Abstract
A novel cobalt(II) complex, namely [Co(tpa)2(H2O)4]·2H2O (1) (Htpa = 5-(1,2,4-triazol-1-yl)pyridine-3-carboxylic acid) has been solvothermally synthesized and structurally characterized by single-crystal X-ray diffraction. Complex 1 was further characterized by elemental analysis, FTIR spectrum, electronic spectrum, X-ray powder diffraction and thermal gravimetric analysis. Structural analysis shows that complex 1 was stabilized via π···π stacking and hydrogen bonding interactions to give a 3D supramolecular framework. Hirshfeld surface analysis showed that O···H (29.5%), H···H (23.8%), N···H (21.5%) and π···π (8.6%) intermolecular contacts are the most important interactions in the crystal of 1. In addition, the synthesized Co(II) complex showed favorable photocatalytic activity for the degradation of methyl orange (MO) under UV light at room temperature. The degradation process of MO was in accordance with the first-order reaction kinetics, and the t1/2 for the reaction is 27.3 min, and the apparent rate constant is k = 2.54 × 10−2 min−1.
1. Introduction
In the last few years, many metal–organic coordination compounds constructed by triazole ligands have been reported; some of them not only have charming molecular structures, but also promising physical properties [1,2,3]. In addition, the structure of coordination complexes will be influenced by the position of substituent groups in the ligands [4]. Recently, we have reported the structures, physical properties and intermolecular interaction analyses of several transition metal complexes derived from a series of rigid triazole-benzoic acid ligands [5,6].
Synthetic dyes used in the textile, leather and printing industry are difficult to degrade due to their stable molecular structure, and some of them are known to be toxic to the human body [7,8]. Common organic dyes, such as methylene blue (MB), congo red (CR), and methyl orange (MO) are widely used in textile, printing and packaging industries and have caused serious natural water pollution. Although different water treatment techniques have been developed to deal with dye pollution, photocatalytic degradation seemed to be the most promising method [8]. Therefore, the synthesis of efficient and environmentally friendly photocatalysts has attracted ever-increasing attention [9,10]. TiO2− and ZnO−based semiconductor materials are the most widely used photocatalysts in recent decades [11,12]. However, the main drawback for these materials is the fast recombination of electrons and hole pairs generated by irradiation [13]. In recent years, a new emerging application of metal–organic complexes as photocatalysts for the degradation of organic dyes has been established [14,15]. For instance, cobalt complexes with different structures have been used to degrade organic dyes in water medium under UV or visible irradiation [16,17].
In continuation of our work, herein we describe the solvothermal synthesis, supramolecular structure, spectroscopic, thermal stability and photocatalytic activity of complex [Co(tpa)2(H2O)4]·2H2O, starting with a triazole ligand 5-(1,2,4-triazol-1-yl)pyridine-3-carboxylic acid (Htpa).
2. Experimental
2.1. Materials and Methods
All starting materials were purchased from commercial sources and used as received. All the organic solvents used in synthesis and analysis were analytical grade and purchased from commercial sources. Methyl orange (MO) was purchased from Sinopharm Chemical Reagent co., Ltd, China. C, H and N elemental analyses were tested on a Vario EL III organic elemental analyzer (Elementar, Langenselbold, Hesse, Germany). FTIR spectrum was obtained from KBr pellet on a Varian 640-IR spectrometer (Varian, Palo Alto, CA, USA) in the range of 400–4000 cm−1. Powder X-ray diffraction patterns were performed on a Rigaku TTR(III) diffractometer (Rigaku, Takatsuki, Osaka, Japan), Cu kα radiation, λ = 1.5406 Å. Thermal analysis was performed on a Mettler Toledo TGA/DSC 1/1600 (Mettler Toledo, Zurich, Switzerland) thermal analyzer from room temperature to 800 °C at a heating rate of 10 °C min−1. UV–Vis absorption spectra were measured on a Persee TU-1901 (Persee, Beijing, China) spectrometer.
2.2. Synthesis of [Co(tpa)2(H2O)4]·2H2O (1)
A mixture of Co(NO3)2·6H2O (43.5 mg, 0.15 mmol) and Htpa (19 mg, 0.1 mmol) was dissolved in 6 mL deionized water, and the mixed solution was stirred for 10 minutes at room temperature. Then, the solution was sealed into a 25 mL Teflon-linear autoclave and heated at 150 °C for 48 h. The reactant mixture was cooled to room temperature at 10 °C per hour. The solution was filtered and left undisturbed for 3 days, and red block crystals of complex 1 were obtained (yield: 55%, based on Htpa ligand). Anal. Calcd. for C16H22CoN8O10 (%): C 35.24, H 4.07, N 20.55; Found (%): C 35.28, H 4.04, N 20.58.
2.3. X-ray Crystallography
Single-crystal X-ray diffraction data for complex 1 was collected on a Bruker Smart Apex II CCD (Bruker, Munich, Bavaria, Germany) area detector using graphite monochromated Mo Kα radiation (λ = 0.71073 Å) at 293(2) K. The crystal structure was solved by the direct method procedure and refined by full-matrix least squares on F2 with the use of the SHELXTL-2014 program [18]. The non-hydrogen atoms were refined anisotropically, and all hydrogen atoms were added theoretically. The crystallographic and refinement data of complex 1 are summarized in Table 1. CCDC deposition number 1947682 contains the supplementary crystallographic data for 1, which can be obtained free of charge via http://www.ccdc.cam.ac.uk/data_request/cif.

Table 1.
Crystal data and structure refinement for complex 1.
2.4. Hirshfeld Surface Analysis
Hirshfeld surface analysis is a powerful technique used to understand the intermolecular interactions within crystals. Hirshfeld surface analyses for complex 1 were carried out using Crystal Explorer 3.1 computer programs, with bond lengths to hydrogen atoms set to standard values. For each point on the Hirshfeld surface, de is the distance from the point to the nearest nucleus external to the surface, and di is the distance to the nearest nucleus internal to the surface [19]. The normalized contact distance (dnorm) is defined as [19,20]
where rivdw and revdw are the van der Waals radii of the atoms.
2.5. Photocatalytic Experiments
The photocatalytic behavior of [Co(tpa)2(H2O)4]·2H2O (1) was tested for the degradation of methyl orange (MO) under UV irradiation at room temperature. A sample of 30 mg powder crystals of complex 1 was immersed in 25 mL of MO solution (10 mg/L). The solution was kept in the dark for 1 h to ensure adsorption equilibrium. Then, the mixed solution was magnetically stirred and exposed to UV irradiation from a 400 W, high-pressure mercury lamp. The mercury lamp was put in the chamber of the annular hollow quartz tube, then the circulating cold water flowed in the hollow quartz tube to ensure that the emitted light was cold. The distance between the lamp and the reaction solution was about 20 cm. At given time intervals, about 3 mL of analytical samples were withdrawn and analyzed by the UV–Vis spectrometer. The control experiment was performed under the same conditions but without catalysts.
3. Results
3.1. Crystal Structure of [Co(tpa)2(H2O)4]·2H2O
Single-crystal X-ray diffraction analysis reveals that complex 1 crystallizes in the triclinic space group P ī. The asymmetry unit of 1 contains one central Co(II) ion, one deprotonated tpa− ligand, two coordinated water molecules, and one lattice water molecule. As shown in Figure 1a, the Co(II) ion is six-coordinated with an N2O4 coordination environment. The Co–O distances are 2.0757(15) and 2.0933(16) Å, and the Co–N bond length is 2.1821(17) Å (Table 2). The carboxylate group of tpa− ligand is deprotonated but not coordinated with the Co(II) ion.
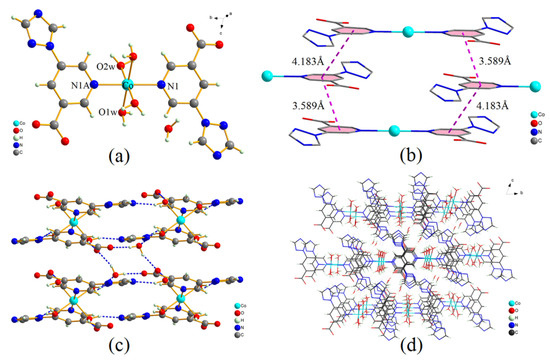
Figure 1.
(a) Coordination environment of Co(II) atom in complex 1, (b) π···π stacking interactions between the adjacent benzene rings, (c) intermolecular H bonds for complex 1, (d) supramolecular framework of complex 1 formed by H bonds and π···π interactions (viewed along a axis).

Table 2.
Selected bond lengths (Å) and angles (°) for complex 1.
As shown in Figure 1b, face-to-face π···π stacking interactions between the adjacent benzene rings can be obviously observed. The centroid to centroid distances between the two adjacent benzene rings are 3.589 and 4.183 Å, respectively. It can be concluded that the π···π interactions play an important role in stabilizing the crystal structure of 1.
Six groups of classical O−H···O and O−H···N hydrogen bonds are found in the packing structure of complex 1. The intermolecular hydrogen bonds are shown in Figure 1c, and the structural parameters of these hydrogen bonds are listed in Table 3. The coordinated water molecules act as H donors to form O−H···N hydrogen bonds with triazole groups (O1W-H1WA···N3), and form O−H···O hydrogen bonds with carboxylate oxygen atoms (O1W-H1WB···O1). However, another coordinated water molecule acts as an H donor to form an O−H···N hydrogen bond with N2 atom of triazole group (O2W-H2WA···N2), and form an O−H···O hydrogen bond with lattice water (O2W-H2WB···O3W). In addition, as the H donor, the lattice water also forms two O−H···O hydrogen bonds with carboxylate oxygen atoms (O3W-H3WA···O1, O3W-H3WB···O2). Moreover, based on intermolecular hydrogen bonds and π···π interactions, complex 1 further forms a stable 3D supramolecular framework (Figure 1d).

Table 3.
Geometrical parameters for the hydrogen bonds in complex 1.
3.2. Hirshfeld Surface of [Co(tpa)2(H2O)4]·2H2O (1)
The 3D Hirshfeld surfaces of complex 1 are illustrated in Figure 2, which maps dnorm, shape index and curvedness [21]. The dnorm Hirshfeld surfaces displayed using a red–white–blue color scheme, where the red spots indicate the close contact interactions, white color indicate contacts around the van der Waals radius distance, and blue color is for longer contacts [19]. As shown in Figure 2a, the deep red spots on the dnorm Hirshfeld surface indicate the close contact interactions, which are mainly responsible for the significant intermolecular O−H···O and O−H···N hydrogen bonds, created by carboxyl groups (spot A), coordinated water (spot B), triazole nitrogen atoms (spot C) and lattice water (spot D). For the shape index plot (Figure 2b), the orange–red areas represent concave regions, indicating that there are hydrogen bonding or π···π stacking interactions in these areas. For the curvedness plot (Figure 2c), the flat areas of the surface indicate interactions between neighboring molecules. In addition, the flat regions above the benzene and triazole rings refer to the strong π···π stacking interactions between the aromatic rings of adjacent molecules.
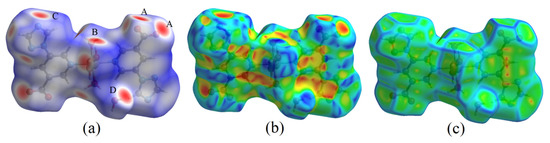
Figure 2.
Hirshfeld surfaces of complex 1 mapped with (a) dnorm, (b) shape index and (c) curvedness.
As shown in Figure 3, a 2D fingerprint plot was performed to identify important intermolecular interactions, which was further decomposed to highlight particular atom pairs’ close contacts. It is found that there are five kinds of intermolecular interactions, including O···H, N···H, H···H, C···H and C···C (π···π) interactions. The O···H/H···O intermolecular interactions comprise 29.5% of the total Hirshfed surface, which appear as two distinct spikes in the 2D fingerprint plot (Figure 3b). It can be inferred from the strongest spike that the O···H interaction is the shortest contact, with a minimum value of (de + di) around 1.7 Å [22]. The N···H/H···N interactions also appear as two sharp spikes, which comprise 21.5% of the total Hirshfed surface (Figure 3c). Apart from those mentioned above, the H···H interactions also have a large contribution to the total Hirshfed surface, comprising 23.8%. It is interesting that C···C interactions are found in the 2D fingerprint plot, which comprised 8.6% of the total Hirshfed surface (Figure 3f). The C···C interactions are mainly caused by the strong π···π stacking interactions between adjacent aromatic rings. From the 2D fingerprint plots, we can also find that the π···π stacking interactions have much longer contact distances when compared with hydrogen bonding interactions. The H bonding and π···π stacking information conveyed by Hirshfeld surface analyses are consistent with the crystal structure analyses.
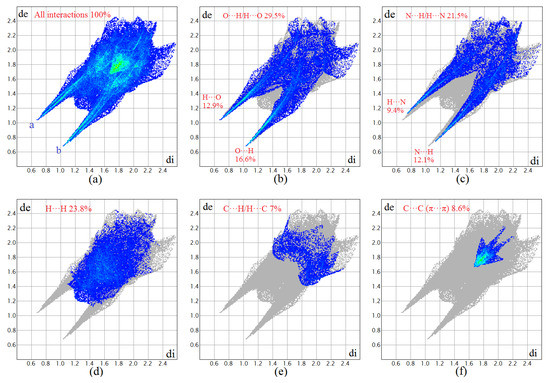
Figure 3.
Fingerprint plots of complex 1: full (a) and resolved into O···H (b), N···H (c), H···H (d), C···H (e) and C···C (f) contacts showing the percentages of contacts contributed to the total Hirshfeld surface area of molecules.
3.3. XRD and Thermal Stability
As shown in Supplementary Materials Figure S1, the experimental XRD pattern agrees well with the simulated pattern generated on the basis of the single-crystal analysis for complex 1, which suggests good phase purity and homogeneity of the synthesized sample. The thermal behavior of complex 1 was studied by gravimetric analysis (TGA). In the TGA curve (Figure S2), the first step takes place in the range of 60–147 °C with a weight loss of 13.0%, which can be attributed to the gradual release of four coordinated H2O (calcd. 13.2%). After that, the skeleton of the complex begins to decompose and a significant weight loss of 22.1% is observed in the range of 264–425 °C, which could be assigned to the decomposition of the triazole group (calcd. 24.9%). Keeping the heat at 900 °C, consecutive decompositions suggest the total destruction of the framework, and at last a residue of CoO remained (obsd. 16.6%, calcd. 13.7%).
3.4. FT-IR and UV-Vis Absorption Spectra
In the FT-IR spectrum of complex 1 (Figure S3), the broad absorption band at about 3300–3500 cm−1 can be assigned to the -OH stretching vibrations of water, including water ligands, lattice water and absorbed water molecules. The bands at 1582 and 1385 cm−1 are attributed to the stretching vibrations of C=C and C=N bonds from benzene and triazole rings [23]. As shown in Figure S4, the UV-Vis absorption spectrum of complex 1 was measured in methanol solution at room temperature (concentrations ca. 10−5 M). Complex 1 has three absorption bands with different intensities, where the two strong absorption bands at 212 and 239 nm can be attributed to the π → π* transition absorption of aromatic rings (C=C), while the weak band at 276 nm can be assigned to the n → π* transition of the triazole group (C=N) [24].
3.5. Photocatalytic Activity
The removal of toxic organic dyes from contaminated water is very important from the perspective of environmental protection. The photocatalytic degradation of methyl orange (MO) in aqueous solution by complex 1 under UV irradiation is shown in Figure 4a. It can be found from the absorption spectra that the absorption intensity of MO solution decreased gradually with the reaction time under the catalysis of complex 1, and the MO is almost completely degraded after 90 min of light irradiation. Degradation efficiencies (D) for MO dye was calculated as D = (A0 − At)/A0 × 100% [25], where A0 is the initial absorbance of MO solution at λmax = 464 nm, and At is the absorbance obtained at t min. As shown in Figure 4b, the degradation efficiency of MO is about 51.9% after reacting for 30 min, and is up to 89.2% after irradiating for 90 min, which indicates the high catalytic efficiency of complex 1. In contrast, the degradation efficiency of MO is only 14.9% after irradiating for 90 min in the absence of complex 1. In addition, we measured the XRD pattern of 1 after the photocatalytic experiment, which was identical to the corresponding one of the original compound (Figure S1), indicating that the crystal structure of complex 1 was stable after photocatalytic reactions. The catalyst was reused three times to study its reusable performance, as shown in Figure 5a. The catalyst retained favorable photocatalytic efficiencies after reused for two cycles, with the degradation efficiency of MO decreased from 89.3% to 80.5%. However, after being reused for three cycles, the degradation efficiency of MO decreased to 65.2%.
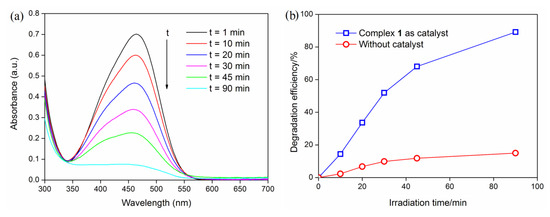
Figure 4.
(a) The UV-Vis absorption spectra of methyl orange (MO) solution during the decomposition reaction under UV irradiation; (b) Degradation efficiencies of MO under UV light.
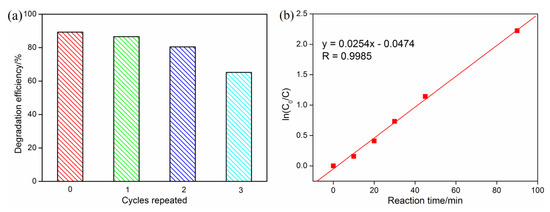
Figure 5.
(a) Reusable degradation activity of complex 1 (irradiation time 90 min, the catalyst was centrifuged, filtered and dried for the next experiment); (b) pseudo-first-order kinetics for the degradation of MO under UV light.
Reaction kinetics studies have shown that the degradation process of MO was in accordance with the pseudo-first-order model, ln(C0/Ct) = k∙t, where C0 and Ct were the concentrations of MO solution at irradiation time t0 and t [26]. As shown in Figure 5b, the ln(C0/Ct) versus t showed a linear relationship, with the correlation coefficient R = 0.9985 (p < 0.0001), and the apparent rate constant k = 2.54 × 10−2 min−1. As an important parameter of the pseudo-first-order reaction, the half-life time t1/2 (calculated as t1/2 = ln2/k) means the time at which C = 0.5 C0 [27,28]. The t1/2 for the photocatalytic reaction of MO is 27.3 min, implying that complex 1 is an effective photocatalyst.
The mechanism of photocatalytic degradation of organic dyes by metal–organic complexes has been described in detail in much of the literature, which proved that these reactions were mainly based on the advanced oxidation of hydroxyl radicals (∙OH) [29]. In aqueous solution, under the excitation of UV light, N–Co charge transfer occurs between the ligands and the metal center of complex 1, promoting electrons from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). Therefore, the highest occupied molecular orbital strongly needs an electron to return to its stable state. The electrons are captured from aqueous solution to generate ·OH radicals. At the same time, the electrons excited to the LUMO are captured by water to generate ·OH radicals [14,29,30]. Finally, the oxidizing ·OH radicals decomposed MO dye to complete the photocatalytic process. Wang and co-workers studied the photocatalytic activity of six different complexes [30]. They observed that the Co(II) complexes show much better photocatalytic activity than that of the Cu(II), Ni(II), Zn(II) and Cd(II) complexes in the degradation of different types of organic dyes. They also pointed out that the different structures and central metal ions of complexes might affect the photocatalytic activity, but the reasons are still unclear. For the synthesized Co(II) complex 1, the N atom of pyridine ring on the ligand coordinate with the cobalt atoms. The pyridine ring is a stable aromatic structure; when the Co(II) complex is excited by UV light, the charges can be distributed over the entire pyridine ring, which promotes the separation of photogenerated electrons and positive charges, making the excited state more stable [30,31]. Besides, in aqueous solution, the presence of four coordination water molecules may contribute to the production of hydroxyl radicals (Figure S5). Moreover, Lin et al. point out that the charge-carrier mobility of materials is affected by π conjugation and π···π stacking [32]. We can infer from the single-crystal structure of complex 1 that the π···π stacking structure makes the photogenerated electrons more stable, which will facilitate the transfer of photogenerated electrons to water to produce hydroxyl radicals.
4. Conclusions
In summary, a novel Co(II) complex [Co(tpa)2(H2O)4]·2H2O (1) has been synthesized using multifunctional triazole derivative as an organic ligand. Hirshfeld surface analysis was used to study the intermolecular interactions, which revealed that the O···H, H···H, N···H and π···π contacts were the most significant interactions in the crystal of complex 1. The hydrogen bonding and π···π stacking information conveyed by Hirshfeld surface analyses were consistent with the crystal structure analyses. Photocatalytic studies revealed that complex 1 could effectively catalyze the degradation of MO dye under UV irradiation, and the degradation process was in accordance with the pseudo-first-order kinetics model.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/10/2/98/s1, Figure S1: XRD patterns of complex 1, Figure S2: TGA curve of complex 1, Figure S3: FT-IR spectrum of complex 1, Figure S4: UV absorption spectrum of complex 1 in methanol solution at room temperature, Figure S5: Possible photocatalytic mechanisms showing the two excited states of Co(II) catalyst under UV excitation.
Author Contributions
D.W. and B.Y. conceived and designed the experiments; T.W. and Y.W. performed X-ray structure determination and analyzed the results; D.W., N.Z. and C.Z. carried out the synthetic and photocatalytic experiments. All authors took part in writing and discussion processes. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (No. 31760190), the Project on applied basic research in Yunnan (No. 2016FB052), and the Research Launch Fund of Southwest Forestry University (No. 111921).
Acknowledgments
The authors would like to thank the forestry engineering postdoctoral station of Southwest Forestry University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.L.; Fu, J.H.; Wei, J.J.; Xu, X.; Li, X.F.; Liu, Q.Y. Noncentrosymmetric organic solid and its zinc coordination polymer with diamonded network prepared from an ionothermal reaction: Syntheses, crystal structures, and second-order nonlinear optics properties. Cryst. Growth Des. 2012, 12, 4663–4668. [Google Scholar] [CrossRef]
- Luo, Y.S.; Chen, J.L.; Zeng, X.H.; Qiu, L.; He, L.H.; Liu, S.J.; Wen, H.R. A highly stable and luminescent mononuclear Cu(I) bis-{5-tert-butyl-3-(6-methyl-2-pyridyl)-1H-1,2,4-triazole} complex. Chin. Chem. Lett. 2017, 28, 1027–1030. [Google Scholar] [CrossRef]
- Yan, Y.T.; Zhang, S.S.; Yang, G.P.; Zhang, W.Y.; Zhang, F.; Cao, F.; Yang, R.F.; Wang, Y.Y. The influence of coordination modes and active sites of a 5-(triazol-1-yl) nicotinic ligand on the assembly of diverse MOFs. Dalton Trans. 2017, 46, 9784–9793. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.F.; Guo, M.; Li, X.L.; Zhao, L.; Sun, Q.F.; Layfield, R.A.; Tang, J.K. From double-shelled grids to supramolecular frameworks. Chem. Commun. 2018, 54, 12097–12100. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Wang, T.; Yan, T.; Du, L.; Zhao, Q.H. Crystal structures and spectroscopic characterizations of two Cd(II) complexes based on [1,2,4]-triazole derivatives. Chin. J. Inorg. Chem. 2017, 33, 1443–1449. [Google Scholar]
- Wang, D.W.; Wang, T.; Yan, T.; Du, L.; Zhao, Q.H. Crystal structure, spectroscopic and thermal properties of copper(II) and manganese(II) coordination polymers based on triazole-benzoic acid ligands. Transit. Met. Chem. 2018, 43, 1–8. [Google Scholar] [CrossRef]
- Harandi, Z.J.; Nasab, S.G.; Teimouri, A. Synthesis and characterisation of magnetic activated carbon/diopside nanocomposite for removal of reactive dyes from aqueous solutions: Experimental design and optimization. Int. J. Environ. Anal. Chem. 2019. [Google Scholar] [CrossRef]
- Cui, J.W.; Hou, S.X.; Li, Y.H.; Cui, G.H. A multifunctional Ni(II) coordination polymer: Synthesis, crystal structure and applications as a luminescent sensor, electrochemical probe, and photocatalyst. Dalton Trans. 2017, 46, 16911–16924. [Google Scholar] [CrossRef]
- Li, Z.R.; Mei, J.X.; Bai, L. Synthesis of C3N4‑decorated ZnO and Ag/ZnO nanoparticles via calcination of ZIF‑8 and melamine for photocatalytic removal of methyl orange. Chem. Pap. 2019, 73, 883–889. [Google Scholar] [CrossRef]
- Li, Z.C.; Ma, J.J.; Zhang, B.; Song, C.X.; Wang, D.B. Crystal phase- and morphology-controlled synthesis of MoO3 materials. CrystEngComm 2017, 19, 1479–1485. [Google Scholar] [CrossRef]
- Foura, G.; Chouchou, N.; Soualah, A.; Kouachi, K.; Guidotti, M.; Robert, D. Fe-doped TiO2 supported on HY zeolite for solar photocatalytic treatment of dye pollutants. Catalysts 2017, 7, 344. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Xu, K.C.; Wu, J.G.; Tan, C.F.; Ho, G.W.; Wei, A.; Hong, M.H. Ag-CuO-ZnO metal-semiconductor multiconcentric nanotubes for achieving superior and perdurable photodegradation. Nanoscale 2017, 9, 11574–11583. [Google Scholar] [CrossRef]
- Wu, Y.; He, J.; Wang, S.Q.; Zou, L.K.; Wu, X.R. Syntheses, crystal structure, and photocatalytic property of two new complexes of an unsymmetrical Schiff base ligand. Inorg. Chim. Acta 2017, 458, 218–223. [Google Scholar] [CrossRef]
- Qin, L.; Xiao, S.L.; Ma, P.J.; Cui, G.H. Synthesis, crystal structures and catalytic properties of Ag(I) and Co(II) 1D coordination polymers constructed from bis(benzimidazolyl)butane. Transit. Met. Chem. 2013, 38, 627–633. [Google Scholar] [CrossRef]
- Li, J.X.; Li, Y.F.; Liu, L.W.; Cui, G.H. Luminescence, electrochemical and photocatalytic properties of sub-micron nickel(II) and cobalt(II) coordination polymers synthesized by sonochemical process. Ultrason. Sonochem. 2018, 41, 196–205. [Google Scholar] [CrossRef]
- Wang, D.W.; Yang, S.L.; Zhuang, C.F.; Wang, Y.; Shi, Z.J. Crystal structure, Hirshfeld surface analysis and photocatalytic activities of a cobalt(III) complex based on acid and alkaline mixed ligands. Transit. Met. Chem. 2019, 44, 455–461. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Zhang, C.G.; Li, Y.; Luo, Y.H.; Sun, B.W. Two novel salts of tris(hydroxymethyl)aminomethane (THAM): Synthesis, crystal structure, thermal and Hirshfeld surfaces analysis. J. Chem. Crystallogr. 2013, 43, 576–584. [Google Scholar] [CrossRef]
- Roy, S.; Harms, K.; Chattopadhyay, S. Synthesis, characterization and photocatalytic activity of a dinuclear thiocyanate bridged cadmium(II) Schiff base complex. Polyhedron 2017, 127, 471–477. [Google Scholar] [CrossRef]
- Luo, Y.H.; Liu, Q.L.; Yang, L.J.; Wang, W.; Ling, Y.; Sun, B.W. Quantitative comparisons between α, β, γ and δ pyrazinamide (PZA) polymorphs. Res. Chem. Intermed. 2015, 41, 7059–7072. [Google Scholar] [CrossRef]
- Seth, S.K.; Maity, G.C.; Kar, T. Structural elucidation, Hirshfeld surface analysis and quantum mechanical study of para-nitro benzylidene methyl arjunolate. J. Mol. Struct. 2011, 1000, 120–126. [Google Scholar] [CrossRef]
- Fang, D.L.; Mo, S.Y.; Wu, K.F.; Huang, Z.J. Synthesis, crystal structures, and properties of three coordination polymers of 5-(1H-imidazol-1-yl) isophthalic acid. Transit. Met. Chem. 2017, 42, 273–283. [Google Scholar] [CrossRef]
- Wang, D.W.; Wang, T.; Du, L.; Zhou, J.; Yan, T.; Zhao, Q.H. Four supramolecular transition metal(II) complexes based on triazole-benzoic acid derivatives: Crystal structure, Hirshfeld surface analysis, spectroscopic and thermal properties. Struct. Chem. 2018, 29, 1013–1023. [Google Scholar] [CrossRef]
- Azam, M.; Al-Resayes, S.; Wabaidur, S.M.; Trzesowska-Kruszynska, A.; Kruszynski, R.; Mohapatra, R.K.; Siddiqui, M.R. Cd(II) complex constructed from dipyridyl imine ligand: Design, synthesis and exploration of its photocatalytic degradation properties. Inorg. Chim. Acta 2018, 471, 698–704. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.L.; Xu, C.X.; Chen, S.F.; Fu, X.L. One-pot synthesis of ZnO2/ZnO composite with enhanced photocatalytic performance for organic dye removal. J. Nanosci. Nanotechnol. 2013, 13, 657–665. [Google Scholar] [CrossRef]
- Mansour, A.M.; Bakry, E.M.; Abdel-Ghani, N.T. Photocatalytic degradation of methylene blue with copper(II) oxide synthesized by thermal decomposition of flubendazole complexes. J. Photoch. Photobiol. A 2016, 327, 21–24. [Google Scholar] [CrossRef]
- Neto, J.O.M.; Bellato, C.R.; Souza, C.H.F.; Silva, R.C.; Rocha, P.A. Synthesis, characterization and enhanced photocatalytic activity of iron oxide/carbon nanotube/Ag-doped TiO2 nanocomposites. J. Braz. Chem. Soc. 2017, 28, 2301–2312. [Google Scholar]
- Zhang, X.T.; Fan, L.M.; Fan, W.L.; Li, B.; Liu, G.Z.; Liu, X.Z.; Zhao, X. Structural diversity, luminescence and photocatalytic properties of six coordination polymers based on designed bifunctional 2-(imidazol-1-yl)terephthalic acid. CrystEngComm 2016, 18, 6914–6925. [Google Scholar] [CrossRef]
- Wang, X.L.; Luan, J.; Lin, H.Y.; Lu, Q.L.; Le, M.; Liu, G.C.; Shao, J.Y. Metal(II)-organic coordination polymers modulated by two isomeric semirigid bis-pyridyl–bis-amide ligands: Structures, fluorescent sensing behavior, and selective photocatalysis. ChemPlusChem 2014, 79, 1691–1702. [Google Scholar] [CrossRef]
- Korala, L.; Germain, J.R.; Chen, E.; Pala, I.R.; Li, D.; Brock, S.L. CdS aerogels as efficient photocatalysts for degradation of organic dyes under visible light irradiation. Inorg. Chem. Front. 2017, 4, 1451–1457. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.D.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1212. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).