Enhanced Electrocatalytic Performance for Selective Glycerol Oxidation to Formic Acid at a Multiphase AuCu-Ag/AgBr Interface
Abstract
1. Introduction
2. Results
2.1. Physicochemical Properties of Catalysts
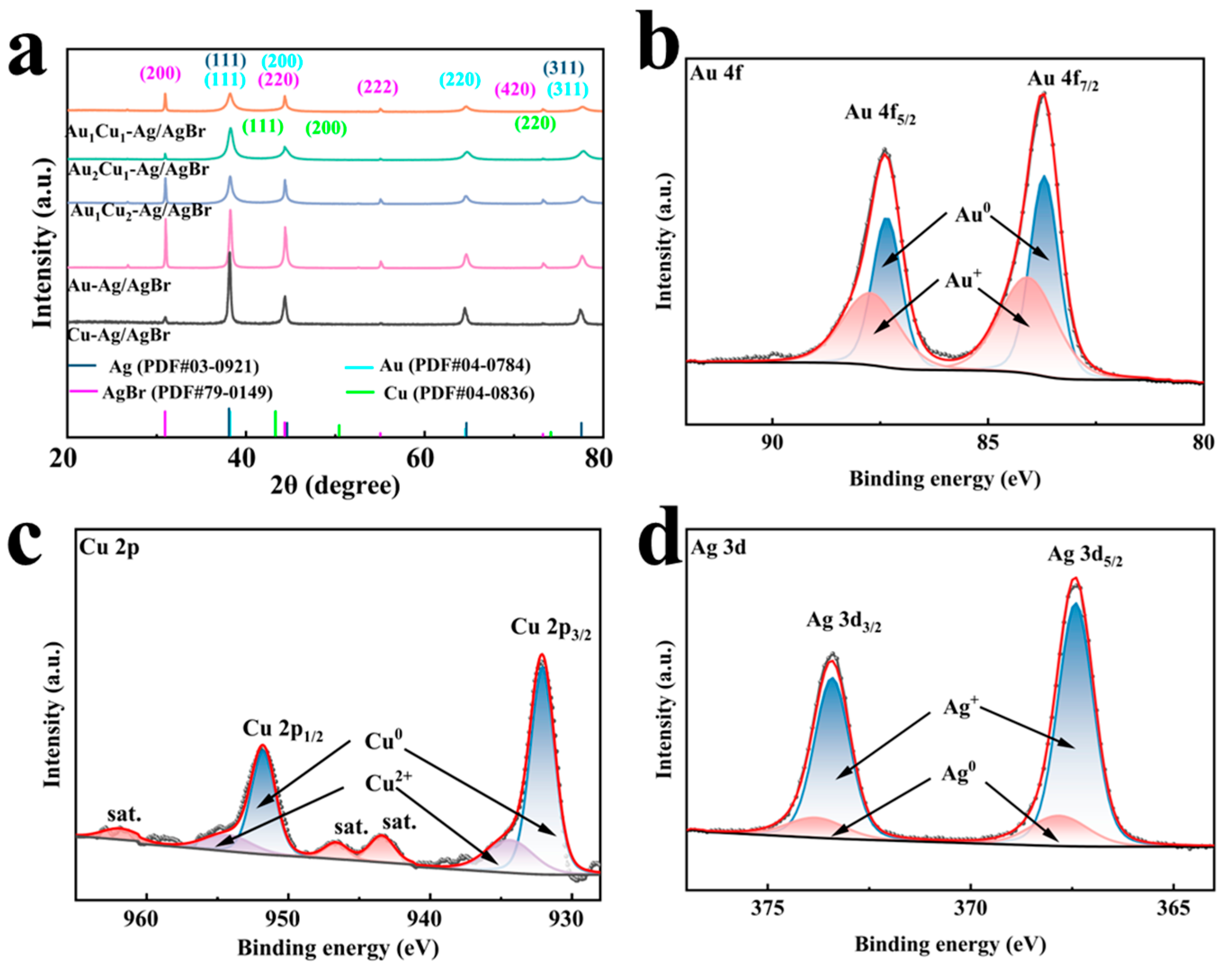
2.1.1. XRD Analysis
2.1.2. Elemental Composition and Chemical State Analysis
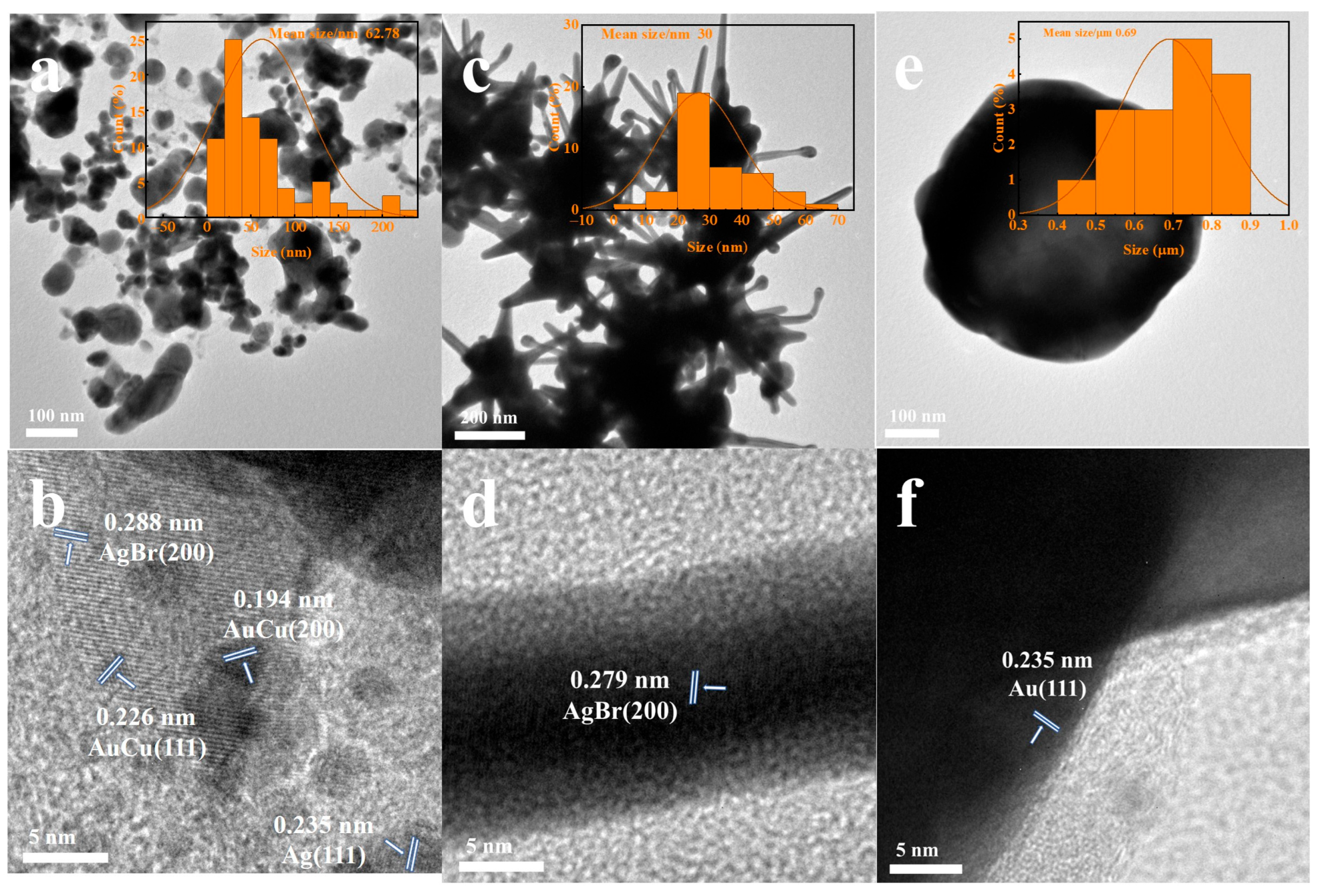
2.1.3. TEM Analysis
2.2. Electro-Oxidation of Glycerol
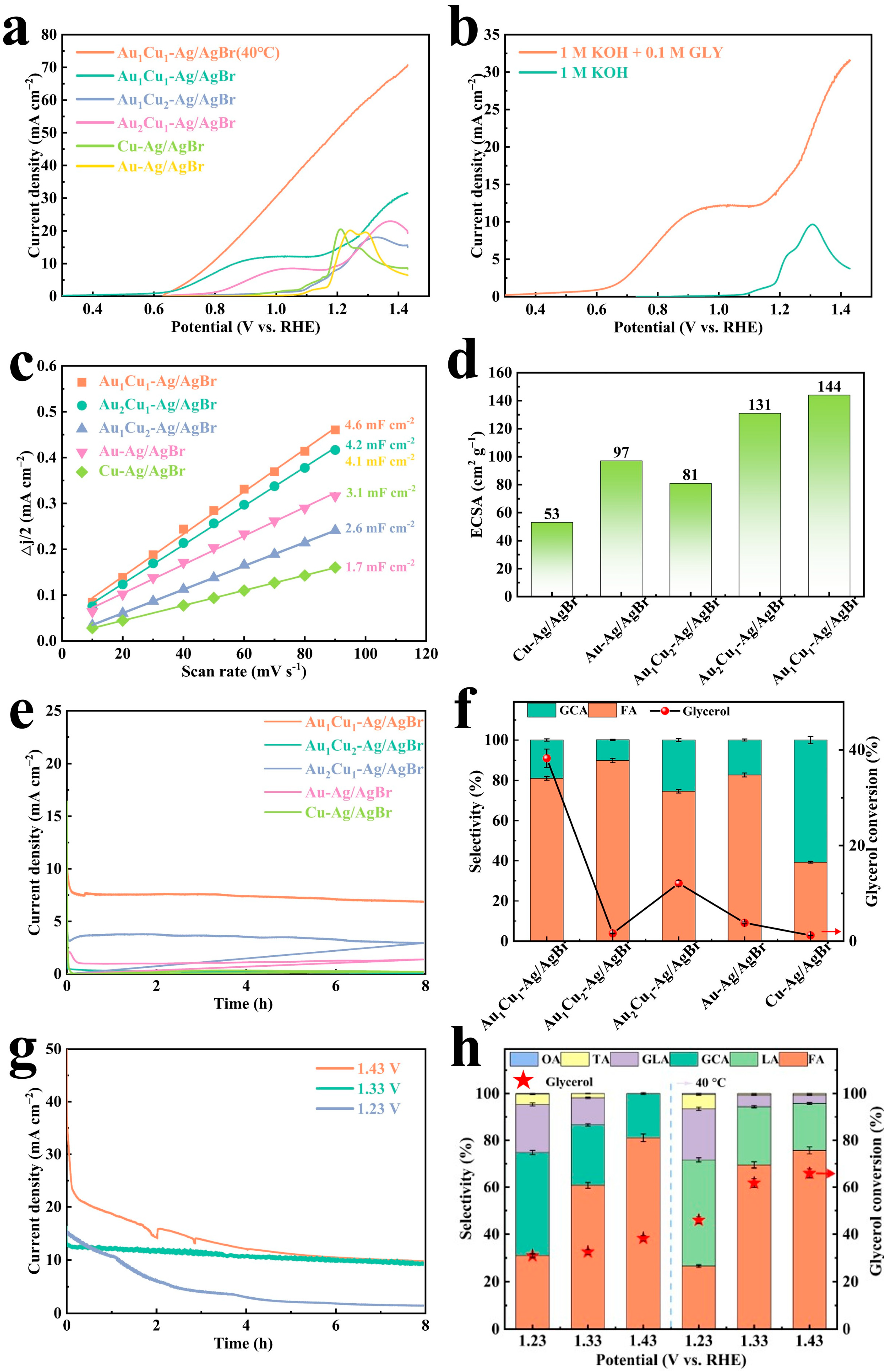
2.2.1. Catalyst Screening Experiments
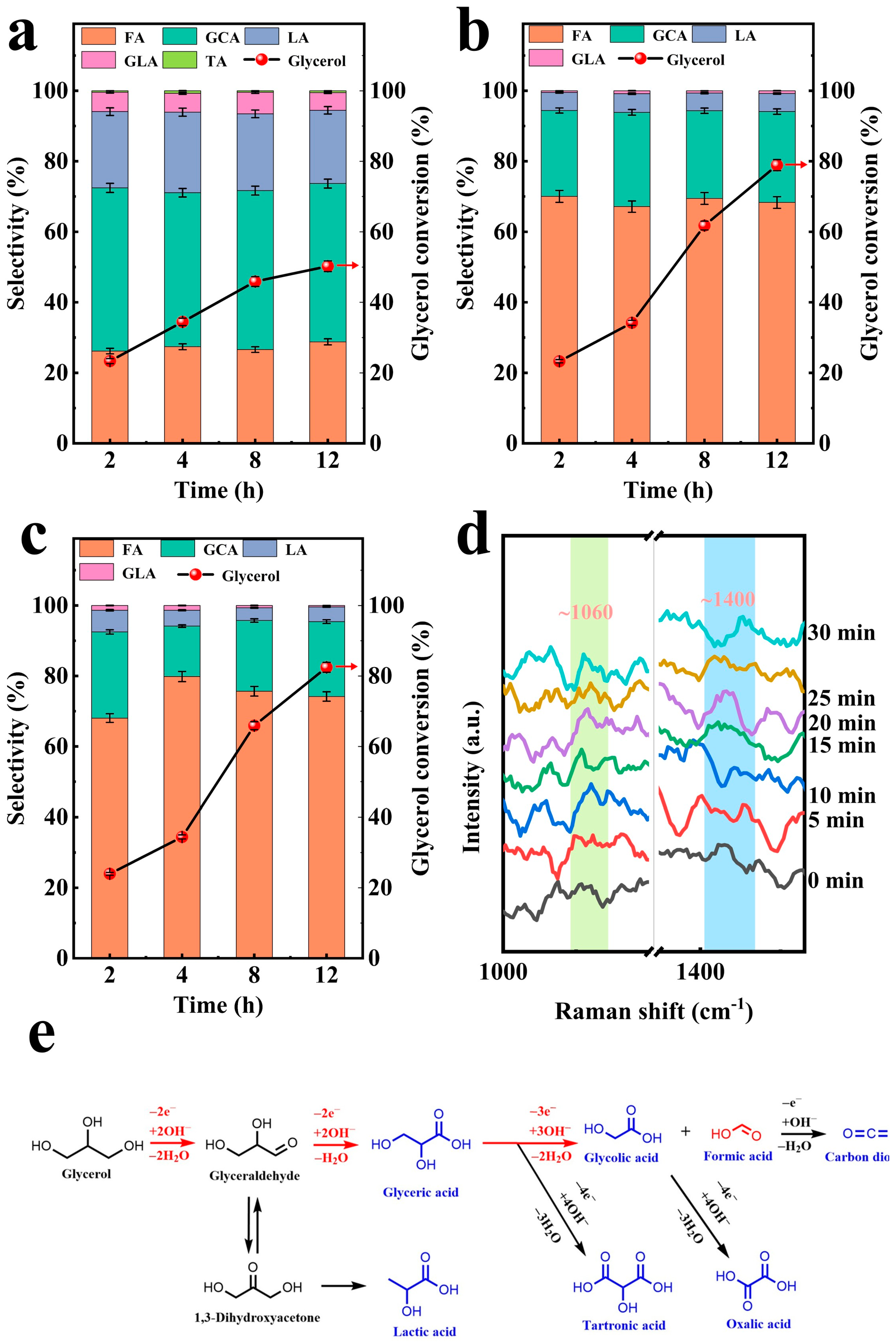
2.2.2. Effect of Single Reaction Parameter
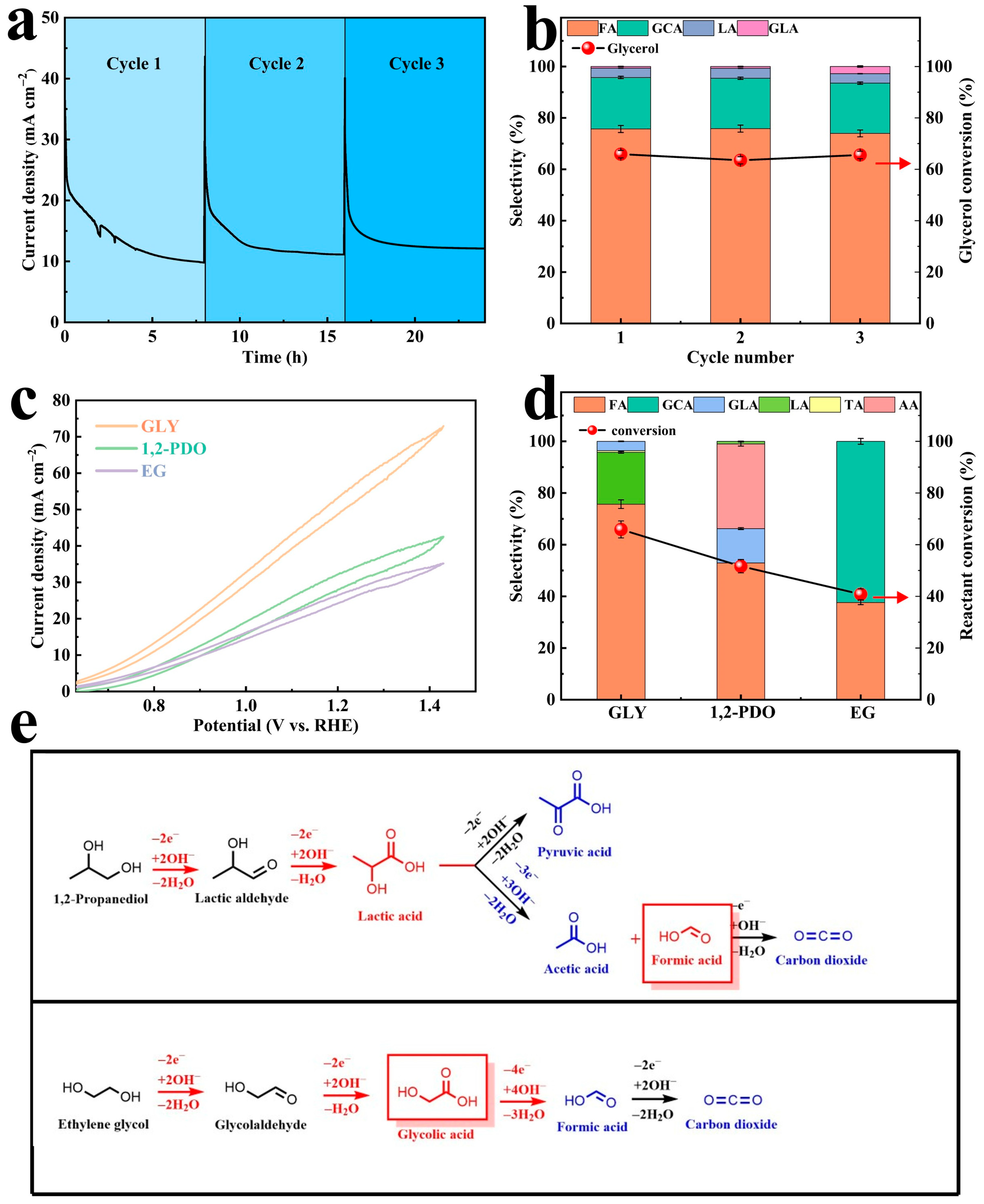
2.2.3. Substrate Scope Investigation
3. Materials and Methods
3.1. Reagents and Materials
3.2. Catalyst Synthesis
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Q.; Eid, K.; Li, W. Heteroatom-Doped Porous Carbon-Based Nanostructures for Electrochemical CO2 Reduction. Nanomaterials 2022, 12, 2379. [Google Scholar] [CrossRef]
- Ma, Q.; Ji, Q.; Chen, L.; Zhu, Z.; Tu, S.; Okonkwo, C.E.; Out, P.; Zhou, C. Multimode ultrasound and ternary deep eutectic solvent sequential pretreatments enhanced the enzymatic saccharification of corncob biomass. Ind. Crops Prod. 2022, 188, 115574. [Google Scholar] [CrossRef]
- Ren, M.; Kong, F.; Zhou, C.; Fakayode, O.A.; Liang, J.; Li, H.; Zhou, M.; Fan, X. Green, one-pot biomass hierarchical utilization strategy for lignin-containing cellulose nanofibrils and fractionated lignin preparation. Ind. Crops Prod. 2023, 203, 117193. [Google Scholar] [CrossRef]
- Nazir, M.J.; Li, G.; Nazir, M.M.; Zulfiqar, F.; Siddique, K.H.M.; Iqbal, B.; Du, D. Harnessing soil carbon sequestration to address climate change challenges in agriculture. Soil Tillage Res. 2024, 237, 105959. [Google Scholar] [CrossRef]
- Eid, K.; Ozoemena, K.I.; Varma, R.S. Unravelling the structure-activity relationship of porous binary metal-based electrocatalysts for green hydrogen evolution reaction. Coord. Chem. Rev. 2025, 523, 216238. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Huang, C.; Jian, L.; Lin, Z.; Lin, L.; Li, C.; Ye, Y. Insight into the influence of plant oils on the composition of diacylglycerol fabricated by glycerolysis and esterification. Ind. Crops Prod. 2023, 204, 117324. [Google Scholar] [CrossRef]
- Fokum, E.; Zabed, H.M.; Ravikumar, Y.; Elshobary, M.E.; Chandankere, R.; Zhang, Y.; Yun, J.; Qi, X. Co-fermentation of glycerol and sugars by Clostridium beijerinckii: Enhancing the biosynthesis of 1,3-propanediol. Food Biosci. 2021, 41, 101028. [Google Scholar] [CrossRef]
- Okonkwo, C.E.; Hussain, S.Z.; Onyeaka, H.; Adeyanju, A.A.; Nwonuma, C.O.; Bashir, A.A.; Farooq, A.; Zhou, C.; Shittu, T.D. Lignin polyphenol: From biomass to innovative food applications, and influence on gut microflora. Ind. Crops Prod. 2023, 206, 117696. [Google Scholar] [CrossRef]
- Gong, C.; Meng, X.; Jin, C.; Yang, M.; Liu, J.; Sheng, K.; Pu, Y.; Ragauskas, A.; Ji, G.; Zhang, X. Green synthesis of cellulose formate and its efficient conversion into 5-hydroxymethylfurfural. Ind. Crops Prod. 2023, 192, 115985. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.; Lakhiar, I.A.; Solangi, K.A.; Qureshi, W.A.; Shaikh, S.A.; Chen, J. Influence of Atomization Nozzles and Spraying Intervals on Growth, Biomass Yield, and Nutrient Uptake of Butter-Head Lettuce under Aeroponics System. Agronomy 2021, 11, 97. [Google Scholar] [CrossRef]
- Pan, S.; Zabed, H.M.; Wei, Y.; Qi, X. Technoeconomic and environmental perspectives of biofuel production from sugarcane bagasse: Current status, challenges and future outlook. Ind. Crops Prod. 2022, 188, 115684. [Google Scholar] [CrossRef]
- Uprety, B.K.; Venkatesagowda, B.; Rakshit, S.K. Current Prospects on Production of Microbial Lipid and Other Value-Added Products Using Crude Glycerol Obtained from Biodiesel Industries. BioEnergy Res. 2017, 10, 1117–1137. [Google Scholar] [CrossRef]
- Xiong, Z.; Liu, J.; Tian, Y.; Wang, Z.; Wang, X.; Shi, T.; Jin, W.; Yuan, L.; Gao, R. Structural and aggregation changes of silver carp myosin induced with alcohols: Effects of ethanol, 1,2-propanediol, and glycerol. Food Chem. 2024, 452, 139542. [Google Scholar] [CrossRef] [PubMed]
- Alaba, P.A.; Lee, C.S.; Abnisa, F.; Aroua, M.K.; Cognet, P.; Pérès, Y.; Wan Daud, W.M.A. A review of recent progress on electrocatalysts toward efficient glycerol electrooxidation. Rev. Chem. Eng. 2021, 37, 779–811. [Google Scholar] [CrossRef]
- Han, X.; Sheng, H.; Yu, C.; Walker, T.W.; Huber, G.W.; Qiu, J.; Jin, S. Electrocatalytic Oxidation of Glycerol to Formic Acid by CuCo2O4 Spinel Oxide Nanostructure Catalysts. ACS Catal. 2020, 10, 6741–6752. [Google Scholar] [CrossRef]
- Shen, L.; Sun, L.; Douthwaite, M.; Akdim, O.; Taylor, S.; Hutchings, G.J. Hollow Au1Cu1(111) Bimetallic Catalyst Promotes the Selective Electrochemical Conversion of Glycerol into Glycolic Acid. ACS Catal. 2024, 14, 11343–11351. [Google Scholar] [CrossRef]
- Morales, D.M.; Jambrec, D.; Kazakova, M.A.; Braun, M.; Sikdar, N.; Koul, A.; Brix, A.C.; Seisel, S.; Andronescu, C.; Schuhmann, W. Electrocatalytic Conversion of Glycerol to Oxalate on Ni Oxide Nanoparticles-Modified Oxidized Multiwalled Carbon Nanotubes. ACS Catal. 2022, 12, 982–992. [Google Scholar] [CrossRef]
- Lyu, N.; Chen, Y.; Guan, A.; Wei, R.; Yang, C.; Huang, Y.; Lv, X.; Hu, C.; Kuang, M.; Zheng, G. Electrocatalytic Glycerol Upgrading into Glyceric Acid on Ni3Sn Intermetallic Compound. Small 2024, 20, e2401872. [Google Scholar] [CrossRef]
- Luo, W.; Tian, H.; Li, Q.; Ma, J.; Sun, W.; Zhu, L.; Wu, H.; Kong, F.; Zhuang, X.; Cui, X.; et al. Controlled Electrocatalytic Glycerol Upgrading to Glyceraldehyde inNear Neutral Media by Cobalt Oxide Lattice Activation. Angew. Chem. Int. Ed 2025, 64, e202505059. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, H.; Xu, S.-M.; Yang, J.; Hao, P.; Cai, X.; Ren, Y.; Xu, M.; Kong, X.; Shao, M.; et al. Electrocatalytic Upcycling of Biomass and Plastic Wastes to Biodegradable Polymer Monomers and Hydrogen Fuel at High Current Densities. J. Am. Chem. Soc. 2023, 145, 6144–6155. [Google Scholar] [CrossRef]
- Shi, K.; Si, D.; Teng, X.; Chen, L.; Shi, J. Enhanced electrocatalytic glycerol oxidation on CuCoN0.6/CP at significantly reduced potentials. Chin. J. Catal. 2023, 53, 143–152. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.; Chen, L.; Shi, J.; He, M. Nickel-molybdenum nitride nanoplate electrocatalysts for concurrent electrolytic hydrogen and formate productions. Nat. Commun. 2019, 10, 5335. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, Q.; Han, Y.; Zhang, D.; Zhang, R.; Song, N.; Wu, X.; Zeng, J.; Yuan, P.; Chen, J.; et al. Strengthening the synergy between oxygen vacancies in electrocatalysts for efficient glycerol electrooxidation. Adv. Mater. 2024, 36, e2401857. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Yu, X.; Chen, L.; Yarley, O.P.N.; Zhou, C. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crops Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Han, E.; Pan, Y.Y.; Li, L.; Cai, J.R. Bisphenol A detection based on nano gold-doped molecular imprinting electrochemical sensor with enhanced sensitivity. Food Chem. 2023, 426, 136608. [Google Scholar] [CrossRef]
- Liao, D.; Zhao, Y.; Zhou, Y.; Yi, Y.; Weng, W.; Zhu, G. Colorimetric detection of organophosphorus pesticides based on Nb2CTx MXene self-reducing PdPt nanozyme integrated with hydrogel and smartphone. J. Food Meas. Charact. 2024, 18, 9223–9232. [Google Scholar] [CrossRef]
- Luo, H.; Titirici, M.M. Rational Engineering of Nanostructured AgM (M = Au, Pt, Pd) Bimetallic Electrodes via Galvanic Replacement for Glycerol Electrolysis. Adv. Eng. Mater. 2025, 27, 2402544. [Google Scholar] [CrossRef]
- Simões, M.; Baranton, S.; Coutanceau, C. Enhancement of catalytic properties for glycerol electrooxidation on Pt and Pd nanoparticles induced by Bi surface modification. Appl. Catal. B Environ. 2011, 110, 40–49. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, L.; Pan, X.; Zhou, Z.; Zheng, Y.; Yang, X.; Zhao, G. Carbon paper supported gold nanoflowers for tunable glycerol electrooxidation boosting efficient hydrogen evolution. Carbon 2022, 203, 88–96. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Qi, J.; Wang, Z.; Li, W. Selective electro-conversion of glycerol to glycolate on carbon nanotube supported gold catalyst. Green Chem. 2012, 14, 2150–2152. [Google Scholar] [CrossRef]
- Gomes, J.F.; Garcia, A.C.; Gasparotto, L.H.S.; de Souza, N.E.; Ferreira, E.B.; Pires, C.; Tremiliosi-Filho, G. Influence of silver on the glycerol electro-oxidation over AuAg/C catalysts in alkaline medium: A cyclic voltammetry and in situ FTIR spectroscopy study. Electrochim. Acta 2014, 144, 361–368. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, R.; Niu, L.; Sun, Y.; Zhang, W.; Wang, H.; Wang, J.; Liu, Y. TiO2 nanorod arrays modified by AuAg alloy nanoparticles for photoelectrocatalytic glycerol conversion and synergistic hydrogen production. Int. J. Hydrogen Energy 2025, 119, 34–44. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Y.; Xi, J. Seed-mediated synthesis of PtxAuy@Ag electrocatalysts for the selective oxidation of glycerol. Appl. Catal. B Environ. 2019, 245, 604–612. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Q.; Yang, J.; Fu, Y.; Shi, Q.; Li, Z.; Zhang, J.; Shao, M.; Duan, X. Selective Electrooxidation of Crude Glycerol to Lactic Acid Coupled With Hydrogen Production at Industrially-Relevant Current Density. Small 2024, 21, e2406782. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Qing, S.; Gao, Z.; Tong, X.; Yang, N. Modulating Electronic Structure of an Au-Nanorod-Core–PdPt-Alloy-Shell Catalyst for Efficient Alcohol Electro-Oxidation. Adv. Energy Mater. 2021, 11, 2100812. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, H.; Lu, X.; Guo, L.; Du, D.; Shan, H.; Geng, L.; Akdim, O.; Huang, X.; Park, G.-S.; et al. Enhanced redox catalysis of electrochemical alcohol oxidation in alkaline medium by using Pt-Cu/C catalyst. J. Alloys Compd. 2022, 926, 166994. [Google Scholar] [CrossRef]
- Garcia, A.C.; Caliman, J.; Ferreira, E.B.; Tremiliosi-Filho, G.; Linares, J.J. Promotional Effect of Ag on the Catalytic Activity of Au for Glycerol Electrooxidation in Alkaline Medium. ChemElectroChem 2015, 2, 1036–1041. [Google Scholar] [CrossRef]
- Lee, C.S.; Aroua, M.K.; Daud, W.A.W.; Cognet, P.; Pérès, Y.; Ajeel, M.A. Selective Electrochemical Conversion of Glycerol to Glycolic Acid and Lactic Acid on a Mixed Carbon-Black Activated Carbon Electrode in a Single Compartment Electrochemical Cell. Front. Chem. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Oh, L.S.; Tan, Y.C.; Song, H.; Kim, H.J.; Oh, J. Enhancing Glycerol Conversion and Selectivity toward Glycolic Acid via Precise Nanostructuring of Electrocatalysts. ACS Catal. 2021, 11, 14926–14931. [Google Scholar] [CrossRef]
- Luo, W.; Tian, H.; Li, Q.; Meng, G.; Chang, Z.; Chen, C.; Shen, R.; Yu, X.; Zhu, L.; Kong, F.; et al. Controllable Electron Distribution Reconstruction of Spinel NiCo2O4 Boosting Glycerol Oxidation at Elevated Current Density. Adv. Funct. Mater. 2024, 34, 202306995. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, W.; Sun, Q.; Liu, X. Raman and infrared spectroscopic quantification of the carbonate concentration in K2CO3 aqueous solutions with water as an internal standard. Geosci. Front. 2021, 12, 1018–1030. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Q.; Zhao, Y.; Feng, Y.; Wang, D.; Schaaf, P.; Guo, G. Wang Efficiently triggering ordered and stable oxidation active sites on Ni-based interfaces by coordination with nitrogen family anions for glycerol electrolysis. Mater. Today Energy 2025, 51, 101874. [Google Scholar] [CrossRef]
- Chauhan, I.; Vijay, P.M.; Ranjan, R.; Patra, K.K.; Gopinath, C.S. Electrocatalytic and Selective Oxidation of Glycerol to Formate on 2D 3d-Metal Phosphate Nanosheets and Carbon-Negative Hydrogen Generation. ACS Mater. Au 2024, 4, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, J.; Wu, Y.; Qi, L.; Tian, X.; Li, M.; Wang, W.; He, R. Interface effect induced electron-deficient Ni sites for efficient glycerol electrooxidation. Chin. Chem. Lett. 2025, 111001. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Gao, F.; Chen, Q.; Li, C.; He, J. The role of iron in Ni-Fe binary catalysts for electrochemical glycerol oxidation. J. Colloid Interface Sci. 2025, 697, 137913. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, C. Hollow CoS/Ni3S2 nanotube arrays for enhanced anodic glycerol electrooxidation-assisted water electrolysis. Appl. Surf. Sci. 2025, 705, 163515. [Google Scholar] [CrossRef]
- Li, Q.; Sun, H.; Liu, Q.; Yang, G.; Pian, Y.; Li, X.; Zheng, Z.; Li, S.; Li, Z.; Liu, J.; et al. Enhancing formic acid and hydrogen co-electrosynthesis via engineering atom arrangement in nickel-boron interstitials. Appl. Catal. B Environ. Energy 2026, 380, 125773. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Fang, K.; Wang, L.; Ni, H.; Yu, J.; Wang, Z.; Wang, J.; Zhao, B. Efficient Glycerol Oxidation on PMo12-Encapsulated MOF-74 Electrocatalyst Coupled With Hydrogen Evolution at Industrial-Level Current Density. Adv. Funct. Mater. 2025, 35, 2502616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Sun, L.; Wang, Z.; Li, S.; Shen, L.; Yin, H. Enhanced Electrocatalytic Performance for Selective Glycerol Oxidation to Formic Acid at a Multiphase AuCu-Ag/AgBr Interface. Catalysts 2025, 15, 831. https://doi.org/10.3390/catal15090831
Jin J, Sun L, Wang Z, Li S, Shen L, Yin H. Enhanced Electrocatalytic Performance for Selective Glycerol Oxidation to Formic Acid at a Multiphase AuCu-Ag/AgBr Interface. Catalysts. 2025; 15(9):831. https://doi.org/10.3390/catal15090831
Chicago/Turabian StyleJin, Jianchuan, Luyao Sun, Zhiqing Wang, Shiyu Li, Lingqin Shen, and Hengbo Yin. 2025. "Enhanced Electrocatalytic Performance for Selective Glycerol Oxidation to Formic Acid at a Multiphase AuCu-Ag/AgBr Interface" Catalysts 15, no. 9: 831. https://doi.org/10.3390/catal15090831
APA StyleJin, J., Sun, L., Wang, Z., Li, S., Shen, L., & Yin, H. (2025). Enhanced Electrocatalytic Performance for Selective Glycerol Oxidation to Formic Acid at a Multiphase AuCu-Ag/AgBr Interface. Catalysts, 15(9), 831. https://doi.org/10.3390/catal15090831






