Abstract
The design of efficient and stable visible-light-driven photocatalysts is paramount for sustainable hydrogen (H2) evolution and the degradation of organophosphorus pesticides, exemplified by dichlorvos (DDVP). In this work, we synthesized a co-catalyst-free nanocomposite photocatalyst composed of Al6Si2O13 (ASO) and Cd8.05Zn1.95S10 (ZCS). By constructing a Type-I heterojunction, the optimized ASO/ZCS-1 nanocomposite (ASO loading ratio: 30%) enhanced visible-light-driven H2 evolution activity (5.1 mmol g−1 h−1), nearly doubling that of pristine ZCS (2.7 mmol g−1 h−1). Stability assessments revealed catalytic durability for ASO/ZCS-1 over five successive cycles, whereas the activity of pure ZCS precipitously declined to 59.7% of its initial level. Additionally, ASO, ZCS, and ASO/ZCS-2 (ASO loading ratio: 50%) demonstrated notable photocatalytic efficiency toward DDVP degradation without any co-catalyst, reducing DDVP concentration to 56.2% (ASO), 18.9% (ASO/ZCS-2), and 38.4% (ZCS), with corresponding degradation stability of 93.8%, 95.1%, and 93.8%, respectively. These results underscore the superior photocatalytic activity and stability of ASO, ZCS, and ASO/ZCS in the remediation of organophosphorus pesticides, with the Type-I heterojunction structure of ASO/ZCS enhancing both degradation activity and stability. Comprehensive characterizations by X-ray photoelectron spectroscopy (XPS), ultraviolet–visible diffuse reflectance spectroscopy (UV–vis DRS), and differential charge density analyses verified the Type-I heterojunction charge-transfer mechanism, effectively suppressing charge recombination and thus improving photocatalytic performance. Consequently, ASO/ZCS nanocomposites exhibit significant promise for broad applications in sustainable H2 production, pollutant degradation, and ensuring food and agricultural product safety.
1. Introduction
With the relentless rise in global energy consumption, escalating environmental pollution, and growing concerns over food safety—particularly from pesticide residues such as dichlorvos (DDVP)—developing highly efficient, stable, and environmentally benign photocatalysts for sustainable hydrogen (H2) production and pollutant degradation has emerged as an imperative research frontier [1,2,3,4,5,6]. Visible-light-driven photocatalytic H2 evolution, leveraging solar irradiation to achieve water splitting, has garnered extensive attention owing to its advantages of cleanliness, efficiency, and sustainability [7,8,9,10,11,12,13,14,15]. Concurrently, the effective degradation of organophosphorus pesticides like DDVP using photocatalysis represents a pivotal approach to safeguarding environmental and food security. Specifically, persistent residues of organophosphorus pesticides not only pose significant ecological threats but also directly compromise human health, thereby necessitating cost-effective, efficient, and environmentally compatible remediation technologies. Nevertheless, conventional single-component photocatalysts, exemplified by g-C3N4 [4], Cu2O [8], and SnS [12], typically exhibit pronounced photogenerated carrier recombination, suboptimal visible-light absorption, and limited stability, markedly constraining their practical utility [16,17,18,19,20,21]. To surmount these intrinsic limitations, recent efforts have increasingly focused on strategies including heterojunction engineering, surface defect modulation, and precise energy-band tailoring to substantially enhance carrier separation, extend visible-light responsiveness, and bolster catalytic stability [22,23,24,25,26,27,28,29]. Accordingly, the present study sets out to rationally construct and mechanistically elucidate a robust, co-catalyst-free Type-I heterojunction photocatalyst capable of simultaneously achieving high-efficiency visible-light H2 evolution and rapid DDVP degradation, thereby addressing intertwined energy and environmental challenges within a unified platform.
Organic-inorganic hybrid materials, particularly those based on semiconductor sulfides like CdS and ZnS, have attracted significant interest due to their notable visible-light responsiveness and improved charge separation capabilities [16,17]. Previous studies have shown that introducing diethylenetriamine (DETA) as a chelating surface modifier markedly increases the solubility of sublimed sulfur (S) and enhances charge transport, thereby boosting catalytic stability and overall performance [14]. Anchored to the photocatalyst surface via strong coordination bonds, DETA suppresses particle aggregation and remains highly dispersed [14,15]; however, its dosage must be carefully optimized, because either excess or deficiency hinders substrate adsorption and electron transfer, ultimately compromising photocatalytic efficiency [16]. Moreover, oxide materials such as Al2O3 [18] and SiO2 [19], known for their exceptional structural robustness and favorable interfacial properties, serve as promising catalyst supports. Their high specific surface areas and tunable surface chemistry further facilitate efficient dispersion of active semiconductor phases, enhancing the accessibility of reactants to catalytic sites. Nonetheless, these single-component systems inherently suffer from rapid electron–hole recombination, thereby limiting their photocatalytic efficacy. To surmount these limitations, designing composite materials with well-defined heterojunction interfaces has become a pivotal strategy. Specifically, Type-I heterojunction structures, characterized by favorable band alignment, profoundly facilitate interfacial electron–hole separation and migration, thus substantially enhancing the photocatalytic performance [30,31,32,33]. Through precise regulation of the interfacial electronic structure, the Type-I built-in electric field effectively suppresses charge-carrier recombination, extends carrier lifetimes, and markedly enhances overall catalytic activity for both photocatalytic H2 evolution and pollutant degradation.
In pursuit of this objective, we have successfully synthesized a novel, co-catalyst-free nanocomposite photocatalyst composed of Al6Si2O13 (ASO) and Cd8.05Zn1.95S10 (ZCS), designated as ASO/ZCS. It is important to note that previous studies have revealed that, without the addition of DETA, S cannot be effectively dispersed, rendering efficient synthesis of the target compound unattainable. Therefore, the addition of DETA is indispensable to ensure the effective dispersion of S and the efficient synthesis of the desired compound [34,35]. The optimized ASO/ZCS-1 composite (with an ASO loading ratio of 30%) exhibited significantly enhanced visible-light-driven H2 evolution activity (5.1 mmol g−1 h−1), nearly doubling that of pure ZCS (2.7 mmol g−1 h−1). Additionally, ASO, ZCS, and ASO/ZCS-2 (ASO loading ratio: 50%) demonstrated superior photocatalytic activity in the degradation of DDVP, achieving remarkable reductions in DDVP concentrations to 56.2% (ASO), 18.9% (ASO/ZCS-2), and 38.4% (ZCS), with corresponding degradation stability values of 93.8%, 95.1%, and 93.8%, respectively. Comprehensive characterization techniques, including X-ray photoelectron spectroscopy (XPS), Ultraviolet–visible diffuse reflectance spectroscopy (UV–vis DRS), and differential charge density analyses, unequivocally confirmed the Type-I heterojunction mechanism, effectively mitigating charge recombination and enhancing catalytic performance. Therefore, the developed ASO/ZCS nanocomposites demonstrate substantial potential for widespread applications in sustainable H2 production, environmental remediation, and ensuring food and agricultural safety.
2. Results
2.1. Diagram of Materials Synthesis Process
Figure 1 illustrates the schematic hydrothermal synthesis route of the ASO/ZCS nanocomposite photocatalyst. In a typical preparation process, with ASO as the substrate, S, zinc chloride (ZnCl2), cadmium chloride dihydrate (CdCl2·2.5H2O), and an appropriate proportion of DETA were fully dissolved to form a precursor solution. The mixture was then subjected to a hydrothermal reaction at 100 °C for 5 h. After centrifugation, washing, and freeze-drying processes, the final ASO/ZCS nanocomposite with a Type-I heterojunction structure was successfully obtained. The specific proportions are listed in Table S1. The schematic clearly demonstrates the formation mechanism of the nanocomposite, involving progressive crystallization of precursor ions (Cd2+, Zn2+, S2−) assisted by DETA, ultimately forming a stable composite interface with the ASO substrate. Detailed experimental parameters can be found in Table S1.
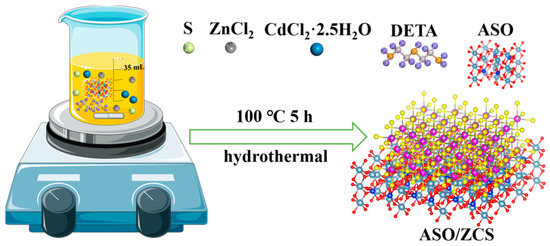
Figure 1.
ASO/ZCS nanocomposites synthesis flowchart.
2.2. Analysis of Phase and Microscopic Morphology
Figure 2a displays the X-ray diffraction (XRD) patterns of the samples. It is evident from the patterns that the characteristic diffraction peaks of the ASO sample align perfectly with the standard reference JCPDS No. 15-0776 for Al6Si2O13, indicating high purity and excellent crystallinity. Meanwhile, the diffraction peaks of the ZCS sample (red curve)—corresponding to the (100), (002), (101), (102), (110), (103), and (200) crystal planes—match well with the standard pattern of Cd8.05Zn1.95S10 (JCPDS No. 40-0835), as illustrated more clearly in Figure S1a, confirming the successful synthesis of ZCS. This indexing and the overall peak profile are consistent with those reported in the literature [36]. However, slight deviations from the standard pattern arise due to preferential crystal growth induced by the incorporation of DETA, which has been confirmed in previous studies. Furthermore, the ASO/ZCS-1 nanocomposite (green curve) exhibits characteristic diffraction peaks corresponding to both ASO and ZCS phases. The ASO/ZCS-2 nanocomposite (blue curve) demonstrates increasingly prominent ASO peaks as ASO content increases. Collectively, these observations confirm the coexistence of both ASO and ZCS phases within the composite materials.
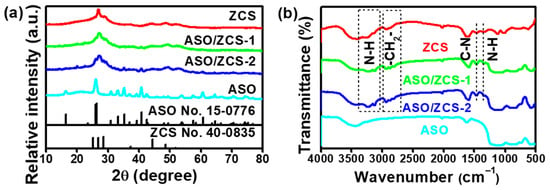
Figure 2.
(a) XRD patterns of ZCS, ASO/ZCS-1, ASO/ZCS-2 and ASO; (b) FT-IR spectra of ZCS, ASO/ZCS-1, ASO/ZCS-2 and ASO showing various characteristic functional groups.
Figure 2b presents the Fourier-transform (FT-IR) spectra of the samples in the 4000–500 cm−1 region. The ZCS spectrum (red) shows a broad band at ~3400 cm−1 and ~1630 cm−1 that originates from water vapor re-adsorbed by the high-surface-area powders and the hygroscopic KBr pellet during sample handling; superimposed on it, sharper absorptions at ~3400 cm−1 and ~1350 cm−1 correspond to the N-H stretching modes of DETA (C4H13N3). The band at ~2900–2700 cm−1 is assigned to –CH2– (from DETA) stretching, while the feature at ~1470 cm−1 arises from C-N (from DETA) stretching, confirming the incorporation of DETA into the ZCS lattice. The ASO/ZCS-1 (green) and ASO/ZCS-2 (blue) composites display the same N-H, –CH2–, and C-N signatures, indicating that DETA is retained in both hybrid systems, whereas these organic bands are absent in the pure ASO sample (cyan), proving that DETA exists only in the ZCS-containing phases.
Figure 3 presents the morphological and elemental distribution characteristics of pure ASO, pure ZCS, and the ASO/ZCS-1 nanocomposite, providing further evidence of the successful formation of the heterojunction structure between ASO and ZCS. As depicted in the scanning electron microscopy (SEM) images (Figure 3a), pure ASO exhibits densely aggregated clusters with particle sizes in the range of hundreds of nanometers. In contrast, pure ZCS (Figure 3b) displays distinct network-like or fibrous structures, characterized by fibers approximately tens of nanometers in diameter and extending several hundred nanometers in length. Such a unique fibrous network greatly enhances the specific surface area and exposure of active catalytic sites, thereby facilitating efficient adsorption and activation of reactant molecules, ultimately accelerating photocatalytic reaction kinetics and efficiency. Figure 3c illustrates the SEM morphology of the ASO/ZCS-1 nanocomposite, with yellow and red arrows indicating ASO and ZCS components, respectively. A clear nanoscale interwoven arrangement confirms the effective coupling between ASO and ZCS, thus demonstrating the successful formation of a closely interconnected heterojunction interface. Importantly, this tightly interwoven heterostructure not only provides abundant catalytic reaction sites but also facilitates rapid interfacial charge migration and effective separation, significantly improving interfacial charge-transfer efficiency and thus substantially boosting overall photocatalytic performance.
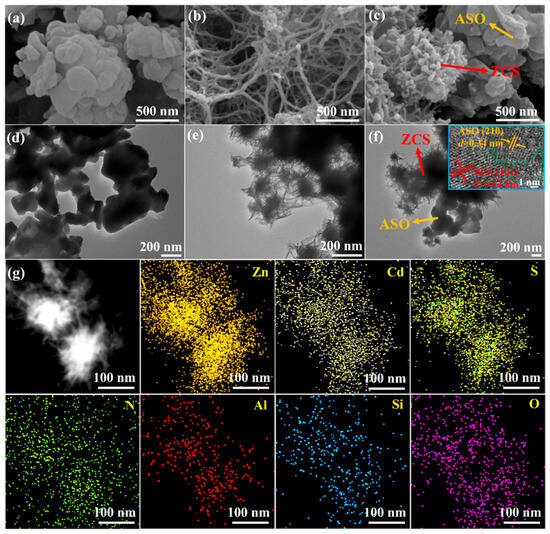
Figure 3.
SEM images of (a) ASO, (b) ZCS, (c) ASO/ZCS-1. TEM images of (d) ASO, (e) ZCS, (f) ASO/ZCS-1 (the inset in (f) is the HRTEM image of ASO/ZCS-1). (g) HAADF-STEM elemental mapping of the ASO/ZCS-1 nanocomposite, displaying the distributions of Zn, Cd, S, N, Al, Si, and O.
Further microstructural insights are provided by transmission electron microscopy (TEM) images shown in Figure 3d–f for ASO, ZCS, and the ASO/ZCS-1 nanocomposite, respectively. Notably, the high-resolution TEM (HRTEM) image inset in Figure 3f distinctly reveals lattice fringes of approximately 0.34 nm and 0.32 nm, corresponding to the (210) plane of ASO and the (101) plane of ZCS, respectively. Additionally, evident lattice distortion at the ASO/ZCS-1 interface underscores the effective formation of the heterojunction, which significantly induces interfacial electric fields and band bending, thereby enhancing spatial separation and efficient transport of photogenerated electron–hole pairs. A clearer view of the heterojunction interface is presented in Figure S1b.
Figure 3g presents the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) elemental mapping of the ASO/ZCS-1 nanocomposite, distinctly demonstrating the homogeneous distribution of Zn, Cd, S, N, Al, Si, and O throughout the composite structure. The uniform presence of nitrogen unequivocally confirms the successful incorporation of DETA into the composite. Such homogeneous elemental dispersion not only strengthens the organic–inorganic coupling between the ASO and ZCS phases but also maximizes the accessibility and utilization of active catalytic sites, thereby accelerating reactant diffusion and facilitating highly efficient interfacial reactions, which ultimately leads to markedly improved overall photocatalytic activity. Overall, Figure 3 clearly elucidates the successful nanoscale integration and structural synergy between ASO and ZCS, providing crucial structural evidence and theoretical guidance for enhanced photocatalytic performance.
2.3. XPS Analysis
Figure S1 and Figure 4 present XPS analyses, including survey spectra and high-resolution core-level spectra, of pure ZCS, pure ASO, and ASO/ZCS-1 nanocomposite materials to elucidate variations in elemental chemical states and interfacial electronic interactions during composite formation. Specifically, Figure S1c confirms the coexistence of ZCS and ASO elements within the ASO/ZCS-1 nanocomposite, whereas Figure S1d presents the N 1s spectrum that verifies DETA incorporation and reveals an ~0.20 eV negative binding-energy shift relative to pristine ZCS, originating from charge transfer at the heterostructure interface.
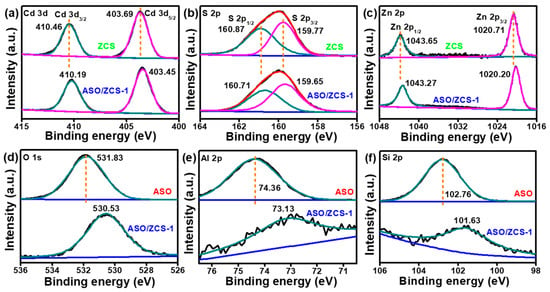
Figure 4.
XPS curves of (a) Cd 3d, (b) S 2p, (c) Zn 2p, (d) O 1s, (e) Al 2p and (f) Si 2p for ZCS, ASO, and ASO/ZCS-1.
Figure 4a–c detail the high-resolution spectra for Cd 3d, S 2p, and Zn 2p. Compared to pristine ZCS, the Cd 3d5/2 and Cd 3d3/2 peaks in ASO/ZCS-1 shift distinctly toward lower binding energies, suggesting increased electron cloud density around Cd atoms due to electron redistribution and interfacial coupling between ZCS and ASO. Similarly, the negative shifts in S 2p peaks imply enhanced chemical bonding interactions between S atoms and neighboring cations, and analogous negative shifts in Zn 2p3/2 and Zn 2p1/2 peaks further confirm substantial electronic coupling at the ASO/ZCS-1 interface.
Additionally, Figure 4d–f illustrate core-level spectra of O 1s, Al 2p, and Si 2p in the ASO component, which also exhibit varying negative shifts in the composite compared to pure ASO. These binding-energy down-shifts reveal strong interfacial contact and effective electron interactions, serving as spectroscopic evidence of a Type-I heterojunction in which photogenerated electrons preferentially migrate from the ASO domains into the ZCS lattice, thereby establishing an internal potential gradient that suppresses carrier recombination. Consequently, the observed interfacial electron redistribution significantly promotes spatial separation and efficient transfer of photogenerated electron–hole pairs, contributing to enhanced photocatalytic H2 production.
Analogously, Figure S2 reveals comparable phenomena. Figure S2a demonstrates that the ASO/ZCS-2 nanocomposite encompasses the full complement of elements from both ASO and ZCS, unequivocally attesting to the coexistence of the two compounds within the hybrid matrix. The N 1s spectrum in Figure S2b further corroborates the successful grafting of DETA. Moreover, Figure S2c–h show conspicuous negative shifts in each core-level binding energy of ASO/ZCS-2 relative to pristine ASO and ZCS, mirroring the observations described above and underscoring the prototypical Type-I heterojunction characteristics. In comparison with ASO/ZCS-1, however, the magnitude of the negative shift in ASO/ZCS-2 is slightly smaller, most likely because the particulate ASO phase tends to aggregate at higher loadings, resulting in interface saturation and partial electrostatic shielding that mitigates the binding-energy shift.
2.4. Photocatalytic H2 Evolution, DDVP Degradation Activity, and Stability Analysis
Figure 5 systematically presents the photocatalytic H2 evolution performance of pure ASO, pure ZCS, and the ASO/ZCS-1 nanocomposite, along with detailed analyses of their band structures and interfacial charge-transfer characteristics. Figure 5a compares the photocatalytic H2 evolution rates of the different materials, revealing that pure ZCS exhibits a H2 evolution rate of approximately 2.7 mmol g−1 h−1, whereas the ASO/ZCS-1 nanocomposite significantly enhances this rate to around 5.1 mmol g−1 h−1, representing an approximate 88.9% improvement compared to pure ZCS. Compared with recent mainstream literature reports, the photocatalytic performance demonstrated in this study stands at the forefront, as shown in Table S2 [17,36,37,38,39,40,41]. In contrast, pure ASO shows negligible H2 evolution activity, generating only trace amounts of H2. This indicates that the single-component ASO is inefficient for H2 evolution, whereas coupling ASO with ZCS markedly improves the photocatalytic performance. Moreover, Figure 5b illustrates the cumulative H2 production over five successive cycles, totaling 300 min of continuous illumination. The ASO/ZCS-1 nanocomposite maintains a consistently higher H2 evolution rate throughout, demonstrating exceptional photocatalytic stability under prolonged visible-light irradiation.
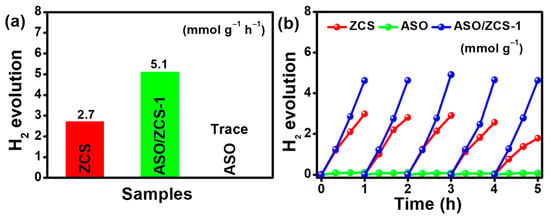
Figure 5.
(a) Photocatalytic H2 evolution activity of ZCS, ASO, and ASO/ZCS-1; (b) stability of H2 evolution for ZCS, ASO, and ASO/ZCS-1.
To investigate whether DETA undergoes leaching or chemical transformation during photocatalytic H2-evolution cycling, XRD and high-resolution N 1s XPS analyses were performed on ZCS before and after H2-evolution cycles (Figure S3). As shown in Figure S3a, the diffraction peaks retain their positions with only minor shifts and a moderate intensity enhancement after cycling, attributable to light-induced micro-recrystallization while the primary crystal structure and grain size remain intact. Figure S3b reveals an almost identical N 1s envelope, with merely a slight binding-energy shift, likewise reflecting subtle recrystallization-related adjustments in the local chemical environment. Collectively, the invariant XRD signatures and the stable N 1s profile demonstrate that DETA remains firmly coordinated to the ZCS surface after repeated cycles, exhibiting neither detectable leaching nor significant photochemical consumption, thereby preserving the structural integrity and cycling durability of the photocatalyst.
Figure 6 illustrates the photocatalytic degradation performance and stability of ASO, ASO/ZCS-2, and ZCS materials towards DDVP. Specifically, Figure 6a–c depict the photocatalytic activity of these catalysts within a single cycle of 140 min. Without employing any cocatalyst, the relative concentrations of DDVP were substantially reduced to 56.2% for ASO, 18.9% for ASO/ZCS-2, and 38.4% for ZCS. Evidently, the heterojunction composite material formed by integrating ASO with ZCS (ASO/ZCS-2) remarkably enhanced DDVP degradation efficacy. Compared with recent mainstream literature reports, our study holds a leading position in pesticide-degradation treatment, as shown in Table S3 [1,42,43,44,45,46]. Moreover, Figure 6d–f evaluate the stability of the photocatalytic degradation of DDVP over repeated cycles totaling 700 min. The data underscore that, even without any cocatalyst, ASO, ASO/ZCS-2, and ZCS demonstrate commendable stability, maintaining photocatalytic efficiencies of 93.8%, 95.1%, and 93.8%, respectively. For clarity, C0 denotes the initial DDVP concentration before illumination, whereas C0–C7 correspond to the residual concentrations measured at the first through eighth sampling intervals; C generically represents any one of these concentrations (C0–C7). Consequently, it is confirmed that the ASO/ZCS nanocomposite not only significantly accelerates the degradation rate of DDVP but also ensures exceptional catalytic stability.
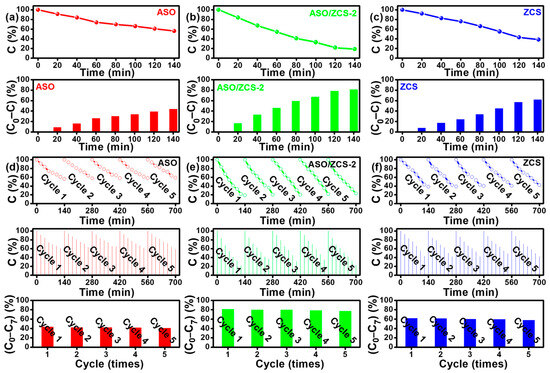
Figure 6.
DDVP degradation efficiency in a single 140 min cycle by (a) ASO, (b) ASO/ZCS-2, and (c) ZCS. Cycling stability of DDVP degradation over five consecutive cycles (700 min total) for (d) ASO, (e) ASO/ZCS-2 and (f) ZCS.
2.5. Optoelectronic Properties and Theoretical Calculations Analysis
Figure 7a displays the UV–vis DRS of the materials. Pure ASO primarily absorbs within the ultraviolet region, while ZCS exhibits distinct absorption in the visible-light range. The ASO/ZCS-1 and ASO/ZCS-2 nanocomposites exhibit absorption profiles intermediate between those of their individual constituents; because ASO/ZCS-1 contains less ASO than ASO/ZCS-2, its optical response leans more toward ZCS, while ASO/ZCS-2 more closely resembles ASO, a trend that is consistent with Figure 7a. Based on the Tauc plots (shown in Figure 7b) and relevant calculations [37] (as provided in Equation (1)), the band gaps of ZCS and ASO are determined to be approximately 2.68 eV and 3.74 eV, respectively, with conduction band positions at about −0.64 eV and −0.67 eV (as provided in Equation (2)) and valence band positions at approximately 2.04 eV and 3.07 eV (as provided in Equation (3)). Consequently, the band alignment at the ASO/ZCS interface clearly conforms to the typical Type-I heterojunction configuration.
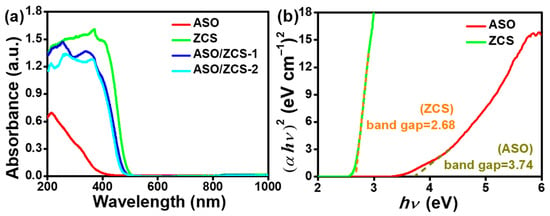
Figure 7.
(a) UV–vis DRS of the samples; (b) corresponding band gap derived from the Tauc plot.
To elucidate interfacial charge transfer at the ASO/ZCS heterojunction, differential charge density isosurfaces (Figure 8) reveal pronounced electron depletion within ASO (cyan and light blue) and accumulation on ZCS (yellow), reflecting the built-in electric field across the interface. The electric field—directed from ZCS toward ASO—drives photogenerated electrons from the higher-energy conduction band of ASO into the lower-lying conduction band of ZCS, while funneling holes into the valence band of ZCS, thereby suppressing electron–hole recombination. Notably, the electric field vector and the electron migration direction are antiparallel, underscoring the negative-charge nature of electron flow relative to the field orientation. This unidirectional carrier migration is characteristic of a Type-I heterojunction with a straddling band alignment: the conduction band of ZCS lies below that of ASO, and the valence band of ASO lies below the valence band of ZCS, confining both electrons and holes within ZCS. Such carrier localization prolongs lifetimes, accelerates interfacial charge-transfer kinetics, and enhances the composite’s photocatalytic activity. Together, these insights highlight the critical role of engineered band offsets and built-in field orientation in guiding charge dynamics and optimizing photocatalytic performance. Furthermore, by concentrating charge carriers at spatially separated active sites, this mechanism also promotes selective surface redox reactions and improves operational stability under illumination.
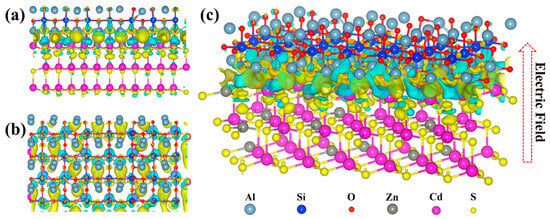
Figure 8.
Differential charge density analysis of the ASO/ZCS nanocomposite, revealing electron transfer at the heterojunction interface: (a) front view; (b) top view; (c) 3D side view.
2.6. Photocatalytic Mechanism
Figure 9 schematically illustrates the Type-I heterojunction photocatalytic mechanism of the ASO/ZCS nanocomposite under visible-light irradiation. Upon illumination with photons possessing energies equal to or greater than the respective band gaps, electrons in the EVB of ASO and ZCS are excited into their ECB, thereby generating electron–hole pairs. Owing to the optimal alignment of energy bands, the photogenerated electrons from the CB of ASO migrate preferentially towards the more negative CB of ZCS, effectively accumulating there and subsequently participating in efficient reduction reactions, such as proton reduction from H+ to H2 and the reduction in molecular oxygen (O2) to form superoxide radicals (·O2−). These reactive species further create a highly reductive environment at the catalytic interface, substantially enhancing photocatalytic H2 production efficiency. Concurrently, the photogenerated holes concentrate in the more positive VB of ZCS, engaging in oxidative reactions by H2O or hydroxide ions (OH−) into reactive hydroxyl radicals (·OH), which, along with the generated·O2−, can effectively oxidize pollutants such as DDVP into harmless substances like CO2 and H2O. Furthermore, the interfacial built-in electric field inherent to the Type-I heterojunction significantly suppresses charge recombination, prolongs carrier lifetimes, and ensures efficient transfer of photogenerated charges toward active reaction sites, thus markedly accelerating the overall catalytic reaction kinetics. Thus, the constructed Type-I heterojunction in ASO/ZCS distinctly elevates photocatalytic H2 production and pollutant degradation efficiencies, providing essential mechanistic insights and design principles for advanced heterostructured photocatalysts in sustainable energy conversion and environmental remediation applications.
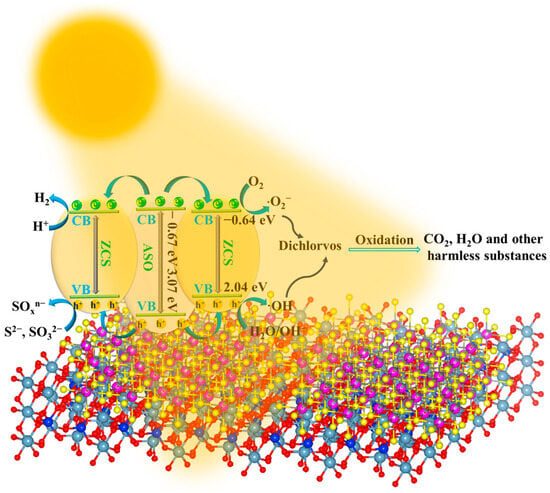
Figure 9.
Proposed Type-I heterojunction photocatalytic mechanism for the ASO/ZCS.
3. Experimental Section
3.1. Materials
The following chemicals were used in this work: Al6Si2O13 (commercially available), distilled water, S, ZnCl2, CdCl2·2.5H2O, sodium sulfide nonahydrate (Na2S·9H2O), sodium bisulfite (Na2SO3), DETA, DDVP.
3.2. Equations
The band gap (Eg), valence band (EVB), and conduction band (ECB) of the photocatalysts can be obtained from the following equation:
(αhv)2 = A(hv − Eg)
EVB = X − Ee + 0.5Eg
ECB = EVB − Eg
Here, α is the absorption coefficient, h represents Planck constant, ν represents light frequency and A is a constant. Ee is a constant with a value of 4.50 eV. The X values of Cd8.05Zn1.95S10 (ZCS) and Al6Si2O13 (ASO) are 5.20 and 5.70 eV, respectively.
3.3. Computational Details
Density function theory (DFT) calculations were performed by using the CP2K-2023.2 package. Perdew–Burke–Ernzerhof (PBE) of functional was used to describe the system. Unrestricted Kohn–Sham DFT was used as the electronic structure method in the framework of the Gaussian and plane waves (GPW) way. The Goedecker–Teter–Hutter (GTH) pseudopotentials and Triple-ζ molecularly optimized basis sets with two polarization functions (TZV2P-MOLOPT-GTH) were used for all elements. The Brillouin zone was sampled at the Γ-point for surface calculations. A plane-wave energy cutoff of 400 Ry (1Ry = 13.606 eV) has been employed. The geometries were optimized using the Broyden–Fletcher–Goldfarb–Shanno (BFGS) algorithm. The convergence threshold of density matrix during self-consistent field (SCF) method was 1 × 10−5 Hartree, and convergence criterion for the forces was set to 4.5 × 10−4 Bohr/Hartree [47]. A vacuum layer of 15 Å was constructed to eliminate interactions between periodic structures of surface models. The van der Waals (vdW) interaction was amended by the DFT-D3 method of Grimme.
3.4. Characterization
All samples were characterized by XRD (Bruker Corporation, Billerica, Massachusetts, USA, Bruker D8 Advance, Cu Kα, λ = 1.5406 Å; 40 kV, 40 mA; 2θ = 5–80°, 0.02° step, 8° min−1), XPS (VG Scientific Ltd., East Grinstead, West Sussex, UK, VG ESCALab MK II, monochromatic Mg Kα, hν = 1253.6 eV; 12 kV, 20 mA; ~1 × 10−8 Pa; survey 50 eV/1.0 eV step, high-resolution 20 eV/0.05 eV step), SEM (Hitachi High-Tech Corporation, Tokyo, Japan, Hitachi S-8100 field-emission, 5 kV; samples sputter-coated with ~10 nm Au), and TEM (FEI Company, Hillsboro, OR, USA, FEI Tecnai G2-20, 200 kV; powder dispersed in ethanol and dropped onto carbon-coated Cu grids). FT-IR infrared spectra were collected on an IR-21 (Shimadzu Corporation, Kyoto, Japan, KBr pellet, 4000–400 cm−1, 4 cm−1 resolution, 32 scans), and UV–vis DRS were measured with a Shimadzu UV-2450 equipped with an integrating sphere (Shimadzu Corporation, Kyoto, Japan, BaSO4 standard, 200–800 nm, 1 nm interval) and converted to Kubelka–Munk units. Photocatalytic H2 evolution was quantified on a GC-126N gas chromatograph under Xe-lamp irradiation (Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China, CEL-HXF300-T3, 300 W). DDVP degradation was tracked using a CXWL-NY-2280-6 detector (Yopusen Instrument Co., Ltd., Huizhou, Guangdong, China).
3.5. Evaluation of Photocatalytic Activity
H2 evolution: A 100 mL three-neck flask containing 50 mg catalyst, 1.60 g Na2SO3, and 4.20 g as sacrificial agents Na2S·9H2O in 50 mL deionized water was purged with high-purity N2 for 30 min before illumination. Gas samples were collected every 20 min during the first hour of irradiation and analyzed offline by gas chromatography. The Na2SO3/Na2S·9H2O pair rapidly consumes photogenerated holes and prevents photocorrosion, thereby steering the photogenerated electrons toward efficient H2 evolution.
DDVP degradation: In a 250 mL colorless beaker, 150 mL of DDVP solution was mixed with 100 mg catalyst by ultrasonic dispersion. The suspension, positioned 10 cm beneath the same xenon lamp, was sampled every 20 min (2.5 mL), centrifuged, and 100 µL of the supernatant was blended with 10 mL buffer. The amount of 2.5 mL aliquot of this mixture received 100 µL of cholinesterase and chromogenic reagent each, was vortexed, and incubated at 37 °C for 15 min; 100 µL substrate was then added and the solution was immediately transferred to a cuvette for absorbance measurement. After blank calibration, inhibition and degradation rates were calculated. The run was halted if abnormal increases or unstable fluctuations appeared. Strict measures prevented cross-contamination, and analytical results were obtained within three minutes of sampling.
4. Conclusions
In this work, a novel Al6Si2O13/Cd8.05Zn1.95S10 (ASO/ZCS) nanocomposite photocatalyst was successfully synthesized, exhibiting significantly enhanced photocatalytic hydrogen (H2) evolution and dichlorvos (DDVP) degradation through a robust Type-I heterojunction. Under visible-light irradiation without any cocatalyst, the optimized ASO/ZCS composite (ASO/ZCS-1, ASO loading ratio: 30%) achieved a remarkable H2 evolution rate of approximately 5.1 mmol g−1 h−1, substantially outperforming pure ASO (trace activity) and pristine ZCS (2.7 mmol g−1 h−1). Additionally, ASO, ZCS, and ASO/ZCS-2 (ASO loading ratio: 50%) exhibited outstanding photocatalytic activity toward DDVP degradation, significantly reducing its relative concentration to 56.2% (ASO), 18.9% (ASO/ZCS-2), and 38.4% (ZCS), respectively. Cycling stability tests further confirmed the sustained catalytic performance of ASO/ZCS over five successive cycles. Comprehensive characterization by X-ray photoelectron spectroscopy (XPS), Ultraviolet–visible diffuse reflectance spectroscopy (UV–vis DRS), and differential charge density analysis validated the effective formation of a stable Type-I heterojunction at the ASO/ZCS interface. This heterostructure facilitated efficient charge separation and migration, markedly suppressed electron–hole recombination, and significantly elevated photocatalytic efficiency. Collectively, these findings underscore the substantial potential of the ASO/ZCS nanocomposite, leveraging its unique Type-I heterojunction mechanism, as an advanced, stable, and efficient catalyst for sustainable H2 production, pollutant remediation, and food safety applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15060564/s1, Figure S1: (a) XRD pattern of ZCS and reference JCPDS card. (b) The HRTEM image of ASO/ZCS-1. (c) XPS survey spectra of ASO, ZCS, and ASO/ZCS-1. (d) XPS spectra of N 1s for ZCS and ASO/ZCS-1. Figure S2: (a) XPS survey spectra of ASO, ZCS, and ASO/ZCS-2. XPS curves of (b) N 1s, (c) Cd 3d, (d) S 2p, (e) Zn 2p, (f) O 1s, (g) Al 2p, and (h) Si 2p for ZCS, ASO, and ASO/ZCS-2. Figure S3: (a) XRD patterns of ZCS before and after photocatalytic cycling; (b) high-resolution N 1s XPS spectra of ZCS before and after cycling. Table S1: The amounts of chemicals for preparing ZCS and ASO/ZCS. Table S2: Comparative hydrogen evolution efficiencies of photocatalysts. Table S3: Comparative pesticide degradation efficiencies of photocatalysts.
Author Contributions
Conceptualization, Z.L. (Zhenhua Li) and Z.L. (Zhen Li); methodology, Z.L. (Zhenhua Li); software, Z.L. (Zhenhua Li) and A.M.; validation, A.M. and W.L.; formal analysis, G.X.; investigation, M.Y.; resources, Z.L. (Zhen Li) and Y.M.; data curation, Z.L. (Zhenhua Li); writing—original draft preparation, Z.L. (Zhenhua Li); writing—review and editing, Z.L. (Zhenhua Li) and Z.L. (Zhen Li); visualization, Z.L. (Zhenhua Li); supervision, Z.L. (Zhen Li); project administration, Z.L. (Zhenhua Li) and Z.L. (Zhen Li); funding acquisition, Z.L. (Zhen Li) and Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from several sources: the National Natural Science Foundation of China (42407636), the Natural Science Research Project for Colleges and Universities in Anhui Province (2023AH051861), the Talent Introduction Foundation of Anhui Science and Technology University (SPYJ202201), the Key Laboratory of materials and surface technology, Ministry of Education (xxx-2024-zc003, xxx-2024-zc002), the Open Project of Engineering Research Center of Biofilm Water Purification and Utilization Technology of Ministry of Education (BWPU2023KF06).
Data Availability Statement
The data presented in this study can be obtained from the corresponding author.
Conflicts of Interest
Author Yaqiang Meng was employed by the company Heilongjiang Environmental Science and Technology Testing Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Tang, Q.; Chen, W.; Lv, Y.; Yang, S.; Xu, Y. Z-scheme hierarchical Cu2S/Bi2WO6 composites for improved photocatalytic activity of glyphosate degradation under visible-light irradiation. Sep. Purif. Technol. 2020, 236, 116243. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Ji, G.; Wang, S.; Mo, C.; Ding, B. Lung regeneration: Diverse cell types and the therapeutic potential. MedComm 2024, 5, e494. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, G.; Kumar, M.; Kumar, A.; Singh, P.; Ansu, A.; Sharma, A.; Alam, T.; Yadav, A.; Dobrota, D. Nanocomposite Marvels: Unveiling Breakthroughs in Photocatalytic Water Splitting for Enhanced Hydrogen Evolution. ACS Omega 2024, 9, 6147–6164. [Google Scholar] [CrossRef]
- Lu, Y.; Zhuang, Z.; Li, L.; Chen, F.; Wei, P.; Yu, Y. Advancements and challenges in g-C3N4/ZnIn2S4 heterojunction photocatalysts. J. Mater. Chem. A 2025, 13, 4718–4745. [Google Scholar] [CrossRef]
- Shuaibu, A.; Hafeez, H.; Mohammed, J.; Dankawu, U.; Ndikilar, C.; Suleiman, A. Progress on g-C3N4 based heterojunction photocatalyst for H2 production via Photocatalytic water splitting. J. Alloys Compd. 2024, 1002, 175062. [Google Scholar] [CrossRef]
- Tahir, M.; Ajiwokewu, B.; Bankole, A.; Ismail, O.; Al-Amodi, H.; Kumar, N. MOF based composites with engineering aspects and morphological developments for photocatalytic CO2 reduction and hydrogen production: A comprehensive review. J. Environ. Chem. Eng. 2023, 11, 109408. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, M.; Wang, Y.; Liu, H.; Manor, B.; Bao, D.; Zhang, L.; Zhou, J. Effects of molecular hydrogen supplementation on fatigue and aerobic capacity in healthy adults: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1094767. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, J.; Guo, W.; Chen, H.; Li, J.; Jing, D.; Luo, B.; Ma, L. A Type-I Heterojunction by Anchoring Ultrafine Cu2O on Defective TiO2 Framework for Efficient Photocatalytic H2 Production. Ind. Eng. Chem. Res. 2023, 62, 1310–1321. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Bo, C.; Sun, Y.; Li, C.; Piao, L. Precise design of TiO2 photocatalyst for efficient photocatalytic H2 production from seawater splitting. Int. J. Hydrogen Energy 2024, 55, 542–549. [Google Scholar] [CrossRef]
- Fan, Q.; Yan, Z.; Li, J.; Xiong, X.; Li, K.; Dai, G.; Jin, Y.; Wu, C. Interfacial-electric-field guiding design of a Type-I FeIn2S4@ZnIn2S4 heterojunction with ohmic-like charge transfer mechanism for highly efficient solar H2 evolution. Appl. Surf. Sci. 2024, 663, 160206. [Google Scholar] [CrossRef]
- Han, S.; Wang, Z.; Yu, J.; Wang, F.; Li, X. Conversion of SOD zeolite into type I porous liquid and preparation of mixed matrix membrane with AO-PIM for efficient gas separation. J. Clean. Prod. 2024, 448, 141737. [Google Scholar] [CrossRef]
- Jia, X.; Lu, Y.; Du, K.; Zheng, H.; Mao, L.; Li, H.; Ma, Z.; Wang, R.; Zhang, J. Interfacial Mediation by Sn and S Vacancies of p-SnS/n-ZnIn2S4 for Enhancing Photocatalytic Hydrogen Evolution with New Scheme of Type-I Heterojunction. Adv. Funct. Mater. 2023, 33, 2304072. [Google Scholar] [CrossRef]
- Jin, N.; Sun, Y.; Shi, W.; Wang, P.; Nagaoka, Y.; Cai, T.; Wu, R.; Dube, L.; Nyiera, H.; Liu, Y.; et al. Type-I CdS/ZnS Core/Shell Quantum Dot-Gold Heterostructural Nanocrystals for Enhanced Photocatalytic Hydrogen Generation. J. Am. Chem. Soc. 2023, 145, 21886–21896. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Lv, J.; Zhang, J.; Zhu, G.; Geng, L.; Liang, C. Efficient Visible-Light-Driven Splitting of Water into Hydrogen over Surface-Fluorinated Anatase TiO2 Nanosheets with Exposed {001} Facets/Layered CdS–Diethylenetriamine Nanobelts. ACS Sustain. Chem. Eng. 2018, 6, 12817–12826. [Google Scholar] [CrossRef]
- Janczak, J. Coordination Properties of Diethylenetriamine in Relation to Zinc Phthalocyanine. Polyhedron 2020, 178, 114313. [Google Scholar] [CrossRef]
- Lv, J.; Liu, J.; Zhang, J.; Dai, K.; Liang, C.; Wang, Z.; Zhu, G. Construction of Organic–Inorganic Cadmium Sulfide/Diethylenetriamine Hybrids for Efficient Photocatalytic Hydrogen Production. J. Colloid Interface Sci. 2018, 512, 77–85. [Google Scholar] [CrossRef]
- Li, W.; Meng, A.; Li, Z.; Zhang, J.; Fu, J. S-scheme CeO2/Cd7.23Zn2.77 S10-DETA heterojunctions for superior cocatalyst-free visible-light photocatalytic hydrogen evolution. J. Cent. South Univ. 2024, 31, 4572–4585. [Google Scholar] [CrossRef]
- Kanchana, V.; Vasanthan, S.; Mayavan, L.; Kistan, A. A Novel Mesoporous Al2O3@Graphene Composite as Photocatalyst for Organic Pollutant Removal. J. Electron. Mater. 2025, 54, 2122–2134. [Google Scholar] [CrossRef]
- Winayu, B.; Shen, B.; Chu, H. Impact of gas composition (CO, H2, and HCl) on chemical looping combustion by SiO2 supported oxygen carriers. Mater. Chem. Phys. 2024, 320, 129475. [Google Scholar] [CrossRef]
- Li, M.; Ren, T.; Li, Y.; Li, S.; He, P.; Xiao, Y.; Chen, J. Constructing CdIn2S4/ZnS type-I band alignment heterojunctions by decorating CdIn2S4 on ZnS microspheres for efficient photocatalytic H2 evolution. Int. J. Hydrogen Energy 2023, 48, 37224–37233. [Google Scholar] [CrossRef]
- Zhang, X.; Puttaswamy, M.; Bai, H.; Hou, B.; Verma, S. CdS/ZnS core-shell nanorod heterostructures co-deposited with ultrathin MoS2 cocatalyst for competent hydrogen evolution under visible-light irradiation. J. Colloid Interface Sci. 2024, 665, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Dai, X.; Song, J.; Pu, K.; Tang, J.; Qin, X.; Wang, F.; Guo, Y.; Zhao, T.; Lei, T. Electronic and optical characteristics of Silicane/GeAs van der Waals heterostructures: Effects of external electric field and biaxial strain: A first-principles study. Phys. E Low-Dimens. Syst. Nanostruct. 2023, 153, 115759. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, F.; Zhao, J.; Zhang, H.; Wang, S. Diffusion-controlled charge separation in conjugated polymer heterojunctions for visible light-driven hydrogen production. Sep. Purif. Technol. 2025, 360, 131255. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Chen, C.; Ma, T.; Zhang, J.; Wang, Z. Transforming the Charge Transfer Mechanism in the In2O3/CdSe-DETA Nanocomposite from Type-I to S-Scheme to Improve Photocatalytic Activity and Stability During Hydrogen Production. Acta Phys.-Chim. Sin. 2022, 39, 2208030. [Google Scholar]
- Xu, D.; Shen, L.; Qin, Z.; Yan, S.; Wang, N.; Wang, J.; Gao, Y. Construction of Reverse Type-II InP/ZnxCd1-xS Core/Shell Quantum Dots with Low Interface Strain to Enhance Photocatalytic Hydrogen Evolution. Inorg. Chem. 2024, 63, 12582–12592. [Google Scholar] [CrossRef]
- Yang, L.; Tian, Q.; Wang, X.; Yang, H.; Meng, A.; Li, Z. Interfacial-Engineered Co3S4/MnCdS Heterostructure for Efficient Photocatalytic Hydrogen Evolution. Sol. RRL 2023, 7, 2300403. [Google Scholar] [CrossRef]
- Yuan, L.; Su, J.; Zhang, M.; Wang, D.; Zhang, H.; Ma, J.; Gong, J. The electronic structures and hydrogen adsorption properties of a new graphene-like AlNC2 monolayer: First-principles calculations. Int. J. Hydrogen Energy 2024, 59, 1054–1062. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, P.; Xue, Y.; Wei, Y. Theoretical Study on Hydrogen Storage and Promoter Effect of Binary Clathrate Hydrates. Chem. J. Chin. Univ. 2024, 45, 20230383. [Google Scholar]
- Zhang, L.; He, J.; Li, N.; Yuan, J.; Li, W.; Liu, P.; Yan, T. Ternary CdS@MoS2-Co3O4 Multiheterojunction Photocatalyst for Boosting Photocatalytic H2 Evolution. ACS Appl. Mater. Interfaces 2023, 15, 43790–43798. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, X.; Wang, H.; Ma, Y.; Huang, H.; Ma, T. Interfacial engineering of Bi2MoO6-BaTiO3 Type-I heterojunction promotes cocatalyst-free piezocatalytic H2 production. Nano Energy 2024, 121, 109206. [Google Scholar] [CrossRef]
- Zhang, S.; Du, S.; Han, Z.; Wang, Y.; Jiang, T.; Wu, S.; Chen, C.; Han, Q.; Suo, S.; Xu, H.; et al. Ohmic-functionalized type I heterojunction: Improved alkaline water splitting and photocatalytic conversion from CO2 to C2H2. Chem. Eng. J. 2023, 471, 144438. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Huang, F.; Xie, H.; Li, Q.; Fang, C. A mechanism study of type I corrosion on the surface of ancient tin rich bronzes. Herit. Sci. 2024, 12, 349. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Li, P.; Yang, Y.; Zhu, L. Pd-CQDs/CdS ternary composite for highly efficient visible light driven H2 evolution under combined action of type I heterojunctions and Schottky junctions. Int. J. Hydrogen Energy 2024, 85, 673–682. [Google Scholar] [CrossRef]
- Huo, Y.; Li, Z.; Zhang, J.; Dai, K.; Liang, C.; Yang, Y. Defect-mediated electron–hole separation in an inorganic–organic CdSxSe1-x-DETA solid solution under amine molecule-assisted fabrication and microwave-assisted method for promoting photocatalytic H2 evolution. Sustain. Energy Fuels 2019, 3, 3550–3560. [Google Scholar] [CrossRef]
- Li, Z.; Jin, D.; Wang, Z. ZnO/CdSe–diethylenetriamine nanocomposite as a step-scheme photocatalyst for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 529, 147071. [Google Scholar] [CrossRef]
- Li, Z.; Meng, A.; Sang, X.; Li, W.; Zhang, J.; Wang, Z. Efficient and Stable Hydrogen Evolution from ZnWO4/Zn1.95Cd8.05S10-DETA via S-Scheme Heterojunction under Visible-Light Irradiation without Co-Catalysts. Int. J. Hydrogen Energy 2024, 51, 777–786. [Google Scholar] [CrossRef]
- Meng, A.; Yang, R.; Li, W.; Li, Z.; Zhang, J. Enhanced photocatalytic hydrogen production through tuning charge transfer in TiO2/CdSxSe1-x-DETA nanocomposites with S-scheme heterojunction structure. J. Mater. 2025, 11, 100919. [Google Scholar]
- Long, Z.; Yang, X.; Huo, X.; Li, X.; Qi, Q.; Bian, X.; Wang, Q.; Yang, F.; Yu, W.; Jang, L. Bioinspired Z-scheme In2O3/C3N4 heterojunctions with tunable nanorod lengths for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2023, 461, 141893. [Google Scholar] [CrossRef]
- Chen, R.; Bai, X.; Luo, Y.; Qian, L.; Ma, M.; She, X. Rational designing of dual-functional photocatalysts for simultaneous hydrogen generation and organic pollutant degradation over Cd0.5Mn0.5S/CoP. Int. J. Hydrogen Energy 2022, 47, 32921–32927. [Google Scholar] [CrossRef]
- Tao, J.; Wang, M.; Liu, G.; Liu, Q.; Lu, L.; Wan, N.; Tang, H.; Qiao, G. Efficient photocatalytic hydrogen evolution coupled with benzaldehyde production over 0D Cd0.5Zn0.5S/2D Ti3C2 Schottky heterojunction. J. Adv. Ceram. 2022, 11, 1117–1130. [Google Scholar] [CrossRef]
- Liu, H.; Tan, P.; Liu, Y.; Zhai, H.; Du, W.; Liu, X.; Pan, J. Ultrafast interfacial charge evolution of the type-II cadmium sulfide/molybdenum disulfide heterostructure for photocatalytic hydrogen production. J. Colloid Interface Sci. 2022, 619, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Li, W.; Li, Z.; Xiong, G.; Pu, X.; Zhang, J.; Li, Z. S-scheme heterojunction BiOBr/Cd8.05Zn1.95S10-DETA nanocomposite photocatalyst for enhanced and stable degradation of dichlorvos. Surf. Interfaces 2025, 68, 106697. [Google Scholar] [CrossRef]
- Li, W.; Meng, A.; Li, C.; Sun, Y.; Zhang, J.; Li, Z. Enhanced efficiency and stability in the degradation of triazophosphorus pesticides by Al6Si2O13/WO2.72 nanocomposites through synergistic action of S-scheme heterojunction and oxygen vacancies. J. Colloid Interface Sci. 2025, 677, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, C.; Yang, R.; Cheng, S.; Sang, X.; Zhang, M.; Zhang, J.; Wang, Z.; Li, Z. Efficient and stable degradation of triazophos pesticide by TiO2/WO3 nanocomposites with S-scheme heterojunctions and oxygen defects. Catalysts 2023, 13, 1136. [Google Scholar] [CrossRef]
- Isari, A.; Moradi, S.; Rezaei, S.; Ghanbari, F.; Dehghanifard, E.; Kakavandi, B. Peroxymonosulfate catalyzed by core/shell magnetic ZnO photocatalyst towards malathion degradation: Enhancing synergy, catalytic performance and mechanism. Sep. Purif. Technol. 2021, 275, 119163. [Google Scholar] [CrossRef]
- Tang, Q.; Luo, X.; Yang, S.; Xu, Y. Novel Z-scheme In2S3/BiVO4 composites with improved visible-light photocatalytic performance and stability for glyphosate degradation. Sep. Purif. Technol. 2020, 248, 117039. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).