Effect of Copper Precursors on the Activity and Hydrothermal Stability of CuII−SSZ−13 NH3−SCR Catalysts
Abstract
1. Introduction
2. Results and Discussion
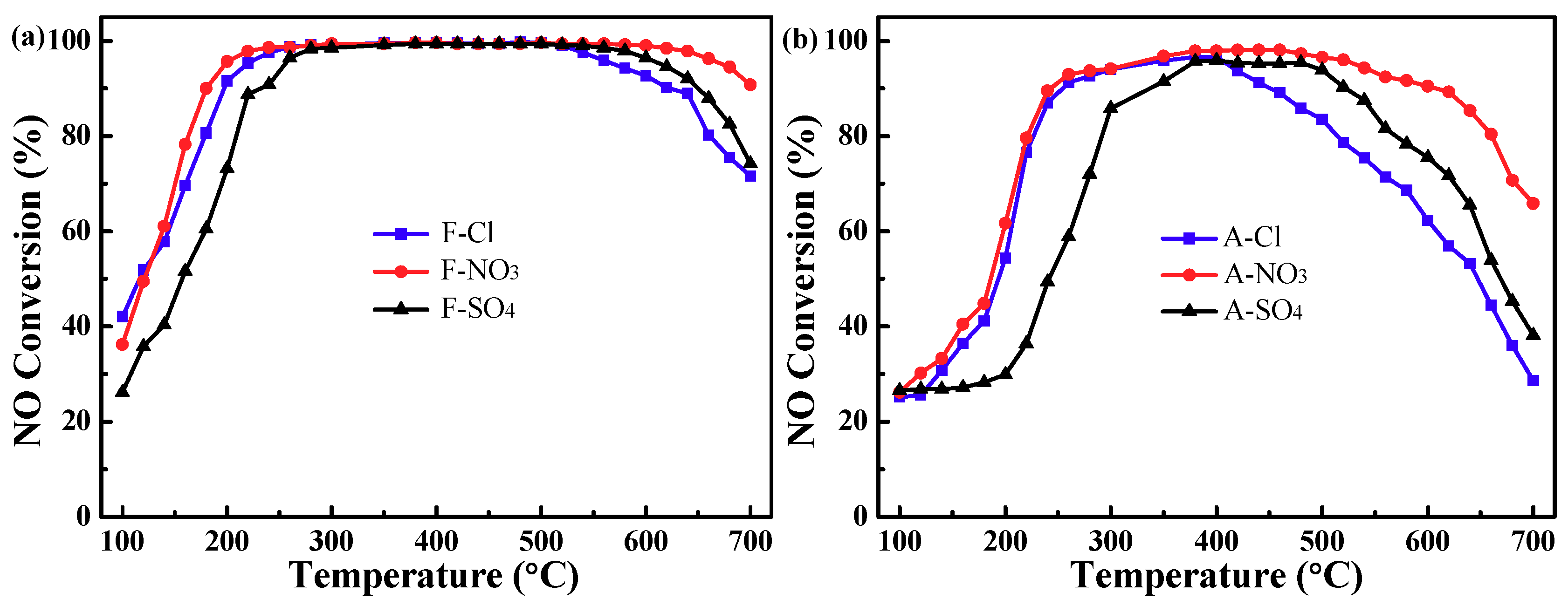
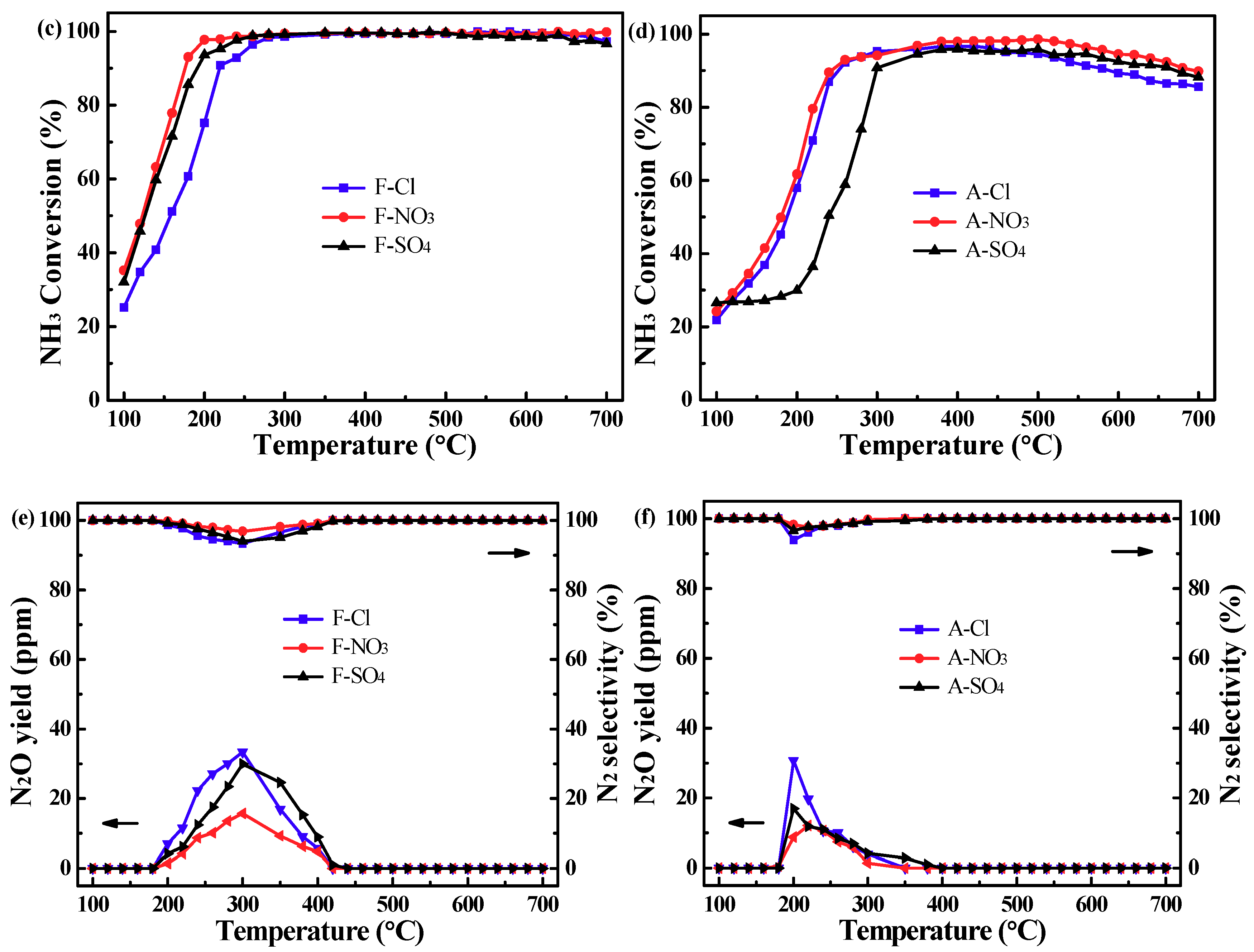
2.1. Catalytic Activity
2.2. Structure and Morphology
2.2.1. X-ray Diffraction (XRD) Results
2.2.2. N2 Adsorption Results
2.2.3. Scanning Electron Microscope (SEM) Results
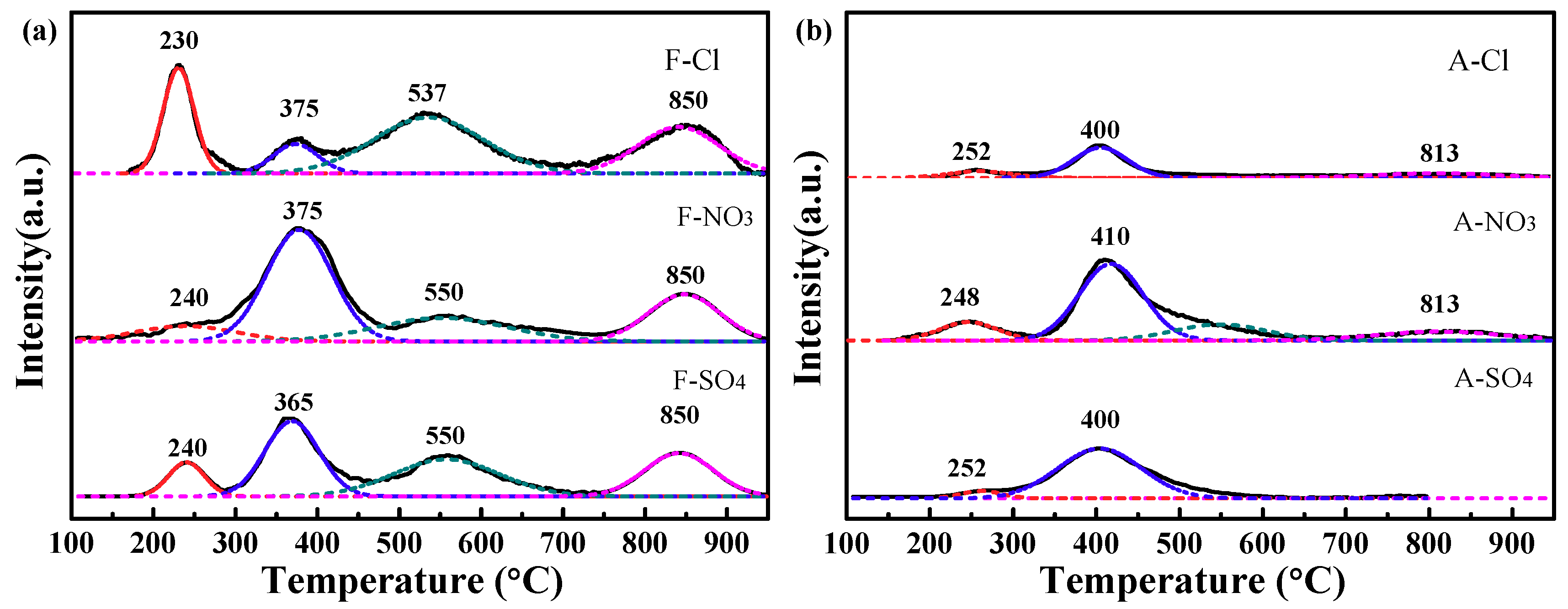
2.2.4. Hydrogen-Temperature-Programmed Reduction (H2−TPR) Results
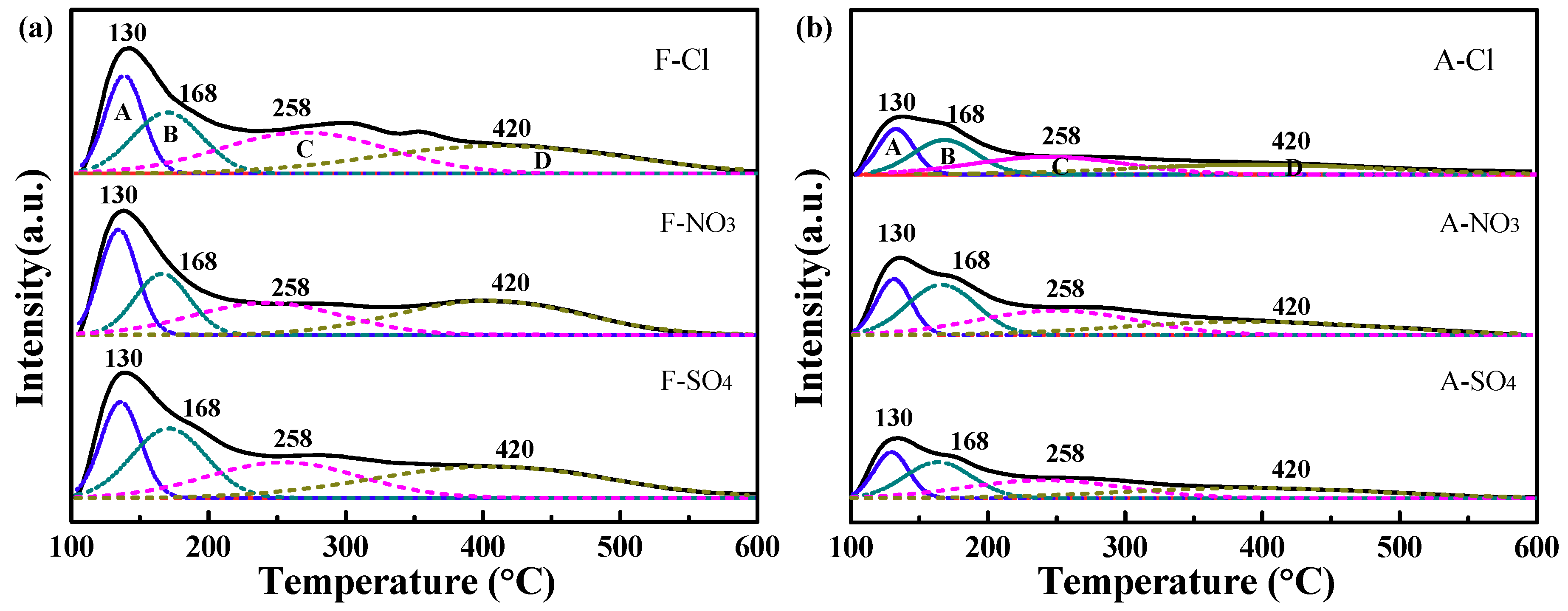
2.2.5. Ammonia Temperature-Programmed Desorption (NH3−TPD) Results
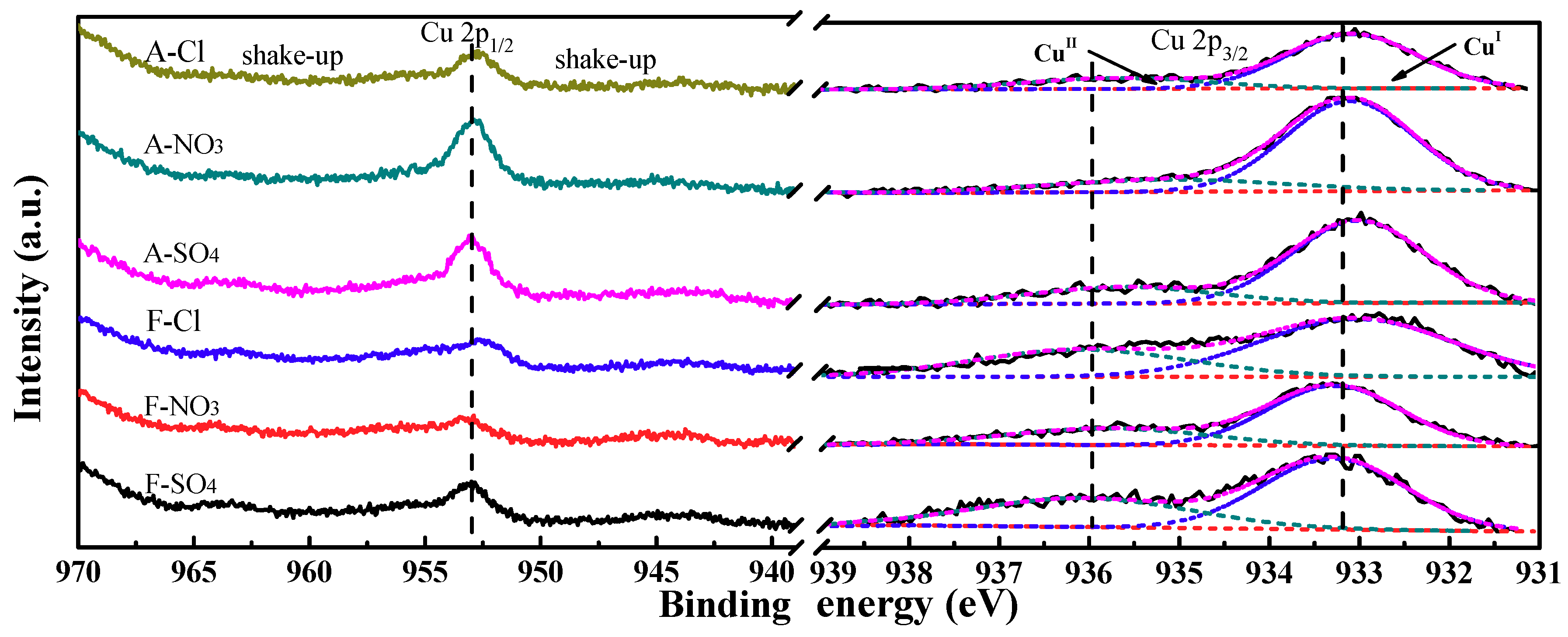
2.2.6. X-ray Photoelectron Spectroscopy (XPS) Results
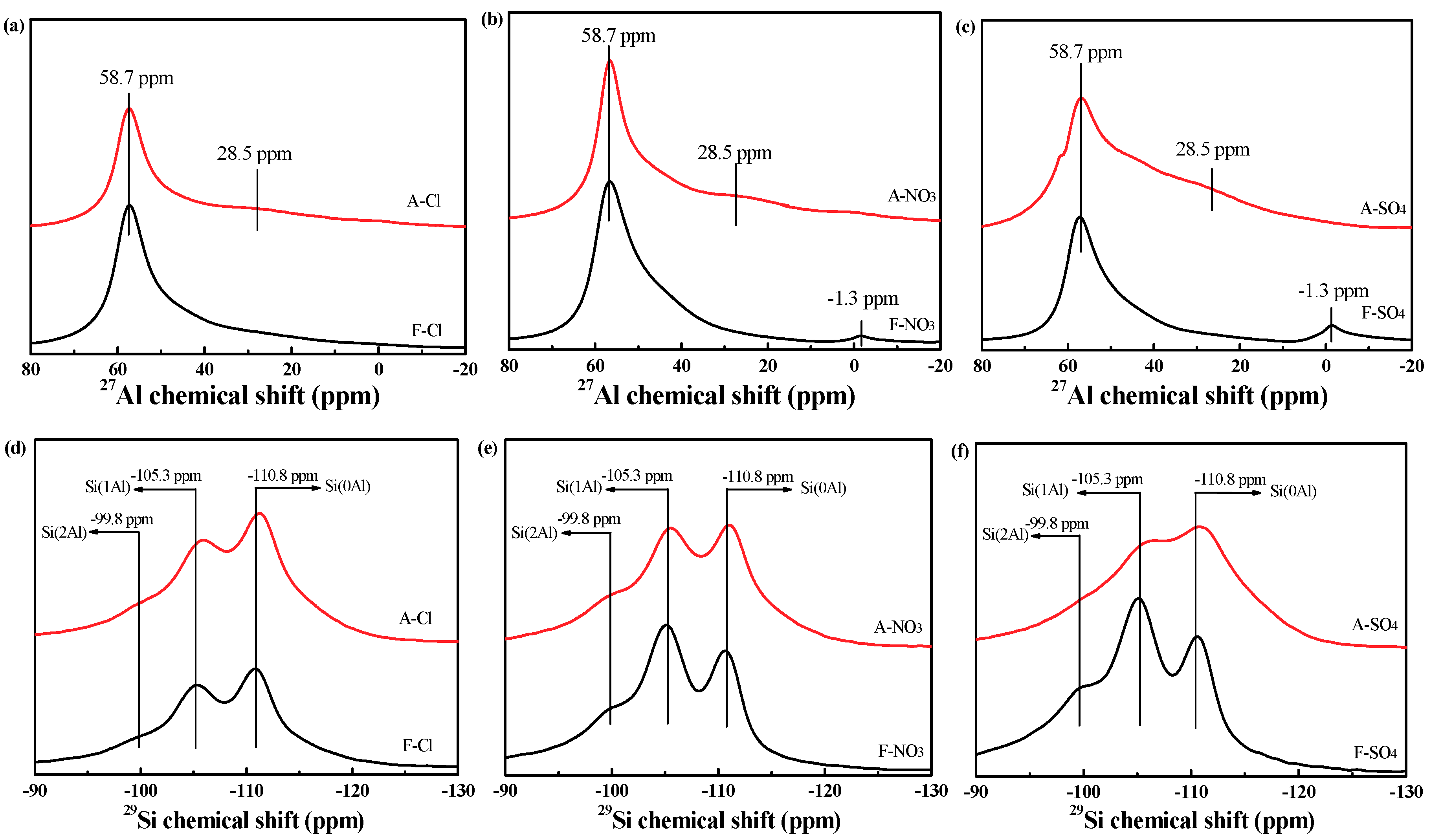
2.2.7. NMR Results
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Hydrothermal Aging Treatment of Catalysts
3.3. Catalyst Activity Evaluation
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, F.; Yu, Y.; He, H. Environmentally-benign catalysts for the selective catalytic reduction of NOx from diesel engines: Structure–activity relationship and reaction mechanism aspects. Chem. Commun. 2014, 50, 8445–8463. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effect of the hydrothermal aging temperature and Cu/Al ratio on the hydrothermal stability of CuSSZ-13 catalysts for NH3-SCR. Catal. Sci. Technol. 2017, 7, 703–717. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Q.; Zhang, Z. Zeolitic materials for DeNOx selective catalytic reduction. ChemCatChem 2018, 10, 29–41. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Z.; Chen, Y.; Bao, W.; Chang, L.; Feng, G. In-situ hydrothermal synthesis of Cu-SSZ-13/cordierite for the catalytic removal of NOx from diesel vehicles by NH3. Chem. Eng. J. 2015, 263, 9–19. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. The role of ceria on the activity and SO2 resistance of catalysts for the selective catalytic reduction of NOx by NH3. Appl. Catal. B 2015, 166, 37–44. [Google Scholar] [CrossRef]
- He, Y.; Ford, M.E.; Zhu, M.; Liu, Q.; Tumuluri, U.; Wu, Z.; Wachs, I.E. Influence of catalyst synthesis method on selective catalytic reduction (SCR) of no by NH3 with V2O5-WO3/TiO2 catalysts. Appl. Catal. B 2016, 193, 141–150. [Google Scholar] [CrossRef]
- Joshi, S.Y.; Kumar, A.; Luo, J.; Kamasamudram, K.; Currier, N.W.; Yezerets, A. New insights into the mechanism of NH3-SCR over Cu- and Fe-zeolite catalyst: Apparent negative activation energy at high temperature and catalyst unit design consequences. Appl. Catal. B 2018, 226, 565–574. [Google Scholar] [CrossRef]
- Xie, K.; Woo, J.; Bernin, D.; Kumar, A.; Kamasamudram, K.; Olsson, L. Insights into hydrothermal aging of phosphorus-poisoned Cu-SSZ-13 for NH3-SCR. Appl. Catal. B 2019, 241, 205–216. [Google Scholar] [CrossRef]
- Cheng, J.; Han, S.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Selective catalytic reduction of NO with NH3 over the Cu/SAPO-34 catalysts derived from different Cu precursors. Microporous Mesoporous Mater. 2019, 278, 423–434. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tran, D.; Burton, S.D.; Szanyi, J.; Lee, J.H.; Peden, C.H.F. Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites. J. Catal. 2012, 287, 203–209. [Google Scholar] [CrossRef]
- Han, L.; Zhao, X.; Yu, H.; Hu, Y.; Li, D.; Sun, D.; Liu, M.; Chang, L.; Bao, W.; Wang, J. Preparation of SSZ-13 zeolites and their NH3-selective catalytic reduction activity. Microporous Mesoporous Mater. 2018, 261, 126–136. [Google Scholar] [CrossRef]
- Shishkin, A.; Kannisto, H.; Carlsson, P.-A.; Härelind, H.; Skoglundh, M. Synthesis and functionalization of SSZ-13 as an NH3-SCR catalyst. Catal. Sci. Technol. 2014, 4, 3917–3926. [Google Scholar] [CrossRef]
- Ren, L.; Zhu, L.; Yang, C.; Chen, Y.; Sun, Q.; Zhang, H.; Li, C.; Nawaz, F.; Meng, X.; Xiao, F.S. Designed copper-amine complex as an efficient template for one-pot synthesis of Cu-SSZ-13 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Chem. Commun. 2011, 47, 9789–9791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qiu, F.; Chang, H.; Li, X.; Li, J. Identification of active sites and reaction mechanism on low-temperature SCR activity over Cu-SSZ-13 catalysts prepared by different methods. Catal. Sci. Technol. 2016, 6, 6294–6304. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, S.; Wu, X.; Shi, Y.; Cao, L.; Liu, L.; Ran, R.; Si, Z.; Liu, J.; Weng, D. Low-temperature solid-state ion-exchange method for preparing Cu-SSZ-13 selective catalytic reduction catalyst. ACS Catal. 2019, 9, 6962–6973. [Google Scholar] [CrossRef]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.; Shih, A.J.; Li, H.; di Iorio, J.R.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef]
- Borfecchia, E.; Beato, P.; Svelle, S.; Olsbye, U.; Lamberti, C.; Bordiga, S. Cu-cha—A model system for applied selective redox catalysis. Chem. Soc. Rev. 2018, 47, 8097–8133. [Google Scholar] [CrossRef] [PubMed]
- Marberger, A.; Petrov, A.W.; Steiger, P.; Elsener, M.; Kröcher, O.; Nachtegaal, M.; Ferri, D. Time-resolved copper speciation during selective catalytic reduction of no on Cu-SSZ-13. Nat. Catal. 2018, 1, 221–227. [Google Scholar] [CrossRef]
- Xu, R.; Pang, W.; Yu, J.; Huo, Q.; Chen, J. Molecular Sieves and Porous Materials Chemietry; Science Press: Beijing, China, 2004. [Google Scholar]
- Kumar, R.; Mukherjee, P.; Pandey, R.K.; Rajmohanan, P.; Bhaumik, A. Role of oxyanions as promoter for enhancing nucleation and crystallization in the synthesis of MFI-type microporous materials. Microporous Mesoporous Mater. 1998, 22, 23–31. [Google Scholar] [CrossRef]
- López-León, T.; Santander-Ortega, M.J.; Ortega-Vinuesa, J.L.; Bastos-González, D. Hofmeister Effects in Colloidal Systems: Influence of the Surface Nature. J. Phys. Chem. C 2008, 112, 16060–16069. [Google Scholar] [CrossRef]
- Toktarev, A.V.; Echevskii, G.V. Hofmeister anion effect on the formation of zeolite beta. Stud. Surf. Sci. Catal. 2008, 174, 167–172. [Google Scholar]
- Leontidis, E. Hofmeister anion effects on surfactant self-assembly and the formation of mesoporous solids. Curr. Opin. Colloid Interface Sci. 2002, 7, 81–91. [Google Scholar] [CrossRef]
- Yang, Z. Hofmeister effects: An explanation for the impact of ionic liquids on biocatalysis. J. Biotechnol. 2009, 144, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Walter, E.D.; Kollar, M.; Wang, Y.; Szanyi, J.; Peden, C.H.F. Understanding ammonia selective catalytic reduction kinetics over Cu/SSZ-13 from motion of the Cu ions. J. Catal. 2014, 319, 1–14. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. Characterization of commercial Cu-SSZ-13 and Cu-SAPO-34 catalysts with hydrothermal treatment forNH3-SCR of NOx in diesel exhaust. Chem. Eng. J. 2013, 225, 323–330. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Zhu, H.; Kwak, J.H.; Peden, C.H.F.; Szanyi, J. In situ DRIFTS-MS studies on the oxidation of adsorbed NH3 by NOx over a Cu-SSZ-13 zeolite. Catal. Today 2013, 205, 16–23. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Z.; Qiao, H.; Yu, H.; Hu, Y.; Chang, L.; Bao, W. Cerium-Stabilized Cu-SSZ-13 Catalyst for the Catalytic Removal of NOx by NH3. Ind. Eng. Chem. Res. 2016, 55, 1174–1182. [Google Scholar] [CrossRef]
- Fan, C.; Chen, Z.; Pang, L.; Ming, S.; Dong, C.; Brou Albert, K.; Liu, P.; Wang, J.; Zhu, D.; Chen, H.; et al. Steam and alkali resistant Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx in diesel exhaust. Chem. Eng. J. 2018, 334, 344–354. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Ren, L.; Shi, X.; Xiao, F.S.; He, H. Excellent performance of one-pot synthesized Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx with NH3. Environ. Sci. Technol. 2014, 48, 566–572. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Li, J. Design and synthesis of core-shell structured meso-Cu-SSZ-13@mesoporous aluminosilicate catalyst for SCR of NOx with NH3: Enhancement of activity, hydrothermal stability and propene poisoning resistance. Appl. Catal. B 2016, 195, 48–58. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Yu, T.; Wang, J.; Shen, M. New insight into Cu/SAPO-34 preparation procedure: Impact of NH4-SAPO-34 on the structure and Cu distribution in Cu-SAPO-34 NH3-SCR catalysts. Appl. Catal. B 2018, 220, 161–170. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, C.; Pang, L.; Ming, S.; Liu, P.; Li, T. The influence of phosphorus on the catalytic properties, durability, sulfur resistance and kinetics of Cu-SSZ-13 for NOx reduction by NH3-SCR. Appl. Catal. B 2018, 237, 116–127. [Google Scholar] [CrossRef]
- Fickel, D.W.; Lobo, R.F. Coordination in Cu-SSZ-13 and Cu-SSZ-16 Investigated by Variable-Temperature XRD. J. Phys. Chem. C 2009, 114, 1633–1640. [Google Scholar] [CrossRef]
- Deka, U.; Juhin, A.; Eilertsen, E.A.; Emerich, H.; Green, M.A.; Korhonen, S.T.; Weckhuysen, B.M.; Beale, A.M. Confirmation of Isolated Cu2+ Ions in SSZ-13 Zeolite as Active Sites in NH3-Selective Catalytic Reduction. J. Phys. Chem. C 2012, 116, 4809–4818. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, R.; Zhou, R. A new insight into active Cu2+ Species Properties in One-Pot Synthesized Cu-SSZ-13 Catalysts for NOx Reduction by NH3. ChemCatChem 2018, 10, 5182–5189. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollár, M.; Szanyi, J.; Peden, C.H.F. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Washton, N.M.; Kollár, M.; Szanyi, J.; Peden, C.H.F. Alkali and Alkaline Earth Cocations on the Activity and Hydrothermal Stability of Cu/SSZ-13 NH3–SCR Catalysts. ACS Catal. 2015, 5, 6780–6791. [Google Scholar] [CrossRef]
- Lezcano-Gonzalez, I.; Deka, U.; van der Bij, H.E.; Paalanen, P.; Arstad, B.; Weckhuysen, B.M.; Beale, A.M. Chemical deactivation of Cu-SSZ-13 ammonia selective catalytic reduction (NH3-SCR) systems. Appl. Catal. B 2014, 154, 339–349. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Feng, G.; Chang, L.; Bao, W. In situ synthesis of CuSAPO-34/cordierite and its selective catalytic reduction of nitrogen oxides in vehicle exhaust: The effect of HF. Fuel 2013, 109, 101–109. [Google Scholar] [CrossRef]
- Chen, B.; Xu, R.; Zhang, R.; Liu, N. Economical Way to Synthesize SSZ-13 with abundant ion-exchanged Cu+ for an extraordinary performance in selective catalytic reduction (SCR) of NOx by ammonia. Environ. Sci. Technol. 2014, 48, 13909–13916. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; et al. Toward rational design of Cu/SSZ-13 selective catalytic reduction catalysts: Implications from atomic-level understanding of hydrothermal stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Wu, L.; Degirmenci, V.; Magusin, P.C.M.M.; Lousberg, N.J.H.G.M.; Hensen, E.J.M. Mesoporous SSZ-13 zeolite prepared by a dual-template method with improved performance in the methanol-to-olefins reaction. J. Catal. 2013, 298, 27–40. [Google Scholar] [CrossRef]
- Prodinger, S.; Derewinski, M.A.; Wang, Y.; Washton, N.M.; Walter, E.D.; Szanyi, J.; Gao, F.; Wang, Y.; Peden, C.H.F. Sub-micron Cu/SSZ-13: Synthesis and application as selective catalytic reduction (SCR) catalysts. Appl. Catal. B 2017, 201, 461–469. [Google Scholar] [CrossRef]
- Martins, G.A.V.; Berlier, G.; Coluccia, S.; Pastore, H.O.; Superti, G.B.; Gatti, G.; Marchese, L. Revisiting the nature of the acidity in chabazite-related Silicoaluminophosphates: Combined FTIR and 29Si MAS NMR study. J. Phys. Chem. C 2007, 111, 330–339. [Google Scholar] [CrossRef]
- Xu, L.; Du, A.; Wei, Y.; Wang, Y.; Yu, Z.; He, Y.; Zhang, X.; Liu, Z. Synthesis of SAPO-34 with only Si(4Al) species: Effect of Si contents on Si incorporation mechanism and Si coordination environment of SAPO-34. Microporous and Mesoporous Mater. 2008, 115, 332–337. [Google Scholar] [CrossRef]
- Barthomeuf, D. Topological model for the compared acidity of SAPOs and SiAl zeolites. Zeolites 1994, 14, 394–401. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, Y.; Zeng, S.; Zhu, L.; Sun, Q.; Zhang, H.; Yang, C.; Meng, X.; Yang, X.; Xiao, F.-S. Design and synthesis of a catalytically active Cu-SSZ-13 zeolite from a copper-amine complex template. Chin. J. Catal. 2012, 33, 92–105. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Z.; Qiao, H.; Han, L.; Bao, W.; Chang, L.; Feng, G.; Liu, W. Influence of aging on in situ hydrothermally synthesized Cu-SSZ-13 catalyst for NH3-SCR reaction. RSC Adv. 2014, 4, 42403–42411. [Google Scholar] [CrossRef]

| Samples | Angle (°) | FWHM | Diameter (nm) | Area (m2) |
|---|---|---|---|---|
| F−Cl | 20.93 | 0.26 | 30.51 | 3024 |
| A−Cl | 20.96 | 0.29 | 27.19 | 1925 |
| F−NO3 | 20.69 | 0.29 | 27.93 | 2620 |
| A−NO3 | 20.87 | 0.34 | 23.64 | 1761 |
| F−SO4 | 20.69 | 0.26 | 31.21 | 2639 |
| A−SO4 | - | - | - | - |
| Samples | SBET (m2/g) | V (cm3/g) | D (nm) |
|---|---|---|---|
| F−Cl | 363.99 | 0.15 | 0.77 |
| A−Cl | 242.15 | 0.10 | 0.67 |
| F−NO3 | 301.64 | 0.13 | 0.65 |
| A−NO3 | 145.84 | 0.08 | 0.56 |
| F−SO4 | 233.44 | 0.12 | 1.07 |
| A−SO4 | 11.64 | 0.01 | 0.49 |
| Samples | H2 Consumption (μmol g−1) | Total H2 Consumption (μmol g−1) | |||
|---|---|---|---|---|---|
| CuII→CuI (CHA Cages) | CuII→CuI (D6R) | CuII→CuI (Total) | CuI→Cu0 | ||
| F−Cl | 53.69 | 28.71 | 82.40 | 163.34 | 245.74 |
| A−Cl | 4.32 | 25.56 | 29.88 | 32.89 | 62.77 |
| F−NO3 | 34.75 | 136.46 | 171.21 | 152.76 | 323.97 |
| A−NO3 | 25.74 | 111.43 | 137.17 | 79.50 | 216.67 |
| F−SO4 | 28.61 | 87.35 | 115.96 | 133.87 | 249.83 |
| A−SO4 | 4.17 | 58.48 | 62.65 | 0 | 62.65 |
| Samples | Adsorbed NH3 Amount (mmol g−1) | Total Amount (mmol g−1) | |||
|---|---|---|---|---|---|
| Physical Adsorption | Weak Lewis Acid Sites | Strong Lewis Acid Sites | Brønsted Acid Sites | ||
| F−Cl | 0.18 | 0.23 | 0.33 | 0.45 | 1.19 |
| A−Cl | 0.08 | 0.10 | 0.17 | 0.21 | 0.56 |
| F−NO3 | 0.20 | 0.21 | 0.32 | 0.54 | 1.27 |
| A−NO3 | 0.14 | 0.19 | 0.21 | 0.28 | 0.82 |
| F−SO4 | 0.18 | 0.23 | 0.31 | 0.47 | 1.19 |
| A−SO4 | 0.09 | 0.11 | 0.16 | 0.23 | 0.59 |
| Samples | Cusur (wt%) | CuII/Cusur | CuII/CuI | Sisur (wt%) | Alsur (wt%) | Si/Alsur |
|---|---|---|---|---|---|---|
| F−Cl | 0.56 | 0.44 | 0.63 | 6.59 | 3.42 | 1.93 |
| A−Cl | 0.29 | 0.15 | 0.35 | 6.30 | 2.66 | 2.37 |
| F−NO3 | 0.34 | 0.30 | 0.61 | 6.36 | 2.44 | 2.61 |
| A−NO3 | 0.57 | 0.17 | 0.39 | 6.66 | 2.95 | 2.26 |
| F−SO4 | 0.26 | 0.38 | 0.52 | 6.94 | 2.50 | 2.78 |
| A−SO4 | 0.77 | 0.24 | 0.35 | 6.65 | 3.36 | 1.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Peng, Z.; Zhang, C.; Liu, M.; Han, L.; Hou, Y.; Huang, Z.; Wang, J.; Bao, W.; Chang, L. Effect of Copper Precursors on the Activity and Hydrothermal Stability of CuII−SSZ−13 NH3−SCR Catalysts. Catalysts 2019, 9, 781. https://doi.org/10.3390/catal9090781
Wang M, Peng Z, Zhang C, Liu M, Han L, Hou Y, Huang Z, Wang J, Bao W, Chang L. Effect of Copper Precursors on the Activity and Hydrothermal Stability of CuII−SSZ−13 NH3−SCR Catalysts. Catalysts. 2019; 9(9):781. https://doi.org/10.3390/catal9090781
Chicago/Turabian StyleWang, Meixin, Zhaoliang Peng, Changming Zhang, Mengmeng Liu, Lina Han, Yaqin Hou, Zhanggen Huang, Jiancheng Wang, Weiren Bao, and Liping Chang. 2019. "Effect of Copper Precursors on the Activity and Hydrothermal Stability of CuII−SSZ−13 NH3−SCR Catalysts" Catalysts 9, no. 9: 781. https://doi.org/10.3390/catal9090781
APA StyleWang, M., Peng, Z., Zhang, C., Liu, M., Han, L., Hou, Y., Huang, Z., Wang, J., Bao, W., & Chang, L. (2019). Effect of Copper Precursors on the Activity and Hydrothermal Stability of CuII−SSZ−13 NH3−SCR Catalysts. Catalysts, 9(9), 781. https://doi.org/10.3390/catal9090781







