Recent Advances in PDI-Based Heterojunction Photocatalysts for the Degradation of Organic Pollutants and Environmental Remediation
Abstract
1. Introduction
2. Principles and Types of Heterojunctions
3. Design and Construction of PDI-Based Heterojunction Photocatalysts
3.1. Properties of PDI
3.2. The Role of PDI in Heterojunctions and Construction Strategies
3.2.1. The Role of PDI in Heterojunctions
3.2.2. The Interface Coupling of PDI Functional Groups with Heterogeneous Materials
3.2.3. Preparation Methods for PDI-Based Composite Photocatalysts
4. Application of PDI-Based Heterojunctions in Pollutant Degradation
4.1. Antibiotics and Drug Residues
| Photocatalyst | Synthesis Method | Antibiotics | Light Source | Time (min) | Efficiency (%) | Type | Reference |
|---|---|---|---|---|---|---|---|
| CNPDI | In situ self-assembly | TC | Visible | 60 | 83.6 | II | Wu et al. [101] |
| CPM | Surface growth, solvothermal | TC | Visible | 60 | 90.0 | II | Li et al. [102] |
| PDI/FM88B | Water bath heating | TC | Visible | 30 | 89.0 | II | Wu et al. [103] |
| I-PDI/PEDOT-M | Surface self-corrosion- assisted rapid spin- coating | TC | Visible | 60 | 73.7 | II | Lu et al. [104] |
| PDI/BiOCl-BiPO4 | Ultrasonic, hydrothermal | TC | Simulated sunlight | 150 | 81.0 | Z | Zhuang et al. [105] |
| PDI/FePc | In situ self-assembly | TC | Visible | 60 | 78.6 | Z | Shi et al. [106] |
| WO3@Cu@PDI | Photo-deposition, water bath heating | TC | Visible | 15 | 75.0 | Z | Zeng et al. [107] |
| PDI/WO3/α-Fe2O3 | PLAL | TC | 254 nm | 150 | 94.2 | Z | Mao et al. [108] |
| PDI/MIL-53(Fe) | Solvent thermal | TC | Visible | 30 | 94.1 | Z | Chen et al. [92] |
| TMOP | Electrospinning | TC | Simulated sunlight | 80 | 91.2 | Z | Sun et al. [109] |
| Ag3PO4/PDIsm | Self-assembly | TC | Visible | 8 | 82.8 | Z | Cai et al. [110] |
| PANI/PDI | In situ growth | TC | Visible | 120 | 70.0 | Z | Dai et al. [90] |
| PDI-Ala/S-C3N4 | In situ self-assembly | TC | Visible | 90 | 90.0 | S | Li et al. [111] |
| IM-NSH-PM | Imprint | TC | Simulated sunlight | 60 | 71.9 | S | Lu et al. [112] |
| PDIs/C, N, S-CeO2 | Oil bath heating | TC | Visible | 30 | 80.1 | S | Jing et al. [113] |
| PDIs/Fe2O3@C | Hydrothermal calcination, oil bath heating | TC | Visible | 8 | 78.9 | S | Jing et al. [114] |
| PDI/ZnFe2O4 | Ultrasonic | TC | Visible | 60 | 66.7 | S | Xu et al. [115] |
| Ag/PCN/UPDI | Self-assembly, photoreduction | OTC | Visible | 150 | 97.5 | S | Xiao et al. [116] |
| g-C3N4/PDI/Co-Fe | Co-precipitation | DOX | Visible | 60 | 96.1 | Z | Li et al. [117] |
| PDI-Urea/BiOBr | Solvent thermal, in situ growth | OFLO | Visible | 150 | 93.0 | S | Wang et al. [118] |
| Bis-PDI-T@TiO2 | Double-solvent phase transfer | CBZ | Visible | 30 | 100.0 | Z | Yang et al. [119] |
| β-PDI/MIL-101(Fe) | Grinding | SMX | Visible | 6 | 99.7 | Z | Jia et al. [120] |
| MIL-101(Fe)-NH2/PDI | Grinding | SMX | Visible | 5 | 99.2 | Z | Jia et al. [121] |
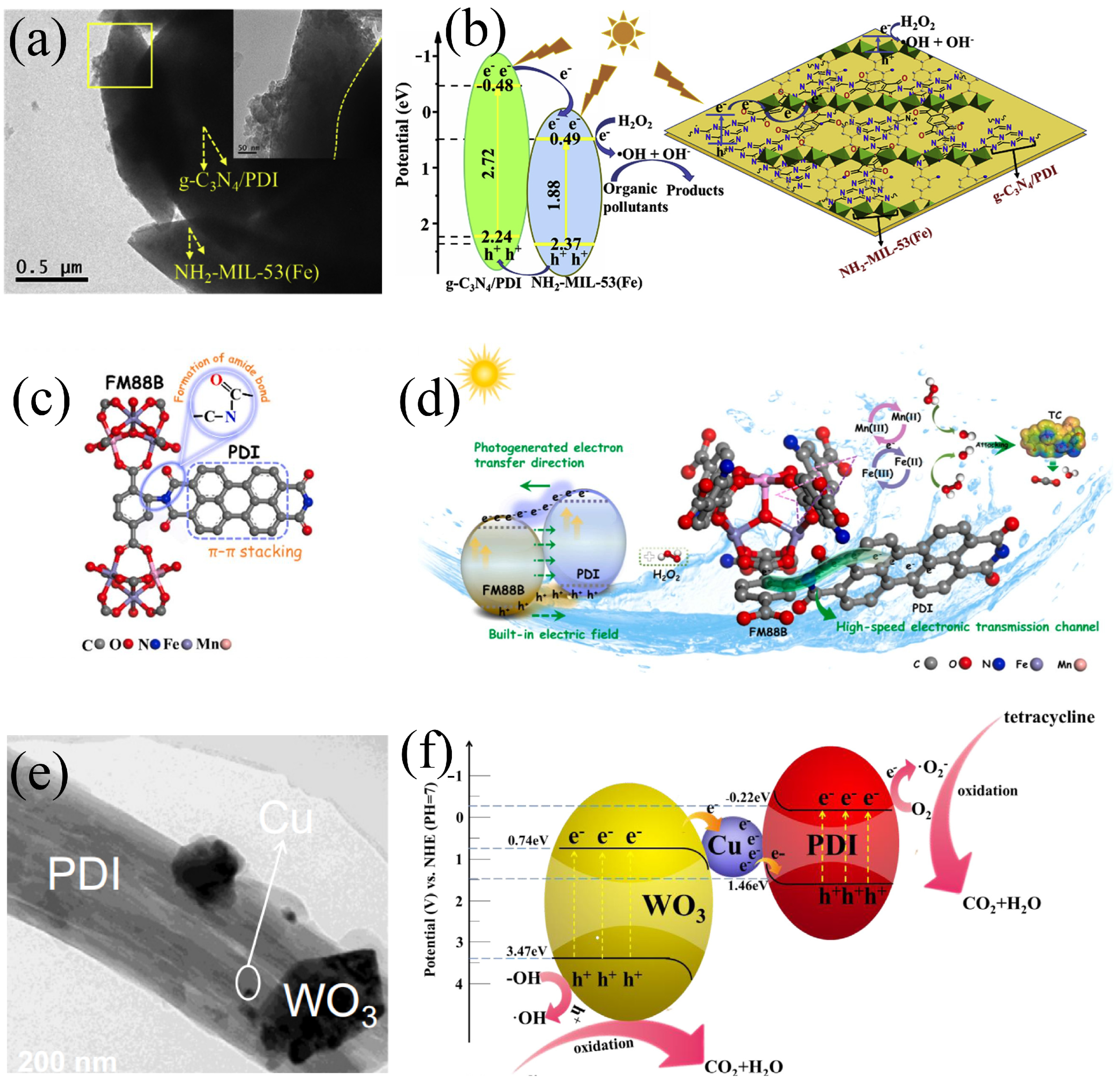
4.2. Phenolic Compounds
| Photocatalyst | Synthesis Method | Phenolic | Light Source | Time (min) | Efficiency (%) | Type | Reference |
|---|---|---|---|---|---|---|---|
| Ag@AgCl/PDI | In situ deposition, photoreduction | PhOH | Visible | 180 | 92.6 | Z | Chen et al. [122] |
| BiOCl/PDI | Water bath heating | PhOH | Simulated sunlight | 180 | 87.0 | Z | Gao et al. [123] |
| TOC-PDI-POSS/g-C3N4 | Solvent exchange | PhOH | Simulated sunlight | 60 | 97.0 | S | Dai et al. [124] |
| BiOBr/Bi4O5Br2/PDI | Self-assembly | BPA | Visible | 75 | 90.0 | II | Wang et al. [125] |
| PDIBr/A10 | Solvent exchange | BPA | Visible | 60 | 71.0 | Z | Zha et al. [126] |
| Bi12O15Cll6@W18O49 @g-C3N4/PDI | Solvent thermal, calcination | BPA | Simulated sunlight | 30 | 100.0 | Z | Zhang et al. [127] |
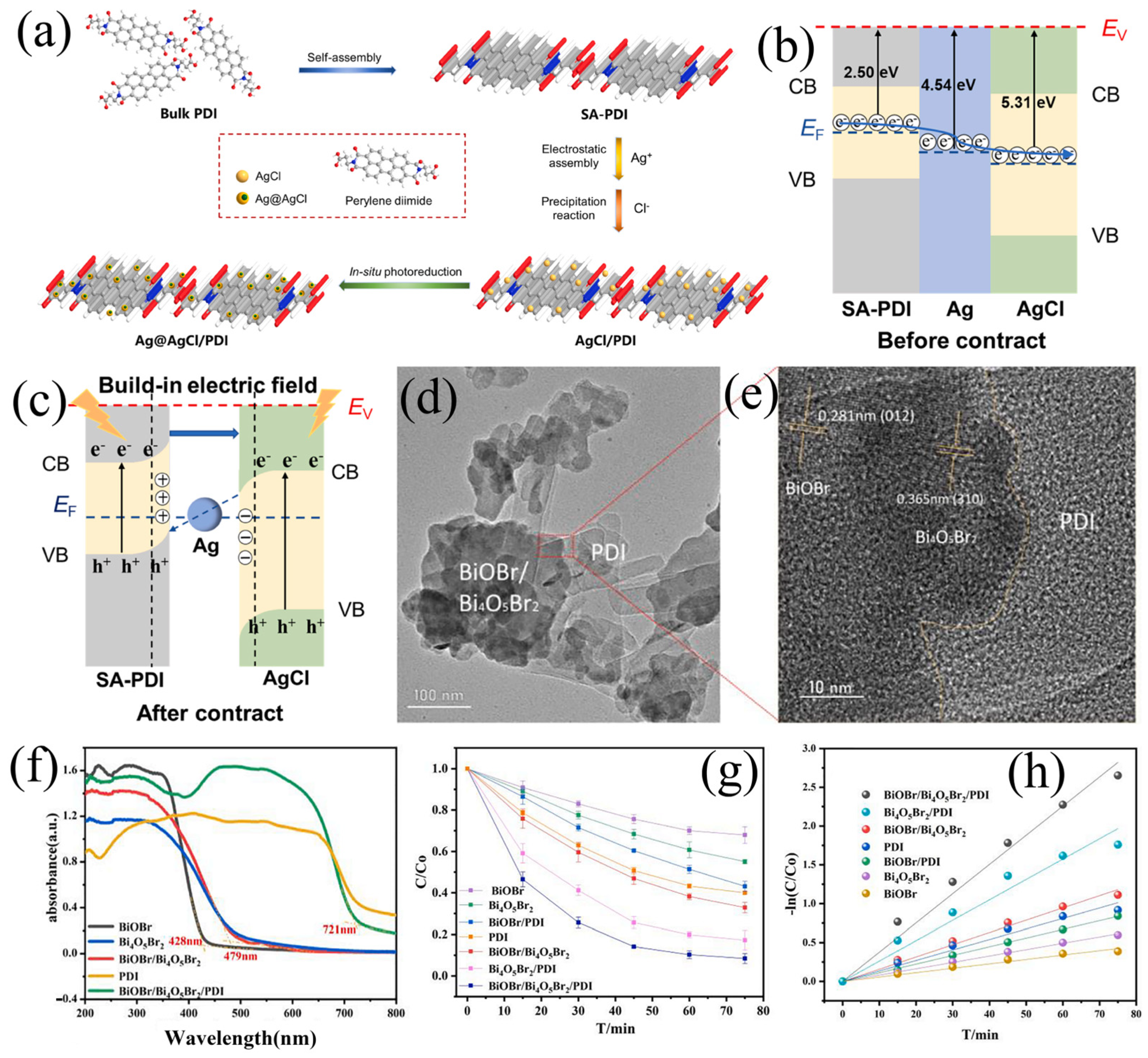
4.3. Industrial Dyes and Other Pollutants
5. Conclusions
6. Prospects
6.1. Functionalization and Modification of Photocatalytic Materials
6.2. Optimization of Light Absorption and Quantum Efficiency
6.3. Enhancement of Heterojunction Interfaces and Stability
6.4. Development and Expansion of Synergistic Catalytic Technologies
6.5. Commercialization-Oriented Innovation
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alsulmi, A.; Mohammed, N.N.; Soltan, A.; Messih, M.A.; Ahmed, M. Engineering S-scheme CuO/ZnO heterojunctions sonochemically for eradicating RhB dye from wastewater under solar radiation. RSC Adv. 2023, 13, 13269–13281. [Google Scholar] [CrossRef] [PubMed]
- Khudhair, E.M.; Ammar, S.H.; Al-Najjar, S.Z.; Al-Jubouri, S.M.; Mahdi, A.S.; Jabbar, Z.H. Facile construction of g-C3N4/MnWO4/NiS heterostructures for photocatalytic degradation of organic contaminates under visible light irradiation. Mater. Lett. 2023, 347, 134599. [Google Scholar] [CrossRef]
- Ponomarev, A.A.; Nurullina, T.S.; Zavatsky, M.D. Remediation of Cr (vi) in water using biosynthesized palladium nano-materials loaded (Shewanella oneidensis) MR-1. Water Conserv. Manag. 2022, 6, 146–153. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Sabbar, H.A.; Khudhair, E.M.; Ammar, S.H.; Batty, S.A.; Khudhair, S.Y.; Mahdi, A.S. Silver oxide-zeolite for removal of an emerging contaminant by simultaneous adsorption-photocatalytic degradation under simulated sunlight irradiation. J. Photochem. Photobiol. A Chem. 2023, 442, 114763. [Google Scholar] [CrossRef]
- Yaseen, D.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, H.; Liu, J.; Xia, Q.; Liu, J.; Liang, X.; Liu, E.; Fan, J. Effective photodegradation of rhodamine B and levofloxacin over CQDs modified BiOCl and BiOBr composite: Mechanism and toxicity assessment. J. Colloid Interface Sci. 2022, 627, 180–193. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Toxicity changes of wastewater during various advanced oxidation processes treatment: An overview. J. Clean. Prod. 2021, 315, 128202. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Al-Jendeel, H.A.; Rashid, S.A.; Al-Batty, S. Green synthesis of porous carbon cross-linked Y zeolite nanocrystals material and its performance for adsorptive removal of a methyl violet dye from water. Micropor. Mesopor. Mater. 2023, 356, 112587. [Google Scholar] [CrossRef]
- Lan, D.; Zhu, H.; Zhang, J.; Li, S.; Chen, Q.; Wang, C.; Wu, T.; Xu, M. Adsorptive removal of organic dyes via porous materials for wastewater treatment in recent decades: A review on species, mechanisms and perspectives. Chemosphere 2022, 293, 133464. [Google Scholar] [CrossRef]
- Zhou, T.; Hou, J.; Tai, M.; Shi, J.; Mi, X.; Hu, B.; Liu, C.; Yan, L.; Liu, L. Polyethyleneimine-induced in-situ chemical epitaxial growth ultrathin 2D/2D graphene carbon nitride intralayer heterojunction with elevating photocatalytic activity: Performances and mechanism insight. Int. J. Hydrogen Energy 2024, 51, 884–896. [Google Scholar] [CrossRef]
- Guo, M.; Ma, Y.; Liu, Z.; Wang, D.; Yang, Y.; Li, X.; Liu, E. Electron, hole and radical competition mechanism of layered porous g-C3N4 for hydrogen generation and organic pollutant degradation. J. Catal. 2024, 430, 115332. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Z.; Lu, P.; Zhou, Y.; Zhou, Y.; Bai, Y.; Yao, J. Cyano-deficient g-C3N4 for round-the-clock photocatalytic degradation of tetracycline: Mechanism and application prospect evaluation. Water Res. 2024, 260, 121936. [Google Scholar] [CrossRef]
- Hayat, A.; Ajmal, Z.; Alzahrani, A.Y.A.; Moussa, S.B.; Khered, M.; Almuqati, N.; Alshammari, A.; Al-Hadeethi, Y.; Ali, H.; Orooji, Y. The photocatalytic H2O2 production: Design strategies, Photocatalyst advancements, environmental applications and future prospects. Coord. Chem. Rev. 2025, 522, 216218. [Google Scholar] [CrossRef]
- Khoo, V.; Ng, S.F.; Haw, C.Y.; Ong, W.J. Additive manufacturing: A paradigm shift in revolutionizing catalysis with 3D printed photocatalysts and electrocatalysts toward environmental sustainability. Small 2024, 20, 2401278. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, D.; Li, J.; Zhi, C.; Zhang, Y.; Liang, L.; Mao, S.; Shi, J.W. Recent research advances of metal organic frameworks (MOFs) based composites for photocatalytic H2 evolution. Coord. Chem. Rev. 2024, 517, 215995. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Tang, Y.; Zhang, X.; Ji, S.; Luo, L.; Jiang, F. Photoinduced RhB-sensitized effect on a novel AgI/BiOCl/biochar photocatalyst to boost its photocatalytic performance for 17α-ethinyl estradiol degradation. Sep. Purif. Technol. 2024, 332, 125774. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Gong, X.; Wang, J. Photocatalytic degradation of chlorinated organic pollutants by ZnS@ ZIF-8 composite through hydrogen peroxide generation by activating dioxygen under simulated sunlight irradiation. J. Colloid Interface Sci. 2024, 654, 1417–1430. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, N.; Wang, L.; Zhao, H.; Zhao, G. In Situ Production of Hydroxyl Radicals via Three-Electron Oxygen Reduction: Opportunities for Water Treatment. Angew. Chem. Int. Ed. 2024, 136, e202407628. [Google Scholar] [CrossRef]
- Bao, Z.; Jiang, Y.; Zhang, Z.; Lv, J.; Shen, W.; Dai, J.; Wang, J.; Cai, J.; Wu, Y. Visible-light-responsive S-vacancy ZnIn2S4/N- doped TiO2 nanoarray heterojunctions for high-performance photoelectrochemical water splitting. J. Mater. Chem. A 2024, 12, 15902–15913. [Google Scholar] [CrossRef]
- Xie, Z.; Saad, A.; Shang, Y.; Wang, Y.; Luo, S.; Wei, Z. Enhanced degradation of micropollutants by visible light photocatalysts with strong oxygen activation ability. Water Res. 2023, 247, 120785. [Google Scholar] [CrossRef]
- Deng, Y.; Shi, Y.; Li, L.; Tang, R.; Zhou, Z.; Xiong, S.; Li, W.; Liu, J.; Huang, Y. Molecular modification: A promising strategy for the design of donor-acceptor-type organic polymers photocatalyst. Appl. Catal. B Environ. 2024, 352, 124043. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, N.; Wang, Y. Construction of Au/ZnWO4/CdS ternary photocatalysts with oxygen vacancy modification for efficient photocatalytic hydrogen production. Adv. Funct. Mater. 2024, 34, 2316604. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Jia, X.; Yang, J.; Miao, X.; Shao, D.; Wu, J.; Song, H. 2D/2D ultrathin polypyrrole heterojunct aerogel with synergistic photocatalytic-photothermal evaporation performance for efficient water purification. Desalination 2024, 574, 117295. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, X.; Li, C. Heterophase Junction Effect on Photogenerated Charge Separation in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2025, 58, 787–798. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Yang, S.; Garcia, H. Metal–organic framework heterojunctions for photocatalysis. Chem. Soc. Rev. 2024, 53, 3002–3035. [Google Scholar] [CrossRef]
- Li, F.; Zhu, G.; Jiang, J.; Yang, L.; Deng, F.; Li, X. A review of updated S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2024, 177, 142–180. [Google Scholar] [CrossRef]
- Ma, C.; Qin, L.; Zhou, T.; Zhang, J. Customized structures of hydrogen-bonded organic frameworks towards photocatalysis. Energy Environ. Sci. 2024, 17, 8992–9026. [Google Scholar] [CrossRef]
- Nie, C.; Wang, X.; Lu, P.; Zhu, Y.; Li, X.; Tang, H. Advancements in S-scheme heterojunction materials for photocatalytic environmental remediation. J. Mater. Sci. Technol. 2024, 169, 182–198. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Liu, W.; Jin, Q.; Lin, H. Piezo-photocatalytic enhanced microplastic degradation on hetero-interpenetrated Fe1−xS/FeMoO4/MoS2 by producing H2O2 and self-Fenton action. Chem. Eng. J. 2025, 508, 160935. [Google Scholar] [CrossRef]
- Li, J.; Xu, N.; Zhang, Y.; Dong, H.; Li, C. Research progress of heterogeneous photocatalyst for H2O2 production: A mini review. Chin. Chem. Lett. 2024, 00, 110470. [Google Scholar] [CrossRef]
- Wang, G.; Lv, S.; Shen, Y.; Li, W.; Lin, L.; Li, Z. Advancements in heterojunction, cocatalyst, defect and morphology engineering of semiconductor oxide photocatalysts. J. Mater. 2024, 10, 315–338. [Google Scholar] [CrossRef]
- Miao, J.; Yang, Y.; Cui, P.; Ru, C.; Zhang, K. Improving charge transfer beyond conventional heterojunction photoelectrodes: Fundamentals, strategies and applications. Adv. Funct. Mater. 2024, 34, 2406443. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Yu, W.; Yin, L.; Liu, X.; Xia, J.; Zhang, N.; Fu, Y.; Yang, G.; Ni, B. Modulating charge carrier transfer channel by 2D/2D Schottky heterojunction of Ti3C2/BiOIO3 for effective photocatalytic degradation of typical antibiotics. Sep. Purif. Technol. 2024, 337, 126393. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, H.; Kang, Y.; Li, X.; Li, Q.; Zhai, P.; Hildebrandt, D.; Liu, X.; Wang, Y.; Qiao, S. Molecular and Heterojunction Device Engineering of Solution-Processed Conjugated Reticular Oligomers: Enhanced Photoelectrochemical Hydrogen Evolution through High-Effective Exciton Separation. Adv. Sci. 2024, 11, 2308535. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zheng, C.; Yan, T.; Jin, Z. Cd0.8Mn0.2S/MoO3 composites with an S-scheme heterojunction for efficient photocatalytic hydrogen evolution. Dalton Trans. 2021, 50, 5360–5369. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Du, H.; Liu, Y.; Shen, C.; Xu, A. Nanoheterostructured photocatalysts for improving photocatalytic hydrogen production. Chin. J. Catal. 2017, 38, 1295–1306. [Google Scholar] [CrossRef]
- Shu, A.; Qin, C.; Li, M.; Zhao, L.; Shangguan, Z.; Shu, Z.; Yuan, X.; Zhu, M.; Wu, Y.; Wang, H. Electric effects reinforce charge carrier behaviour for photocatalysis. Energy Environ. Sci. 2024, 17, 4907–4928. [Google Scholar] [CrossRef]
- Lu, N.; Yan, X.; Wu, B.; Kobayashi, H.; Li, R. A universal molecular oxygen-mediated photocatalysis strategy to boost visible-light induced hydrogen evolution through partial water splitting. Appl. Catal. B Environ. 2025, 360, 124536. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Naz, M.; Ahmad, M.; Ahmed, E.; Liu, Y.; Hussain, A.; Iqbal, S.; Ullah, S. Recent advances and challenges in 2D/2D heterojunction photocatalysts for solar fuels applications. Adv. Colloid Interface Sci. 2022, 304, 102661. [Google Scholar] [CrossRef]
- Zhu, B.; Sun, J.; Zhao, Y.; Zhang, L.; Yu, J. Construction of 2D S-scheme heterojunction photocatalyst. Adv. Mater. 2024, 36, 2310600. [Google Scholar] [CrossRef] [PubMed]
- Akinoglu, E.M.; Hoogeveen, D.A.; Cao, C.; Simonov, A.N.; Jasieniak, J.J. Prospects of Z-scheme photocatalytic systems based on metal halide perovskites. ACS Nano 2021, 15, 7860–7878. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Li, J.; Wen, Z.; Lu, R.; Zhang, Q.; Jin, H.; Zhang, L.; Chen, Y.; Wang, S. Halide perovskite materials for photo (electro) chemical applications: Dimensionality, heterojunction, and performance. Adv. Energy Mater. 2022, 12, 2004002. [Google Scholar] [CrossRef]
- Wang, L.; Bie, C.; Yu, J. Challenges of Z-scheme photocatalytic mechanisms. Trends Chem. 2022, 4, 973–983. [Google Scholar] [CrossRef]
- Huang, X.; Du, R.; Zhang, Y.; Ren, J.; Yang, Q.; Wang, K.; Ni, Y.; Yao, Y.; Soomro, R.; Guo, L. Modulating charge oriented accumulation via interfacial chemical-bond on In2O3/Bi2MoO6 heterostructures for photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2024, 664, 33–44. [Google Scholar] [CrossRef]
- Chang, P.; Wang, Y.; Wang, Y.; Zhu, Y. Current trends on In2O3 based heterojunction photocatalytic systems in photocatalytic application. Chem. Eng. J. 2022, 450, 137804. [Google Scholar] [CrossRef]
- Leelavathi, H.; Muralidharan, R.; Abirami, N.; Tamizharasan, S.; Sankeetha, S.; Kumarasamy, A.; Arulmozhi, R. Construction of step-scheme g-C3N4/Co/ZnO heterojunction photocatalyst for aerobic photocatalytic degradation of synthetic wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130449. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Liu, E.; Fan, J. Novel S-scheme 2D/2D BiOBr/g-C3N4 heterojunctions with enhanced photocatalytic activity. Chin. J. Catal. 2021, 42, 1519–1529. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Qi, K.; Imparato, C.; Almjasheva, O.; Khataee, A.; Zheng, W. TiO2-based photocatalysts from type-II to S-scheme heterojunction and their applications. J. Colloid Interface Sci. 2024, 675, 150–191. [Google Scholar] [CrossRef]
- Zhou, T.; Ma, Y.; Feng, H.; Lu, Y.; Che, G.; Liu, C.; Lan, Y. COFs-Based Metal-Free Heterojunctions for Solar-to-Chemical Energy Conversion. Adv. Funct. Mater. 2024, 34, 2409396. [Google Scholar] [CrossRef]
- Chen, R.; Gan, W.; Guo, J.; Lu, Y.; Ding, S.; Liu, R.; Zhang, M.; Sun, Z. Internal electric field and oxygen vacancies synergistically boost S-scheme VO/BiOCl-TiO2 heterojunction film for photocatalytic degradation of norfloxacin. Chem. Eng. J. 2024, 489, 151260. [Google Scholar] [CrossRef]
- Rao, V.N.; Ahn, C.W.; Lee, Y.; Shankar, M.V.; Kwon, H.; Kim, K.; Rezakazemi, M.; Kim, S.j.; Yang, J.M. Insights into excitons manipulation in metal chalcogenides based Nano-heterojunction Photocatalysts: A breakthrough in green hydrogen production. Coord. Chem. Rev. 2025, 522, 216176. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Z.; Yang, Q.; Wang, L.; Xing, Y. Review on g-C3N4-based S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2022, 125, 128–144. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Q.; Huang, X.; Ma, Q.; Shao, J.; Shen, Q. Recent progress of acenaphthylene-imide-fused polycyclic aromatic hydrocarbons: Synthesis and application. Dye. Pigment. 2021, 185, 108877. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, G.; Ge, C.; Liu, J.; Zhou, L.; Liu, C.; Gao, X.; Narita, A.; Zou, Y.; Hu, Y. Electron-deficient contorted polycyclic aromatic hydrocarbon via one-pot annulative π-extension of perylene diimide. Org. Lett. 2022, 24, 2414–2419. [Google Scholar] [CrossRef]
- Chen, S.; Slattum, P.; Wang, C.; Zang, L. Self-assembly of perylene imide molecules into 1D nanostructures: Methods, morphologies, and applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef]
- Zhou, C.; Xia, W.; Huang, D.; Cheng, M.; Zhang, H.; Cai, T.; Xiong, W.; Yang, Y.; Song, B.; Wang, W.; et al. Strategies for enhancing the perylene diimide photocatalytic degradation activity: Method, effect factor, and mechanism. Environ. Sci. Nano 2021, 8, 602–618. [Google Scholar] [CrossRef]
- Fan, Q.; Cheng, K.; Yang, Z.; Zhang, R.; Yang, M.; Hu, X.; Ma, X.; Bu, L.; Lu, X.; Xiong, X. Perylene-diimide-based nanoparticles as highly efficient photoacoustic agents for deep brain tumor imaging in living mice. Adv. Mater. 2014, 27, 843. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Wang, Z.Y.; Zhang, R.; Yu, L. Acceleration of biotic decolorization and partial mineralization of methyl orange by a photo-assisted n-type semiconductor. Chemosphere 2022, 291, 132846. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Blaney, L.; Cagnetta, G.; Huang, J.; Wang, B.; Wang, Y.; Deng, S.; Yu, G. Degradation of ofloxacin by perylene diimide supramolecular nanofiber sunlight-driven photocatalysis. Environ. Sci. Technol. 2019, 53, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zajaczkowski, W.; Velpula, G.; Jänsch, D.; Graf, R.; Marszalek, T.; Parekh, S.H.; Zagranyarski, Y.; Mali, K.; Wagner, M. Transformation from helical to layered supramolecular organization of asymmetric perylene diimides via multiple intermolecular hydrogen bonding. Chem. Sci. 2020, 11, 4960–4968. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, L.; Wang, J.; Zhu, Y.; Pu, Y.; Dai, W. Photocatalytic degradation of tetracycline antibiotics using three-dimensional network structure perylene diimide supramolecular organic photocatalyst under visible-light irradiation. Appl. Catal. B Environ. 2020, 277, 119122. [Google Scholar] [CrossRef]
- Langhals, H.; Jona, W.; Einsiedl, F.; Wohnlich, S. Self-dispersion: Spontaneous formation of colloidal dyes in water. Adv. Mater. 1998, 10, 1022–1024. [Google Scholar] [CrossRef]
- Goerl, D.; Zhang, X.; Würthner, F. Molecular assemblies of perylene bisimide dyes in water. Angew. Chem. Int. Ed. 2012, 51, 6328–6348. [Google Scholar] [CrossRef]
- Liu, K.; Xu, Z.; Yin, M.; Yang, W.; He, B.; Wei, W.; Shen, J. A multifunctional perylenediimide derivative (DTPDI) can be used as a recyclable specific Hg2+ ion sensor and an efficient DNA delivery carrier. J. Mater. Chem. B 2014, 2, 2093–2096. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, K.; Yu, J.; Sun, H.; Tang, J.; Shen, J.; Müllen, K.; Yang, W.; Yin, M. A unique perylene-based DNA intercalator: Localization in cell nuclei and inhibition of cancer cells and tumors. Small 2014, 10, 4087–4092. [Google Scholar] [CrossRef]
- Kohl, C.; Weil, T.; Qu, J.; Müllen, K. Towards highly fluorescent and water-soluble perylene dyes. Chem. Eur. J. 2004, 10, 5297–5310. [Google Scholar] [CrossRef]
- Zhong, L.; Xing, F.; Shi, W.; Yan, L.; Xie, L.; Zhu, S. Synthesis, spectra, and electron-transfer reaction of aspartic acid-functionalized water-soluble perylene bisimide in aqueous solution. ACS Appl. Mater. Interfaces 2013, 5, 3401–3407. [Google Scholar] [CrossRef]
- Peneva, K.; Mihov, G.; Nolde, F.; Rocha, S.; Hotta, J.-i.; Braeckmans, K.; Hofkens, J.; Uji-i, H.; Herrmann, A.; Müllen, K. Water-soluble monofunctional perylene and terrylene dyes: Powerful labels for single-enzyme tracking. Angew. Chem. Int. Ed. 2008, 47, 3372–3375. [Google Scholar] [CrossRef] [PubMed]
- Battagliarin, G.; Davies, M.; Mackowiak, S.; Li, C.; Müllen, K. Ortho-functionalized perylenediimides for highly fluorescent water-soluble dyes. Chem Phys Chem 2012, 13, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Müllen, K.; Yin, M. Water-soluble perylenediimides: Design concepts and biological applications. Chem. Soc. Rev. 2016, 45, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, G.; Yang, B.; Ji, Q.; Xiang, W.; He, H.; Xu, Z.; Qi, C.; Li, S.; Yang, S. Review on application of perylene diimide (PDI)-based materials in environment: Pollutant detection and degradation. Sci. Total Environ. 2021, 780, 146483. [Google Scholar] [CrossRef]
- Sun, T.; Song, J.; Jia, J.; Li, X.; Sun, X. Real roles of perylenetetracarboxylic diimide for enhancing photocatalytic H2-production. Nano Energy 2016, 26, 83–89. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, G.; Lan, L.; Chen, J.; Zhu, X.; Duan, J.; Cong, S.; Li, Z.; Wang, Y.; Wang, Z.J. n-Type glycolated imide-fused polycyclic aromatic hydrocarbons with high capacity for liquid/solid-electrolyte-based electrochemical devices. Adv. Funct. Mater. 2023, 33, 2300012. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, X.; Dong, Y.; Zhang, N.; Wang, H.; Li, X.; Ren, X.; Ma, H.; Wei, Q. Self-assembled perylene diimide (PDI) nanowire sensitized In2O3@MgIn2S4 S-scheme heterojunction as photoelectrochemical biosensing platform for the detection of CA15–3. Anal. Chem. 2024, 96, 13197–13206. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Song, R.; Zhang, Y.; Ma, Y.; Wan, J.; Zhang, M.; Cui, H.; Yang, H.; Chen, X. Morphology engineering and photothermal effect derived from perylene diimide based derivative for boosting photocatalytic hydrogen evolution of ZnIn2S4. J. Colloid Interface Sci. 2022, 628, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Bai, Y.; Wang, X.; Fu, H.; Zhao, P.; Zhu, P.; Yu, J. Self-standing perylene diimide covalent organic framework membranes for trace TMA sensing at room temperature. J. Colloid Interface Sci. 2024, 663, 262–269. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, D.; Zhu, Y. Deep degradation of pollutants by perylene diimide supramolecular photocatalyst with unique bi-planar π-π conjugation. Chem. Eng. J. 2022, 438, 135667. [Google Scholar] [CrossRef]
- Che, W.; Sun, C.; Wu, Z.; Sun, Y.; Shang, Q. Efficient separation of photo-generated carriers for in-situ induction of PDI cation radicals to enhance the photocatalytic performance of PDI supramolecules. J. Clean. Prod. 2024, 453, 142235. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; Lu, Y.; Hu, J.; Feng, J.; Shang, H.; Sun, B.; Jiang, W. Molecular design of perylene diimide derivatives for photocatalysis. ACS Catal. 2025, 15, 1829–1840. [Google Scholar] [CrossRef]
- Fan, Y.; Kong, C.; Zhang, L.; Wu, H.; Li, J.; Guo, J.; Yi, Q. Enhancing photocatalytic hydrogen evolution performance for D-π-A conjugated polymers based on the perylene diimide. Sep. Purif. Technol. 2025, 355, 129721. [Google Scholar] [CrossRef]
- Zhao, P.; Hu, Y.; An, X.; Ji, R.; Liu, H.; Zhao, H.; Song, W.; Dong, Y.; Wang, X. Polymeric PDI-based photocatalytic nanoarchitectures promoting the performance of thin film composite membrane for forward osmosis water purification. Chem. Eng. J. 2023, 476, 146747. [Google Scholar] [CrossRef]
- Dong, T.; Dong, G.; Han, K.; Chen, C.; Hu, J.; Uvdal, K. All-organic heterojunctions used for the excellent photocatalytic H2O2 synthesis: The key role of bay-position Cl in PDI. Appl. Catal. B Environ. 2024, 354, 124144. [Google Scholar] [CrossRef]
- Yang, S.; Deng, X.; Chen, P.; Li, G.; Wang, Q.; Wang, Q.; Yin, S.-F. Bridges engineering manipulated exciton dissociation and charge separation in small acceptors of PDI supramolecular for boosting photocatalytic nitrogen fixation. Chem. Eng. J. 2022, 441, 136084. [Google Scholar] [CrossRef]
- Yang, J.; Miao, H.; Wei, Y.; Li, W.; Zhu, Y. π-π Interaction between self-assembled perylene diimide and 3D graphene for excellent visible-light photocatalytic activity. Appl. Catal. B Environ. 2019, 240, 225–233. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Jiang, W.; Yao, W.; Yang, H.; Zhu, Y. Self-assembled perylene diimide based supramolecular heterojunction with Bi2WO6 for efficient visible-light-driven photocatalysis. Appl. Catal. B Environ. 2018, 232, 175–181. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, J.; Wang, Z.; Zhu, Y. Enhanced visible photocatalytic oxidation activity of perylene diimide/g-C3N4 n-n heterojunction via π-π interaction and interfacial charge separation. Appl. Catal. B Environ. 2020, 271, 118933. [Google Scholar] [CrossRef]
- Dai, W.; Jiang, L.; Wang, J.; Pu, Y.; Zhu, Y.; Wang, Y.; Xiao, B. Efficient and stable photocatalytic degradation of tetracycline wastewater by 3D polyaniline/perylene diimide organic heterojunction under visible light irradiation. Chem. Eng. J. 2020, 397, 125476. [Google Scholar] [CrossRef]
- Wang, S.; Xia, Y.; Yan, G.; Chen, M.; Wang, X.; Wu, L.; Liang, R. PDI bridged MIL-125(Ti)-NH2 heterojunction with frustrated Lewis pairs: A promising photocatalyst for Cr(VI) reduction and antibacterial application. Appl. Catal. B Environ. 2022, 317, 121798. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, W.; Liu, Y.; Dong, W.; Cai, T.; Tang, L.; Li, J.; Li, W. Unique MIL-53(Fe)/PDI Supermolecule Composites: Z-Scheme Heterojunction and Covalent Bonds for Uprating Photocatalytic Performance. ACS Appl. Mater. Interfaces 2021, 13, 16364–16373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, J.; Yang, X.; Hu, A.; Yang, Y.; Guo, Y. Fabrication of a perylene tetracarboxylic diimide-graphitic carbon nitride heterojunction photocatalyst for efficient degradation of aqueous organic pollutants. ACS Appl. Mater. Interfaces 2018, 11, 588–602. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Ji, L.; Luo, Q.; Duan, Y.; An, J.; Chen, X.; Zhang, Y.; Ren, J.; Wang, D. Resin with short-range π-π stacking aggregates for an efficient photocatalyst. Chem. Eng. J. 2022, 433, 134502. [Google Scholar] [CrossRef]
- Miao, H.; Yang, J.; Wei, Y.; Li, W.; Zhu, Y. Visible-light photocatalysis of PDI nanowires enhanced by plasmonic effect of the gold nanoparticles. Appl. Catal. B Environ. 2018, 239, 61–67. [Google Scholar] [CrossRef]
- Yang, J.; Miao, H.; Jing, J.; Zhu, Y.; Choi, W. Photocatalytic activity enhancement of PDI supermolecular via π-π interaction and energy level adjusting with graphene quantum dots. Appl. Catal. B Environ. 2021, 281, 119547. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.; Wang, B.; Yang, R.; Zhu, S.; Song, Y.; Hua, Y.; Yan, J.; Cheng, M.; Xu, H.; et al. Noble-metal-free Co-N-graphene/PDI for significant enhancement of photocatalytic performance. J. Alloys Compd. 2022, 925, 166370. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, M.; Li, W.; Yang, J.; Miao, H.; Zhang, Z.; Zhu, Y. Enhanced photocatalytic activity of PTCDI-C60 via π-π interaction. Appl. Catal. B Environ. 2018, 238, 302–308. [Google Scholar] [CrossRef]
- Zhang, F.; Li, W.; Jiang, T.; Li, X.; Shao, Y.; Ma, Y.; Wu, J. Real roles of perylene diimides for improving photocatalytic activity. RSC Adv. 2020, 10, 23024–23037. [Google Scholar] [CrossRef]
- Sheng, Y.; Miao, H.; Jing, J.; Yao, W.; Zhu, Y. Perylene diimide anchored graphene 3D structure via π-π interaction for enhanced photoelectrochemical degradation performances. Appl. Catal. B Environ. 2020, 272, 118897. [Google Scholar] [CrossRef]
- Wu, G.; Tai, G.; Li, G.; Lu, J.; Pan, Y.; Han, J.; Xing, W. Self-assembled perylene diimide decorated g-C3N4 heterojunction catalyst with strong interfacial charge transfer through π-π interaction for efficient boosted photocatalytic degradation of tetracycline. Surf. Interfaces 2024, 53, 105008. [Google Scholar] [CrossRef]
- Li, Y.; Fang, Y.; Cao, Z.; Li, N.; Chen, D.; Xu, Q.; Lu, J. Construction of g-C3N4/PDI@MOF heterojunctions for the highly efficient visible light-driven degradation of pharmaceutical and phenolic micropollutants. Appl. Catal. B Environ. 2019, 250, 150–162. [Google Scholar] [CrossRef]
- Wu, M.; Yang, H.; Wu, Q.; He, Z.; Wang, S. Directional and rapid electron transfer in perylene diimide modified iron- manganese bimetallic metal-organic frameworks for enhanced photo-Fenton process. J. Environ. Chem. Eng. 2024, 12, 112246. [Google Scholar] [CrossRef]
- Lu, Z.; Li, B.; Wei, B.; Zhou, G.; Xu, Y.; Zhang, J.; Chen, H.; Hua, S.; Wu, C.; Liu, X. NMP-induced surface self-corrosion- assisted rapid spin-coating method for synthesizing imprinted heterojunction photocatalyst anchored membrane towards high-efficiency selective degradation tetracycline. Sep. Purif. Technol. 2023, 314, 123609. [Google Scholar] [CrossRef]
- Zhuang, H.; Wang, F.; Shi, K.; Yang, K. Designed Synthesis of PDI/BiOCl-BiPO4 Composited Material for Boosted Photocatalytic Contaminant Degradation. Catalysts 2023, 13, 688. [Google Scholar] [CrossRef]
- Shi, K.; Zhou, M.; Wang, F.; Li, X.; Huang, W.; Lu, K.; Yang, K.; Yu, C. Perylene diimide/iron phthalocyanine Z-scheme heterojunction with strong interfacial charge transfer through π-π interaction: Efficient photocatalytic degradation of tetracycline hydrochloride. Chemosphere 2023, 329, 138617. [Google Scholar] [CrossRef]
- Zeng, W.; Cai, T.; Liu, Y.; Wang, L.; Dong, W.; Chen, H.; Xia, X. An artificial organic-inorganic Z-scheme photocatalyst WO3@Cu@PDI supramolecular with excellent visible light absorption and photocatalytic activity. Chem. Eng. J. 2020, 381, 122691. [Google Scholar] [CrossRef]
- Mao, Z.; Luo, P.; Ling, J.; Zhu, X.; Sun, K.; Cao, Y.; Zhu, D.; Liu, W. Laser preparation of dual Z-scheme heterojunctions PDI/WO3/α-Fe2O3 to enhance adsorption-photocatalytic synergistic degradation. J. Alloys Compd. 2025, 1011, 178195. [Google Scholar] [CrossRef]
- Sun, F.; Xie, Y.; Xu, D.; Liu, F.; Qi, H.; Ma, Q.; Yang, Y.; Yu, H.; Yu, W.; Dong, X. Electrospun self-supporting double Z-scheme tricolor-typed microfiber oriented-heterostructure photocatalyst with highly effective hydrogen evolution and organic pollutants degradation. J. Environ. Chem. Eng. 2023, 11, 109169. [Google Scholar] [CrossRef]
- Cai, T.; Zeng, W.; Liu, Y.; Wang, L.; Dong, W.; Chen, H.; Xia, X. A promising inorganic-organic Z-scheme photocatalyst Ag3PO4/PDI supermolecule with enhanced photoactivity and photostability for environmental remediation. Appl. Catal. B Environ. 2020, 263, 118327. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Huang, J.; He, C.; Feng, Z.; Chen, Z.; Wan, L.; Deng, F. All Organic S-Scheme Heterojunction PDI-Ala/S-C3N4 Photocatalyst with Enhanced Photocatalytic Performance. Acta Phys. Chim. Sin. 2020, 37, 2010030. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, Y.; Wang, P.; Xu, Y.; Zhang, J.; Wei, B.; Zhou, G.; Liu, X.; Huang, Y.; Wu, C. High-throughput imprinted non-metal S-scheme heterojunction self-cleaning membrane with tight adhesion via dopamine for selective photodegradation of TC. J. Environ. Chem. Eng. 2023, 11, 109745. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Xie, M.; Liu, Y.; Du, X.; Hu, J. Photothermal-assisted S-scheme PDIs/C, N, S-CeO2 derived from MOF-808 (Ce) heterojunction for photocatalytic removal of antibiotics. J. Alloys Compd. 2024, 979, 173568. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Xie, M.; Liu, Y.; Du, X.; Hu, J. Rational construction of visible-light-driven perylene diimides/Fe2O3@C derived from MIL-88A (Fe) heterojunction with S-scheme electron transfer pathway to activate peroxymonosulfate for degradation of antibiotics. J. Colloid Interface Sci. 2024, 659, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, X.; Yan, H.; Wang, P.; Song, M.; Ma, C.; Chen, Z.; Chu, J.; Liu, X.; Lu, Z. Hydrochloric acid-mediated synthesis of ZnFe2O4 small particle decorated one-dimensional Perylene Diimide S-scheme heterojunction with excellent photocatalytic ability. Chin. J. Catal. 2022, 43, 1111–1122. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Z.; Li, M.; Liu, Q.; Liu, X.; Wang, Y. Efficient Charge Separation in Ag/PCN/UPDI Ternary Heterojunction for Optimized Photothermal-Photocatalytic Performance via Tandem Electric Fields. Small 2024, 20, 2306692. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Gao, C.; Wen, Q.; Ma, X.; Song, F.; Zhou, J. Photocatalysis and peroxymonosulfate activation by dual Z-scheme g-C3N4/PDI/Co-Fe Prussian blue analogue for doxycycline hydrochloride removal. J. Environ. Chem. Eng. 2025, 13, 115422. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Deng, F.; Liu, J.; Gao, X.; Huang, J.; Xu, J.; Feng, Z.; Chen, Z.; Han, L. Novel organic/inorganic PDI-Urea/BiOBr S-scheme heterojunction for improved photocatalytic antibiotic degradation and H2O2 production. Chin. Chem. Lett. 2022, 33, 5200–5207. [Google Scholar] [CrossRef]
- Yang, L.; Hao, X.; Yu, D.; Zhou, P.; Peng, Y.; Jia, Y.; Zhao, C.; He, J.; Zhan, C.; Lai, B. High visible-light catalytic activity of Bis-PDI-T@TiO2 for activating persulfate toward efficient degradation of carbamazepine. Sep. Purif. Technol. 2021, 263, 118384. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.; Duan, L.; Gao, Q.; Zhang, H.; Li, S.; Li, M. Activation of persulfate by β-PDI/MIL-101(Fe) photocatalyst under visible light toward efficient degradation of sulfamethoxazole. Chem. Eng. J. 2024, 481, 148588. [Google Scholar] [CrossRef]
- Jia, Y.; Duan, L.; Li, H.; Zhang, C.; Gao, Q.; Zhang, H.; Li, S.; Li, M. Fast removal of sulfamethoxazole by MIL-101(Fe)–NH2/perylene diimide activated persulfate under visible light. Sep. Purif. Technol. 2025, 358, 130292. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Shen, X.; Zhang, Y.; Lou, Y.; Pan, C.; Zhu, Y.; Xu, J. A plasmonic Z-scheme Ag@AgCl/PDI photocatalyst for the efficient elimination of organic pollutants, antibiotic resistant bacteria and antibiotic resistance genes. Appl. Catal. B Environ. 2023, 324, 122220. [Google Scholar] [CrossRef]
- Gao, X.; Gao, K.; Li, X.; Shang, Y.; Fu, F. Hybrid PDI/BiOCl heterojunction with enhanced interfacial charge transfer for a full-spectrum photocatalytic degradation of pollutants. Catal. Sci. Technol. 2020, 10, 372–381. [Google Scholar] [CrossRef]
- Dai, S.; Xu, Y.; Zhang, W.; Li, S.; Guo, Q.Y.; Cui, J.; Song, Y.; Yuan, J.; Peng, W.; Huang, M. S-scheme enhanced photocatalysis on titanium oxide clusters functionalized with soluble perylene diimides. J. Mater. Chem. A 2022, 10, 20248–20253. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Wang, J.; Li, A.; Corvini, P. BiOBr/Bi4O5Br2/PDI constructed for visible-light degradation of endocrine disrupting chemicals: Synergistic effects of bi-heterojunction and oxygen evolution. Chem. Eng. J. 2022, 433, 133622. [Google Scholar] [CrossRef]
- Zha, K.; Li, L.; Zhang, J.; Tang, S.; Li, X.; Hai, J.; Fan, D.; Li, M.; Liu, Y.; Lu, Z. Investigation the influence of bay substitution with perylene diimide on the photocatalytic performance of perylene–diimide/TiO2 composites. J. Photochem. Photobiol. A Chem. 2024, 451, 115517. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Gu, P.Y.; Ji, R.; Jin, L.; Zhou, S.; He, J.; Chen, D.; Xu, Q.; Lu, J. Preparation of a Bi12O15Cl6@W18O49@g-C3N4/PDI heterojunction with dual charge transfer paths and its photocatalytic performance for phenolic pollutants. Sep. Purif. Technol. 2022, 287, 120539. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, L.; Zhang, Y. Preparation of organic-inorganic PDI/BiO2-x photocatalyst with boosted photocatalytic performance. J. Taiwan Inst. Chem. Eng. 2022, 132, 104111. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, S.; Zhang, W.; Shi, L. Facile preparation of PDI nano-rods coupled with AgBr for enhanced photocatalytic performance. Opt. Mater. 2024, 147, 114656. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, L.; Yao, L.; Cui, L. The boosted photocatalytic activity over perylene diimide modified Bi2O4 hybrid photocatalyst with internal electric field. Mater. Res. Bull. 2022, 146, 111589. [Google Scholar] [CrossRef]
- Mardiroosi, A.; Mahjoub, A.R.; Fakhri, H.; Boukherroub, R. Design and fabrication of a perylene dimiide functionalized g-C3N4@UiO-66 supramolecular photocatalyst: Insight into enhancing the photocatalytic performance. J. Mol. Struct. 2021, 1246, 131244. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Wang, Z.; Yao, L.; Shi, L. Preparation of 1D/1D perylene diimide nano-rod modified Bi4O7 rod with highly active photocatalytic performance. Opt. Mater. 2023, 138, 113734. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Y.; Shen, Y.; Zhang, Y.; Men, D.; Qiu, L.; Xu, X.; Xi, J.; Li, P.; Duo, S. g-C3N4/PDI@ZnIn2S4 2D/2D organic– inorganic hybrid heterojunction with enhanced visible light photocatalytic property. Chem. Phys. Lett. 2023, 833, 140936. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Deng, J.; Li, L.; Zhou, Z.; Zheng, J.; Su, L.; Yang, L. π-π Stacked step-scheme PDI/g-C3N4/TiO2@Ti3C2 photocatalyst with enhanced visible photocatalytic degradation towards atrazine via peroxymono- sulfate activation. Chem. Eng. J. 2022, 427, 131809. [Google Scholar] [CrossRef]
- Ren, Y.; Tian, Y.; Lu, Y.; Nie, D.; Zhu, H.; Yang, X. Z-scheme H-PDI supermolecule/NH2-MIL-101(Fe) for enhanced malathion degradation: Mechanism, pathway, and toxicity assessment. J. Environ. Chem. Eng. 2024, 12, 114358. [Google Scholar] [CrossRef]
- Chen, R.; Lou, H.; Pang, Y.; Yang, D.; Qiu, X. Enhancing Pollutant Mineralization through Organic–Inorganic Defect-Transit Dual S-scheme with a Robust Internal Electric Field. Small 2024, 20, 15. [Google Scholar] [CrossRef]
- Ji, Q.; Cheng, X.; Kong, X.; Sun, D.; Wu, Y.; Xu, Z.; Liu, Y.; Duan, X.; He, H.; Li, S.; et al. Visible-light activation of persulfate ions by Z-scheme perylene diimide/MIL-101(Cr) heterojunction photocatalyst towards efficient degradation of iohexol. Chem. Eng. J. 2022, 435, 134947. [Google Scholar] [CrossRef]
- Ren, J.; Meng, Y.; Zhang, X.; Gao, Y.; Liu, L.; Zhou, X.; Zhang, Z.; Zeng, L.; Ke, J. Self-assembled perylene diimide modified NH2-UiO-66 (Zr) construct n-n heterojunction catalysts for enhanced Cr (VI) photocatalytic reduction. Sep. Purif. Technol. 2022, 296, 121423. [Google Scholar] [CrossRef]
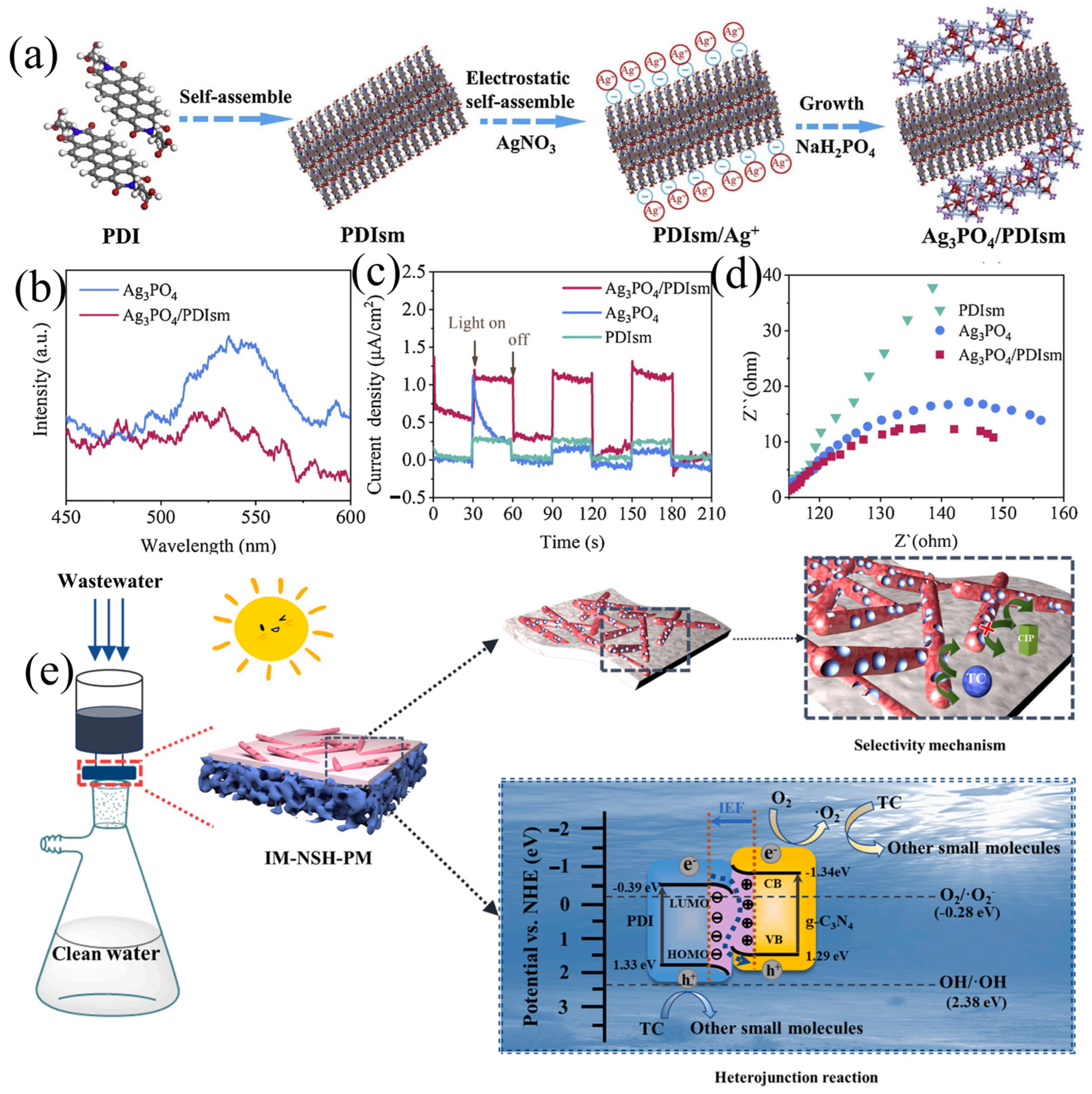
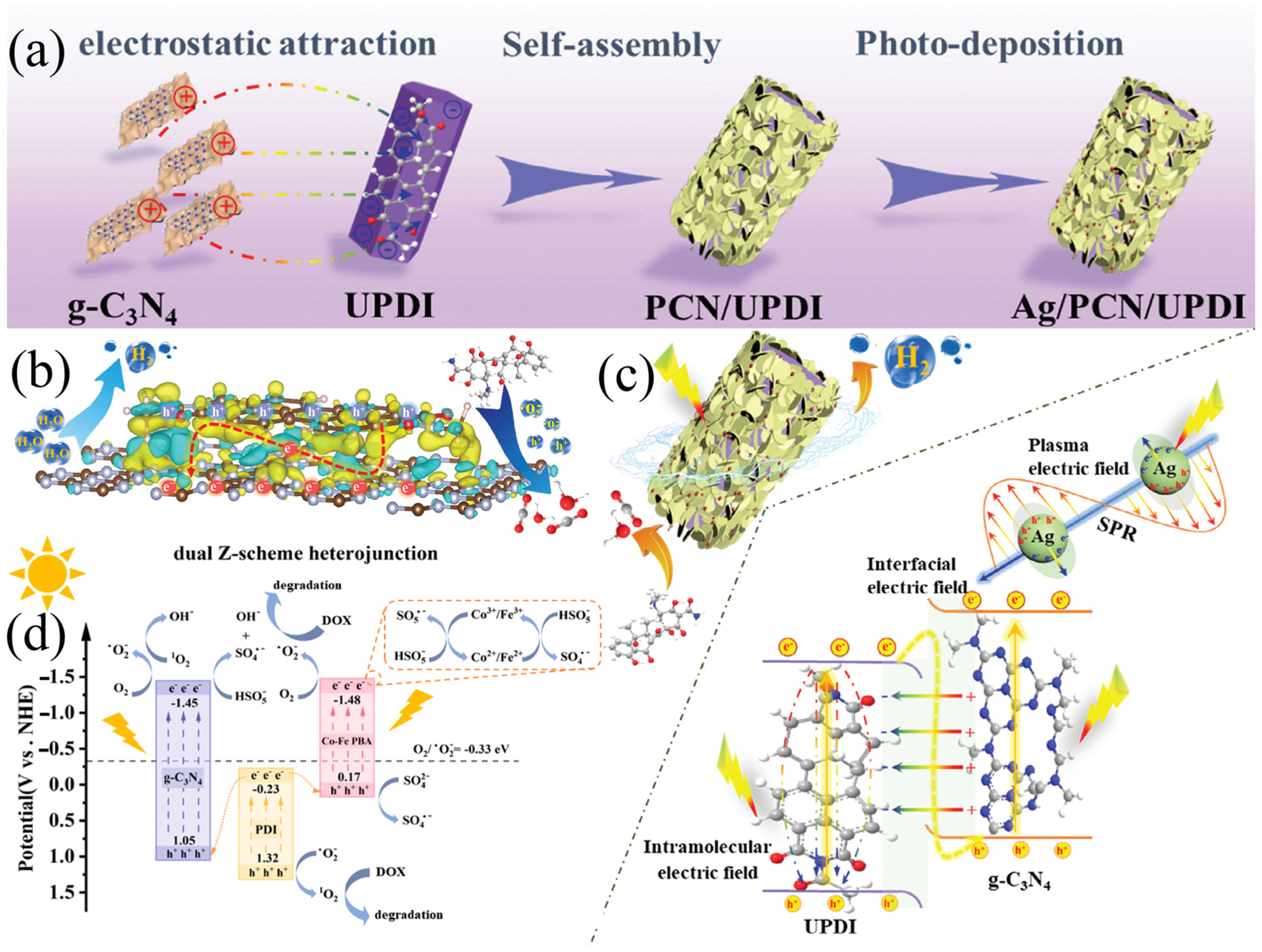
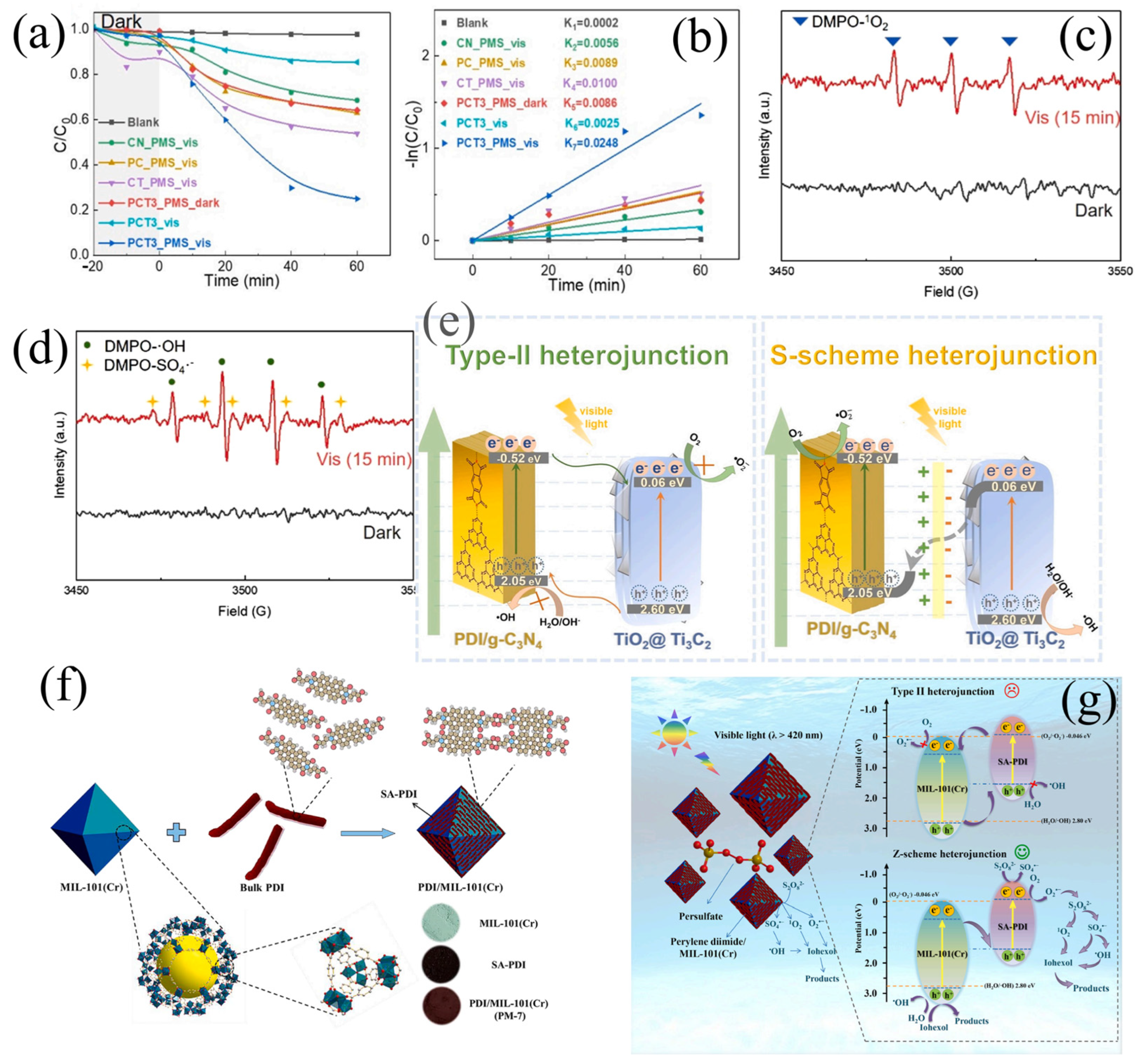
| Photocatalyst | Synthesis Method | Pollutants | Light Source | Time (min) | Efficiency (%) | Type | Reference |
|---|---|---|---|---|---|---|---|
| PDI/BiO2-x | Ultrasonic, self-assembly | RhB | Visible | 20 | 98.7 | II | Zhang et al. [128] |
| PDISA/AgBr | Co-precipitation | RhB | Visible | 20 | 97.8 | II | Xu et al. [129] |
| PDI/Bi2O4 | Water bath heating, ultrasonic | RhB | Visible | 25 | 98.6 | II | Zhang et al. [130] |
| PCN@UiO-66 | Solvent thermal | RhB | Visible | 140 | 99.0 | II | Mardiroosi et al. [131] |
| SAPDI/Bi4O7 | Self-assembly | RhB | Visible | 20 | 87.6 | II | Zhang et al. [132] |
| g-C3N4/PDI@ZnIn2S4 | Oil bath heating | RhB | Visible | 120 | 83.9 | Z | Zhu et al. [133] |
| PDI/g-C3N4/TiO2@Ti3C2 | Calcination | ATZ | Visible | 60 | 75.0 | S | Tang et al. [134] |
| H-PDI/NH2-MIL-101(Fe) | Hydrothermal | MA | Simulated sunlight | 180 | 91.1 | Z | Ren et al. [135] |
| In2O3/PDI/In2S3 | Self-assembly | SL | 420 nm | 80 | 80.9 | S | Chen et al. [136] |
| PDI/MIL-101(Cr) | Water bath heating | IOH | Visible | 35 | 100.0 | Z | Ji et al. [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Lou, J.; Huang, Y.; Chen, Y. Recent Advances in PDI-Based Heterojunction Photocatalysts for the Degradation of Organic Pollutants and Environmental Remediation. Catalysts 2025, 15, 565. https://doi.org/10.3390/catal15060565
Song X, Lou J, Huang Y, Chen Y. Recent Advances in PDI-Based Heterojunction Photocatalysts for the Degradation of Organic Pollutants and Environmental Remediation. Catalysts. 2025; 15(6):565. https://doi.org/10.3390/catal15060565
Chicago/Turabian StyleSong, Xiaofang, Jiahui Lou, Yaqiong Huang, and Yijiang Chen. 2025. "Recent Advances in PDI-Based Heterojunction Photocatalysts for the Degradation of Organic Pollutants and Environmental Remediation" Catalysts 15, no. 6: 565. https://doi.org/10.3390/catal15060565
APA StyleSong, X., Lou, J., Huang, Y., & Chen, Y. (2025). Recent Advances in PDI-Based Heterojunction Photocatalysts for the Degradation of Organic Pollutants and Environmental Remediation. Catalysts, 15(6), 565. https://doi.org/10.3390/catal15060565






