water 2021, 13, 1051. [Google Scholar] [CrossRef]
- Bhuyan, B.; Paul, B.; Purkayastha, D.D.; Dhar, S.S.; Behera, S. Facile Synthesis and Characterization of Zinc Oxide Nanoparticles and Studies of Their Catalytic Activity towards Ultrasound-Assisted Degradation of Metronidazole. Mater. Lett. 2016, 168, 158–162. [Google Scholar] [CrossRef]
- El-Kemary, M.; El-Shamy, H.; El-Mehasseb, I. Photocatalytic Degradation of Ciprofloxacin Drug in Water Using ZnO Nanoparticles. J. Lumin. 2010, 130, 2327–2331. [Google Scholar] [CrossRef]
- Zhang, Z.; Zada, A.; Cui, N.; Liu, N.; Liu, M.; Yang, Y.; Jiang, D.; Jiang, J.; Liu, S. Synthesis of Ag Loaded ZnO/BiOCl with High Photocatalytic Performance for the Removal of Antibiotic Pollutants. Crystals 2021, 11, 981. [Google Scholar] [CrossRef]
- Majumder, S.; Chatterjee, S.; Basnet, P.; Mukherjee, J. ZnO Based Nanomaterials for Photocatalytic Degradation of Aqueous Pharmaceutical Waste Solutions—A Contemporary Review. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100386. [Google Scholar] [CrossRef]
- Semeraro, P.; Bettini, S.; Sawalha, S.; Pal, S.; Licciulli, A.; Marzo, F.; Lovergine, N.; Valli, L.; Giancane, G. Photocatalytic Degradation of Tetracycline by ZnO/γ-Fe2O3 Paramagnetic Nanocomposite Material. Nanomaterials 2020, 10, 1458. [Google Scholar] [CrossRef]
- Jalili-Jahani, N.; Rabbani, F.; Fatehi, A.; MusaviHaghighi, T. Rapid One-Pot Synthesis of Ag-Decorated ZnO Nanoflowers for Photocatalytic Degradation of Tetracycline and Product Analysis by LC/APCI-MS and Direct Probe ESI-MS. Adv. Powder Technol. 2021, 32, 3075–3089. [Google Scholar] [CrossRef]
- Hashem, E.M.; Hamza, M.A.; El-Shazly, A.N.; Abd El-Rahman, S.A.; El-Tanany, E.M.; Mohamed, R.T.; Allam, N.K. Novel Z-Scheme/Type-II CdS@ZnO/g-C3N4 Ternary Nanocomposites for the Durable Photodegradation of Organics: Kinetic and Mechanistic Insights. Chemosphere 2021, 277, 128730. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Komchoo, N.; Senasu, T.; Piriyanon, J.; Youngme, S.; Hemavibool, K.; Nanan, S. Silver Decorated ZnO Photocatalyst for Effective Removal of Reactive Red Azo Dye and Ofloxacin Antibiotic under Solar Light Irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127034. [Google Scholar] [CrossRef]
- Mccally, M.; Solomon, G.M.; Schettler, T. Environment and Health: 6. Endocrine Disruption and Potential Human Health Implications. CMAJ 2000, 163, 1471–1476. [Google Scholar]
- Ji, B.; Zhang, J.; Zhang, C.; Li, N.; Zhao, T.; Chen, F.; Hu, L.; Zhang, S.; Wang, Z. Vertically Aligned ZnO@ZnS Nanorod Chip with Improved Photocatalytic Activity for Antibiotics Degradation. ACS Appl. Nano Mater. 2018, 1, 793–799. [Google Scholar] [CrossRef]
- Jingyu, H.; Ran, Y.; Zhaohui, L.; Yuanqiang, S.; Lingbo, Q.; NtiKani, A. In-Situ Growth of ZnO Globular on g-C3N4 to Fabrication Binary Heterojunctions and Their Photocatalytic Degradation Activity on Tetracyclines. Solid State Sci. 2019, 92, 60–67. [Google Scholar] [CrossRef]
- Ramasamy, B.; Jeyadharmarajan, J.; Chinnaiyan, P. Novel Organic Assisted Ag-ZnO Photocatalyst for Atenolol and Acetaminophen Photocatalytic Degradation under Visible Radiation: Performance and Reaction Mechanism. Environ. Sci. Pollut. Res. 2021, 28, 39637–39647. [Google Scholar] [CrossRef]
- Piña-Pérez, Y.; Aguilar-Martínez, O.; Acevedo-Peña, P.; Santolalla-Vargas, C.E.; Oros-Ruíz, S.; Galindo-Hernández, F.; Gómez, R.; Tzompantzi, F. Novel ZnS-ZnO Composite Synthesized by the Solvothermal Method through the Partial Sulfidation of ZnO for H2 Production without Sacrificial Agent. Appl. Catal. B Environ. 2018, 230, 125–134. [Google Scholar] [CrossRef]
- Costa, L.N.; Nobre, F.X.; Lobo, A.O.; de Matos, J.M.E. Photodegradation of Ciprofloxacin Using Z-Scheme TiO2/SnO2 Nanostructures as Photocatalyst. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100466. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.D.; Purohit, L.P. RGO supported ZnO/SnO2 Z-scheme heterojunctions with enriched ROS production towards enhanced photocatalytic mineralization of phenolic compounds and antibiotics at low temperature. J. Colloid Interface Sci. 2023, 632, 196–215. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, B.; Cao, B.; Ma, W. Morphology-controlled Fabrication of SnO2/ZnO Nanocomposites with Enhanced Photocatalytic Performance. Photochem. Photobiol. 2019, 95, 1131–1141. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; Şahin, S.; Kazan, E.S. One-Pot Green Preparation of Deep Eutectic Solvent-Assisted ZnO/GO Nanocomposite for Cefixime Trihydrate Photocatalytic Degradation under UV-A Irradiation. Biomass Convers. Biorefin. 2022, 12 (Suppl. 1), 73–86. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Sobhani, S.; Shekari, H. Photocatalytic Degradation of Azithromycin Using GO@Fe3O4/ZnO/SnO2 Nanocomposites. J. Clean. Prod. 2019, 232, 127–136. [Google Scholar] [CrossRef]
- Mahmud, S. One-Dimensional Growth of Zinc Oxide Nanostructures from Large Micro-Particles in a Highly Rapid Synthesis. J. Alloy. Compd. 2011, 509, 4035–4040. [Google Scholar] [CrossRef]
- Tran, M.L.; Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Hybridizing Ag-Doped ZnO Nanoparticles with Graphite as Potential Photocatalysts for Enhanced Removal of Metronidazole Antibiotic from Water. J. Environ. Manag. 2019, 252, 109611. [Google Scholar] [CrossRef]
- Sabouni, R.; Gomaa, H. Photocatalytic Degradation of Pharmaceutical Micro-Pollutants Using ZnO. Environ. Sci. Pollut. Res. 2019, 26, 5372–5380. [Google Scholar] [CrossRef]
- Yasir, M.; Šopík, T.; Ali, H.; Kimmer, D.; Sedlařík, V. Green synthesis of titanium and zinc oxide nanoparticles for simultaneous photocatalytic removal of estrogens in wastewater. In Proceedings of the NANOCON 2021—International Conference on Nanomaterials, Brno, Czech Republic, 20–22 October 2021; pp. 189–196. [Google Scholar] [CrossRef]
- Finčur, N.; ŠojićMerkulov, D.; Putnik, P.; Despotović, V.; Banić, N.; Lazarević, M.; Četojević-Simin, D.; Agbaba, J.; Abramović, B. Environmental Photocatalytic Degradation of Antidepressants with Solar Radiation: Kinetics, Mineralization, and Toxicity. Nanomaterials 2021, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Gurke, R.; Eckert, H.; Kühn, K.; Fauler, J.; Cuniberti, G. Photocatalytic Degradation of Pharmaceuticals Present in Conventional Treated Wastewater by Nanoparticle Suspensions. J. Environ. Chem. Eng. 2016, 4, 287–292. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Kudlek, E.; Dudziak, M. Influence of the Catalyst Type (TiO2 and ZnO) on the Photocatalytic Oxidation of Pharmaceuticals in the Aquatic Environment. Desalination Water Treat. 2016, 57, 1552–1563. [Google Scholar] [CrossRef]
- Divband, B.; Khatamian, M.; Eslamian, G.R.K.; Darbandi, M. Synthesis of Ag/ZnO Nanostructures by Different Methods and Investigation of Their Photocatalytic Efficiency for 4-Nitrophenol Degradation. Appl. Surf. Sci. 2013, 284, 80–86. [Google Scholar] [CrossRef]
- Cheng, J.; Shen, Y.; Chen, K.; Wang, X.; Guo, Y.; Zhou, X.; Bai, R. Flower-like Bi2WO6/ZnO Composite with Excellent Photocatalytic Capability under Visible Light Irradiation. Chin. J. Catal. 2018, 39, 810–820. [Google Scholar] [CrossRef]
- Mills, A.; Wiser, R.; Millstein, D.; Carvallo, J.P.; Gorman, W.; Seel, J.; Jeong, S. The Impact of Wind, Solar, and Other Factors on the Decline in Wholesale Power Prices in the United States. Appl. Energy 2021, 283, 116266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).