Abstract
Bis(NHC) ligand precursors, L1, based on trans-1,2-diaminocyclohexane were designed and synthesized. To introduce chirality at the hydroxyamide side arm on the NHC of L1, a chiral β-amino alcohol, such as enantiopure leucinol, was used. Cu-catalyzed asymmetric conjugate addition reactions of cyclic and acyclic enones with Et2Zn were selected to evaluate the performance of L1 as a chiral ligand. For the reaction of cyclic enone, a combination of [bis(trimethylsilyl)acetylene]-(hexafluoroacetylacetonato)copper(I) (Cu(hfacac)(btmsa)) with a (±)-trans-1,2-cyclohexanediamine-based bis(NHC) ligand precursor, (rac; S,S)-L1, which was prepared from (S)-leucinol, was the most effective. Thus, treating 2-cyclohexen-1-one (3) with Et2Zn in the presence of catalytic amounts of Cu(hfacac)(btmsa) and (rac; S,S)-L1 afforded (R)-3-ethylcyclohexanone ((R)-4) with 97% ee. Similarly, use of (rac; R,R)-L1, which was prepared from (R)-leucinol, produced (S)-4 with 97% ee. Conversely, for the asymmetric 1,4-addition reaction of the acyclic enone, optically pure (−)-trans-1,2-cyclohexanediamine-based bis(NHC) ligand precursor, (R,R; S,S)-L1, worked efficiently. For example, 3-nonen-2-one (5) was reacted with Et2Zn using the CuOAc/(R,R; S,S)-L1 catalytic system to afford (R)-4-ethylnonan-2-one ((R)-6) with 90% ee. Furthermore, initially changing the counterion of the Cu precatalyst between an OAc and a ClO4 ligand on the metal reversed the facial selectivity of the approach of the substrates. Thus, the conjugate addition reaction of 5 with Et2Zn using the Cu(ClO4)2/(R,R; S,S)-L1 catalytic system, afforded (S)-6 with 75% ee.
1. Introduction
Synthesizing efficient chiral ligands for asymmetric catalysis is currently a major challenge in synthetic organic chemistry [,,,]. In recent years, N-heterocyclic carbenes (NHCs) have become recognized as versatile ligands [,,,,,,,,,,,,,,,,,,,,]. There have been several recent reports on chiral bis(NHC) ligands (Figure 1) [,,,,].
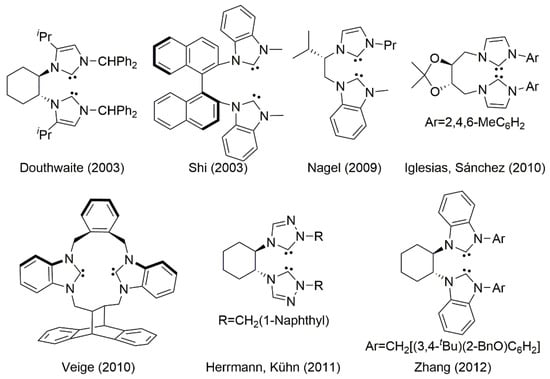
Figure 1.
Selected examples of chiral bis-N-heterocyclic carbenes bis(NHC)s.
Douthwaite et al. synthesized a chiral bis(NHC)-Pd(II) complex from optically pure trans-1,2-diaminocyclohexane [,]. In 2003, Shi et al. developed a bidentate axially chiral bis(NHC)-Rh(III) complex []. They succeeded in creating a large variety of asymmetric transformations by using this versatile chiral ligand [,,,,,,,,,,,,,]. Nagel et al. showed the enantioselective transfer hydrogenation of ketones catalyzed by Rh(I) and Ir(I) complexes derived from (S)-valinol [,]. Iglesias and Sánchez et al. reported the asymmetric hydrogenation of alkenes with bis(NHC) derived from tartaric acid [,]. A chiral palladium allyl complex bearing bis(NHC)/cyclophane ligand for 1,4-addition of phenylboronic acid to 2-cyclohexen-1-one was synthesized by Veige et al. [,].
To the best of our knowledge, there are two bis(NHC) ligands based on chiral trans-1,2-diaminocyclohexane for asymmetric catalytic transformations (Figure 1). Herrmann and Kühn et al. demonstrated the enantioselective hydrogenation of alkenes catalyzed by Rh(I) complexes derived from 1,2,4-triazole and [,]. Zhang et al. reported asymmetric catalytic Suzuki–Miyaura couplings employing a bis(NHC)-Pd(II) complex [,,,]. However, the appearance of an efficient asymmetric catalytic system using a chiral bis(NHC) ligand remains rare.
Introducing an appropriate hemilabile chiral donor group at the NHC side arm provides a chelating NHC-based ligand [,,,,,,,]. As part of our research program on developing chiral NHC ligand precursors, we have reported a Cu-catalyzed enantioselective conjugate addition reaction of enone with Et2Zn, using a chiral hydroxyamide-functionalized azolium ligand precursor [,,,,,,,,]. Much attention has been given to the 1,4-addition reaction [,,,]. We previously synthesized a new chiral azolium salt, (rac; S,S)-L1, which serves as a bis(NHC) ligand precursor []. The (rac; S,S)-L1 was prepared by using (±)-trans-1,2-diaminocyclohexane ((rac)-1) and (S)-leucinol ((S)-2) (Scheme 1). Thus, (rac; S,S)-L1 was obtained as a 1:1 diastereoisomeric mixture and used for the catalytic asymmetric reaction. Importantly, a highly enantioselective Cu-catalyzed conjugate addition reaction of cyclic enone with dialkylzinc in the presence of (rac; S,S)-L1 was successfully achieved [].
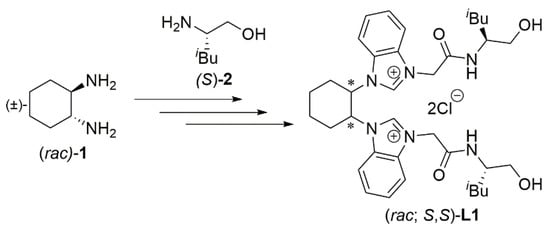
Scheme 1.
Previously reported bis(NHC) ligand precursor.
However, the 1,4-addition reaction employing the bis(NHC) ligand precursor derived from an optically active trans-1,2-diaminocyclohexane (1) remains unclarified. In this paper, we show the synthesis of the corresponding bis(NHC) ligand precursor L1 from a single enantiomer of trans-1,2-diaminocyclohexane ((‒)-1 or (+)-1). Systematic studies on the catalytic asymmetric 1,4-addition reaction using this promising class of enantiomerically pure bis(NHC) ligand are described.
2. Results and Discussion
2.1. Catalytic Asymmetric Conjugate Addition Reaction of Cyclic Enone
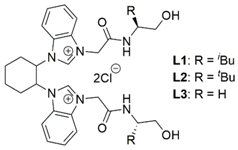
Enantiomerically pure L1 was synthesized from enantiopure trans-1,2-diaminocyclohexane ((R,R)-1 or (S,S)-1) []. To introduce chirality at the hydroxyamide side arm at L1, we chose (S)-leucinol ((S)-2) or (R)-leucinol ((R)-2) as a chiral source. In this study, we wanted to determine the influence of the chirality of the cyclohexanediamine skeleton of L1 on the catalytic asymmetric transformation. Thus, four sets of bis(NHC) azolium ligands were synthesized (Scheme 2).
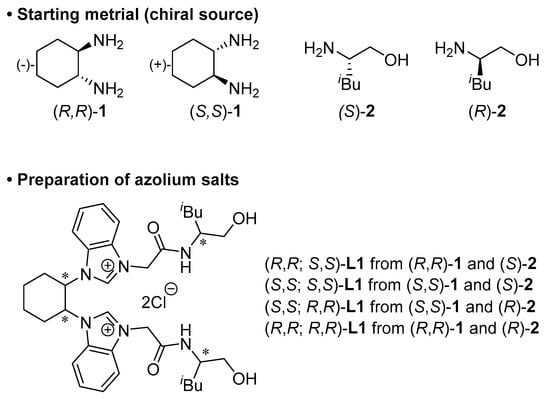
Scheme 2.
Preparation of a series of chiral ligands L1 used in this study.
The previously reported bis(NHC) azolium ligand (rac; S,S)-L1 consists of a 1:1 diastereoisomeric mixture of (R,R; S,S)-L1 and (S,S; S,S)-L1 (Scheme 1) []. With a set of diastereomerically and enantiomerically pure azolium salts in hand (Scheme 2), we performed an NMR study of these compounds (Figure 2, Figure 3 and Figure 4). Initially, temperature dependence was observed in the 1H NMR spectrum of the bis(NHC) azolium precursor (Figure 2). For example, 1H NMR signals of (R,R; R,R)-L1 at 25 °C were extremely broad, indicating that several conformers, slowly interconverting on the NMR timescale, were present at room temperature. Conversely, increasing the temperature (to 80 °C) provided sharp, well-defined signals in the NMR spectra (Figure 2). Thus, a fully assignable NMR spectrum was obtained.
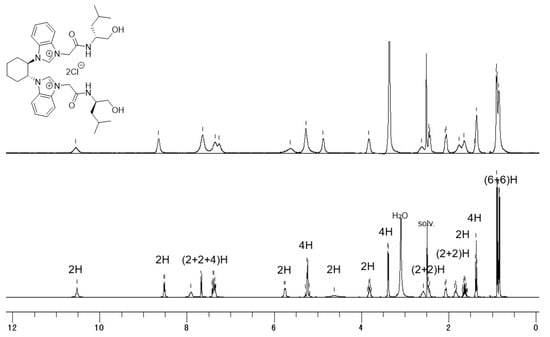
Figure 2.
1H NMR spectrum of (R,R; R,R)-L1 in (CD3)2SO at 25 °C (upper) and 80 °C (lower).
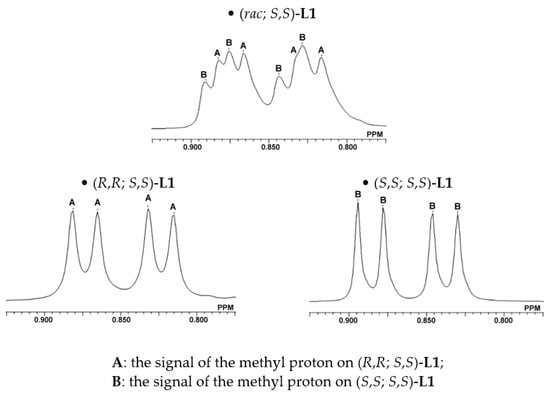
Figure 3.
1H NMR spectrum of the methyl proton on selected L1 (at 80 °C).
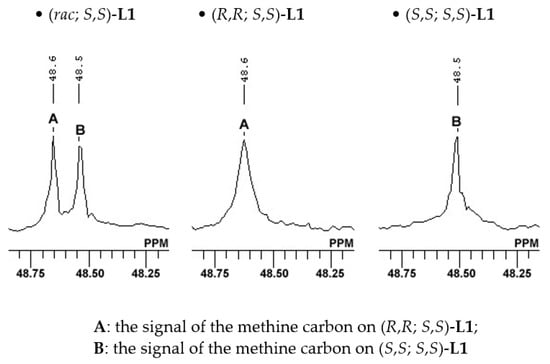
Figure 4.
13C NMR spectrum of the methine carbon adjacent to isobutyl substituent.
Furthermore, the methyl group signals of the isobutyl substituent in the 1H NMR spectra of a series of azolium salts L1 was considered. Figure 3 shows the expanded spectra of the methyl group regions of (rac; S,S)-L1, (R,R; S,S)-L1 and (S,S; S,S)-L1. Their C-H resonances appeared at δ = 0.87/0.82 ppm (J = 6.9 Hz) for (R,R; S,S)-L1 and δ = 0.88/0.83 ppm (J = 6.9 Hz) for (S,S; S,S)-L1. Thus, the methyl group signals in (rac; S,S)-L1 could be assigned, as shown in Figure 3.
Similarly, in the 13C NMR spectrum of (rac; S,S)-L1, two signals were observed in the methine carbon adjacent to the isobutyl substituent (Figure 4). This is because of the presence of a diastereoisomeric mixture. The 13C NMR spectra of the diastereoisomerically pure isomers revealed that the signal at δ 48.6 ppm could be attributed to the methine carbon on (R,R; S,S)-L1 and that at δ 48.5 ppm, it could be attributed to the methine carbon on (S,S; S,S)-L1.
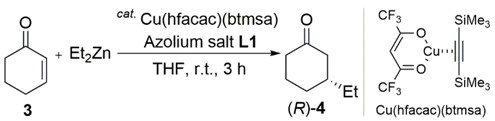
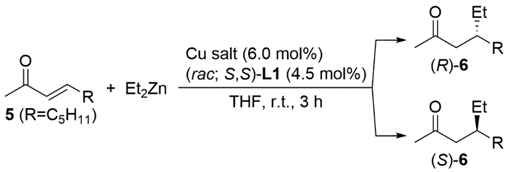
To test the performance of the chiral bis(NHC) ligand precursors L1, the conjugate addition reaction of 2-cyclohexen-1-one (3) with Et2Zn catalyzed by [bis(trimethylsilyl)acetylene](hexafluoro-acetylacetonato)copper(I) (Cu(hfacac)(btmsa)), combined with L1, was examined (Table 1).
In our previous publication, we briefly described a highly stereoselective transformation that was achieved using (rac; S,S)-L1 []. Thus, the reaction of 3 with Et2Zn in the presence of catalytic amounts of Cu(hfacac)(btmsa) and (rac; S,S)-L1 in THF at room temperature afforded (R)-3-ethylcyclohexanone ((R)-4) in 99% yield, with excellent enantioselectivity (97% ee) (Table 1, entry 2). We then investigated the 1,4-addition reaction using the diastereomerically and enantiomerically pure ligand. Notably, use of (R,R; S,S)-L1 in place of (rac; S,S)-L1 under these reaction conditions resulted in a lower yield (57%) of (R)-4 with poor enantioselectivity (16% ee) (entry 1). As with (R,R; S,S)-L1, a combination of Cu(hfacac)(btmsa) and (S,S; S,S)-L1 led to (R)-4 in 34% yield with 26% ee (entry 3).
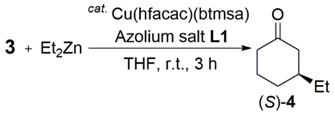
Next, we synthesized (rac; R,R)-L1, (S,S; R,R)-L1 and (R,R; R,R)-L1 (Scheme 2). Clearly, the Cu-catalyzed 1,4-addition reaction of 3 with Et2Zn in the presence of (rac; R,R)-L1 afforded (S)-4 with 97% ee (Table 1, entry 5). Conversely, it was difficult to obtain the desired 1,4-adduct, (S)-4, with high yield and enantioselectivity by using (S,S; R,R)-L1 or (R,R; R,R)-L1 (entries 4 and 6). These results strongly suggest that the chirality of the hydroxyamide side arm plays a critical role in the asymmetric 1,4-addition reaction of 3 with Et2Zn.
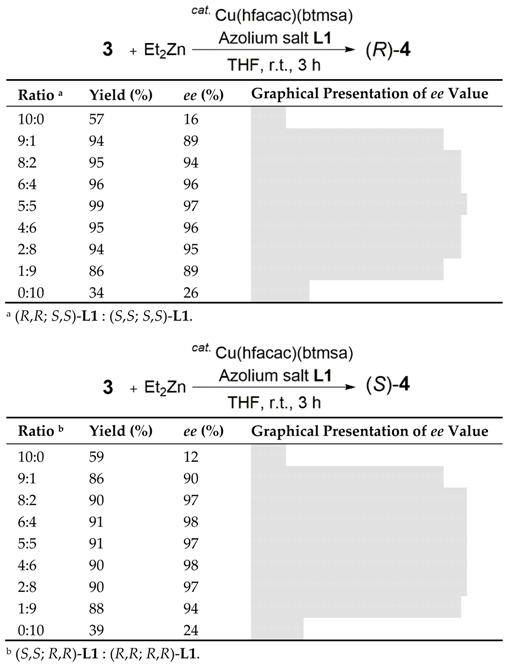
It is noteworthy that the asymmetric catalytic 1,4-addition reaction occurred efficiently when using a 1:1 mixture of (R,R; S,S)-L1 and (S,S; S,S)-L1 ((rac; R,R)-L1). To highlight the importance of both components in the catalytic reaction, we then varied the ratio of (R,R; S,S)-L1 and (S,S; S,S)-L1 (Table 2).

Table 2.
Influence of the ratio of chiral ligands L1 in the conjugate addition reaction of 3.
We carefully prepared various mixtures of (R,R; S,S)-L1 and (S,S; S,S)-L1 (with (R,R; S,S)-L1/(S,S; S,S)-L1 ratios of 10:0, 9:1, 8:2, 6:4, 5:5, 4:6, 2:8, 1:9 and 0:10). Notably, 3 was reacted with Et2Zn catalyzed by Cu(hfacac)(btmsa) in the presence of a 9:1 mixture to afford (R)-4 in 94% yield with 89% ee (Table 2). Use of (R,R; S,S)-L1 alone resulted in poor enantioselectivity (16% ee) of (R)-4. The best result (99% yield, 97% ee) was obtained at the 5:5 ratio. Furthermore, surprisingly, the yield and stereoselectivity of the 1,4-addition reaction using a 1:9 mixture were comparable to those obtained by using a 9:1 mixture. These results suggest that the presence of a small amount of each component of these two chiral ligands is enough to achieve an optimum asymmetric catalytic performance. This will be discussed later (vide infra; see Table 3).

Table 3.
Effect of the amount of chiral ligand and ligand structure on the conjugate addition reaction of 3 a.
The same experiment was conducted using (S,S; R,R)-L1 and (R,R; R,R)-L1 to provide (S)-4 in the Cu-catalyzed reaction of 3 with Et2Zn (Table 2). In a similar manner, a 9:1 or 1:9 mixture facilitated the highly enantioselective conjugate addition reaction to afford (S)-4 with 90% or 94% ee, respectively. These results confirm the importance of the presence of both stereoisomers of the cyclohexanediamine moiety in the chiral ligands for reaction efficiency and stereoselectivity.
The relationship between the catalyst ee and the product ee was also investigated (Figure 5). Various mixtures of (rac; S,S)-L1 and (rac; R,R)-L1 were carefully prepared. The enantioselective conjugate addition reaction of cyclic enone 3 with Et2Zn provided sufficient chiral amplification (Figure 5).
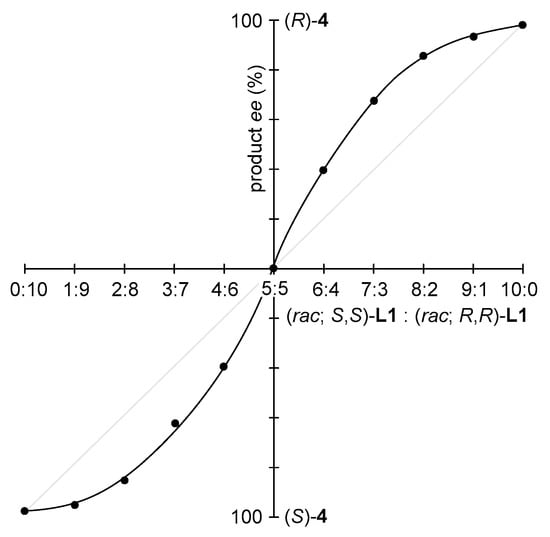
Figure 5.
Chiral amplification in the conjugate addition reaction of 3 using (rac; S,S)-L1 and (rac; R,R)-L1.
As shown in Table 2, we have clearly demonstrated that a 9:1 or 1:9 mixture of (R,R; S,S)-L1 and (S,S; S,S)-L1 promotes the enantioselective catalytic reaction. These reactions were performed in the presence of 6.0 mol % of Cu salt and 4.5 mol % of the chiral ligand mixtures. We assumed that an excess of (R,R; S,S)-L1 would not be needed in the catalytic reaction with a 9:1 mixture of (R,R; S,S)-L1 and (S,S; S,S)-L1 and that a small amount of a 1:1 mixture of these ligands, with respect to the Cu precatalyst, would be sufficient to facilitate the catalytic reaction. This assumption prompted us to study the asymmetric 1,4-addition reaction of 3 with Et2Zn catalyzed by Cu(hfacac)(btmsa), with a reduced amount of the chiral ligand mixtures (Table 3).
As expected, the 1,4-addition reactions with Cu/ligand ratios of 1:0.75, 1:0.3, 1:0.225, and 1:0.15 occurred in a similar manner to afford the corresponding adduct with 97%, 93%, 96%, and 97% ee, respectively (entries 1–4). These results indicate that the same catalytic active species were probably formed. It is also noteworthy that the loading of chiral ligand could be dramatically reduced.
No reaction was observed in the absence of L1. Additionally, two bis(NHC) ligands, L2 and L3, were synthesized to investigate the effect of the ligand structure on the catalytic performance. Changing the alkyl substituent at the chiral carbon center of the ligand from the isobutyl group ((rac; S,S)-L1) to the more sterically hindered tert-butyl group (L2) led to the conjugate adduct, (S)-4, in lower yield and enantioselectivity with the opposite configuration (Table 3, entry 6). This might be due to the highly hindered tert-butyl group blocking the approach of the reagents. Furthermore, L3 was synthesized from 1-aminoethanol. However, it was difficult to react 3 with Et2Zn in the presence of Cu(hfacac)(btmsa) in combination with L3 (entry 7).
The catalytic performance of the Cu(hfacac)(btmsa)/(rac; R,R)-L1 system in the asymmetric 1,4-addition reaction of various cyclic enones was investigated, as described in our previous publication [].
2.2. Catalytic Asymmetric Conjugate Addition Reaction of Acyclic Enone
In contrast to the conjugate addition reactions of organometallic compounds with cyclic enones, few highly enantioselective reactions of acyclic enones have been reported [,,,,,,,,,,,,]. We then focused on studying the Cu-catalyzed asymmetric 1,4-addition reaction of acyclic enone with Et2Zn using this promising class of bis(NHC) chiral ligand L1.
In our previous publication, we briefly reported that 3-nonen-2-one (5) was reacted with Et2Zn catalyzed by Cu(hfacac)(btmsa) and (rac; S,S)-L1 at room temperature to afford (R)-4-ethylnonan-2-one ((R)-6) in 90% yield with moderate stereoselectivity (49% ee) (Table 4, entry 1) []. Replacing Cu(hfacac)(tmsa) with cyclooctadiene(hexafluoro-2,4-pentanedionato)copper(I) (Cu(hfacac)(cod)) improved the stereoselectivity (61% ee) of the 1,4-addition reaction (entry 2).

Table 4.
Switching of stereoselectivity in the conjugate addition reaction of acyclic enone 5 a.
Encouraged by this success, we continued to test various Cu precatalysts for further reaction optimization (Table 4). Although the reaction was catalyzed by bis(hexafluoroacetylacetonato)-copper(II) (Cu(hfacac)2) and (rac; S,S)-L1 to afford (R)-6 with moderate enantioselectivity (53% ee), CuOCOCF3 did not work efficiently (entries 3 and 4). The promising result was achieved when the reaction was performed with CuOAc. Thus, the combination of CuOAc and (rac; S,S)-L1 catalyzed the reaction to furnish the corresponding adduct, (R)-6, in 78% yield and with 60% ee (entry 5). Despite achieving the same enantioselectivity (63% ee), Cu(OAc)2 decreased the product yield (entry 6).
Interestingly, during this study, we found that simply changing the copper catalyst precursor with the same ligand reversed the stereochemistry. The reaction of 5 with Et2Zn using the CuCl2/(rac; S,S)-L1 catalytic system afforded (R)-6 in 50% yield and with 39% ee (entry 7). In contrast, Cu(OTf)2 in place of CuCl2 led to the 1,4-adduct, (S)-6, with the opposite configuration with 33% ee (entry 8). Developing a synthetic methodology for switching enantioselectivity is an essential and challenging research topic. Recently, many papers have been published to provide a comprehensive overview of the importance of stereodivergent catalytic transformations [,,,,,,].
Based on this finding, we decided to evaluate several Cu salts. CuOTf·0.5C6H6 and Cu(NO3)2 resulted in racemic 6 (Table 4, entries 9 and 10). Cu(ClO4)2 led to a marked increase in the enantioselectivity of the 1,4-addition reaction to provide (S)-6 as a major product (entry 11). Previously, we showed that the reversal of enantioselectivity was achieved in the Cu-catalyzed conjugate addition reaction of cyclic enone using a mono-NHC azolium ligand L4 with a chiral hydroxyamide side-arm (Figure 6) []. For example, 3 was reacted with Et2Zn catalyzed by Cu(OTf)2/L4 to afford (S)-4, whereas (R)-4 was obtained in the same reaction with the Cu(acac)2/L4 catalytic system []. However, no reversal of enantioselectivity was observed in the Cu-catalyzed 1,4-addition reaction of acyclic enone when changing the Cu precatalyst in the presence of L4 []. Therefore, to the best of our knowledge, this is the first example of switchable enantioselectivity in a catalytic conjugate addition reaction of acyclic enone, with the same chiral ligand.
Figure 6.
Previously reported chiral ligand L4.
The combination of transition metal complex and chiral ligand can provide a chiral environment for the metal, where high enantioselectivity requires only small differences in transition state energies in the catalytic reaction. The transition metal complex consists of a cationic metal and an achiral counter anion. While the chiral ligand design is critical to achieving a highly stereoselective catalytic reaction, the choice of the achiral counter anion of the transition metal complex is also an important factor. In the literature, it appears that changing the achiral counter anion of the metal catalyst precursor has sometimes switched the stereochemistry of the catalytic reaction [,,,,,,,].
Table 5 summarizes the switching of enantioselectivity in the asymmetric 1,4-addition reaction of 5 with Et2Zn catalyzed by CuOAc or Cu(ClO4)2 using the chiral bis(NHC) ligand precursor, (rac; S,S)-L1, under selected reaction conditions. To optimize the reaction conditions, various reaction parameters, including the ratio of Cu salt/chiral ligand, solvents and reaction temperatures were screened with CuOAc (entries 1–8) or Cu(ClO4)2 (entries 9–16). Further evaluations of the reaction parameters with both catalytic systems are presented in Tables S1 and S2, respectively (see Supplementary Materials).

Table 5.
Switching of stereoselectivity in the conjugate addition reaction of 5 under selected conditions a.
In the CuOAc/(rac; S,S)-L1 catalytic system, a 1:1 ratio of Cu/ligand was most effective (entries 1–3). We thus investigated the solvent effect on the reaction in the presence of 4 mol% of a 1:1 mixture of CuOAc and (rac; S,S)-L1 (entries 3–5; also see Table S1). Although slightly lower enantioselectivity was observed in the catalytic reaction with Et2O, a promising result was achieved with 1,2-dimethoxyethane (DME) (entries 4 and 5). Furthermore, a decrease in the reaction temperature to 0 °C improved the enantioselectivity (73–78% ee), even though a longer reaction time was needed (entries 6 and 7). The desired product (R)-6 was obtained in 69% yield with 75% ee when the reaction was performed in DME at −10 °C for 48 h (entry 8). The facial selectivities of the 1,4-addition reaction using the Cu(ClO4)2/(rac; S,S)-L1 catalytic system were reversed, compared to those of the CuOAc-catalyzed reaction (Table 5, entries 9–17). Compound 5 was reacted with Et2Zn catalyzed by 6 mol% of Cu(ClO4)2 and 4.5 mol% of (rac; S,S)-L1 in THF at room temperature to afford (S)-6 in 31% yield with 50% ee (entry 9). Changing the Cu/ligand ratio from 6/4.5 to 4.5/6 decreased the ee value (entry 10). Therefore, the reaction was performed in the presence of a catalytic amount of Cu(ClO4)2/(rac; S,S)-L1 (6/3 mol%) to explore the effect of solvent (entries 11–13). Among the solvents examined, THF gave moderate enantioselectivity, providing (S)-6 with 46% ee (entry 11). Fortunately, performing the catalytic reaction at a lower temperature remarkably increased the stereoselectivity (entries 14–16). Thus, treatment of 5 with Et2Zn in the presence of Cu(ClO4)2 and (rac; S,S)-L1 in THF at 0 °C for 24 h produced (S)-6 in 89% yield with 70% ee (entry 14). Almost the same result was observed for the catalytic 1,4-addition reaction in DME at 0 °C for 24 h (entry 17).
In the Cu-catalyzed conjugate addition reaction of acyclic enone using the bis(NHC) ligand, we described successful enantioselectivity reversal by simply changing of the counter anion of the Cu salt. We then assumed that combining copper chloride with a silver salt facilitates the catalytic reaction and that a silver salt additive would control the setereoselectivity of the 1,4-addition reaction (Table 6).

Table 6.
Switching of stereoselectivity in the CuCln-catalyzed conjugate addition reaction of 5 in the presence of an additive.
First, we tested the reaction of 5 with Et2Zn catalyzed by CuCl (4 mol%) in the presence of (rac; S,S)-L1 (4 mol%). This reaction afforded (R)-6 in lower enantioselectivity, compared with the reaction using the CuOAc/(rac; S,S)-L1 catalytic system (entries 1 and 2). As expected, adding AgOAc (4 mol%) to the CuCl-catalyzed reaction led to a remarkable increase in the stereoselectivity (86% ee) (entry 3). This result was comparable to that of the CuOAc-catalyzed reaction, meaning that the acetate anion would play an important role through interaction with a copper ion having the (rac; S,S)-L1 ligand. Based on this assumption, an excess of AgOAc additive, with respect to the CuCl catalyst, was employed. The catalytic 1,4-addition reaction with a 1:1.5 molar ratio of CuCl/AgOAc provided (R)-6 in 54% yield with 85% ee, whereas a greater excess (8 mol%) of AgOAc decreased its catalytic activity (entries 4 and 5).
Next, to test the influence of AgClO4, the reaction of 5 with Et2Zn catalyzed by CuCl2 (6 mol%) in the presence of (rac; S,S)-L1 (3 mol%) was performed as a control experiment. As with the CuCl-catalyzed reaction, the combination of CuCl2 with (rac; S,S)-L1 produced (R)-6 (Table 6, entry 2 vs. entry 7). Interestingly, adding AgClO4 to this catalytic system dramatically affected the facial selectivity of the approach of the substrates to the Cu species. Thus, the reaction of 5 with Et2Zn in the presence of catalytic amounts of CuCl2 (6 mol%), (rac; S,S)-L1 (3 mol%), and AgClO4 (12 mol%) afforded (S)-6 in 93% yield with 68% ee (entry 8). This result was comparable to that of the Cu(ClO4)2-catalyzed reaction, as expected (entry 6 vs. entry 8). Using excess AgClO4 (15 mol%) did not drastically affect the outcome (entry 9). The reversal of enantioselectivity was also achieved in the CuCl2-catalyzed conjugate reaction, using half the amount of AgClO4 (6 mol%) (entry 10).
To evaluate the substrate scope and limitations of the developed catalytic reactions, several α,β-unsaturated enones were investigated (Table 7). The conjugate addition reaction of 5-methyl-3-hexen-2-one (7) with Et2Zn in the presence of catalytic amounts of CuOAc and (rac; S,S)-L1 yielded (S)-4-ethyl-5-methylhexan-2-one ((S)-8) with 69% ee (entry 3). In this reaction, a somewhat lower yield was probably due to steric hindrance in the substrate. In contrast, the 1,4-addition reaction of 7 catalyzed by Cu(ClO4)2 gave reversed enantioselectivity, affording (R)-8 in 44–59% yield with 80–82% ee (entries 4 and 5). Additionally, when 7 was allowed to react with Et2Zn using the combined catalytic system comprising Cu(ClO4)2 and the bis(NHC) azolium ligand having a tert-butyl group, (rac; S,S)-L2, (R)-8 was obtained, with satisfactory enantioselectivity (75% ee) (entry 6).

Table 7.
Exploring substrate scope.
Switching of stereoselectivity was also observed in the 1,4-addition reaction of benzalacetone (9). Treatment of 9 with Et2Zn in the presence of catalytic amounts of CuOAc and (rac; S,S)-L1 afforded (S)-4-phenylhexan-2-one ((S)-10) in 55% yield and 63% ee (entry 7), whereas (R)-10 was obtained using Cu(ClO4)2 catalyst combined with the same ligand (entry 8). The results of the conjugate addition reaction of chalcone (11) differ from those for the reaction of 9. Although the CuOAc/(rac; S,S)-L1 system catalyzed the reaction of 11 with Et2Zn to produce (S)-1,3-diphenylpentan-1-one ((S)-12) with 55% ee (entry 9), the Cu(ClO4)2/(rac; S,S)-L1 catalytic system gave poor yield and stereoselectivity of (R)-12 (entry 10).
The performance of both catalytic systems for in the Cu-catalyzed conjugate addition of cyclic enone were evaluated (Table 7, entries 11 and 12). As mentioned above, we previously reported successful enantioselectivity reversal of the Cu-catalyzed 1,4-addition reaction of 3 with Et2Zn using the mono-NHC azolium ligand, L4 (Figure 6), by changing the Cu precatalyst from Cu(OTf)2 to Cu(acac)2 []. The bis(NHC) azolium ligand, (rac; S,S)-L1, was suitable for achieving the enantioselectivity switch in the 1,4-addition reaction of both acyclic and cyclic enones. The combination of CuOAc and (rac; S,S)-L1 catalyzed the reaction of 3 with Et2Zn to afford the corresponding adduct (R)-4 in 38% yield with 57% ee (entry 11). The facial selectivity of the 1,4-addition reaction using the Cu(ClO4)2/(rac; S,S)-L1 catalytic system was reversed compared to that of the reaction using CuOAc (entry 12).
As shown in Table 2, a mixture of (R,R; S,S)-L1 and (S,S; S,S)-L1 promoted the Cu-catalyzed conjugate addition reaction of cyclic enone with Et2Zn, and using (R,R; S,S)-L1 or (S,S; S,S)-L1 alone led to a poor stereoselectivity. Based on this finding, we next investigated the catalytic reaction of acyclic enone using a set of diastereomerically and enantiomerically pure azolium salts, (R,R; S,S)-L1 and (S,S; S,S)-L1 (Table 8).

Table 8.
Effect of chiral azolium ligand L1 in the conjugate addition reaction of 5.
Compound 5 was treated with Et2Zn in the presence of catalytic amounts of CuOAc and (R,R; S,S)-L1 in DME at 0 °C for 24 h to afford (R)-6 in good yield and stereoselectivity (79% yield, 88% ee, entry 1). This is in contrast to the catalytic reaction of cyclic enone, where the Cu/(R,R; S,S)-L1 catalytic system was inert (see Table 2). It is noteworthy that (R,R; S,S)-L1 showed a superior catalytic performance compared a diastereomeric mixture of ligands, such as (rac; S,S)-L1, in the 1,4-addition reaction of acyclic enone (entry 1 vs. entry 2). In contrast to the catalytic conjugate addition reaction of 5 using (R,R; S,S)-L1, the reaction using (S,S; S,S)-L1 proceeded, with difficulty, to produce the desired (R)-6 (17% yield and 48% ee, entry 3). These results suggest that (S,S; S,S)-L1 acts as a catalyst poison in the catalytic reaction with (rac; S,S)-L1. Additionally, an excellent ee value of 91% of (R)-6 was obtained in the conjugate addition reaction of 5 with Et2Zn using 4 mol% of CuOAc and 3 mol% of (R,R; S,S)-L1 (entry 4).
Next, in the switchable enantioselective reaction using Cu(ClO4)2 precatalyst to provide (S)-6, the performances of (R,R; S,S)-L1 and (S,S; S,S)-L1 were examined (Table 8, entries 5–7). With 6 mol% of Cu(ClO4)2, 3 mol% of chiral ligand containing (R,R; S,S)-L1 and/or (S,S; S,S)-L1 was employed in the conjugate addition reaction. These experiments showed the formation of the desired (S)-6 with almost the same yields and enantioselectivities (66–75% ee) in each of the catalytic reactions (entries 5–7). These results differed from those of the CuOAc/L1 catalytic system (entries 1–3). It could be assumed that the chiral hydroxyamide side arm of the bis(NHC) ligand would play an important role in the Cu(ClO4)2-catalyzed asymmetric conjugate addition reaction.
Finally, we highlighted the greater reactivity of the 1,2-diaminocyclohexane-based bis(NHC) ligand L1 compared with those of chiral ligands based on other bis(NHC) skeletons. We synthesized three chiral ligands L5, L6 and L7 from 1,2-diaminoethane, 1,3-diaminobenzene and 1,2-benzenedimethanamine, respectively (Table 9).

Table 9.
Effect of chiral ligand structure on switching of stereoselectivity in the conjugate addition reaction of 5.
The reaction of 5 with Et2Zn catalyzed by CuOAc in the presence of 1,2-diaminoethane-based bis(NHC) ligand L5 provided the corresponding 1,4-adduct, (R)-6, in poor yield and stereoselectivity (entry 1). When the same reaction was conducted using the Cu(ClO4)2/L5 catalytic system, the enantioselectivity of the reaction was reversed, affording (S)-6 in 73% yield and 58% ee (entry 2). However, no reversal of enantioselectivity was observed in the catalytic reactions using 1,3-diaminobenzene-based chiral ligand L6 (entries 3 and 4). Additionally, treatment of 5 with Et2Zn catalyzed by CuOAc combined with 1,2-benzenedimethanamine-based ligand L7 gave an almost racemic mixture of conjugate adduct 6 (entry 5). Conversely, the Cu(ClO4)2/L7 catalytic system offered an efficient enantioselective protocol for the 1,4-addition reaction of 5 with Et2Zn to afford (S)-6 in 87% yield and 69% ee (entry 6). Based on the screening test using a series of bis(NHC) ligands, the 1,2-diaminocyclohexane-derived skeleton is critically important for successful enantioselectivity switching.
3. Experimental
3.1. General Procedures
All chemicals were obtained from commercial sources and were used as received. 1H and 13C NMR spectra were recorded on spectrometers at 400 and 100 MHz, respectively. Chemical shifts were reported in ppm relative to TMS for 1H and 13C NMR spectra. CDCl3 and (CD3)2SO were used as the NMR solvent. Thin-layer chromatography (TLC) analysis was performed with glass-backed plates, pre-coated with silica gel and examined under UV (254 nm) irradiation. Flash column chromatography was executed on silica gel 60 (230–400; particle size: 0.040–0.063 nm).
3.2. Procedure for Preparation of Azolium Salt L1
Azoles were synthesized from trans-1,2-cyclohexanediamine (1) according to the literature procedure [,]. The reaction mixture of azole (485 mg, 1.5 mmol) and α-chloroacetamide (580 mg, 3.0 mmol), derived from chloroacetyl chloride and luecinol, was heated to 110 °C and stirred for 2 days. After the reaction, the solvent was removed under reduced pressure. The desired product was isolated from the crude residue by column chromatography (SiO2, CH2Cl2/CH3OH = 8/2) to yield the light yellow solution after removing the solvent. The residue was dissolved in methanol, and then activated carbon (ca. 1 g) was added. After 3 h, the activated carbon was removed by filtration. After removing the CH3OH in vacuo from the filtrate, the azolium salt L1 was purified by reprecipitation using CH3OH and CH3CO2C2H5 affording white solid (yield: 710 mg, 62%). Compounds (rac; S,S)-L1, (rac; R,R)-L1, L5 and L6 were reported in our previous publication [].
(R,R; S,S)-L1. White solid. 1H-NMR ((CD3)2SO, 400 MHz): δ 10.66 (s, 2H, NCHN), 8.60 (d, J = 8.2 Hz, 2H, NCCH), 7.94 (br, 2H, NH), 7.69 (d, J = 7.8 Hz, 2H, NCCH), 7.39–7.33 (m, 4H, NCCHCH), 5.81–5.79 (m, 2H, NCH), 5.31 (d, J = 16.0 Hz, 2H, NCH2CO), 5.23 (d, J = 16.0 Hz, 2H, NCH2CO), 4.69 (br, 2H, OH), 3.86–3.78 (m, 2H, NHCH)), 3.41 (br, 4H, CH2OH), 2.61 (br, 2H), 2.46–2.43 (m, 2H), 2.07–2.05 (m, 2H), 1.87–1.85 (m, 2H), 1.67–1.57 (m, 2H, CHiBu), 1.37 (t, J = 7.1 Hz, 4H, CH2iBu), 0.87 (d, J = 6.9 Hz, 6H, CH3iBu), 0.82 (d, J = 6.9 Hz, 6H, CH3iBu); 13C-NMR (100 MHz): δ 163.1 (CO), 142.5 (NCHN), 130.2, 129.9, 126.3, 126.2, 112.7 (NC), 112.4 (NC), 63.1 (CH2OH), 60.0 (CH2CO), 49.8 (NCH), 48.6 (NHCH), 39.5, 30.7, 23.9 (CH2iBu), 23.5 (CHiBu), 22.6 (CH3iBu), 21.6 (CH3iBu).
(S,S; S,S)-L1. White solid. 1H-NMR ((CD3)2SO, 400 MHz): δ 10.65 (s, 2H, NCHN), 8.63 (d, J = 8.7 Hz, 2H, NCCH), 8.00 (br, 2H, NH), 7.69 (d, J = 7.8 Hz, 2H, NCCH), 7.41–7.34 (m, 4H, NCCHCH), 5.84–5.82 (m, 2H, NCH), 5.30 (d, J = 16.0 Hz, 2H, NCH2CO), 5.25 (d, J = 16.0 Hz, 2H, NCH2CO), 4.68 (br, 2H, OH), 3.85–3.77 (m, 2H, NHCH), 3.39 (br, 4H, CH2OH), 2.61 (br, 2H), 2.46–2.43 (m, 2H), 2.06–2.04 (m, 2H), 1.88–1.86 (m, 2H), 1.70–1.56 (m, 2H, CHiBu), 1.37 (t, J = 7.3 Hz, 4H, CH2iBu), 0.88 (d, J = 6.9 Hz, 6H, CH3iBu), 0.83 (d, J = 6.9 Hz, 6H, CH3iBu); 13C-NMR (100 MHz): δ 163.1 (CO), 142.5 (NCHN), 130.3, 129.8, 126.3, 112.7 (NC), 112.5 (NC), 63.2 (CH2OH), 60.1 (CH2CO), 49.8 (NCH), 48.5 (NHCH), 39.6, 30.7, 24.0 (CH2iBu), 23.5 (CHiBu), 22.6 (CH3iBu), 21.6 (CH3iBu).
(S,S; R,R)-L1. White solid. 1H-NMR ((CD3)2SO, 400 MHz): δ 10.65 (s, 2H, NCHN), 8.59 (d, J = 8.5 Hz, 2H, NCCH), 7.93 (br, 2H, NH), 7.69 (d, J = 7.6 Hz, 2H, NCCH), 7.39–7.32 (m, 4H, NCCHCH), 5.81–5.79 (m, 2H, NCH), 5.30 (d, J = 15.7 Hz, 2H, NCH2CO), 5.22 (d, J = 15.7 Hz, 2H, NCH2CO), 4.68 (br, 2H, OH), 3.86–3.78 (m, 2H, NHCH), 3.42–3.39 (m, 4H, CH2OH), 2.61 (br, 2H), 2.46–2.43 (m, 2H), 2.07–2.05 (m, 2H), 1.87–1.85 (m, 2H), 1.67–1.56 (m, 2H, CHiBu), 1.37 (t, J = 7.0 Hz, 4H, CH2iBu), 0.87 (d, J = 6.7 Hz, 6H, CH3iBu), 0.82 (d, J = 6.7 Hz, 6H, CH3iBu); 13C-NMR (100 MHz): δ 163.1 (CO), 142.5 (NCHN), 130.2, 129.9, 126.3, 126.2, 112.7 (NC), 112.3 (NC), 63.1 (CH2OH), 60.1 (CH2CO), 49.8 (NCH), 48.6 (NHCH), 39.5, 30.8, 23.9 (CH2iBu), 23.5 (CHiBu), 22.6 (CH3iBu), 21.6 (CH3iBu).
(R,R; R,R)-L1. White solid. 1H-NMR ((CD3)2SO, 400 MHz): δ 10.53 (s, 2H, NCHN), 8.52 (d, J = 8.5 Hz, 2H, NCCH), 7.91 (br, 2H, NH), 7.68 (d, J = 8.1 Hz, 2H, NCCH), 7.43–7.34 (m, 4H, NCCHCH), 5.77–5.75 (m, 2H, NCH), 5.26 (d, J = 16.0 Hz, 2H, NCH2CO), 5.22 (d, J = 16.0 Hz, 2H, NCH2CO), 4.63 (br, 2H, OH), 3.86–3.78 (m, 2H, NHCH), 3.43–3.36 (m, 4H, CH2OH), 2.58 (br, 2H), 2.47–2.44 (m, 2H), 2.09–2.06 (m, 2H), 1.86–1.82 (m, 2H), 1.69–1.57 (m, 2H, CHiBu), 1.38 (t, J = 7.0 Hz, 4H, CH2iBu), 0.89 (d, J = 6.7 Hz, 6H, CH3iBu), 0.85 (d, J = 6.7 Hz, 6H); 13C-NMR (DMSO, 100 MHz): δ 163.1 (CO), 142.5 (NCHN), 130.3, 129.8, 126.3, 112.7 (NC), 112.5 (NC), 63.1 (CH2OH), 60.1 (CH2CO), 49.8 (NCH), 48.5 (NHCH), 39.6, 30.7, 24.0 (CH2iBu), 23.5 (CHiBu), 22.6 (CH3iBu), 21.6 (CH3iBu).
L2. White solid. 1H-NMR ((CD3)2SO, 400 MHz): δ 10.69 (br, 2H, NCHN), 8.48 (br, 2H, NCCH), 7.96 (br, 2H, NH), 7.72 (br, 2H, NCCH), 7.35 (br, 4H, NCCHCH), 5.80 (br, 2H, NCH), 5.43–5.31 (m, 4H, NCH2CO), 4.55 (br, 2H, OH), 3.65–3.60 (m, 4H, CH2OH), 3.48–3.45 (m, 2H, NHCH), 2.61 (br, 2H), 2.50–2.44 (m, 2H), 2.02 (br, 2H), 1.85 (br, 2H), 0.88 (m, 18H, CH3tBu). Because of the presence of the diastereoisomeric mixture, so many broad signals appeared in 1H-NMR, that the failure of 13C-NMR measurement in (CD3)2SO was observed. Elemental analysis: Calculated for C36H52Cl2N6O4·3H2O: C, 57.06; H, 7.72; N, 11.09. Found: C, 56.74; H, 7.41; N, 10.86%. M.p. 204-216 °C (decompose).
L3. White solid. 1H-NMR ((CD3)2SO, 400 MHz): δ 10.65 (s, 2H, NCHN), 8.86 (br, 2H, NCCH), 8.00 (br, 2H, NH), 7.70 (d, J = 7.7 Hz, 2H, NCCH), 7.40–7.30 (m, 4H, NCCHCH), 5.83 (br, 2H, NCH), 5.30 (d, J = 16.3 Hz, 2H, NCH2CO), 5.24 (d, J = 16.3 Hz, 2H, NCH2CO), 4.77 (br, 2H, OH), 3.49–3.47 (m, 4H, CH2OH), 3.22–3.19 (m, 4H, NH(CH2)2OH), 2.61 (br, 2H), 2.44–2.41 (m, 2H), 2.03–2.01 (m, 2H), 1.85 (br, 2H). Failure of 13C-NMR measurement in (CD3)2SO was observed. Elemental analysis: Calculated for C28H36Cl2N6O4·3H2O: C, 52.09; H, 6.56; N, 13.02. Found: C, 51.67; H, 6.36; N, 12.88%. M.p. 86.4–89.5 °C.
L7. White solid. 1H-NMR ((CD3)2SO, 400 MHz) δ 9.94 (s, 2H, NCHN), 8.71 (d, J = 8.5 Hz, 2H), 8.02–7.97 (m, 4H, NH), 7.71–7.64 (m, 4H), 7.41–7.38 (m, 2H), 7.16–7.14 (m, 2H), 6.17 (s, 4H, NCH2C), 5.42 (d, J = 16.4 Hz, 2H, NCH2CO), 5.35 (d, J = 16.4 Hz, 2H, NCH2CO), 4.88 (br, 2H, OH), 3.83–3.77 (m, 2H, NHCH), 3.40–3.32 (br, 4H, CH2OH), 1.64–1.57 (m, 2H, CHiBu), 1.36–1.30 (m, 4H, CH2iBu), 0.86 (d, J = 6.4 Hz, 6H, CH3iBu), 0.79 (d, J = 6.4 Hz, 6H, CH3iBu); 13C-NMR (100 MHz) δ 164.0 (CO), 143.9 (NCHN), 132.2, 131.6, 130.8, 129.3, 128.4, 126.9, 126.8, 114.1 (NC), 113.7 (NC), 63.5 (CH2OH), 49.7, 48.8, 47.5, 39.5 (CH2iBu), 24.2 (CHiBu), 23.3 (CH3iBu), 21.8 (CH3iBu). Elemental analysis: Calculated for C38H50Cl2N6O4·1.5H2O: C, 59.91; H, 7.15; N, 11.03. Found: C, 60.13; H, 6.96; N, 10.95%. M.p. 165–174 °C (decompose).
3.3. General Procedure for Cu-Catalyzed Asymmetric Reaction of Enone with Et2Zn
Cu salt and azolium salt were added to anhydrous THF. After stirring at room temperature for 1 h, the mixture was cooled to 0 °C. Then, Et2Zn (3 mmol, 1 M in hexanes, 3 mL) was added to the reaction vessel. After the resulting mixture was stirred at room temperature for 30 min, a solution of enone (1 mmol) in anhydrous THF was added dropwise over a period of 10 min. After stirring at room temperature for 3 h, the reaction was quenched with 10% HCl aq. The product yield was determined by GC using internal standard technique. The enantiomeric excess was measured by the chiral GC (see Supplementary Materials). The conjugate adduct was isolated as follows: After quenching the reaction mixture with 10% HCl aq., the resulting mixture was extracted with diisopropyl ether (3 × 10 mL) and dried over Na2SO4. The product was purified by silica gel column chromatography (hexane/Et2O).
4. Conclusions
We demonstrated that a chiral bis(NHC) ligand precursor L1, which is a dibenzimidazolium salt having a hydroxyamide side arm, efficiently performs Cu-catalyzed asymmetric conjugate addition of Et2Zn to cyclic or acyclic enones. In the catalytic 1,4-addition reaction of cyclic enone 3 with Et2Zn, an excellent enantioselectivity (up to 97% ee) was achieved with the Cu(hfacac)(btmsa)/(rac; S,S)-L1 catalytic system. Further investigations revealed that a 9:1 or 1:9 mixture of (R,R; S,S)-L1 and (S,S; S,S)-L1 promoted the highly enantioselective catalytic reaction. A potential application of the Cu/L1 catalytic system was investigated in the enantioselective 1,4-addition reaction of acyclic enones with Et2Zn. Interestingly, the stereoselectivity switching was observed in the Cu-catalyzed 1,4-addition reaction of 5 with Et2Zn in the presence of L1 when changing the counter anion on the Cu precatalyst.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/9/780/s1: NMR charts; Selected chiral GC and LC traces in the catalytic reactions; Table S1: CuOAc-catalyzed reaction of 5 with E2Zn yielding (R)-6; Table S2: Cu(ClO4)2-catalyzed reaction of 5 with E2Zn yielding (S)-6.
Author Contributions
Conceptualization, Methodology and Writing—original draft preparation, S.S.; Investigation—experiments and analyses, A.I., S.K., and Y.I.; Writing—review and editing, S.S.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, G.; Zhang, W. Renaissance of pyridine-oxazolines as chiral ligands for asymmetric catalysis. Chem. Soc. Rev. 2018, 47, 1783–1810. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Müller, D.; Schlepphorst, C.; Glorius, F. Privileged chiral N-heterocyclic carbene ligands for asymmetric transition-metal catalysis. Chem. Soc. Rev. 2017, 46, 4845–4854. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-L. Privileged Chiral Ligands and Catalysts; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Yoon, T.P.; Jacobsen, E.N. Privileged Chiral Catalysis. Science 2003, 299, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.-H.; Crudden, C.M. N-Heterocyclic Carbenes in Materials Chemistry. Chem. Rev. 2019, 119, 4986–5056. [Google Scholar] [CrossRef] [PubMed]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, L.; Sun, P.; Shi, L.; Yue, C.; Li, F. Reusable N-Heterocyclic Carbene Complex Catalysts and Beyond: A Perspective on Recycling Strategies. Chem. Rev. 2018, 118, 9843–9929. [Google Scholar] [CrossRef] [PubMed]
- Díez-González, S. N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, 2nd ed.; RSC-Publishing: Cambridge, UK, 2017. [Google Scholar]
- Lazreg, F.; Nahra, F.; Cazin, C.S.J. Copper-NHC complexes in catalysis. Coord. Chem. Rev. 2015, 293–294, 48–79. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Schaper, L.A.; Hock, S.J.; Herrmann, W.A.; Kühn, F.E. Synthesis and Application of Water-Soluble NHC Transition-Metal Complexes. Angew. Chem. Int. Ed. 2013, 52, 270–289. [Google Scholar] [CrossRef]
- Hamadd, F.B.; Sun, T.; Xiao, S.; Verpoor, F. Olefin metathesis ruthenium catalysts bearing unsymmetrical heterocylic carbenes. Coord. Chem. Rev. 2013, 257, 2274–2292. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.-J.; Wang, W.; Li, S.; Shi, M. Chiral NHC-metal-based asymmetric catalysis. Coord. Chem. Rev. 2012, 256, 804–853. [Google Scholar] [CrossRef]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; César, V. Synthetic Routes to N-Heterocyclic Carbene Precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef] [PubMed]
- Melaimi, M.; Soleilhavoup, M.; Bertrand, G. Stable Cyclic Carbenes and Related Species beyond Diaminocarbenes. Angew. Chem. Int. Ed. 2010, 49, 8810–8849. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Casely, I.J. F-Block N-Heterocyclic Carbene Complexes. Chem. Rev. 2009, 109, 3599–3611. [Google Scholar] [CrossRef]
- De Frémont, P.; Marion, N.; Nolan, S.P. Carbenes: Synthesis, properties, and organometallic chemistry. Coord. Chem. Rev. 2009, 253, 862–892. [Google Scholar] [CrossRef]
- Díez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. Well-Defined N-Heterocyclic Carbenes-Palladium(II) Precatalysts for Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-Heterocyclic Carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef]
- Glorius, F. N-Heterocyclic Carbenes in Transition Metal Catalysis; Springer: Berlin, Germany, 2007. [Google Scholar]
- Kantchev, E.A.B.; O’Brien, C.J.; Organ, M.G. Palladium Complexes of N-Heterocyclic Carbenes as Catalysts for Cross-Coupling Reactions-A Synthetic Chemist’s Perspective. Angew. Chem. Int. Ed. 2007, 46, 2768–2813. [Google Scholar] [CrossRef]
- Nolan, S.P. N-Heterocyclic Carbenes in Synthesis; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Herrmann, W.A. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Bourissou, D.; Guerret, O.; Gabbaï, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–91. [Google Scholar] [CrossRef] [PubMed]
- Boratynski, P.J.; Nowak, A.E.; Skarźewski, J. New Chiral Benzimidazoles Derived from 1,2-Diaminocyclohexane. Synthesis 2015, 47, 3797–3804. [Google Scholar]
- Fliedel, C.; Braunstein, P. Recent advances in S-functionalized N-heterocyclic carbene ligands: From the synthesis of azolium salts and metal complexes to applications. J. Organomet. Chem. 2014, 751, 286–300. [Google Scholar] [CrossRef]
- Poyatos, M.; Mata, J.A.; Peris, E. Complexes with Poly(N-heterocyclic carbene) Ligands: Structural Features and Catalytic Applications. Chem. Rev. 2009, 109, 3677–3707. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.E.; Jahnke, M.C. Heterocyclic Carbenes: Synthesis and Coordination Chemistry. Angew. Chem. Int. Ed. 2008, 47, 3122–3172. [Google Scholar] [CrossRef] [PubMed]
- Peris, E.; Crabtree, R.H. Recent homogeneous catalytic applications of chelate and pincer N-heterocyclic carbenes. Coord. Chem. Rev. 2004, 248, 2239–2246. [Google Scholar] [CrossRef]
- Hodgson, R.; Douthwaite, R.E. Synthesis and asymmetric catalytic application of chiral imidazolium–phosphines derived from (1R, 2R)-trans-diaminocyclohexane. J. Organomet. Chem. 2005, 690, 5822–5831. [Google Scholar] [CrossRef]
- Bonnet, L.G.; Douthwaite, R.E.; Hodgson, R. Synthesis of Constrained-Geometry Chiral Di-N-Heterocyclic Carbene Ligands and Their Silver(I) and Palladium(II) Complexes. Organometallics 2003, 22, 4384–4386. [Google Scholar] [CrossRef]
- Duan, W.-L.; Shi, M.; Rong, G.-B. Synthesis of novel axially chiral Rh-NHC complexes derived from BINAM and application in the enantioselective hydrosilylation of methyl ketones. Chem. Commun. 2003, 39, 2916–2917. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, M. Catalytic Enantioselective Addition of Cyclic β-Keto Esters with Activated Olefins and N-Boc Imines Using Chiral C2-Symmetric Cationic Pd2+ N-Heterocyclic Carbene (NHC) Diaqua Complexes. Organometallics 2010, 29, 2831–2834. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, M. Catalytic asymmetric addition of arylboronic acids to N-Boc imines generated in situ using C2-symmetric cationic N-heterocyclic carbenes (NHCs) Pd2+ diaquo complexes. Tetrahedron 2010, 66, 2619–2623. [Google Scholar] [CrossRef]
- Cao, S.-H.; Shi, M. Axially chiral C2-symmetric N-heterocyclic carbene (NHC) palladium complex-catalyzed asymmetric α-hydroxylation of β-keto esters. Tetrahedron Asymmetry 2010, 21, 2675–2680. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Q.; Zhang, X.; Zhang, T.; Shi, M. Axially chiral C2-symmetric N-heterocyclic carbene (NHC) palladium complexes-catalyzed asymmetric arylation of aldehydes with arylboronic acids. Tetrahedron Asymmetry 2010, 21, 1928–1935. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, R.; Zhang, T.; Shi, M. Asymmetric 1,4-Addition of Arylboronic Acids to 2,3-Dihydro-4-pyridones Catalyzed by Axially Chiral NHC-Pd(II) Complexes. J. Org. Chem. 2010, 75, 3935–3937. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, F.; Shi, M. Synthesis of Chiral Bis(N-heterocyclic carbene) Palladium and Rhodium Complexes with 1,10-Biphenyl Scaffold and Their Application in Asymmetric Catalysis. Organometallics 2009, 28, 4416–4420. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.; Shi, M. Chiral Bis(NHC)-Palladium(II) Complex Catalyzed and Diethylzinc-Mediated Enantioselective Umpolung Allylation of Aldehydes. Organometallics 2009, 28, 2640–2642. [Google Scholar] [CrossRef]
- Ma, G.-N.; Zhang, T.; Shi, M. Catalytic Enantioselective Arylation of N-Tosylarylimines with Arylboronic Acids Using C2-Symmetric Cationic N-Heterocyclic Carbene Pd2+ Diaquo Complexes. Org. Lett. 2009, 11, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, M. Efficient chirality switching in the asymmetric addition of indole to N-tosylarylimines in the presence of axially chiral cyclometalated bidentate N-heterocyclic carbene palladium(II) complexes. Tetrahedron Asymmetry 2009, 20, 119–123. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, M. Chiral Bidentate Bis(N-Heterocyclic Carbene)-Based Palladium Complexes Bearing Carboxylate Ligands: Highly Effective Catalysts for the Enantioselective Conjugate Addition of Arylboronic Acids to Cyclic Enones. Chem. Eur. J. 2008, 14, 3759–3764. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, M.; Zhao, M. Bis(NHC)-Pd(II) complexes as highly efficient catalysts for allylation of aldehydes with allyltributyltin. Tetrahedron 2008, 64, 2412–2418. [Google Scholar] [CrossRef]
- Xu, Q.; Gu, X.; Liu, S.; Dou, Q.; Shi, M. The Use of Chiral BINAM NHC-Rh(III) Complexes in Enantioselective Hydrosilylation of 3-Oxo-3-arylpropionic Acid Methyl or Ethyl Esters. J. Org. Chem. 2007, 72, 2240–2242. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, J.-J.; Xu, Q.; Shi, M. Axially Chiral NHC-Pd(II) Complexes in the Oxidative Kinetic Resolution of Secondary Alcohols Using Molecular Oxygen as a Terminal Oxidant. Org. Lett. 2007, 9, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Qian, H.-X. A new dimeric bidentated NHC-Pd(II) complex from trans-cyclohexane-1,2-diamine for Suzuki reaction and Heck reaction. Tetrahedron 2005, 61, 4949–4955. [Google Scholar] [CrossRef]
- Diez, C.; Nagel, U. Chiral iridium(I) bis(NHC) complexes as catalysts for asymmetric transfer hydrogenation. Appl. Organomet. Chem. 2010, 24, 509–516. [Google Scholar] [CrossRef]
- Nagel, U.; Diez, C. Modular Synthesis of a New Type of Chiral Bis(carbene) Ligand from L-Valinol and Iridium(I) and Rhodium(I) Complexes Thereof. Eur. J. Inorg. Chem. 2009, 2009, 1248–1255. [Google Scholar] [CrossRef]
- Villaverde, G.; Corma, A.; Iglesias, M.; Sánchez, F. Chiral NHC-Complexes with Dioxolane Backbone Heterogenized on MCM-41. Catalytic Activity. ChemCatChem 2011, 3, 1320–1328. [Google Scholar] [CrossRef]
- Arnanz, A.; González-Arellano, C.; Juan, A.; Villaverde, G.; Corma, A.; Iglesias, M.; Sánchez, F. New chiral ligands bearing two N-heterocyclic carbene moieties at a dioxolane backbone. Gold, palladium and rhodium complexes as enantioselective catalysts. Chem. Commun. 2010, 46, 3001–3003. [Google Scholar] [CrossRef]
- Jeletic, M.S.; Lowry, R.J.; Swails, J.M.; Ghiviriga, I.; Veige, A.S. Synthesis and characterization of κ-2-bis-N-heterocyclic carbene rhodium(I) catalysts: Application in enantioselective arylboronic acid addition to cyclohex-2-enones. J. Organomet. Chem. 2011, 696, 3127–3134. [Google Scholar] [CrossRef]
- Jeletic, M.S.; Ghiviriga, I.; Abboud, K.A.; Veige, A.S. A new chiral di-N-heterocyclic carbene (NHC) cyclophane ligand and its application in palladium enantioselective catalysis. Dalton Trans. 2010, 39, 6392–6394. [Google Scholar] [CrossRef]
- Gigler, P.; Bechlars, B.; Herrmann, W.A.; Kühn, F.E. Hydrosilylation with Biscarbene Rh(I) Complexes: Experimental Evidence for a Silylene-Based Mechanism. J. Am. Chem. Soc. 2011, 133, 1589–1596. [Google Scholar] [CrossRef]
- Riederer, S.K.U.; Bechlars, B.; Herrmann, W.A.; Kühn, F.E. Chiral N-heterocyclic biscarbenes based on 1,2,4-triazole as ligands for metal-catalyzed asymmetric synthesis. Dalton Trans. 2011, 40, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; He, Y.; Yu, J.; Zhang, D. N,N’-Bisaryl Substituted Chiral Linker-Bridged Bis(N-Heterocyclic Carbene) Palladium Complexes: Design, Synthesis, and Catalytic Properties. Organometallics 2017, 36, 1372–1382. [Google Scholar] [CrossRef]
- Zhang, D.; He, Y.; Tang, J. Chiral linker-bridged bis-N-heterocyclic carbenes: Design, synthesis, palladium complexes, and catalytic properties. Dalton Trans. 2016, 45, 11699–11709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Tang, J.; Gu, J.; Wang, Q.; Sun, P.; Zhang, D. Chiral 1,2-Cyclohexane-Bridged Bis-NHC Palladium Catalysts for Asymmetric Suzuki-Miyaura Coupling: Synthesis, Characterization, and Steric Effects on Enantiocontrol. Organometallics 2014, 33, 876–884. [Google Scholar] [CrossRef]
- Shigeng, G.; Tang, J.; Zhang, D.; Wang, Q.; Chen, Z.; Weng, L. Synthesis, structure, and catalytic activity of palladium complexes with new chiral cyclohexane-1,2-based di-NHC-ligands. J. Organomet. Chem. 2012, 700, 223–229. [Google Scholar] [CrossRef]
- Khose, V.N.; John, M.E.; Pandey, A.D.; Karnik, A.V. Chiral benzimidazoles and their applications in stereodiscrimination processes. Tetrahedron Asymmetry 2017, 28, 1233–1289. [Google Scholar] [CrossRef]
- Zhao, D.; Candish, L.; Paul, D.; Glorius, F. N-Heterocyclic Carbenes in Asymmetric Hydrogenation. ACS Catal. 2016, 6, 5978–5988. [Google Scholar] [CrossRef]
- Tornatzky, J.; Kannenberg, A.; Blechert, S. New catalysts with unsymmetrical N-heterocyclic carbene ligands. Dalton Trans. 2012, 41, 8215–8225. [Google Scholar] [CrossRef]
- Brown, M.K.; May, T.L.; Baxter, C.A.; Hoveyda, A.H. All-Carbon Quaternary Stereogenic Centers by Enantioselective Cu-Catalyzed Conjugate Additions Promoted by a Chiral N-Heterocyclic Carbene. Angew. Chem. Int. Ed. 2007, 46, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Douthwaite, R.E. Metal-mediated asymmetric alkylation using chiral N-heterocyclic carbenes derived from chiral amines. Coord. Chem. Rev. 2007, 251, 702–717. [Google Scholar] [CrossRef]
- Gade, L.H.; Bellemin-Laponnaz, S. Mixed oxazoline-carbenes as stereodirecting ligands for asymmetric catalysis. Coord. Chem. Rev. 2007, 251, 718–725. [Google Scholar] [CrossRef]
- César, V.; Bellemin-Laponnaz, S.; Gade, L.H. Chiral N-heterocyclic carbenes as stereodirecting ligands in asymmetric catalysis. Chem. Soc. Rev. 2004, 33, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.C.; Burgess, K. Chiral N-heterocyclic carbene-transition metal complexes in asymmetric catalysis. Tetrahedron Asymmetry 2003, 14, 951–961. [Google Scholar] [CrossRef]
- Nakano, Y.; Sakaguchi, S. Inversions in asymmetric conjugate addition reaction of cyclic enones catalyzed by the Cu/NHC-AgX system: Factors affecting the stereoselective formation of both enantiomers. J. Organomet. Chem. 2017, 846, 407–416. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakano, Y.; Shibata, N.; Sakaguchi, S. Enantioselectivity switch in copper-catalyzed conjugate addition reactions under the influence of a chiral N-heterocyclic carbene-silver complex. RSC Adv. 2016, 6, 7755–7759. [Google Scholar] [CrossRef]
- Kondo, J.; Harano, A.; Dohi, K.; Sakaguchi, S. C2-symmetric functionalized azolium salt from serine ester for Cu-catalyzed asymmetric conjugate addition reaction. J. Mol. Catal. A Chem. 2014, 395, 66–71. [Google Scholar] [CrossRef]
- Dohi, K.; Kondo, J.; Yamada, H.; Arakawa, R.; Sakaguchi, S. Functionalized N-Heterocyclic Carbene Ligands for Dual Enantioselective Control in the Cu-Catalyzed Conjugate Addition of Dialkylzinc Compounds to Acyclic Enones. Eur. J. Org. Chem. 2012, 2012, 7143–7152. [Google Scholar] [CrossRef]
- Yoshimura, M.; Shibata, N.; Kawakami, M.; Sakaguchi, S. Ligand design for dual enantioselective control in Cu-catalyzed asymmetric conjugate addition of R2Zn to cyclic enone. Tetrahedron 2012, 68, 3512–3518. [Google Scholar] [CrossRef]
- Shibata, N.; Yoshimura, M.; Yamada, H.; Arakawa, R.; Sakaguchi, S. Hydroxy-amide Functionalized Azolium Salts for Cu-Catalyzed Asymmetric Conjugate Addition: Stereocontrol Based on Ligand Structure and Copper Precatalyst. J. Org. Chem. 2012, 77, 4079–4086. [Google Scholar] [CrossRef]
- Shibata, N.; Okamoto, M.; Yamamoto, Y.; Sakaguchi, S. Reversal of Stereoselectivity in the Cu-Catalyzed Conjugate Addition Reaction of Dialkylzinc to Cyclic Enone in the Presence of a Chiral Azolium Compound. J. Org. Chem. 2010, 75, 5707–5715. [Google Scholar] [CrossRef]
- Harano, A.; Sakaguchi, S. A new C2-symmetric azolium compound for Cu-catalyzed asymmetric conjugate addition of R2Zn to cyclic enone. J. Organomet. Chem. 2011, 696, 61–67. [Google Scholar] [CrossRef]
- Okamoto, M.; Yamamoto, Y.; Sakaguchi, S. A new approach to switching of enantioselectivity in NHC–Cu-catalyzed conjugate addition of alkylzincs to cyclic enones. Chem. Commun. 2009, 45, 7363–7365. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Matsubara, R. Recent topics on catalytic asymmetric 1,4-addition. Tetrahedron Lett. 2017, 58, 1793–1805. [Google Scholar] [CrossRef]
- Alexakis, A.; Krause, N.; Woodward, S. Copper-Catalyzed Asymmetric Synthesis; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Jerphagnon, T.; Pizzuti, M.G.; Minnaard, A.J.; Feringa, B.L. Recent advances in enantioselective copper-catalyzed 1,4-addition. Chem. Soc. Rev. 2009, 38, 1039–1075. [Google Scholar] [CrossRef] [PubMed]
- Alexakis, A.; Bäckvall, J.E.; Krause, N.; Pàmies, O.; Diéguez, M. Enantioselective Copper-Catalyzed Conjugate Addition and Allylic Substitution Reactions. Chem. Rev. 2008, 108, 2796–2823. [Google Scholar] [CrossRef] [PubMed]
- Kamihigashi, S.; Shibata, N.; Sakaguchi, S. Cu-Catalyzed Asymmetric Conjugate Addition of Dialkylzincs to Enones Using a (±)-trans-1,2-Cyclohexanediamine-Based Bis(NHC) Derived from L-Leucinol. Synlett 2014, 25, 2933–2937. [Google Scholar] [CrossRef]
- Endo, K.; Yakeishi, S.; Hamada, D.; Shibata, T. Functionalized BINOL-mono-PHOS for Multinuclear Cu-Catalysts in Asymmetric Conjugate Addition of Organozinc Reagents. Chem. Lett. 2013, 42, 547–549. [Google Scholar] [CrossRef]
- Wang, L.; Meng, W.; Zhu, C.-L.; Zheng, Y.; Nie, J.; Ma, J.-A. The Long-Arm Effect: Influence of Axially Chiral Phosphoramidite Ligands on the Diastereo- and Enantioselectivity of the Tandem 1,4-Addition/Fluorination. Angew. Chem. Int. Ed. 2011, 50, 9442–9446. [Google Scholar] [CrossRef]
- Uchida, T.; Katsuki, T. Construction of a new type of chiral bidentate NHC ligands: Copper-catalyzed asymmetric conjugate alkylation. Tetrahedron Lett. 2009, 50, 4741–4743. [Google Scholar] [CrossRef]
- García, J.M.; González, A.; Kardak, B.G.; Odriozola, J.M.; Oiarbide, M.; Razkin, J.; Palomo, C. Copper-Catalyzed Enantioselective Conjugate Addition of Dialkylzinc Reagents to α’-Oxy Enones. Chem. Eur. J. 2008, 14, 8768–8771. [Google Scholar] [CrossRef]
- Šebesta, R.; Pizzuti, M.G.; Minnaard, A.J.; Feringa, B.L. Copper-Catalyzed Enantioselective Conjugate Addition of Organometallic Reagents to Acyclic Dienones. Adv. Synth. Catal. 2007, 349, 1931–1937. [Google Scholar] [CrossRef]
- Li, K.; Alexakis, A. Copper-Catalyzed Enantioselective Intramolecular Conjugate Addition/Trapping Reactions: Synthesis of Cyclic Compounds with Multichiral Centers. Chem. Eur. J. 2007, 13, 3765–3771. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, Y.; Cao, P.; Wang, M.; Li, L.; Li, D.; Liao, J. Copper-catalyzed enantioselective conjugate addition of diethylzinc to acyclic enones with chiral sulfoxideephosphine ligands. Tetrahedron 2016, 72, 2707–2711. [Google Scholar] [CrossRef]
- Harutyunyan, S.R.; López, F.; Browne, W.R.; Correa, A.; Peňa, D.; Badorrey, R.; Meetsma, A.; Minnaard, A.J.; Feringa, B.L. On the Mechanism of the Copper-Catalyzed Enantioselective 1,4-Addition of Grignard Reagents to α,β-Unsaturated Carbonyl Compounds. J. Am. Chem. Soc. 2006, 128, 9103–9118. [Google Scholar] [CrossRef] [PubMed]
- Hajra, A.; Yoshikai, N.; Nakamura, E. Aminohydroxyphosphine Ligand for the Copper-Catalyzed Enantioselective Conjugate Addition of Organozinc Reagents. Org. Lett. 2006, 8, 4153–4155. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yamamoto, Y.; Katagiri, K.; Danjo, H.; Yamaguchi, K.; Imamoto, T. P-Chiral o-Phosphinophenol as a P/O Hybrid Ligand: Preparation and Use in Cu-Catalyzed Asymmetric Conjugate Addition of Diethylzinc to Acyclic Enones. J. Org. Chem. 2005, 70, 9009–9012. [Google Scholar] [CrossRef] [PubMed]
- Börner, C.; Dennis, M.R.; Sinn, E.; Woodward, S. Copper-Catalysed Asymmetric 1,4-Addition of Organozinc Compounds to Linear Aliphatic Enones Using 2,2′-Dihydroxy 3,3′-Dithioether Derivatives of 1,1′-Binaphthalene. Eur. J. Org. Chem. 2001, 2001, 2435–2446. [Google Scholar] [CrossRef]
- Börner, C.; König, W.A.; Woodward, S. Enone structure as a probe to Lewis acid carbonyl binding in copper-catalysed asymmetric conjugate addition. Tetrahedron Lett. 2001, 42, 327–329. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Nájera, C.; Yus, M. Stereodivergent Catalysis. Chem. Rev. 2018, 118, 5080–5200. [Google Scholar] [CrossRef]
- Blanco, V.; Leigh, D.A.; Marcos, V. Artificial switchable catalysts. Chem. Soc. Rev. 2015, 44, 5341–5370. [Google Scholar] [CrossRef]
- Kim, H.Y.; Oh, K. Stereodivergent Asymmetric Reactions Catalyzed by Brucine Diol. Synlett 2015, 26, 2067–2087. [Google Scholar]
- Escorihuela, J.; Burguete, M.I.; Luis, S.V. New advances in dual stereocontrol for asymmetric reactions. Chem. Soc. Rev. 2013, 42, 5595–5617. [Google Scholar] [CrossRef] [PubMed]
- Bartók, M. Unexpected Inversions in Asymmetric Reactions: Reactions with Chiral Metal Complexes, Chiral Organocatalysts, and Heterogeneous Chiral Catalysts. Chem. Rev. 2010, 110, 1663–1705. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hayashi, M. New Approach for Complete Reversal of Enantioselectivity Using a Single Chiral Source. Synthesis 2008, 2008, 3361–3376. [Google Scholar]
- Zanoni, G.; Castronovo, F.; Franzini, M.; Vidari, G.; Giannini, E. Toggling enantioselective catalysis-a promising paradigm in the development of more efficient and versatile enantioselective synthetic methodologies. Chem. Soc. Rev. 2003, 32, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cao, P.; Wang, B.; Jia, T.; Lou, Y.; Wang, M.; Liao, J. Copper(I)-Catalyzed Asymmetric Pinacolboryl Addition of N-Bocimines Using a Chiral Sulfoxide−Phosphine Ligand. Org. Lett. 2015, 17, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Spahn, E.; Albright, A.; Shevlin, M.; Pauli, L.; Pfaltz, A.; Gawley, R.E. Double-Asymmetric Hydrogenation Strategy for the Reduction of 1,1-Diaryl Olefins Applied to an Improved Synthesis of CuIPhEt, a C2-Symmetric N-Heterocyclic Carbenoid. J. Org. Chem. 2013, 78, 2731–2735. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.-Y.; Chen, F.; Qin, J.; He, Y.-M.; Fan, Q.-H. Asymmetric Hydrogenation of 2,4-Disubstituted 1,5-Benzodiazepines Using Cationic Ruthenium Diamine Catalysts: An Unusual Achiral Counteranion Induced Reversal of Enantioselectivity. Angew. Chem. Int. Ed. 2012, 51, 5706–5710. [Google Scholar] [CrossRef]
- Kim, H.Y.; Li, J.-Y.; Kim, S.; Oh, K. Stereodivergency in Catalytic Asymmetric Conjugate Addition Reactions of Glycine (Ket)imines. J. Am. Chem. Soc. 2011, 133, 20750–20753. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, L.; Zhou, Y.; Nie, Z.; Luo, S.; Cheng, J.-P. Asymmetric Binary Acid Catalysis: A Regioselectivity Switch between Enantioselective 1,2- and 1,4-Addition through Different Counteranions of InIII. Angew. Chem. Int. Ed. 2011, 50, 6610–6614. [Google Scholar] [CrossRef]
- Frölander, A.; Moberg, C. Ag+-Assisted Hydrosilylation: Complementary Behavior of Rh and Ir Catalysts (Reversal of Enantioselectivity). Org. Lett. 2007, 9, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Kozlowski, M.C.; Murry, J.A.; Burgey, C.S.; Campos, K.R.; Connell, B.T.; Staples, R.J. C2-Symmetric Copper(II) Complexes as Chiral Lewis Acids. Scope and Mechanism of Catalytic Enantioselective Aldol Additions of Enolsilanes to (Benzyloxy)acetaldehyde. J. Am. Chem. Soc. 1999, 121, 669–685. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).