Abstract
Persistent reactive azo dyes released from textile finishing are a serious threat to water systems, but effective methods using sunlight to break them down are still limited. Everzol Yellow 3RS (EY-3RS) is particularly recalcitrant: past studies have relied almost exclusively on physical adsorption onto natural or modified clays and zeolites, and no photocatalytic pathway employing engineered nanomaterials has been documented to date. This study reports the synthesis, characterization, and performance of a visible-active ternary nanocomposite, Cu4O3/ZrO2/TiO2, prepared hydrothermally alongside its binary (Cu4O3/ZrO2) and rutile TiO2 counterparts. XRD, FT-IR, SEM-EDX, UV-Vis, and PL analyses confirm a heterostructured architecture with a narrowed optical bandgap of 2.91 eV, efficient charge separation, and a mesoporous nanosphere-in-matrix morphology. Photocatalytic tests conducted under midsummer sunlight reveal that the ternary catalyst removes 91.41% of 40 ppm EY-3RS within 100 min, markedly surpassing the binary catalyst (86.65%) and TiO2 (81.48%). Activity trends persist across a wide range of operational variables, including dye concentrations (20–100 ppm), catalyst dosages (10–40 mg), pH levels (3–11), and irradiation times (up to 100 min). The material retains ≈ 93% of its initial efficiency after four consecutive cycles, evidencing good reusability. This work introduces the first nanophotocatalytic strategy for EY-3RS degradation and underscores the promise of multi-oxide heterojunctions for solar-driven remediation of colored effluents.
1. Introduction
Environmental pollution is one of the most significant issues facing humanity and the primary cause of disease along with mortality in the environment. The industrial revolution and development have become indispensable in today’s world [1]. A wide range of contaminants, including dyes, pesticides, surfactants, oil and grease, antibiotics, various metal ions, and auxiliary chemicals, are typically present in industrial effluents. These effluents cause serious health effects, including infectious and carcinogenic diseases. According to the WHO, ambient air in both cities and villages caused more than 4 billion premature deaths in 2016 [2]. Development and environmental pollution are directly related. Water pollution, greenhouse gas emissions, and other public health-related challenges are exacting a toll on humankind [3]. Ten to fifty per cent of the dyes are lost during the colouring process in the various industries and are released into wastewater, resulting in colourful effluents. For instance, it’s estimated that over 100,000 synthetic dyes are produced annually worldwide, totaling more than 700,000 tons, which generates a substantial amount of wastewater [4]. Since organic pollutants are resistant, traditional biological therapy techniques are unsuccessful. In addition to biological therapy, physical techniques such as coagulation, ultrafiltration, and adsorption have also been used to get rid of these industrial pollutants but these methods only successfully move the contaminants from one phase of the water to another, causing secondary contamination that calls for further solid waste treatment and adsorbent regeneration, both of which can be expensive to handle [5]. Thus, the need for practical, novel, and cost-effective methods for handling industrial wastewater rich in pollutants is urgent.
To separate and remove pollutants from wastewater, various technologies have been proposed, out of which photocatalysis has gained interest due to its excellent efficiency and low cost. Photocatalytic processes are environmentally acceptable strategies for treating organic pollutants without causing secondary pollution. Among many catalysts, semiconductor photocatalysts are highly active in the photodegradation of organic dyes under UV and visible light irradiation [6]. Photocatalytic activity occurs when a complex absorbs photons with sufficient energy to overcome band gap thresholds. This causes an excited electron to be promoted to the conduction band, allowing for the reduction of electron acceptors, such as H2 and O2. Electron holes, which remain in the valence band, can oxidize substrates adsorbed on the surface. Oxidation of the surrounding environment facilitates single-electron interactions that promote sequential degradation reactions [7]. The photocatalytic activity of the compounds can be enhanced efficiently using a combination of individual semiconductor materials (as well as doping with different metals) to form binary/ternary nanocomposites. A ternary nanocomposite is composed of three distinct chemicals that work together to enhance their overall properties. Novel three-dimensional ternary nanocomposites with lower activation energy offer potential for enhanced photocatalytic degradation. Titanium dioxide has been extensively explored in photocatalysis due to its unique properties, including photocatalytic activity and photostability. TiO2, in its original or modified form, is the most often used metal oxide photocatalyst and is found in many current photoactive nanocomposite materials. TiO2, with a bandgap of 3.2 eV, usually appears in three crystalline forms. Anatase (the most common commercial form) and rutile (the thermodynamically stable phase) are widely employed in photocatalysis. The rutile phase has a bandgap of approximately 3.0 eV. However, rutile is octahedral and has four edges instead of four corners [8]. Titanium dioxide nanoparticles (NPs) are distinguished by their strong oxidizing capabilities at room temperature and pressure, resistance to photo corrosion, long-term chemical stability, a range of acceptable physical and optical characteristics, and industrial availability [9]. The conduction and valence bands of titanium dioxide produce electrons and positive holes when exposed to light. The holes could oxidize organic molecules by forming hydroxyl radicals or by reacting directly with them. Additionally, the electrons can produce reduction products through reactions with organic molecules. Oxygen has a crucial role since it can react with the electrons produced by photolysis [10].
When scientists found that a substance’s size influences its chemical, electrical, mechanical, and optical properties, they realized how essential NMs (nanomaterials) are. The unique properties of nanomaterials have garnered considerable attention. Different synthetic methods like hydrothermal [11], sol-gel [12], co-precipitation [13], oxidative polymerization [14] and electrodeposition [15] are used to synthesize ternary metal oxide. Conventional synthesis techniques have several disadvantages, including prolonged reaction times, heterogeneity in particle size, and the formation of multiple by-products. The hydrothermal method is therefore extensively used due to its ability to synthesize nanomaterials with definite morphology and dimensions under high pressure, necessitating adequate reaction temperatures, convenient and cost-effective compared to alternative methods [16]. A lot of work has been done on titanium nanoparticles for their strong oxidizing capabilities at room temperature and pressure, resistance to photo corrosion, long-term chemical stability, a range of acceptable physical and optical characteristics, and industrial availability. Titanium based nanomaterial for the degradation of many industrial dyes are reported in this era with good photocatalytic efficiency [17,18]. According to our best knowledge a very few data is available on selected dye and no report on this photocatalyst. This research will be able to synthesize such titanium-based ternary metal oxide that has shown maximum degrading efficiency for industrial pollutants, mainly EY-3RS. This research study is anticipated to contribute positively to the possible commercialization and will be effective for the reduction of environmental pollution.
2. Results and Discussion
2.1. Characterization of the Nanocomposites
XRD analysis provides information on the phase, purity, and crystallinity of pure TiO2, Cu4O3/ZrO2 and Cu4O3/ZrO2/TiO2 nanocomposites. The diffraction peaks observed in the pattern can be attributed to the monoclinic structure of ZrO2 (space group: P21/c), the tetragonal structure of TiO2(space group: P42/mnm), and the tetragonal structure of Cu4O3 (space group:141/amd). It confirmed the presence of rutile, baddeleyite and paramelaconite phases of TiO2, ZrO2 and Cu4O3 nanocomposites, respectively. The distinct diffraction peaks of Cu4O3, TiO2, and ZrO2 are resolved, and no peaks corresponding to impurities or secondary phases are observed. These XRD results provide further evidence for the synthesis of the hetero structured material (Cu4O3-ZrO2-TiO2). The standard JCPDS patterns of rutile (01-075-1750), baddeleyite (01-072-0597), and paramelaconite (1-083-1665) match the recorded XRD data. From Figure 1 diffraction angles of pure TiO2 peak were observed at 2θ = 27.43°, 36.08°, 39.19°, 41.24°, 44.04°, 54.32°, 56.62°, 62.75°, 64.04°, 69.01° and 69.80° corresponding to hkl values (110), (101), (200), (111), (210), (211), (220), (002), (310), (301) and (112) respectively [19,20,21,22,23]. Two phases (Cu4O3 & ZrO2) for binary nanocomposites were observed in the XRD pattern. Diffraction angles for Cu4O3 peaks were noticed at 2θ = 28.08°, 36.14°, 43.83°, 44.14°, 54.67°, 58.07°, 63.72° and 66.68° corresponding to crystal plane values (112), (004), (220), (213), (303), (224), (400) and (411) respectively [24,25,26,27,28]. Whereas peaks for ZrO2 were observed at 2θ = 24.19°, 27.84°, 31.18°, 33.81°, 34.06°, 35.45°, 38.98°, 44.89°, 45.05°, 48.75°, 53.58°, 56.57°, 59.17°, 60.76°, 68.21° and 70.06° analogous to crystal plane values (110), (111), (−111), (002), (020), (102), (210), (202), (−211), (022), (−202), (−212), (302), (−113), (−132), and (322) respectively [29,30,31,32]. Overall, three phases were observed in the ternary nanocomposite material. The presence of these three phases confirmed the formation of the hetero structure Cu4O3/ZrO2/TiO2 ternary nanocomposite. Figure 1 displays the X-ray diffraction pattern of as-prepared pure TiO2, binary Cu4O3/ZrO2 and ternary Cu4O3/ZrO2/TiO2 nanocomposites. Crystallite sizes (coherent scattering domains) were estimated with the Scherrer Equation (1) applied to the main diffraction peaks after instrumental-broadening correction.
where D is the crystallite size, k is the constant (≈0.9), λ the X-ray wavelength (Cu Kα = 0.15406 nm), β the full width at half-maximum (FWHM, in radians) of a diffraction peak corrected for instrumental broadening, and θ the Bragg angle [33]. The average D values are 28 ± 2 nm for TiO2, 22 ± 2 nm for Cu4O3/ZrO2, and 19 ± 1 nm for Cu4O3/ZrO3/TiO2, indicating progressive size refinement upon binary and ternary assembly.
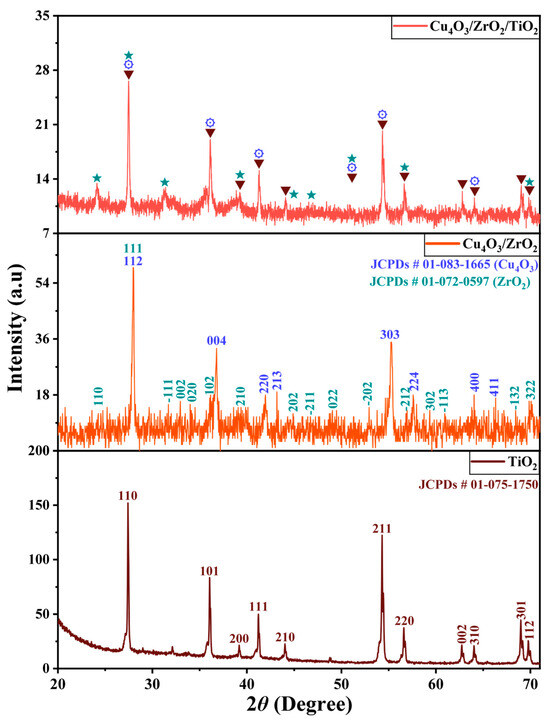
Figure 1.
Powder X-ray diffraction patterns of rutile TiO2, binary Cu4O3/ZrO2, and ternary Cu4O3/ZrO2/TiO2 nanocomposites. Peaks marked with ▼ (down-pointing triangles) arise from rutile TiO2 (JCPDS 01-075-1750); peaks marked with ⚙ (gear symbols) originate from tetragonal Cu4O3 (paramelaconite, JCPDS 01-083-1665); and peaks marked with ★ (stars) correspond to monoclinic ZrO2 (baddeleyite, JCPDS 01-072-0597).
FTIR is an analytical technique used to investigate functional groups and chemical bonds of different organic and inorganic materials by analyzing their corresponding absorption of infrared radiation [34]. The FTIR spectra of pure TiO2, binary Cu4O3/ZrO2, and Cu4O3/ZrO2/TiO2 nanocomposites are displayed in Figure 2. The prepared nanocomposites exhibit absorption bands in the range of 500 cm−1 to 4000 cm−1. The absorption peak between 3416 and 3312 cm−1 in all spectra can be attributed to the O-H stretching vibration of the hydroxyl group, most likely due to adsorbed water on the photo catalysts’ surfaces [1,35]. The hydroxyl groups capture holes under sunlight irradiation, forming hydroxyl radicals that boost photocatalytic effectiveness. The absence of a peak at 2900 cm−1 confirmed that no C-H group was present, indicating the complete calcination of the nanocomposites. In the TiO2 spectrum, bands around 1049, 879 and 818 cm−1 represent the stretching vibration of short Ti-O bonds involving nonbridging oxygen [36,37,38]. The peaks from 1510–1386 cm−1 indicated the presence of stretching vibrations of Ti-O-Ti [37]. Broad and intense peaks at 1087 and 1089 cm−1 in binary and ternary nanocomposite spectra were mainly due to the stretching vibrations of Cu-O in the nanocomposites [39,40,41]. Relatively similar peaks at 834 cm−1 and 826 cm−1 in the spectra of binary and ternary nanocomposites correspond to Zr-O vibrations [42]. Peaks at 589 and 592 cm−1 in binary and ternary nanocomposites spectra were assigned to Zr-O absorption [43].
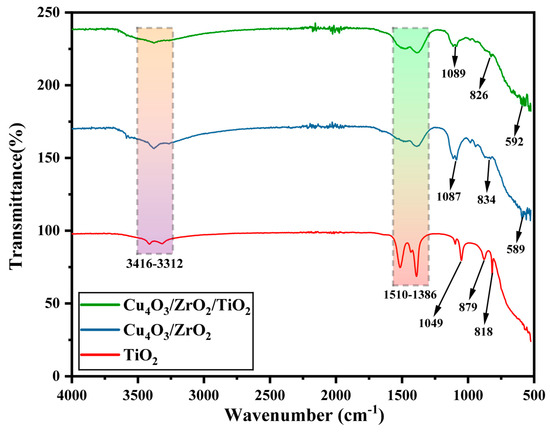
Figure 2.
FTIR spectra of rutile TiO2, binary Cu4O3/ZrO2, and ternary Cu4O3/ZrO2/TiO2 nanocomposites recorded in the 500–4000 cm−1 range.
Room temperature photoluminescence (PL) spectra (λexc = same for all samples) of pure TiO2, binary Cu4O3/ZrO2 and ternary Cu4O3/ZrO2/TiO2 nanocomposites exhibit a one emission band centred at around 580 nm, as shown in Figure 3. Although the precise origin of this band can vary among Ti- and Cu-oxide systems, its relative intensity is a reliable qualitative indicator of electron-hole recombination probability [44,45]. The pure phase of TiO2 has the largest PL intensity (8.3 × 102 a.u.), which means that the recombination ratio of electron-hole is the highest and has poor photocatalytic activity. The binary Cu4O3/ZrO2 nanocomposite manifests an approximate 40% decrease in PL intensity, indicating enhanced charge separation through interfacial (step) heterojunctions. Interestingly, the ternary Cu4O3/ZrO2/TiO2 nanocomposite exhibits the highest quenching (about 2.1 × 102 a.u.), providing an effective way to suppress radiative recombination because of the synergetic effect of the three oxides [46,47,48,49]. This progressive PL quenching is consistent with an improved photocatalytic activity; a lower recombination is translated into longer lifetime of the charge carriers and higher contents of the reactive oxygen species (•OH/•O2−) responsible for the degradation of EY-3RS. Thus, the PL analysis confirms enhanced charge carrier separation in the synthesized ternary heterostructure, and this accounts for the high value of the kinetic rate constant and dye removal efficiency.
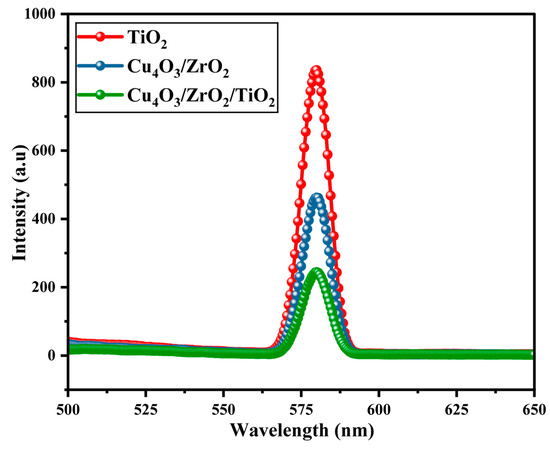
Figure 3.
Photoluminescence (PL) spectra of rutile TiO2, binary Cu4O3/ZrO2, and ternary Cu4O3/ZrO2/TiO2 nanocomposites.
Figure 4a–c shows a comparison of the UV-visible absorption spectra of pure TiO2, binary Cu4O3/ZrO2 and ternary Cu4O3/ZrO2/TiO2 nanocomposites. Rutile TiO2 exhibits the anticipated sharp absorption edge in the ultra-violet (UV) (λ < 364 nm) with just a deep tail into the visible, consistent with its directly measured wide band gap [50,51,52]. The addition of ZrO2 and Cu4O3 significantly increases the baseline absorbance and shifts the absorption envelope to longer wavelengths (381 nm in the binary and 416 nm in the ternary nanocomposite). The broad shoulder at 390–420 nm in the nanocomposites is attributed to charge-transfer transitions among O 2p, Cu 3d, and Ti 3d states. The tail extending into the visible regime suggests the creation of defect-related mid-gap states, as well as interfacial charge-transfer bands. This red-shifted and enhanced absorption profile suggests an increased ability to utilize the solar spectrum, a prerequisite to effective visible-light photocatalysis. The corresponding Tauc plots, Figure 4d–f, were drawn based on the direct-allowed transitions assumption (αhv)2 versus hv, calculated using Equation (2).
where h is Planck’s constant; n is the transition exponent, whose value depends on the nature of the electronic transition (n = 2 for direct and n = ½ for indirect transitions); A is a proportionality constant; ν is the photon frequency; and Eg denotes the optical band-gap energy. The high-energy edge can be linearly extrapolated to optical band-gap energies (Eg) of 3.42 eV in TiO2, 3.12 eV in Cu4O3/ZrO2 [53,54] and 2.91 eV in Cu4O3/ZrO2/TiO2 [53,55,56]. The spectral red shift is supported by the progressive 0.51 eV narrowing of the band gap of TiO2 to the ternary nanocomposite, which can be attributed to two synergistic effects: (i) partial replacement of Ti4+ by Zr4+/Cu+ alters the lattice potential, creating shallow acceptor states at the vicinity of the valence band; and (ii) the intimate heterojunction formation leads to orbital overlap (Cu 3d O 2p Ti 3d), effectively reducing the energy needed to promote electrons by photon The narrower band gap (≈2.9 eV) makes the ternary nanocomposite employ a considerable portion of the visible light (λ < 426 nm), which is consistent with its subsequently reported higher photocatalytic activity. In general, the UV-vis/Tauc analysis indicates that TiO2 engineering with Cu4O3 and ZrO2 not only enhances light absorption but also modifies the electronic structure to the visible regime. The reduced Eg and enhanced absorption window explain the improved production of photo-excited charge carriers, supporting the maximum EY-3RS degradation performance of the Cu4O3/ZrO2/TiO2 nanocomposite.
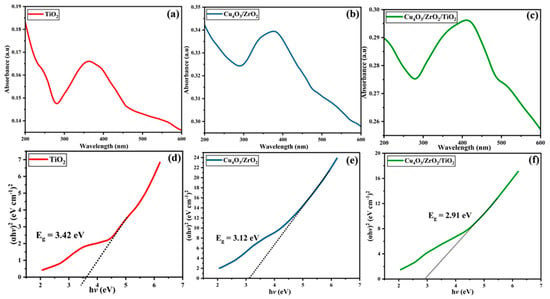
Figure 4.
(a–c) UV-visible absorption spectra of rutile TiO2, binary Cu4O3/ZrO2, and ternary Cu4O3/ZrO2/TiO2 nanocomposites, showing progressive redshifts, (d–f) Corresponding Tauc plots (αhν)2 vs. hν used to estimate direct optical band gaps of rutile TiO2, binary Cu4O3/ZrO2, and ternary Cu4O3/ZrO2/TiO2 nanocomposites.
Figure 5 shows low- and high-magnification SEM micrographs of the hydrothermally synthesized nanocomposites; Figure 5a,b present the binary Cu4O3/ZrO2 sample, whereas Figure 5c,d correspond to the ternary Cu4O3/ZrO2/TiO2 material. Figure 5a at 1 µm resolution shows that the binary catalyst consists of strongly sintered, cauliflower-like agglomerates composed of sub-100 nm primary grains. The aggregates have few interparticle voids, implying a relatively porous network, though there is some densification, which may complicate the diffusion of reactants. At higher magnification, 500 nm, Figure 5b shows that the major crystallites are quasi-spherical (35–60 nm) and necked together; this close intergrain contact should be advantageous to the transport of electrons across the heterojunction, but further sintering can decrease available surface area [57].
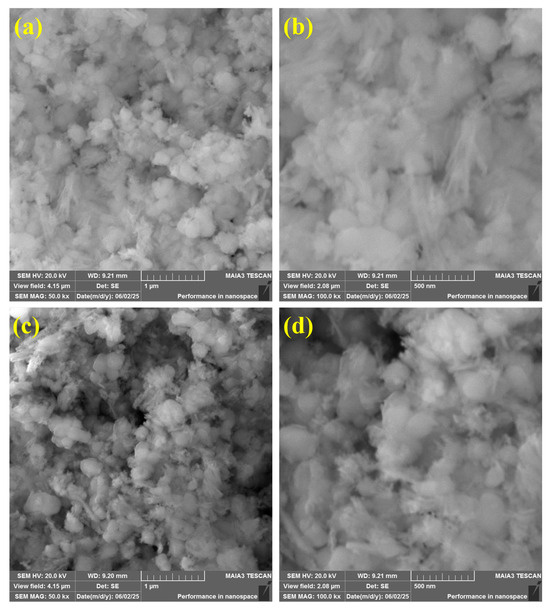
Figure 5.
Scanning electron micrographs of the synthesized nanocomposites; (a,b) Binary Cu4O3/ZrO2 recorded at 50 kx and 100 kx magnifications (scale bars = 1 µm and 500 nm, respectively), (c,d) Ternary Cu4O3/ZrO2/TiO2 at the same magnifications.
Conversely, the ternary nanocomposite Figure 5c,d forms a much looser texture, comprising quasi-spherical nanoparticles (nanospheres) essentially embedded in sponge-like scaffold. The low-magnification image shows fluffy agglomerates with many meso-/macroporous channels that permeate the framework [58,59]. It has been verified by high-magnification observation that the primary nanospheres (25–50 nm) are evenly dispersed but connected by the porous matrix, which generates abundant interstitial pores that increase the overall surface area and reduce the diffusion length of dye molecules. The small grain size and highly porous structure are properties of the synergistic nucleation, where TiO2 addition retards the growth of Cu4O3 and ZrO2 crystallites during calcination, whereas the lattice mismatch at the multi-oxide interfaces inhibits coalescence. Such a textural improvement, coupled with the previously established bandgap narrowing and PL quenching, equips the Cu4O3/ZrO2/TiO2 nanocomposite with a large number of surface-accessible active sites and an efficient charge-migration network, which is the underlying reason behind its high EY-3RS degradation activity.
The energy-dispersive X-ray spectrum of the ternary Cu4O3/ZrO2/TiO2 nanocomposite (Figure 6) displays only the characteristic lines of O, Ti, Zr and Cu, confirming that the target elements are successfully incorporated without adventitious impurities. The intense peak at ≈approximately 0.52 keV (O Kα), together with the strong Ti Kα/Kβ doublet at 4.51/4.93 keV, indicates that titanium and lattice oxygen are the dominant constituents, consistent with the TiO2 backbone observed in XRD. Two sets of Cu signals are evident: the Cu Lα line at ≈0.93 keV and the Cu Kα/Kβ pair at 8.04/8.90 keV, unambiguously verifying the presence of copper oxide clusters. The Zr L series confirm Zr incorporation at 2.04 keV (Lα), and 2.24 keV (Lβ). The carbon, nitrogen, or other metallic contaminant peaks are not detectable throughout the 0–13 keV window, indicating high chemical purity and the efficiency of organic elimination during the calcination process. This elemental confirmation, coupled with the preceding structural and optical characterizations, supports the efficacy of the Cu-Zr-Ti oxide framework for the photocatalytic degradation of EY-3RS [58].
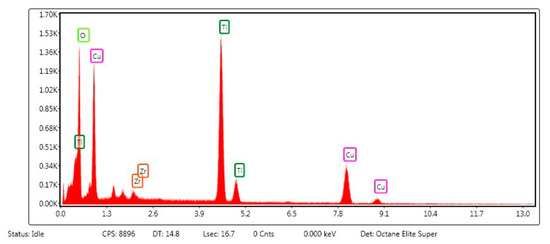
Figure 6.
Energy-dispersive X-ray spectrum of the Cu4O3/ZrO2/TiO2 nanocomposite.
2.2. Photocatalytic Activity of Pure, Binary and Ternary Nanocomposites
The sunlight-driven degradation of EY-3RS was monitored for the pure TiO2, Cu4O3/ZrO2, and Cu4O3/ZrO2/TiO2 photocatalysts, as well as the catalyst-free control. After a 30-min dark adsorption phase, during which the EY-3RS absorbance at 430 nm fell by <1%, the suspensions were exposed to midsummer solar irradiance. In the blank run (no catalyst), only 0.98% decolorization occurred after 100 min, confirming that self-photolysis of EY-3RS is negligible. Figure 7a records the time-resolved UV–Vis spectra for the Cu4O3/ZrO2/TiO2 photocatalyst. The intense azo band, centred at 430 nm, decays steadily, falling by more than an order of magnitude over 100 min, while no new peaks emerge, indicating the progressive cleavage of the -N=N- and aromatic π-frameworks, followed by further degradation of the intermediates. The bar chart in Figure 7b compares overall decolorization efficiencies after 100 min: TiO2 (81.48%) < Cu4O3/ZrO2 (86.65%) < Cu4O3/ZrO2/TiO2 (91.41%). The marked improvement obtained upon passing from the binary to the ternary system corroborates earlier optical and PL evidence that Cu- and Zr-induced heterojunctions shorten charge-migration paths, suppress e-/h+ recombination, and extend the photo-response into the visible region. Collectively, these results demonstrate that rational multi-oxide engineering yields a robust, earth-abundant catalyst capable of removing more than 90% of a representative textile dye within 100 min of natural sunlight irradiation.
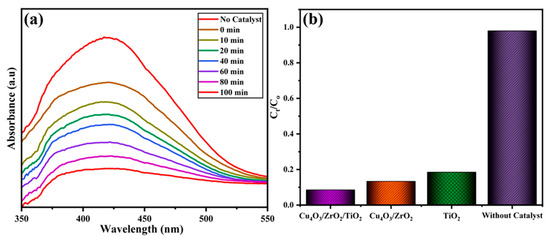
Figure 7.
(a) UV-Vis absorption spectra for time-dependent photocatalytic degradation of EY 3RS using Cu4O3/ZrO2/TiO2 photocatalyst (b) bar graph representing the Ct/C0 of EY 3RS using different photocatalysts.
2.2.1. Effect of Dye Concentration
Figure 8 illustrates the dependence of degradation efficiency (%D) on the EY-3RS loading (20–100 ppm) for the three photocatalysts. At the lowest concentration (20 ppm), removal efficiencies are highest: 89.99% for TiO2, 93.43% for Cu4O3/ZrO2, and 96.15% for Cu4O3/ZrO2/TiO2, because the active sites are plentiful and the solution is optically thin. As the dye concentration rises, %D declines monotonically, falling to 80.22%, 84.55% and 87.11% for the respective catalysts at 100 ppm. The trend is well documented for heterogeneous photo-oxidation and is attributed to three, mutually reinforcing factors: (i) surface saturation, whereby an excess of adsorbed dye blocks catalytic sites and restricts •OH generation; (ii) inner-filter/shielding effects, in which the dense dye layer absorbs incident photons before they reach the catalyst surface, lowering the photon flux available for charge separation; and (iii) radical or hole scavenging by intermediates, whose concentration necessarily increases at higher substrate loadings and competes with the parent dye for reactive oxygen species [60]. Although all three materials follow the same qualitative pattern, the ternary heterostructure consistently outperforms the binary and pure TiO2 at every concentration, confirming that its larger surface area and more efficient charge separation partly offset the detrimental effects of surface crowding and light attenuation.
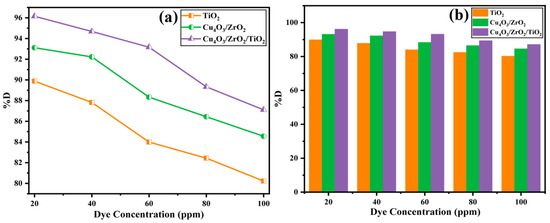
Figure 8.
Effect of dye concentration vs. % degradation values of EY 3RS ((a) line graph, (b) bar graph).
2.2.2. Effect of Catalyst Loading
The influence of catalyst dosage on EY-3RS removal is displayed in Figure 9. Raising the loading from 5 to 25 mg (0.17 → 0.83 g L−1) steadily enhances degradation for all three materials, consistent with the well-established principle that a larger amount of photocatalyst provides (i) a greater illuminated surface area and (ii) more photogenerated e−/h+ pairs available to produce reactive oxygen species [17]. At the lowest dose (5 mg) color removal is modest, 55.22% (TiO2), 58.36% (Cu4O3/ZrO2) and 63.34% (Cu4O3/ZrO2/TiO2)-because photon absorption and the number of accessible active sites are limited. Doubling the mass to 10 mg increases the %D to 67.34%, 72.47%, and a pronounced 89.38% for the ternary nanocomposite, the latter reflecting its higher intrinsic activity and larger external surface area. Further increments to 15 mg and 20 mg provide diminishing, yet still positive, returns: the efficiency levels off near 74.62% (TiO2), 78.65% (binary), and 94.56% (ternary) at 20 mg. Beyond this point, the curves plateau, indicating that light scattering and particle agglomeration begin to offset the benefits of additional surface. Consequently, 20 mg (0.67 g L−1) is taken as the optimum loading for all subsequent experiments; higher doses confer no measurable advantage but would increase sludge-handling costs in practical applications [61]. Throughout the entire range, the ternary Cu4O3/ZrO2/TiO2 photocatalyst outperforms both the binary and rutile TiO2, underscoring the role of its heterojunction in maintaining high quantum efficiency even at suboptimal catalyst densities.
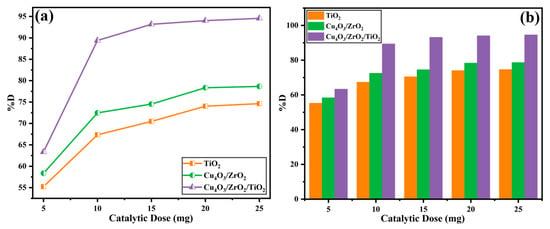
Figure 9.
Effect of photocatalytic dose vs. % degradation values of EY3RS ((a) line graph, (b) bar graph).
2.2.3. Effect of Solution pH
Figure 10 depicts the influence of pH (3–11) on EY-3RS removal after 60 min of irradiation. All three catalysts exhibit their highest activities in strongly acidic medium: 84.97% (TiO2), 88.42% (Cu4O3/ZrO2), and 95.91% (Cu4O3/ZrO2/TiO2) at pH 3. This behavior is governed by the point-of-zero charge (pHZPC) of Ti-, Zr- and Cu-oxides, which lies near 5–6. Below pHZPC, the oxide surface is positively charged, whereas EY-3RS carries negative sulfonate groups; the resulting electrostatic attraction favors dye adsorption and accelerates interfacial electron-transfer steps [62,63]. Shifting the pH from 3 to 5 and 7 produces only a modest decline (≤5 percentage points), indicating that the catalysts remain effective as long as the surface charge is still slightly positive or neutral. In contrast, a sharp decline in activity occurs once the medium becomes alkaline: at pH 9, the degradation efficiencies drop to 18.87% (TiO2), 22.09% (binary), and 30.48% (ternary), and further decrease at pH 11. Two phenomena account for this drop. First, above pHZPC, the catalyst surface acquires a negative charge, generating Coulombic repulsion with the anionic dye and lowering surface coverage. Second, alkaline conditions convert •OH generation pathways into less reactive HO2−/O2− routes, diminishing the steady-state concentration of oxidative radicals [64]. Despite the common trend, the ternary Cu-Zr-Ti oxide consistently outperforms the other two samples at every pH, reflecting its superior light harvesting and charge-separation efficiencies, which partially compensate for the electrostatic penalty encountered in near-neutral and alkaline media.
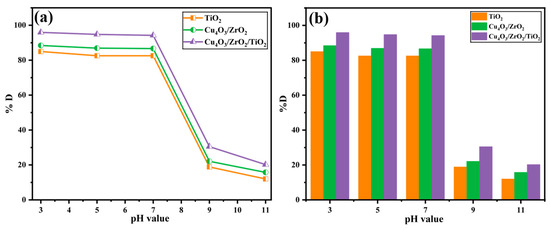
Figure 10.
Influence of pH vs. % degradation values of EY3RS ((a) line graph, (b) bar graph).
2.2.4. Effect of Irritation Time
The photocatalytic degradation of the EY-3RS solution increased significantly with increasing irradiation time, as shown in Figure 11. A longer time results in more photon absorption by the catalyst, due to which the generation of electron-hole pairs increases, resulting in a higher %D being observed [65,66]. To measure the photocatalytic activities of the prepared materials, all suspensions were first held in the dark for 30 min to reach an adsorption-desorption equilibrium. During the equilibration time, there was less than 5% loss of absorbance at 430 nm, suggesting there was minimal photolysis or spontaneous hydrolysis of EY-3RS such that any subsequent disappearance of the compound was due to photocatalysis. Irradiation (t = 0) initiates a rapid acceleration phase that spans the first 20 min: the ternary Cu4O3/ZrO2/TiO2 nanocomposite removes 80.42% of the dye, while the binary Cu4O3/ZrO2 and rutile TiO2 achieve 68.87% and 55.76%, respectively. Such a significant initial increase is possible due to the large number of fresh surface sites, the high initial medium-irradiation photon flux, and the effective diffusion of dye molecules from a bulk solution to the catalyst surface. The ternary photocatalyst, with the narrowest band gap of 2.91 eV and most effective electron-hole separation, produces the highest content of ROS (•OH, •O2−) in this time window, which makes the fastest decay in dye concentration [67]. Beyond 20 min, the reaction enters a deceleration phase in which the removal rate steadily tapers. By 60 min, the decolorization plateaus at 90.19% for the ternary, 84.36% for the binary, and 79.68% for TiO2. Several factors contribute to this gradual slowdown: (i) adsorption sites become partially saturated with dye residues and reaction intermediates, limiting the further uptake of intact EY-3RS molecules; (ii) as the dye concentration dwindles, the probability of dye–radical encounters drops; and (iii) continuous photon absorption leads to a non-negligible recombination of photogenerated carriers, particularly in TiO2, thereby lowering the steady-state radical population. The ternary nanocomposite is least affected by these limitations, owing to its porous nanosphere-in-matrix architecture that promotes mass transfer and its multi-oxide interfacial fields that keep electron-hole recombination at a minimum [68].
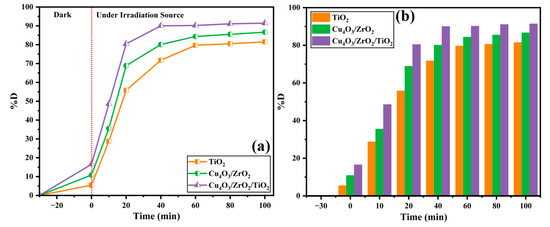
Figure 11.
Effect of irradiation time vs. % degradation values of EY 3RS ((a) line graph, (b) bar graph).
With the reaction time further increasing to 100 min, the degradation profiles approached asymptotic curves. After optimization (40 ppm EY-3RS, 30 mg catalyst, pH 3), the maximum decolourization efficiencies were 91.41% for Cu4O3/ZrO2/TiO2, 86.65% for Cu4O3/ZrO2 and 81.48% for bare TiO2. The nearly 20-percentage-point advantage of the ternary catalyst over rutile TiO2 underscores the critical role of bandgap tailoring and heterojunction engineering in sustaining high radical fluxes and maintaining catalytic activity as the reaction approaches completion. These results also show that most of the practicable decolorization is achieved within the first hour, an important consideration for designing treatment residence times in potential field applications.
2.3. Photocatalytic Degradation Mechanism of Everzol Yellow 3RS
When an oxide-based semiconductor photocatalyst (SC) is irradiated with photons whose energy equals or exceeds its bandgap, electrons from the valence band (VB) are promoted to the conduction band (CB), leaving charge-compensating holes in the VB. The photogenerated electrons and holes quickly migrate to the particle surface, where they participate in a suite of redox steps that collectively mineralize the dye [69]. A brief dark-adsorption stage, used in all experiments, pre-concentrates dye molecules at the catalyst surface and ensures that subsequent spectral changes originate from true photodegradation rather than from mere physisorption [70]. Under illumination, there may be synergic effect of both photocatalyst as well as dye, because the under studied dye has the ability to absorb in visible region and can inject an electron to the conduction band leading to formation of unstable dye cation radical [71,72]. The CB electrons react preferentially with dissolved molecular oxygen, forming superoxide radicals (•O2−). Sequential proton-coupled electron transfers convert •O2− to hydroperoxyl radicals (•HO2), hydrogen peroxide (H2O2), and, finally, highly oxidizing hydroxyl radicals (•OH). In parallel, VB holes oxidize surface-adsorbed water or hydroxide to additional •OH or can attack the dye directly. These reactive oxygen species, together with the VB holes, cleave the azo (-N=N-) bridge and aromatic π-systems of EY-3RS, yielding progressively smaller intermediates that are further oxidized to CO2, and H2O, as demonstrated in Figure 12. The nanosphere-in-porous-matrix morphology of the catalyst provides abundant adsorption sites, shortens charge-migration distances, accelerates reactant diffusion, and further retards charge recombination [70,73]. These synergistic structural and electronic features account for the observed 91% removal of EY-3RS within 100 min, compared with <1% self-photolysis under identical conditions.
SC + hν → e−CB + h+VB
e−CB + O2 → •O2•
•O2− + H+ → •HO2
•HO2 + e−CB + H+ → H2O2
H2O2 + e−CB → •OH + OH−
h+VB + H2O → •OH + H+
h+VB + EY-3RS → Oxidized Intermediates
•OH/•O2− + Intermediates → CO2 + H2O
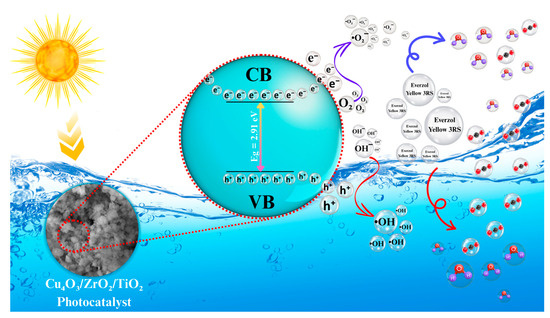
Figure 12.
Possible photocatalytic mechanism proposed for EY-2RS degradation over the Cu4O3/ZrO2/TiO2 nanocomposite.
The mechanistic key elementary steps are summarized: photon absorption and charge separation, oxygen reduction to radical species, water oxidation, direct hole attack, and successive degradation of dye fragments into harmless end-products [74].
2.4. Reusability of Material
Long-term durability is a decisive criterion for translating a laboratory photocatalyst into an industrial water-treatment process. The reusability of the Cu4O3/ZrO2/TiO2 nanocomposite was therefore investigated in four consecutive degradation cycles carried out under the optimized conditions (40 ppm EY-3RS, 30 mg catalyst, pH 3, 100 min sunlight). After each run, the solid was recovered by centrifugation, rinsed thoroughly with de-ionised water, followed by ethanol to remove adsorbed organics, and oven-dried at 60 °C overnight before being reused. As summarized in Figure 13, the removal efficiencies recorded in the successive 1–4 cycles are 91.4%, 88.1%, 86.5% and 84.9%, respectively. In other words, the catalyst maintains ≈ approximately 93% of its initial activity after four uses, with a cumulative loss of 6.5% points, translating to an average drop of only 1.6% points per cycle. The small performance decrement most likely stems from (i) partial fouling of surface sites by tightly bound intermediates that are not entirely removed during washing and (ii) the unavoidable loss of a few milligrams of powder during each recovery step. Overall, the data indicates that the ternary nanocomposite can be recycled multiple times with minimal loss in photocatalytic efficiency, a crucial prerequisite for the cost-effective deployment of sunlight-driven dye-remediation processes. In the ternary composite, each oxide plays a complementary role: ZrO2 supplies the most electrons and therefore drives O2 reduction most efficiently [75]; Cu4O3, with the most positive valence band, furnishes high-energy holes that favor •OH generation and direct dye oxidation [76]; TiO2 acts as the central light-harvesting scaffold and an electron-transfer bridge that promotes interfacial charge separation between ZrO2 and Cu4O3. The synergistic coupling of these functions sustains a high steady-state concentration of reactive oxygen species while suppressing e−/h+ recombination [77]. Table 1 represents the comparison of documented studies with synthesized nanomaterials.
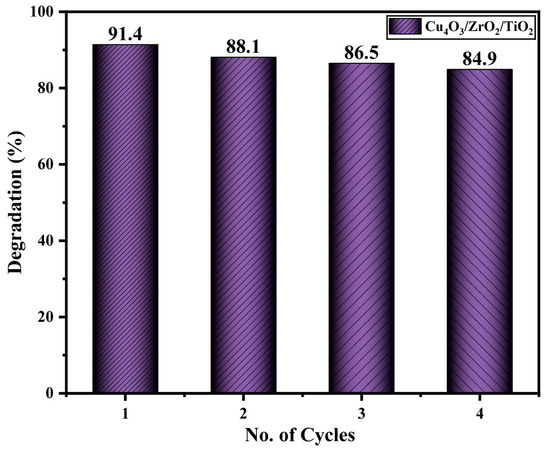
Figure 13.
Degradation efficiency retained by the ternary Cu4O3/ZrO2/TiO2 photocatalyst across four consecutive reuse cycles.

Table 1.
Comparison of current study with already reported work.
3. Materials and Methods
3.1. Materials Used
The chemicals used during the research were Titanium (IV) butoxide (Ti(OBu)4, Copper acetate Cu(CH3COO)2, Zirconium chloride (ZrOCl2.8H2O), and urea (CH4N2O). All these chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), with a purity of 99.9%. All the aqueous solutions were prepared in distilled water. For pH adjustment, sodium hydroxide and hydrochloric acid solutions were used. For photo catalytic purposes, Hydrogen peroxide (H2O2) as an oxidant and EY 3RS (C46H28ClN10Na5O15S6), MW = 1303.60Da, absorption λmax = 430 nm), as a target pollutant, were also obtained from Sigma Aldrich.
3.2. Synthesis of Ternary Nanocomposites
The synthesis of ternary nanomaterials was performed using the hydrothermal method with slight modifications [82]. A precursor solution was prepared by dissolving 5 mmol titanium (IV) butoxide (Ti(OBu)4, 5 mmol zirconium chloride (ZrOCl2.8H2O), 5 mmol copper acetate and 1.126 mmol urea in 90 mL of distilled water, followed by vigorous stirring for 15 min at 600 rpm (at room temperature). The solution was poured into a 100 mL Teflon-lined autoclave and put in the oven at 140 °C for 4 h. The product was then allowed to cool, followed by washing with distilled water, and subsequently placed on a hot plate at 60 °C overnight for drying. Finally, the ternary nanomaterials were calcinated in air at 350 °C for 3 h, as illustrated in Figure 14.
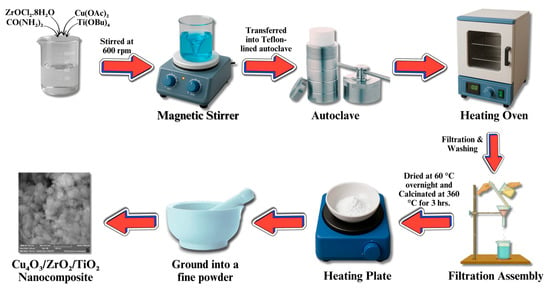
Figure 14.
Schematic representation of ternary Cu4O3/ZrO2/TiO2 nanocomposite synthesis.
3.3. Photocatalytic Activity Experiment
Everzol Yellow 3RS (Reactive Yellow 176) dye is a dye used in the textile industry. The chemical structure of Reactive Yellow 176 dye is given in Figure 15 [83]. The dye was mixed with distilled water and then used for photocatalytic studies.
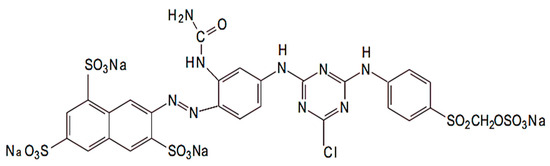
Figure 15.
Synthetic structure of EY-3RS dye.
Photocatalytic degradation of EY-3RS was evaluated under natural sunlight for three catalysts-rutile TiO2, the binary Cu4O3/ZrO2 heterostructure, and the ternary Cu4O3/ZrO2/TiO2 nanocomposite. For each run, 30 mL of the required initial dye solution concentration (20–100 ppm) was transferred to a jacket-free 100 mL borosilicate flask fitted with a magnetic bar. The pH was adjusted to the desired value (3, 5, 7, 9, or 11) with 0.1 M HCl or NaOH, after which the weighed catalyst (10, 20, 30, or 40 mg; 0.33–1.33 g/L) was ultrasonically dispersed for 5 min. The suspension was then magnetically stirred in the dark for 40 min at 25 ± 1 °C (400 rpm) to establish adsorption–desorption equilibrium; during this period, the absorbance at λmax = 430 nm fell by <5%, confirming negligible photolysis or hydrolysis. After dark equilibration, the flask was exposed to natural sunlight typical of Gujrat, Punjab (mid-June, clear-sky global horizontal irradiance ≈ 850–900 W m−2 at solar noon) and irradiation continued for up to 100 min. Aliquots of 1 mL were withdrawn at 0, 10, 20, 40, 60, 80, and 100 min, centrifuged to remove residual solids, and the absorbance of the supernatant was recorded using a UV–Vis spectrophotometer. Because absorbance in the Beer–Lambert range is proportional to concentration, the degradation efficiency was calculated directly from Equation (2) [69].
where Ao is the initial dye concentration (mg L−1) and At is the concentration of dye at time t (mg L−1). The traditional method of identifying each reaction parameter separately as an independent variable was used to increase degradation efficiency. Changing one parameter at a time within a specific range while keeping all other parameters constant is the random screening approach. The effect of each modification on the response factor (%D) is then explained. The goal is to optimize the process by evaluating how changing parameters such as EY 3RS concentration, NPs concentration, pH, and irradiation time affect the %D values. Individual levels of parameters, including 20–100 ppm EY 3RS concentration, 10–40 mg NPs concentration, 3–11 pH, and 20–100 min irradiation period, were investigated experimentally. %D values were analyzed to determine the maximum efficiency and improve the reaction conditions.
4. Conclusions
A visible-light-active ternary nanocomposite, Cu4O3/ZrO2/TiO2, was successfully synthesized by a one-step hydrothermal route and benchmarked against its binary (Cu4O3/ZrO2) and rutile TiO2 counterparts for the photocatalytic removal of the textile dye Everzol Yellow 3RS. Structural (XRD, FT-IR), morphological (SEM-EDX) and optical (UV-Vis, PL) characterization confirmed the formation of a well-coupled heterojunction with a narrowed band gap of 2.91 eV, a nanosphere-in-porous-matrix architecture and markedly suppressed electron-hole recombination. Under midsummer natural sunlight, the ternary photocatalyst achieved 91.41% decolorization of a 40-ppm dye solution within 100 min, outperforming the binary (86.65%) and TiO2 (81.48%) by wide margins. Systematic parameter studies revealed that the nanocomposite retained its activity over a range of 20–100 ppm dye loadings, 10–40 mg catalyst dosages, and pH levels of 3–7. Notably, the optimum conditions (40 ppm, 30 mg, pH 3) yielded the highest degradation rate. Reusable tests demonstrated a loss of only 6.5 percentage points after four cycles, indicating their excellent operability. As far as we know, this is the first report on nanophotocatalytic route of EY-3RS removal. The study thus offers a feasible and cost-effective approach for sunlight-driven dye-enriched textile effluent treatment, demonstrating the overall capability of the multioxide heterojunction for industrial wastewater treatment.
Author Contributions
Conceptualization, M.A.R.; Data curation, S. and M.S.; Formal analysis, Z.A.S. and M.F.; Funding acquisition, W.A.E.-F.; Investigation, A.G. and Z.A.S.; Methodology, S. and S.A.; Project administration, M.A.R.; Resources, M.A.R.; Supervision, M.A.R.; Validation, N.B.H., Z.A.S. and W.A.E.-F.; Writing—original draft, Z.A.S. and N.B.H.; Writing—review & editing, M.A.R. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2503).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data is available upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, M.; Singh, J.; Rawat, M.; Sharma, J.; Singh, P.P. Enhanced photocatalytic degradation of hazardous industrial pollutants with inorganic–organic TiO2–SnO2–GO hybrid nanocomposites. J. Mater. Sci. Mater. Electron. 2019, 30, 13389–13400. [Google Scholar] [CrossRef]
- Siddique, H.M.A.; Kiani, A.K. Industrial pollution and human health: Evidence from middle-income countries. Environ. Sci. Pollut. Res. 2020, 27, 12439–12448. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wang, Z.; Li, J. The effect of urbanization on environmental pollution in rapidly developing urban agglomerations. J. Clean. Prod. 2019, 237, 117649. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Chandrasekaran, A. Recent advances in TiO2/ZnS-based binary and ternary photocatalysts for the degradation of organic pollutants. Sci. Total Environ. 2023, 868, 161525. [Google Scholar] [CrossRef] [PubMed]
- Salari, H. Efficient photocatalytic degradation of environmental pollutant with enhanced photocarrier separation in novel Z-scheme a-MnO2 nanorod/a-MoO3 nanocomposites. J. Photochem. Photobiol. A Chem. 2020, 401, 112787. [Google Scholar] [CrossRef]
- Kanan, S.; Moyet, M.A.; Arthur, R.B.; Patterson, H.H. Recent advances on TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catal. Rev. 2020, 62, 1–65. [Google Scholar] [CrossRef]
- Tahir, N.; Zahid, M.; Bhatti, I.A.; Mansha, A.; Naqvi, S.A.R.; Hussain, T. Metal oxide-based ternary nanocomposites for wastewater treatment. In Aquananotechnology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 135–158. [Google Scholar]
- Lavrynenko, O.; Zahornyi, M.; Paineau, E.; Yu, P.O. Synthesis of active binary and ternary TiO2-based nanocomposites for efficient dye photodegradation. Appl. Nanosci. 2023, 13, 7365–7377. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Shahat, A.M.; El-Hossary, F.; Ghitas, A.; Abd El-Rahman, A.; Ebnalwaled, A. Low-temperature hydrothermal synthesis of titanium dioxide nanoparticles for photocatalytic applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1171, 012008. [Google Scholar] [CrossRef]
- Imoisili, P.E.; Jen, T.-C.; Safaei, B. Microwave-assisted sol–gel synthesis of TiO2-mixed metal oxide nanocatalyst for degradation of organic pollutant. Nanotechnol. Rev. 2021, 10, 126–136. [Google Scholar] [CrossRef]
- Rakhi, C.; Preetha, K. An investigation on effect of complexing agents on zirconium based ternary metal oxide nanoparticles by co-precipitation method. J. Mater. Sci. Mater. Electron. 2019, 30, 19587–19597. [Google Scholar] [CrossRef]
- Tyagi, N.; Ashraf, W.; Mittal, H.; Fatima, T.; Khanuja, M.; Singh, M.K. A facile synthesis of ternary hybrid nanocomposite of WS2/ZnO/PPy: An efficient photocatalyst for the degradation of chromium hexavalent. Dye. Pigment. 2023, 210, 110998. [Google Scholar] [CrossRef]
- Kamble, C.; Jadhav, V.V.; Mane, R.S. Electrodeposition of metal oxide nanostructures. In Solution Methods for Metal Oxide Nanostructures; Elsevier: Amsterdam, The Netherlands, 2023; pp. 197–220. [Google Scholar]
- Suganya, S.; Alam, M.M.; Kousi, F.; Ramalingam, G.; Prabhu, M.R.; Sudhahar, S. Facile one-pot synthesis of ternary Ni-Mn-Zn oxide nanocomposites for high-performance hybrid supercapacitors. J. Energy Storage 2023, 71, 108176. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Ghoshal, D.; Mondal, D.; Paul, B.K.; Bose, N.; Das, S.; Basu, M. Visible light driven degradation of brilliant green dye using titanium based ternary metal oxide photocatalyst. Results Phys. 2019, 12, 1850–1858. [Google Scholar] [CrossRef]
- Muthamilarasu, A.; Sivakumar, S.; Divya, G.; Sivakumar, M.; Sakthi, D. NiO/CuO/TiO2 ternary composites: Development, physicochemical characterization and photocatalytic degradation study over reactive orange 30 solutions under solar light irradiation. Adv. Mater. Sci. 2022, 22, 36–54. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interface Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef]
- Kerrami, A.; Khezami, L.; Bououdina, M.; Mahtout, L.; Modwi, A.; Rabhi, S.; Bensouici, F.; Belkacemi, H. Efficient photodegradation of azucryl red by copper-doped TiO2 nanoparticles—Experimental and modeling studies. Environ. Sci. Pollut. Res. 2021, 28, 57543–57556. [Google Scholar] [CrossRef] [PubMed]
- S Altimari, U.; Alkadir, K.A.; H Abdulrazzak, F.; F Alkaim, A. Improve the Medical Properties of Nanocomposite Metal Oxide by Increase the Activity. J. Nanostructures 2021, 11, 568–576. [Google Scholar]
- Delsouz Khaki, M.R.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Enhanced UV–Visible photocatalytic activity of Cu-doped ZnO/TiO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 5480–5495. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Preparation and characterization of ZnO–TiO2 nanocomposite for photocatalytic disinfection of bacteria and detoxification of cyanide under visible light. Mater. Res. Bull. 2011, 46, 1586–1592. [Google Scholar] [CrossRef]
- Pilliadugula, R.; Nithya, C.; Krishnan, N.G. Influence of Ga2O3, CuGa2O4 and Cu4O3 phases on the sodium-ion storage behaviour of CuO and its gallium composites. Nanoscale Adv. 2020, 2, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, S.; Aman, A.K.; Karim, S.; Kumar, S.; Kar, M. Study on physical properties of Ayurvedic nanocrystalline Tamra Bhasma by employing modern scientific tools. J. Ayurveda Integr. Med. 2019, 10, 88–93. [Google Scholar] [CrossRef]
- Giri, S.D.; Mahajani, S.M.; Suresh, A.; Sarkar, A. Electrochemical reduction of CO2 on activated copper: Influence of surface area. Mater. Res. Bull. 2020, 123, 110702. [Google Scholar] [CrossRef]
- Jagadish, K.A.; Kekuda, D. Thermal annealing effect on phase evolution, physical properties of DC sputtered copper oxide thin films and transport behavior of ITO/CuO/Al Schottky diodes. Appl. Phys. A 2024, 130, 315. [Google Scholar] [CrossRef]
- Nurulhuda, A.; Warikh, A.; Hafizzal, Y. Sol gel synthesis and characterization studies of Cupromanganite CaCu3Mn4O12. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012154. [Google Scholar] [CrossRef]
- Wnuk, R.; Novak, P.; Stępień, M.; Jaworska, L.; Marek, I.; Noga, P.; Skrzekut, T.; Małecki, S. Activation energies of oxidation in air for SPS-sintered zirconium and its alloys. Arch. Civ. Mech. Eng. 2025, 25, 1–14. [Google Scholar] [CrossRef]
- Patel, R.; Fakeeha, A.H.; Kasim, S.O.; Sofiu, M.L.; Ibrahim, A.A.; Abasaeed, A.E.; Kumar, R.; Al-Fatesh, A.S. Optimizing yttria-zirconia proportions in Ni supported catalyst system for H2 production through dry reforming of methane. Mol. Catal. 2021, 510, 111676. [Google Scholar] [CrossRef]
- Shu, X.; Lu, X.; Fan, L.; Yang, R.; Ding, Y.; Pan, S.; Zhou, P.; Wu, Y. Design and fabrication of Gd2Zr2O7-based waste forms for U3O8 immobilization in high capacity. J. Mater. Sci. 2016, 51, 5281–5289. [Google Scholar] [CrossRef]
- Żurowski, R.; Zygmuntowicz, J.; Piotrkiewicz, P.; Wachowski, M.; Szczypiński, M.M. ZTA pipes with a gradient structure-effect of the rheological the behavior of ceramic suspensions on the gradient structure and characterized of the obtained products. Materials 2021, 14, 7348. [Google Scholar] [CrossRef]
- Asiltürk, M.; Sayılkan, F.; Erdemoğlu, S.; Akarsu, M.; Sayılkan, H.; Erdemoğlu, M.; Arpaç, E. Characterization of the hydrothermally synthesized nano-TiO2 crystallite and the photocatalytic degradation of Rhodamine B. J. Hazard. Mater. 2006, 129, 164–170. [Google Scholar] [CrossRef]
- Prasad, R.D.; Sarvalkar, P.D.; Prasad, N.; Prasad, S.R.; Prasad, R.S.; Prasad, R.B.; Prasad, R.R.; Desai, C.; Vaidya, A.K.; Teli, B. A Review on Spectroscopic Techniques for Analysis of Nanomaterials and Biomaterials. ES Energy Environ. 2024, 27, 1264. [Google Scholar] [CrossRef]
- Divya, G.; Sivakumar, S.; Sakthi, D.; Priyadharsan, A.; Arun, V.; Kavitha, R.; Boobas, S. Developing the NiO/CuTiO3/ZnO ternary semiconductor heterojunction for harnessing photocatalytic activity of reactive dye with enhanced durability. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4480–4490. [Google Scholar] [CrossRef]
- Qamar, M.; Yoon, C.; Oh, H.; Lee, N.; Park, K.; Kim, D.; Lee, K.; Lee, W.; Kim, S. Preparation and photocatalytic activity of nanotubes obtained from titanium dioxide. Catal. Today 2008, 131, 3–14. [Google Scholar] [CrossRef]
- Durai, S.V.; Kumar, E.; Muthuraj, D. Investigations on structural, optical, and impedance spectroscopy studies of titanium dioxide nanoparticles. Bull. Chem. Soc. Ethiop. 2021, 35, 151–160. [Google Scholar] [CrossRef]
- Ouda, A.A.; Alosfur, F.K.M.; Ridha, N.J.; Abud, S.H.; Umran, N.M.; Al-aaraji, H.H.; Madlool, R.A. Facile method to synthesis of anatase TiO2 nanorods. J. Phys. Conf. Ser. 2018, 1032, 012038. [Google Scholar] [CrossRef]
- David, S.A.; Vedhi, C. Synthesis and characterization of Co3O4-CuO-ZrO2 ternary nanoparticles. Int. J. Chem Tech Res. 2017, 10, 905–912. [Google Scholar]
- Batool, M.; Qureshi, M.Z.; Hashmi, F.; Mehboob, N.; Shah, A.S. Congo red azo dye removal and study of its kinetics by aloe vera mediated copper oxide nanoparticles. Indones. J. Chem. 2019, 19, 626–637. [Google Scholar] [CrossRef]
- Wahba, M.A.; Badawy, A.A. Novel Zr–Cu–Fe nanocomposite metal oxides: Structural, magnetic and composition activity effects on photodegradation of phenols. J. Sol-Gel Sci. Technol. 2020, 94, 637–647. [Google Scholar] [CrossRef]
- Mersian, H.; Alizadeh, M.; Hadi, N. Synthesis of zirconium doped copper oxide (CuO) nanoparticles by the Pechini route and investigation of their structural and antibacterial properties. Ceram. Int. 2018, 44, 20399–20408. [Google Scholar] [CrossRef]
- Rahmawati, L.; Kurniawan, R.; Prasetyo, N.; Sudiono, S.; Syoufian, A. Copper-and-Nitrogen-Codoped Zirconium Titanate (Cu-N-ZrTiO4) as a Photocatalyst for Photo-Degradation of Methylene Blue under Visible-Light Irradiation. Indones. J. Chem. 2023, 23, 416–424. [Google Scholar] [CrossRef]
- Afzal, S.; Naeem, R.; Sherino, B.; Nabi, N.; Behlil, F.; Julkapli, N.M. Impact of chitosan on CS/TiO2 composite system for enhancing its photocatalytic performance towards dye degradation. Desalination Water Treat. 2023, 283, 274–279. [Google Scholar] [CrossRef]
- Katoh, R.; Takahashi, K.; Sugawa, K. Quantum Yields of Photoluminescence of TiO2 Photocatalysts. J. Phys. Chem. C 2022, 126, 20954–20959. [Google Scholar] [CrossRef]
- Gurushantha, K.; Anantharaju, K.; Kottam, N.; Keshavamurthy, K.; Ravikumar, C.; Surendra, B.; Murugan, A.; Murthy, H.A. Synthesis of ZrO2: Dy3+ nanoparticles: Photoluminescent, photocatalytic, and electrochemical sensor studies. Adsorpt. Sci. Technol. 2022, 2022, 5664344. [Google Scholar] [CrossRef]
- Uma, H.B.; Kumar, M.S.V.; Ananda, S. Semiconductor-assisted photodegradation of textile dye, photo-voltaic and antibacterial property of electrochemically synthesized Sr-doped CuO nano photocatalysts. J. Mol. Struct. 2022, 1264, 133110. [Google Scholar] [CrossRef]
- Bunea, R. The Effect of Annealing Temperatures and Inert/Reactive Gasses on Optical Properties of Cu2O and CuO Thin Films. Ph.D. Thesis, University of Central Florida, Orlando, FL, USA, 2021. [Google Scholar]
- Zheng, J.; Sun, L.; Jiao, C.; Shao, Q.; Lin, J.; Pan, D.; Naik, N.; Guo, Z. Hydrothermally synthesized Ti/Zr bimetallic MOFs derived N self-doped TiO2/ZrO2 composite catalysts with enhanced photocatalytic degradation of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126629. [Google Scholar] [CrossRef]
- Kuriechen, S.K.; Murugesan, S.; Paul Raj, S. Mineralization of Azo Dye Using Combined Photo-Fenton and Photocatalytic Processes under Visible Light. J. Catal. 2013, 2013, 104019. [Google Scholar] [CrossRef]
- Lim, P.F.; Leong, K.H.; Sim, L.C.; Abd Aziz, A.; Saravanan, P. Amalgamation of N-graphene quantum dots with nanocubic like TiO2: An insight study of sunlight sensitive photocatalysis. Environ. Sci. Pollut. Res. 2019, 26, 3455–3464. [Google Scholar] [CrossRef] [PubMed]
- Nur, A.S.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Process Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Wahba, M.A.; Yakout, S.M. Microwave-synthesized ZrO2/ZnO heterostructures: Fast and high charge separation solar catalysts for dyes-waste degradation. J. Sol-Gel Sci. Technol. 2022, 104, 330–341. [Google Scholar] [CrossRef]
- Bashir, A.; Farooq, M.; Malik, A.; Naseem, S.; Bhatti, A.S. UV-a treatment of ZrO2 thin films fabricated by environmental friendlier water-based solution processing: Structural and optical studies. Coatings 2021, 11, 821. [Google Scholar] [CrossRef]
- Anandan, K.; Rajesh, K.; Rajendran, V. Enhanced optical properties of spherical zirconia (ZrO2) nanoparticles synthesized via the facile various solvents mediated solvothermal process. J. Mater. Sci. Mater. Electron. 2017, 28, 17321–17330. [Google Scholar] [CrossRef]
- Ansari, F.; Sheibani, S.; Fernandez-García, M. Surface modification of Cu2O-CuO photocatalyst on Cu wire through decorating with TiO2 nanoparticles for enhanced visible light photocatalytic activity. J. Alloys Compd. 2022, 919, 165864. [Google Scholar] [CrossRef]
- Yitagesu, G.B.; Leku, D.T.; Seyume, A.M.; Workneh, G.A. Biosynthesis of TiO2/CuO and Its Application for the Photocatalytic Removal of the Methylene Blue Dye. ACS Omega 2024, 9, 41301–41313. [Google Scholar] [CrossRef]
- Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Ahamed, M. Chemical synthesis, characterization, and anticancer potential of CuO/ZrO2/TiO2/RGO nanocomposites against human breast (MCF-7) cancer cells. RSC Adv. 2024, 14, 37697–37708. [Google Scholar] [CrossRef]
- Ruíz-Santoyo, V.; Marañon-Ruiz, V.F.; Romero-Toledo, R.; González Vargas, O.A.; Pérez-Larios, A. Photocatalytic degradation of rhodamine B and methylene orange using TiO2-ZrO2 as nanocomposite. Catalysts 2021, 11, 1035. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Raju, G.S.R.; Pavitra, E.; Kwak, C.H.; Han, Y.-K.; Huh, Y.S. Controlled synthesis of hierarchical α-nickel molybdate with enhanced solar-light-responsive photocatalytic activity: A comprehensive study on the kinetics and effect of operational factors. Ceram. Int. 2019, 45, 12041–12052. [Google Scholar] [CrossRef]
- Mehrabad, J.T.; Rad, F.A. Exploring the photocatalytic activity of magnesium and copper-doped titanium dioxide nano catalyst through synthesis and characterization. Adv. J. Chem. A 2024, 7, 374–385. [Google Scholar]
- Raghav, R.; Aggarwal, P.; Srivastava, S. Tailoring oxides of copper-Cu2O and CuO nanoparticles and evaluation of organic dyes degradation. AIP Conf. Proc. 2016, 1724, 020078. [Google Scholar]
- Kosmulski, M. The significance of the points of zero charge of zirconium (hydr) oxide reported in the literature. J. Dispers. Sci. Technol. 2002, 23, 529–538. [Google Scholar] [CrossRef]
- Rasheed, S.; Batool, Z.; Intisar, A.; Riaz, S.; Shaheen, M.; Kousar, R. Enhanced photodegradation activity of cuprous oxide nanoparticles towards Congo red for water purification. Desalination Water Treat. 2021, 227, 330–337. [Google Scholar] [CrossRef]
- Biru, M.; Qaderi, J.; Mamat, C.; Jalil, A.A. Preparation and characterization of copper, iron, and nickel doped titanium dioxide photocatalysts for decolorization of methylene blue. Sains Malays. 2021, 50, 135–149. [Google Scholar] [CrossRef]
- Prasannalakshmi, P.; Shanmugam, N. Fabrication of TiO2/ZnS nanocomposites for solar energy mediated photocatalytic application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 1–10. [Google Scholar] [CrossRef]
- Soleimani, F.; Rahmani, M.B. Optimizing Photocatalytic Efficiency for MB Dye Degradation Through Sol-Gel Synthesized ZrO2/Anatase-TiO2 Nanocomposites. J. Clust. Sci. 2025, 36, 1–16. [Google Scholar] [CrossRef]
- Supriya, G.; Douglas, S.P. Effective Visible Light Photodegradation of Alizarin red S dye and Antibacterial Activity Studies with Magnetically Separable NiFe2O4/TiO2/ZrO2 Ternary Nanocomposites. Results Surf. Interfaces 2025, 20, 100566. [Google Scholar] [CrossRef]
- Arjun, A.; Dharr, A.; Raguram, T.; Rajni, K. Study of copper doped zirconium dioxide nanoparticles synthesized via sol–gel technique for photocatalytic applications. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4989–4998. [Google Scholar] [CrossRef]
- Abdi, J.; Yahyanezhad, M.; Sakhaie, S.; Vossoughi, M.; Alemzadeh, I. Synthesis of porous TiO2/ZrO2 photocatalyst derived from zirconium metal organic framework for degradation of organic pollutants under visible light irradiation. J. Environ. Chem. Eng. 2019, 7, 103096. [Google Scholar] [CrossRef]
- Mitoraj, D.; Lamdab, U.; Kangwansupamonkon, W.; Pacia, M.; Macyk, W.; Wetchakun, N.; Beranek, R. Revisiting the problem of using methylene blue as a model pollutant in photocatalysis: The case of InVO4/BiVO4 composites. J. Photochem. Photobiol. A Chem. 2018, 366, 103–110. [Google Scholar] [CrossRef]
- Rochkind, M.; Pasternak, S.; Paz, Y. Using dyes for evaluating photocatalytic properties: A critical review. Molecules 2014, 20, 88–110. [Google Scholar] [CrossRef]
- Turki Jalil, A.; Emad Al Qurabiy, H.; Hussain Dilfy, S.; Oudah Meza, S.; Aravindhan, S.; M Kadhim, M.; M Aljeboree, A. CuO/ZrO2 nanocomposites: Facile synthesis, characterization and photocatalytic degradation of tetracycline antibiotic. J. Nanostructures 2021, 11, 333–346. [Google Scholar]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.F.; Trevelin, L.C.; Lima, A.S.; Meloni, G.N.; Bertotti, M.; Hammer, P.; Bertazzoli, R.; Lanza, M.R. Synthesis and characterization of ZrO2/C as electrocatalyst for oxygen reduction to H2O2. Electrocatalysis 2017, 8, 189–195. [Google Scholar] [CrossRef]
- Baran, T.; Visibile, A.; Busch, M.; He, X.; Wojtyla, S.; Rondinini, S.; Minguzzi, A.; Vertova, A. Copper oxide-based photocatalysts and photocathodes: Fundamentals and recent advances. Molecules 2021, 26, 7271. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Araque, D.; Ramírez-Ortega, D.; Acevedo-Peña, P.; Tzompantzi, F.; Calderón, H.A.; Gómez, R. Interfacial charge-transfer process across ZrO2-TiO2 heterojunction and its impact on photocatalytic activity. J. Photochem. Photobiol. A Chem. 2017, 335, 276–286. [Google Scholar] [CrossRef]
- Raghavan, N.; Thangavel, S.; Venugopal, G. Enhanced photocatalytic degradation of methylene blue by reduced graphene-oxide/titanium dioxide/zinc oxide ternary nanocomposites. Mater. Sci. Semicond. Process. 2015, 30, 321–329. [Google Scholar] [CrossRef]
- Yadav, S.; Jilani, A.; Sachan, S.; Kumar, P.; Ansari, S.A.; Afzal, M.; Ansari, M.O. Highly Efficient Visible-Light-Driven Photocatalysis of Rose Bengal Dye and Hydrogen Production Using Ag@Cu/TiO2 Ternary Nanocomposites. Chemistry 2024, 6, 489–505. [Google Scholar] [CrossRef]
- Mohamed, S.; Ma’amor, A.; Abdullah, F.Z.; Muhd Julkapli, N. Solar-driven photodegradation of synthetic dyes by ternary of titanium oxide-copper oxide-chitosan catalyst. J. Phys. Chem. Solids 2023, 181, 111517. [Google Scholar] [CrossRef]
- Bibi, S.; Shah, S.S.; Muhammad, F.; Siddiq, M.; Kiran, L.; Aldossari, S.A.; Sheikh Saleh Mushab, M.; Sarwar, S. Cu-doped mesoporous TiO2 photocatalyst for efficient degradation of organic dye via visible light photocatalysis. Chemosphere 2023, 339, 139583. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Lin, L.-Y. Synthesis of ternary metal oxides for battery-supercapacitor hybrid devices: Influences of metal species on redox reaction and electrical conductivity. ACS Appl. Energy Mater. 2018, 1, 2979–2990. [Google Scholar] [CrossRef]
- Shen, Y.; Han, S.; Xu, Q.; Wang, Y.; Xu, Z.; Zhao, B.; Zhang, R. Optimizing degradation of Reactive Yellow 176 by dielectric barrier discharge plasma combined with TiO2 nano-particles prepared using response surface methodology. J. Taiwan Inst. Chem. Eng. 2016, 60, 302–312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).