Abstract
A linear regression model is presented in this study to determine the pre-exponential factor and interfacial energy of the crystallized substance based on classical nucleation theory using the metastable zone width data. The nucleation event is assumed corresponding to a point at which the total number density of the nuclei has reached a fixed (but unknown) value. One equation is derived for any temperature-dependent functional form of the solubility. Another equation is derived for the van’t Hoff solubility expression. The pre-exponential factor and interfacial energy obtained from these two equations are found consistent for the studied systems, including glutamic acid, glycine, and 3-nito-1,2,4-triazol-5-one. The results obtained from these two equations are also compared with those obtained from the integral method and classical 3D nucleation theory approach.
1. Introduction
The nucleation behavior in a supersaturated solution is closely related to the induction time or metastable zone width (MSZW) measurements [1,2,3]. As opposed to a lag time during the temperature-decreasing process for the prepared supersaturated solution at a higher temperature lowered to the desired constant temperature in the induction time measurements, there is no lag time for the supersaturated solution cooled at a constant cooling rate in the MSZW measurements. Thus, the MSZW data should be more reliable than the induction time data in determination of the nucleation rate for a system.
In classical nucleation theory (CNT) [1,2,3], the nucleation rate of a crystallization system depends on both the pre-exponential factor and interfacial energy, which are usually determined using induction time data by assuming , where is the nucleation rate and is the induction time [4,5,6,7,8,9,10]. Due to the complicated data interpretation method, determination of the pre-exponential factor and interfacial energy using MSZW data has long been a challenging task.
Based on the Nyvlt’s approach [11,12], Sangwal [13,14,15,16] proposed a self-consistent Nyvlt-like equation using the power-law nucleation rate, leading to a linear relationship between and , where is the initial saturated temperature, is the MSZW, and is the cooling rate. Similarly, using the nucleation rate based on CNT, Sangwal [13,14,15,16] developed a linear relationship of versus . Kashchiev et al. [17] presented a general expression for the total volume and number of crystallites as functions of the cooling rate for progressive nucleation based on CNT. They verified that the linear dependence of on is the linear approximation to their presented equation. For instantaneous nucleation, a more simplified model is derived by Kashchiev et al. [18] which also yields a linear relationship between and . Kubota [19] proposed a model based on progressive nucleation to account for the MSZW limit corresponding to a point at which the number density of accumulated crystals has reached a fixed (but unknown) value during the cooling process. The simple power-law form of nucleation rate is adopted in the Kubota’s model, leading to a linear relationship of versus for the MSZW data.
The above-mentioned models have been widely applied in the literature to correlate MSZW with cooling rate in various crystallization systems [20,21,22,23,24,25,26,27]. Although the pre-exponential factor and interfacial energy can provide important information in understanding the nucleation behavior, the pre-exponential factor and interfacial energy of the crystallized substance are usually not determined in these studies. Recently, some researchers [28,29,30] investigated the relationship between MSZW and process parameters in terms of the pre-exponential factor and interfacial energy using classical 3D nucleation theory approach based on the Sangwal’s theory [13,14,15,16]. Shiau and Lu [31,32,33] developed an integral model to determine the pre-exponential factor and interfacial energy based on CNT using the MSZW data. As the nonlinear regression along with numerical integration involved in the integral model is complicated, the objective of this work is to present a linear regression model to determine the pre-exponential factor and interfacial energy of the crystallized substance based on CNT using the MSZW data.
2. Theory
2.1. Integral Method
The nucleation rate based on CNT is expressed as [1,2,3]
where is the nucleation pre-exponential factor, is the interfacial energy, is the Boltzmann constant, and is the molecular volume.
To interpret the onset of nucleation based on the progressive nucleation observed in the induction time and MSZW measurements, the nucleation event is assumed corresponding to a point at which the total number density of the nuclei has reached a fixed (but unknown) value, [19,30,31,32]. Thus, one can derive at the induction time, which is consistent with reported in the literature [1]. As nuclei are continuously born during the cooling process, Shiau and Lu [31,32,33] derived at the MSZW limit:
where depends on the measurement device and on the substance.
If the solution is cooled at a constant rate defined as:
one obtains during the cooling process. Substituting Equations (1) and (3) into Equation (2) yields [31,32,33]:
where, as shown in Figure 1, is the initial saturated temperature at , is the maximum supercooling temperature at , is the MSZW, is the saturated concentration at , is the temperature-dependent supersaturation during the cooling process, and is the temperature-dependent solubility. Note that remains unchanged before the onset of nucleation. As usually decreases with decreasing temperature, increases with decreasing temperature; and subsequently defined in Equation (1) increases during the cooling process.
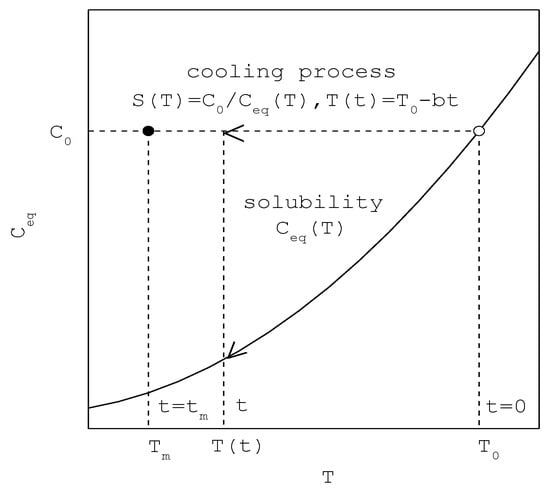
Figure 1.
A schematic diagram showing the increasing of supersaturation during the cooling process for the saturated concentration (○ represents the starting point and ● represents the nucleation point at a given ).
Shiau and Lu [31,32,33] proposed the following nonlinear regression procedure to determine two parameters, and , in Equation (4) from the experimental data of versus : (a) Guess ; (b) determine for each pair of versus data by numerical integration; (c) calculate ; (d) calculate the coefficient of variation among all . For the guessed , is defined as:
where is the number of data points . For the guessed , among all is defined as:
The same procedure from (a) to (d) is repeated by guessing various values of until the optimal with the minimum is found. Then, the corresponding calculated from the optimal is taken as the optimal . If is known, can be determined.
2.2. Linearized Integral Method
As the nonlinear regression along with numerical integration involved in Equation (4) is complicated, a simplified linear regression model is proposed in this study to extend the applicability of integral model as follows. Based on the trapezoidal rule for the numerical integration, Equation (2) is approximated as:
Here, represents the total time required during the cooling process from to at a constant cooling rate . and represent the nucleation rate at and , respectively. Note that due to .
As shown in Figure 1, increases gradually from 1 at during the cooling process. As defined in Equation (1), starts from at and increases gradually as temperature decreases from to . The nucleation rate at can be expressed as:
with
where is the saturated concentration at and is the supersaturation at .
Substituting Equation (8) into Equation (7) yields:
Taking logarithm on both sides of Equation (10) gives:
By rearranging Equation (11), the linearized integral method I is expressed as:
A plot of versus at a given should give a straight line, the slope and intercept of which permit determination of and , respectively, without the knowledge of .
If the temperature-dependent solubility is described in terms of the van’t Hoff equation [1], one obtains:
where is the heat of dissolution and is the gas constant. By substituting Equation (13) into Equation (12), linearized integral method II is expressed as:
Similarly, a plot of versus at a given should give a straight line, the slope and intercept of which permit determination of and , respectively, without the knowledge of .
It should be noted in the application of Equations (12) and (14) that is not influenced by the chosen value of although needs to be determined based on . Based on the study of 28 inorganic systems, Mersmann and Bartosch [34] concluded that the minimum detectable volume fraction of nuclei in solution corresponds to with the minimum detectable size of . The intermediate value, , was adopted at the detection of the nucleation point for the Lasentec focus beam reflectance measurements reported by Lindenberg and Mazzotti [35] and for the turbidity measurements reported by Shiau et al. [31,32,33,36]. If the uniform-sized spherical nuclei of with are assumed for simplicity, corresponds to [32].
2.3. Classical 3D Nucleation Theory Approach
Sangwal [13,14,15,16] related with the rate of change of solution supersaturation as:
where , , and is a constant defined as the number of clusters per unit volume. The value of is governed by the aggregation and diffusion processes in the solution.
If is slightly greater than 1, one obtains:
Combining Equations (13) and (16) yields:
Substituting Equation (9) into Equation (17) gives:
By combining Equations (8), (15) and (18), Sangwal [13,14,15,16] derived the classical 3D nucleation theory approach as:
where:
A plot of versus at a given should give a straight line, the slope and intercept of which permit determination of and , respectively. Subsequently, the values of and can be calculated without the knowledge of .
It should be noted in the application of Equation (19) that is not influenced by the chosen value of although needs to be determined based on . Sangwal [14] proposed that the upper limit of may be estimated from solute concentration in the saturated solution. For example, , the saturated concentration for aqueous glycine solutions at 308.15 K (), corresponds to based on the number of solute molecules per unit solution volume, which is close to proposed by Sangwal [14].
3. Results and Discussion
The experimental MSZW data for three crystallization systems, including glutamic acid, glycine and, 3-nito-1,2,4-triazol-5-one (NTO), reported in the literature are analyzed as follows. Figure 2a shows that the MSZW data of aqueous glutamic acid solutions fitted to linearized integral method I Equation (12) at various , where the original experimental MSZW data listed in Table 1 are taken from Shiau and Lu [32] in a 200 mL vessel. The solubility of glutamic acid in water is taken as ; [37]. Note that for glutamic acid. Figure 2b shows the same MSZW data of aqueous glutamic acid solutions fitted to linearized integral method II Equation (14) at various , where is used for the van’t Hoff solubility equation. For comparison, Figure 2c,d show the same MSZW data of aqueous glutamic acid solutions fitted to the classical 3D approach Equation (19) and integral method Equation (4), respectively, at various .
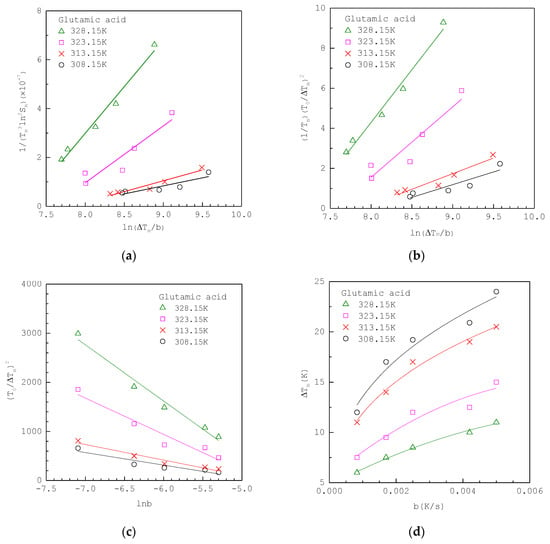
Figure 2.
The experimental MSZW data of aqueous glutamic acid solutions at various (a) fitted to Equation (12); (b) fitted to Equation (14); (c) fitted to Equation (19); (d) fitted to Equation (4). The MSZW data are taken from Shiau and Lu [32].

Table 1.
The experimental MSZW data of aqueous glutamic acid solutions taken from Shiau and Lu [32], where is the initial saturated temperature at the corresponding initial saturated concentration .
Figure 3a shows that the MSZW data of aqueous glycine solutions fitted to linearized integral method I Equation (12) at various , where the original experimental MSZW data listed in Table 2 are taken from Shiau [38] in a 200 mL vessel. The solubility of glycine in water is taken as ; [39]. Note that for glycine. Figure 3b shows the same MSZW data of aqueous glycine solutions fitted to linearized integral method II Equation (14) at various , where is used for the van’t Hoff solubility equation. For comparison, Figure 3c,d show the same MSZW data of aqueous glycine solutions fitted to classical 3D approach Equation (19) and integral method Equation (4), respectively, at various .
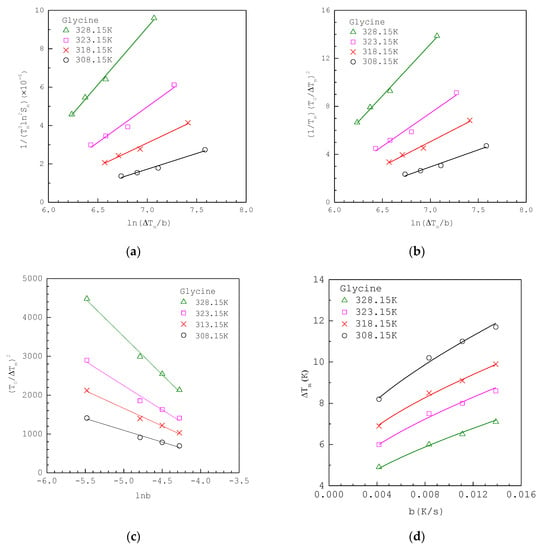
Figure 3.
The experimental MSZW data of aqueous glycine solutions at various (a) fitted to Equation (12); (b) fitted to Equation (14); (c) fitted to Equation (19); (d) fitted to Equation (4). The MSZW data are taken from Shiau [38].

Table 2.
The experimental MSZW data of aqueous glycine solutions taken from Shiau [38], where is the initial saturated temperature at the corresponding initial saturated concentration .
Figure 4a shows that the MSZW data of aqueous NTO solutions fitted to linearized integral method I Equation (12) at various , where the original experimental MSZW data listed in Table 3 are taken from Kim et al. [40] in a 300 mL vessel. The solubility of NTO in water is taken as ; [40]. Note that for NTO. Figure 4b shows the same MSZW data of aqueous NTO solutions fitted to linearized integral method II Equation (14) at various , where is used for the van’t Hoff solubility equation. For comparison, Figure 4c,d show the same MSZW data of aqueous NTO solutions fitted to the classical 3D approach Equation (19) and integral method Equation (4), respectively, at various .
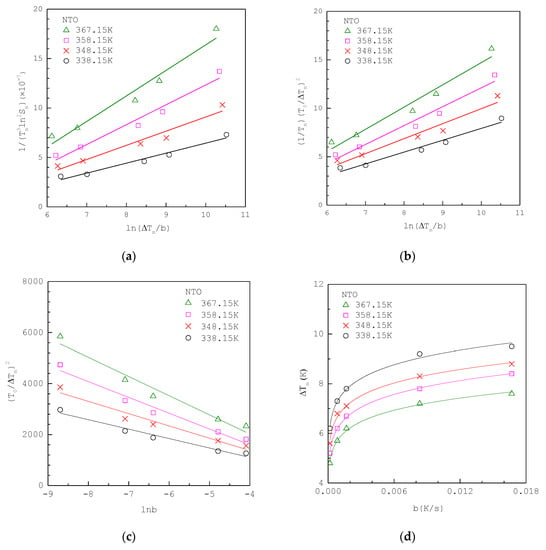
Figure 4.
The experimental MSZW data of aqueous NTO solutions at various (a) fitted to Equation (12); (b) fitted to Equation (14); (c) fitted to Equation (19); (d) fitted to Equation (4). The MSZW data are taken from Kim et al. [40].

Table 3.
The experimental MSZW data of aqueous NTO solutions taken from Kim et al. [40], where is the initial saturated temperature at the corresponding initial saturated concentration .
The fitted results for glutamic acid, glycine, and NTO are listed in Table 4, Table 5 and Table 6. As integral method Equation (4) is numerically integrated without any approximations in this study, and obtained from integral method Equation (4) represent the exact solution to Equation (2) based on the nucleation event assumed corresponding to a point at which the total number density of the nuclei has reached . For all three studied systems, as opposed to obtained from classical 3D approach Equation (19) compared with that obtained from integral method Equation (4), obtained from linearized integral methods Equations (12) and (14) is closer to that obtained from integral method Equation (4) at each condition. Furthermore, obtained from linearized integral methods Equations (12) and (14) is also consistent with that obtained from integral method Equation (4) at each condition. As and are two different parameters, determined from Equations (4), (12) and (14) based on is not strictly comparable with that determined from Equation (19) based on . It should be noted in Table 4, Table 5 and Table 6 that and (or ) are determined first without the knowledge of (or ). Consequently, is not influenced by the chosen value of (or ) although needs to be determined based on (or ). For example, if the chosen value of (or ) is increased by ten times, is also increased by ten times at each condition while remains unchanged.

Table 4.
The fitted results of and at various for aqueous glutamic acid solution based on and .

Table 5.
The fitted results of and at various for aqueous glycine solutions based on and .

Table 6.
The fitted results of and at various for aqueous NTO solutions based on and .
As compared in Table 4, Table 5 and Table 6, and obtained from linearized integral method I Equation (12) are consistent with those obtained from linearized integral method II Equation (14) at each condition for all three studied systems. Thus, both equations can be applied to determine and of the crystallized substance using the MSZW data. As opposed to the temperature-dependent solubility required for linearized integral method I Equation (12), only the value of is required in application of linearized integral method II Equation (14).
4. Conclusions
A linear regression method is proposed in this work to determine the pre-exponential factor and interfacial energy based on CNT using the MSZW data. Linearized integral method I Equation (12) is derived for any temperature-dependent functional form of the solubility while linearized integral method II Equation (14) is derived for the van’t Hoff temperature-dependent solubility. Only the value of is required in application of linearized integral method II Equation (14), as opposed to the temperature-dependent solubility required for linearized integral method I Equation (12). The experimental MSZW data for all three studied systems, including glutamic acid, glycine, and NTO, are fitted well to these two equations. The pre-exponential factor and interfacial energy obtained from linearized integral method I Equation (12) are consistent with those obtained from linearized integral method II Equation (14) for these systems.
As the integral method is numerically integrated without any approximations, the pre-exponential factor and interfacial energy obtained from the integral method represent the exact values based on the nucleation event assumed corresponding to a point at which the total number density of the nuclei has reached a fixed value. As opposed to the interfacial energy obtained from classical 3D nucleation theory approach compared with that from the integral method, the interfacial energy obtained from linearized integral methods Equations (12) and (14) is closer to that from the integral method at each condition. Furthermore, the pre-exponential factor obtained from linearized integral method Equations (12) and (14) is also consistent with that from the integral method at each condition.
Funding
The author would like to thank Chang Gung Memorial Hospital (CMRPD2G0242) and the Ministry of Science and Technology of Taiwan (MOST108-2221-E-182-034) for financial support of this research.
Conflicts of Interest
The author declares no conflict of interest.
Notation
| nucleation pre-exponential factor | |
| cooling rate | |
| initial saturated concentration of solutes at | |
| saturated concentration of solutes | |
| number of clusters per unit volume | |
| minimum detectable number density of generated nuclei | |
| minimum detectable volume fraction of generated nuclei | |
| nucleation rate | |
| nucleation rate at | |
| nucleation rate at | |
| Boltzmann constant | |
| volume shape factor | |
| molar mass | |
| Avogadro number | |
| gas constant | |
| supersaturation ratio | |
| supersaturation ratio at | |
| temperature | |
| initial saturated temperature | |
| temperature at tm | |
| time | |
| induction time | |
| time at the MSZW limit | |
| Greek letters | |
| crystal density | |
| volume of the solute molecule | |
| surface energy | |
| heat of dissolution | |
| MSZW | |
References
- Mullin, J.W. Crystallization; Butterworth-Heinemann: Oxford, UK, 1993. [Google Scholar]
- Kashchiev, D. Nucleation: Basic Theory with Applications; Butterworth-Heinemann: Oxford, UK, 2000. [Google Scholar]
- Kashchiev, D.; van Rosmalen, G.M. Review: Nucleation in solutions revisited. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Sohnel, O.; Mullin, J.W. A method for the determination of precipitation induction periods. J. Cryst. Growth 1978, 44, 377–382. [Google Scholar] [CrossRef]
- Lancia, A.; Musmarra, D.; Prisciandaro, M. Measuring induction period for calcium sulfate dihydrate precipitation. AIChE J. 1999, 45, 390–396. [Google Scholar] [CrossRef]
- Granberg, R.A.; Ducreux, C.; Gracin, S.; Rasmuson, A.C. Primary nucleation of paracetamol in acetone–water mixtures. Chem. Eng. Sci. 2001, 56, 2305–2313. [Google Scholar] [CrossRef]
- Omar, W.; Mohnicke, M.; Ulrich, J. Determination of the solid liquid interfacial energy and thereby the critical nucleus size of paracetamol in different solvents. Cryst. Res. Technol. 2006, 41, 337–343. [Google Scholar] [CrossRef]
- Yang, H.; Rasmuson, A.C. Nucleation of butyl paraben in different solvents. Cryst. Growth Des. 2013, 13, 4226–4238. [Google Scholar] [CrossRef]
- You, S.; Zhang, Y.; Zhang, Y. Nucleation of ammonium aluminum sulfate dodecahydrate from unseeded aqueous solution. J. Cryst. Growth 2015, 411, 24–29. [Google Scholar] [CrossRef]
- Du, G.; Sun, Z.; Xian, Y.; Jing, H.; Chen, H.; Yin, D. The nucleation kinetics of ammonium metavanadate precipitated by ammonium chloride. J. Cryst. Growth 2016, 441, 117–123. [Google Scholar] [CrossRef]
- Nyvlt, J. Kinetics of crystallization in solution. J. Cryst. Growth 1968, 3/4, 377–383. [Google Scholar] [CrossRef]
- Nyvlt, J.; Sohnel, O.; Matuchova, M.; Broul, M. The Kinetics of Industrial Crystallization; Academia: Prague, Czech Republic, 1985. [Google Scholar]
- Sangwal, K. A novel self-consistent Nyvlt-like equation for metastable zone width determined by the polythermal method. Cryst. Res. Technol. 2009, 44, 231–247. [Google Scholar] [CrossRef]
- Sangwal, K. New approach to analyze metastable zone width determined by the polythermal method: Physical interpretation of various parameters. Cryst. Growth Des. 2009, 9, 942–950. [Google Scholar] [CrossRef]
- Sangwal, K. Effects of impurities on the metastable zone width of solute-solvent systems. J. Cryst. Growth 2009, 311, 4050–4061. [Google Scholar] [CrossRef]
- Sangwal, K. On the effects of impurities on the metastable zone width of phosphoric acid. J. Cryst. Growth 2010, 312, 3316–3325. [Google Scholar] [CrossRef]
- Kashchiev, D.A.; Borissova, A.; Hammond, R.B.; Roberts, K.J. Effect of cooling rate on the critical undercooling for crystallization. J. Cryst. Growth 2010, 312, 698–704. [Google Scholar] [CrossRef]
- Kashchiev, D.A.; Borissova, A.; Hammond, R.B.; Roberts, K.J. Dependence of the critical undercooling for crystallization on the cooling rate. J. Phys. Chem. 2010, 114, 5441–5446. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N. A new interpretation of metastable zone widths measured for unseeded solutions. J. Cryst. Growth 2008, 310, 629–634. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, G.; Zhou, X. Effects of different organic acids on solubility and metastable zone width of zinc lactate. J. Chem. Eng. Data 2012, 57, 2963–2970. [Google Scholar] [CrossRef]
- Peng, J.; Dong, Y.; Wang, L.; Li, L.; Li, W.; Feng, H. Effect of impurities on the solubility, metastable zone width, and nucleation kinetics of borax decahydrate. Ind. Eng. Chem. Res. 2014, 53, 12170–12178. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Chai, J.; Xu, J.; Zhang, Z.; Qian, G.; Zhou, X. Nucleation kinetics of lovastatin in different solvents from metastable zone widths. Chem. Eng. Sci. 2015, 133, 62–69. [Google Scholar] [CrossRef]
- Jiang, X.; Ruan, X.; Xiao, W.; Lu, D.; He, G. A novel membrane distillation response technology for nucleation detection, metastable zone width measurement and analysis. Chem. Eng. Sci. 2015, 134, 671–680. [Google Scholar] [CrossRef]
- Wang, L.; Feng, H.; Dong, Y.; Peng, J.; Li, W. Solubility and metastable zone width of aqueous sodium dichromate dehydrate solutions in the presence of sodium chromate additive. J. Cryst. Growth 2016, 454, 105–110. [Google Scholar] [CrossRef]
- Luo, M.; Liu, C.; Xue, J.; Li, P.; Yu, J. Determination of metastable zone width of potassium sulfate in aqueous solution by ultrasonic sensor and FBRM. J. Cryst. Growth 2017, 469, 144–153. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Zhang, M.; Guo, M.; Xu, S.; Yin, Q. Determination of metastable zone and induction time of analgin for cooling crystallization. Chin. J. Chem. Eng. 2017, 25, 313–318. [Google Scholar] [CrossRef]
- Mastan, T.H.; Lenka, M.; Sarkar, D. Nucleation kinetics from metastable zone widths for sonocrystallization of L-phenylalanine. Ultrason. Sonochem. 2017, 36, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, J.; Zhang, K.; Wu, S.; Li, S.; Li, K.; Yu, B.; Gong, J. Nucleation behavior of eszopiclone-butyl acetate solutions from metastable zone widths. Chem. Eng. Sci. 2016, 155, 248–257. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, J.; Chen, H.; Du, W.; Wang, X. Effects of succinic acid and adipic acid on the metastable width of glutaric acid in acetic acid. J. Cryst. Growth 2019, 507, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Peng, J.; Wang, X.; Dong, Y.; Li, W. Effects of CO32− and OH− on the solubility, metastable zone width and nucleation kinetics of borax decahydrate. R. Soc. Open Sci. 2019, 6, 181862. [Google Scholar] [CrossRef]
- Shiau, L.D.; Lu, T.S. A model for determination of the interfacial energy from the measured metastable zone width data by the polythermal method. J. Cryst. Growth 2014, 402, 267–272. [Google Scholar] [CrossRef]
- Shiau, L.D.; Lu, T.S. A model for determination of the interfacial energy from the induction time or metastable zone width data based on turbidity measurements. CrystEngComm. 2014, 16, 9743–9752. [Google Scholar] [CrossRef]
- Shiau, L.D. Comparison of the interfacial energy and pre-exponential factor calculated from the induction time and metastable zone width data based on classical nucleation theory. J. Cryst. Growth 2016, 450, 50–55. [Google Scholar] [CrossRef]
- Mersmann, A.; Bartosch, K. How to predict the metastable zone width. J. Cryst. Growth 1998, 183, 240–250. [Google Scholar] [CrossRef]
- Lindenberg, C.; Mazzotti, M. Effect of temperature on the nucleation kinetics of α L-glutamic acid. J. Cryst. Growth 2009, 311, 1178–1184. [Google Scholar] [CrossRef]
- Shiau, L.D. Determination of the nucleation and growth kinetics from aqueous L-glycine solutions from the turbidity induction time data. Crystals 2018, 8, 403. [Google Scholar] [CrossRef]
- Emanuel, M.; Alexander, A. Solubilities of L-glutamic acid, 3-nitrobenzoic acid, p-toluic acid, calcium-L-lactate, calcium gluconate, magnesium-DL-aspartate, and magnesium-L-lactate in water. J. Chem. Thermodyn. 2002, 34, 1127–1136. [Google Scholar]
- Shiau, L.D. The temperature dependence of the pre-exponential factor and interfacial energy for aqueous glycine solutions based on the metastable zone width data. J. Cryst. Growth 2018, 496–497, 18–23. [Google Scholar] [CrossRef]
- Park, K.; Evans, J.M.B.; Myerson, A.S. Determination of solubility of polymorphs using differential scanning calorimetry. Cryst. Growth Des. 2003, 3, 991–995. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, M.J.; Lee, J.M.; Kim, S.H.; Kim, H.S.; Park, B.S. Solubility, density, and metastable zone width of the 3-nito-1,2,4-triazol-5-one + water system. J. Chem. Eng. Data 1998, 43, 65–68. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).