Solar-Driven Selective Benzyl Alcohol Oxidation in Pickering Emulsion Stabilized by CNTs/GCN Hybrids Photocatalyst
Abstract
1. Introduction
2. Results and Discussion
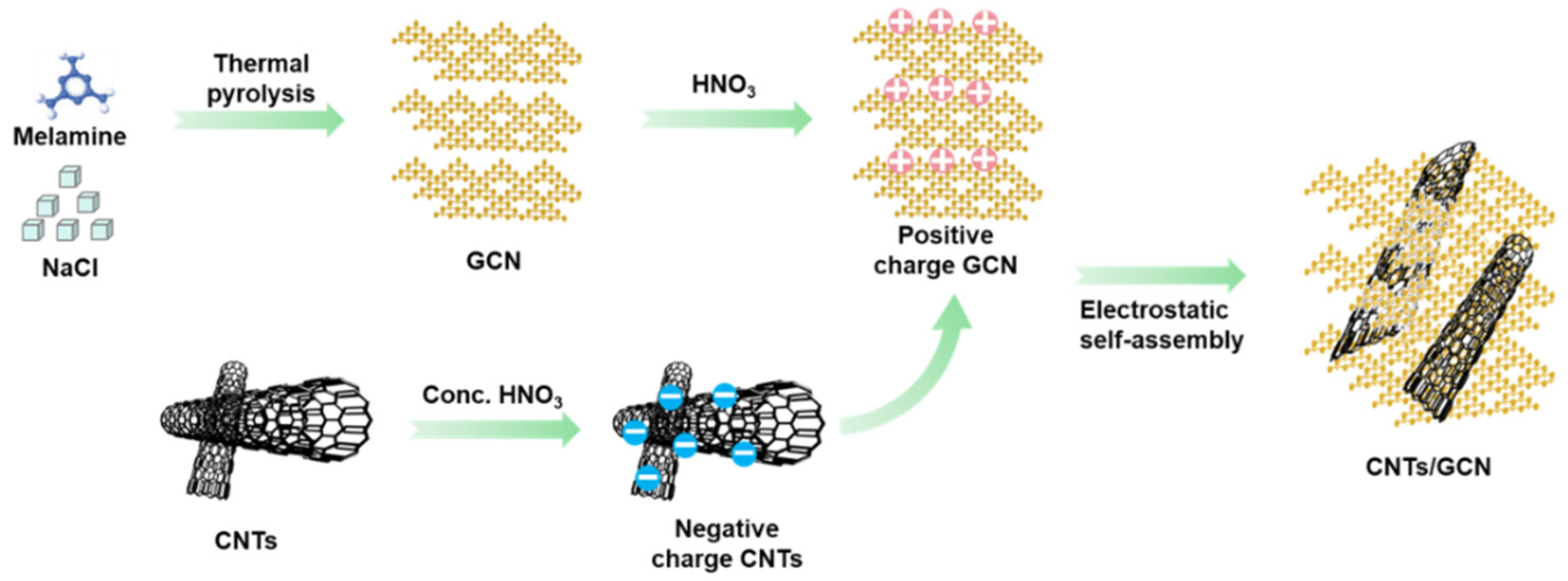
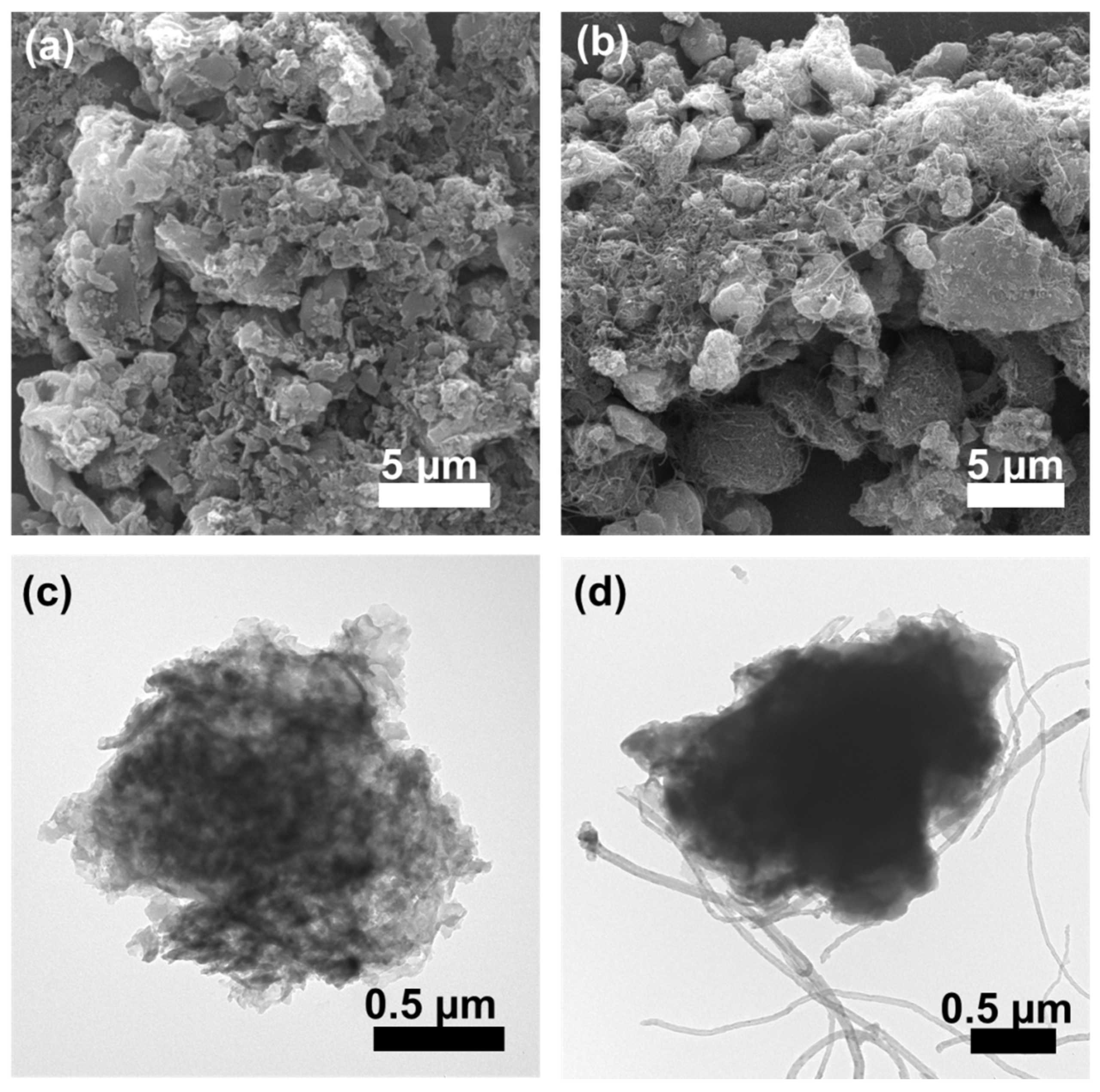
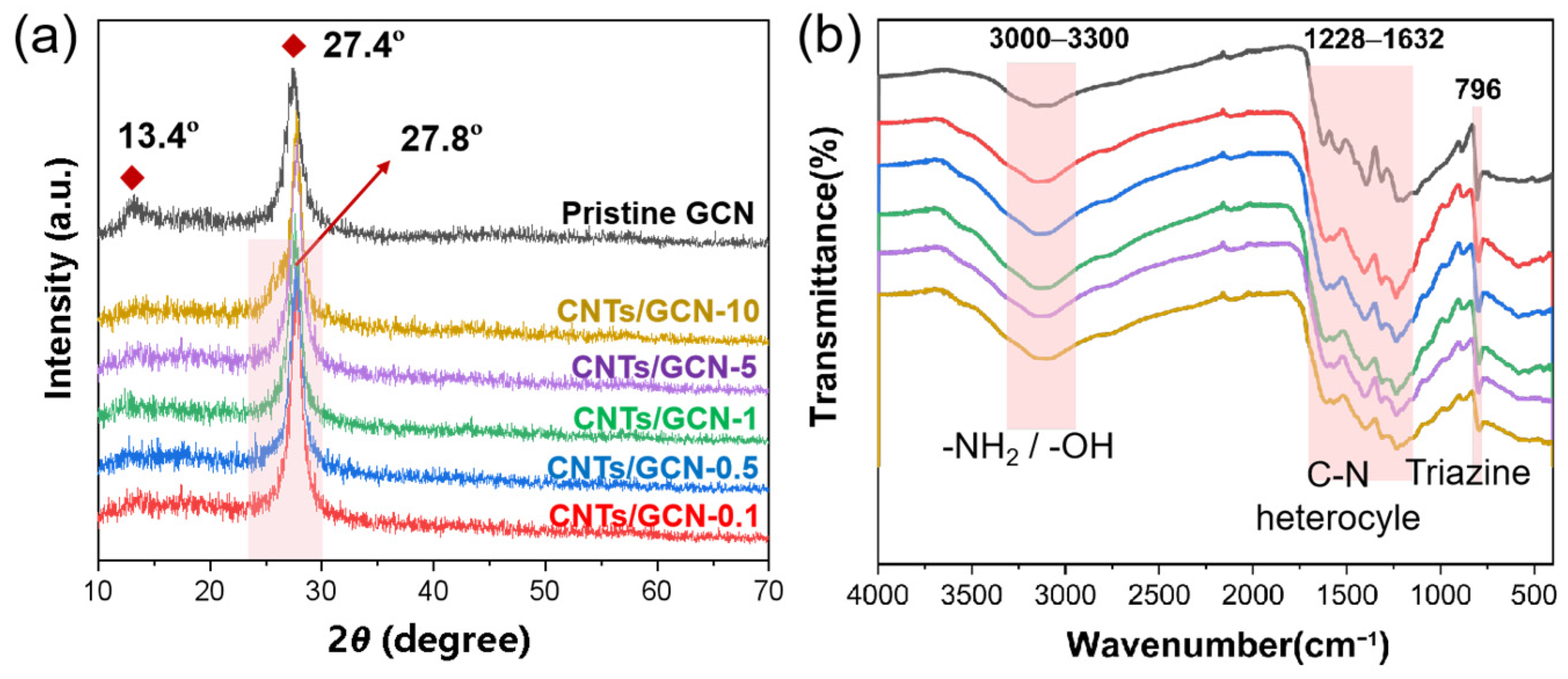
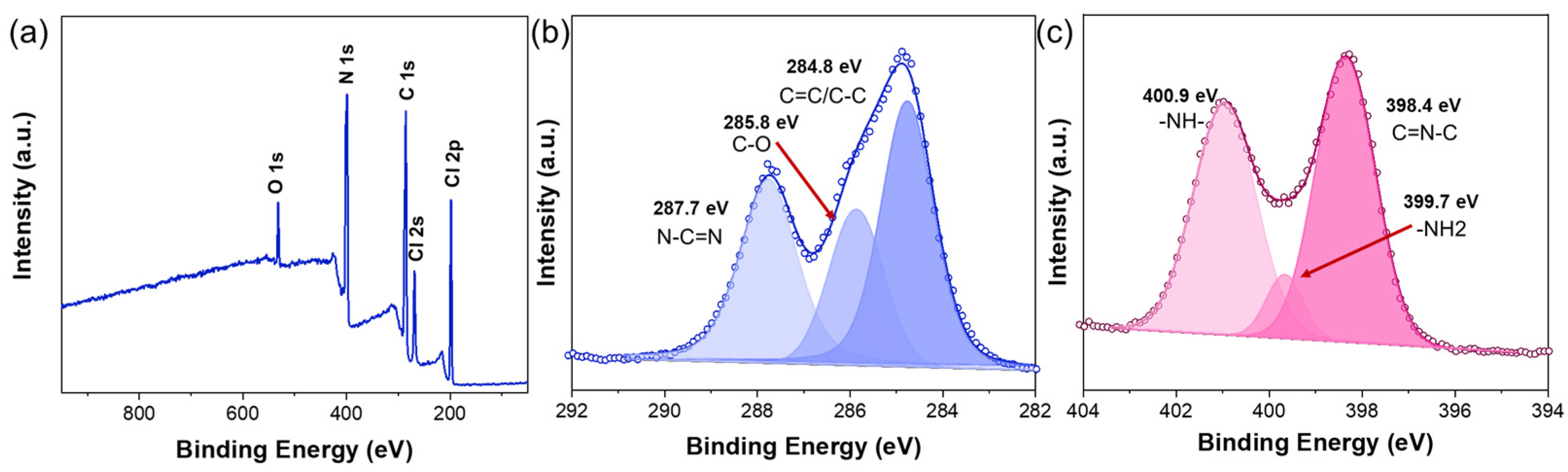
2.1. Morphology and Structure
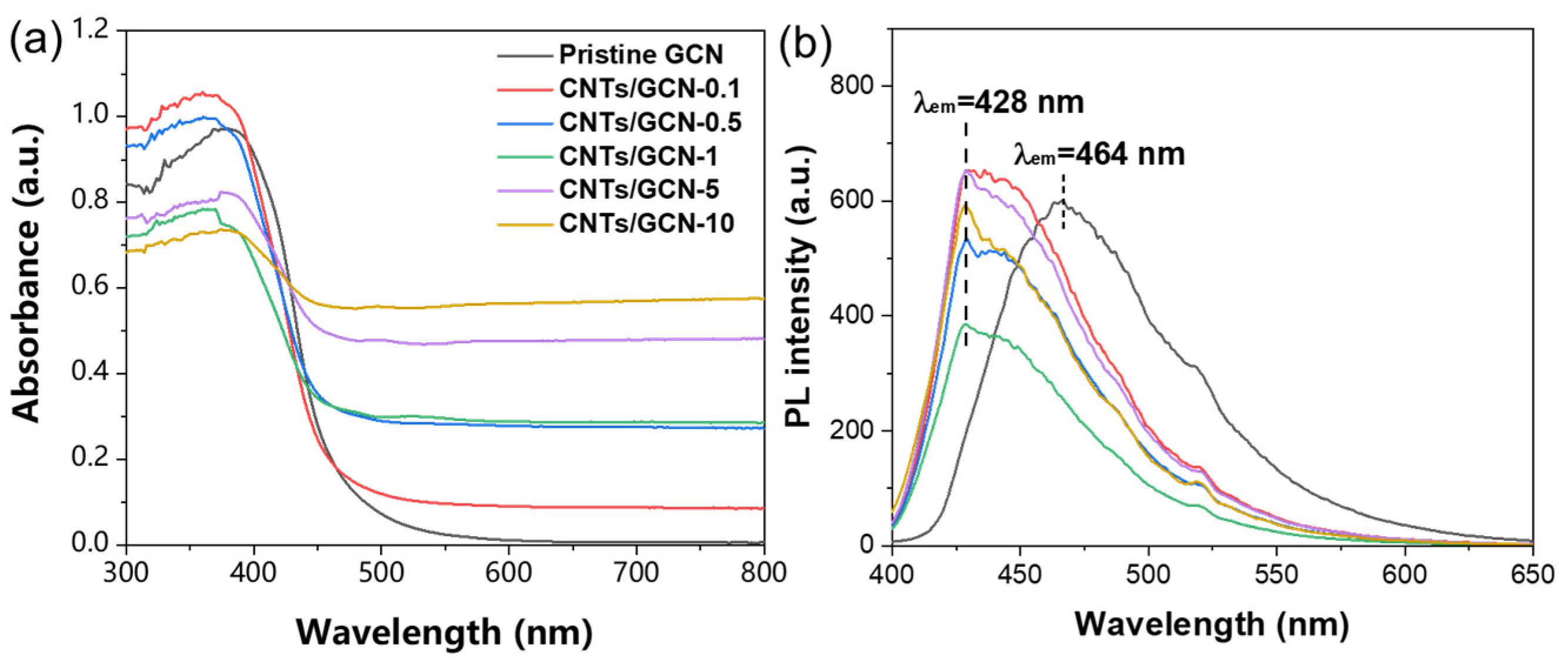
2.2. Optical Properties
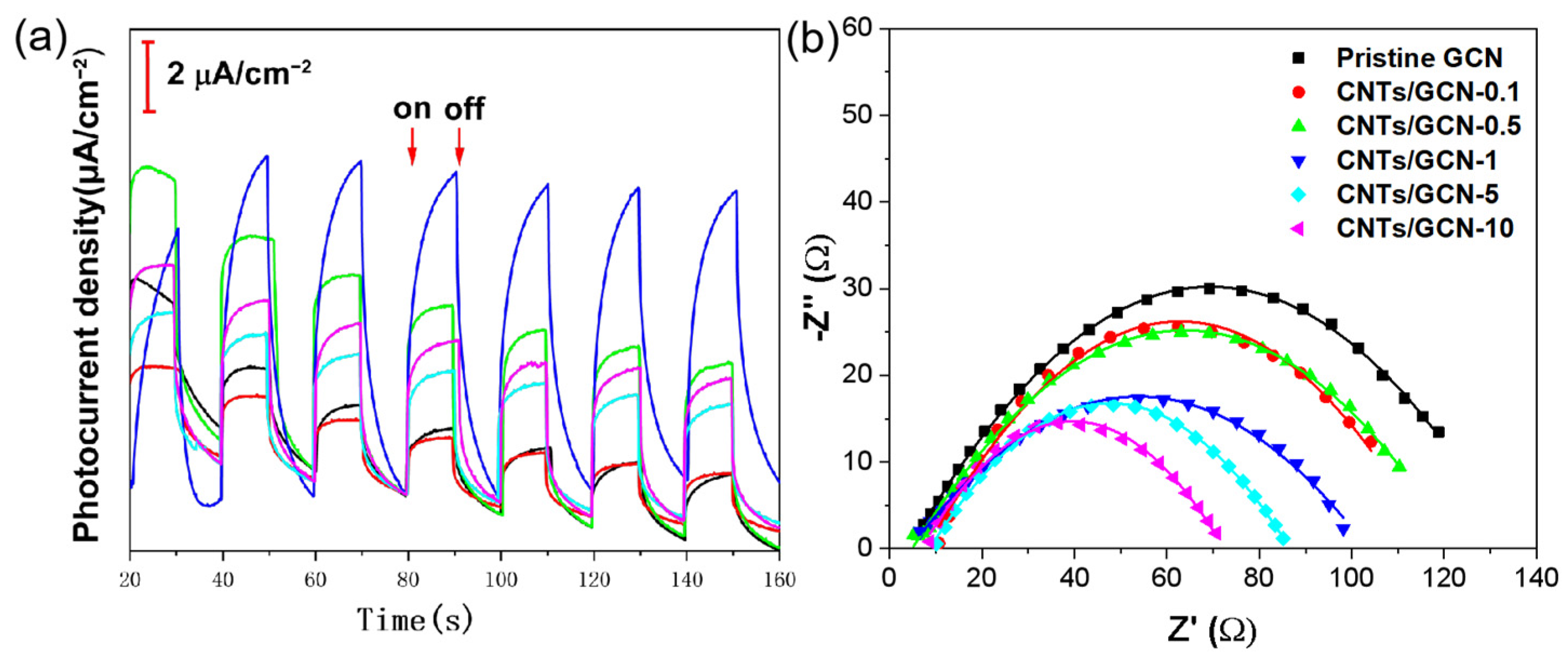
2.3. Photoelectrochemical Performance
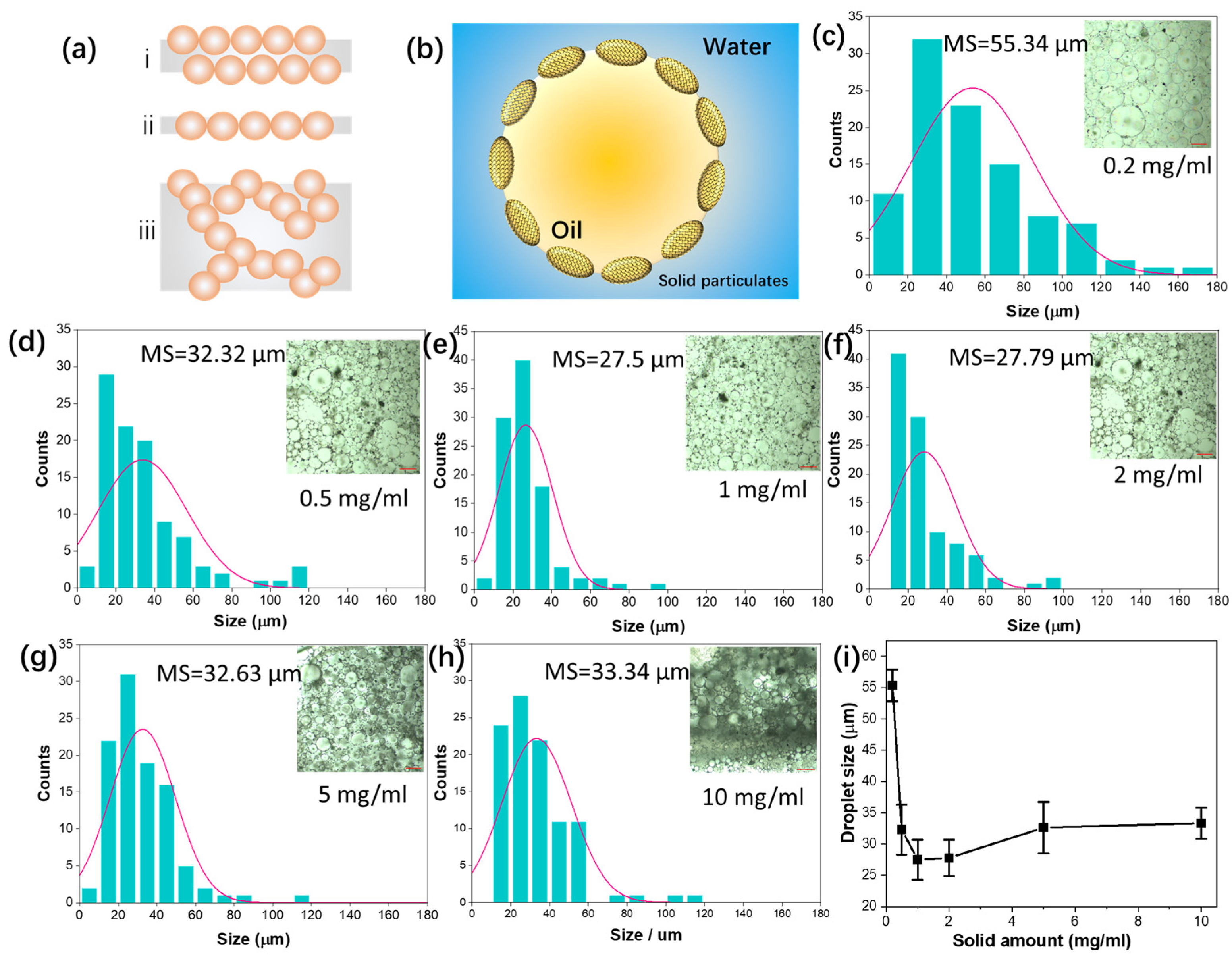
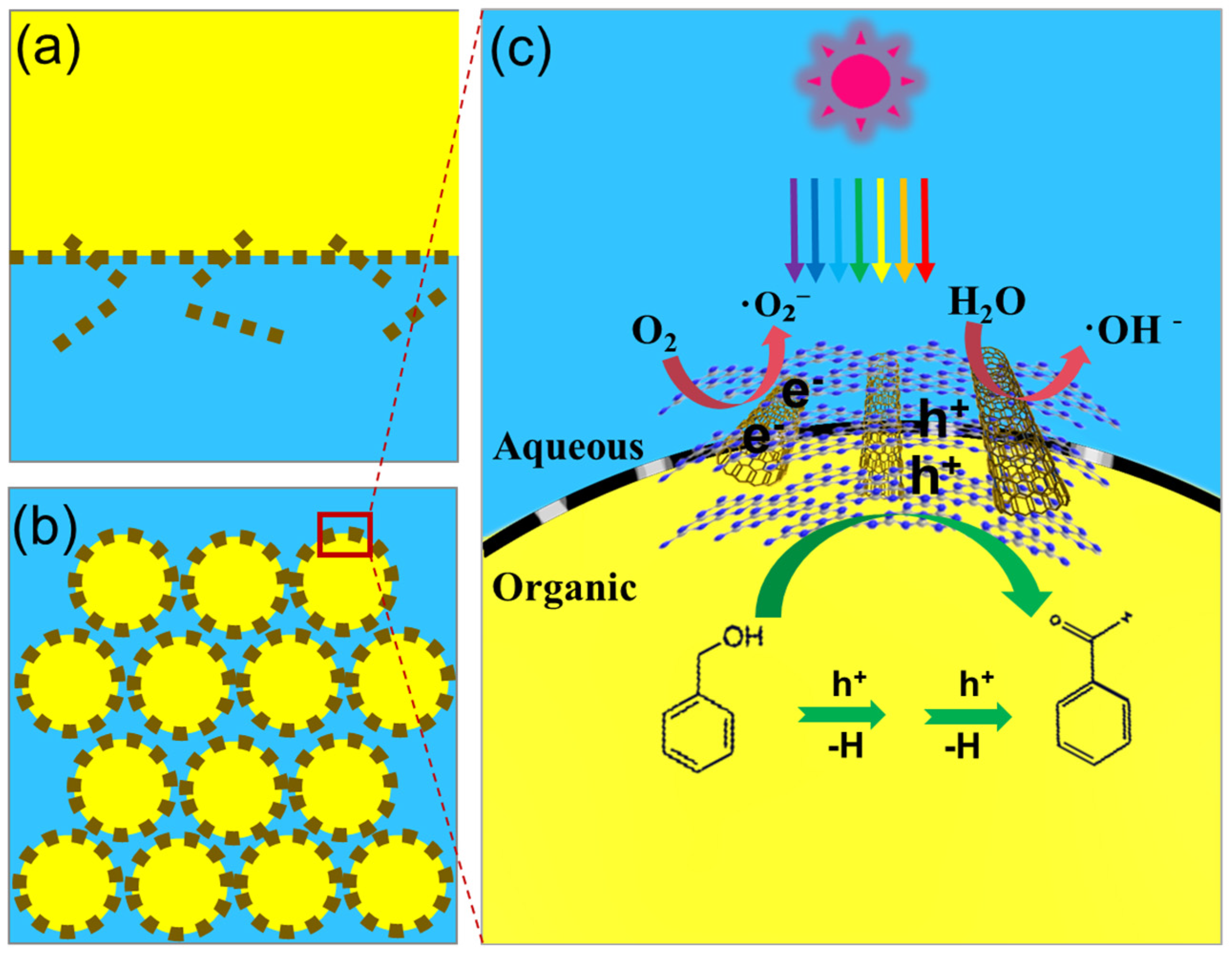
2.4. CNTs/GCN Stabilized Pickering Emulsion
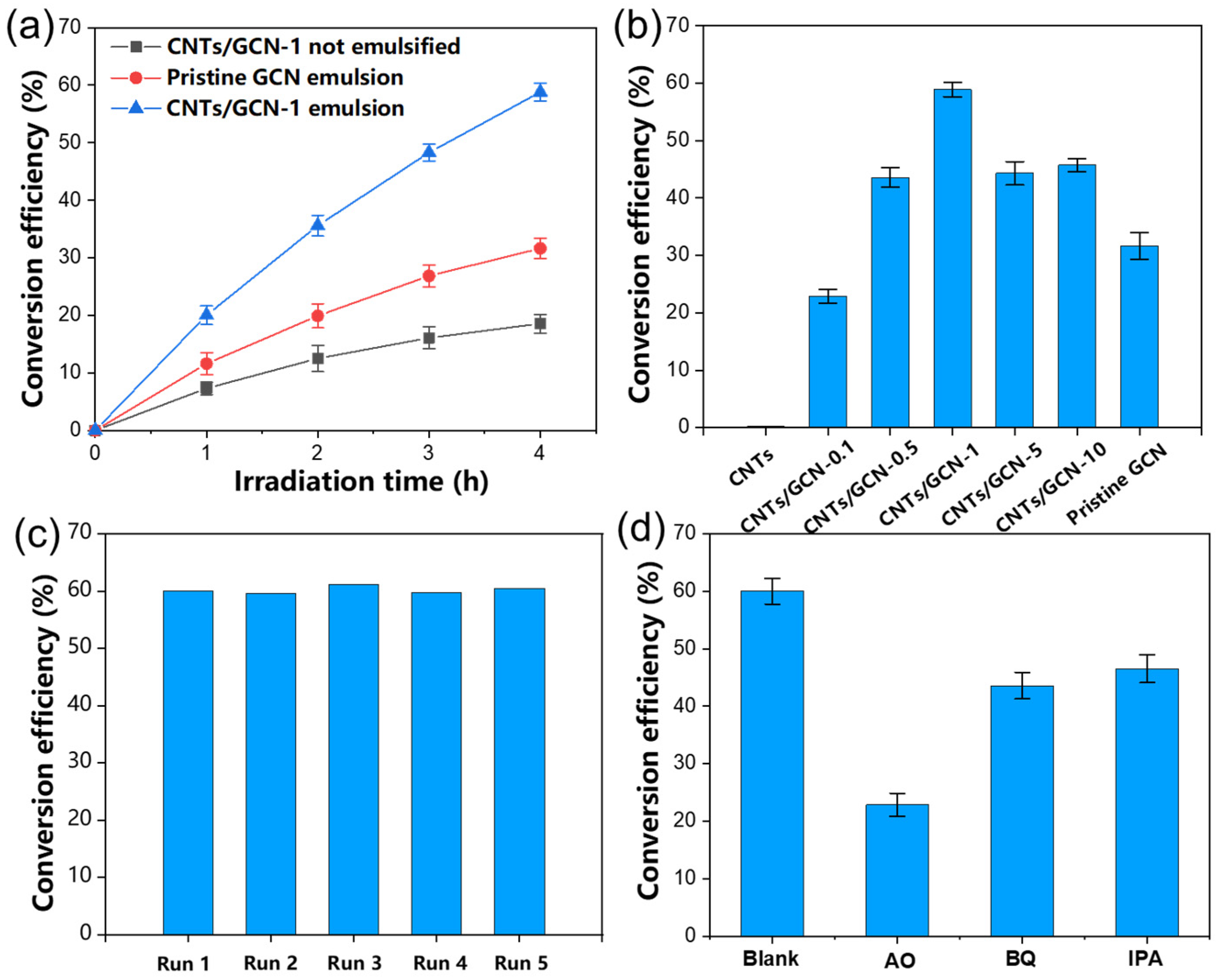
2.5. Photocatalytic Benzyl Alcohol Oxidation in Pickering Emulsion
2.6. Proposed Photocatalytic Mechanism
3. Experimental Section
3.1. Chemicals
3.2. Preparation of CNTs/GCN Hybrid Material
3.2.1. Synthesis of GCN
3.2.2. Synthesis of CNTs/GCN Hybrid
3.3. Characterization
3.4. Photoelectrochemical Measurement
3.5. Preparation of Pickering Emulsion
3.6. Photocatalytic Benzyl Alcohol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Linsebigler, L.A.; Lu, G.; Yates, T.J. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 2002, 95, 735–758. [Google Scholar] [CrossRef]
- Giovanni, A.; Vincenzo, A.; Mario, P.; Leonardo, P. Photocatalysis: A promising route for 21st century organic chemistry. Chem. Comm. 2007, 33, 3425–3437. [Google Scholar]
- Wen, J.; Lin, R.; Wu, Y.; Hu, H.; Liu, Z.; Zhou, H.; Ouyang, X. Qualified interlayer modifier for organic solar cells with optimized interfacial topography and boosted efficiency based on biomass-derived acid. Chem. Eng. J. 2022, 450, 138169. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhu, X.; Guan, M.; Gong, R.; Gong, X.; Dai, C.; Tang, J. A Conjugated polymer coupled with graphitic carbon nitride for boosting photocatalytic hydrogen generation under visible light. Sustain. Energy Fuels 2023, 7, 1537–1543. [Google Scholar] [CrossRef]
- Gong, X.; Yu, S.; Guan, M.; Zhu, X.; Xue, C. Pyrene-functionalized polymeric carbon nitride for photocatalytic carbonate reduction with simultaneous biphasic oxidation of alkenes. Mater. Chem. A 2019, 7, 7373–7379. [Google Scholar] [CrossRef]
- Gong, R.; Yang, X.; Liu, G.; Dong, Z.; Guan, M.; Gong, X.; Tang, J. Tailored polymeric carbon nitride coupled with Bi2O3 for constructing Z-Scheme heterojunction with enhanced photocatalytic activity. ChemPhotoChem. 2024, 8, e202300255. [Google Scholar] [CrossRef]
- Yang, X.; Gong, R.; Dong, Z.; Liu, G.; Han, Y.; Hou, Y.; Li, Y.; Guan, M.; Gong, X.; Tang, J. Polymeric Carbon Nitride-CNTs-Ferric Oxide All-Solid Z-Scheme Heterojunction with Improved Photocatalytic Activity towards Organic Dye Removal. Catalysts 2024, 14, 516. [Google Scholar] [CrossRef]
- Jingsan, X.; Markus, A. The Performance of Nanoparticulate Graphitic Carbon Nitride as an Amphiphile. J. Am. Chem. Soc. 2017, 139, 6026–6029. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Li, Q.; Liu, X.; Zou, H.; Yang, H. Metal-Nanoparticles-Loaded Ultrathin g-C3N4 Nanosheets at Liquid-Liquid Interfaces for Enhanced Biphasic Catalysis. ACS Appl. Mater. Interfaces 2021, 13, 47236–47243. [Google Scholar] [CrossRef]
- Hong, C.Y.; Shin, H.D.; Uhm, S.H. Super-hydrophobicity of multi-walled carbon nanotubes treated by a glow discharge. Surf. Coat. Technol. 2007, 201, 5025–5029. [Google Scholar] [CrossRef]
- Xu, Z.; Li, H.; Fu, M. Nitrogen-doped carbon nanotubes synthesized by pyrolysis of nitrogen-rich metal phthalocyanine derivatives for oxygen reduction. J. Mater. Chem. 2012, 22, 18230–18236. [Google Scholar] [CrossRef]
- Rivadulla, F.; Mateo-Mateo, C.; Correa-Duarte, M.A. Layer-by-Layer Polymer Coating of Carbon Nanotubes: Tuning of Electrical Conductivity in Random Networks. J. Am. Chem. Soc. 2010, 132, 3751–3755. [Google Scholar] [CrossRef]
- Dai, H. Carbon nanotubes: Opportunities and challenges. Sur. Sci. 2002, 500, 218–241. [Google Scholar] [CrossRef]
- Nikolaos, K.; Nikos, T.; Dimitrios, T. Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Yu, J.; Ma, T.; Liu, S. Enhanced photocatalytic activity of mesoporous TiO2 aggregates by embedding carbon nanotubes as electron-transfer channel. Phys. Chem. Chem. Phys. 2011, 13, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Jiang, L.Y. Investigation of uniaxial stretching effects on the electrical conductivity of CNT-polymer nanocomposites. Phys. D Appl. Phys. 2014, 47, 405103. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Zhu, J.; Li, Y.; He, C.; Ren, X.; Zhang, P.; Mi, H. Enhanced photocatalytic H2 production independent of exciton dissociation in crystalline carbon nitride. Appl. Catal. B-Environ. Energy 2023, 338, 123049. [Google Scholar] [CrossRef]
- Yuan, B.; Chu, Z.; Li, G. Water-Soluble Ribbon-Like Graphitic Carbon Nitride (g-C3N4): Green Synthesis, Self-Assembly and Unique Optical Properties. Mater. Chem. 2014, 2, 8212–8215. [Google Scholar] [CrossRef]
- Georg, D.S.; Siegmar, R.; Patrick, D.; Andrew, M.; Ralf, G.; Lothar, L.; Norbert, N. Modification of Single-Walled Carbon Nanotubes by Hydrothermal Treatment. Chem. Mater. 2003, 15, 3314–3319. [Google Scholar] [CrossRef]
- Ma, T.; Dai, S.; Jaroniec, M.; Qiao, S. Graphitic Carbon Nitride Nanosheet–Carbon Nanotube Three- Dimensional Porous Composites as High-Performance Oxygen Evolution Electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 7281–7285. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Kessler, K.F.; Zheng, Y.; Schwarz, D. Functional carbon nitride materials-design strategies for electrochemical devices. Nat. Rev. Mater. 2017, 2, 17030. [Google Scholar] [CrossRef]
- Guirguis, A.; Polaki, S.R.; Sahoo, G.; Ghosh, S.; Kamruddin, M.; Merenda, A.; Chen, X.; Maina, J.W.; Szekely, G.; Dumee, L. Engineering high-defect densities across vertically-aligned graphene nanosheets to induce photocatalytic reactivity. Carbon 2020, 168, 32–41. [Google Scholar] [CrossRef]
- Nobuaki, T.; Hiromasa, N.; Satoshi, K.; Morinobu, E.; Tsuneo, F. Photochemical deposition of Ag nanoparticles on multi walled carbon nanotubes. Carbon 2009, 47, 2752–2754. [Google Scholar]
- Usman, M.; Zeb, Z.; Ullah, H.; Suliman, M.H.; Humayun, M.; Ullah, L.; Shah, S.N.A.; Ahmed, U.; Saeed, M. A review of metal-organic frameworks/graphitic carbon nitride composites for solar-driven green H2 production, CO2 reduction, and water purification. J. Environ. Chem. Eng. 2022, 10, 107548. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S.; Jin, W. Recent advances in non-metal modification of graphitic carbon nitride for photocatalysis: A historic review. Catal. Sci. Technol. 2016, 6, 7002–7023. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Dong, F.; Reshak, A.H.; Ye, L.; Pinna, N.; Zeng, C.; Zhang, T.; Huang, H. Chlorine intercalation in graphitic carbon nitride for efficient photocatalysis. Appl. Catal. B Environ. 2017, 203, 465–474. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Zhang, Y.; Yang, Z.; Zhu, A.; Dionysiou, D.D. Enhancement of the Cr(VI) adsorption and photocatalytic reduction activity of g-C3N4 by hydrothermal treatment in HNO3 aqueous solution. Appl. Catal. A Gen. 2016, 521, 9–18. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Dresselhaus, G.; Saito, R.; Souza, F.A.G.; Pimenta, M.A. Raman Spectroscopy of Nanoscale Carbons And of An Isolated Carbon Nanotube. J. Mol. Cryst. Liq. Cryst. 2002, 387, 21–29. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Yousefzadeh, S.; Fathi, B. Construction of carbon nanotube-g-C3N4 nanocomposite photoanode for the enhanced photoelectrochemical activity in water splitting. J. Electroanal. Chem. 2020, 878, 114580. [Google Scholar] [CrossRef]
- Liu, T.; Pasumarthi, V.; Laporte, C.; Feng, Z.; Li, Q.; Yang, J.; Li, C.; Dupuis, M. Bimodal hole transport in bulk BiVO4 from computation. J. Mater. Chem. A 2018, 6, 3714–3723. [Google Scholar] [CrossRef]
- Yang, T.; Dai, S.; Tan, H.; Zong, Y.; Liu, Y.; Chen, J.; Zhang, K.; Wu, P.; Zhang, S.; Xu, J.; et al. Mechanism of Photoluminescence in Ag Nanoclusters: Metal-Centered Emission versus Synergistic Effect in Ligand-Centered Emission. J. Phys. Chem. C 2019, 123, 18638–18645. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, Q.; Li, B.; Li, X.; Chen, M.; Zhang, M.; Feng, Y.; Ding, Y. Aminated silicon dioxide enriching iron-containing polyoxometalate catalyst confined in CdS for efficient H2 evolution. Appl. Catal. B Environ. 2022, 304, 120998. [Google Scholar] [CrossRef]
- Eric, D. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid. Interface 2009, 15, 40–49. [Google Scholar]
- Tian, Y.; Chang, B.; Lu, J.; Fu, J.; Xi, F.; Dong, X. Hydrothermal synthesis of graphitic carbon nitride-Bi2WO6 heterojunctions with enhanced visible light photocatalytic activities. ACS App. Mater. Interfaces 2013, 5, 7079–7085. [Google Scholar] [CrossRef]
- Binks, B.P.; Clint, J.H. Solid wettability from surface energy components: Relevance to pickering emulsions. Langmuir 2002, 18, 1270–1273. [Google Scholar] [CrossRef]
- Binks, B.P.; Rodrigues, J.A. Inversion of emulsions stabilized solely by ionizable nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 441–444. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Liu, Y.; Ren, M.; Du, J.; Chen, A.; Li, F. Suppressing interlayer-gliding and Jahn-Teller effect in P2-type layered manganese oxide cathode via Mo doping for sodium-ion batteries. Chem. Eng. J. 2021, 426, 130813. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, B.; Liu, H.; Du, X.; Sun, F.; Xia, X. Nanoscale Pickering emulsion food preservative films/coatings: Compositions, preparations, influencing factors, and applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13279. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, X.; Liu, X.; Xu, L.; Liu, B.; Zhang, J.; Xu, H.; Han, Z.; Li, G. In-situ exfoliation and assembly of 2D/2D g-C3N4/TiO2(B) hierarchical microflower: Enhanced photo-oxidation of benzyl alcohol under visible light. Carbon 2022, 196, 401–409. [Google Scholar] [CrossRef]
- Kisch, H.; Bahnemann, D. Best Practice in Photocatalysis: Comparing Rates or Apparent Quantum Yields. J. Phys. Chem. Lett. 2015, 6, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zheng, C.; Lu, M.; Zhang, L.; Liu, F.; Zuo, X.; Nan, J. Deficient Bi24O31Br10 as a highly efficient photocatalyst for selective oxidation of benzyl alcohol into benzaldehyde under blue LED irradiation. Appl. Catal. B Environ. 2018, 228, 142–157. [Google Scholar] [CrossRef]
- Lima, M.J.; Tavares, P.B.; Silva, A.M.T.; Silva, C.G.; Faria, J.L. Selective photocatalytic oxidation of benzyl alcohol to benzaldehyde by using metal-loaded g-C3N4 photocatalysts. Catal. Today 2017, 287, 70–77. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. g-C3N4-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Hou, Y.; Gong, X.; Zhang, Y.; Wang, M.; Ivanovich, P.V.; Guan, M.; Tang, J. Solar-Driven Selective Benzyl Alcohol Oxidation in Pickering Emulsion Stabilized by CNTs/GCN Hybrids Photocatalyst. Catalysts 2025, 15, 753. https://doi.org/10.3390/catal15080753
Han Y, Hou Y, Gong X, Zhang Y, Wang M, Ivanovich PV, Guan M, Tang J. Solar-Driven Selective Benzyl Alcohol Oxidation in Pickering Emulsion Stabilized by CNTs/GCN Hybrids Photocatalyst. Catalysts. 2025; 15(8):753. https://doi.org/10.3390/catal15080753
Chicago/Turabian StyleHan, Yunyi, Yuwei Hou, Xuezhong Gong, Yu Zhang, Meng Wang, Pekhyo Vasiliy Ivanovich, Meili Guan, and Jianguo Tang. 2025. "Solar-Driven Selective Benzyl Alcohol Oxidation in Pickering Emulsion Stabilized by CNTs/GCN Hybrids Photocatalyst" Catalysts 15, no. 8: 753. https://doi.org/10.3390/catal15080753
APA StyleHan, Y., Hou, Y., Gong, X., Zhang, Y., Wang, M., Ivanovich, P. V., Guan, M., & Tang, J. (2025). Solar-Driven Selective Benzyl Alcohol Oxidation in Pickering Emulsion Stabilized by CNTs/GCN Hybrids Photocatalyst. Catalysts, 15(8), 753. https://doi.org/10.3390/catal15080753






