Abstract
The escalating concerns about global warming and energy shortages have presented an urgent need for efficient and environmentally sustainable hydrogen production methods. This work presents an efficient Ni3S2/ZnIn2S4 (ZIS) composite photocatalyst synthesized via a hydrothermal process, demonstrating enhanced performance for hydrogen evolution and benzyl alcohol oxidation under visible-light irradiation. Specifically, the optimized 3.2% Ni3S2/ZIS composite achieves hydrogen and benzaldehyde production rates of 4.342 mmol g–1 h–1 and 4.213 mmol g–1 h–1, respectively, 1.79 and 1.76 times greater than those of pristine ZIS. The system exhibits excellent selectivity, producing benzaldehyde as the sole by-product, and maintains stability over multiple reaction cycles. Mechanistic studies reveal that Ni3S2 facilitates charge separation and accelerates reaction dynamics by providing conductive channels and enhancing catalytic activity at the ZIS interface. These findings highlight the potential of Ni3S2/ZIS composites as cost-effective, scalable, and noble-metal-free photocatalysts for hydrogen production and green chemical synthesis, offering a promising pathway toward energy sustainability.
1. Introduction
Hydrogen has become increasingly crucial in modern energy systems as an ideal energy carrier due to its cleanliness and efficiency, making it an essential player in reducing fossil fuel dependence and mitigating environmental pollution to support sustainable energy transition [1,2,3,4,5]. In this context, photoelectrocatalytic hydrogen production has gained significant attention, especially since Fujishima and Honda discovered the water splitting through titanium dioxide electrodes under ultraviolet light in 1972 [6,7]. This breakthrough in photocatalytic technology opened a new era for green hydrogen production, leveraging renewable energy sources (e.g., solar energy) and offering cost advantages [8,9,10,11]. However, photocatalytic water splitting faces intrinsic challenges, including efficiency limitation due to high energy barriers, slow reaction rates, and the production of oxygen as a by-product with limited utility [12,13,14,15,16]. While hole sacrificial agents are often used to enhance charge separation and catalytic activity, most are toxic, increase costs, and waste the oxidation capacity of photogenerated holes [17,18,19,20,21].
As an alternative strategy, replacing sacrificial agents with high-value-added organic conversions in photocatalytic hydrogen production systems appears to have significant potential to simultaneously achieve the production of fine chemicals using green energy [22,23]. To this end, benzyl alcohol (BA) has emerged as a leading candidate because of two key advantages [24]. First of all, the oxidation of BA requires less energy to enable higher hydrogen yields even at lower light intensities [25,26]. Secondly, benzaldehyde, a by-product formed during the photocatalytic reaction of BA, is a valuable industrial raw material used for pharmaceuticals, pesticides, and fine chemicals [27]. This dual benefit provides strong motivation to develop efficient, reusable photocatalytic systems for hydrogen production and BA oxidation, offering a pathway that integrates high efficiency, economic viability, and environmental sustainability that can be established [28,29,30].
Given that photocatalytic redox reactions are known to occur on semiconductor surfaces via photogenerated holes and electrons [31,32,33,34], the formation of a heterojunction between two or more materials with compatible band alignments is an effective scheme to enhance photocatalytic efficiency by reducing the e−/h+ recombination rate and improving e−/h+ pair separation between the materials [35,36,37,38]. Towards this goal, ZnIn2S4 (ZIS), a ternary metal chalcogenide with unique photoelectrocatalytic properties and high stability, has been widely studied [39,40]; its layered structure allows modification and coupling with other semiconductor catalysts, facilitating the development of photocatalytic systems with enhanced hydrogen production performance [41]. For example, Li et al. constructed a Z-scheme heterostructure of sulfur vacancies ZIS and MoSe2 with high hydrogen evolution rate through photocatalytic water splitting [42]; Guan et al. developed a 2D/2D CuInS2/ZnIn2S4 heterojunction photocatalyst to enhance solar hydrogen production through improved charge separation [43]; Yang et al. developed an adaptive S vacancy (Vs) and atomic Cu-doped ZnIn2S4 nanosheet photocatalyst that enhances charge separation and creates gradient channels for hydrogen migration [44]. Nevertheless, ZIS-based hybrid photocatalysts still face challenges in achieving stable overall water-splitting reactions, and their explorations in photocatalytic BA splitting remain very limited [45,46].
In the past few years, earth-abundant and low-cost nickel-based sulfides, such as NiS, NiS2, and Ni3S2, have emerged as rising candidates for water splitting, primarily due to the relatively high electronegativity (2.5) of sulfur when compared to most other metals [47]. Among these, the Ni3S2 electrocatalyst has received significant attention for its high conductivity and unique structural properties [48]. While Ni3S2 possesses limited exposed active sites and unbalanced hydrogen evolution reaction/oxygen evolution reaction performance, it is considered a promising co-catalyst for enhancing heterogeneous interfaces and increasing the density of active sites [49]. Here, we demonstrate a facile method for synthesizing Ni3S2/ZIS composite photocatalysts using a hydrothermal approach. The integration of Ni3S2 into ZIS not only significantly improves the separation of photogenerated charge carriers but also decreases the hydrogen reduction overpotential, which leads to enhanced visible-light photocatalytic activity for BA dissociation to produce hydrogen and benzaldehyde. The 3.2% Ni3S2/ZIS composite catalyst was used for simultaneous photocatalytic hydrogen production and BA oxidation to benzaldehyde under light with activities of 4.342 and 4.213 mmol g–1 h–1, respectively, which is 1.79 times and 1.76 times higher than that of pure ZIS. No other by-products were detected in the reaction process of this photocatalytic system except for the generation of benzaldehyde. Our results demonstrate that the Ni3S2/ZIS composite offers a novel route for efficient and nonprecious hybrid photocatalysts to the sustainable production of hydrogen and benzaldehyde through BA splitting.
2. Results and Discussion
The schematic in Figure 1 illustrates the synthesis of the Ni3S2/ZIS heterostructure, a highly efficient photocatalyst designed to selectively convert benzyl alcohol to hydrogen and benzaldehyde. The synthesis process involves a hydrothermal method to integrate Ni3S2 with ZIS. Initially, ZIS nanosheets are synthesized through a solvothermal process, followed by the in situ growth of Ni3S2 nanoparticles on the ZIS surface under controlled hydrothermal conditions. This approach ensures precise control over the interface and morphology of the heterostructure, which is critical for optimizing charge separation and enhancing catalytic performance. As a result, the Ni3S2/ZIS photocatalyst demonstrates exceptional efficiency in converting benzyl alcohol to hydrogen and benzaldehyde, underscoring its potential for applications in green chemical synthesis and renewable energy.
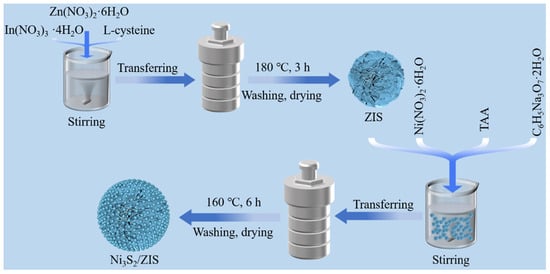
Figure 1.
Schematic flow chart of hydrothermal synthesis of Ni3S2/ZIS.
The X-ray diffraction (XRD) patterns of Ni3S2, ZIS, and Ni3S2-modified ZIS (Ni3S2/ZIS) composites with varying Ni3S2 contents (1.9%, 3.2%, 4.8%, and 6.4%) are shown in Figure 2a and Figure S1. The five main diffraction peaks of Ni3S2 were observed at 30.2°, 35.7°, 45.7°, 48.8°, and 53.5°, corresponding to the (110), (003), (202), (113), and (122) facets of Ni3S2 (JCPDS No. 44-1418), respectively. For ZIS, distinct diffraction peaks at 21.6°, 27.7°, 47.2°, 52.4°, and 55.6° can be ascribed to the (006), (102), (110), (116), and (022) planes, respectively, corresponding to the hexagonal phase of ZIS (JCPDS No. 65-2023) [50]. After doping with a small amount of Ni3S2, it is shown that the crystal structure of ZIS does not change significantly after doping with a small amount of Ni3S2 (Figure 2b). No prominent diffraction peaks related to Ni3S2 were detected in the Ni3S2/ZIS sample, which may be due to the low content of Ni3S2, so the diffraction peak of Ni3S2 at low doping may be masked by the main peak of ZIS, resulting in a signal that is too weak to be detected. In addition, the pore size distribution curves and nitrogen adsorption–desorption isotherms for ZIS and 3.2% Ni3S2/ZIS composites are investigated. As shown in Figure 2c, the pore size distributions of the samples range from 2 to 40 nm, consistent with those of pristine ZIS. While the 3.2% Ni3S2/ZIS photocatalyst also exhibits pristine ZIS-like type IV isotherms [51], the introduction of Ni3S2 records an over 20% increase in specific surface area, which is crucial for improving the photocatalytic efficiency by providing more adsorption reaction sites.
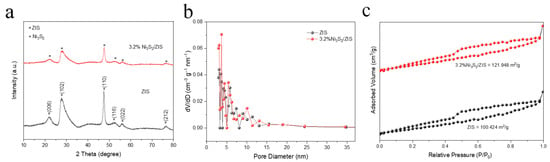
Figure 2.
(a) XRD patterns of Ni3S2, ZIS, and 3.2% Ni3S2/ZIS. (b) Nitrogen adsorption–desorption isotherms for ZIS and 3.2% Ni3S2/ZIS. (c) ZIS and 3.2% Ni3S2/ZIS pore size distribution.
SEM and HRTEM analyses are performed to further examine the morphology of the prepared catalysts. While pure ZIS exhibits a distinctive flower-like structure composed of densely packed nanosheets (Figure 3a), the Ni3S2-modified ZIS composite retains the nanosheet architecture. Still, it displays rougher surfaces, confirming the successful deposition of Ni3S2 on ZIS (Figure 3b). HRTEM imaging of the 3.2% Ni3S2/ZIS composite (Figure 3c) reveals a well-defined lamellar structure with tight interfacial contact between ZIS and Ni3S2, consistent with the SEM observations. Elemental mapping images (Figure 3e–h) show the homogenous distribution of Zn, In, S, and Ni elements across the composite, with no evident phase separation. This uniform dispersion of Ni3S2 on the ZIS surface suggests effective integration, critical for enhancing photocatalytic performance.
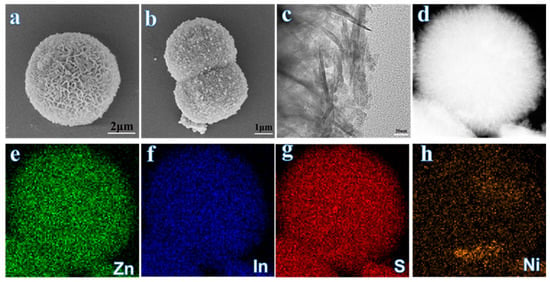
Figure 3.
(a,b) SEM images of (a) ZIS and (b) 3.2% Ni3S2/ZIS. (c) High-resolution TEM image of 3.2% Ni3S2/ZIS. (d) HAADF-STEM image of 3.2% Ni3S2/ZIS. (e–h) STEM-EDS mapping of 3.2% Ni3S2/ZIS.
To investigate the chemical states of the elements and the interaction between ZIS and Ni3S2, X-ray photoelectron spectroscopy (XPS) is performed (Figure 4 and Figure S2). For pure ZIS, the Zn 2p3/2 and Zn 2p1/2 peaks are located at 1021.28 eV and 1045.03 eV, respectively. Upon modification with Ni3S2, these peaks shift slightly to lower binding energies at 1020.98 eV and 1044.43 eV (Figure 4a), indicating an enhanced electron density on the ZIS surface due to Ni3S2 incorporation and improved separation of photogenerated electron–hole pairs. For In 3d peaks (Figure 4b), the In 3d5/2 and In 3d3/2 peaks of pristine ZIS appear at 444.63 eV and 452.08 eV, respectively, but shift to 444.93 eV and 452.48 eV in the 3.2% Ni3S2/ZIS composite, highlighting surface charge redistribution due to interaction with Ni3S2. Moreover, while the S 2p3/2 and S 2p1/2 peaks of ZIS are observed at 161.23 eV and 162.43 eV, respectively (Figure 4c), the S 2p3/2 peak in the Ni3S2-modified samples shifts slightly to 161.48 eV, indicating electronic coupling between sulfur atoms and Ni3S2 [52]. Figure 4d shows the Ni 2p XPS spectra of the 3.2% Ni3S2/ZIS composite material and pure Ni3S2. By comparison, it can be seen that the binding energies of Ni 2p3/2 and Ni 2p1/2 in the composite material decreased from 855.33 eV and 872.84 eV to 854.89 eV and 872.4 eV, respectively, and the positions of the satellite peaks also changed, indicating that the chemical environment and valence state of Ni have been adjusted. The reduction in binding energy indicates that ZIS, as an electron acceptor, accepts electrons from Ni3S2, reducing the oxidation state of Ni from the Ni2+ part in pure Ni3S2 to possibly close to Ni2+ or between Ni1+ and Ni2+. This interfacial electron transfer improves the redox properties and electron separation efficiency of the material, thereby enhancing the performance of the composite material in photocatalytic benzyl alcohol hydrogen production and benzaldehyde synthesis [53]. Overall, XPS results demonstrate that Ni3S2 effectively modulates the surface electronic structure of ZIS, enhances interfacial electron transfer, and inhibits photogenerated electron–hole recombination to allow for potentially improved photocatalytic performance.
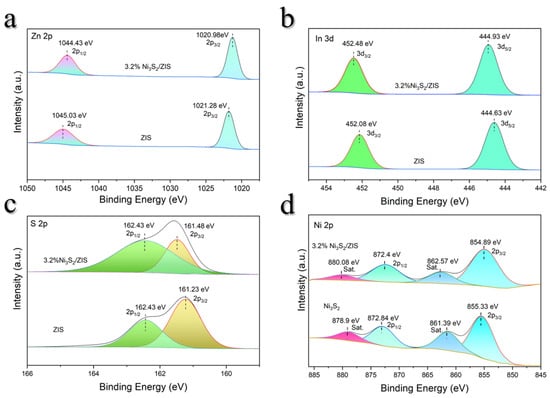
Figure 4.
ZIS and 3.2% Ni3S2/ZIS high-resolution XPS spectra of (a) Zn 2p, (b) In 3d, (c) S 2p (d) Ni3S2 and 3.2% Ni3S2/ZIS high-resolution XPS spectra of Ni 2p.
The optical properties of the photocatalysts are evaluated by UV-vis absorption spectroscopy (Figure S3). Compared with pure ZIS, the 3.2% Ni3S2/ZIS photocatalyst illustrates an obvious enhancement in absorption intensity, demonstrating improved optical response and a broader spectral range through the incorporation of Ni3S2. Additionally, the band gap energy values are calculated using the Kubelka–Munk function [54]. The calculated band gap values for ZIS and 3.2% Ni3S2/ZIS are 2.35 eV and 2.15 eV [55], respectively (Figure 5a). The narrower band gap of 3.2% Ni3S2/ZIS further validates its improved visible light absorption, advantageous for photocatalytic applications. Furthermore, we determined the conduction band positions of ZIS and 3.2% Ni3S2/ZIS using Mott–Schottky diagrams. The measured flat-band potentials of ZIS and 3.2% Ni3S2/ZIS were found to be −1.69 V and −0.81 V (relative to Ag/AgCl), respectively (Figure 5b). By adjusting the flat-band potentials by 0.88 V, the conduction band positions relative to the NHE were calculated. As illustrated in Figure 4c, the introduction of Ni3S2 resulted in a more positive valence band (VB) position in the 3.2% Ni3S2/ZnIn2S4 composite compared to pristine ZIS. This more positive VB potential significantly facilitates the oxidation of benzyl alcohol, as the photogenerated holes are now stronger oxidants. The efficient consumption of these holes in the oxidation half-reaction suppresses the recombination of electron–hole pairs. Consequently, more electrons are available in the conduction band for the reduction of H+, leading to the observed enhancement in H2 production. Photoluminescence spectra (PL) can illustrate the role of photogenerated carrier separation on the composites, and the samples are analyzed at an excitation wavelength of 300 nm. As revealed in Figure 5d, all samples display emission peaks near 610 nm, with pure ZIS exhibiting the highest emission intensity, indicative of strong charge carrier recombination. Conversely, the reduced PL intensity in 3.2% Ni3S2/ZIS indicates effective suppression of electron–hole recombination due to Ni3S2 loading. This improvement could enable more photogenerated electrons to participate in the photocatalytic reaction and thereby enhance the overall photocatalytic performance.
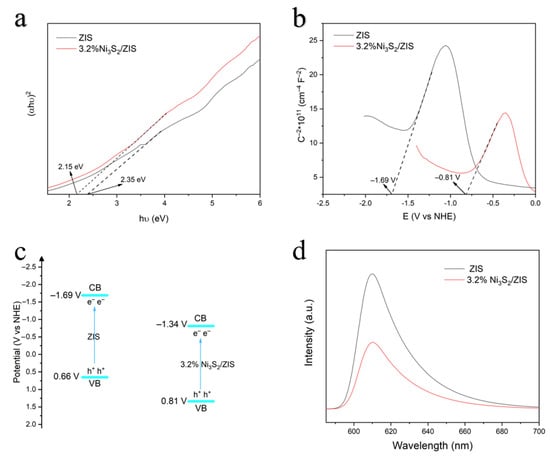
Figure 5.
(a) The bandgaps of ZIS and 3.2% Ni3S2/ZIS were determined from the UV-vis spectra using the Kubelka–Munk function. (b) Mott–Schottky plots of ZIS and 3.2% Ni3S2/ZIS. (c) The flat and conduction bands of ZIS and 3.2% Ni3S2/ZIS. (d) Steady-state photoluminescence (PL) spectra of ZIS and 3.2% Ni3S2/ZIS.
To further investigate the photocatalytic mechanism of these composite photocatalysts, photoelectrochemical measurements, including electrochemical impedance spectroscopy (EIS) and transient photocurrent response, are conducted. The EIS results (Figure 6a) reveal semicircular Nyquist plots for all samples. Generally speaking, a smaller semicircular radius corresponds to a lower charge transfer impedance, which facilitates carrier mobility. From this perspective, the 3.2% Ni3S2/ZIS photocatalyst exhibits a notably reduced impedance compared to pure ZIS, indicating enhanced electron–hole transfer and separation, a key factor in promoting photocatalytic hydrogen evolution. Transient photocurrent responses of FTO electrodes coated with different catalysts under 300 W xenon lamp irradiation (Figure 6b) show that all samples produce photocurrent signals. The higher photocurrent density recorded from the 3.2% Ni3S2/ZIS photocatalyst over that of pure ZIS reflects improved light absorption and enhanced generation and separation of charge carriers, highlighting the efficacy of Ni3S2 loading in boosting photocatalytic performance.

Figure 6.
(a) Nyquist plot of electrochemical impedance spectra of ZIS and 3.2% Ni3S2/ZIS. (b) Transient photocurrent response of ZIS and 3.2% Ni3S2/ZIS.
The photocatalytic performance of the prepared Ni3S2/ZIS composites was evaluated by measuring hydrogen production rates under visible light irradiation. As shown in Figure 7a,b, pure ZIS exhibited a hydrogen evolution rate of 2.422 mmol g–1 h–1. Control experiments using only Ni3S2 showed no hydrogen evolution, confirming that Ni3S2 alone lacks intrinsic photocatalytic activity for hydrogen generation. Interestingly, the incorporation of Ni3S2 into ZIS significantly enhanced photocatalytic performance, as evidenced by the volcano-like trend observed in Figure 7b,c. The hydrogen evolution rate increased with Ni3S2 loading, peaking at 4.342 mmol g–1 h–1 at an optimal Ni3S2 content of 3.2% due to the decreased recombination of photogenerated electron–hole pairs. A similar trend was observed in the BA conversion rate, which reached a maximum of 4.213 mmol g–1 h–1 at the same Ni3S2 loading. These results represent improvements of 1.79 fold and 1.76 fold, respectively, compared to pristine ZIS, highlighting the synergistic role of Ni3S2 in boosting photocatalytic activity. However, beyond the optimal loading of 3.2% Ni3S2, the hydrogen evolution rate declined, likely due to excessive Ni3S2 coverage on the ZIS surface, which blocked active sites and impeded light absorption. Product analysis confirmed that benzaldehyde was the sole reaction product, with no detectable by-products, demonstrating an exceptional selectivity of the system. The strong correlation between hydrogen production and BA conversion rates indicates that the dehydrogenation of BA to benzaldehyde proceeds with high efficiency, achieving a 69% conversion rate after 24 h using the 3.2% Ni3S2/ZIS composite (Table S1). To contextualize our findings within the current literature, we have compiled the performance of recently reported photocatalytic systems for this reaction (Table S2). This comparison clearly demonstrates that our optimized Ni3S2/ZnIn2S4 photocatalyst shows a highly competitive, and in most cases superior, performance. Furthermore, stability and reusability tests demonstrated the practical viability of the photocatalyst, as shown in Figure S4. Over four consecutive reaction cycles spanning four days, the 3.2% Ni3S2/ZIS composite retained its hydrogen production efficiency with minimal loss, demonstrating its excellent stability and reusability. Figure S5 depicts the XRD spectrum of the composite catalyst 3.2% Ni3S2/ZIS before and after the cyclic experiment. Notably, there are no discernible distinctions between the two spectra. These findings, combined with the high performance, selectivity, and durability of the composite, statistically proved the potential of the 3.2% Ni3S2/ZIS composite as a sustainable and efficient photocatalyst for hydrogen production and green chemical synthesis under visible light.
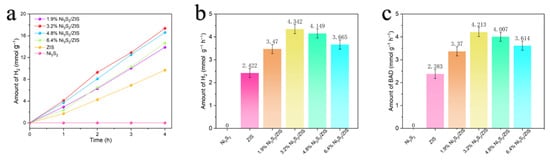
Figure 7.
(a) Trend of H2 evolution with time. (b) Ni3S2, ZIS, and x Ni3S2/ZIS H2 evolution rates (x is the weight percentage of Ni3S2 to ZIS). (c) Ni3S2, ZIS, and x Ni3S2/ZIS BAD evolution rates.
To elucidate the mechanism of photocatalytic BA decomposition, controlled experiments with quenchers are conducted to identify the active species in the photocatalytic system. As summarized in Table 1, the introduction of the hole scavenger triethanolamine (TEOA) significantly reduces benzaldehyde production from 16.906 mmol g–1 to 7.936 mmol g–1, while the hydrogen production rate remains nearly unchanged. This suggests that photogenerated holes are primarily involved in the oxidation of BA in the photocatalytic process. On the contrary, the addition of the electron scavenger AgNO3 (0.1 mmol) results in a complete cessation of hydrogen production, demonstrating that photogenerated electrons are essential for the reduction of H+ to H2 in the system. These findings confirm that the photocatalytic reaction involves distinct roles for photogenerated holes in BA oxidation and photogenerated electrons in hydrogen evolution, emphasizing their synergistic contribution to the overall photocatalytic mechanism [56].

Table 1.
Quenching experiments for photocatalytic benzyl alcohol oxidation and hydrogen evolution.
To further confirm the generation of free radicals during photocatalysis, electron spin resonance (EPR) spectroscopy is conducted using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a radical trapping agent [57]. As shown in Figure 8a,b, no radical signals are observed for either photocatalyst under dark conditions. However, under visible light irradiation, six characteristic signals corresponding to carbon-centered radicals, specifically α-hydroxybenzyl radicals, are detected for both ZIS and 3.2% Ni3S2/ZIS photocatalysts, suggesting that visible light activates the C-H bond in BA to induce radical formation [58]. Importantly, the DMPO-Cα radical signal intensity is significantly higher for the 3.2% Ni3S2/ZIS composite than for ZIS alone, which unravels the critical role of the Ni3S2 co-catalyst in accelerating C-H activation and dehydrogenation, thereby improving the performance of the composite photocatalytic system.
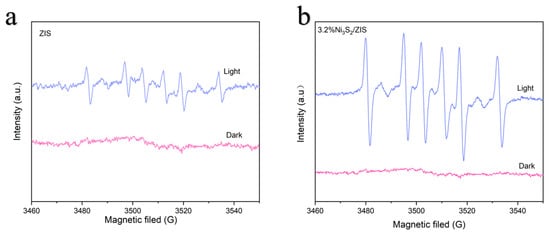
Figure 8.
Spin-trapped EPR spectra with and without visible light irradiation. (a) ZIS, (b) 3.2% Ni3S2/ZIS.
Based on the above findings, we propose a rational photocatalytic mechanism for the Ni3S2/ZIS composite, as illustrated in Figure 9. Firstly, the photocatalyst absorbs incident light energy to generate photogenerated electron–hole pairs, through which the efficient separation of charge carriers prevents their recombination. Following that, the photogenerated holes interact with BA molecules adsorbed on the catalyst surface, oxidizing them to benzaldehyde while releasing hydrogen ions. Simultaneously, the photogenerated electrons participate in the reduction of protons to produce hydrogen [59]. The incorporation of Ni3S2 significantly enhances charge separation efficiency and facilitates electron migration through conductive channels while confining photogenerated holes to the catalyst surface. This improved charge carrier dynamics underpins the superior photocatalytic activity of Ni3S2/ZIS over the pristine ZIS.

Figure 9.
(a) Oxidation and reduction reactions of benzyl alcohol during photocatalysis. (b) Diagram illustrating the photocatalytic synthesis of benzaldehyde and hydrogen generation using Ni3S2/ZIS as the photocatalyst.
3. Experiment
3.1. Materials
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O, CP), nickel nitrate hexahydrate (Ni(NO3)2·6H2O, AR), L-cysteine (C3H7NO2S, BR), benzyl alcohol (C7H8O, 99.0%), acetonitrile (C2H3N, 99.8%), triethanolamine (C6H15NO3, AR), silver nitrate (AgNO3, AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Indium nitrate tetrahydrate (In(NO3)2·4H2O, 99.9%), thioacetamide (C2H5NS, 99%), sodium citrate dihydrate (C6H5Na3O7·2H2O, 99.0%) were supported by Macklin Chemical Reagent Co., Ltd. (Shanghai, China). Ultrapure water, with an electrical resistivity of 18.2 MΩ·cm, was employed as the experimental solvent.
3.2. Synthesis of ZIS Photocatalyst
For the synthesis of ZIS, 3.5 mmol of zinc nitrate hexahydrate, 7 mmol of indium nitrate tetrahydrate, and 28 mmol of L-cysteine were dissolved in 40 mL of deionized water. The solution was sonicated and magnetically stirred for 30 min at room temperature until it became clear. The resulting solution was then transferred to a 100 mL Teflon-lined stainless-steel autoclave and heated at 180 °C for 3 h. After cooling the autoclave to room temperature, the product was collected by centrifugation, washed several times with deionized water and ethanol, and dried in an oven at 60 °C for 12 h.
3.3. Synthesis of the Heterogeneous Ni3S2/ZIS
To prepare the Ni3S2/ZIS composite, 0.24 mmol of nickel nitrate hexahydrate and 500 mg of sodium citrate dihydrate were first dissolved in 35 mL of deionized water and stirred for 30 min. Subsequently, 0.6 g of the synthesized ZIS was added to the solution and dispersed by stirring for 2 h. Afterward, 5 mL of an aqueous solution containing 0.32 mmol of TAA was gradually added, and the mixture was stirred for another hour. The resulting solution was transferred to a 100 mL Teflon-lined stainless-steel autoclave and heated at 160 °C for 6 h. After cooling to room temperature, the product was collected by centrifugation, washed multiple times with deionized water and ethanol, and dried at 60 °C for 12 h. To obtain different ratios of Ni3S2 in ZIS, the mass of Ni3S2 was kept constant, and the ratios were adjusted by varying the mass of ZIS.
3.4. Characterization
Scanning electron microscopy (SEM) analyses were performed using a Zeiss Supra 55 (Zeiss, Oberkochen, Germany) high-resolution field emission scanning electron microscope at 5.0 kV. Powder X-ray diffraction (XRD) patterns were obtained using Cu-Kα radiation on a Bruker D8 X-ray diffractometer (Bruker AXS, Karlsruhe, Germany). Morphological and elemental profiles were analyzed using a high-resolution transmission electron microscope (HRTEM, Tecnai G2 F30 S-TWIN). Fluorescence spectra were measured using an F-7000 fluorescence spectrophotometer (Hitachi, Japan) for photoluminescence (PL) spectroscopy. X-ray photoelectron spectroscopy (XPS) was performed using an ESCALAB 250 Xi electron spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Nitrogen adsorption–desorption measurements were carried out using an Autosorb IQ3 instrument (Quantachrome, Boynton Beach, FL, USA) to analyze the Brunner–Emmett–Taylor (BET) surface area and pore size of the samples. The absorbance of the samples was measured using a UV-visible near-infrared absorption spectrophotometer (Cary 5000, Varian, Palo Alto, CA, USA), and barium sulfate was used as a blank. The formation of free radicals during the photocatalytic reaction was analyzed using EPR (Bruker A300-10/12, Billerica, MA, USA).
3.5. Photoelectrochemical Testing
Electrochemical tests were performed in a three-electrode system. 5 mg of the material was dissolved with 25 μL of Nafion solution in ethanol and uniformly coated on a conductive glass substrate as a working electrode with an effective area of 1 cm2. A platinum sheet was used as the counter electrode, the Ag/AgCl electrode was used as the reference electrode, and the electrolyte was a 0.1 M sodium sulfate solution. Electrochemical impedance spectroscopy (EIS) measurements were performed on a Gamry Reference 3000 workstation (Gamry Instruments, Warminster, PA, USA) with an applied voltage of 0.2 V and a frequency range of 105 Hz to 0.05 Hz. Photocurrent measurements were also performed using a Gamry Reference 3000 workstation under 300 W xenon lamp illumination (China Education Au-light, Beijing, China).
3.6. Photocatalytic Hydrogen Production Test
The photocatalytic reaction system employed a 100 mL quartz reactor with a 300 W xenon arc lamp as the light source. In the experiment, 20 mg of the prepared catalyst was uniformly dispersed in 40 mL of benzyl alcohol solution by ultrasonic treatment. To ensure an oxygen-free environment in the system, nitrogen was used as a protective atmosphere, and the reactor is evacuated to remove air by a vacuum pump for 15 min. Subsequently, the mixture was then stirred and irradiated from the top using a xenon arc lamp at a light intensity of 250 mW cm−2. The generated hydrogen was analyzed hourly during the reaction using a hydrogen chromatograph equipped with a thermal conductivity detector (GC-7920, Beijing CEJ Tech. Co., Ltd., Beijing, China, 5A sieve column, and N2 as carrier). To compare the performance of different catalysts, catalytic hydrogen production experiments with other catalysts were carried out under the same experimental conditions.
In addition, to assess the conversion and selectivity of benzyl alcohol oxidation for the production of benzaldehyde, the experiments were performed by adding 20 mg of photocatalyst, 20 μL of benzyl alcohol, and 50 mL of acetonitrile to the reaction system and subjected to light irradiation for 24 h (see Table S1 for details). At the end of the reaction, the supernatant was separated by centrifugation and analyzed using hydrogen chromatography (GC, Agilent 7890A-7697A, Tokyo, Japan). The conversion of benzyl alcohol and selectivity of benzaldehyde are calculated according to Equations (1) and (2), respectively.
where C0 represents the initial concentration of benzyl alcohol, C1 represents the remaining concentration of benzyl alcohol after the reaction, and C2 represents the concentration of benzaldehyde after the reaction.
Conversion = (C0 − C1)/C0 × 100%
Selectivity = C2/(C0 − C1) × 100%
4. Conclusions
In summary, this study presents the synthesis and application of a Ni3S2/ZIS composite photocatalyst with remarkable photocatalytic performance for simultaneous hydrogen production and BA oxidation under visible-light irradiation. The optimized 3.2% Ni3S2/ZIS composite demonstrated significantly enhanced photocatalytic activity compared to pristine ZIS, achieving hydrogen and benzaldehyde production rates of 4.342 mmol g–1 h–1 and 4.213 mmol g–1 h–1, respectively. These results represent an improvement of approximately 1.8-fold, attributed to the synergistic effects of Ni3S2, which enhance charge separation, suppress electron–hole recombination, and improve interfacial charge transfer. Mechanistic studies confirmed that Ni3S2 plays a crucial role in modulating the surface electronic structure and facilitating efficient photocatalytic redox reactions. Additionally, the system exhibited exceptional selectivity for benzaldehyde as the sole by-product and retained its stability across multiple reaction cycles, demonstrating its practicality for sustainable hydrogen production and green chemical synthesis. This work highlights the potential of Ni3S2/ZIS composites as cost-effective and scalable photocatalysts, paving the way for the development of noble-metal-free systems in renewable energy applications and fine chemical production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15090830/s1, Figure S1: XRD patterns of x-Ni3S2/ZIS (x is the weight percentage of Ni3S2 to ZIS); Table S1: Photocatalytic conversion of benzyl alcohol and selectivity of benzaldehyde. References [60,61,62,63,64,65,66] are cited in Supplementary Materials.
Author Contributions
H.W.: Investigation, Methodology, Writing—original draft. C.Z.: Supervision, Writing—reviewing and editing, Funding acquisition. S.Y.: Conceptualization, Supervision, Writing - reviewing & editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the National Natural Science Foundation of China (21801219), the “Qing-Lan” Project of Jiangsu Province, the top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP), and the start-up fund from Yangzhou University.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, F.; Fu, H.; Yang, X.; Xiong, S.; Wang, S.; Gu, F.; An, X. Efficient TaON/CdZnS nanorods with enhanced interfacial charge transfer for visible-light-driven hydrogen evolution. Powder Technol. 2024, 437, 119540. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Rosen, M.A.; Koohi-Fayegh, S. The prospects for hydrogen as an energy carrier: An overview of hydrogen energy and hydrogen energy systems. Energy Ecol. Environ. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An overview of hydrogen production: Current status, potential, and challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Xu, Y.; Du, C.; Zhou, C.; Yang, S. Ternary noble-metal-free heterostructured NiS-CuS-C3N4 with near-infrared response for enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 4084–4094. [Google Scholar] [CrossRef]
- Arunachalam, P.; Nagai, K.; Amer, M.S.; Ghanem, M.A.; Ramalingam, R.J.; Al-Mayouf, A.M. Recent developments in the use of heterogeneous semiconductor photocatalyst based materials for a visible-light-induced water-splitting system—A brief review. Catalysts 2021, 11, 160. [Google Scholar] [CrossRef]
- Bessegato, G.G.; Guaraldo, T.T.; Brito, J.F.D.; Brugnera, M.F.; Zanoni, M.V.B. Achievements and Trends in Photoelectrocatalysis: From Environmental to Energy Applications. Electrocatalysis 2015, 6, 415–441. [Google Scholar] [CrossRef]
- Rej, S.; Hejazi, S.M.H.; Badura, Z.; Zoppellaro, G.; Kalytchuk, S.; Kment, Š.; Fornasiero, P.; Naldoni, A. Light-Induced Defect Formation and Pt Single Atoms Synergistically Boost Photocatalytic H2 Production in 2D TiO2-Bronze Nanosheets. ACS Sustain. Chem. Eng. 2022, 10, 17286–17296. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shen, C.; Xu, Y.; Zhong, Y.; Wang, C.; Yang, S.; Wang, G. An improved metal-to-ligand charge transfer mechanism for photocatalytic hydrogen evolution. ChemSusChem 2019, 12, 4221–4228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Du, C.; Zhao, Q.; Zhou, C.; Yang, S. Visible light-driven the splitting of ethanol into hydrogen and acetaldehyde catalyzed by fibrous AgNPs/CdS hybrids at room temperature. J. Taiwan Inst. Chem. E. 2019, 109, 182–189. [Google Scholar] [CrossRef]
- Zaman, N.; Iqbal, N.; Noor, T. Advances and challenges of MOF derived carbon-based electrocatalysts and photocatalyst for water splitting: A review. Arab. J. Chem. 2022, 15, 103906. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting-superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Lv, H.; Wu, H.; Zheng, J.; Kong, Y.; Xing, X.; Wang, G.; Liu, Y. Engineering of direct Z-scheme ZnIn2S4/NiWO4 heterojunction with boosted photocatalytic hydrogen production. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131384. [Google Scholar] [CrossRef]
- Liu, Y.; Du, C.; Zhou, C.; Yang, S. One-step synthesis of hierarchical AuNPs/Cd0.5Zn0.5S nanoarchitectures and their application as an efficient photocatalyst for hydrogen production. J. Ind. Eng. Chem. 2019, 72, 338–345. [Google Scholar] [CrossRef]
- Xu, Y.; Du, C.; Steinkruger, J.D.; Zhou, C.; Yang, S. Microwave-assisted synthesis of AuNPs/CdS composite nanorods for enhanced photocatalytic hydrogen evolution. J. Mater. Sci. 2019, 54, 6930–6942. [Google Scholar] [CrossRef]
- Ling, G.Z.S.; Ng, S.-F.; Ong, W.-J. Tailor-engineered 2D cocatalysts: Harnessing electron-hole redox center of 2D g-C3N4 photocatalysts toward solar-to-chemical conversion and environmental purification. Adv. Funct. Mater. 2022, 32, 2111875. [Google Scholar] [CrossRef]
- Kampouri, S.; Stylianou, K.C. Dual-functional photocatalysis for simultaneous hydrogen production and oxidation of organic substances. ACS Catal. 2019, 9, 4247–4270. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Nian, P.; Ma, H.; Hou, J.; Zhang, Y. Facile preparation of high-performance hydrochar/TiO2 heterojunction visible light photocatalyst for treating Cr(VI)-polluted water. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132775. [Google Scholar] [CrossRef]
- Ji, C.; Du, C.; Steinkruger, J.D.; Zhou, C.; Yang, S. In-situ hydrothermal fabrication of CdS/g-C3N4 nanocomposites for enhanced photocatalytic water splitting. Mater. Lett. 2019, 240, 128–131. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Q.; Du, C.; Sun, S.; Steinkruger, J.D.; Zhou, C.; Yang, S. Synergistic effect of dual particle-size AuNPs on TiO2 for efficient photocatalytic hydrogen evolution. Nanomaterials 2019, 9, 499. [Google Scholar] [CrossRef]
- Shang, W.; Li, Y.; Huang, H.; Lai, F.; Roeffaers, M.B.J.; Weng, B. Synergistic redox reaction for value-added organic transformation via dual-functional photocatalytic systems. ACS Catal. 2021, 11, 4613–4632. [Google Scholar] [CrossRef]
- Wang, Y.; Vogel, A.; Sachs, M.; Sprick, R.S.; Wilbraham, L.; Moniz, S.J.A.; Godin, R.; Zwijnenburg, M.A.; Durrant, J.R.; Cooper, A.I.; et al. Current understanding and challenges of solar-driven hydrogen generation using polymeric photocatalysts. Nat. Energy 2019, 4, 746–760. [Google Scholar] [CrossRef]
- Tang, D.; Lu, G.; Shen, Z.; Hu, Y.; Yao, L.; Li, B.; Zhao, G.; Peng, B.; Huang, X. A review on photo-, electro- and photoelectro- catalytic strategies for selective oxidation of alcohols. J. Energy Chem. 2023, 77, 80–118. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Xu, Z.; Yue, W.; Zhou, L.; Lei, J.; Zhang, J. A new breakthrough in photocatalytic hydrogen evolution by amorphous and chalcogenide enriched cocatalysts. Chem. Eng. J. 2023, 455, 140601. [Google Scholar] [CrossRef]
- Zhang, X.-R.; Ye, H.; Liang, P.Y.; Han, P.C.; Sun, Y. Zinc indium sulfide-based photocatalysts for selective organic transformations. ChemCatChem 2024, 16, e202301553. [Google Scholar] [CrossRef]
- Gupta, S.; Fernandes, R.; Patel, R.; Spreitzer, M.; Patel, N. A review of cobalt-based catalysts for sustainable energy and environmental applications. Appl. Catal. A Gen. 2023, 661, 119254. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Mamba, B.B. TiO2-based photocatalysis: Toward visible light-responsive photocatalysts through doping and fabrication of carbon-based nanocomposites. Crit. Rev. Solid State Mater. Sci. 2017, 42, 295–346. [Google Scholar] [CrossRef]
- Li, J.L.; Li, Z.X.; Wei, Y.; Wang, J.; Huang, W.Y.; Zhang, J.L.; Yang, K.; Lu, K.Q. ZnIn2S4 Nanosheet Arrays on Co9S8 Hollow Nanotubes for the Photoredox Coupling of Benzyl Alcohol Oxidation with H2 Evolution. ACS Appl. Nano Mater. 2024, 7, 20849–20857. [Google Scholar] [CrossRef]
- Zhu, T.; Ye, X.; Zhang, Q.; Hui, Z.; Wang, X.; Chen, S. Efficient utilization of photogenerated electrons and holes for photocatalytic redox reactions using visible light-driven Au/ZnIn2S4 hybrid. J. Hazard. Mater. 2019, 367, 277–285. [Google Scholar] [CrossRef]
- Ning, X.; Meng, S.; Fu, X.; Ye, X.; Chen, S. Efficient utilization of photogenerated electrons and holes for photocatalytic selective organic syntheses in one reaction system using a narrow band gap CdS photocatalyst. Green Chem. 2016, 18, 3628–3639. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Yin, S.-N.; Feng, L.; Zang, Y.; Xue, H. In situ construction of fibrous AgNPs/g-C3N4 aerogel toward light-driven COx-free methanol dehydrogenation at room temperature. Chem. Eng. J. 2018, 334, 2401–2407. [Google Scholar] [CrossRef]
- Ji, C.; Yin, S.-N.; Sun, S.; Yang, S. An in situ mediator-free route to fabricate Cu2O/g-C3N4 type-II heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 2018, 434, 1224–1231. [Google Scholar] [CrossRef]
- Chachvalvutikul, A.; Luangwanta, T.; Pattisson, S.; Hutchings, G.J.; Kaowphong, S. Enhanced photocatalytic degradation of organic pollutants and hydrogen production by a visible light-responsive Bi2WO6/ZnIn2S4 heterojunction. Appl. Surf. Sci. 2021, 544, 148885. [Google Scholar] [CrossRef]
- Hassan, J.Z.; Raza, A.; Qumar, U.; Li, G. Recent advances in engineering strategies of Bi-based photocatalysts for environmental remediation. Sustain. Mater. Techno. 2022, 33, e00478. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Ye, Z.; Ma, C.; Wang, H.; Huo, P.; Shi, W.; Yan, Y. Transfer charge and energy of Ag@CdSe QDs-rGO core-shell plasmonic photocatalyst for enhanced visible light photocatalytic activity. ACS Appl. Mater. Interfaces 2015, 7, 28231–28243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, S.; Yin, S.-N.; Xue, H. Over two-orders of magnitude enhancement of the photocatalytic hydrogen evolution activity of carbon nitride via mediator-free decoration with gold-organic microspheres. Chem. Commun. 2017, 53, 11814–11817. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, A.; Zheng, G.; Mashifana, T.; Dhiman, P.; Sharma, G.; Stadler, F.J. Current scenario in ternary metal indium sulfides-based heterojunctions for photocatalytic energy and environmental applications: A Review. Mater. Today Commun. 2023, 36, 106741. [Google Scholar] [CrossRef]
- Wang, X.; Sun, K.; Gu, S.; Zhang, Y.; Wu, D.; Zhou, X.; Gao, K.; Din, Y. Construction of a novel electron transfer pathway by modifying ZnIn2S4 with α-MnO2 and Ag for promoting solar H2 generation. Appl. Surf. Sci. 2021, 549, 149341. [Google Scholar] [CrossRef]
- Zheng, X.; Song, Y.; Liu, Y.; Yang, Y.; Wu, D.; Yang, Y.; Feng, S.; Li, J.; Liu, W.; Shen, Y.; et al. ZnIn2S4-based photocatalysts for photocatalytic hydrogen evolution via water splitting. Coord. Chem. Rev. 2023, 475, 214898. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Huang, J.; Li, S.; Meng, A.; Li, Z. Interfacial chemical bond and internal electric field modulated Z-scheme Sv-ZnIn2S4/MoSe2 photocatalyst for efficient hydrogen evolution. Nat. Commun. 2021, 12, 4112. [Google Scholar] [CrossRef]
- Guan, Z.; Pan, J.; Li, Q.; Li, G.; Yang, J. Boosting visible-light photocatalytic hydrogen evolution with an efficient CuInS2/ZnIn2S4 2D/2D heterojunction. ACS Sustain. Chem. Eng. 2019, 7, 7736–7742. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Si, Y.; Li, B.; Deng, F.; Yang, L.; Liu, X.; Dai, W.; Luo, S. Gradient hydrogen migration modulated with self-adapting S vacancy in copper-doped ZnIn2S4 nanosheet for photocatalytic hydrogen evolution. ACS Nano 2021, 15, 15238–15248. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Song, Y.; Gao, M.; Zhang, W. Synergistic coupling of photocatalytic H2 evolution and selective oxidation of benzyl alcohol over ZnIn2S4/Ni in aqueous solution. Appl. Catal. A Gen. 2024, 683, 119855. [Google Scholar] [CrossRef]
- Chong, W.-K.; Ng, B.-J.; Tan, L.-L.; Chai, S.-P. A compendium of all-in-one solar-driven water splitting using ZnIn2S4-based photocatalysts: Guiding the path from the past to the limitless future. Chem. Soc. Rev. 2024, 53, 10080–10146. [Google Scholar] [CrossRef]
- Jiang, N.; Tang, Q.; Sheng, M.; You, B.; Jiang, D.-E.; Sun, Y. Nickel sulfides for electrocatalytic hydrogen evolution under alkaline conditions: A case study of crystalline NiS, NiS2, and Ni3S2 nanoparticles. Catal. Sci. Technol. 2016, 6, 1077–1084. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Pang, Q.; Zhang, J.Z. Core/shell cable-like Ni3S2 nanowires/N-doped graphene-like carbon layers as composite electrocatalyst for overall electrocatalytic water splitting. Chem. Eng. J. 2020, 401, 126045. [Google Scholar] [CrossRef]
- Zhao, Y.; You, J.; Wang, L.; Bao, W.; Yao, R. Recent advances in Ni3S2-based electrocatalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 39146–39182. [Google Scholar] [CrossRef]
- Tan, M.; Ma, Y.; Yu, C.; Luan, Q.; Li, J.; Liu, C.; Dong, W.; Su, Y.; Qiao, L.; Gao, L.; et al. Boosting photocatalytic hydrogen production via interfacial engineering on 2D ultrathin Z-scheme ZnIn2S4/g-C3N4 heterojunction. Adv. Funct. Mater. 2022, 32, 2111740. [Google Scholar] [CrossRef]
- Li, H.; Chong, B.; Xu, B.; Wells, N.; Yan, X.; Yang, G. Nanoconfinement-induced conversion of water chemical adsorption properties in nanoporous photocatalysts to improve photocatalytic hydrogen evolution. ACS Catal. 2021, 11, 14076–14086. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Feng, Y.; Meng, F.; Ma, C.; Yan, Y.; Meng, M. Synergy between van der waals heterojunction and vacancy in ZnIn2S4/g-C3N4 2D/2D photocatalysts for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 277, 119254. [Google Scholar] [CrossRef]
- Cui, Y.; Pan, Y.-X.; Qin, H.; Cong, H.-P.; Yu, S.-H. A noble-metal-free CdS/Ni3S2@C nanocomposite for efficient visible-light-driven photocatalysis. Small Methods 2018, 2, 1800029. [Google Scholar] [CrossRef]
- Su, H.; Lou, H.; Zhao, Z.; Zhou, L.; Pang, Y.; Xie, H.; Rao, C.; Yang, D.; Qiu, X. In-situ Mo doped H2 wrapped MoO3 S-scheme heterojunction via Mo-S bonds to enhance photocatalytic HER. Chem. Eng. J. 2022, 430, 132770. [Google Scholar] [CrossRef]
- Chaudhari, N.S.; Bhirud, A.P.; Sonawane, R.S.; Nikam, L.K.; Warule, S.S.; Raneb, V.H.; Kale, B.B. Ecofriendly hydrogen production from abundant hydrogen sulfide using solar light-driven hierarchical nanostructured ZnIn2S4 photocatalyst. Green Chem. 2011, 13, 2500–2506. [Google Scholar] [CrossRef]
- Zhang, J.; Du, C.; Zhou, C.; Yang, S. Highly efficient splitting of benzyl alcohol for the production of hydrogen and benzaldehyde using synergistic CuNi/CdS photocatalysts. J. Ind. Eng. Chem. 2023, 122, 292–302. [Google Scholar] [CrossRef]
- Xing, F.; Zeng, R.; Cheng, C.; Liu, Q.; Huang, C. POM-incorporated ZnIn2S4 Z-scheme dual-functional photocatalysts for cooperative benzyl alcohol oxidation and H2 evolution in aqueous solution. Appl. Catal. B Environ. 2022, 306, 121087. [Google Scholar] [CrossRef]
- Cao, X.; Han, T.; Peng, Q.; Chen, C.; Li, Y. Modifications of heterogeneous photocatalysts for hydrocarbon C-H bonds activation and selective conversion. Chem. Commun. 2020, 56, 13918–13932. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhang, H.; Xu, J.; Zhou, C.; Qu, G.; Yang, S. Significant augmentation of hydrogen and benzaldehyde production through mediator-free in-situ synthesis of CuNi/CN photocatalysts for benzyl alcohol splitting. Fuel 2024, 370, 131827. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Wei, Y.; Xie, L.; Chen, H.-Q.; Wang, J.; Yang, K.; Zou, L.-X.; Deng, T.; Lu, K.-Q. Decorating CdS with cobaltous hydroxide and graphene dual cocatalyst for photocatalytic hydrogen production coupled selective benzyl alcohol oxidation. Mol. Catal. 2024, 553, 113738. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, X.; Zhang, Z.; Ye, X.; Zhang, T.; Zeng, W.; Guan, X.; Guo, L. Photocatalytic hydrogen production coupled with selective benzyl alcohol oxidation via WOx/CdS S-scheme heterojunction. Int. J. Hydrogen Energy 2024, 74, 31–38. [Google Scholar] [CrossRef]
- Guo, P.; Liu, X.; You, C.; Zhang, B.; Zhang, P.; Xiong, Z.; Li, H.; Zhang, H.; Wang, R.; Zhang, Z.; et al. Controllable construction of defect-mediated Z-scheme heterojunction for dual-functional cooperative photocatalysis of benzyl alcohol conversion and hydrogen evolution. App. Catal. B Environ. 2025, 366, 125011. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Y.; Wang, J.; Wu, Y.-H.; Weng, Z.; Huang, W.; Yang, K.; Zhang, J.-L.; Li, Q.; Lu, K.-Q.; et al. Rationally designed dual cocatalysts on ZnIn2S4 nanoflowers for photoredox coupling of benzyl alcohol oxidation with H2 evolution. J. Mater. Chem. A 2024, 12, 18986–18992. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Fang, M.; Xia, X.; Liu, Y. Efficient spatial separation of charge carriers over CoS1+x cocatalyst modified MIL-88B(Fe)/ZnIn2S4 S-scheme heterojunctions for photoredox dual reaction and insight into the charge-transfer mechanism. Sep. Purif. Technol. 2023, 305, 122509. [Google Scholar] [CrossRef]
- Sehrawat, P.; Mehta, S.K.; Kansal, S.K. Synergistic enhancement of photocatalytic activity in ZnS/P-doped-MoS2 composite for hydrogen generation simultaneously oxidation of benzyl alcohol through water splitting and dye degradation. Int. J. Hydrogen Energy 2024, 80, 573–585. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Wang, S.; Zhu, X.; Chen, Y.; Cai, Z.; Xu, L.; Zhao, Y. Electron energy band and function-oriented micro-nano structures integrated reactors for boosting photocatalytic hydrogen production coupled with selective benzyl alcohol oxidation. J. Photoch. Photobio. A 2025, 116652. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).