Abstract
There is much interest and focus on solid forms of famciclovir. However, in spite of the abundance of reported differences in oral bioavailability, compressibility, and other physical–chemical properties of the various crystal forms of this drug, very little precise structural analysis is available in the literature to date. The form used in the commercial formulation is the anhydrous form I. Patents and patent applications report three different anhydrous crystalline forms on the basis of unindexed powder diffraction patterns. Single-crystal and variable-temperature X-ray diffraction experiments using the commercially available anhydrous form of famciclovir were carried out and led not only to the crystal structure determination of the anhydrous form I, but also to discovery of a new crystal form of anhydrous famciclovir from powder data.
1. Introduction
Herpes Simplex Virus (HSV), better known as the virus family responsible for cold sores (HSV-1) and genital herpes (HSV-2) is one of the most widely distributed type of viruses among animals and humans [1]. According to recent surveys seropositivity for HSV-1 and HSV-2 ranges from 50–80% to 4–24% of adults in Europe, respectively [1]. The presence of this type of virus in living organisms probably dates back millions of years, and one of its most notable features is that HSV infections are life-long [1]. Once infection takes place, often, periods of latency are followed by phases of activity in a random manner, the classical signs of recurrent herpes. Frequent bouts are therefore possible and consequently, good, and effective medication is necessary.
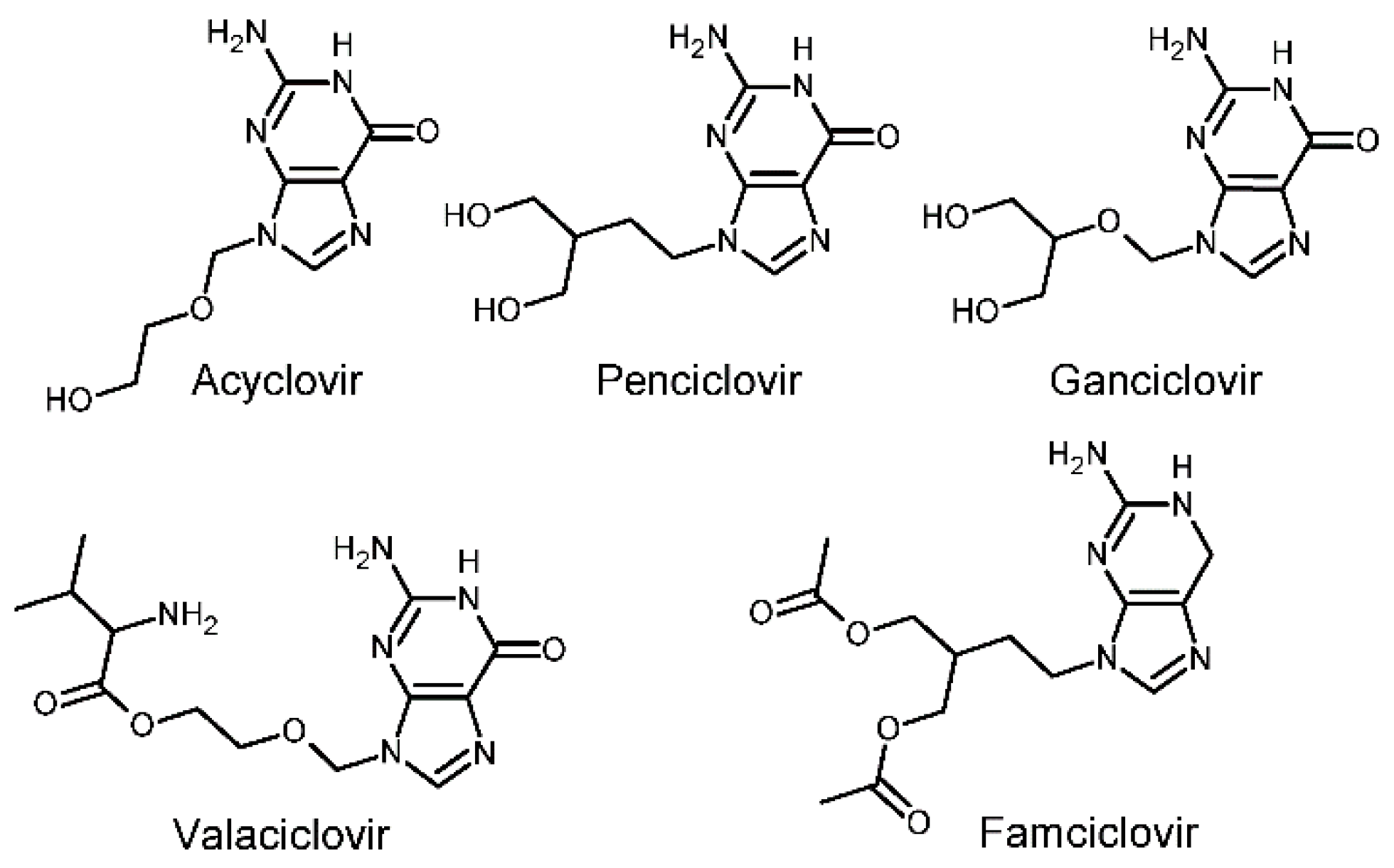
Forms of antiviral chemotherapyexist since 1959. However, it was the discovery of the API (active pharmaceutical ingredient) Acyclovir (ACV) by the Wellcome group in 1977, which lead to a first highly effective and selective antiviral drug [2,3]. The mechanism of action is a fascinating chain of interactions of the viral thymidine kinase and the viral DNA chain with ACV acting as an “obligate DNA chain terminator”. Further development has taken place and so the prodrugs of ACV and Penciclovir (PCV), Valaciclovir (VACV), and famciclovir (FCV), are currently the only therapies for both HSV-1 and HSV-2 available worldwide [1,4]. Figure 1 shows molecular diagrams of the five most used guanosine nucleoside analogues.
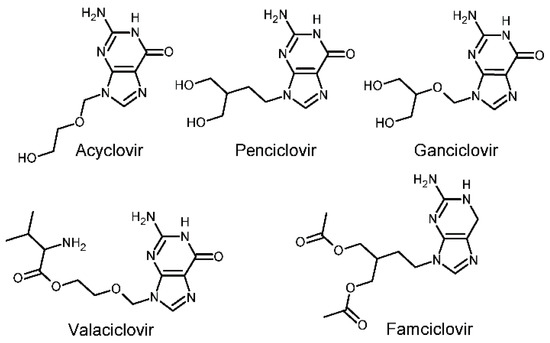
Figure 1.
Molecular diagrams of the five most known guanosine nucleoside analogues used as anti-virals.
The development of prodrugs was pivotal due to of the limited bioavailability of ACV and PCV. Both, FCV and VACV have proven to be superior and more patient-friendly when taken orally because of the much improved bioavailability [1,4]. Within the group of guanosine nucleoside analogues, famciclovir, stands out as it (i) exhibits an extraordinary safety profile [1]; (ii) shows activity in reducing the potential for latency [1]; (iii) is also used to treat Epstein-Barr virus (EBV) and hepatitis B virus (HBV) [2] infections; and (iv) together with the parent drug acyclovir, it is discussed as a possible candidate for anti-tumor activity when coordinated to platinum [5]. Since its first appearance in journals and patents during the 1980s numerous reviews and further patent applications can be found in the literature [1,3,4,6,7,8,9].
This stands in strong contrast to the structural data that is currently available of this widely-used drug family. Apart from various crystal structure analyses reported of acyclovir, [10,11,12] very little or no structural data is available for other guanosine nucleoside analogues which are APIs [13,14,15]. This is very surprising given the interest and focus on solid forms of famciclovir; e.g., the occurrence of different crystalline forms of famciclovir during the manufacturing and drying process [16,17,18]. Co-crystallisations with electron accepting molecules were carried out [19] and potential anti-tumor agents were synthesized using famciclovir [5]. However, in spite of reported differences in oral bioavailability, compressibility, or other physical-chemical properties, no precise structural analysis was reported in the literature to our knowledge. The above mentioned differences in properties most likely originate from the conformational alterations of the famciclovir molecule in the crystal lattice, which then lead to polymorphism (observed), differences in dissolution of the compound in different media (reported) and the way it can be compressed (reported).
So far, there is only one crystal structure of the hydrated form of famciclovir reported in the literature (CCDC refcode YACHAL) [15]. However, the form used to produce the commercially-available drug is the anhydrous form I, ideally in >99% purity. Patents and patent applications report three different anhydrous forms on the basis of unindexed powder diffraction patterns [9,16,17,18,20]. The polymorphism observed is a result of the drying process of famciclovir which is a major obstacle during the manufacturing process [16,18]. The main difficulty is that melting point of the wet product (initial water content is 40% in weight) is below the boiling temperature of water and increases only slowly by drying to ca. 102 °C. This requires a slow and stepwise drying process from 30 °C to 80 °C as melting of the drug has to be avoided to prevent the occurrence of other polymorphic forms.
Crystal structure determination of the various anhydrous forms of famciclovir would provide vital information about the conformational changes of the molecules in their different solid forms and thus, it could inspire new pathways for the production of one particular crystal form.
In order to fill this knowledge gap, we carried out variable-temperature diffraction experiments using the commercially available anhydrous form of famciclovir as starting compound. Our investigations led not only to the crystal structure determination of form I, but also to the structure determination of a new crystal form of famciclovir from powder data.
2. Results and Discussion
2.1. Powder Diffraction Studies
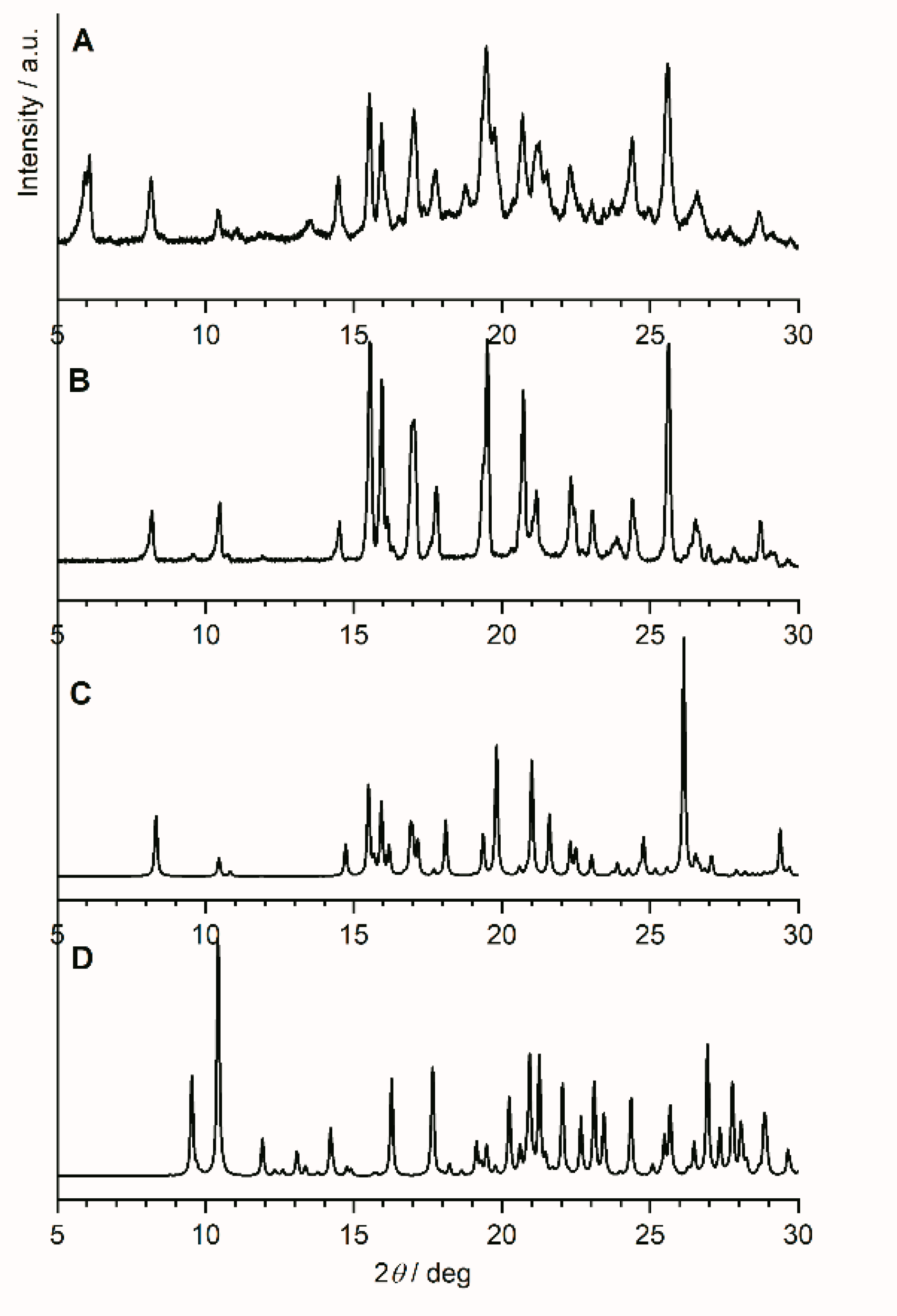
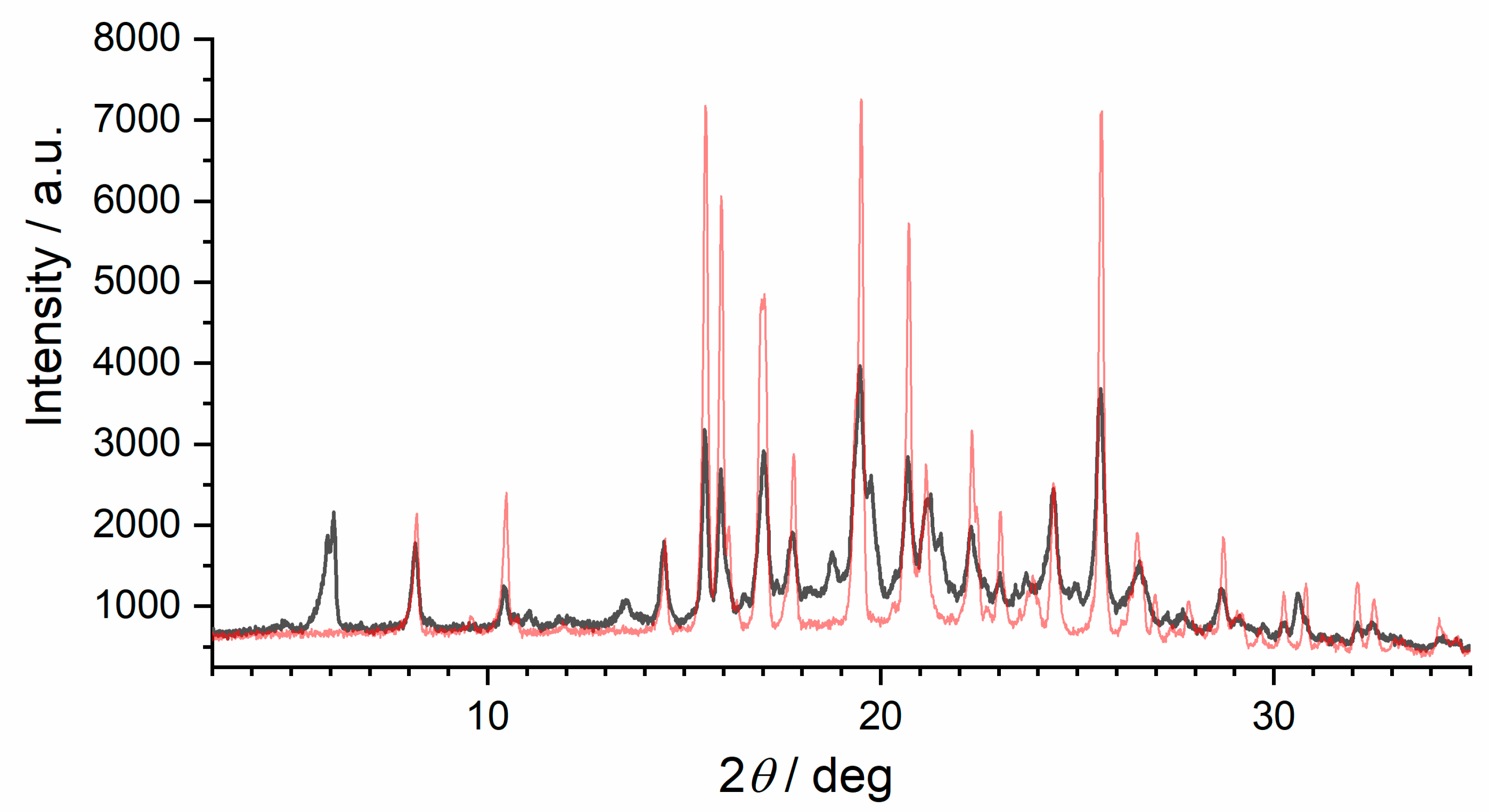
Variable temperature X-ray diffraction studies have been carried out on the commercially available anhydrous famciclovir. Data sets were collected in this order at 25 °C, 70 °C, 110 °C, 25 °C, −50 °C, and 25 °C. Thus, room temperature data was collected at the beginning, after heating and after cooling. Figure 2 shows the diffraction pattern of famciclovir after (Figure 2A) and before one heating and cooling cycle (Figure 2B), the calculated pattern of anhydrous famciclovir from single-crystal data (Figure 2C) discussed below, and the calculated pattern of the monohydrate of famcicolovir (Figure 2D) [15], also reported under the structure code YACHAL in the Cambridge Crystallographic Database.
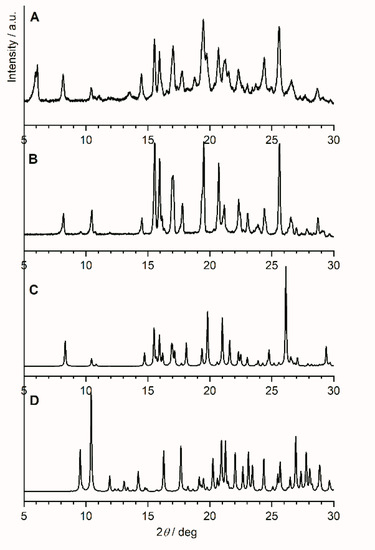
Figure 2.
Powder X-ray diffraction patterns of famciclovir (FAM). (A) After and (B) before heating and cooling from RT to 110 °C/−50 °C at a heating rate of 6 °C/min, (C) the pattern of anhydrous famciclovir calculated from single-crystal data discussed in chapter 2.2 (vide infra), and (D) the calculated pattern of the hydrated form of famcicolovir reported under the structure code YACHAL in the Cambridge Crystallographic Database and published by Harnden et al. [15].
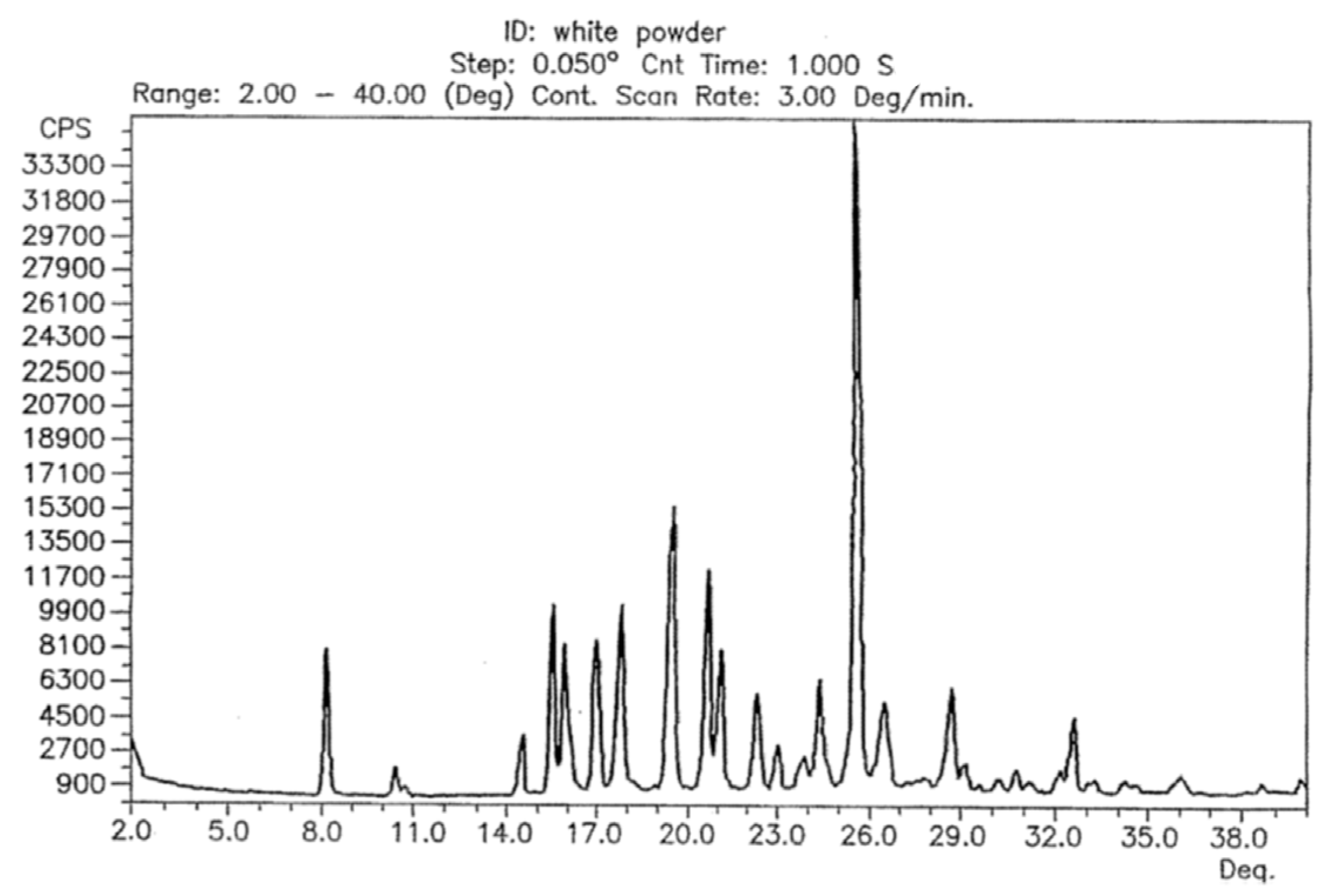
The reflection positions in the powder patterns of the reported hydrated form compared to the in-house collected data from powder and single-crystal data are clearly different proving that the compound did not take on water during storage or during the variable temperature data collection. The powder pattern derived from single-crystal data and the one from the initial data collection before the start of the variable temperature dependent study are very similar with regards to the relative reflection positions. A noticeable shift and difference in intensities does exist, however (Please see Figure S1 in the supplementary information). These differences were expected as (i) single-crystal data was recorded at a lower temperature in order to obtain sufficiently good data, (ii) the commercially obtained compound is likely to contain both impurities and traces of different crystalline forms for the reasons explained above, and (iii) the plate like shape of the crystals results in preferred orientation. Despite that, it is safe to say at this point that the bulk powder of the commercially available solid consists of the same crystalline form determined from one single crystal found in the solid. The powder pattern of the untreated solid is also identical to the anhydrous form I reported in a patent by Dolitzky et al. [18] (Figure 3) and is therefore also referred as form I throughout this paper.
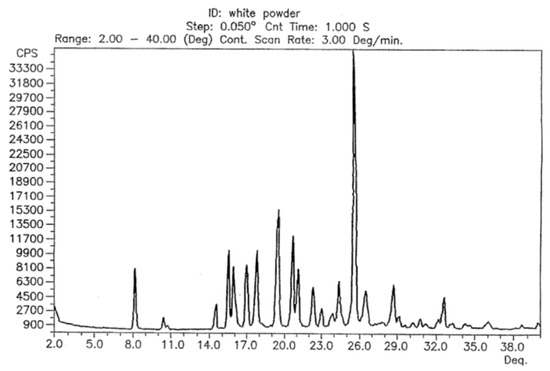
Figure 3.
Powder X-ray pattern of form I as reported by Dolitzky et al. [18]. Permission obtained from Prof. Dr O. Reany.
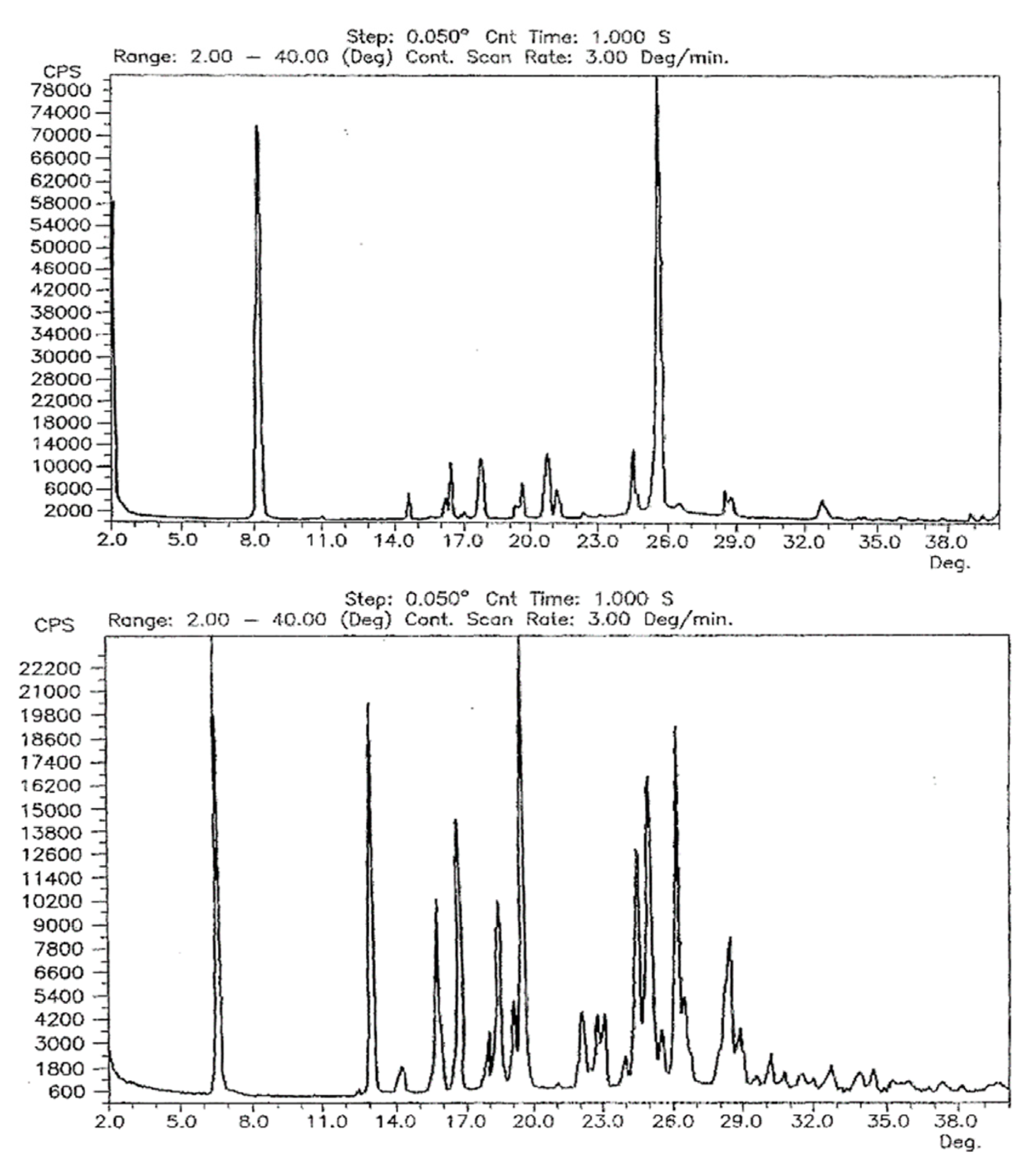
Comparison of the powder patterns before and after heating shows distinct differences, e.g., the additional intensities between 5° and 6° 2θ or the slightly different and additional reflection positions from 15° to 25° 2θ. Furthermore, comparison with the other two polymorphs reported by Dolitzky et al. [18] (form II and III, see Figure 4) showed that the resulting powder pattern (Figure 2A) is again different and thus represents a fourth polymorphic form of famciclovir, form IV.
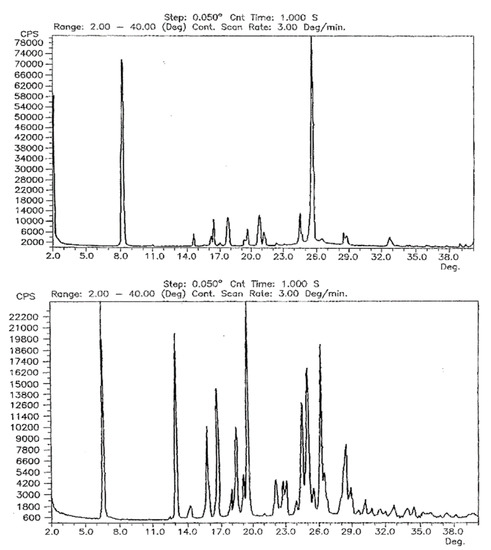
Figure 4.
Powder X-ray patterns of form II (left) and form III (right) as reported by Dolitzky et al. [18]. Permission obtained from Prof. Dr O. Reany.
Form IV formed after heating form I in a capillary to 110 °C directly followed by cooling to −50 °C at a rate of 6 °C/min. A comparison of form I and form IV is shown in Figure 5. Structure determination of form IV is currently underway.
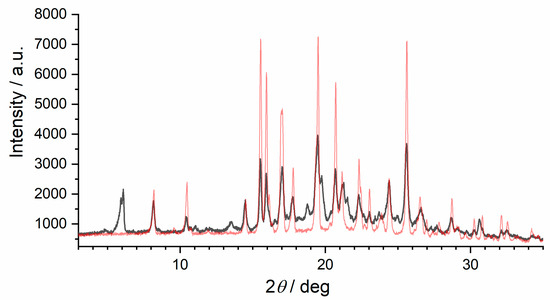
Figure 5.
Powder X-ray patterns showing the appearance of the new polymorph form IV: comparison of form I (red) and the powder pattern after heating form I in a capillary to 110 °C followed by cooling to −50 °C (black).
2.2. Single-Crystal Diffraction
Single crystals of famciclovir have been found in the untreated commercial product and multiple datasets were recorded of the very fragile plate-like crystals. The calculated powder pattern of the determined structure coincides very well with the patterns of the bulk powder and that of form I (vide supra) of anhydrous famciclovir.
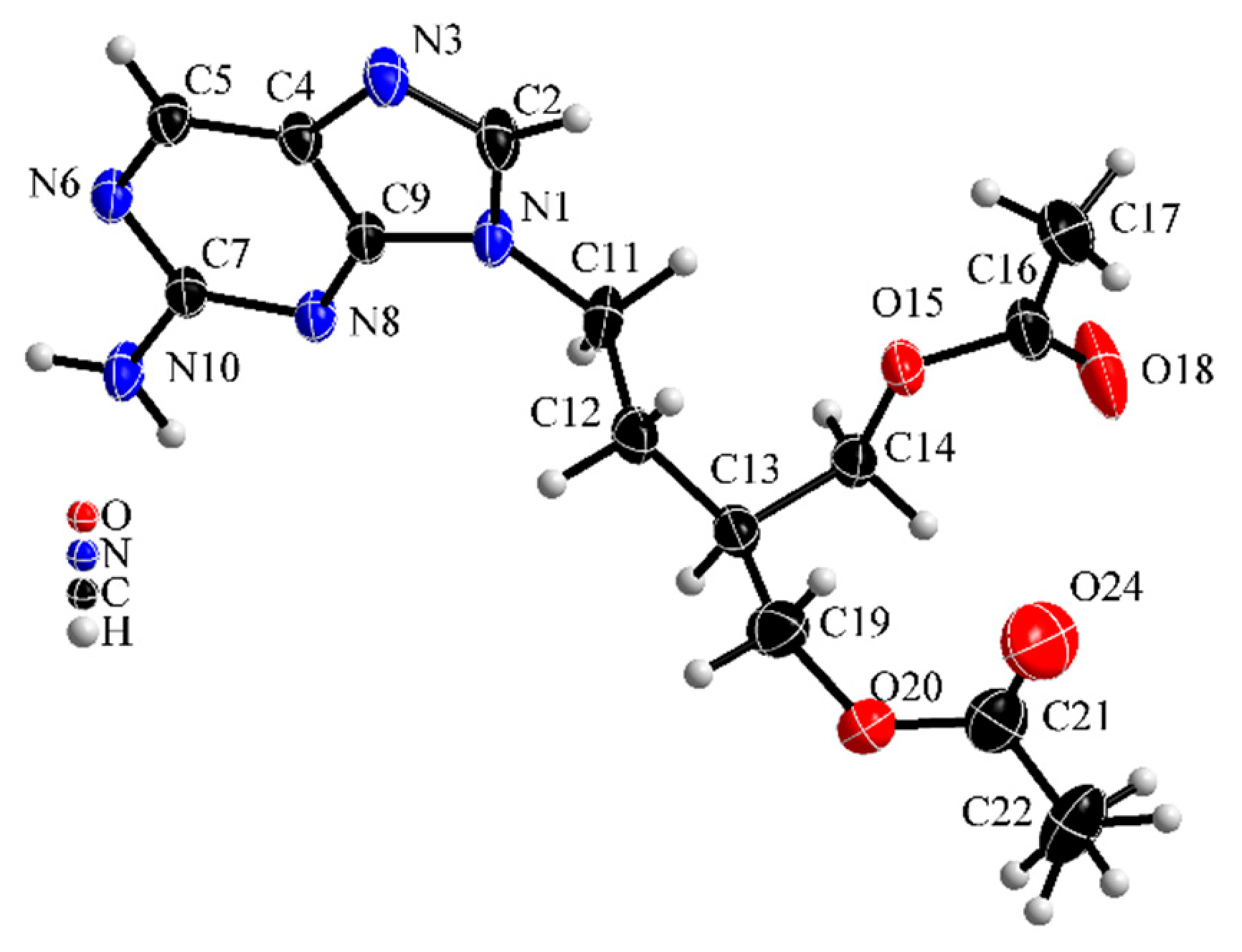
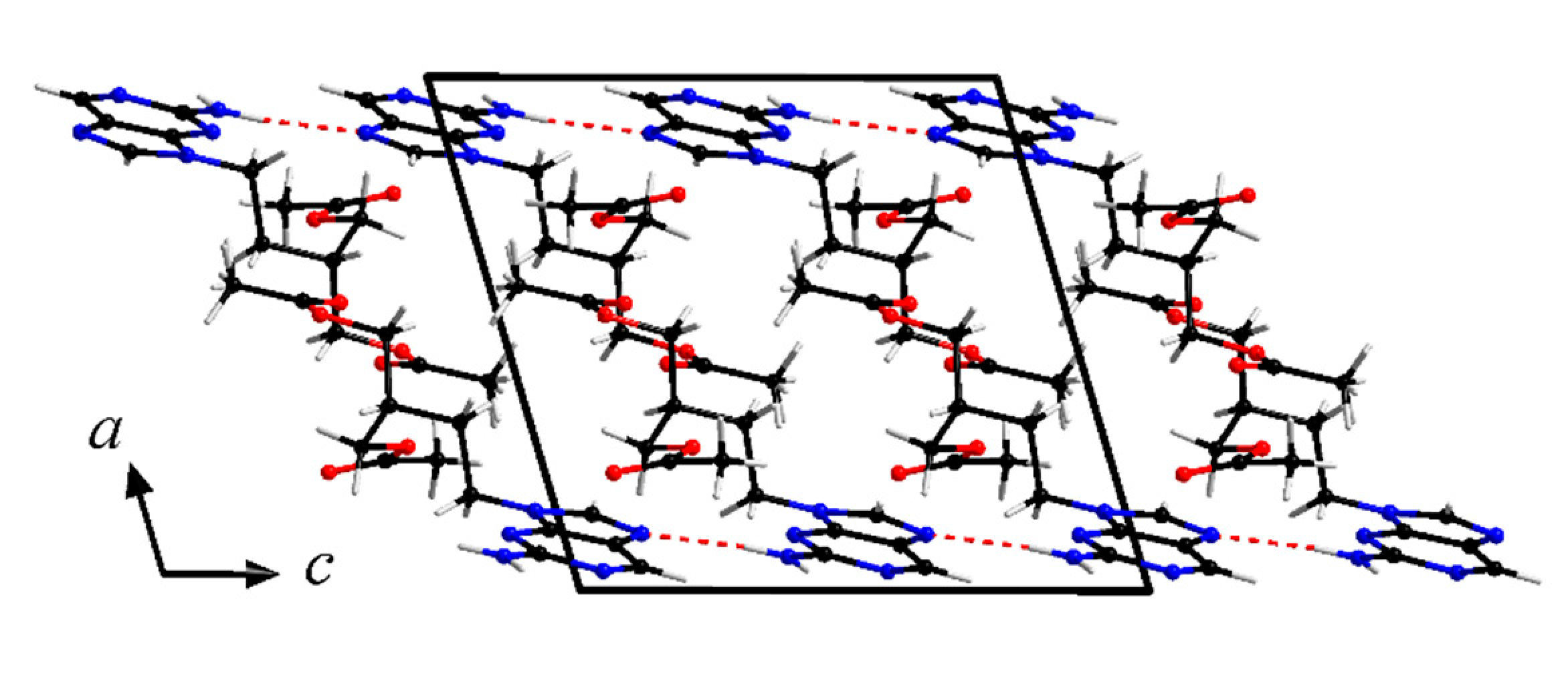
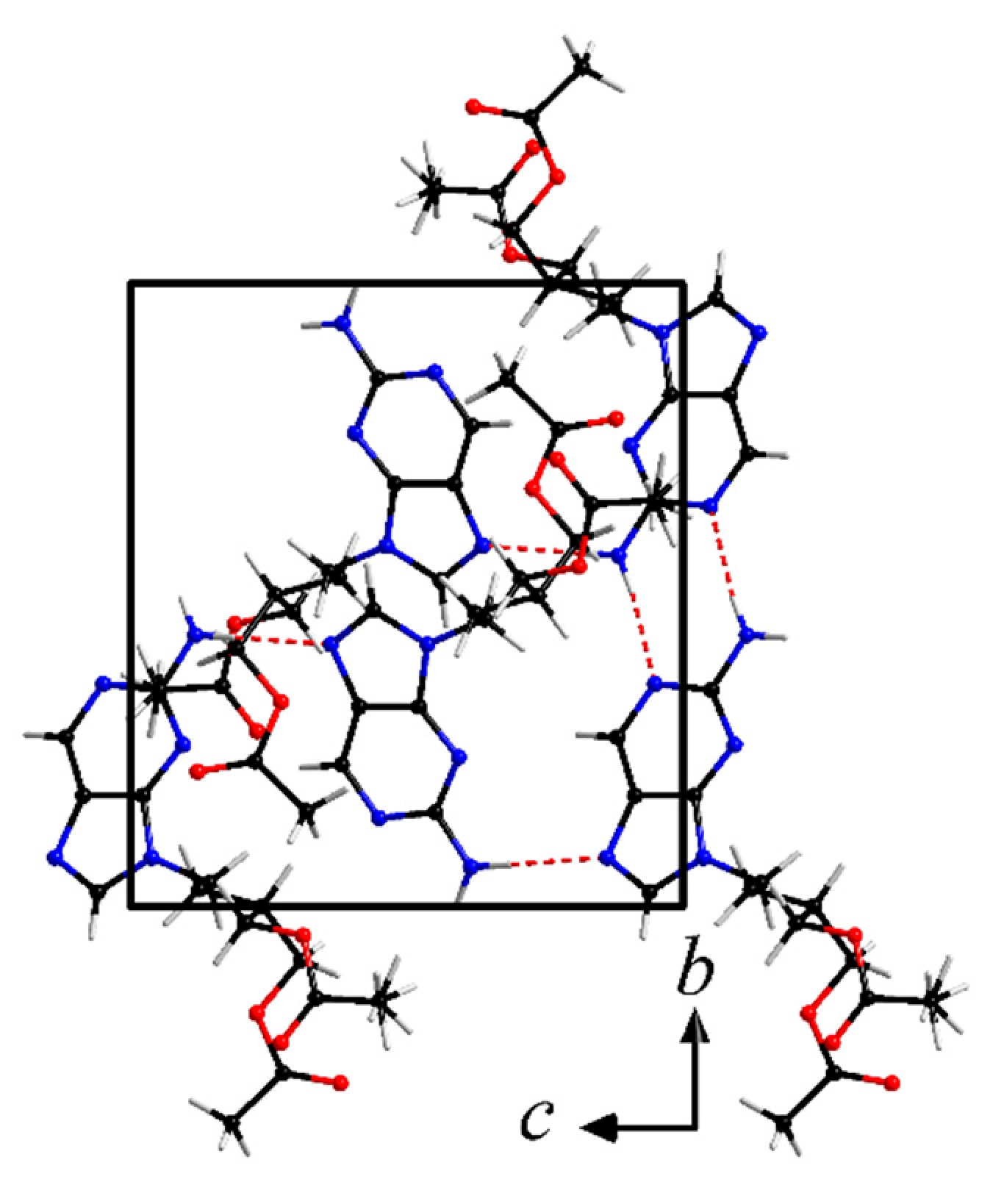
Figure 6 shows the molecular structure of famciclovir lifted out of the determined structure and Figure 7 and Figure 8 show packing diagrams of the crystal structure along cell axes a and b, respectively.
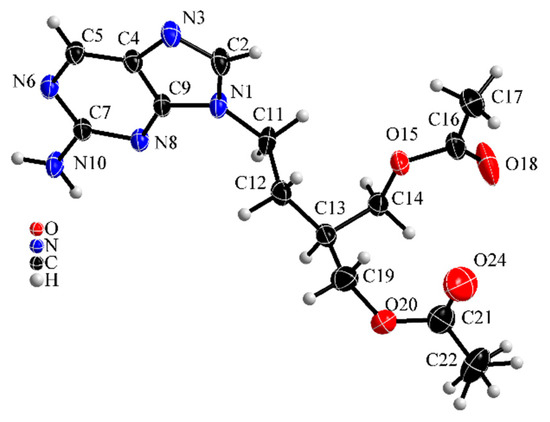
Figure 6.
Molecular structure of anhydrous famciclovir (form I). Thermal ellipsoids are drawn on 50% level.

Figure 7.
Crystal structure of anhydrous famciclovir (form I). View along cell axis b. Hydrogen bond interactions are shown as red dotted lines.

Figure 8.
Crystal structure of anhydrous famciclovir (form I). View along cell axis a. Hydrogen bond interactions are shown as red dotted lines.
Form I forms two characteristic hydrogen bonds in the solid state, two parallel N-H···N interactions with d(DH-A) = 2.180(19)Å and 2.187(19)Å between N10 and N6/N3, respectively. Further C-H···N/O interactions are present in the structure and are responsible for a typical arrangement of purine containing compounds with large side chains (Table 1). The planar purine backbones form double layers, across which are the parallel N-H···N interactions. This arrangement provides enough space for the side chains to orient themselves towards each other, in a roughly ladder-like manner.

Table 1.
Hydrogen bonds in crystal structure of anhydrous famciclovir (form I).
3. Conclusions
This study showed how the combination of both cooling and heating cycles can influence the formation of different crystal forms. Whereas forms II and III were reported to have been discovered serendipitously following different methods for drying hydrated famciclovir to obtain the anhydrous form, form IV only seems to form after combined cooling and heating. Structure determination and further analysis (e.g., variable temperature RAMAN and simultaneous coupled thermal analysis) are underway to scale up the targeted synthesis of form IV and explain its formation. Table 2 summarizes the different forms of Famiclovir and the information obtained about each form in the literature so far.

Table 2.
The different crystalline forms of anhydrous Famiclovir and the information obtained about each form in the literature.
Compared to the only other crystal structure known in the literature, famciclovir monohydrate (CCDC code YACHAL [15]), the molecular arrangement in the newly determined structure of form I is very different. In YACHAL the additional water molecule is heavily involved in hydrogen bonding, resulting in a typical herringbone motif, whereas form I of anhydrous famciclovir forms a double layer of the purine backbone with the side chains being oriented towards each other. It is logical that such different hydrogen bonding must have an impact on the physical properties of the API and thus, patent literature discusses different methods to obtain anhydrous famciclovir in such detail.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/11/2/129/s1, Crystallographic data as well as tables reporting bond distances and angles of form I of anhydrous famciclovir.
Author Contributions
Conceptualization, methodology, validation, formal analysis, investigation, L.V.-Z. and U.B.; resources, L.V.-Z., writing—original draft preparation, U.B.; writing—review and editing, L.V.-Z.; visualization, U.B. funding acquisition, L.V.-Z. and U.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would also like to acknowledge the project: “Setting up of transdisciplinary research and knowledge exchange (TRAKE) complex at the University of Malta (ERDF.01.124)” which is being co-financed through the European Union through the European Regional Development Fund 2014–2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hodge, R.A.V.; Field, H.J. Antiviral agents for herpes simplex virus. Adv. Pharmacol. 2013, 67, 1–38. [Google Scholar]
- Bacon, T.H. Famciclovir, from the bench to the patient—A comprehensive review of preclinical data. Int. J. Antimicrob. Agents 1996, 7, 119–134. [Google Scholar] [CrossRef]
- Harnden, M.R.; Jarvest, R.L.; Boyd, M.R.; Sutton, D.; Vere Hodge, R.A. Prodrugs of the selective antiherpesvirus agent 9-[4-hydroxy-3-(hydroxymethyl)but-1-yl]guanine (BRL 39123) with improved gastrointestinal absorption properties. J. Med. Chem. 1989, 32, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Mackman, R.L.; Cihlar, T. Prodrug strategies in the design of nucleoside and nucleotide antiviral therapeutics. Annu. Rep. Med. Chem. 2004, 39, 305–321. [Google Scholar]
- Cerasino, L.; Intini, F.P.; Kobe, J.; de Clercq, E.; Natile, G. Synthesis and stereochemical characterisation of platinum(II) complexes with the antiviral agents penciclovir and famciclovir. Inorg. Chim. Acta 2003, 344, 174–182. [Google Scholar] [CrossRef]
- Field, H.J.; Vere Hodge, R.A. Recent developments in anti-herpesvirus drugs. Br. Med. Bull. 2013, 106, 213–249. [Google Scholar] [CrossRef] [PubMed]
- Jarvest, R.L.; Sutton, D.; Vere Hodge, R.A. Famciclovir. Discovery and development of a novel antiherpesvirus agent. Pharm. Biotechnol. 1998, 11, 313–343. [Google Scholar] [PubMed]
- Cirelli, R.; Herne, K.; McCrary, M.; Lee, P.; Tyring, S.K. Famciclovir: Review of clinical efficacy and safety. Antivir. Res. 1996, 29, 141–151. [Google Scholar] [CrossRef]
- Harnden, M.R.; Jarvest, R.L. Purine Derivatives. U.S. Patent US5246937A, 21 September 1993. [Google Scholar]
- Terada, K.; Kurobe, H.; Ito, M.; Yoshihashi, Y.; Yonemochi, E.; Fujii, K.; Uekusa, H. Polymorphic and pseudomorphic transformation behaviornof acyclovir based on thermodynamics and crystallography. J. Therm. Anal. Calorim. 2013, 113, 1261. [Google Scholar] [CrossRef]
- Birnbaum, G.I.; Cygler, M.; Shugar, D. Conformational features of acyclonucleosides: Structure of acyclovir, an antiherpes agent. Can. J. Chem. 1984, 62, 2646. [Google Scholar] [CrossRef]
- Birnbaum, G.I.; Cygler, M.; Kusmierek, J.T.; Shugar, D. Structure and conformation of the potent antiherpes agent 9-(2-hydroxyethoxymethyl)guanine (acycloguanosine). Biochem. Biophys. Res. Comm. 1981, 103, 968. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, F.-J.; Li, N.; Liu, Y.-C.; Chen, Z.-F. 9-[4-Hydroxy-3-(hydroxymethyl)butyl]-guanine monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, 2878. [Google Scholar] [CrossRef]
- Kawamura, T.; Hirayama, N. Crystal Structure of Ganciclovir. Anal. Sci. X-ray Struct. Anal. Online 2009, 25, 51. [Google Scholar] [CrossRef][Green Version]
- Harnden, M.R.; Jarvest, R.L.; Slawin, A.M.Z.; Williams, D.J. Crystal and Molecular Structures of the Antiviral Acyclonucleoside 9-[4-Hydroxy-3-(hydroxymethyl)butyl]guanine (BRL39123, Penciclovir) and its Prodrug 9-[4-Acetoxy-3-(acetoxymethyl)butyl]-2-aminopurine (BRL 42810, Famciclovir). Nucleos. Nucleot. 1990, 9, 499. [Google Scholar] [CrossRef]
- Eisen-Nevo, H.; Maidan-Hanoch, D. Drying Process for Preparing Crystalline Solid Famciclovir. WIPO (PCT) WO2005116031A1, 8 December 2005. [Google Scholar]
- Campbell, K.C.; Greenway, M.J.; Hancock, S.A. Pharmaceutical Compositions Containing Famciclovir Monohydrate. WIPO (PCT) WO9729108A1, 14 August 1997. [Google Scholar]
- Dolitzky, B.-Z.; Wizel, S.; Reany, O.; Shammai, J. Crystalline Solid Famciclovir forms I, II, III and Preparation Thereof. WIPO (PCT) WO2004018470A2, 4 March 2004. [Google Scholar]
- Gaballa, A.S.; Teleb, S.M.; Nour, E.-M. Preparation and spectroscopic studies on charge-transfer complexes of famciclovir drug with different electron acceptors. J. Mol. Struct. 2012, 1024, 32–39. [Google Scholar] [CrossRef]
- Darmuzey, O.; Macleod, G.; Cengic, D.; Stokes, K.M. Solid Pharmaceutical Dosage form. WIPO (PCT) WO2008140461A1, 20 November 2008. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).