Abstract
Metal–organic frameworks (MOFs) have become a hot topic in various research fields nowadays. And MOF-derived metal oxides prepared by the sacrificial template method have been widely applied as catalysts for pollutant removal. Accordingly, we prepared a series of MOF-derived MnOx catalysts with diverse morphologies (rod-like, flower-like, slab-like) via the pyrolysis of MOF precursors, and the as-prepared MnOx catalysts demonstrated superior performance compared to the one prepared using the co-precipitation method. MnOx-II, with a flower-like structure, exhibited excellent activity for formaldehyde (HCHO) catalytic ozonation at room temperature, reaching complete HCHO conversion at O3/HCHO of 1.5 and more than 90% CO2 selectivity at an O3/HCHO ratio of 2.5. On the basis of various characterization methods, it was clarified that the enhanced catalytic performance of MnOx-II benefited from its larger BET surface area, abundant oxygen vacancies, better redox ability at lower temperature, and more Lewis acid sites. The H2O resistance and stability tests were also conducted. Furthermore, DFT calculations substantiated the enhanced adsorption of HCHO and O3 on oxygen vacancies, while in–situ DRIFTS measurements elucidated the degradation pathway of HCHO during catalytic ozonation through detected intermediates.
1. Introduction
Driven by the accelerating urbanization and industrialization trends, the emission of gaseous pollutants has increased significantly, causing great damage to air quality worldwide. Typically, volatile organic compounds (VOCs), as crucial atmospheric pollutants, consistently induce adverse health effects and air quality issues, like dust haze and photochemical smog [1]. Common in indoor environments, formaldehyde (HCHO) usually leads to respiratory problems and lung damage, and worse yet, long exposure to HCHO even causes leukemia and cancers [2]. To mitigate the adverse effect of HCHO on the atmosphere and public health, developing efficient control means has become imperative.
Catalytic ozonation is a desirable method for pollution treatment, which can mineralize VOCs into harmless products (H2O and CO2) without secondary pollution. With the promotion of ozone (O3), the reaction temperature is further lowered due to its strong oxidizability and produced free radicals (·O2–, ·O2–, ·OH) [3]. Moreover, the selection of catalysts with superior catalytic activity is important to impel the proceeding of the catalytic ozonation reaction. To date, numerous catalysts have been employed for HCHO degradation, involving precious metals (Au, Pt, Pd, etc.) [4,5,6,7] and non-precious metals (Mn, Co, etc.) [8,9]. However, the exorbitant price and deactivation tendency of precious metals restrict their practical application. Hence, efficient and economical catalysts are urgently required for HCHO removal.
Manganese oxides (MnOx) have been widely applied to pollutant treatment as promising materials because of their low cost, multiple valence states, and diverse shapes [10]. Rational design strategies for better catalytic activity of MnOx have been extensively investigated, such as regulating oxygen vacancies [11], adjusting morphologies [12], and altering preparation methods [13]. Zhao et al. [14] reported that the catalytic ozonation on MnOx (O3/HCHO=3:1) promoted the HCHO conversion (~100%) at ambient temperature, confirming the unique ability of ozone in promoting the performance of MnOx under low temperature. Saputra et al. [15] systematically designed the morphologies of α-Mn2O3 (cubic, octahedral, truncated-octahedral), and the catalyst with a cubic shape exhibited higher catalytic activity for its bigger specific surface area and abundant active surface facets. Wang et al. [12] prepared several MnOx catalysts with distinct morphologies (rod-, wire-, tubular-, and flower-like), and rod-like α-MnO2 achieved complete toluene oxidation at lower temperatures, relating to its plentiful oxygen vacancies and reliable redox ability. Accordingly, the morphological control of MnOx catalysts might prove to be critical to optimize catalytic performance.
Recently, MOFs have been intensively investigated in the catalysis field for some attractive advantages, including large surface area, controllable morphology, and high porosity [16,17,18,19,20]. With plenty of electron-deficient open metal sites, MOFs have strong interaction with VOCs and can easily adsorb and degrade pollution gases, which brings a broader prospect for VOC removal [21]. Furthermore, MOFs can be pyrolyzed as sacrificial precursors at high temperature in an aerobic atmosphere to fabricate diverse porous metal oxides, which can inherit the porosity and well-defined morphology of pristine MOFs [22]. By selecting appropriate precursors and control strategies, diverse morphologies of MOF-derived nano-structure catalysts can be rationally designed. Zhang et al. [23] researched the activity of Mn2O3 obtained from Mn-MOFs with different precursors (MIL-100, MOF-74, BTC). The catalyst derived from the cubic Mn-MIL-100 precursor performed well, associating with its higher Mn3+/Mn4+ ratio and plentiful surface oxygen species. Li et al. [24] fabricated MOF-derived MnOx-m catalysts with a nanorod-self-assembly hollow microsphere structure. Compared with other derivatives, the MnOx-m catalysts presented superior catalytic performance due to their abundant exposed active sites, desirable redox ability, and plentiful active oxygen species. These studies illustrate that the catalyst performance is highly associated with morphological structures. However, there have been few studies on the catalytic ozonation of HCHO over MOF-derived MnOx catalysts with different morphologies so far.
Herein, a series of MOFs-derived MnOx catalysts with diverse structures were synthesized in this work via the pyrolysis of different MOFs precursors. The catalytic activity was evaluated through the measurement of HCHO conversion and CO2 selectivity during the catalytic ozonation reaction at ambient temperature. Meanwhile, all kinds of characterization methods were adopted to illustrate the structure-activity relationship. Moreover, the intermediates generated during HCHO ozonation were also detected through in-situ DRIFTS analysis. Integrated with DFT calculation, the reaction mechanism was further clarified.
2. Results and Discussion
2.1. Catalytic Ozonation of HCHO
Figure 1a presents the catalytic performance of MnOx catalysts during HCHO ozonation at room temperature by varying O3/HCHO ratios (0.5~3.0). As shown in Equation (1), the complete mineralization of HCHO with ozone required a theoretical O3/HCHO ratio of 2.0. Clearly, the increase in the O3/HCHO ratio significantly improved the HCHO conversion of the catalysts. The O3/HCHO ratio corresponding to 90% HCHO conversion (O90) was the following sequence: MnOx-II (1.1) < MnOx-I (1.3) < MnOx-III (1.4) < MnOx-IV (1.8). Compared to the MOF-derived MnOx catalysts, MnOx-IV prepared by the co-precipitation method had undesirable catalytic activity, getting a 100% conversion at O3/HCHO of 3.0. Meanwhile, MnOx-II with a flower-like structure exhibited remarkably higher HCHO conversion, achieving 100% HCHO conversion when the O3/HCHO ratio was 1.5. It was worth mentioning that almost no O3 residual was observed during the catalytic tests.
HCHO + (2 − x)O3 → (1 − x)CO2 + xCO + (2 − x)O2 + H2O
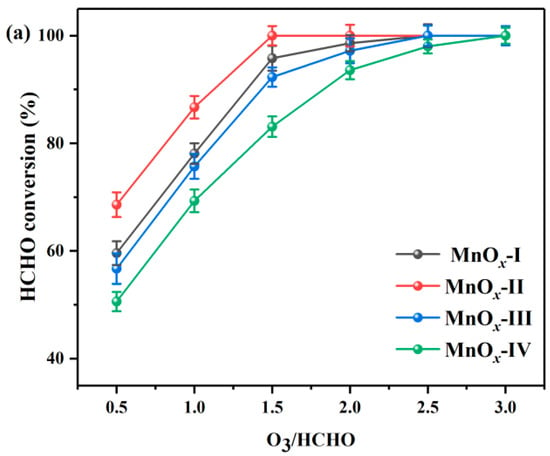
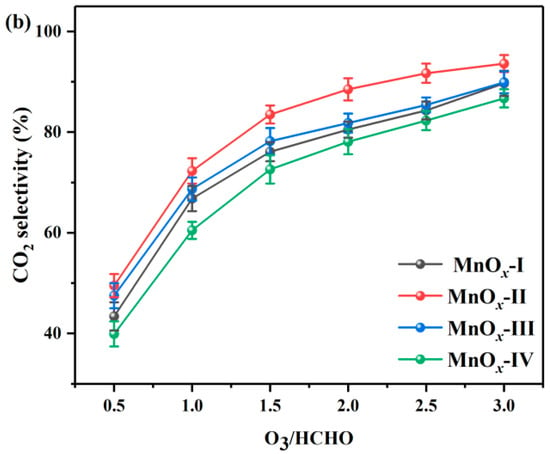
Figure 1.
(a) HCHO conversion and (b) CO2 selectivity during catalytic ozonation of HCHO over synthesized catalysts. (HCHO initial concentration: 30 ppm, T = 30 °C).
To reduce hazardous intermediates and promote green environmental protection, environmentally friendly CO2 and H2O are regarded as ideal final products. Consequently, the CO2 selectivity results of MnOx catalysts are shown in Figure 1b. Obviously, for all the catalysts, the CO2 selectivity had a nearly linear increase with the O3/HCHO ratio. And on the whole range of O3 input, MnOx-II maintained the highest CO2 selectivity, which attained above 90% CO2 selectivity at an O3/HCHO ratio of 2.5, and the order for the CO2 selectivity was as follows: MnOx-II > MnOx-III > MnOx-I > MnOx-IV.
2.2. Catalyst Characterization
2.2.1. Crystalline Structures
The crystalline phases of the MnOx catalysts were identified through XRD analysis (Figure 2). And the diffraction peaks at 23.1°, 32.9°, 38.2°, 45.1°, 49.3°, 55.1°, and 65.7° were ascribed to the (2 1 1), (2 2 2), (4 0 0), (3 3 2), (4 3 1), (4 4 0), and (6 2 2) planes of α-Mn2O3 (PDF#71-0636), while the weak peaks at 18.1°, 28.8°, and 36.6° were indexed to the (2 0 0), (3 1 0), and (4 0 0) plane of MnO2 (PDF#44-0141) [25]. Compared with other MnOx catalysts, MnOx-I and MnOx-II displayed a relatively weaker diffraction intensity, implying their smaller particle sizes, which introduced sufficient lattice defects and enhanced catalytic performance [26]. Moreover, the crystallite size was calculated by the Scherrer formula, and the crystallite sizes of MnOx-I, MnOx-II, MnOx-III, and MnOx-IV were 35.6 nm, 32.3 nm, 36.7 nm, and 37.2 nm, respectively. MnOx-II showed the smallest crystallite size.
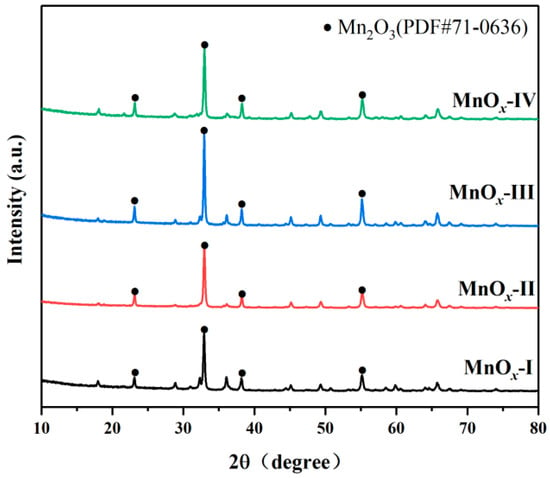
Figure 2.
XRD patterns of prepared catalysts.
To explore the corresponding morphologies of MOF precursors and MOF-derived MnOx catalysts, the images of SEM characterization are depicted in Figure 3. Clearly, MnOx-I presented a rod-like structure composed of multiple stacked nanorods, while MnOx-II exhibited a flower-like structure with ordered assembly nanosheets, and MnOx-III consisted of many stacked slabs. Prepared by the co-precipitation method, MnOx-IV showed an amorphous structure with agglomerated nanoparticles, which might result in a reduction in surface area. After calcination, the surface of MOF-derived MnOx catalysts became rougher, and a lot of voids appeared, attributable to the thermolysis of organic ligands under thermal treatment. Fortunately, the morphologies of MOF precursors were basically retained on MOF-derived MnOx catalysts, and the generated surface defects on porous structures accelerated both active site formation and VOCs–catalyst contact efficiency [27].
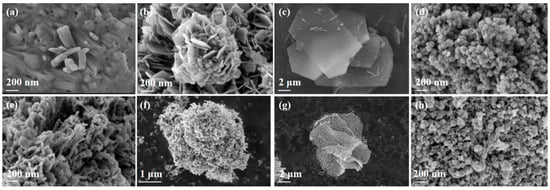
Figure 3.
SEM images of (a) MnOx-I, (b) MnOx-II, (c) MnOx-III, (d) MnOx-IV before calcination; and (e) MnOx-I, (f) MnOx-II, (g) MnOx-III, (h) MnOx-IV after calcination.
2.2.2. Textual Properties
To explore the textual properties of MnOx catalysts, their N2 adsorption–desorption isotherms (Type IV, H3 hysteresis loop) and BJH pore size distributions are depicted (Figure S1). The BET surface area and pore volume are arranged in the sequence of MnOx-II > MnOx-I > MnOx-III > MnOx-IV (Table 1), in accord with the HCHO conversion sequence. Higher surface area usually promotes the generation of surface active sites, the adsorption of VOCs, and the activation of ozone [28,29]. With the optimal catalytic activity, MnOx-II possessed a higher surface area and pore volume, 20.0 m2·g−1 and 0.079 cm3·g−1, respectively. Moreover, MnOx-IV exhibited the least favorable catalytic activity in HCHO degradation, with lower surface area and pore volume.

Table 1.
Textual properties of prepared catalysts.
2.2.3. Surface Properties
To research the valence states of elements on the catalyst surface, the XPS curves are depicted in Figure 4, and the content of elements after deconvolution is presented in Table 2. As displayed in Figure 4a, the binding energies at 641.4 and 643.3 eV were ascribed to Mn3+ and Mn4+ species, respectively [30,31]. The Mn3+/Mn4+ ratio declined as the sequence MnOx-II (1.85) > MnOx-I (1.72) > MnOx-III (1.48) > MnOx-IV (1.44), fitting with the order of HCHO conversion. The existence of Mn3+ ions could promote the formation of oxygen vacancies through a charge balance mechanism [32]. Thus, a higher Mn3+/Mn4+ ratio usually suggests more oxygen vacancies on the catalyst surface, which can promote the decomposition of ozone and the catalytic ozonation of VOCs [33].
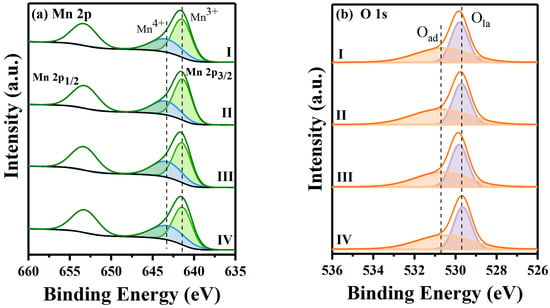
Figure 4.
XPS spectra of (a) Mn 2p and (b) O 1 s for synthesized catalysts.

Table 2.
XPS results of synthesized catalysts.
The O 1s spectra identified lattice oxygen species (Ola) at 529.7 eV and adsorbed oxygen species (Oad) at 530.7 eV, respectively (Figure 4b). Moreover, the ratio of Oad/Ola ranked in order of MnOx-II (1.23) > MnOx-I (1.10) > MnOx-III (1.07) > MnOx-IV (1.03). Compared to Ola species, Oad species showed higher oxygen mobility, which could accelerate oxygen vacancy generation and promote the reactant activation process [34,35].
To sum up, MnOx-II had the highest Mn3+ and Oad contents, which was conducive to increased oxygen vacancies and accelerated oxygen mobility. Different from the MOF-derived MnOx catalysts, MnOx-IV presented a lower Mn3+ and Oad proportion, fitting with its undesirable catalytic efficiency. Moreover, the results of XPS analysis aligned with the HCHO conversion order, indicating the critical role of oxygen vacancies in affecting catalytic performance.
2.2.4. Temperature Programmed Studies
A Raman spectrum study was performed to explore the oxygen vacancies in MnOx catalysts (Figure 5a). The characteristic peaks at 630~650 cm−1 corresponded to the v2(Mn−O) stretching vibration of [MnO6] chains, while Mn−O bond strength usually correlates to oxygen vacancies formation [36]. Based on Hooke’s law, the strength of the Mn−O bond was determined by the following equation (Equation (2)):
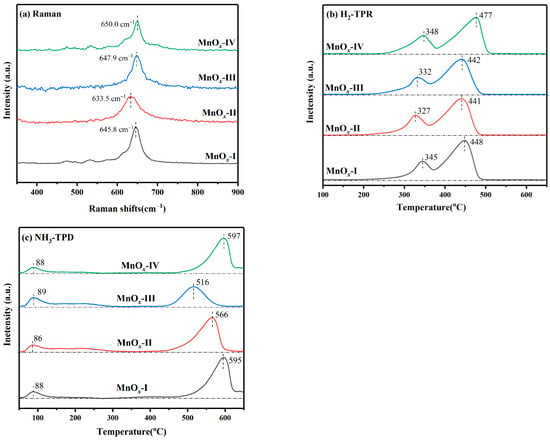
Figure 5.
(a) Raman; (b) H2-TPR; (c) NH3-TPD profiles of synthesized catalysts.
The ω value of catalysts decreased in the order: MnOx-IV (650.0 cm−1) > MnOx-III (647.9 cm−1) > MnOx-I (645.8 cm−1) > MnOx-II (633.5 cm−1). Performing well in catalytic activity, MnOx-II showed the lowest ω value, which corresponded to the lowest strength. Weaker Mn−O bond strength indicates an increase in oxygen vacancy defects as well as higher oxygen mobility because of the easily stripped oxygen species, which can facilitate HCHO deep oxidation [37,38]. Hence, increasing oxygen vacancy concentration usually enhances catalytic performance, consistent with the XPS analysis results.
H2-TPR measurement was implemented to assess the reducibility of synthesized MnOx catalysts, with relevant profiles presented in Figure 5b. Clearly, the curves of catalysts presented two obvious reduction peaks, while the peak at 300~400 °C belonged to Mn4+ to Mn3+ transformation (MnO2 → Mn2O3) and another one (400~500 °C) was assigned to Mn3+ to Mn2+ reduction (Mn2O3 → MnO), respectively, corresponding to the main existence of Mn4+ and Mn3+ from XPS analysis [39,40]. For the peaks at lower temperature (300~400 °C), the temperature of reduction peaks ranked as MnOx-II < MnOx-III < MnOx-I < MnOx-IV. Lower reduction temperature usually means better reducibility, which can promote oxygen species migration and mobility [41,42]. The reduction temperature of MnOx-IV was relatively higher, corresponding to its weak reducibility and poor catalytic activity. With better redox ability at lower temperatures, the low-temperature activity of MnOx-II would be greatly promoted.
To analyze the acidity of MnOx catalysts, the NH3-TPD profiles are presented in Figure 5c. With desorption temperature increasing, the two peaks were ascribed to Lewis acid sites (physisorbed NH3 and NH4+) and Brönsted acid sites (desorption of NH4+), respectively [43]. Generally, surface acidity is of great importance in the removal procedure of VOCs, covering reactant adsorption, ozone decomposition, and resultant desorption [44]. Notably, Lewis acid sites are closely correlated to reactive oxygen species formation and C–C bond breaking, which facilitate the further reaction of HCHO [45,46]. With desirable catalytic activity, MnOx-II showed a lower desorption temperature (86 °C) and more Lewis acid sites, signifying its better acidity at lower temperatures, which would facilitate the degradation of HCHO indoors.
2.3. Effect of H2O
As an inevitable component in indoor air, water vapor (H2O) is known to be a possible influence factor for catalytic performance. In the long-time H2O resistance test, two different concentrations of H2O (RH = 50% and RH = 100%) were added to reaction gas (Figure 6). Meanwhile, the HCHO conversion and outlet CO2 selectivity over MnOx-II catalyst at 30 °C were monitored during the test, with an O3/HCHO ratio of 2.0. With the addition of a low concentration of H2O (RH = 50%), the HCHO conversion was basically unchanged after 2 h. Subsequently, introducing a higher concentration of H2O (RH = 100%), the HCHO conversion slightly decreased to a stable platform at ~97% and then gradually recovered to ~99% after stopping H2O. Evidently, H2O of high concentration resulted in catalyst poisoning to a certain extent, and the slight deactivation was fortunately reversible. In addition, the outlet CO2 selectivity obviously increased when introducing H2O, as the generated OH active radicals may be conducive to the complete oxidation of HCHO [47]. Moreover, the stability test was conducted, and the HCHO conversion was maintained at ~100% for 7 h, demonstrating the desirable stability of MnOx-II.
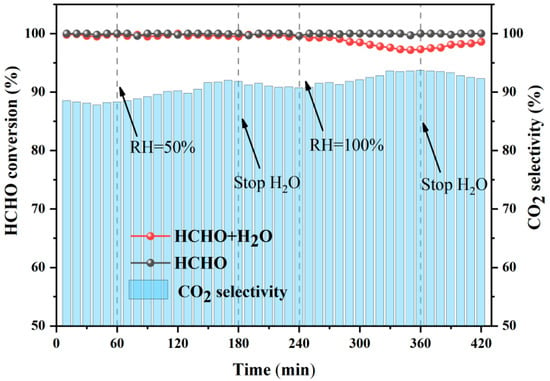
Figure 6.
Effect of H2O on HCHO catalytic ozoantion over MnOx-II catalyst.
2.4. In-Situ DRIFTS Measurement
To study the HCHO ozonation reaction mechanism on MnOx-II catalysts, in situ DRIFTS spectra were collected to record the evolution of intermediate products. As shown in Figure 7a, the dynamic changes in the HCHO adsorption process (30 ppm HCHO+N2) at ambient temperature were conducted with the change in reaction time. Typically, the peaks located between 800 and 1200 cm−1 were identified as DOM species [48]. While the bands at 1579 cm−1 and 1376 cm−1 were ascribed to the asymmetric and symmetric (COO) stretch vibration of formates, respectively. Also, the band at 2858 cm−1 was associated with various hydrocarbon vibrations [49]. Additionally, the peaks at 3690 and 1350 cm−1 belonged to hydroxyl groups and carbonate species, respectively [50]. The weak inverse peaks of hydroxyl groups may reflect the utilization of OH groups and the continuous regeneration, possibly facilitating the further conversion of DOM species [51].
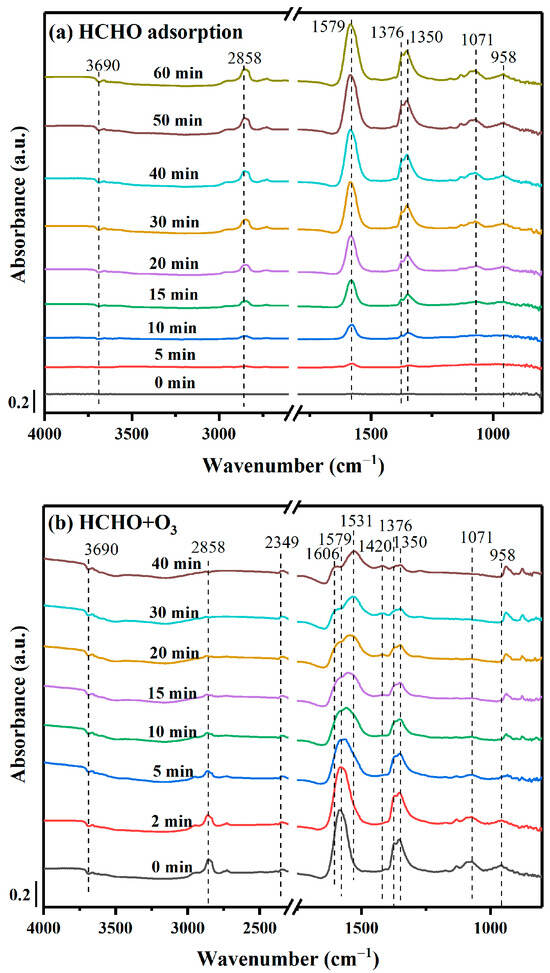
Figure 7.
In-situ DRIFTS analysis of HCHO degradation mechanism on MnOx-II catalyst (Reaction conditions: (a) 30 ppm HCHO; (b) addition of 90 ppm O3).
When the adsorption of HCHO was saturated, 90 ppm O3 was added to the feed gas. Clearly, the intensity of DOM bands (1071 and 958 cm−1) gradually disappeared, as presented in Figure 7b. Simultaneously, the peaks of formate species (1376, 1579, and 2858 cm−1) decreased with time, and the peaks corresponding to different modes of carbonate species, such as hydrocarbonate species (1606 cm−1), bicarbonate species (1420 cm−1) and monodentate carbonate species (1531 cm−1) emerged [52]. Moreover, the band that appeared at 2349 cm−1 corresponded to CO2 formation as the ideal final product. And the band of hydroxyl groups (3690 cm−1) was basically unchanged, which may be replenished by the generated H2O during the HCHO catalytic ozonation.
2.5. Theoretical Calculation and Reaction Mechanism
For heterogeneous catalysis, the adsorption of reactants on MnOx catalysts with α-Mn2O3 structure was an important process [53]. To find out the role of oxygen vacancies in the reactant adsorption procedure, the adsorption energies and bond lengths of O3 and HCHO on the α-Mn2O3 (1 1 1) surface were evaluated (Figure 8). With an oxygen vacancy (V0), the calculated O3 adsorption energy (−0.46 eV) and Mn-O distance (1.935 Å) were lower than the original ones (Eads = −0.34 eV, LMn-O = 1.951 Å). As for the adsorption of HCHO, the adsorption energy was −0.36 eV, higher than the configuration with V0 (Eads = −0.52 eV), and the Mn–O bond length was in the same situation (LMn-O = 2.44 Å, LMn-O = 2.34 Å with V0). Typically, more negative adsorption energies and shorter bond lengths always indicate an enhanced interaction between reactants and catalytic surfaces [54]. Thus, the existence of oxygen vacancies may promote reactant adsorption and consequently accelerate catalytic reaction.
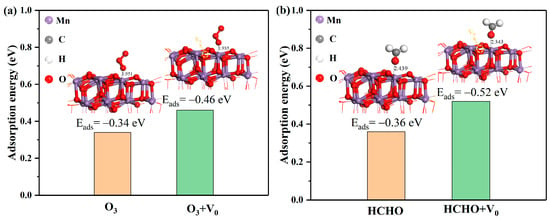
Figure 8.
The Eads and bond length of (a) O3 and (b) HCHO on the α-Mn2O3 (1 1 1) surface (V0 referred to oxygen vacancy).
In summary, coupling in situ DRIFTS with DFT analysis, a feasible reaction route of HCHO catalytic ozonation is depicted in Figure 9. Firstly, with the promotion of oxygen vacancies, the adsorption process of HCHO and O3 on surface reactive sites of MnOx catalysts occurred. And then the active oxygen species immediately converted HCHO into DOM species, while the surface reactive hydroxyl groups were related to the formation of formate species. Then, on the surface oxygen vacancies, O3 was quickly decomposed to O2 molecules and reactive oxygen radicals (O*). Finally, formate species were further oxidized into carbonic acid and decomposed to form CO2 and H2O, producing harmless degradation products.
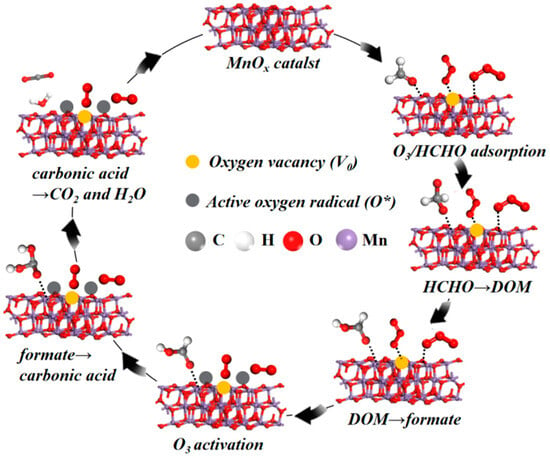
Figure 9.
Possible route for HCHO catalytic ozonation on MnOx catalysts.
3. Materials and Methods
3.1. Catalyst Preparation
For the synthesis of MnOx-I, 5 mmol Mn(CH3COO)2·4H2O (Aladdin, Shanghai, China) and 15 mmol H3BTC (Aladdin, China) were placed in deionized (DI) water and ethanol (Sinopharm, China), respectively. Next, they would be vigorously blended and kept under constant stirring (25 °C, 15 h).
To obtain MnOx-II, 5 mmol Mn(CH3COO)2·4H2O and a certain amount of PVP (Aladdin, China) were dispersed in a mixture solution (vol ratio of C2H5OH and H2O was 2:1) (A solution). Next, 10 mmol H3BTC was dissolved in the same C2H5OH/H2O (2:1) solution (B solution). Subsequently, after the thorough mixing of A and B, the admixture was left to stand for 12 h at ambient temperature.
During the typical synthesis of MnOx-III, 0.1 mol/L Mn(CH3COO)2·4H2O (5 mmol) solution and 0.1 mol/L 8-hydroxyquinoline (10 mmol) (Aladdin, Shanghai, China) solution were mixed vigorously and maintained for 5 h at normal temperature.
Typically, the MnOx-IV was synthesized by the co-precipitation method for comparison via the intensive mixing of Mn(CH3COO)2·4H2O solution and NaOH (Sinopharm, Beijing, China) solution.
All the samples were separated through centrifugation and washed with DI water and ethanol. After being totally dried and then annealed at 500 °C for 3 h, the obtained catalysts were ground to 40~60 mesh. In summary, the synthesis scheme of MOF-derived MnOx catalysts is presented in Figure 10.
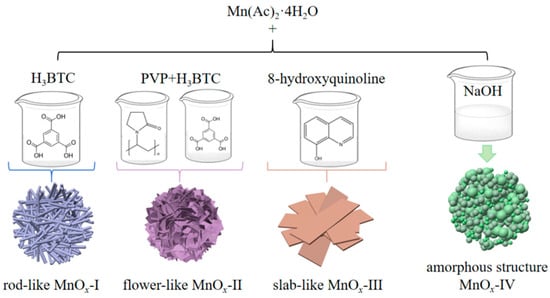
Figure 10.
The preparation scheme of MOFs-derived MnOx catalysts.
3.2. Catalytic Activity
The catalytic ozonation of HCHO over synthesized catalysts was performed on an experimental setup, as depicted in Figure S2. Meanwhile, the detailed procedures were summarized in Supporting Information (Text S1).
The catalytic activity was evaluated by conversion efficiencies of HCHO and O3, and the selectivity of CO and CO2, which were calculated with the following equations:
where [HCHO]initial and [O3]initial are the initial concentration of HCHO and O3, unit ppm, respectively. [CO]outlet, [CO2]outlet and [O3]outlet are the outlet concentration of CO, CO2 and O3, unit ppm, respectively.
3.3. Catalyst Characterization
The samples were evaluated by XRD, SEM, XPS, BET, NH3-TPD, H2-TPR, and in situ DRIFTS characterizations. And the materials in detail are presented in Supporting Information (Text S2).
3.4. DFT Calculation
The DFT calculations were conducted with the CASTEP package using Material Studio software 2019 [55]. And the exchange–correlation interaction was processed with the generalized gradient approximation (GGA) using the Perdew–Burke–Enzerhof (PBE) function. A 650 eV cutoff energy was adopted for all DFT calculations. From XRD analysis, a an α-Mn2O3 phase of MnOx catalysts was confirmed, and the Mn2O3 (1 1 1) surface was cleaved, as shown in Figure S3 [56,57]. And a 16 Å vacuum layer was introduced in the z-direction to eliminate the next layer’s influence. The adsorption energy (Eads) of adsorbates on the catalyst surface was determined by the equation below:
where E(adsorbate-catalyst) is the total energy of catalyst-adsorbate system, Ecatalyst is the total energy of catalyst, and Eadsorbate is the total energy of the gas-phase molecule.
Eads = E(adsorbate-catalyst) − (Ecatalyst + Eadsorbate)
4. Conclusions
To completely remove HCHO under room temperature, the catalytic ozonation method with higher efficiency and fewer byproducts is an efficient way. Herein, a series of efficient MOF-derived MnOx catalysts with distinct shapes were fabricated via a facile sacrificial template strategy for HCHO catalytic ozonation. The flower-like MnOx-II catalyst showed excellent catalytic activity (100% HCHO conversion, O3/HCHO = 1.5) and higher CO2 selectivity (above 90%, O3/HCHO = 2.5), while the MnOx-IV prepared by the co-precipitation method exhibited poor catalytic performance. The unique flower-like structure of MnOx-II endows it with expanded surface area and abundant surface active sites, facilitating the catalytic ozonation process of HCHO. Moreover, its higher Mn3+ and Oad content promoted the formation of oxygen vacancies, as verified by Raman spectra. Therefore, the presence of oxygen vacancies appears crucial for catalysis, accelerating reactant adsorption, ozone decomposition, and complete oxidation of HCHO. In addition, the better acidity and reducibility, sufficient active oxygen species, and improved oxygen mobility also contributed to the excellent activity of MnOx-II. In stability and H2O tolerance experiments, MnOx-II maintained ~100% HCHO conversion without H2O and ~97% HCHO conversion when RH = 100%, respectively. Based on DFT calculation, the crucial role of oxygen vacancies in promoting HCHO and O3 adsorption process was elucidated. Moreover, through the detection of intermediate products, a feasible catalytic mechanism was raised (HCHO, DOM, formates, H2CO3, H2O+CO2, successively).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15080752/s1, Figure S1: N2 adsorption-desorption isotherms and distribution curves of pore size of synthesized catalysts.; Figure S2: Schematic of experimental set-up; Figure S3: Schematic structure model of Mn2O3 (1 1 1) surface.
Author Contributions
Y.S.: investigation, conceptualization, methodology, validation, writing original draft. Y.Z. (Yiwei Zhang): writing—review and editing, conceptualization. Y.H.: project administration, resources, methodology. W.W.: conceptualization, supervision, project administration. Y.Z. (Yanqun Zhu): writing—review and editing. Z.W.: writing—review and editing, methodology, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the “Pioneer” and “Leading Goose” R&D Program of Zhejiang (2023C03126), the National Natural Science Foundation of China (52125605), and the Fundamental Research Funds for the Central Universities (2022ZFJH04).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hequet, V.; Raillard, C.; Debono, O.; Thévenet, F.; Locoge, N.; Le Coq, L. Photocatalytic oxidation of VOCs at ppb level using a closed-loop reactor: The mixture effect. Appl. Catal. B Environ. 2018, 226, 473–486. [Google Scholar] [CrossRef]
- Ji, J.; Lu, X.; Chen, C.; He, M.; Huang, H. Potassium-modulated δ-MnO2 as robust catalysts for formaldehyde oxidation at room temperature. Appl. Catal. B Environ. 2020, 260, 118210. [Google Scholar] [CrossRef]
- He, C.; Wang, Y.; Li, Z.; Huang, Y.; Liao, Y.; Xia, D.; Lee, S. Facet engineered α-MnO2 for efficient catalytic ozonation of odor CH3SH: Oxygen vacancy-induced active centers and catalytic mechanism. Environ. Sci. Technol. 2020, 54, 12771–12783. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Tanaka, K.I. Perfect catalytic oxidation of formaldehyde over a Pt/TiO2 catalyst at room temperature. Catal. Commun. 2005, 6, 211–214. [Google Scholar] [CrossRef]
- Qu, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. 3D gold-modified cerium and cobalt oxide catalyst on a graphene aerogel for highly efficient catalytic formaldehyde oxidation. Small 2019, 15, 1804415. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lv, D.; Wu, J.; Xiao, J.; Xi, H.; Xia, Q.; Li, Z. A new MOF-505@GO composite with high selectivity for CO2/CH4 and CO2/N2 separation. Chem. Eng. J. 2017, 308, 1065–1072. [Google Scholar] [CrossRef]
- Chen, M.; Yin, H.; Li, X.; Qiu, Y.; Cao, G.; Wang, J.; Yang, X.; Wang, P. Facet-and defect-engineered Pt/Fe2O3 nanocomposite catalyst for catalytic oxidation of airborne formaldehyde under ambient conditions. J. Hazard. Mater. 2020, 395, 122628. [Google Scholar] [CrossRef]
- Guan, S.; Li, W.; Ma, J.; Lei, Y.; Zhu, Y.; Huang, Q.; Dou, X. A review of the preparation and applications of MnO2 composites in formaldehyde oxidation. J. Ind. Eng. Chem. 2018, 66, 126–140. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, Y.; Li, R.; Huang, T.; Cao, J.J.; Shen, Z.; Lee, S.C. Formaldehyde oxidation over Co@N-doped carbon at room temperature: Tunable Co size and intensified surface electron density. ACS EST Eng. 2021, 1, 917–927. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, J.; Jiang, W.; Zhang, Z.; Wang, J.; Zhu, Y.; Zhang, Q. Surface oxygen vacancy induced α-MnO2 nanofiber for highly efficient ozone elimination. Appl. Catal. B Environ. 2017, 209, 729–737. [Google Scholar] [CrossRef]
- Wang, F.; Dai, H.; Deng, J.; Bai, G.; Ji, K.; Liu, Y. Manganese oxides with rod-, wire-, tube-, and flower-like morphologies: Highly effective catalysts for the removal of toluene. Environ. Sci. Technol. 2012, 46, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Lin, F.; Cai, B.; Li, G.; Zhang, L.; Wang, Z.; Yan, B.; Wang, Y.; Chen, G. Catalytic ozonation of CH2Cl2 over hollow urchin-like MnO2 with regulation of active oxygen by catalyst modification and ozone promotion. J. Hazard. Mater. 2022, 436, 129217. [Google Scholar] [CrossRef] [PubMed]
- Dezhi, Z.; Tianying, D.; Xiaosong, L.I.; Jinglin, L.I.U.; Chuan, S.H.I.; Aimin, Z.H.U. Ozone catalytic oxidation of HCHO in air over MnOx at room temperature. Chin. J. Catal. 2012, 33, 396–401. [Google Scholar] [CrossRef]
- Tadéa, M.O.; Wanga, S. Shape-controlled activation of peroxymonosulfate by single crystal-Mn2O3 for catalytic phenol degradation in aqueous solution. Appl. Catal. B Environ. 2014, 154, 246–251. [Google Scholar]
- Zhao, Q.; Du, Q.; Yang, Y.; Zhao, Z.; Cheng, J.; Bi, F.; Shi, X.; Xu, J.; Zhang, X. Effects of regulator ratio and guest molecule diffusion on VOCs adsorption by defective UiO-67: Experimental and theoretical insights. Chem. Eng. J. 2022, 433, 134510. [Google Scholar] [CrossRef]
- Huang, C.; Ji, Q.; Zhang, H.; Wang, Y.; Wang, S.; Liu, X.; Guo, Y.; Zhang, C. Ru-incorporated Co3O4 nanoparticles from self-sacrificial ZIF-67 template as efficient bifunctional electrocatalysts for rechargeable metal-air battery. J. Colloid Interface Sci. 2022, 606, 654–665. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Zhao, S.; Bi, F.; Song, L.; Liu, N.; Xu, J.; Wang, Y.; Zhang, X. Stable black phosphorus encapsulation in porous mesh-like UiO-66 promoted charge transfer for photocatalytic oxidation of toluene and o-dichlorobenzene: Performance, degradation pathway, and mechanism. ACS Catal. 2022, 12, 8069–8081. [Google Scholar] [CrossRef]
- Bi, F.; Zhao, Z.; Yang, Y.; Liu, Q.; Huang, W.; Huang, Y.; Zhang, X. Efficient degradation of toluene over ultra-low Pd supported on UiO-66 and its functional materials: Reaction mechanism, water-resistance, and influence of SO2. Environ. Funct. Mater. 2022, 1, 166–181. [Google Scholar] [CrossRef]
- Yuan, K.; Song, T.; Wang, D.; Zhang, X.; Gao, X.; Zou, Y.; Dong, H.; Tang, Z.; Hu, W. Effective and Selective Catalysts for Cinnamaldehyde Hydrogenation: Hydrophobic Hybrids of Metal–Organic Frameworks, Metal Nanoparticles, and Micro-and Mesoporous Polymers. Angew. Chem. Int. Ed. 2018, 57, 5708–5713. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.H.; Kwon, E.E.; Szulejko, J.E. Metal–organic frameworks for the control and management of air quality: Advances and future direction. J. Mater. Chem. A 2016, 4, 345–361. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal–organic framework-derived nanoporous metal oxides toward supercapacitor applications: Progress and prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, X.; Bi, F.; Lu, G.; Wang, Y. Highly efficient Mn2O3 catalysts derived from Mn-MOFs for toluene oxidation: The influence of MOFs precursors. Mol. Catal. 2020, 482, 110701. [Google Scholar] [CrossRef]
- Li, J.; Mo, S.; Ding, X.; Huang, L.; Zhou, X.; Fan, Y.; Zhang, Y.; Fu, M.; Xie, Q.; Ye, D. Hollow cavity engineering of MOFs-derived hierarchical MnOx structure for highly efficient photothermal degradation of ethyl acetate under light irradiation. Chem. Eng. J. 2023, 464, 142412. [Google Scholar] [CrossRef]
- Cheng, L.; Men, Y.; Wang, J.; Wang, H.; An, W.; Wang, Y.; Duan, Z.; Liu, J. Crystal facet-dependent reactivity of α-Mn2O3 microcrystalline catalyst for soot combustion. Appl. Catal. B Environ. 2017, 204, 374–384. [Google Scholar] [CrossRef]
- Mei, J.; Shen, Y.; Wang, Q.; Shen, Y.; Li, W.; Zhao, J.; Chen, J.; Zhang, S. Roles of oxygen species in low-temperature catalytic o-xylene oxidation on MOF-derived bouquetlike CeO2. ACS Appl. Mater. Interfaces 2022, 14, 35694–35703. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Deng, W.; Han, J.; Qin, L.; Zhao, B.; Guo, L.; Xing, F. Study on the structure-activity relationship of Fe-Mn oxide catalysts for chlorobenzene catalytic combustion. Chem. Eng. J. 2020, 395, 125172. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Q.; Shan, C.; Lu, S.; Su, Y.; Han, R.; Song, C.; Ji, N.; Ma, D.; Liu, Q. Enhanced acetone oxidation over the CeO2/Co3O4 catalyst derived from metal– organic frameworks. ACS Appl. Mater. Interfaces 2020, 12, 28139–28147. [Google Scholar] [CrossRef]
- Yang, P.; Yang, S.; Shi, Z.; Meng, Z.; Zhou, R. Deep oxidation of chlorinated VOCs over CeO2-based transition metal mixed oxide catalysts. Appl. Catal. B Environ. 2015, 162, 227–235. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, S.; Zhang, R.; Tu, J.; Fei, T.; Zhang, T. Effect of cation substitution on the gas-sensing performances of ternary spinel MCo2O4 (M = Mn, Ni, and Zn) multishelled hollow twin spheres. ACS Appl. Mater. Interfaces 2019, 11, 28023–28032. [Google Scholar] [CrossRef]
- Pei, W.; Liu, Y.; Deng, J.; Zhang, K.; Hou, Z.; Zhao, X.; Dai, H. Partially embedding Pt nanoparticles in the skeleton of 3DOM Mn2O3: An effective strategy for enhancing catalytic stability in toluene combustion. Appl. Catal. B Environ. 2019, 256, 117814. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, P.; Chen, L. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures. Appl. Catal. B Environ. 2016, 189, 210–218. [Google Scholar] [CrossRef]
- Gopi, T.; Swetha, G.; Shekar, S.C.; Ramakrishna, C.; Saini, B.; Krishna, R.; Rao, P.V.L. Catalytic decomposition of ozone on nanostructured potassium and proton containing δ-MnO2 catalysts. Catal. Commun. 2017, 92, 51–55. [Google Scholar] [CrossRef]
- Li, J.R.; Zhang, W.P.; Li, C.; He, C. Efficient catalytic degradation of toluene at a readily prepared Mn-Cu catalyst: Catalytic performance and reaction pathway. J. Colloid Interface Sci. 2021, 591, 396–408. [Google Scholar] [CrossRef]
- Nawaz, F.; Cao, H.; Xie, Y.; Xiao, J.; Chen, Y.; Ghazi, Z.A. Selection of active phase of MnO2 for catalytic ozonation of 4-nitrophenol. Chemosphere 2017, 168, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; An, X.; Shi, J.; Shangguan, W.; Hao, X.; Xu, G.; Tang, B.; Abudula, A.; Guan, G. Generation of abundant defects in Mn-Co mixed oxides by a facile agar-gel method for highly efficient catalysis of total toluene oxidation. Appl. Catal. B Environ. 2021, 282, 119560. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Li, N.; Xing, X.; Yang, H.; Zhang, F.; Cheng, J.; Zhang, Z.; Hao, Z. Surface properties enhanced MnxAlO oxide catalysts derived from MnxAl layered double hydroxides for acetone catalytic oxidation at low temperature. Appl. Catal. B Environ. 2019, 251, 295–304. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Chen, J.; Zhong, F. Mn2O3/γ-Al2O3 catalysts synergistic double dielectric barrier discharge (DDBD) degradation of toluene, ethyl-acetate and acetone. Chemosphere 2021, 284, 131299. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Z.; Li, Y.; Leng, X.; Zhang, T.; Yuan, F.; Niu, X.; Zhu, Y. Synthesis of Ce-MnOx hollow microsphere with hierarchical structure and its excellent catalytic performance for toluene combustion. Appl. Catal. B Environ. 2019, 245, 502–512. [Google Scholar] [CrossRef]
- Wang, D.T.; Guo, N.; Jiang, L.X.; Lian, S.Z.; Wang, Z.W. Metal organic frameworks derived metal oxides prepared by oxygen vacancy engineering with the enhanced catalytic activity for toluene oxidation. J. Environ. Chem. Eng. 2022, 10, 108798. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, W.; Wang, Y.; Guo, L.; Ishihara, T. A comparative study of the catalytic oxidation of chlorobenzene and toluene over Ce-Mn oxides. Mol. Catal. 2018, 459, 61–70. [Google Scholar] [CrossRef]
- Shao, J.; Lin, F.; Wang, Z.; Liu, P.; Tang, H.; He, Y.; Cen, K. Low temperature catalytic ozonation of toluene in flue gas over Mn-based catalysts: Effect of support property and SO2/water vapor addition. Appl. Catal. B Environ. 2020, 266, 118662. [Google Scholar] [CrossRef]
- Sun, P.; Wang, W.; Dai, X.; Weng, X.; Wu, Z. Mechanism study on catalytic oxidation of chlorobenzene over MnxCe1-xO2/H-ZSM5 catalysts under dry and humid conditions. Appl. Catal. B Environ. 2016, 198, 389–397. [Google Scholar] [CrossRef]
- Weng, X.; Sun, P.; Long, Y.; Meng, Q.; Wu, Z. Catalytic oxidation of chlorobenzene over MnxCe1-xO2/HZSM-5 catalysts: A study with practical implications. Environ. Sci. Technol. 2017, 51, 8057–8066. [Google Scholar] [CrossRef]
- Tian, M.; Guo, X.; Dong, R.; Guo, Z.; Shi, J.; Yu, Y.; Cheng, M.; Albilali, R.; He, C. Insight into the boosted catalytic performance and chlorine resistance of nanosphere-like meso-macroporous CrOx/MnCo3Ox for 1, 2-dichloroethane destruction. Appl. Catal. B Environ. 2019, 259, 118018. [Google Scholar] [CrossRef]
- Tang, H.; Wang, Z.; Shao, J.; Lin, F.; Liu, P.; He, Y.; Zhu, Y. Catalytic decomposition of residual ozone over cactus-like MnO2 nanosphere: Synergistic mechanism and SO2/H2O interference. ACS Omega 2022, 7, 9818–9833. [Google Scholar] [CrossRef]
- Li, C.; Domen, K.; Maruya, K.I.; Onishi, T. Spectroscopic identification of adsorbed species derived from adsorption and decomposition of formic acid, methanol, and formaldehyde on cerium oxide. J. Catal. 1990, 125, 445–455. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Rong, S.; Wang, H.; Zhang, P. Cerium modified birnessite-type MnO2 for gaseous formaldehyde oxidation at low temperature. Appl. Catal. B Environ. 2017, 211, 212–221. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, F.; Zhai, Y.; Ariga, H.; Yi, N.; Liu, Y.; Asakura, K.; Flytzani-Stephanopoulos, M.; He, H. Alkali-metal-promoted Pt/TiO2 opens a more efficient pathway to formaldehyde oxidation at ambient temperatures. Angew. Chem. Int. Ed. 2012, 51, 9628–9632. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Cheng, B.; Jiang, C. Co3O4 nanorod-supported Pt with enhanced performance for catalytic HCHO oxidation at room temperature. Appl. Surf. Sci. 2017, 404, 426–434. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Jiang, Z.; Yang, R.; Jiang, Z.; Pan, Y.; Shangguan, W. New insight into the enhanced activity of ordered mesoporous nickel oxide in formaldehyde catalytic oxidation reactions. J. Catal. 2018, 361, 370–383. [Google Scholar] [CrossRef]
- Collins, S.S.; Cittadini, M.; Pecharromán, C.; Martucci, A.; Mulvaney, P. Hydrogen spillover between single gold nanorods and metal oxide supports: A surface plasmon spectroscopy study. Acs Nano 2015, 9, 7846–7856. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, Y.; Liu, J.; Ding, J. Mechanistic insights into benzene oxidation over CuMn2O4 catalyst. J. Hazard. Mater. 2022, 431, 128640. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.; Probert, M.A.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Xin, Y.; Cheng, L.; Lv, Y.; Jia, J.; Han, D.; Zhang, N.; Wang, J.; Zhang, Z.; Cao, X.M. Experimental and theoretical insight into the facet-dependent mechanisms of NO oxidation catalyzed by structurally diverse Mn2O3 nanocrystals. ACS Catal. 2021, 12, 397–410. [Google Scholar] [CrossRef]
- Yan, H.; Shen, Q.; Sun, Y.; Zhao, S.; Lu, R.; Gong, M.; Liu, Y.; Zhou, X.; Jin, X.; Yang, C.; et al. Tailoring facets of α-Mn2O3 microcrystalline catalysts for enhanced selective oxidation of glycerol to glycolic acid. ACS Catal. 2021, 11, 6371–6383. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).