Recent Developments in the Suzuki–Miyaura Reaction Using Nitroarenes as Electrophilic Coupling Reagents
Abstract
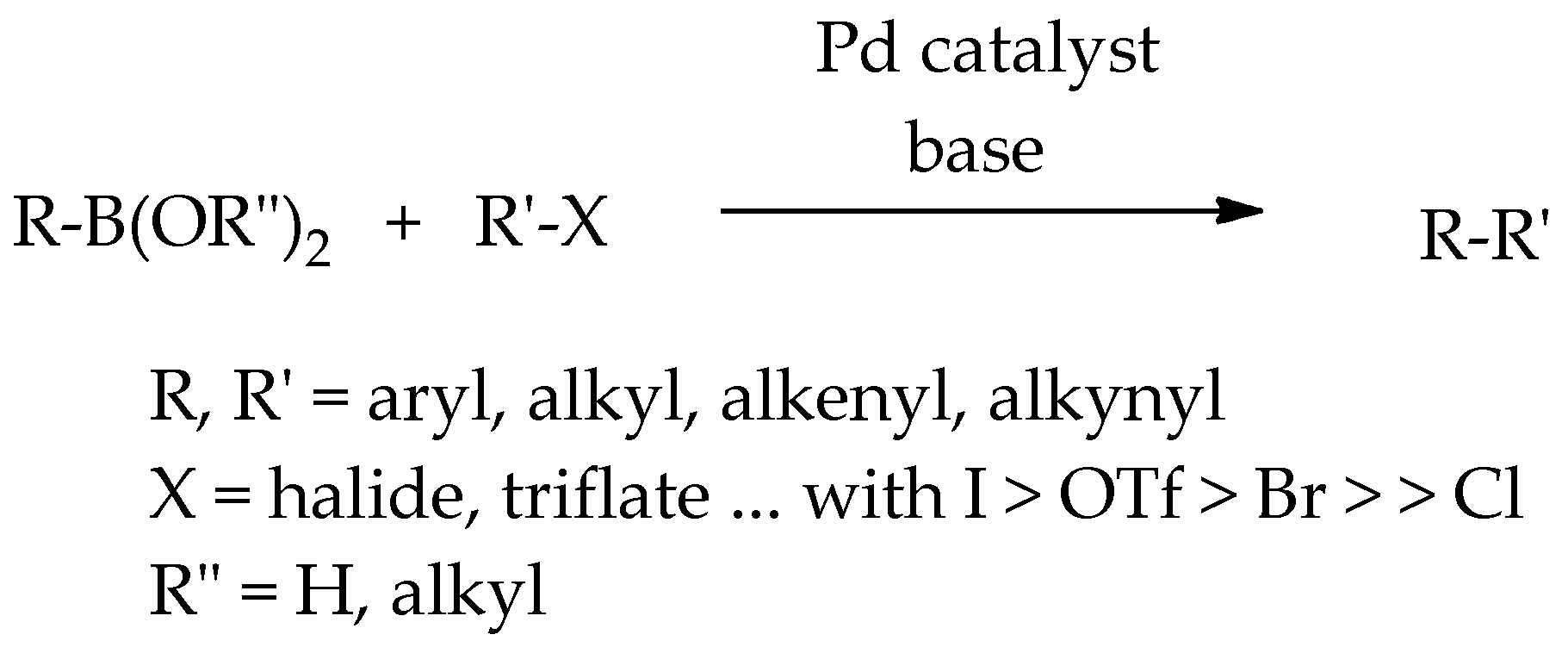
1. Introduction
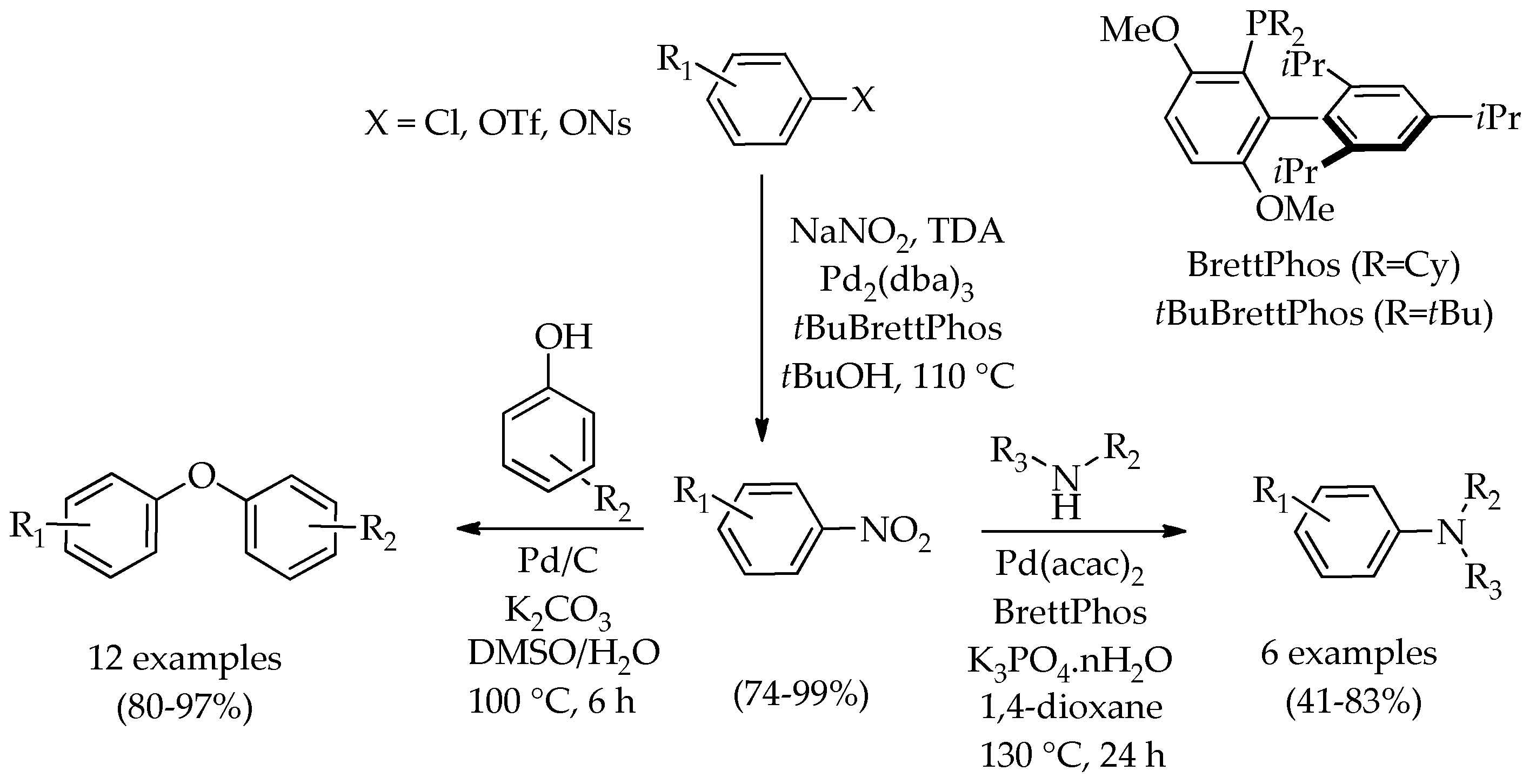
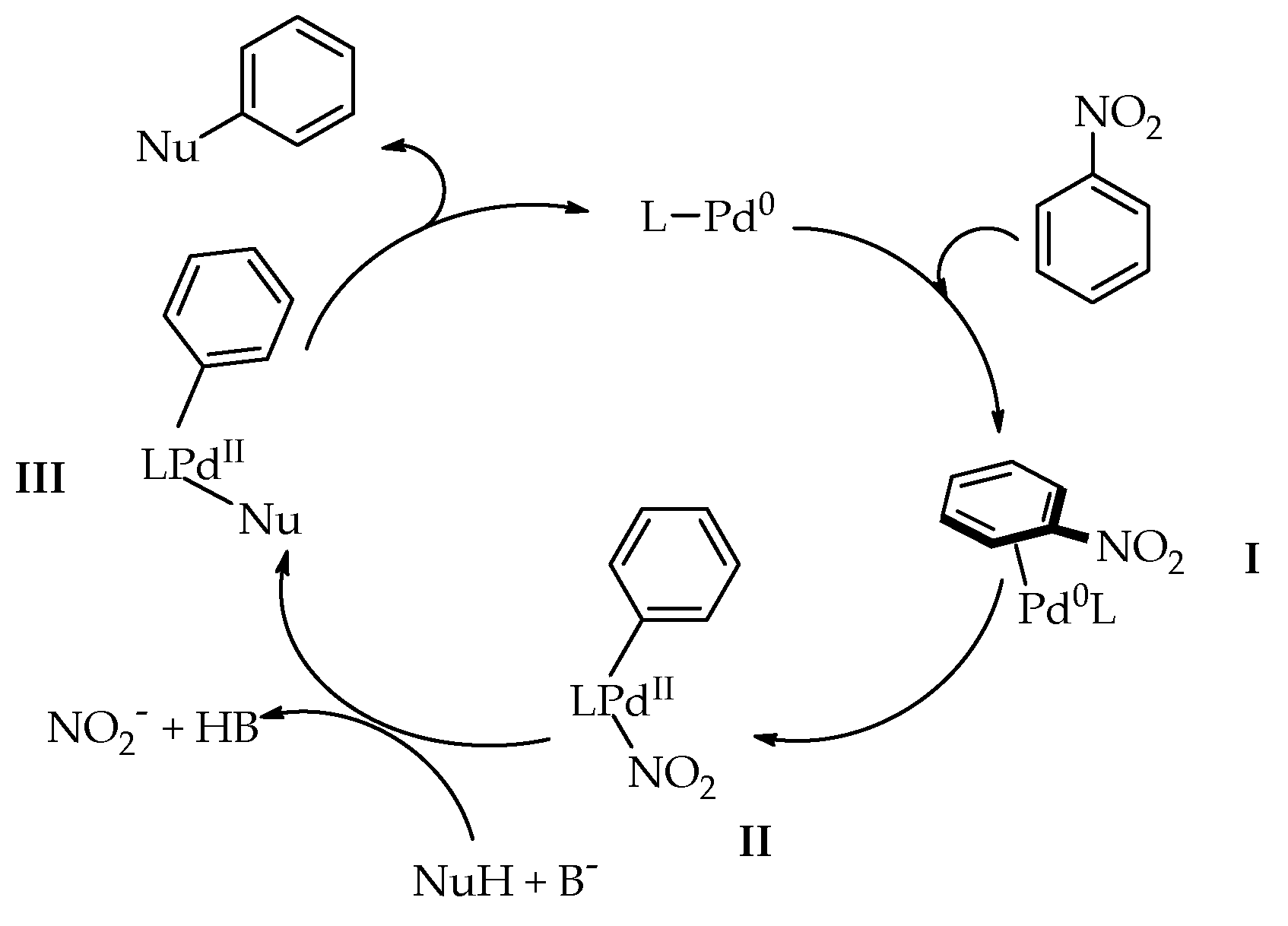
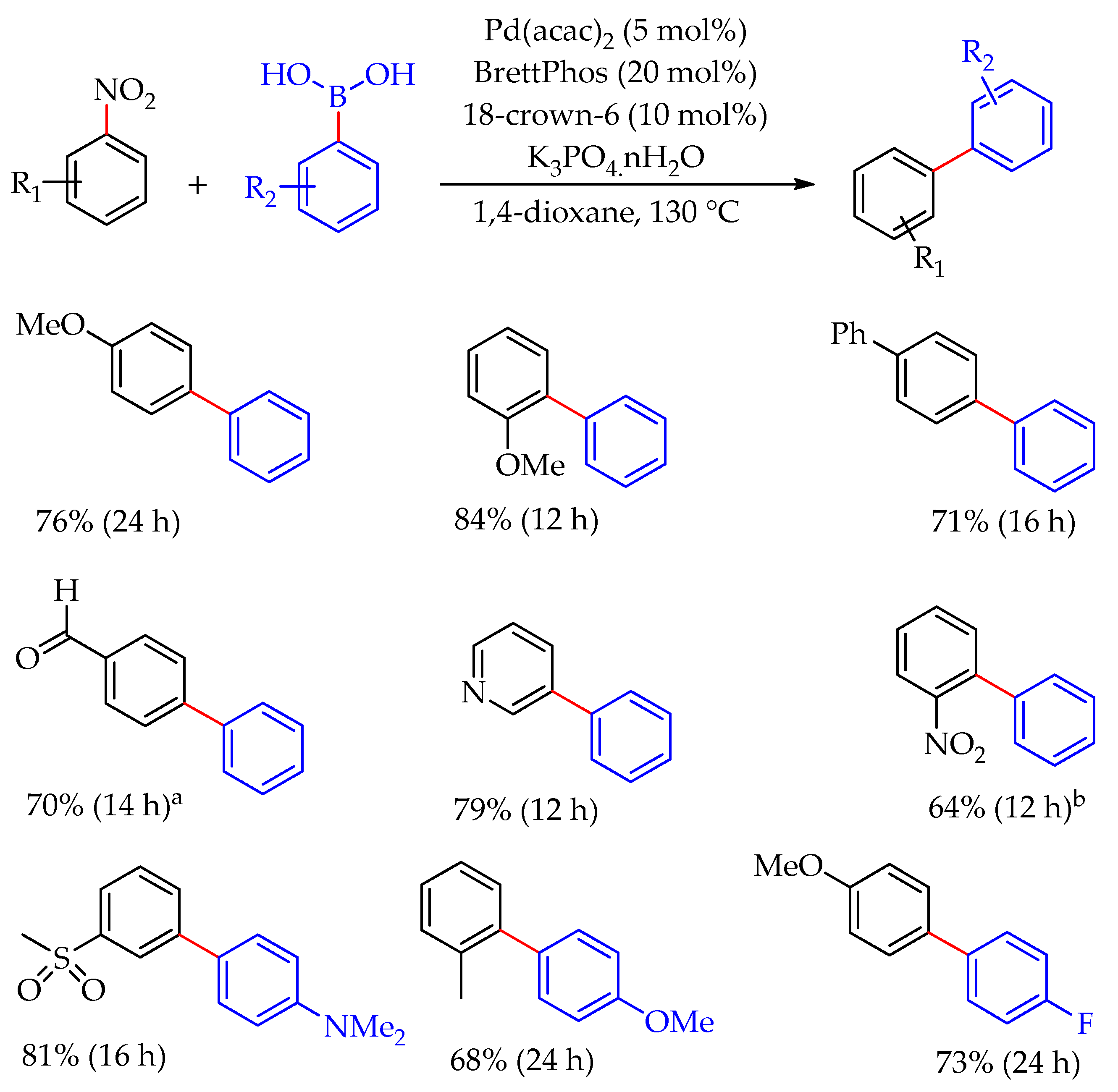
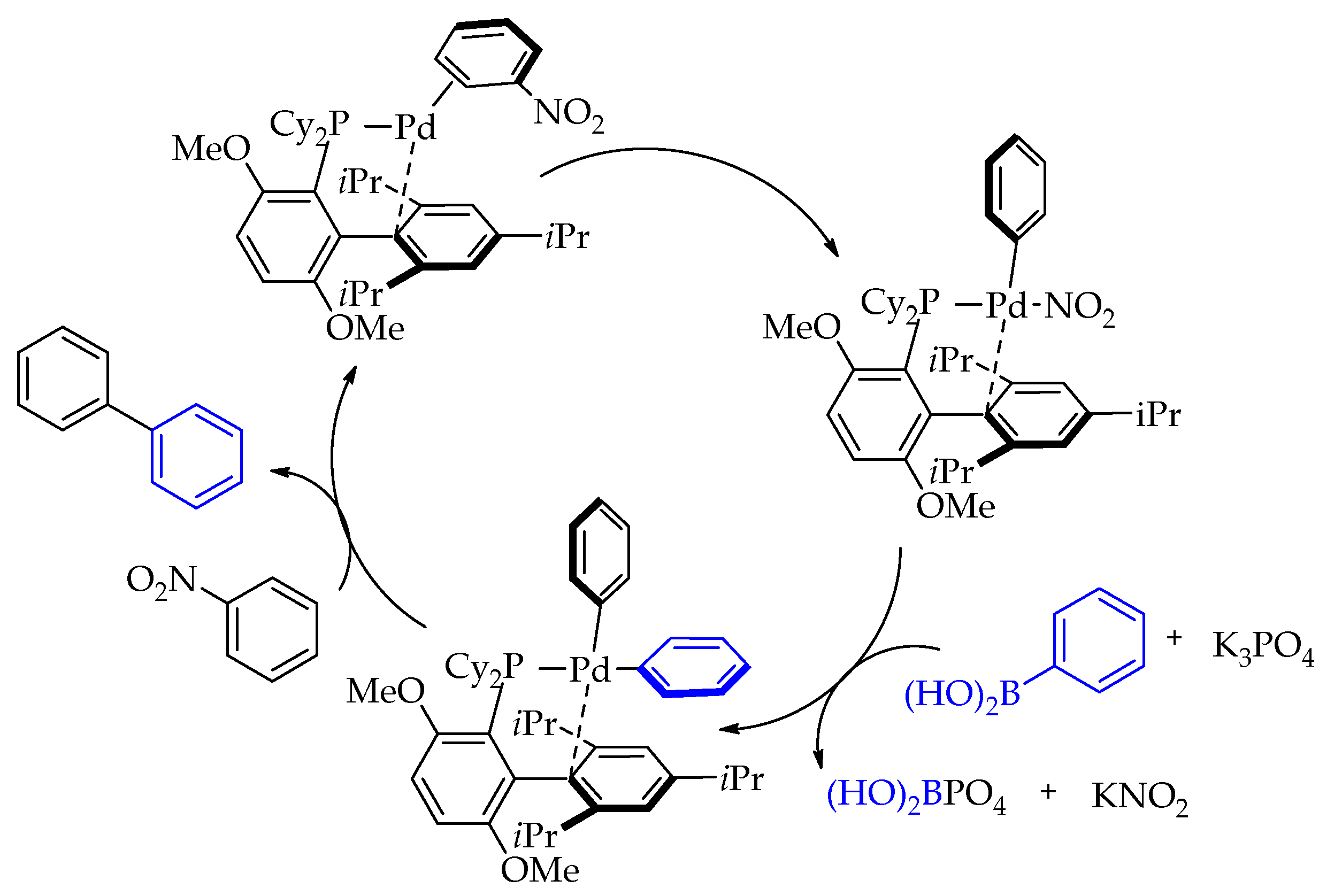
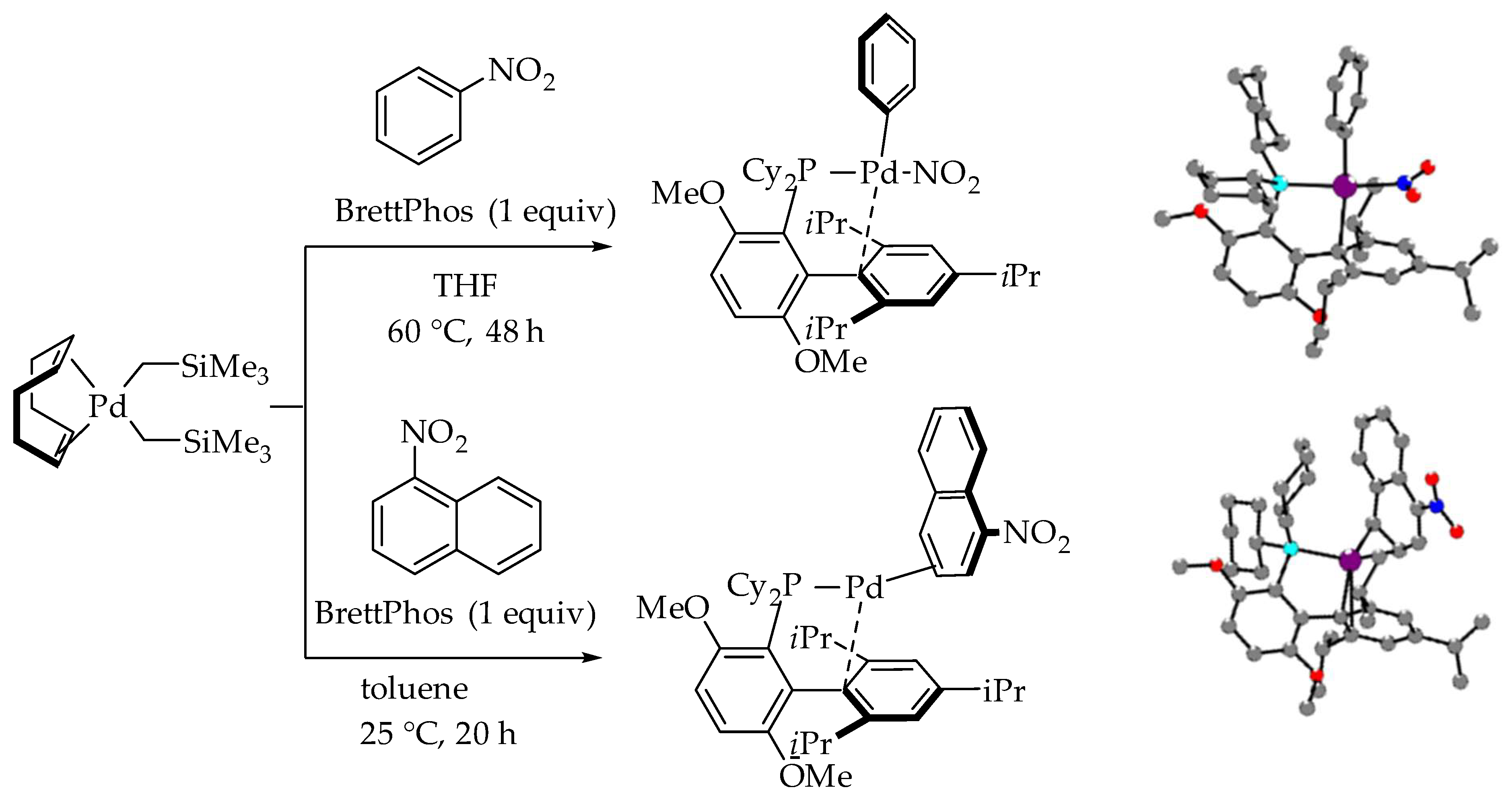
2. Discussion
3. Conclusions
Funding
Conflicts of Interest
Abbreviations of Ligands
| Brett Phos | (2-(Dicyclohexylphosphino)3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl) |
| SPhos | (2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl) |
| RuPhos | (2-Dicyclohexylphosphino-2′,6′-diisopropoxybiphenyl) |
| PCy3 | (Tricyclohexylphosphine) |
| IPr | (1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) |
References
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Cross-Coupling Reactions of Organoboranes: An Easy Way to Construct C-C Bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 3437–3440. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc. Chem. Commun. 1979, 866–867. [Google Scholar] [CrossRef]
- Miyaura, N.; Yanagi, T.; Suzuki, A. The palladium-catalyzed cross-coupling reaction of phenylboronic acid with haloarenes in the presence of bases. Synth. Commun. 1981, 11, 513–519. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Suzuki, A. Synthetic studies via the cross-coupling reaction of organoboron derivatives with organic halides. Pure Appl. Chem. 1991, 63, 419–422. [Google Scholar] [CrossRef]
- Norberg, A.M.; Sanchez, L.; Maleczka, R.E., Jr. Aryl-aryl cross-couplings that avoid the preparation of haloaromatics. Curr. Opin. Drug Discov. Dev. 2008, 11, 853–869. [Google Scholar]
- Dikova, A.; Cheval, N.P.; Blanc, A.; Weibel, J.-M.; Pale, P. Aryl and heteroaryl nosylates as stable and cheap partners for Suzuki–Miyaura cross-coupling reactions. Tetrahedron 2016, 72, 1960–1968. [Google Scholar] [CrossRef]
- Cheval, N.P.; Dikova, A.; Blanc, A.; Weibel, J.-M.; Pale, P. Vinyl Nosylates: An Ideal Partner for Palladium-Catalyzed Cross-Coupling Reactions. Chem. Eur. J. 2013, 19, 8765–8768. [Google Scholar] [CrossRef] [PubMed]
- Han, F.-S. Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: A remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef] [PubMed]
- Payard, P.-A.; Perego, L.A.; Ciofini, I.; Grimaud, L. Taming Nickel-Catalyzed Suzuki–Miyaura Coupling: A Mechanistic Focus on Boron-to-Nickel Transmetalation. ACS Catal. 2018, 8, 4812–4823. [Google Scholar] [CrossRef]
- Maluenda, I.; Navarro, O. Recent developments in the Suzuki–Miyaura reaction: 2010–2014. Molecules 2015, 20, 7528–7557. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, A.E.; Jones, B. Kinetics and mechanism of some electrophilic benzene-substitution reactions. Trans. Faraday Soc. 1941, 37, 726–743. [Google Scholar] [CrossRef]
- Zheng, X.-W.; Ding, J.-C.; Chen, J.-X.; Gao, W.-X.; Liu, M.-C.; Wu, H.-Y. The Coupling of Arylboronic Acids with Nitroarenes Catalyzed by Rhodium. Org. Lett. 2011, 13, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Liu, M.; Zheng, X.; Ding, J.; Wu, H. Ligand-free copper-catalyzed coupling of nitroarenes with arylboronic acids. Green Chem. 2012, 14, 912–916. [Google Scholar] [CrossRef]
- Bahekar, S.S.; Sarkate, A.P.; Wadhai, V.M.; Wakte, P.S.; Shinde, D.B. CuI catalyzed CS bond formation by using nitroarenes. Catal. Commun. 2013, 41, 123–125. [Google Scholar] [CrossRef]
- Yang, Y. Palladium-Catalyzed Cross-Coupling of Nitroarenes. Angew. Chem. Int. Ed. 2017, 56, 15802–15804. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Hegedus, L.S. Palladium(0)-catalyzed allylic alkylation and amination of allylnitroalkanes. J. Am. Chem. Soc. 1982, 104, 3727–3729. [Google Scholar] [CrossRef]
- Inoue, F.; Kashihara, M.; Yadav, M.R.; Nakao, Y. Buchwald-Hartwig Amination of Nitroarenes. Angew. Chem. Int. Ed. 2017, 56, 13307–13309. [Google Scholar] [CrossRef] [PubMed]
- Begum, T.; Mondal, M.; Borpuzari, M.P.; Kar, R.; Gogoi, P.K.; Bora, U. Palladium-on-Carbon-Catalyzed Coupling of Nitroarenes with Phenol: Biaryl Ether Synthesis and Evidence of an Oxidative-Addition-Promoted Mechanism. Eur. J. Org. Chem. 2017, 2017, 3244–3248. [Google Scholar] [CrossRef]
- Fors, B.P.; Buchwald, S.L. Pd-Catalyzed Conversion of Aryl Chlorides, Triflates, and Nonaflates to Nitroaromatics. J. Am. Chem. Soc. 2009, 131, 12898–12899. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.R.; Nagaoka, M.; Kashihara, M.; Zhong, R.-L.; Miyazaki, T.; Sakaki, S.; Nakao, Y. The Suzuki–Miyaura Coupling of Nitroarenes. J. Am. Chem. Soc. 2017, 139, 9423–9426. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.-L.; Nagaoka, M.; Nakao, Y.; Sakaki, S. How to Perform Suzuki–Miyaura Reactions of Nitroarene or Nitrations of Bromoarene Using a Pd0 Phosphine Complex: Theoretical Insight and Prediction. Organometallics 2018, 37, 3480–3487. [Google Scholar] [CrossRef]
- El-Berjawi, R.; Hudhomme, P. Synthesis of a perylenediimide-fullerene C60 dyad: A simple use of a nitro leaving group for a Suzuki–Miyaura coupling reaction. Dyes Pigments 2018, 159, 551–556. [Google Scholar] [CrossRef]
- Fernández-Lázaro, F.; Zink-Lorre, N.; Sastre-Santos, Á. Perylenediimides as non-fullerene acceptors in bulk-heterojunction solar cells (BHJSCs). J. Mater. Chem. A 2016, 4, 9336–9346. [Google Scholar] [CrossRef]
- Venkateswararao, A.; Liu, S.-W.; Wong, K.-T. Organic polymeric and small molecular electron acceptors for organic solar cells. Mater. Sci. Eng. R Rep. 2018, 124. [Google Scholar] [CrossRef]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, H.S.; Guo, X.; Facchetti, A.; Yan, H. Material insights and challenges for non-fullerene organic solar cells based on small molecular acceptors. Nat. Energy 2018, 3, 720–731. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J. Visible light-harvesting perylenebisimide–fullerene (C60) dyads with bidirectional “ping-pong” energy transfer as triplet photosensitizers for photooxidation of 1,5-dihydroxynaphthalene. Chem. Commun. 2012, 48, 3751–3753. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Xu, L.; Zhang, D.; Zhou, Y.; Zheng, Y.; Fu, Q.; Jiang, X.-F.; Lu, F. D-A dyad and D-A-D triad incorporating triphenylamine, benzanthrone and perylene diimide: Synthesis, electrochemical, linear and nonlinear optical properties. Chem. Phys. Lett. 2017, 682, 133–139. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocard, L.; Hudhomme, P. Recent Developments in the Suzuki–Miyaura Reaction Using Nitroarenes as Electrophilic Coupling Reagents. Catalysts 2019, 9, 213. https://doi.org/10.3390/catal9030213
Rocard L, Hudhomme P. Recent Developments in the Suzuki–Miyaura Reaction Using Nitroarenes as Electrophilic Coupling Reagents. Catalysts. 2019; 9(3):213. https://doi.org/10.3390/catal9030213
Chicago/Turabian StyleRocard, Lou, and Piétrick Hudhomme. 2019. "Recent Developments in the Suzuki–Miyaura Reaction Using Nitroarenes as Electrophilic Coupling Reagents" Catalysts 9, no. 3: 213. https://doi.org/10.3390/catal9030213
APA StyleRocard, L., & Hudhomme, P. (2019). Recent Developments in the Suzuki–Miyaura Reaction Using Nitroarenes as Electrophilic Coupling Reagents. Catalysts, 9(3), 213. https://doi.org/10.3390/catal9030213