Stability and Deactivation Behavior of Cuprous Acetylide Containing Catalysts in Reppe Ethynylation
Abstract
1. Introduction
2. Results and Discussion
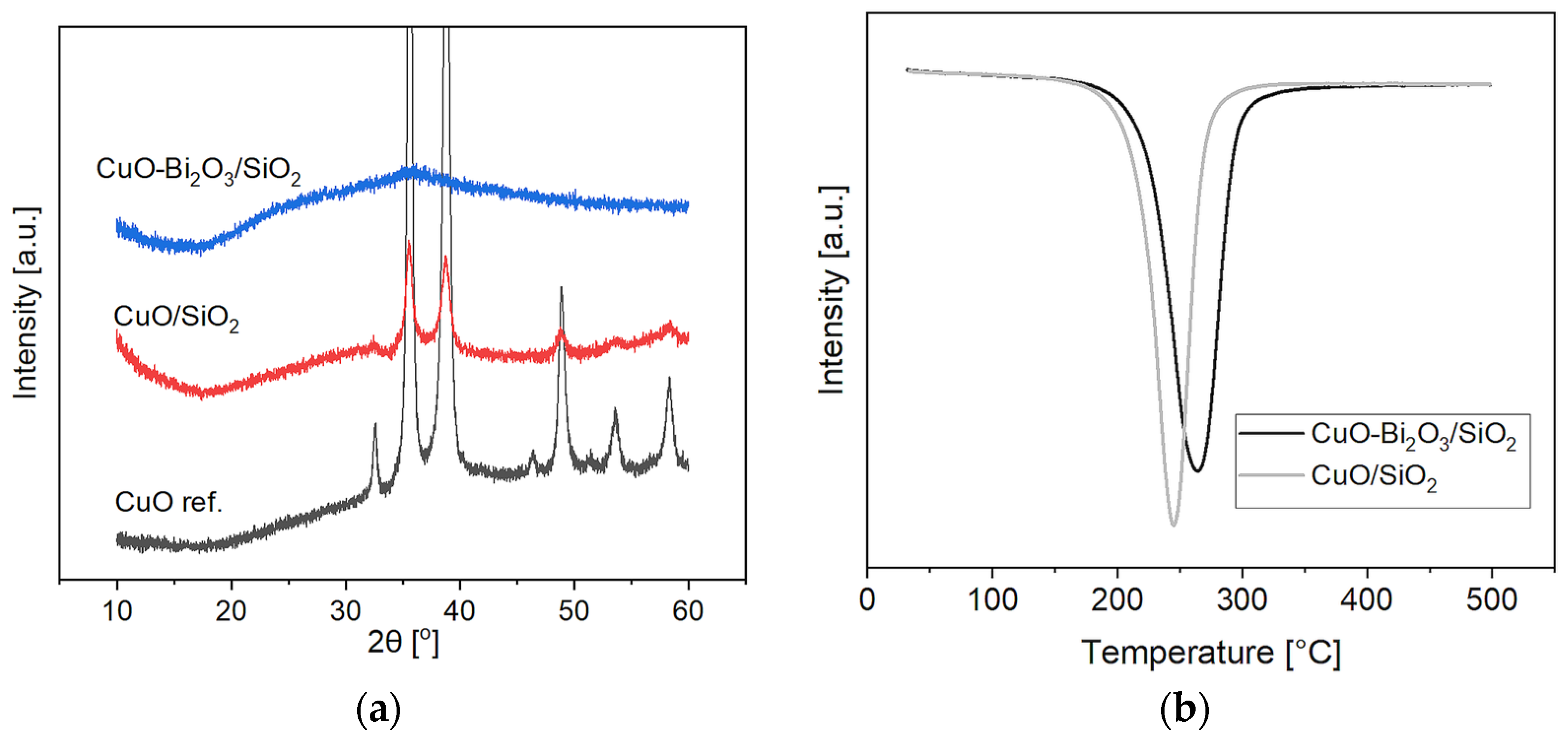
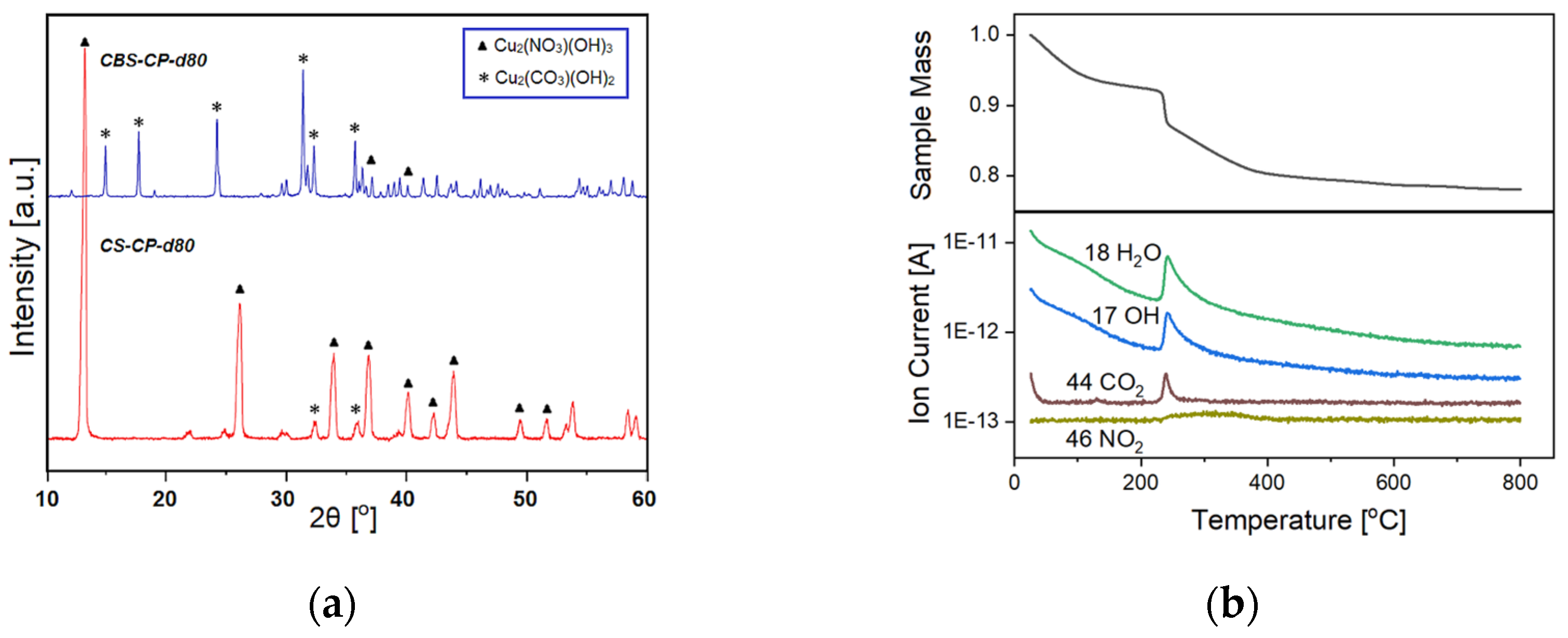
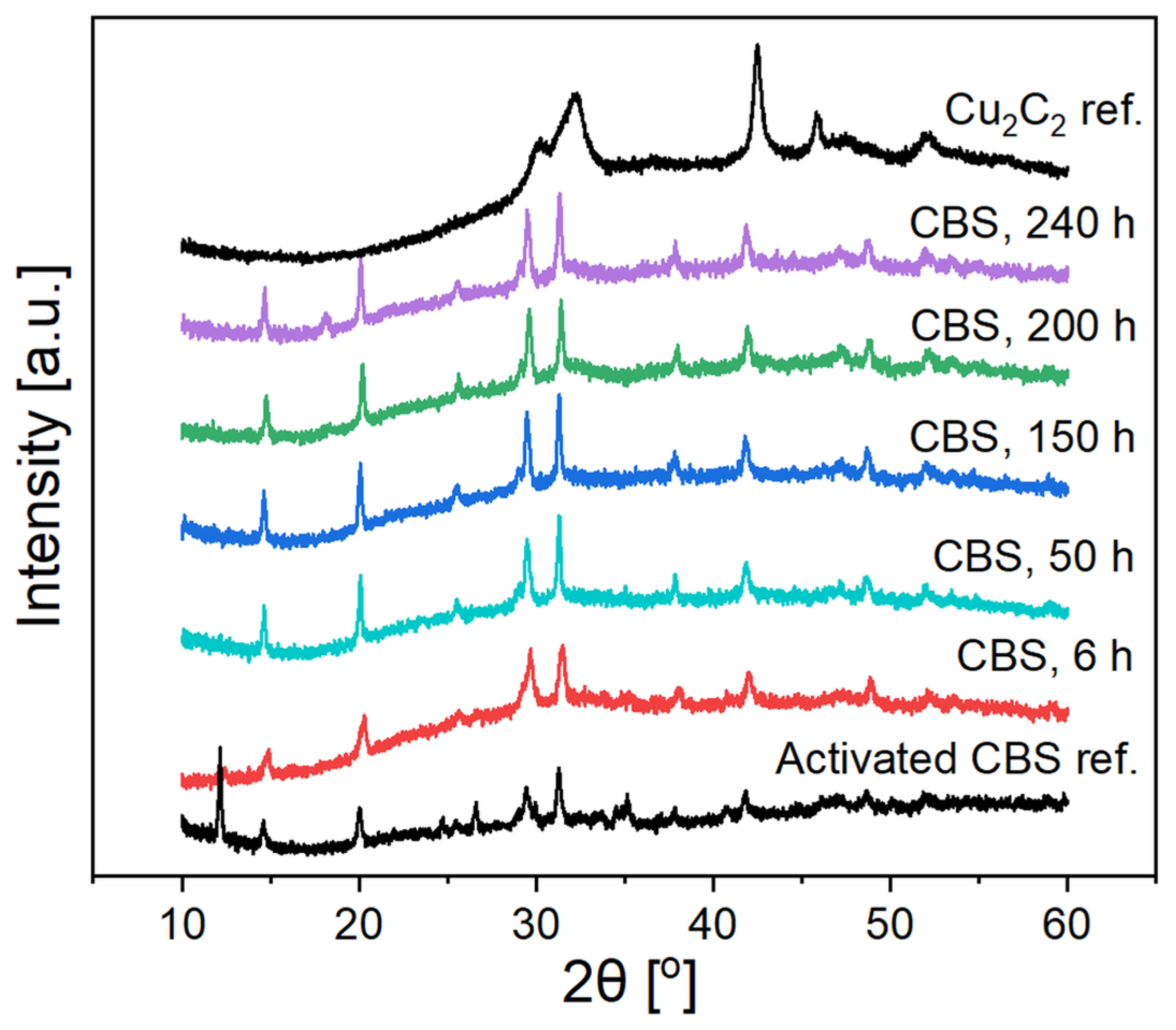
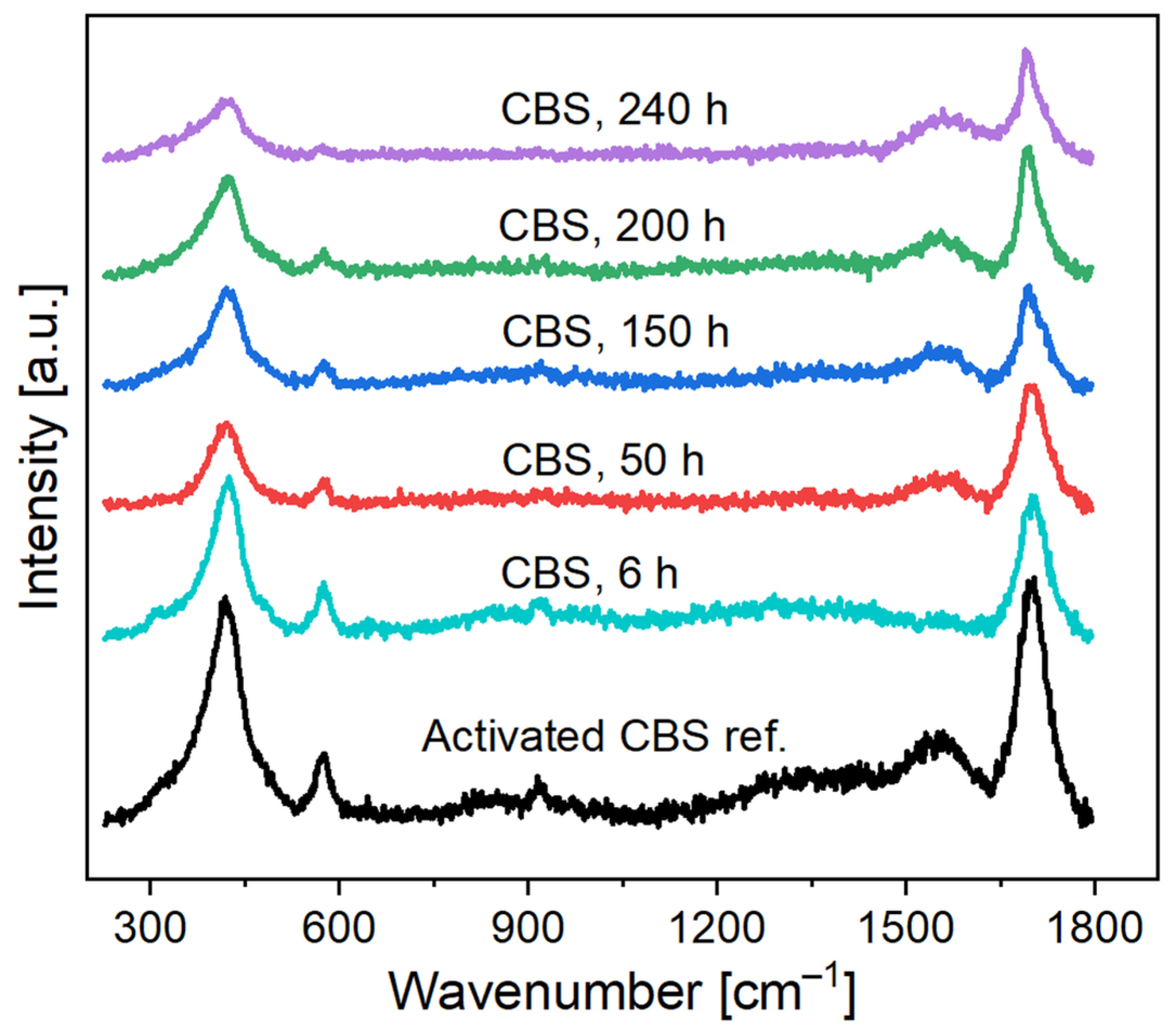
2.1. Structural Characterization of the Pre-Catalyst and of the Activated Catalyst—Cuprous Acetylide Formation
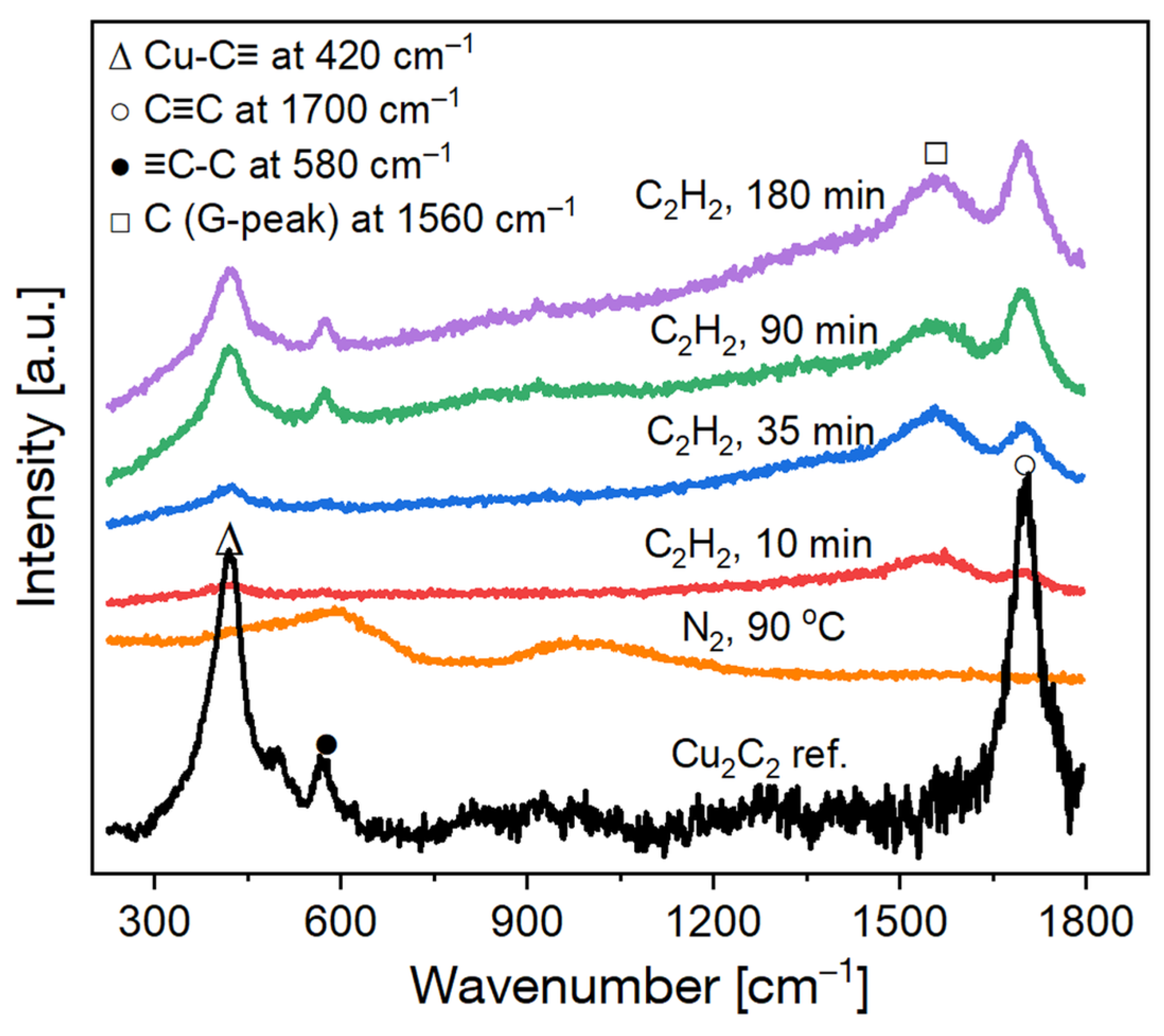
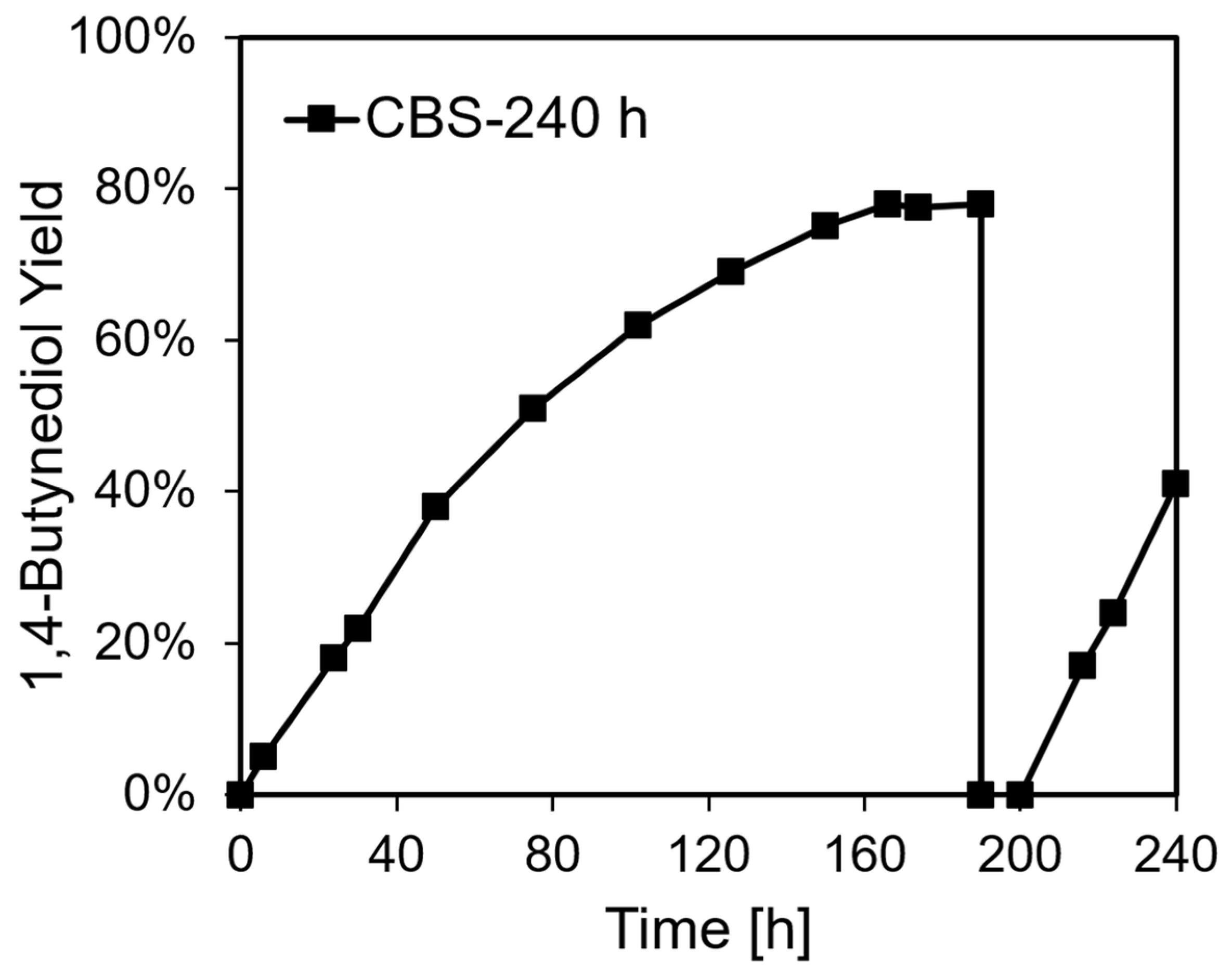
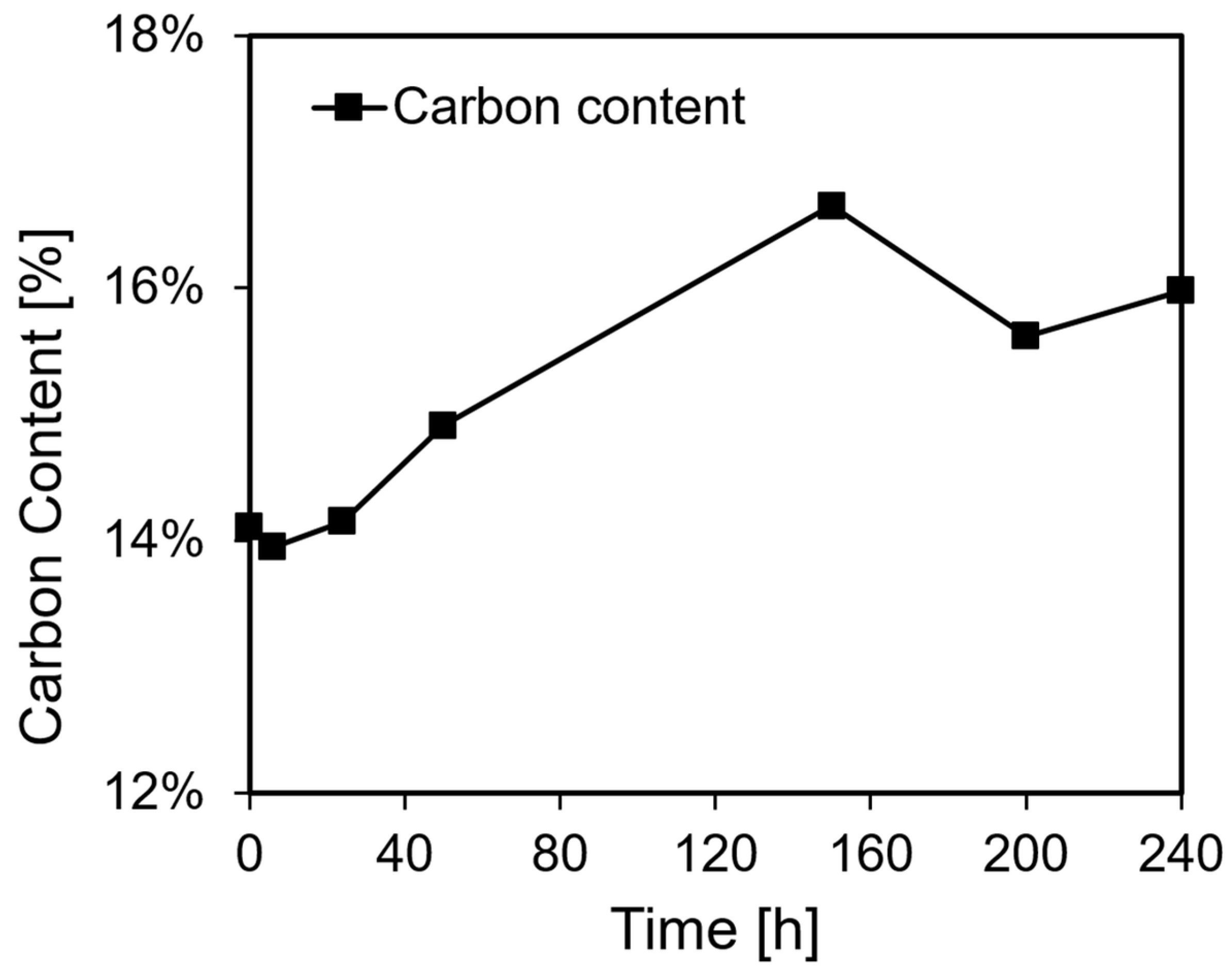
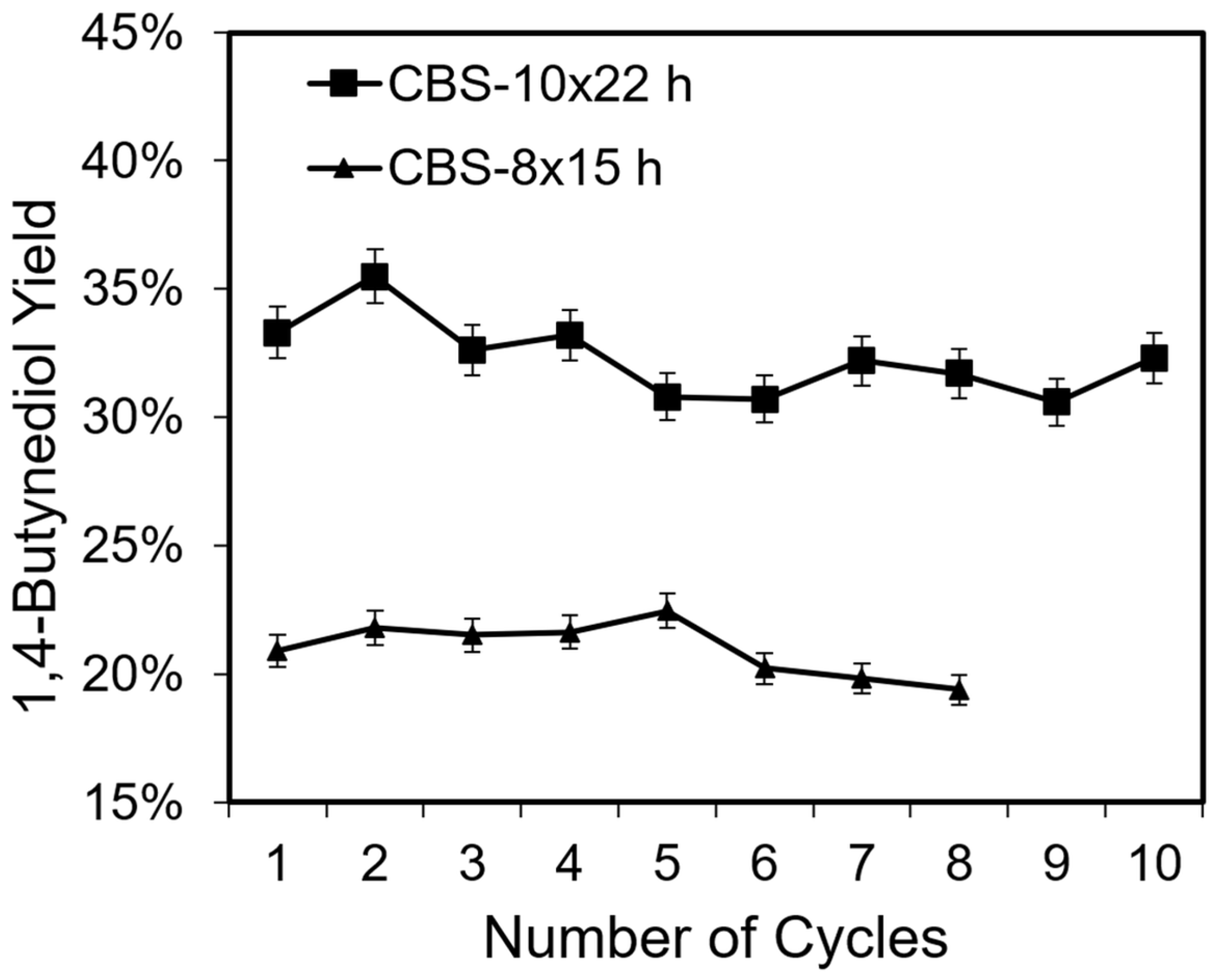
2.2. Catalysis, Structural Changes, and Deactivation of Cuprous Acetylide in the Ethynylation Reaction
3. Materials and Methods
3.1. Preparation of Ethynylation Catalysts
3.2. Catalytic Ethynylation Procedures
3.3. Sample Preparation, Characterization, and Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
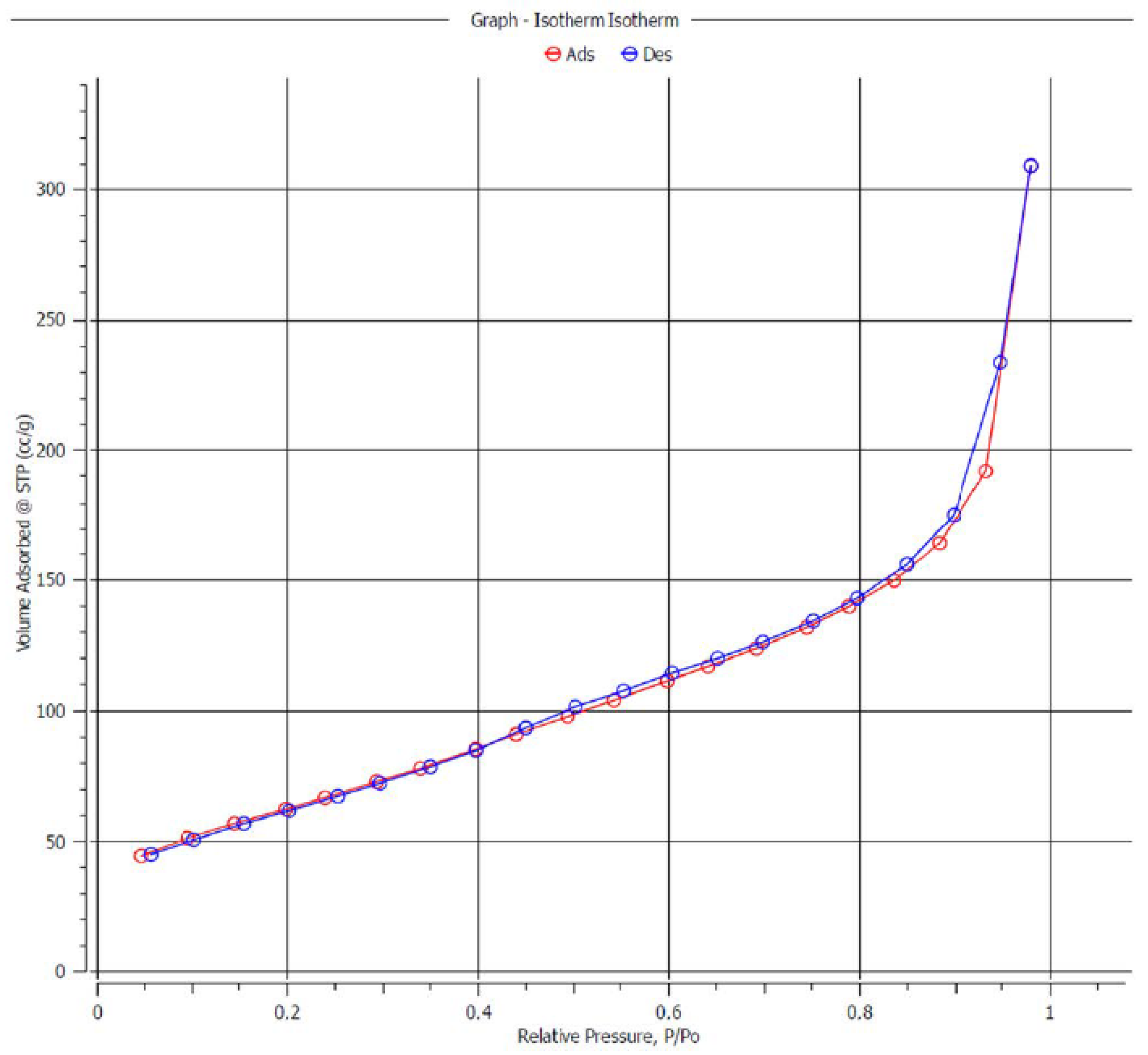
Appendix A. Catalysis and Characteristics of CuO(-Bi2O3)/SiO2 Pre-Catalyst
References
- Reppe, W.; Steinhofer, A.; Spaenig, H.; Locker, K. Production of Alkinols. U.S. Patent 2300969, 3 November 1942. [Google Scholar]
- Hanford, W.E.; Fuller, D.L. Acetylene Chemistry. Ind. Eng. Chem. 1948, 40, 1171–1177. [Google Scholar] [CrossRef]
- Reppe, W. Chemie und Technik der Acetylen-Druck-Reaktionen I-IV. Chem. Ing. Tech. 1950, 22, 361–373. [Google Scholar] [CrossRef]
- Reppe, W. Äthinylierung 0, I-VI. Justus Liebigs Ann. Der Chem. 1955, 596, 158–224. [Google Scholar]
- Luo, P.; Li, X. Application and Market of 1,4-butanediol Production of Reppe Method in China. Am. J. Chem. Eng. 2021, 9, 34. [Google Scholar] [CrossRef]
- Yang, W.; Peng, W.; Li, H.; Mao, J.; Qian, L.; Zhang, Q. Catalytic ethynylation of formaldehyde for selective propargyl alcohol production using the copper metal organic framework HKUST-1. New J. Chem. 2024, 48, 9082–9089. [Google Scholar] [CrossRef]
- Voronin, V.V.; Ledovskaya, M.S.; Bogachenkov, A.S.; Rodygin, K.S.; Ananikov, V.P. Acetylene in Organic Synthesis: Recent Progress and New Uses. Molecules 2018, 23, 2442. [Google Scholar] [CrossRef]
- Sitte, N.A.; Ghiringhelli, F.; Shevchenko, G.A.; Röminger, F.; Hashmi, A.S.K.; Schaub, T. Copper-Catalysed Synthesis of Propargyl Alcohol and Derivatives from Acetylene and other Terminal Alkynes. Adv. Synth. Catal. 2022, 364, 2227–2234. [Google Scholar] [CrossRef]
- Evans, E. The Production and Use of Butadiene and Isobutylene. In Introduction to Petroleum Chemicals; Pergamon Press: Oxford, UK, 1961; p. 18. [Google Scholar]
- Rode, C.V. Catalytic Hydrogenation of 2-Butyne-1, 4-diol: Activity, Selectivity and Kinetics Studies. J. Jpn. Pet. Inst. 2008, 51, 119–133. [Google Scholar] [CrossRef]
- Tanielyan, S.K.; More, S.R.; Augustine, R.L.; Schmidt, S.R. Continuous Liquid-Phase Hydrogenation of 1,4-Butynediol to High-Purity 1,4-Butanediol over Particulate Raney Nickel Catalyst in a Fixed Bed Reactor. Org. Process. Res. Dev. 2017, 21, 327–335. [Google Scholar] [CrossRef]
- Hort, E. Ethynylation Catalyst and Method of Producing Alkynols by Low-Pressure Reactions. U.S. Patent 3920759A, 18 November 1975. [Google Scholar]
- Hort, E. Ethynylation Catalyst and Process for Producing Alkynols. U.S. Patent 4002694A, 1 November 1977. [Google Scholar]
- Meng, L.; Li, H.; Liu, B.; Duan, R.; Yuan, S. Hierarchical Malachite Microsphere Catalyst in the Ethynylation of Formaldehyde for 1, 4-Butynediol Synthesis. ACS Omega 2025, 10, 12054–12061. [Google Scholar] [CrossRef]
- Madon, R.; Nagel, P.; Hedrick, S.; Thakur, D. Novel Ethynylation Catalyst and Method of Making Same. U.S. Patent US20150258534A1, 17 September 2014. [Google Scholar]
- Guo, H.; Sun, B. Preparation Method of Unsupported Copper-Bismuth Catalyst Applied to Preparation of 1, 4-Butynediol. CN Patent 106964385A, 21 July 2017. [Google Scholar]
- Yang, G.; Yu, Y.; Tahir, M.U.; Ahmad, S.; Su, X.; Xie, Y.; Wang, J. Promotion effect of Bi species in Cu/Bi/MCM-41 catalysts for 1,4-butynediol synthesis by ethynylation of formaldehyde. React. Kinet. Mech. Catal. 2019, 127, 425–436. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, Z.; Hao, Q.; Ban, L.; Li, H.; Zhao, Y.; Jiang, Z. Enhancing the Ethynylation Performance of CuO-Bi2O3 Nanocatalysts by Tuning Cu-Bi Interactions and Phase Structures. Catalysts 2019, 9, 35. [Google Scholar] [CrossRef]
- Chen, J.; Yang, G.; Gao, F.; Wang, R. The preparation of CuM/SiO2 (M=Bi, Mg, Mn) catalysts applied in ethynylation of formaldehyde for 1, 4-butynediol synthesis: The positive interface effect of CuO and Bi2O3. Dalton Trans. 2025, 54, 774–783. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Zhao, S.; Wang, B.; Ma, X.; Gong, J. A copper–phyllosilicate core–sheath nanoreactor for carbon–oxygen hydrogenolysis reactions. Nat. Commun. 2013, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ma, X.; Xu, H.; Ge, Q. Comparative study of silica-supported copper catalysts prepared by different methods: Formation and transition of copper phyllosilicate. Catal. Sci. Technol. 2016, 6, 4151–4158. [Google Scholar] [CrossRef]
- Xu, C.; Chen, G.; Zhao, Y.; Liu, P.; Duan, X.; Gu, L.; Fu, G.; Yuan, Y.; Zheng, N. Interfacing with Silica Boosts the Catalysis of Copper. Nat. Commun. 2018, 9, 3367. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Kawi, S. Preparation, characterization and catalytic application of phyllosilicate: A review. Catal. Today 2020, 339, 3–23. [Google Scholar] [CrossRef]
- Yang, G.; Gao, F.; Yang, L. The Importance of Copper-Phyllosilicate Formed in CuO/SiO2 Catalysts in the Ethynylation of Formaldehyde for 1, 4-Butynediol Synthesis. React. Chem. Eng. 2023, 8, 881–890. [Google Scholar] [CrossRef]
- Kirchner, J. Ethynylation Catalyst Catalyst Preparation and Process. U.S. Patent 3650985A, 21 March 1972. [Google Scholar]
- Sun, F.; Wang, G. Investigation of an Accidental Explosion Caused by Reaction Runaway of a Mixture Containing Copper Acetylide and Butynediol. J. Loss Prev. Process. Ind. 2019, 62, 103967. [Google Scholar] [CrossRef]
- Miller, S.; Penny, E. Hazards in Handling Acetylene in Chemical Processes Particularly Under Pressure; Institution of Chemical Engineers-Symposium on Chemical Process Hazards: Rugby, UK, 1960. [Google Scholar]
- Oxley, J.C.; Smith, J.L.; Steinkamp, F.L.; Gorawara, J.; Kanazirev, V. Study on Exposing Supported Copper Compounds to Acetylene. Acs Chem. Heal. Saf. 2017, 24, 26–33. [Google Scholar] [CrossRef]
- Judai, K.; Nishijo, J.; Nishi, N. Self-Assembly of Copper Acetylide Molecules into Extremely Thin Nanowires and Nanocables. Adv. Mater. 2006, 18, 2842–2846. [Google Scholar] [CrossRef]
- Evano, G.; Al, E. Turning Unreactive Copper Acetylides into Remarkably Powerful and Mild Alkyne Transfer Reagents by Oxidative Umpolung. ChemInform 2014, 50, 10008–10018. [Google Scholar] [CrossRef]
- Makarem, A.; Berg, R.; Rominger, F.; Straub, B.F. A Fluxional Copper Acetylide Cluster in CuAAC Catalysis. Angew. Chem. Int. Ed. Engl. 2015, 54, 7431–7435. [Google Scholar] [CrossRef] [PubMed]
- Díez-González, S. Copper(I)-Acetylides: Access, Structure, and Relevance in Catalysis. Adv. Organomet. Chem. 2016, 66, 93–141. [Google Scholar]
- Rasmussen, S.C. Cuprene: A historical curiosity along the path to polyacetylene. Bull. Hist. Chem. 2017, 42, 63–78. [Google Scholar] [CrossRef]
- Bruhm, T.; Abram, A.; Häusler, J.; Thomys, O.; Köhler, K. Walter Reppe Revival–Identification and Genesis of Copper Acetylides Cu2C2 as Active Species in Ethynylation Reactions. Chem.–A Eur. J. 2021, 27, 16834–16839. [Google Scholar] [CrossRef]
- Rosenthal, U. To “Dress” the “Naked” Acetylide with Metal Complexes: How and Why? Angew. Chem. Int. Ed. 2003, 42, 1794–1798. [Google Scholar] [CrossRef]
- Brameld, V.F.; Clark, M.T.; Seyfang, A.P. Copper Acetylides. J. Soc. Chem. Ind. 1947, 66, 346–353. [Google Scholar] [CrossRef]
- Hudson, B.S. Polyacetylene: Myth and Reality. Materials 2018, 11, 242. [Google Scholar] [CrossRef]
- Singh, R.; Tiwari, P.; Sharma, B.; Guerrero-Perilla, C.; Coy-Barrera, E. Analysis of Polyacetylenes. In Recent Advances in Natural Products Analysis; Silva, S., Nabavi, A., Saeedi, S.F., Nabavi, M.M.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 707–722. [Google Scholar] [CrossRef]
- Cataldo, F. Structural Relationships between Dicopper Diacetylide and Dicopper Acetylide. Eur. J. Solid State Inorg. Chem. 1998, 35, 11. [Google Scholar] [CrossRef]
- Cataldo, F. A Method for Synthesizing Polyynes in Solution. Carbon 2005, 43, 2792–2800. [Google Scholar] [CrossRef]
- Cataldo, F.; Casari, C.S. Synthesis, Structure and Thermal Properties of Copper and Silver Polyynides and Acetylides. J. Inorg. Organomet. Polym. Mater. 2007, 17, 641–651. [Google Scholar] [CrossRef]
- Cataldo, F.; Compagnini, G.; Scandurra, A.; Strazzulla, G. Silver and Copper Polyynides: A Study with HPLC, FT-IR and XPS Spectroscopy. Fuller. Nanotub. Carbon Nanostructures 2008, 16, 126–141. [Google Scholar] [CrossRef]
- Cataldo, F. The role of Raman spectroscopy in the research on sp-hybridized carbon chains: Carbynoid structures polyynes and metal polyynides. J. Raman Spectrosc. 2007, 39, 169–176. [Google Scholar] [CrossRef]
- Judai, K.; Numao, S.; Nishijo, J.; Nishi, N. In situ Preparation and Catalytic Activation of Copper Nanoparticles from Acetylide Molecules. J. Mol. Catal. A Chem. 2011, 347, 28–33. [Google Scholar] [CrossRef]
- Bruhm, T.; Kong, L.; Abram, A.; Thomys, O.; Köhler, K. Interrelationship of Cuprous Acetylide Cu2C2 Formation and Catalytic Performance in the Reppe Ethynylation of Formaldehyde. ChemCatChem 2025, 17, e202500565. [Google Scholar] [CrossRef]
- Ess, M.N.; Bladt, H.; Mühlbauer, W.; Seher, S.I.; Zöllner, C.; Lorenz, S.; Brüggemann, D.; Nieken, U.; Ivleva, N.P.; Niessner, R. Reactivity and Structure of Soot Generated at Varying Biofuel Content and Engine Operating Parameters. Combust. Flame 2016, 163, 157–169. [Google Scholar] [CrossRef]

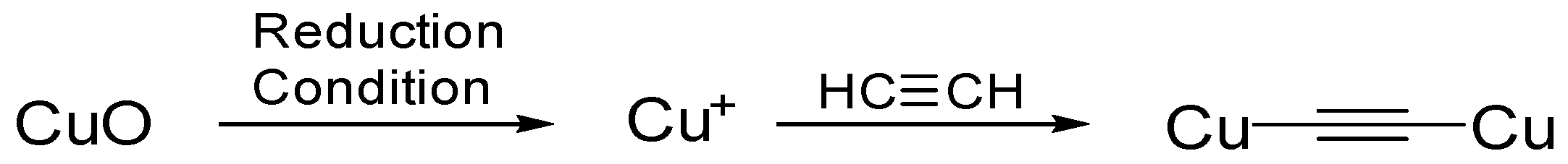

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Köhler, K. Stability and Deactivation Behavior of Cuprous Acetylide Containing Catalysts in Reppe Ethynylation. Catalysts 2025, 15, 829. https://doi.org/10.3390/catal15090829
Kong L, Köhler K. Stability and Deactivation Behavior of Cuprous Acetylide Containing Catalysts in Reppe Ethynylation. Catalysts. 2025; 15(9):829. https://doi.org/10.3390/catal15090829
Chicago/Turabian StyleKong, Lingdi, and Klaus Köhler. 2025. "Stability and Deactivation Behavior of Cuprous Acetylide Containing Catalysts in Reppe Ethynylation" Catalysts 15, no. 9: 829. https://doi.org/10.3390/catal15090829
APA StyleKong, L., & Köhler, K. (2025). Stability and Deactivation Behavior of Cuprous Acetylide Containing Catalysts in Reppe Ethynylation. Catalysts, 15(9), 829. https://doi.org/10.3390/catal15090829





