Selective CO Hydrogenation Over Bimetallic Co-Fe Catalysts for the Production of Light Paraffin Hydrocarbons (C2–C4): Effect of Space Velocity, Reaction Pressure and Temperature
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Characterization
3.3. Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, B.; Chen, Z.; Chen, Y.; Ma, X. Syngas methanation over Ni/SiO2 catalyst prepared by ammonia-assisted impregnation. Int. J. Hydrog. Energy 2017, 42, 27073–27083. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.; Hong, U.G.; Gil Seo, J.; Jung, J.C.; Koh, D.J.; Lim, H.; Byun, C.; Song, I.K. Methane production from carbon monoxide and hydrogen over nickel–alumina xerogel catalyst: Effect of nickel content. J. Ind. Eng. Chem. 2011, 17, 154–157. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Li, J.; Zhang, M.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Ni/Al2O3 catalysts for CO methanation: Effect of Al2O3 supports calcined at different temperatures. J. Energy Chem. 2013, 22, 919–927. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M. Production of synthetic natural gas (SNG) from coal and dry biomass—A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.; Wang, X.; Yin, S.; Leng, F.; Zhang, F.; Lin, H.; Wang, S. Catalytic methanation of syngas over Ni-based catalysts with different supports. Chin. J. Chem. Eng. 2017, 25, 602–608. [Google Scholar] [CrossRef]
- Inui, T.; Sakamoto, A.; Takeguchi, T.; Ishigaki, Y. Synthesis of highly calorific gaseous fuel from syngas on cobalt-manganese-ruthenium composite catalysts. Ind. Eng. Chem. Res. 1989, 28, 427–431. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, H.; Choi, H.S.; Lee, D.-W.; Lee, K.-Y. Co-Mn-Ru/Al2O3 catalyst for the production of high-calorific synthetic natural gas. Korean J. Chem. Eng. 2015, 32, 2220–2226. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, D.-W.; Kim, H.; Choi, H.S.; Lee, K.-Y. Fe–Zn catalysts for the production of high-calorie synthetic natural gas. Fuel 2015, 159, 259–268. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, D.-W.; Lee, K.-Y. Production of high-calorie synthetic natural gas using copper-impregnated iron catalysts. J. Mol. Catal. A Chem. 2016, 425, 190–198. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, K.-Y. Effect of surface composition of Fe catalyst on the activity for the production of high-calorie synthetic natural gas (SNG). Korean J. Chem. Eng. 2017, 34, 320–327. [Google Scholar] [CrossRef]
- Jo, S.B.; Chae, H.J.; Kim, T.Y.; Lee, C.H.; Oh, J.U.; Kang, S.-H.; Kim, J.W.; Jeong, M.; Lee, S.C.; Kim, J.C. Selective CO hydrogenation over bimetallic Co-Fe catalysts for the production of light paraffin hydrocarbons (C2-C4): Effect of H2/CO ratio and reaction temperature. Catal. Commun. 2018, 117, 74–78. [Google Scholar] [CrossRef]
- Jo, S.B.; Kim, T.Y.; Lee, C.H.; Kang, S.-H.; Kim, J.W.; Jeong, M.; Lee, S.C.; Kim, J.C. Hybrid catalysts in a double-layered bed reactor for the production of C2–C4 paraffin hydrocarbons. Catal. Commun. 2019, 127, 29–33. [Google Scholar] [CrossRef]
- Lee, J.; Kang, S. Formation behaviours of mixed gas hydrates including olefin compounds. Chem. Eng. Trans. 2013, 32, 1921–1926. [Google Scholar]
- Griboval-Constant, A.; Butel, A.; Ordomsky, V.V.; Chernavskii, P.A.; Khodakov, A.; Khodakov, A. Cobalt and iron species in alumina supported bimetallic catalysts for Fischer–Tropsch reaction. Appl. Catal. A Gen. 2014, 481, 116–126. [Google Scholar] [CrossRef]
- Lögdberg, S.; Tristantini, D.; Borg, Ø.; Ilver, L.; Gevert, B.; Järås, S.; Blekkan, E.A.; Holmén, A. Hydrocarbon production via Fischer–Tropsch synthesis from H2-poor syngas over different Fe-Co/γ-Al2O3 bimetallic catalysts. Appl. Catal. B Environ. 2009, 89, 167–182. [Google Scholar] [CrossRef]
- Rytter, E.; Holmen, A. Deactivation and Regeneration of Commercial Type Fischer-Tropsch Co-Catalysts—A Mini-Review. Catalysts 2015, 5, 478–499. [Google Scholar] [CrossRef]
- Gao, J.; Gu, F.; Zhong, Z.; Liu, Q.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. RSC Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Lv, X.; Li, Z. Co hydrogenation combined with water-gas-shift reaction for synthetic natural gas production: A thermodynamic and experimental study. Int. J. Coal Sci. Technol. 2018, 5, 439–451. [Google Scholar] [CrossRef]
- Yang, J.; Ma, W.; Chen, D.; Holmén, A.; Davis, B.H. Fischer–Tropsch synthesis: A review of the effect of CO conversion on methane selectivity. Appl. Catal. A Gen. 2014, 470, 250–260. [Google Scholar] [CrossRef]
- Novak, S. Secondary effects in the Fischer-Tropsch synthesis. J. Catal. 1982, 77, 141–151. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Kirchner, J.; Anolleck, J.K.; Lösch, H.; Kureti, S. Methanation of CO2 on iron based catalysts. Appl. Catal. B Environ. 2018, 223, 47–59. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2017, 293, 89–96. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; De Jong, K.P. Catalysts for Production of Lower Olefins from Synthesis Gas: A Review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
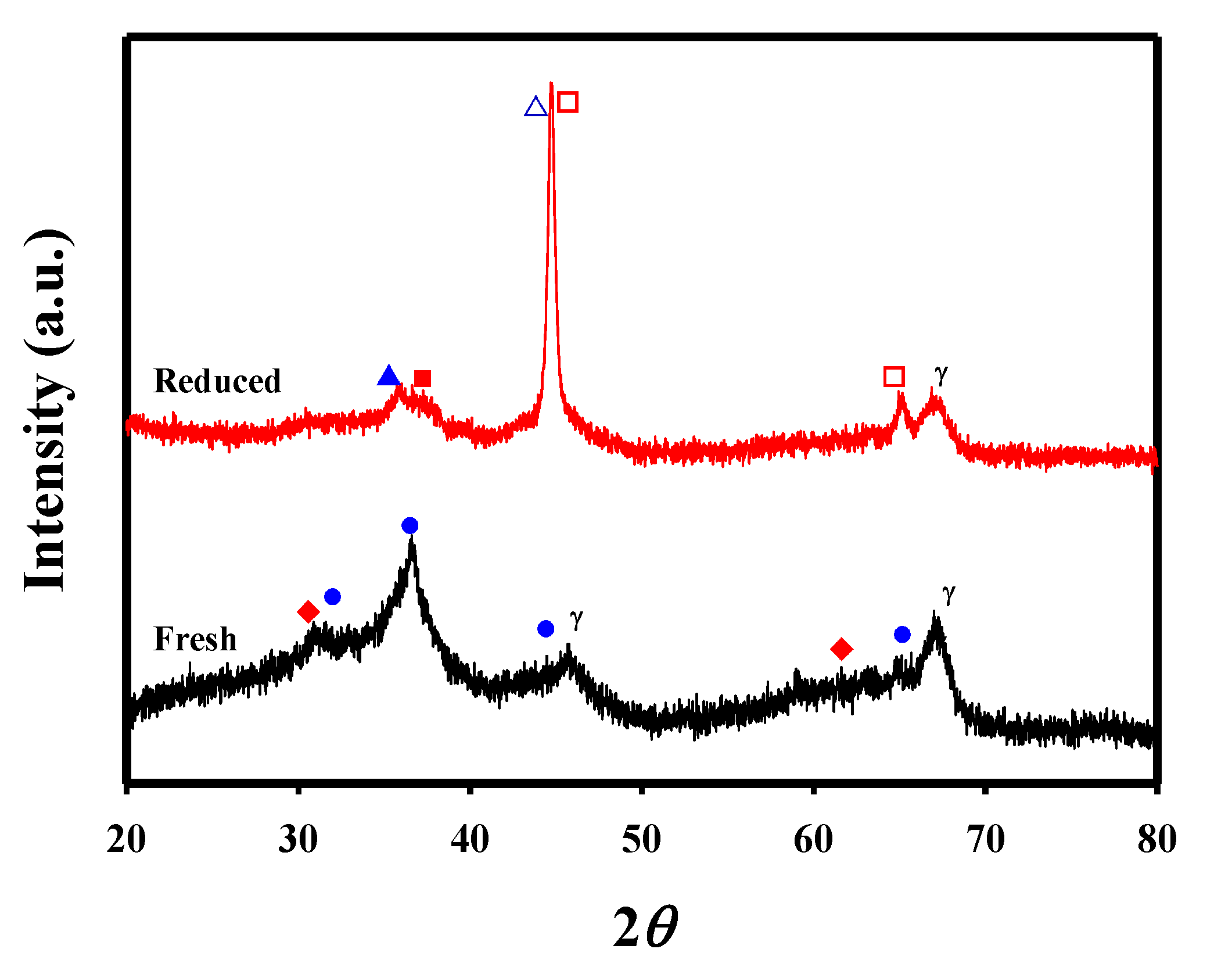
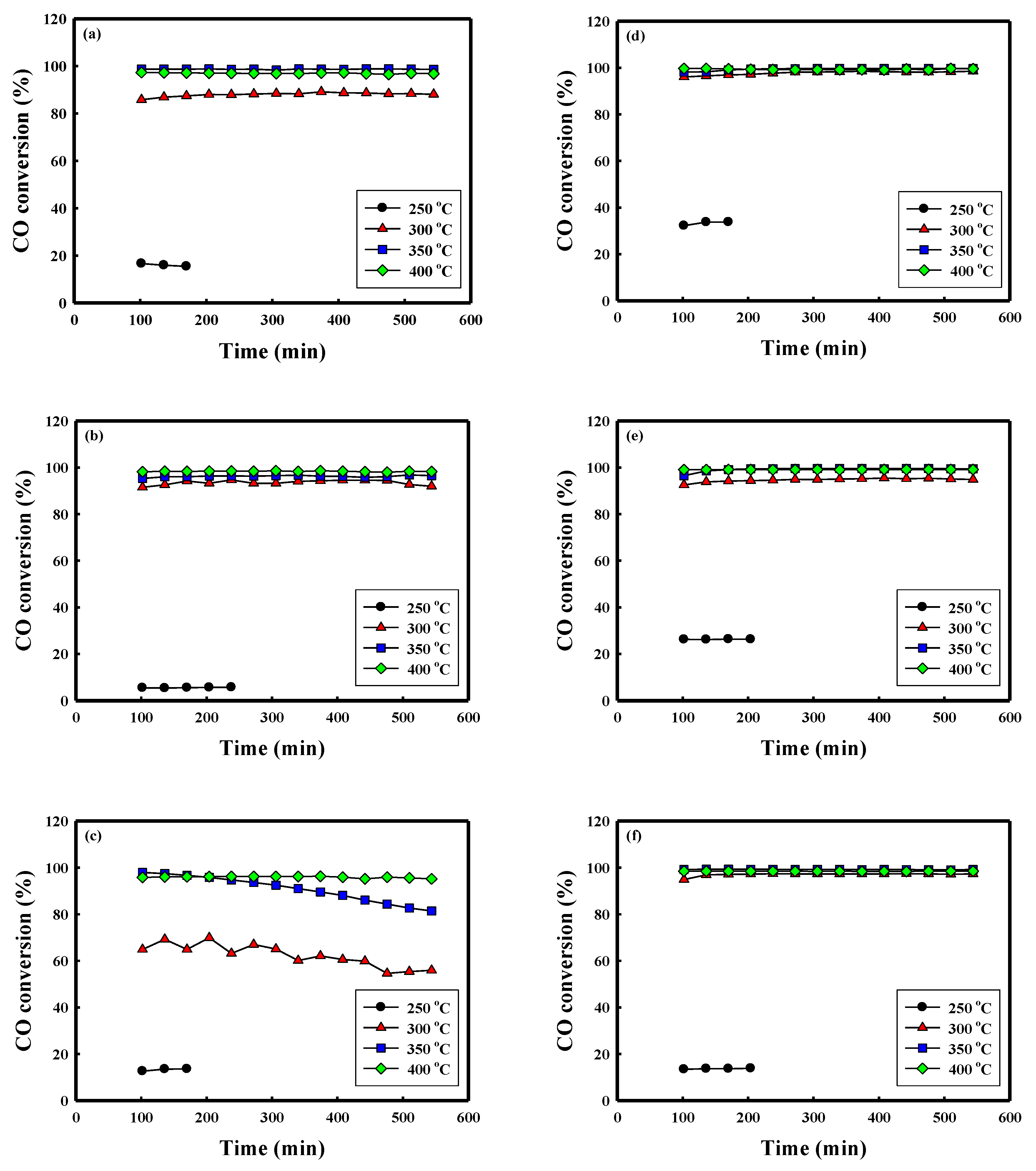
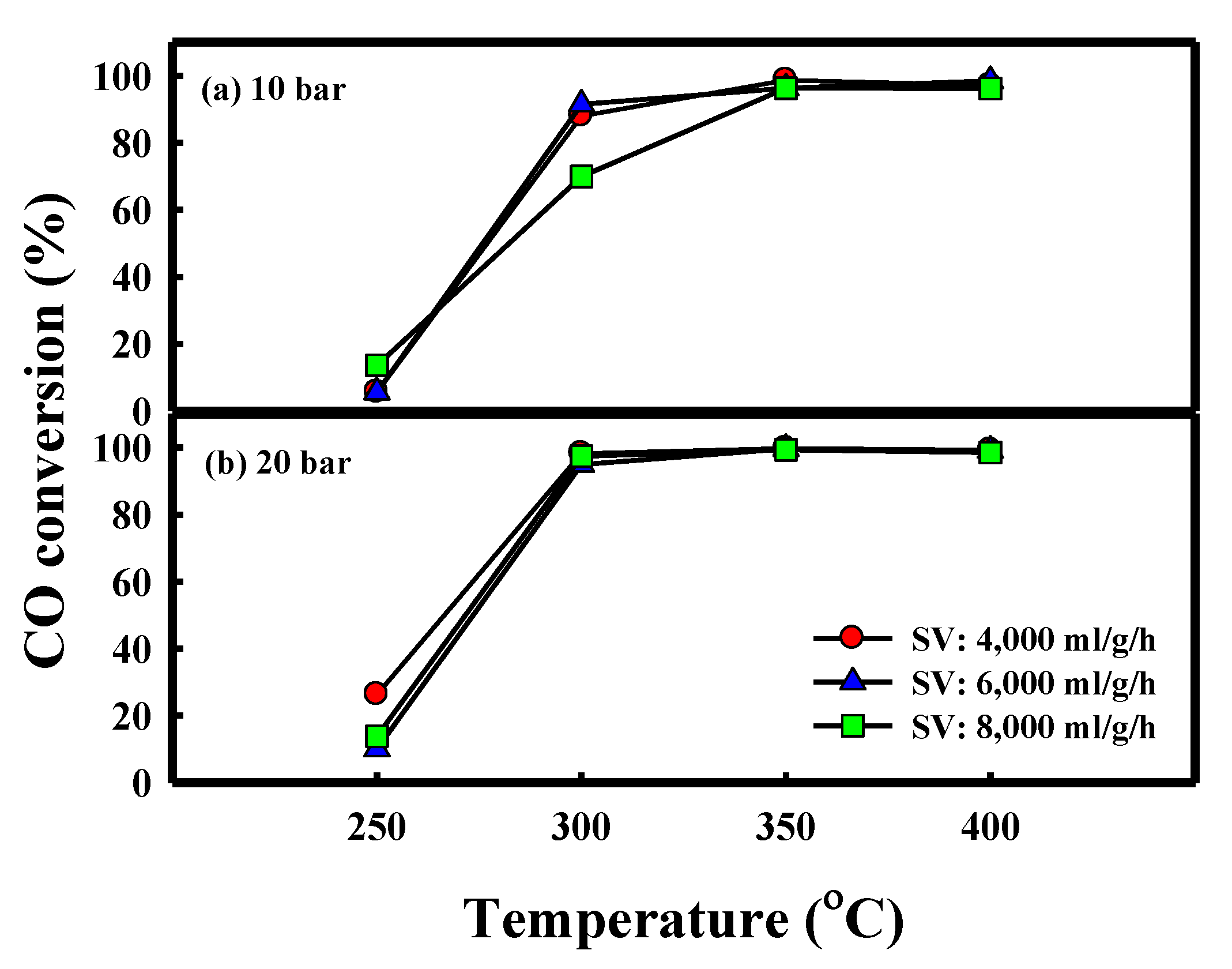
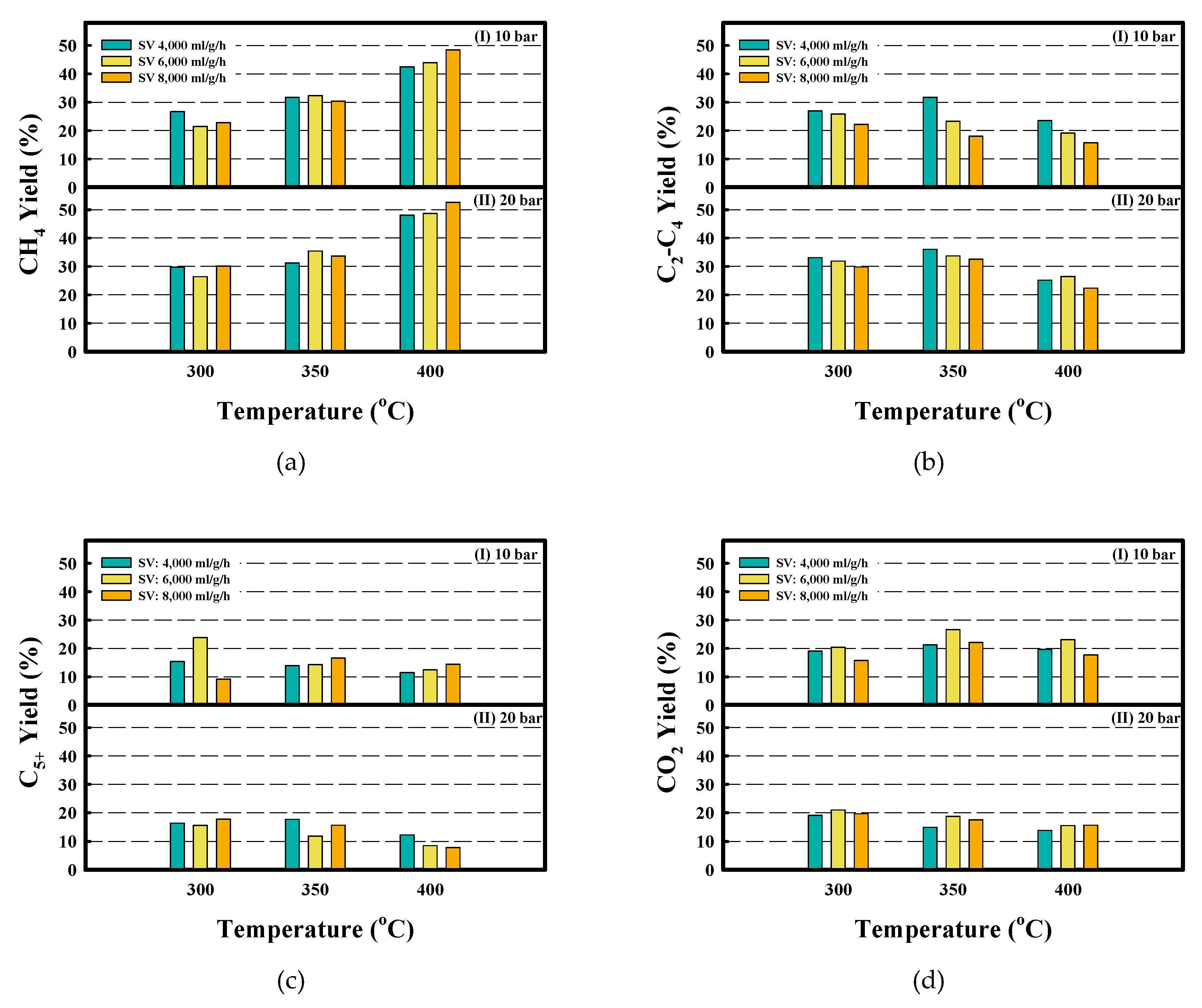
| Metal Content (wt.%) a | Textural Properties | Crystallite Size (nm) c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Co | Fe | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) b | Fresh | Reduced | |||
| CoO | Fe2O3 | Co0 | Fe0 | ||||||
| γ-Al2O3 | - | - | 156.9 | 0.23 | 5.9 | - | - | - | - |
| 5Co-15Fe/γ-Al2O3 | 5.2 | 14.1 | 40.6 | 0.09 | 4.6 | 4.7 | - | - | 20 |
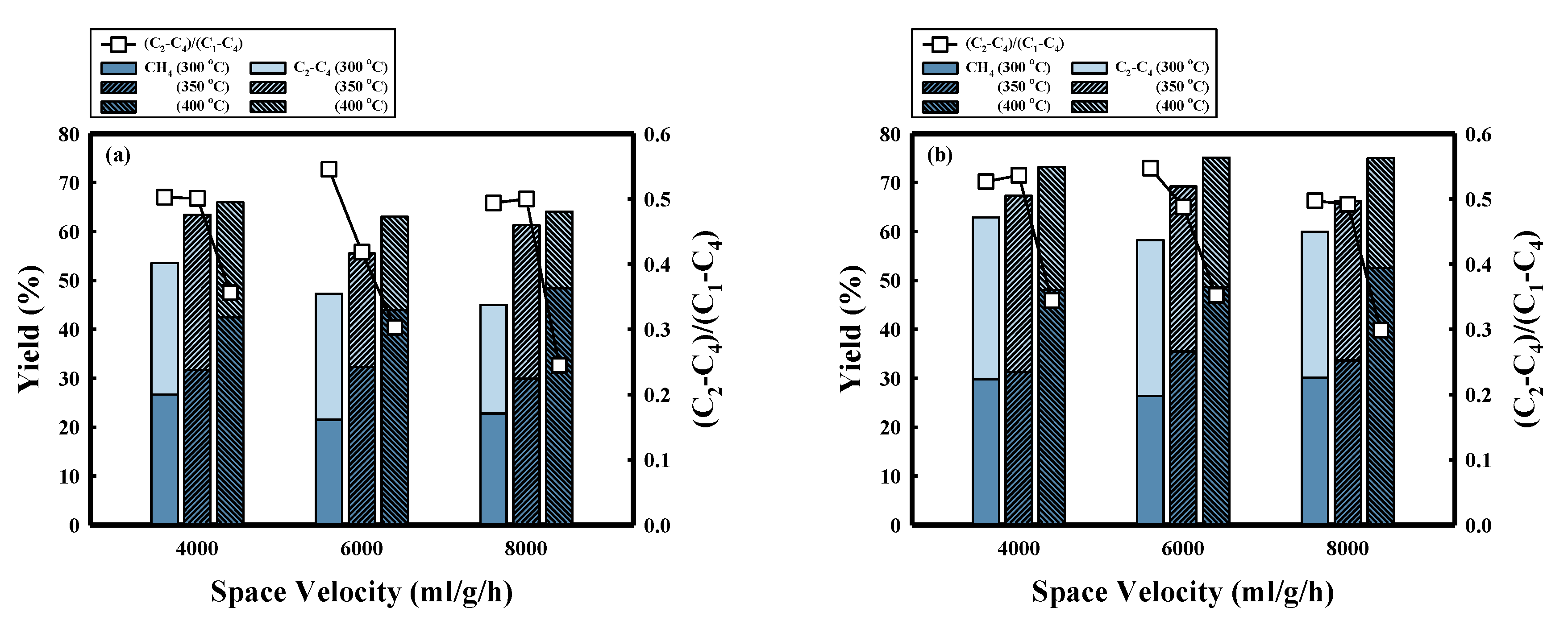
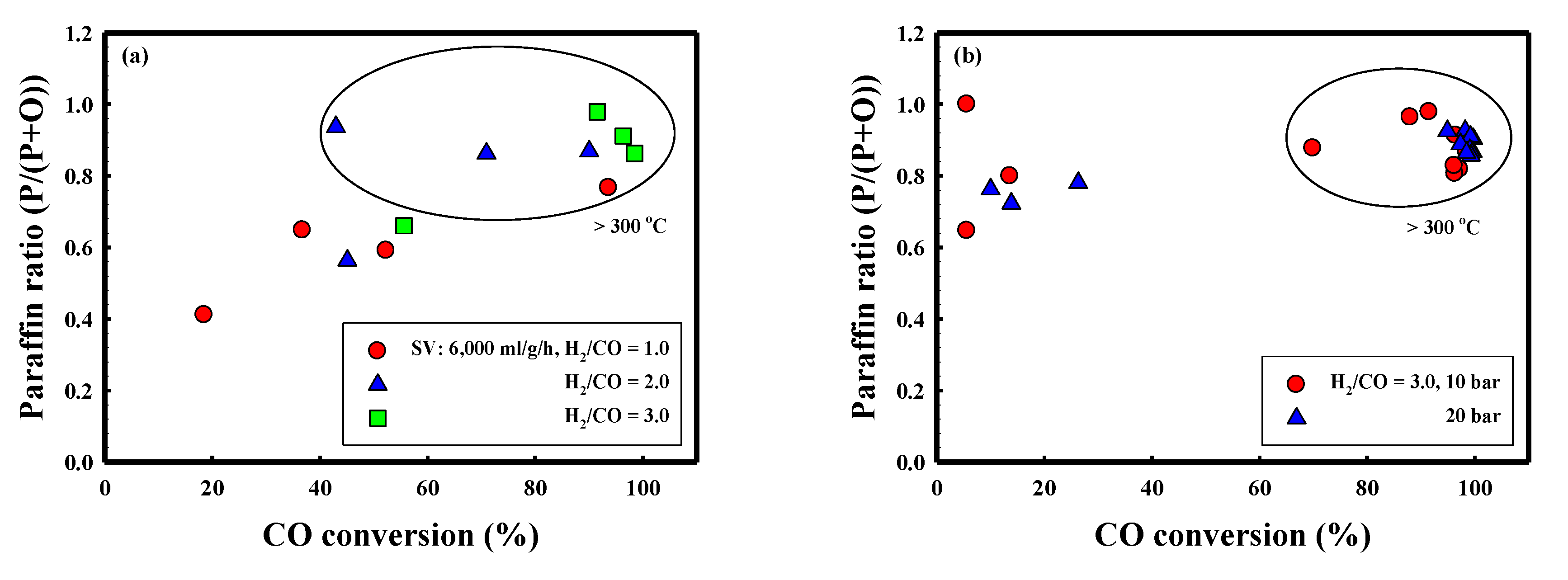
| P (bar) | SV (ml/g/h) | T (°C) | Conversion | Yield (%) | (C2–C4)/(C1–C4) | P/(P+O) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CO | H2 | CH4 | C2–C4 | C5+ | CO2 | |||||
| 10 | 4000 | 300 | 88.0 ± 1.2 | 43.1 ± 0.5 | 26.7 ± 0.9 | 26.9 ± 1.8 | 15.4 ± 0.3 | 19.1 ± 0.1 | 0.50 | 0.96 |
| 350 | 98.7 ± 0.1 | 49.3 ± 0.2 | 31.7 ± 2.1 | 31.8 ± 0.6 | 13.9 ± 2.5 | 21.2 ± 0.2 | 0.50 | 0.89 | ||
| 400 | 97.2 ± 0.2 | 51.7 ± 0.2 | 42.5 ± 1.5 | 23.5 ± 1.8 | 11.5 ± 0.5 | 19.7 ± 0.4 | 0.36 | 0.82 | ||
| 6000 | 300 | 91.5 ± 1.0 | 38.2 ± 0.9 | 21.5 ± 0.7 | 25.8 ± 0.6 | 23.8 ± 1.6 | 20.4 ± 0.4 | 0.55 | 0.98 | |
| 350 | 96.4 ± 0.2 | 40.0 ± 0.8 | 32.3 ± 0.4 | 23.2 ± 0.3 | 14.3 ± 0.7 | 26.6 ± 0.2 | 0.42 | 0.91 | ||
| 400 | 98.5 ± 0.0 | 47.6 ± 0.2 | 43.9 ± 0.5 | 19.1 ± 1.5 | 12.4 ± 2.1 | 23.0 ± 0.2 | 0.30 | 0.87 | ||
| 8000 | 300 | 77.1 ± 15.1 | 35.8 ± 6.6 | 22.8 ± 1.1 | 22.2 ± 2.2 | 9.2 ± 3.2 | 15.7 ± 3.3 | 0.49 | 0.88 | |
| 350 | 96.3 ± 4.9 | 31.7 ± 2.0 | 29.9 ± 1.4 | 31.4 ± 1.1 | 15.5 ± 1.6 | 19.5 ± 0.8 | 0.51 | 0.81 | ||
| 400 | 96.2 ± 0.2 | 55.3 ± 1.2 | 48.4 ± 0.3 | 15.7 ± 0.3 | 14.4 ± 0.9 | 17.7 ± 0.3 | 0.24 | 0.83 | ||
| 20 | 4000 | 300 | 98.2 ± 0.1 | 50.8 ± 0.1 | 29.8 ± 0.0 | 33.1 ± 0.0 | 16.3 ± 0.1 | 19.1 ± 0.1 | 0.53 | 0.93 |
| 350 | 99.7 ± 0.1 | 57.0 ± 0.2 | 31.2 ± 0.4 | 36.1 ± 0.6 | 17.6 ± 0.4 | 14.9 ± 0.2 | 0.54 | 0.90 | ||
| 400 | 99.2 ± 0.1 | 62.2 ± 0.6 | 48.0 ± 1.6 | 25.2 ± 1.6 | 12.2 ± 0.6 | 13.8 ± 0.1 | 0.34 | 0.91 | ||
| 6000 | 300 | 90.5 ± 0.3 | 42.6 ± 0.2 | 26.4 ± 0.3 | 31.9 ± 0.2 | 15.6 ± 0.5 | 21.0 ± 0.0 | 0.55 | 0.93 | |
| 350 | 99.6 ± 0.1 | 53.0 ± 0.1 | 35.5 ± 0.2 | 33.8 ± 0.2 | 11.8 ± 0.4 | 18.7 ± 0.0 | 0.49 | 0.87 | ||
| 400 | 99.1 ± 0.0 | 60.3 ± 0.1 | 48.7 ± 0.7 | 26.5 ± 0.6 | 8.5 ± 1.0 | 15.5 ± 0.1 | 0.35 | 0.87 | ||
| 8000 | 300 | 97.2 ± 0.2 | 48.8 ± 0.2 | 30.2 ± 0.4 | 29.8 ± 0.8 | 17.7 ± 0.4 | 19.6 ± 0.0 | 0.50 | 0.89 | |
| 350 | 99.3 ± 0.1 | 53.7 ± 0.3 | 33.7 ± 0.8 | 32.6 ± 1.4 | 15.6 ± 1.8 | 17.5 ± 0.4 | 0.49 | 0.86 | ||
| 400 | 98.5 ± 0.1 | 58.7 ± 0.3 | 52.6 ± 1.3 | 22.4 ± 0.9 | 7.8 ± 1.7 | 15.6 ± 0.1 | 0.30 | 0.86 | ||
| Catalysts | H2/CO | Reaction Condition | CO Conv. (%) | Yield | P/(P+O) | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2–C4 | C5+ | CO2 | ||||||
| 5Co-15Fe/γ-Al2O3 | 3.0 | SV: 6000 ml/g/h, 300 °C, 10 bar | 91.5 | 21.5 | 25.8 | 23.8 | 20.4 | 0.98 | This study |
| 3.0 | SV: 4000 ml/g/h, 350 °C, 20 bar | 99.7 | 31.2 | 36.1 | 17.6 | 14.9 | 0.90 | This study | |
| 10Co-6Mn-2Ru/γ-Al2O3 a | 3.0 | SV: 6000 ml/g/h, 300 °C, 10 bar | 99.7 | 60.6 | 24.5 | 4.8 | 10.9 | 0.96 | [8] |
| 10Co-6Mn-2.5Ru/γ-Al2O3 b | 3.0 | SV: 6000 ml/g/h, 250 °C, 10 bar | 100.0 | 53.0 | 23.0 | 8.6 | n/a g | n/a g | [7] |
| 20Co-16Mn/γ-Al2O3 c | 3.0 | SV: 6000 ml/g/h, 250 °C, 10 bar | 92.0 | 53.0 | 24.0 | 5.8 | n/a g | n/a g | [7] |
| FC15 d | 3.0 | SV: 6000 ml/g/h, 300 °C, 10 bar | 97.5 | 21.5 | 35.7 | 12.9 | 27.4 | 0.58 | [9] |
| FZ5 e | 3.0 | SV: 6000 ml/g/h, 300 °C, 10 bar | 89.9 | 19.1 | 35.3 | 24.1 | 24.1 | 0.76 | [8] |
| FZ10 f | 3.0 | SV: 6000 ml/g/h, 300 °C, 10 bar | 98.2 | 23.7 | 40.0 | 12.7 | 21.9 | 0.70 | [8] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.B.; Kim, T.Y.; Lee, C.H.; Woo, J.H.; Chae, H.J.; Kang, S.-H.; Kim, J.W.; Lee, S.C.; Kim, J.C. Selective CO Hydrogenation Over Bimetallic Co-Fe Catalysts for the Production of Light Paraffin Hydrocarbons (C2–C4): Effect of Space Velocity, Reaction Pressure and Temperature. Catalysts 2019, 9, 779. https://doi.org/10.3390/catal9090779
Jo SB, Kim TY, Lee CH, Woo JH, Chae HJ, Kang S-H, Kim JW, Lee SC, Kim JC. Selective CO Hydrogenation Over Bimetallic Co-Fe Catalysts for the Production of Light Paraffin Hydrocarbons (C2–C4): Effect of Space Velocity, Reaction Pressure and Temperature. Catalysts. 2019; 9(9):779. https://doi.org/10.3390/catal9090779
Chicago/Turabian StyleJo, Seong Bin, Tae Young Kim, Chul Ho Lee, Jin Hyeok Woo, Ho Jin Chae, Suk-Hwan Kang, Joon Woo Kim, Soo Chool Lee, and Jae Chang Kim. 2019. "Selective CO Hydrogenation Over Bimetallic Co-Fe Catalysts for the Production of Light Paraffin Hydrocarbons (C2–C4): Effect of Space Velocity, Reaction Pressure and Temperature" Catalysts 9, no. 9: 779. https://doi.org/10.3390/catal9090779
APA StyleJo, S. B., Kim, T. Y., Lee, C. H., Woo, J. H., Chae, H. J., Kang, S.-H., Kim, J. W., Lee, S. C., & Kim, J. C. (2019). Selective CO Hydrogenation Over Bimetallic Co-Fe Catalysts for the Production of Light Paraffin Hydrocarbons (C2–C4): Effect of Space Velocity, Reaction Pressure and Temperature. Catalysts, 9(9), 779. https://doi.org/10.3390/catal9090779







