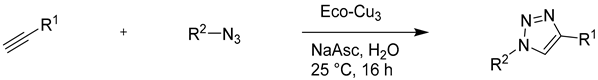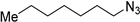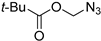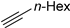Abstract
The abundance of Cu-contaminated effluents and the serious risk of contamination of the aquatic systems combine to provide strong motivating factors to tackle this environmental problem. The treatment of polluted effluents by rhizofiltration and biosorption is an interesting ecological alternative. Taking advantage of the remarkable ability of the selected plants to bioconcentrate copper into roots, these methods have been exploited for the decontamination of copper-rich effluents. Herein, we present an overview on the utility of the resulted copper-rich biomass for the preparation of novel bio-sourced copper-based catalysts for copper-mediated reactions: from the bioaccumulation of copper in plant, to the preparation and full analysis of the new Eco-Cu catalysts, and their application in selected key reactions. The hydrolysis of a thiophosphate, an Ullmann-type coupling leading to N- and O-arylated compounds, and a CuAAC “click” reaction, all performed under green and environmentally friendly conditions, will be described.
1. Introduction
Numerous of the greatest successes of organometallic and inorganic chemistry are based on the use of metal complexes for catalysis and organic synthesis [1,2]. In many cases, such metal complexes, based most frequently on precious metals such as platinoids, allow the creation of new C–C or C–H bonds or the cleavage of H–H bonds, etc. Wilkinson [3,4] and, more recently, Noyori and coworkers [5] have developed remarkably reactive rhodium and ruthenium complexes for use in catalytic asymmetric hydrogenations of C=C or C=O bonds. Nowadays, many C–C, C–N, and C–O coupling reactions; C–H activations; and metal catalysed redox reactions are widely used in organic processes [6]. However, the main limitation of these catalytic systems remains the cost and availability of the metal used [6].
Therefore, the current challenges rely precisely in the use of new metal catalytic systems replacing platinoids by more abundant, cheap, and readily available metals [7]. In that scenario, copper is a transition metal that perfectly meets these criteria [8]. Indeed, the literature testifies a broad range of reactivity of copper species (Figure 1). One of the most famous is the ability of copper to promote coupling reactions [9,10], among them the Ullmann-type reactions [11,12,13,14]. Indeed, the literature offers many methodologies for the formation of C–N bonds, such as the arylation of amines [15,16,17], the arylation and vinylation of N-heterocycles [18], aromatic amidation (Goldberg reaction) [19,20], and azidation [21]. These Ullmann-type reactions have been extended to the formation of C–O bonds that led to the synthesis of diaryl ethers [22], to the aryloxylation of vinyl halides [23,24], or to the cross-coupling of aryl halides with aliphatic alcohols [25]. Likewise, formations of C–C bonds were reported, including cross-coupling with terminal acetylene [26,27,28,29], the arylation of activated methylene compounds [30], and cyanation [31]. Worth mentioning are also the C–S bond formation (the synthesis of bisaryl- and arylalkyl-thioethers [32,33] and the assembly of aryl sulfones [34]) and the C–P bond formation by copper catalysis [35]. Another key part of copper chemistry is the 1,3-dipolar cycloaddition, in particular the Cu-catalysed Azide-Alkyne Cycloaddition (CuAAC) “click” [36,37]. Indeed, this reaction represented the prime example of the concept of “click” chemistry, developed by Sharpless et al. [38]. This reaction had a huge influence on drug discovery and became very popular in biological and medicinal science as a ligation tool [39]. Copper can also be a versatile catalyst and promote reductions (hydrosilylation of ketones [40]) or oxidations (cleavage/oxidation of benzylidene acetals [41]). As last examples, copper catalyses the protection/deprotection transformations of important functional groups such as the formation of isopropylidene acetals [42], the deprotection of thioacetals, and selective ester hydrolysis [43].
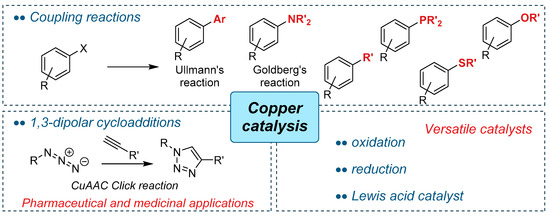
Figure 1.
A representation of different reactivities of copper species.
Furthermore, in line with the current trends in sustainable and green chemistry, heterogenous catalysts for copper-based reactions were reported in the literature. This area of research continues to evolve as a more suitable approach because of its crucial advantages such as the easy separation of products from catalyst, a high stability of the heterogenous catalysts, and, most importantly, their recyclability. In that aspect, the selected examples of heterogenous copper catalysts employing Cu/C (copper-on-charcoal) [44,45], copper powder [46], magnetite-supported copper nanoparticles [47], Cu/ligand catalyst immobilized on silica [48], CuI immobilised on MOF [49], alumina-supported CuO [50], and recent mesoporous copper supported on manganese oxide material (meso Cu/MnOx) [51] as well as copper oxide catalysts supported on three dimensional mesoporous aluminosilicates [52] are certainly worth mentioning. Additionally, representative examples of Ullmann-type reactions using organic (bio)polymers [53,54,55], or zeolites [56], as supports should be mentioned.
Copper-catalysed reactions have found wide applications for the synthesis of natural products, biomolecules, and precursors of advanced materials [57,58,59]. Heterocycles have been efficiently prepared through copper-catalysed cascade or multicomponent reactions [60,61,62,63,64], some of which have led to the synthesis of pharmaceutically important compounds [65,66,67].
Given the importance of copper-based catalysis, the purpose of this review is to discuss the recent advances in the preparation of the first fully bio-based, ecological copper catalysts, named Eco-Cu, and their application in organic synthesis.
In a first part, this review will focus on the preparation of the ecocatalysts based on the use of Cu-rich biomasses and remedial phytotechnologies (i.e., rhizofiltration [68,69,70] or biosorption [71]) in order to decontaminate metal-polluted water. This part highlights the possibility and feasibility to efficiently reconcile copper-catalysis for organic synthesis with the protection of the environment, including the preservation or remediation of aquatic systems. Rhizofiltration refers to the approach in which aquatic plant roots are used to purify contaminated water through metabolically mediated process, whereas biosorption can be defined as the ability of biological materials to accumulate pollutants from wastewater through physicochemical pathways based on different mechanisms including absorption, adsorption, ion exchange, surface complexation, or precipitation.
In a second part of this review, the full characterisation of the copper ecocatalysts will be described. Polymetallic compositions, morphology, Lewis acid properties, and oxidation’s states of reported ecocatalysts will be detailed in order to rationalise and predict their reactivity.
Finally, the synthetic potential of Eco-Cu will be illustrated through three major applications of copper catalysis: (i) the hydrolysis of the thiophosphate group in an important example of parathion [68], (ii) the Ullmann coupling for N- and O-arylation [69], and finally, (iii) the CuAAC “click” reaction performed under green and environmentally friendly conditions [70].
2. Phytoaccumulation of Copper
There are different ways to phytoaccumulate metallic elements. In this part, we will focus on the rhizofiltration and biosorption that have been used by Grison et al. to prepare copper ecocatalysts [69,70,71].
2.1. Preparation via Rhizofiltration
The rhizofiltration of copper was studied with three aquatic plants: Bacopa monierri, Lolium multifolium, and Eichhornia crassipes [69,70]. When choosing the plants, two criteria were selected: i) the biomass should be insoluble in water and ii) the structure of the biomass should be based on carbon-containing aromatic compounds and contain many carboxylate groups naturally present in the material of plant origin. Table 1 presents the concentration of copper in each plant after rhizofiltration and the bioconcentration factor of each accumulation. The bioconcentration rates of Cu in the roots were evaluated by ICP-MS (Inductively Coupled Plasma Mass Spectrometry).

Table 1.
The copper concentration in roots and bioconcentration factor (BCF) calculation.
The BCF is the ratio of the amount of copper accumulated in the roots to the concentration of the element left in the effluent after rhizofiltration. For all species, the initial concentrations of copper were the same. The BCF results showed that each studied aquatic plant was capable of bioconcentrating copper in large quantities. E. crassipes, however, was found to be the most efficient compared to B. monnieri and L. multiflorum, the latter being the least effective of the three species for this bioaccumulation. The E. crassipes also has the advantage of having a very important root biomass, and therefore, this plant represents the best candidate for rhizofiltration in order to prepare a large quantity of Cu-rich ecocatalysts.
2.2. Preparation by Biosorption
Biosorption is using natural waste, abundant and rich in tannin biomasses, as a metal accumulator. The same authors recently illustrated this methodology by using coffee grounds to accumulate different metals, in particular copper [71]. In order to increase their adsorption capacity and affinity with the metal, the coffee grounds were functionalised with citric acid. This modification was described as easily doable and important for the efficiency of the biosorbant but also for its preservation. Table 2 depicts the biosorption of different copper solution by analysing the quantity of the element in the effluent, before and after accumulation.

Table 2.
The biosorption of copper using functionalized coffee grounds. 1
It appeared that the maximum biosorption capacity of the functionalised coffee grounds was very high. These data could be advantageously compared to the values reported in the literature by Cerino-Córdova et al. [72]. The authors described the accumulation of copper with closely related materials. The coffee grounds’ functionalisation’s conditions are different in both studies. In Cerino-Córdova’s work, the esterification reaction occurred in aqueous medium, compared to Grison’s conditions in ethanol. In addition, the biosorption tests were carried out at different pH, slightly acidic (pH = 5) in Cerino-Córdova’s work, where a large part of the carboxylate functions was protonated, and this fact limited the biosorption of the copper. Under Grison’s conditions, the biosorption was faster (2 hours compared to 5 days) at a neutral pH. The biosorption seemed then faster than the precipitation of Cu2+ ions. The biosorption method was, therefore, ideal for recycling Cu(II) species from homogeneous catalysis reactions.
3. Preparation and Characterisation of the Copper Ecocatalyst, Eco-Cu
3.1. Preparation via Rhizofiltration
An interesting recovery of Cu-rich biomasses or Cu-rich coffee grounds is the preparation of bio-based catalysts, called Eco-Cu. A sequence of treatments was developed for the reproductible preparation of Eco-Cu [69,70]. After a controlled thermal treatment under air, the organic matter was converted into CO2 and H2O. The mineral residue polymetallic oxidized species were treated under acidic condition (HCl in this case) in order to form metallic chloride species. The mineral compositions of the resulting Eco-Cu were measured by ICP-MS (Inductively Coupled Plasma Mass Spectrometry) (Table 3).

Table 3.
The mineral compositions of the different Eco-Cu.
It appeared that the functionalized coffee grounds could absorb by biosorption more copper element than the plants by rhizofiltration. This made coffee grounds a promising material for recycling copper in effluents. However, the lack of physiological metallic elements (for example Fe and K) in it decreased the catalytic activity, in comparison to the Eco-Cu prepared from plants. Therefore, the Infrared (IR) study of the Lewis and Brønsted acidic character of Eco-Cu4 was not carried out. The polymetallic composition of the other Eco-Cu was clearly an advantage.
3.2. Identification of the Degree of Oxidation
Usually, X-ray Photoelectron Spectrometry (XPS) allows the determination of the oxidation state of a metallic centre. In the case of copper salts, the XPS analysis did not allow to identify the oxidation’s state, as the method induces the reduction of Cu(II) salts to Cu(I). The oxidation’ states were established by different colourimetric methods, the oxime [73] and ammonia tests [74].
Both tests supported the presence of Cu(II) species in all catalysts.
3.3. Direct-Injection Mass Spectrometric Analysis of Eco-Cu3
In order to specify the nature of the copper(II) chloride salts present in Eco-Cu3, direct injection electrospray mass spectrometry operating in negative ion mode (MS-ESI) analyses were carried out. From Eco-Cu3, two copper chloride species were detected: CuCl32− and CuCl2−. During the experiment, an electrochemical reduction of Cu(II) into Cu(I) was observed. This led to the conclusion that Eco-Cu3 consisted of a mixture of CuCl42− and CuCl3−.
3.4. Morphology Study of Eco-Cu3 by BET Analyses
BET analyses were performed in order to study the morphology of the new materials. The surface properties of the Eco-Cu3 catalyst were determined by nitrogen sorption. The material has a relatively low porosity with an specific surface area SBET of 12.0 m2.g−1 and a mesoporous volume Vmeso of 0.07 cm3.g−1. An absorption of nitrogen appeared at high P/P0 values, indicating a broad pore size distribution and an average pore size greater than 10 nm, due in part to intergranular porosity. Overall, bulk copper(I) catalyst exhibit a small surface area (for example 1.9 m2.g−1 with CuO). However copper nanoparticles with a defined size can have a greater surface area (136 m2.g−1), which allows for enhanced catalytic activity in an Ullmann reaction [9].
3.5. XRD Analysis of the Eco-Cu3 Catalyst
X-ray Diffraction (XRD) analyses were performed in order to determine the crystalline structure of the different complexes in the Eco-Cu3 catalyst. No crystalline form of copper was observed. However, a variety of salts such as K6Fe2O5, MnO, NaCl, and CaSO4 were detected. Among them, K6Fe2O5, a polymetallic species, was reported by Shanks et al. as an efficient catalyst for the ethylbenzene dehydrogenation into styrene [75].
3.6. Analysis of the Acidic Properties of the Eco-Cu
3.6.1. Lewis and Brønsted Acidic Character
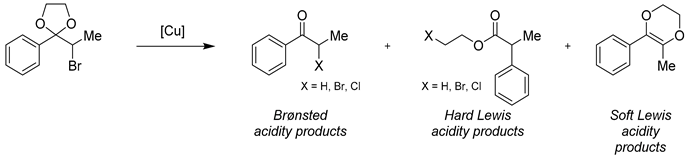
The Lewis and Brønsted acid properties of the ecocatalyst Eco-Cu3 were examined and compared to commercially available copper chloride. A first method of acidic properties analysis was based on the IR study of pyridine sorption/desorption at 25 °C and then 150 °C in order to distinguish the physisorbed pyridine from pyridine coordinately bonded to Lewis acid sites [76,77]. Pyridine is commonly used as a probe to evaluate the acidity of known Lewis and Brønsted acids by controlling its infrared absorption bands observed between 1400 and 1660 cm−1. Absorption bands between 1445–1460 cm−1 are characteristic for strongly bound Lewis acid sites of pyridine. The frequencies of these absorption bands were similar in the four catalysts (Table 4). Only in the case of Eco-Cu2 the values were lower, suggesting a weaker Lewis acid character for this ecocatalyst. It was concluded that the Lewis acid character of Eco-Cu1,3, anhydrous CuCl2, and CuCl2·2H2O was similar. Surprisingly, in the case of Eco-Cu1 and Eco-Cu3, we have also observed absorption bands at 1529 and 1530 cm−1. This observation was interpreted as a proof for the unique Brønsted acid character of that ecocatalyst, not observed either in the case on commercially available copper chloride.

Table 4.
The IR spectra of adsorbed pyridine on commercial anhydrous CuCl2, on CuCl2·2H2O, and on Eco-Cu1–3.
3.6.2. Analysis of the Lewis and Brønsted Acid Properties by the Corma Method
The results and information obtained during IR analysis were supported by the results of Corma’s test performed in the related work. This method introduced by Corma et al. consists of studying the rearrangement pathway of the cyclic α-bromopropiophenone acetal in the presence of a catalyst [78]. The proportion of the rearrangement’s products provides useful information evaluating the hardness of the Lewis acid character of the catalyst (Table 5). The formation of an ester results from the opening of the acetal by a hard Lewis acid. In contrast, the formation of the cyclic product proceeds from the action of a soft Lewis acid. Moreover, the Brønsted acidity of the catalyst can be evaluated by measuring the amount of propiophenone formed. The results using Corma’s method for Eco-Cu3 and CuCl2 catalysts are presented below.

Table 5.
The analysis of the acidic properties of the copper catalysts by Corma’s method.
The first remarkable observation was the high reactivity of Eco-Cu3 compared to both anhydrous and hydrated CuCl2. In the presence of Eco-Cu3, the complete conversion of the starting material was observed, while the use of both cupric chlorides led to a lower conversion, 49% and 65% depending on their degree of hydration. It appeared that the Brønsted acidic character was slightly higher for Eco-Cu3 than in the case of the two commercially available copper chlorides, which supports the results obtained by IR. Finally, the formation of the heterocycle resulting from a soft acidity was observed only in the case of commercial copper chlorides. From this study, it was concluded that the Eco-Cu3 was a stronger and harder Lewis acid than the commercially available cupric chlorides. This reactivity was rationalised with the polymetallic composition of the ecocatalysts and with the presence of other elements known to be hard Lewis acids, such as iron or calcium species, as shown by ICP-MS analysis.
4. Study of the Synthetic Potential of the Eco-Cu Catalysts
4.1. Cu-Catalysed Hydrolysis of Thiophosphates
The copper-catalysed hydrolysis of thiophosphates using an Eco-Cu has been reported using an example of environmental interest, namely the catalysed decomposition of parathion [68]. Discovered by Schrader, this compound has been widely used as an insecticide and acaricide agent [79]. However, parathion was recently found to be highly neurotoxic for many other organisms, as a good inhibitor of the enzyme acetylcholinesterase [80,81]. The transformation of parathion into paraoxon in the liver or in the environment is even more dangerous [82]. Therefore, their first identified objective was the development of a method to decompose such biocides (parathion here) in order to decontaminate humid or aquatic zones while being environmentally friendly technologies. A first difficulty was related to the chemical nature of this type of biocide. Indeed, the presence of the sulphur atom of the thiophosphate group is responsible for the much higher stability compared to a phosphoric ester [83]. Therefore, the proposed strategy for the rapid decomposition of parathion in aqueous media was based on the use the natural affinity of sulphur to coordinate with the Cu(II) ions. Copper should act as an electrophilic activator, weakening the P=S bond and facilitating the nucleophilic attack of water (Scheme 1). To experimentally confirm the utility of the Losfled strategy, the ecocatalyst (Eco-Cu1) has been used as the source of copper. The Eco-Cu1 was prepared from the biomass of a metallophyte plant, Bacopa monierri, able to bioaccumulate cooper during rhizofiltration of industrial effluents polluted with copper [69]. During the hydrolysis of parathion, the pH is usually important, especially to follow the formation of p-nitrophenolate. In the presented case, the pH during hydrolysis was close to 6 and could not promote the hydrolysis.
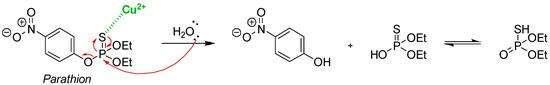
Scheme 1.
The copper-catalysed hydrolysis of parathion.
Therefore, this developed process proposed the remediation of two kinds of pollution: (i) metallic pollution by Cu(II) salts where rhizofiltration can naturally clean contaminated water and (ii) pollution with organophosphate. This method is bio-based: The Eco-Cu1 are prepared from Bacopa monierri and copper, the latter being overconcentrated because of anthropogenic metallurgy. The ecocatalysts are then used as a catalyst in the hydrolysis, and thus in the neutralization, of the toxic biocide parathion. A kinetic study of the hydrolysis of parathion was performed to evaluate the catalytic effect of these new plant-based copper species, according to the mechanism indicated above (Scheme 1). The progress of copper-catalysed hydrolysis of parathion was monitored by phosphorus nuclear magnetic resonance spectroscopy (31P NMR) in order to unambiguously distinguish and quantify the numerous phosphorylated compounds present in the reaction mixture. Each phosphorylated compound was identified according to its chemical shift on the 31P NMR spectra which was strongly dependent from the electronic environment around the phosphorus atom (Figure 2).
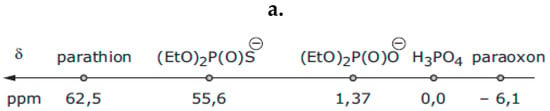
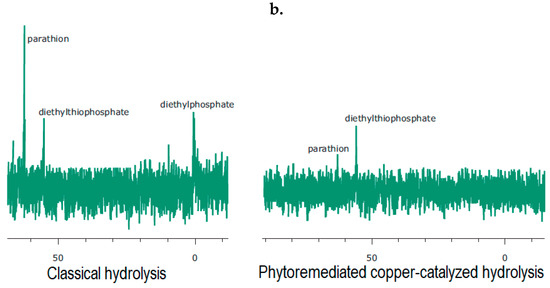
Figure 2.
(a) The 31P NMR chemical shift of the phosphorylated species and (b) the 31P NMR study of the hydrolysis of parathion with commercially available Cu2+ species (on the left) and the Eco-Cu prepared from the plant Bacopa monnieri after the rhizofiltration of copper(II) (on the right).
The analysis of the 31P NMR spectra was demonstrative after 30 hours of stirring: the catalyst promoted the hydrolysis of parathion (29% remained instead of 56%, based on the integration of the signals on the 31P NMR) and the formation of diethyl thiophosphate. This decomposition was unambiguous, and no trace of paraoxon was observed (Figure 2).
4.2. Copper-Catalysed Ullmann Coupling Reactions
As mentioned in the introduction, copper-catalysed coupling reactions are widely used in organic syntheses. The application of the Eco-Cu catalysts was investigated for Ullmann-type reactions [69]. Despite considerable efforts studying the Ullmann reaction, the development of ligands that are more efficient, more air and moisture stable, recyclable, and easy to prepare is still under investigation in order to facilitate this coupling reaction under green conditions. Copper-catalysed Ullmann coupling frequently requires harsh conditions, specific ligands, and large amounts of Cu for the reaction to occur. In this context, the catalytic potential of Eco-Cu was, therefore, particularly appealing.
4.2.1. N-Arylation Ullmann-type Reaction
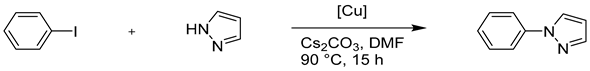
One of the particularities of Eco-Cu is their polymetallic structure. Those catalysts were, therefore, compared to the catalysts described by Taillefer et al. who have developed one of the few examples of bimetallic catalysis [84,85]. In the following described work, the N-arylation reaction carried out with pyrazole and iodobenzene was chosen as the comparative model (Table 6) [69]. Each Eco-Cu was found to be efficient for this reaction. A direct correlation between the coupling efficiency and the copper concentration in the catalyst was established. Importantly, at equal amounts of copper, Eco-Cu were found to be more active than CuCl2. Only 1 mol. % of copper was enough to observe a good reactivity, which is a very low catalytic loading compared with reported studies on copper-catalysed coupling (10 to 20 mol. %). These results are described as an important advantage from the practical, environmental, and economic point of view. Additionally, Eco-Cu3, which contains more copper than the other ecocatalysts, was found to be also the most active of all tested Eco-Cu.

Table 6.
A comparison of the catalytic activity of Eco-Cu and CuCl2.
Similar reactions under ligand-free conditions, reported in the literature, required a considerably higher loading of copper and harsher conditions, for example, 20 mol. % of Cu, 8 h at 120 °C in propionitrile as described by Hu et al. [86] or 10 mol. % of Cu, 24 h at 120 °C in DMF as described by Zhang et al. [87]. In comparison, the same authors reported the N-arylation of pyrazole with iodobenzene using excess tetraethylenepentamine (TEPA) (2 equiv.) as a base, and 10 mol. % of the copper catalyst was still needed or tetrabutylammonium bromide (TBAB) (3 equiv.) and a reaction time of 12 h at 125 °C was needed [88]. The copper loading could be reduced to 5 mol. % in some cases using unusual copper sources such as CuO hollow nanospheres immobilized on acetylene black [89] or Cu nanoparticles [90]. However, both protocols required harsh conditions: 18 h at 180 °C and 18 h at 150 °C. Finally, the loading of 0.08 mol. % of copper for the N-arylation of pyrazole with iodobenzene under ligand-like conditions was reported by Bolm et al. [91]. However, this method required the presence of 1,2-dimethylethylenediamine (DMEDA) (20 mol. %); plus, the reactions were carried out at 135 °C for 24 h. It appeared that the use of Eco-Cu catalysts represented an improvement over the methods found in the literature. Once again, the performance of the ecocatalysts was explained by their polymetallic composition and, more precisely, by the presence of alkaline metals (Na+ and K+) and salts with Lewis acid properties (Ca2+, Mg2+, and Fe3+). This hypothesis was in agreement with the work of Zhang et al. who demonstrated the advantages of inorganic salt particles in transition metal-catalysed coupling reactions [92]. Partial negative charges on the salt surface created an electron donor effect and increased the electron density around the metal centres. In addition, the anions on the surface of the material have a similar influence on the aryl halide. The combination of these effects favoured the oxidative addition step and improved the reaction rate. In addition, according to Fan et al., the presence of Lewis acids would favour the polarization of the aryl-halogen bond [93]. This effect resulted in a significant increase of the cross-coupling rate [94]. Finally, these results were consistent with the previous work reported by Grison and coworkers on Heck-Mizoroki and Suzuki-Miyaura cross-coupling reactions with Eco-Pd catalysts, characterized by an excellent dispersion of the active centres on the mineral matrix of Na, K, Ca, Mg, and Fe [95].
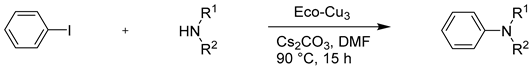
This Ullmann-type reaction was extended to other azole derivatives but with some limitations concerning aniline and secondary amines such as pyrrolidine and morpholine as nucleophiles (Table 7).

Table 7.
The extension of the Ullmann reaction to other nitrogen nucleophiles.
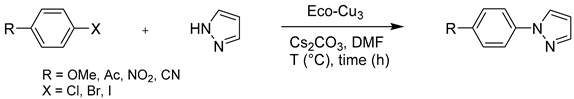
The study of the scope of the reaction showed a good applicability of the method with a large variety of aryl halides, substituted by electron withdrawing or donating groups (Table 8). The use of DMF as a solvent was required; only γ-valerolactone could replace DMF, but lower conversions were observed. The Eco-Cu3 promoted efficient coupling reactions with aryl iodide, bromide, and even chloride. The yields were good to excellent when the temperature was correctly adjusted. As expected, the presence of an electron donating group such as p-OMe resulted in a decrease of reactivity (entry 2). However, by increasing the temperature of the reaction from 90 to 110 °C (entry 3), the aryl halide, even substituted with an electron donating group, could react efficiently with the pyrazole partner. Finally, the presence of electron-withdrawing groups facilitated the coupling reaction (entries 4–9).

Table 8.
The extension of the Ullmann reaction to other halogen derivatives.
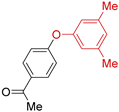
4.2.2. O-Arylation Ullmann-type Reaction
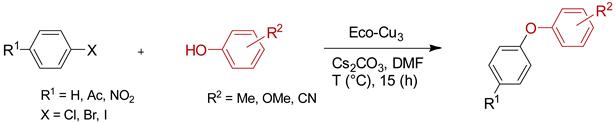
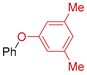
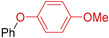
The creation of C–O bonds by Ullmann reaction is less reported in the literature than the N-arylation. The most conventional approach is based on the reaction of aryl bromides with phenols in the presence of ligands and a high catalyst loading (10–30 mol. %) and at elevated temperatures (50–110 °C). In the case of the use of aryl chlorides, even higher temperatures are necessary (135 °C to 160 °C). Therefore, the study of the potential application of Eco-Cu catalysts for that reaction was interesting (Table 9).

Table 9.
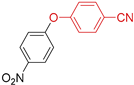
The Eco-Cu3-catalysed O-arylation.
The O-arylation reactions catalysed by Eco-Cu3 were found to be very efficient in the presence of Cs2CO3 as a base at 110–130 °C. The reaction did not require the presence of any ligand, and only 1 mol. % of copper was sufficient to reach very good yields (51–98%) and a large substrate scope. The high potential of Eco-Cu to catalyse efficiently Ullmann-type coupling reactions has been demonstrated. Importantly N- and O-arylation reactions did not work without the Eco-Cu catalyst or with CuO [96].
4.3. The Copper(I)-Catalysed Alkyne-Azide Cycloaddition (CuAAC) “Click” Reaction
As a last huge application of copper catalysis, the feasibility with Eco-Cu of 1,3-dipolar cycloaddition between azides and alkynes was investigated [70]. This important reaction was the first example of "click chemistry" where the mechanism of "fusion" between two molecules represents a perfect atom economy [97]. This reaction fits perfectly into the current trends in green chemistry. It is also a very good method of access to substituted 1,2,3-triazoles. Finally, CuAAC has led to many applications in the fields of organic synthesis [98], medicinal chemistry [99,100], molecular biology [99,101], and materials science [102]. The “click” chemistry is increasingly used for the labelling of biomacromolecules [39,103,104] or as functional tool to understand complex biological systems [105,106]. The abovementioned facts illustrate the current interest for synthesizing triazoles using aqueous systems. Additionally, in the context of green chemistry, the development of ligand-free catalysts is an additional challenge. Ligand free catalysis represents a double advantage in terms of cost (the high cost of the ligands limits the industrial interest of the process) and purification (the delicate separation step between the ligand and the product is avoided). The Eco-Cu represented a very good alternative for the development of new sustainable and environmentally friendly catalytic processes. This possibility was demonstrated by of Eco-Cu3 catalyzed azide-alkyne cycloaddition (CuAAC) in aqueous medium [70]. Additionally, for the first time, it has been shown that a homogeneous Cu-based catalyst, Eco-Cu3, can be recycled by the rhizofiltration technique from the reaction mixture and then reused.
4.3.1. Application of Eco-Cu in the CuAAC Reaction
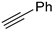
The reaction of benzyl azide with propargyl alcohol was chosen as the illustrative example. During the optimisation of the reaction, it appeared that sodium ascorbate, a natural and aqueous soluble reducing agent, could reduce in situ Eco-Cu3 into a Cu(I) active catalyst in only few minutes at room temperature. The Eco-Cu3 was found to be very efficient for this reaction, using a mixture of triethyl amine and sodium ascorbate (NaAsc). Different solvent systems were investigated (Table 10): Very good conversions were obtained in green solvents, such as water, ethanol, isopropanol, or 2-methyltetrahydrofuran.

Table 10.
The influence of the solvent on the CuAAC Click reaction with Eco-Cu3.
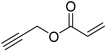
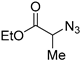
The polymetallic composition of Eco-Cu3 did not affect the interactions between the copper site and the substrate. Additionally, the ecocatalyst displayed a very high selectivity, and no formation of by-products was observed. The reaction led exclusively to 1,4-substituted triazole. It has to be noted that no Glaser coupling product (diynes) was detected under aerobic conditions. However, in the presence of K2CO3 under air, the only observed reaction is the Glaser coupling product, 2,4-hexadiyne-1,6-diol, resulting from the homocoupling of the alkyne. The most efficient conditions reported in the literature for CuAAC involved the use of CuSO4 under aqueous conditions or CuI, CuBr, Cu(OAc)2, or Cu in organic solvents [107]. The effectiveness of CuCl or CuCl2/sodium ascorbate was rarely described [102]. The use of Eco-Cu3 ensured very simple experimental conditions and a large application of the method to aliphatic, aromatic, and functionalised substrates such as propargyl ester, alkenyl, or substrates containing amines or alcohol groups (Table 11). Worth mentioning is the activity of the Eco-Cu3, comparable to homogeneous Cu catalysts [108], and recently to the heterogeneous catalysts described by Taskin et al. [109]. The clear advantage of this methodology reported here with Eco-Cu was the simplicity of the reaction treatment and isolation of products. No purification was necessary: A simple addition of 2-methyltetrahydrofurane or ethyl acetate allowed the isolation of the products with a high purity.

Table 11.
A substrate scope of the Eco-Cu-catalysed CuAAC reaction.
4.3.2. Recycling and Reuse of the Ecocatalysts
An ecotechnology (rhizofiltration or biosorption) was performed with the plant E. crassipes of the waste aqueous phase after treatment of the CuAAC reaction reported above. It has been reported that the plant could bioaccumulate the metals from the first ecocatalyst used. During such processes, 95% and 53% of the Cu were bioaccumulated, respectively (rhizofiltration of biosorption). After the usual thermal and acidic treatment, the recycled ecocatalysts Eco-Cu4 and Eco-Cu5 were obtained and characterised (Figure 3). The ICP-MS analysis revealed a significant level of iron (1.35 wt. %), that represented more than a half of the amount of copper (2.29 wt. %). An X-ray analysis revealed that the Eco-Cu5 structure was very close to the one of Eco-Cu3. The catalytic activity of the recycled Eco-Cu5 was tested in a CuAAC reaction using benzylazide and phenylacetylene as substrates. Under the optimised conditions (described above), the reaction remained quantitative. This result was noteworthy as it is one of the rare cases of the recycling and reuse of a homogeneous catalyst. The ecological conditions and the effectiveness of the methodology are, therefore, remarkable.
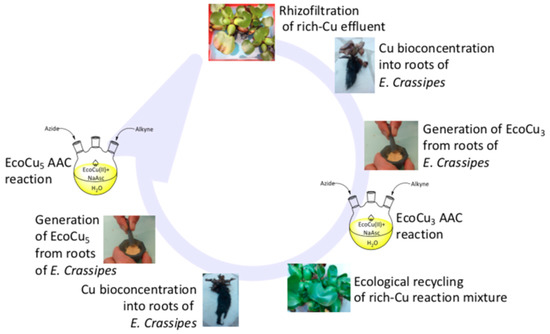
Figure 3.
The recycling and reuse of the Eco-Cu3.
5. Conclusions
The first examples of Cu catalysts of plant origin, Eco-Cu, were presented in this review. These catalysts resulted from the bioaccumulation of copper by living (rhizofiltration) or dead (biosorption) aquatic plants. Among the aquatic plants used, the Eichhornia crassipes was found to be the most efficient to such purposes and, in each case, allowed the innovative recovery of Cu from copper-rich effluents. The transformation of Cu-rich roots led to Eco-Cu catalysts. The polymetallic composition of the Eco-Cu was established as the reason for their interesting, original, and unique catalytic activity. The utility of the Eco-Cu was demonstrated through three main reactions for copper catalysis: (i) the catalytic hydrolysis of the thiophosphate group; (ii) the Ullmann-type reactions and their application for N- and O-arylation; and (iii) finally, the CuAAC “click” reaction. These reactions were very simple and did not require the presence of an amine or ligands. Importantly, the obtained products did not require any additional purification. A very important aspect of the use of Eco-Cu was the possibility of their recovery and reuse. The recovery of the catalyst could be effectively preformed via ecological recycling of the reaction mixture through a second cycle of bioaccumulation. The recycled Eco-Cu has a similar catalytic activity. This easy recovery of Eco-Cu is another asset in terms of green catalysis.
Author Contributions
Writing—review and editing, T.K.O., P.A., and C.G.
Acknowledgments
We thank the Université de Montpellier and the CNRS for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brandsma, L.; Vasilevsky, S.F.; Verkruijsse, H.D. Application of transition metal catalysts in organic synthesis; Springer: Berlin, Germany; New York, NY, USA, 1999; ISBN 978-3-642-60328-0. [Google Scholar]
- Beller, M.; Bolm, C. (Eds.) Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals, 2nd rev. and enl. ed.; WILEY-VCH: Weinheim, Germany, 2004; ISBN 978-3-527-30613-8. [Google Scholar]
- Osborn, J.A.; Wilkinson, G.; Mrowca, J.J. Tris(triphenylphosphine)halorhodium(I). In Inorganic Syntheses; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; ISBN 978-0-470-13241-8. [Google Scholar]
- Perea-Buceta, J.E.; Fernández, I.; Heikkinen, S.; Axenov, K.; King, A.W.T.; Niemi, T.; Nieger, M.; Leskelä, M.; Repo, T. Diverting Hydrogenations with Wilkinson’s Catalyst towards Highly Reactive Rhodium(I) Species. Angew. Chem. Int. Ed. 2015, 54, 14321–14325. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R.; Ohkuma, T.; Kitamura, M.; Takaya, H.; Sayo, N.; Kumobayashi, H.; Akutagawa, S. Asymmetric hydrogenation of .beta.-keto carboxylic esters. A practical, purely chemical access to .beta.-hydroxy esters in high enantiomeric purity. J. Am. Chem. Soc. 1987, 109, 5856–5858. [Google Scholar] [CrossRef]
- Zhou, Q.-L. Transition-Metal Catalysis and Organocatalysis: Where Can Progress Be Expected? Angew. Chem. Int. Ed. 2016, 55, 5352–5353. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.R.; Schindler, C.S. Catalyst: Sustainable Catalysis. Chem 2017, 2, 313–316. [Google Scholar] [CrossRef]
- Chemler, S.R. Copper catalysis in organic synthesis. Beilstein J. Org. Chem. 2015, 11, 2252–2253. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Blanchard, N. (Eds.) Copper-Mediated Cross-Coupling Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 978-1-118-69065-9. [Google Scholar]
- Beletskaya, I.P.; Cheprakov, A.V. Copper in cross-coupling reactions: The post-Ullmann chemistry. Coord. Chem. Rev. 2004, 248, 2337–2364. [Google Scholar] [CrossRef]
- Ullmann, F.; Bielecki, J. Ueber Synthesen in der Biphenylreihe. Ber. Dtsch. Chem. Ges. 1901, 34, 2174–2185. [Google Scholar] [CrossRef]
- Ullmann, F. Ueber symmetrische Biphenylderivate. Justus Liebigs Ann. Chem. 1904, 332, 38–81. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S. Recent advancement of Ullmann-type coupling reactions in the formation of C–C bond. ChemTexts 2016, 2, 17. [Google Scholar] [CrossRef]
- Antilla, J.C.; Buchwald, S.L. Copper-Catalyzed Coupling of Arylboronic Acids and Amines. Org. Lett. 2001, 3, 2077–2079. [Google Scholar] [CrossRef] [PubMed]
- Kwong, F.Y.; Klapars, A.; Buchwald, S.L. Copper-Catalyzed Coupling of Alkylamines and Aryl Iodides: An Efficient System Even in an Air Atmosphere. Org. Lett. 2002, 4, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Kwong, F.Y.; Buchwald, S.L. Mild and Efficient Copper-Catalyzed Amination of Aryl Bromides with Primary Alkylamines. Org. Lett. 2003, 5, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Antilla, J.C.; Klapars, A.; Buchwald, S.L. The Copper-Catalyzed N-Arylation of Indoles. J. Am. Chem. Soc. 2002, 124, 11684–11688. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I. Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator. Ber. Dtsch. Chem. Ges. 1906, 39, 1691–1692. [Google Scholar] [CrossRef]
- Strieter, E.R.; Bhayana, B.; Buchwald, S.L. Mechanistic Studies on the Copper-Catalyzed N-Arylation of Amides. J. Am. Chem. Soc. 2009, 131, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Jiao, N. Copper-Catalyzed C–H Azidation of Anilines under Mild Conditions. J. Am. Chem. Soc. 2012, 134, 18924–18927. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, J.-F.; Doye, S.; Buchwald, S.L. A General Copper-Catalyzed Synthesis of Diaryl Ethers. J. Am. Chem. Soc. 1997, 119, 10539–10540. [Google Scholar] [CrossRef]
- Wan, Z.; Jones, C.D.; Koenig, T.M.; Pu, Y.J.; Mitchell, D. Vinyl aryl ethers from copper-catalyzed coupling of vinyl halides and phenols. Tetrahedron Lett. 2003, 44, 8257–8259. [Google Scholar] [CrossRef]
- Ma, D.; Cai, Q.; Xie, X. CuI/N,N-Dimethylglycine-Catalyzed Cross-Coupling Reaction of Vinyl Halides with Phenols and its Application to the Assembly of Substituted Benzofurans. Synlett 2005, 11, 1767–1770. [Google Scholar] [CrossRef]
- Wolter, M.; Nordmann, G.; Job, G.E.; Buchwald, S.L. Copper-Catalyzed Coupling of Aryl Iodides with Aliphatic Alcohols. Org. Lett. 2002, 4, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, D.; Zhang, Z. Novel Synthesis of 2-Oxo-3-butynoates by Copper-Catalyzed Cross-Coupling Reaction of Terminal Alkynes and Monooxalyl Chloride. J. Org. Chem. 2003, 68, 10172–10174. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Wang, Y.-J.; Cheng, J.-H.; Lee, C.-F. Copper-Catalyzed Coupling of Alkynes with Alkenyl Halides. Synlett 2012, 23, 930–934. [Google Scholar] [CrossRef]
- Liwosz, T.W.; Chemler, S.R. Copper-Catalyzed Oxidative Heck Reactions between Alkyltrifluoroborates and Vinyl Arenes. Org. Lett. 2013, 15, 3034–3037. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Yoon, S.-K.; Kim, Y.-M. Copper-Catalyzed Coupling Reaction of Terminal Alkynes with Aryl- and Alkenyliodonium Salts. Org. Lett. 2001, 3, 2697–2699. [Google Scholar] [CrossRef] [PubMed]
- Okuro, K.; Furuune, M.; Miura, M.; Nomura, M. Copper-catalyzed coupling reaction of aryl and vinyl halides with terminal alkynes. Tetrahedron Lett. 1992, 33, 5363–5364. [Google Scholar] [CrossRef]
- Okamoto, K.; Watanabe, M.; Sakata, N.; Murai, M.; Ohe, K. Copper-Catalyzed C–H Cyanation of Terminal Alkynes with Cyanogen Iodide. Org. Lett. 2013, 15, 5810–5813. [Google Scholar] [CrossRef] [PubMed]
- Uyeda, C.; Tan, Y.; Fu, G.C.; Peters, J.C. A New Family of Nucleophiles for Photoinduced, Copper-Catalyzed Cross-Couplings via Single-Electron Transfer: Reactions of Thiols with Aryl Halides Under Mild Conditions (O °C). J. Am. Chem. Soc. 2013, 135, 9548–9552. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-J.; Zhao, Y.-Q.; Feng, T.; Feng, Y.-S. Chan–Lam-Type S-Arylation of Thiols with Boronic Acids at Room Temperature. J. Org. Chem. 2012, 77, 2878–2884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, D. Synthesis of Aryl Sulfones via l-Proline-Promoted CuI-Catalyzed Coupling Reaction of Aryl Halides with Sulfinic Acid Salts. J. Org. Chem. 2005, 70, 2696–2700. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.; Jiang, L.; Buchwald, S.L. Copper-Catalyzed C−P Bond Construction via Direct Coupling of Secondary Phosphines and Phosphites with Aryl and Vinyl Halides. Org. Lett. 2003, 5, 2315–2318. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Stanley, L.M.; Sibi, M.P. Enantioselective Copper-Catalyzed 1,3-Dipolar Cycloadditions. Chem. Rev. 2008, 108, 2887–2902. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Zarafshani, Z. Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide–alkyne “click” chemistry. Adv. Drug Deliv. Rev. 2008, 60, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Lower, A.; Noson, K. Copper(I) Hydride-Catalyzed Asymmetric Hydrosilylation of Heteroaromatic Ketones. Org. Lett. 2002, 4, 4045–4048. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Igarashi, T.; Yanagisawa, Y.; Kawauchi, N.; Hashimoto, H.; Yoshimura, J. Oxidative Cleavage of 4,6-O-Benzylidene Ring with t-Butyl Hydroperoxide and Copper(II) Chloride. Preparation of Methyl 4-O- and 6-O-Benzoylhexopyranoside Derivatives. Chem. Lett. 1988, 17, 1699–1702. [Google Scholar] [CrossRef]
- Curtis, E.J.C.; Jones, J.K.N. SOME OPEN-CHAIN DERIVATIVES OF GLUCOSE AND MANNOSE. Can. J. Chem. 1960, 38, 890–895. [Google Scholar] [CrossRef]
- Kocieński, P.J. Protecting Groups, 3rd ed.; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 2005; ISBN 978-3-13-135603-1. [Google Scholar]
- Lipshutz, B.H.; Unger, J.B.; Taft, B.R. Copper-in-charcoal (Cu/C) promoted diaryl ether formation. Org. Lett. 2007, 9, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.R.; Butterworth, R.; Dann, S.E.; Heaney, H.; Stubbs, E.C. Copper-in-Charcoal Revisited: Delineating the Nature of the Copper Species and Its Role in Catalysis. ACS Catal. 2015, 5, 793–796. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, X.-R.; Chang, N.-H.; Wang, J.; Wei, J.-F.; Shi, X.- Y.; Chen, Z.-G. A facile and practical copper powder-catalyzed, organic solvent-and ligand-free Ullmann amination of aryl halides. J. Org. Chem. 2011, 76, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.N.; Bankar, S.R.; Mhaske, G.R.; Kadam, S.S.; Murade, D.K.; Bhorkade, S.B.; Rathi, A.K.; Bundaleski, N.; Teodoro, O.M.; Zboril, R. Iron oxide-supported copper oxide nanoparticles (Nanocat-Fe-CuO): magnetically recyclable catalysts for the synthesis of pyrazole derivatives, 4-methoxyaniline, and Ullmann-type condensation reactions. ACS Sustainable Chem. Eng. 2014, 2, 1699–1706. [Google Scholar] [CrossRef]
- Benyahya, S.; Monnier, F.; Taillefer, M.; Man, M.W.C.; Bied, C.; Ouazzani, F. Efficient and Versatile Sol-Gel Immobilized Copper Catalyst for Ullmann Arylation of Phenols. Adv. Synth. Catal. 2008, 350, 2205–2208. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, B.; Ma, T.; Jiang, H.; Li, Y. Ligand-free coupling of phenols and alcohols with aryl halides by a recyclable heterogeneous copper catalyst. RSC Adv. 2012, 2, 5528–5530. [Google Scholar] [CrossRef]
- Ling, P.; Li, D.; Wang, X. Supported CuO/γ-Al2O3 as heterogeneous catalyst for synthesis of diaryl ether under ligand-free conditions. J. Mol. Catal. A: Chem. 2012, 357, 112–116. [Google Scholar] [CrossRef]
- Mullick, K.; Biswas, S.; Kim, C.; Ramprasad, R.; Angeles-Boza, A.M.; Suib, S.L. Ullmann Reaction Catalyzed by Heterogeneous Mesoporous Copper/Manganese Oxide: A Kinetic and Mechanistic Analysis. Inorg. Chem. 2017, 56, 10290–10297. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, V.; Gowsika, J.; Pandurangan, A.; Sabarathinam, S.; Lu, F. An efficient copper catalyzed 3D mesoporous aluminosilicate for the synthesis of dibenzodiazonines in the Ullmann cross-coupling reaction. New J. Chem. 2018, 42, 13065–13073. [Google Scholar]
- Benyahya, S.; Monnier, F.; Wong Chi Man, M.; Bied, C.; Ouazzan, F.; Taillefer, M. Sol–gel immobilized and reusable copper catalyst for arylation of phenols from aryl bromides. Green Chem. 2009, 11, 1121–1123. [Google Scholar]
- Bhadra, S.; Sreedhar, B.; Ranu, B.C. Recyclable Heterogeneous Supported Copper-Catalyzed Coupling of Thiols with Aryl Halides: Base-Controlled Differential Arylthiolation of Bromoiodobenzenes. Adv. Synth. Catal. 2009, 351, 2369–2378. [Google Scholar] [CrossRef]
- Bodhak, C.; Kundu, A.; Pramanik, A. An efficient and recyclable chitosan supported copper(II) heterogeneous catalyst for C–N cross coupling between aryl halides and aliphatic diamines. Tet. Lett. 2015, 56, 419–424. [Google Scholar] [CrossRef]
- Garnier, T.; Danel, M.; Magné, V.; Pujol, A.; Bénéteau, V.; Pale, P.; Chassaing, S. Copper(I)–USY as a Ligand-Free and Recyclable Catalyst for Ullmann-Type O-, N-, S-, and C-Arylation Reactions: Scope and Application to Total Synthesis. J. Org. Chem. 2018, 83, 6408–6422. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Theunissen, C.; Pradal, A. Impact of copper-catalyzed cross-coupling reactions in natural product synthesis: the emergence of new retrosynthetic paradigms. Nat. Prod. Rep. 2013, 30, 1467. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Z. Development and Applications of the Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) as a Bioorthogonal Reaction. Molecules 2016, 21, 1393. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Fu, H. Copper-Catalyzed Cascade Synthesis of Alkyl 6-Aminobenzimidazo[2,1-a]isoquinoline-5-carboxylates. J. Org. Chem. 2011, 76, 4600–4605. [Google Scholar] [CrossRef] [PubMed]
- Murru, S.; Mondal, P.; Yella, R.; Patel, B.K. Copper(I)-Catalyzed Cascade Synthesis of 2-Substituted 1,3-Benzothiazoles: Direct Access to Benzothiazolones. Eur. J. Org. Chem. 2009, 31, 5406–5413. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Jamir, L.; Guin, S.; Patel, B.K. Copper(I)-Catalyzed Cascade Synthesis of 2-Arylsulfanyl- arylcyanamides. Adv. Synth. Catal. 2010, 352, 2538–2548. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Liu, M.; Ding, J.; Gao, W.; Huang, X.; Wu, H. Unexpected Copper-Catalyzed Cascade Synthesis of Quinazoline Derivatives. J. Org. Chem. 2013, 78, 11342–11348. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fu, Y.; Fu, H.; Jiang, Y.; Zhao, Y. Highly efficient copper-catalyzed cascade synthesis of quinazoline and quinazolinone derivatives. Chem. Commun. 2008, 47, 6333–6335. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Yuan, Q.; Ma, D. Synthesis of 1,2-Disubstituted Benzimidazoles by a Cu-Catalyzed Cascade Aryl Amination/Condensation Process. Angew. Chem. Int. Ed. 2007, 46, 2598–2601. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Bao, W. Copper-Catalyzed Cascade Addition/Cyclization: An Efficient and Versatile Synthesis of N-Substituted 2-Heterobenzimidazoles. J. Org. Chem. 2009, 74, 5618–5621. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. Copper-Catalyzed Synthesis of Benzimidazoles via Cascade Reactions of o-Haloacetanilide Derivatives with Amidine Hydrochlorides. J. Org. Chem. 2008, 73, 7841–7844. [Google Scholar] [CrossRef] [PubMed]
- Losfeld, G.; Escande, V.; Mathieu, T.; Grison, C. Phytoextraction et biodégradation dynamisée: une approche interdisciplinaire inventive au service de l’environnement. Techniques de l’ingénieur Innovations en énergie et environnement 2011, IN 135, 1–8. [Google Scholar]
- Clavé, G.; Garel, C.; Poullain, C.; Renard, B.-L.; Olszewski, T.K.; Lange, B.; Shutcha, M.; Faucon, M.-P.; Grison, C. Ullmann reaction through ecocatalysis: insights from bioresource and synthetic potential. RSC Adv. 2016, 6, 59550–59564. [Google Scholar] [CrossRef]
- Clavé, G.; Garoux, L.; Boulanger, C.; Hesemann, P.; Grison, C. Ecological Recycling of a Bio-Based Catalyst for Cu Click Reaction: A New Strategy for a Greener Sustainable Catalysis. ChemistrySelect 2016, 1, 1410–1416. [Google Scholar] [CrossRef]
- Grison, C.; Carrasco, D.; Stanovych, A. Method for the Production of a Material of Plant Origin That Is Rich in Phenolic Acids, Comprising at Least One Metal, for Carrying Out Organic Synthesis Reactions. PCT Int. Appl. (2018) WO 2018178374 A1, 4 October 2018. [Google Scholar]
- Cerino-Córdova, F.J.; Díaz-Flores, P.E.; García-Reyes, R.B.; Soto-Regalado, E.; Gómez-González, R.; Garza-González, M.T.; Bustamante-Alcántara, E. Biosorption of Cu(II) and Pb(II) from aqueous solutions by chemically modified spent coffee grains. Int. J. Environ. Sci. Technol. 2013, 10, 611–622. [Google Scholar] [CrossRef]
- Poddar, S.N. Ortho-hydroxy acetophenone oxime as an analytical reagent. Part I. Z. Anal. Chem. 1957, 154, 254–259. [Google Scholar] [CrossRef]
- Mehlig, J. Colorimetric Determination of Copper with Ammonia. Ind. Eng. Chem. Anal. Ed. 1941, 13, 533–535. [Google Scholar] [CrossRef]
- Ndlela, S.C.; Shanks, B.H. Reduction Behavior of Potassium-Promoted Iron Oxide under Mixed Steam/Hydrogen Atmospheres. Ind. Eng. Chem. Res. 2006, 45, 7427–7434. [Google Scholar] [CrossRef]
- Parry, E.P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Zaki, M.I.; Hasan, M.A.; Al-Sagheer, F.A.; Pasupulety, L. In situ FTIR spectra of pyridine adsorbed on SiO2–Al2O3, TiO2, ZrO2 and CeO2: General considerations for the identification of acid sites on surfaces of finely divided metal oxides. Colloids Surf., A 2001, 190, 261–274. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Primo, A.; Domenech, A. A test reaction to assess the presence of Brönsted and the softness/hardness of Lewis acid sites in palladium supported catalysts. New J. Chem. 2004, 28, 361–365. [Google Scholar] [CrossRef]
- Chambers, H.W.; Meek, E.C.; Chambers, J.E. Chapter 64—Chemistry of Organophosphorus Insecticides. In Hayes’ Handbook of Pesticide Toxicology (Third Edition); Krieger, R., Ed.; Academic Press: New York, NY, USA, 2010; pp. 1395–1398. ISBN 978-0-12-374367-1. [Google Scholar]
- Wilson, B.W. Chapter 68—Cholinesterases. In Hayes’ Handbook of Pesticide Toxicology (Third Edition); Krieger, R., Ed.; Academic Press: New York, NY, USA, 2010; pp. 1457–1478. ISBN 978-0-12-374367-1. [Google Scholar]
- Wogram, J.; Sturm, A.; Segner, H.; Liess, M. Effects of parathion on acetylcholinesterase, butyrylcholinesterase, and carboxylesterase in three-spined stickleback (Gasterosteus aculeatus) following short-term exposure. Environ. Toxicol. Chem. 2001, 20, 1528–1531. [Google Scholar] [CrossRef]
- Jan, Y.-H.; Richardson, J.R.; Baker, A.A.; Mishin, V.; Heck, D.E.; Laskin, D.L.; Laskin, J.D. Novel approaches to mitigating parathion toxicity: targeting cytochrome P450-mediated metabolism with menadione: Redox cycling inhibits parathion metabolism. Ann. N. Y. Acad. Sci. 2016, 1378, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R. On the Mechanism of Thiamine Action. IV.1 Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Taillefer, M.; Xia, N.; Ouali, A. Efficient Iron/Copper Co-Catalyzed Arylation of Nitrogen Nucleophiles. Angew. Chem. Int. Ed. 2007, 46, 934–936. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Giacovazzi, R.; Ouali, A.; Taillefer, M.; Jutand, A. Activation of aryl halides by Cu0/1,10-phenanthroline: Cu0 as precursor of CuI catalyst in cross-coupling reactions. Chem. Commun. 2008, 45, 6051–6053. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Xing, L.; Wang, X.; Cheng, C.; Su, D.; Hu, Y. Highly Practical “Ligand-Free-Like” Copper-Catalyzed N-Arylation of Azoles in Lower Nitrile Solvents. Adv. Synth. Catal. 2008, 350, 1253–1257. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Zhang, B.; Zhang, M. Direct N-Arylation of Azaheterocycles with Aryl Halides under Ligand-free Condition. Chin. J. Chem. 2012, 30, 2389–2393. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Yang, L.; Zhang, M. N-Arylation of heterocycles promoted by tetraethylenepentamine in water. Tetrahedron 2013, 69, 6230–6233. [Google Scholar] [CrossRef]
- Kim, A.Y.; Lee, H.J.; Park, J.C.; Kang, H.; Yang, H.; Song, H.; Park, K.H. Highly Efficient and Reusable Copper-Catalyzed N-Arylation of Nitrogen-Containing Heterocycles with Aryl Halides. Molecules 2009, 14, 5169–5178. [Google Scholar] [CrossRef] [PubMed]
- Son, S.U.; Park, I.K.; Park, J.; Hyeon, T. Synthesis of Cu2O coated Cu nanoparticles and their successful applications to Ullmann-type amination coupling reactions of aryl chlorides. Chem. Commun. 2004, 7, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.-F.; Correa, A.; Carril, M.; Norrby, P.-O.; Bolm, C. Copper-Catalyzed Cross-Couplings with Part-per-Million Catalyst Loadings. Angew. Chem. Int. Ed. 2009, 48, 5691–5693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Song, J.; Liu, H.; Shi, J.; Ma, J.; Fan, H.; Wang, W.; Zhang, P.; Han, B. Acceleration of Suzuki coupling reactions by abundant and non-toxic salt particles. Green Chem. 2014, 16, 1198–1201. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, H.; Cheng, S.; Ren, Z.; Hu, Z.; Wang, Z. Lewis Acid-Promoted Suzuki Reaction using Palladium Chloride Anchored on a Polymer as a Catalyst. Aust. J. Chem. 2008, 61, 610–614. [Google Scholar] [CrossRef]
- Sud, A.; Deshpande, R.M.; Chaudhari, R.V. Rate enhancement in palladium catalyzed Heck reactions by Lewis acid promoters. Catal. Commun. 2007, 8, 183–186. [Google Scholar] [CrossRef]
- Garel, C.; Renard, B.-L.; Escande, V.; Galtayries, A.; Hesemann, P.; Grison, C. C–C bond formation strategy through ecocatalysis: Insights from structural studies and synthetic potential. Appl. Catal. A-Gen. 2015, 504, 272–286. [Google Scholar] [CrossRef]
- Drapeau, M.P.; Ollevier, T.; Taillefer, M. On the Frontier Between Nucleophilic Aromatic Substitution and Catalysis. Chem. Eur. J. 2014, 20, 5231–5236. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef] [PubMed]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-Triazoles as Pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.S.; Finn, M.G. Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Lallana, E.; Fernandez-Trillo, F.; Sousa-Herves, A.; Riguera, R.; Fernandez-Megia, E. Click Chemistry with Polymers, Dendrimers, and Hydrogels for Drug Delivery. Pharm. Res. 2012, 29, 902–921. [Google Scholar] [CrossRef] [PubMed]
- Aragão-Leoneti, V.; Campo, V.L.; Gomes, A.S.; Field, R.A.; Carvalho, I. Application of copper(I)-catalysed azide/alkyne cycloaddition (CuAAC) ‘click chemistry’ in carbohydrate drug and neoglycopolymer synthesis. Tetrahedron 2010, 66, 9475–9492. [Google Scholar] [CrossRef]
- Kele, P.; Li, X.; Link, M.; Nagy, K.; Herner, A.; Lőrincz, K.; Béni, S.; Wolfbeis, O.S. Clickable fluorophores for biological labeling—with or without copper. Org. Biomol. Chem. 2009, 7, 3486–3490. [Google Scholar] [CrossRef] [PubMed]
- Grammel, M.; Hang, H.C. Chemical reporters for biological discovery. Nat. Chem. Biol. 2013, 9, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Luz, I.; Llabrés i Xamena, F.X.; Corma, A. Bridging homogeneous and heterogeneous catalysis with MOFs: “Click” reactions with Cu-MOF catalysts. J. Catal. 2010, 276, 134–140. [Google Scholar] [CrossRef]
- Taskin, O.S.; Dadashi-Silab, S.; Kiskan, B.; Weber, J.; Yagci, Y. Highly Efficient and Reusable Microporous Schiff Base Network Polymer as a Heterogeneous Catalyst for CuAAC Click Reaction. Macromol. Chem. Phys. 2015, 216, 1746–1753. [Google Scholar] [CrossRef]
- Díez-González, S.; Nolan, S.P. [(NHC)2Cu]X Complexes as Efficient Catalysts for Azide–Alkyne Click Chemistry at Low Catalyst Loadings. Angew. Chem. Int. Ed. 2008, 47, 8881–8884. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; García-Álvarez, J. Glycerol: a biorenewable solvent for base-free Cu(I)-catalyzed 1,3-dipolar cycloaddition of azides with terminal and 1-iodoalkynes. Highly efficient transformations and catalyst recycling. Green Chem. 2014, 16, 3515–3521. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).