Improving the Thermal Stability of Xylanase XynASP from Aspergillus Saccharolyticus JOP 1030-1 Through Modular Assembly
Abstract
1. Introduction
2. Results
2.1. Mutation Design Based on Consensus Sequences
2.2. Thermal Stability Characteristics of the Chimerics
2.3. Thermal Stability Characteristics of the Single Mutants
2.4. Integration of Chimeric Z2 with Mutant A144T/V198M and Their Enzymatic Characterization
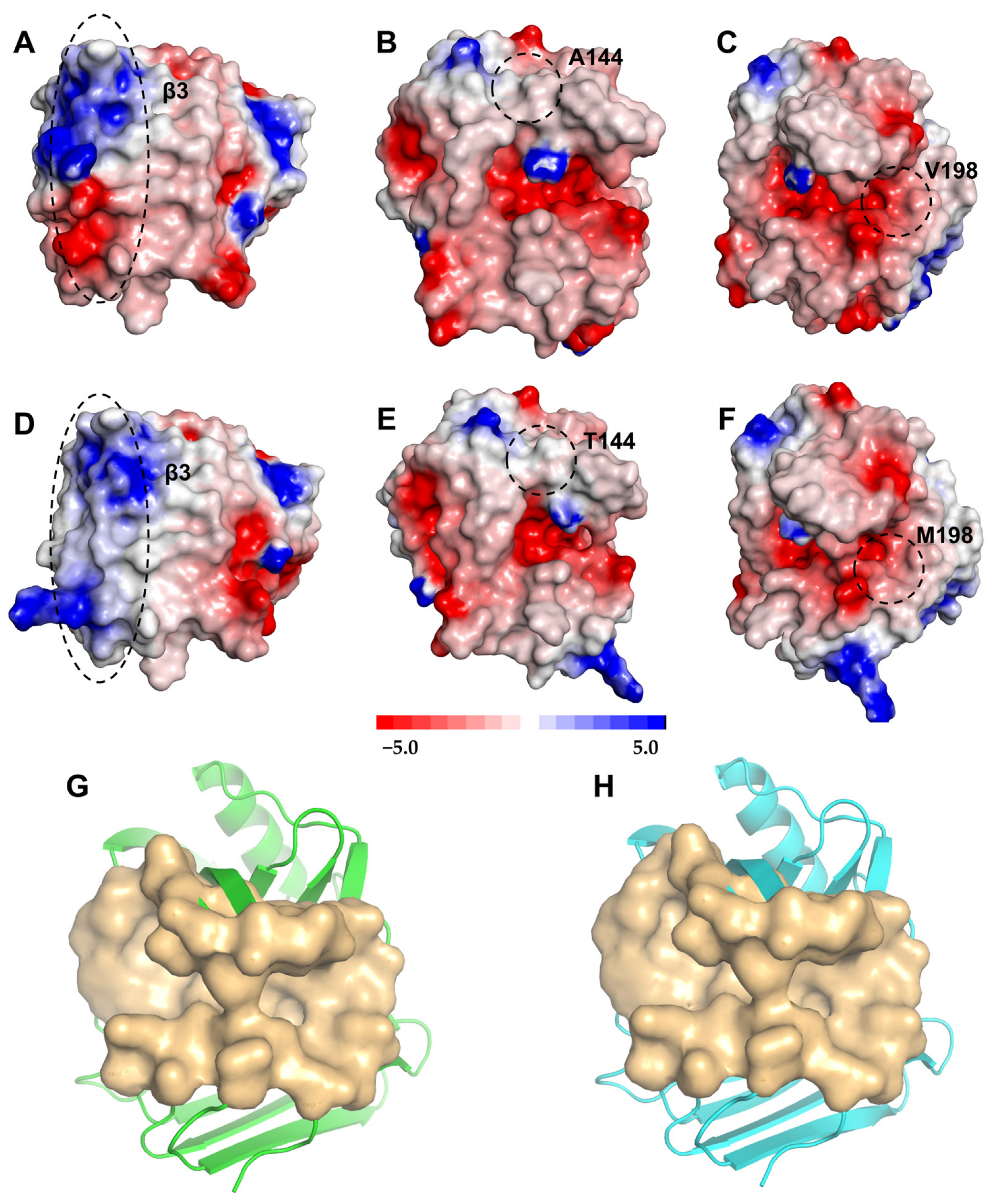
2.5. Structural Analysis of XynASP and ZM
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Site-Directed Mutagenesis
4.3. Protein Expression and Purification
4.4. Recombinant Xylanase Activity Assay
4.5. Enzymatic Property and Kinetic Parameter Measurements
4.6. Structural Analysis and Molecular Dynamics Simulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nezhad, N.G.; Rahman, R.N.Z.R.; Normi, Y.M.; Oslan, S.N.; Shariff, F.M.; Leow, T.C. Recent advances in simultaneous thermostability-activity improvement of industrial enzymes through structure modification. Int. J. Biol. Macromol. 2023, 232, 123440. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shi, Z.; Tian, W.; Liu, M.; Huang, S.; Liu, X.; Yin, H.; Wang, L. A thermostable and CBM2-linked GH10 xylanase from Thermobifida fusca for paper bleaching. Front. Bioeng. Biotechnol. 2022, 10, 939550. [Google Scholar] [CrossRef]
- Nakamura, T.; Takita, T.; Kuwata, K.; Mizutani, K.; Mikami, B.; Nakamura, S.; Yasukawa, K. Activity-stability trade-off observed in variants at position 315 of the gh10 xylanase xynr. Sci. Rep. 2024, 14, 7767. [Google Scholar] [CrossRef]
- Talens-Perales, D.; Jimenez-Ortega, E.; Sanchez-Torres, P.; Sanz-Aparicio, J.; Polaina, J. Phylogenetic, functional and structural characterization of a gh10 xylanase active at extreme conditions of temperature and alkalinity. Comput. Struct. Biotechnol. J. 2021, 19, 2676–2686. [Google Scholar] [CrossRef]
- Paës, G.; Berrin, J.; Beaugrand, J. Gh11 xylanases: Structure/function/properties relationships and applications. Biotechnol. Adv. 2012, 30, 564–592. [Google Scholar] [CrossRef]
- Boonyapakron, K.; Keiser, B.; Prabmark, K.; Aiewviriyasakul, K.; Arunrattanamook, N.; Jaruwat, A.; Chitnumsub, P.; Li, J.Y.; Wong, T.S.; Zhao, X.Q.; et al. Hyperthermophilic xylanase and thermophilicity analysis by molecular dynamic simulation with quantum mechanics. Appl. Microbiol. Biotechnol. 2024, 108, 526. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, C.; Dong, W.; Lu, H.; Yang, Y.; Li, W.; Xu, Y.; Li, X. Enhanced thermostability of xylanase xyna via computationally designed assembly of multiple n-terminal disulfide bridges. Process Biochem. 2024, 138, 67–78. [Google Scholar] [CrossRef]
- Phuyal, M.; Budhathoki, U.; Bista, D.; Shakya, S.; Shrestha, R.; Shrestha, A.K. Xylanase-producing microbes and their real-world application. Int. J. Chem. Eng. 2023, 2023, 3593035. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, J.; Liu, Y.; Chen, X.; Gao, T.; Li, N.; Huang, W.; Wu, M. Directed modification of a ghf11 thermostable xylanase ausm for enhancing inhibitory resistance towards syxip-i and application of ausmpkk in bread making. Foods 2023, 12, 3574. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, L.; Xu, Y.; Lu, H.; Wu, Q.; Li, W.; Zhang, C.; Li, X. Three molecular modification strategies to improve the thermostability of xylanase xyna from streptomyces rameus l2001. Foods 2023, 12, 879. [Google Scholar] [CrossRef]
- Glekas, P.D.; Kalantzi, S.; Dalios, A.; Hatzinikolaou, D.G.; Mamma, D. Biochemical and Thermodynamic Studies on a Novel Thermotolerant GH10 Xylanase from Bacillus safensis. Biomolecules 2022, 12, 790. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhang, Z.; Yang, J.; Turunen, O.; Xiong, H. High-temperature behavior of hyperthermostable thermotoga maritima xylanase xyn10b after designed and evolved mutations. Appl. Microbiol. Biotechnol. 2022, 106, 2017–2027. [Google Scholar] [CrossRef]
- You, S.; Zha, Z.; Jing, L.; Zhang, W.; Bai, Z.; Hu, Y.; Wang, X.; Chen, Y.; Chen, Z.; Wang, J.; et al. Improvement of xyl10c_ Δ n catalytic performance through loop engineering for lignocellulosic biomass utilization in feed and fuel industries. Biotechnol. Biofuels 2021, 14, 195. [Google Scholar] [CrossRef]
- Yin, X.; Li, J.F.; Wang, J.Q.; Tang, C.D.; Wu, M.C. Enhanced thermostability of a mesophilic xylanase by N-terminal replacement designed by molecular dynamics simulation. J. Sci. Food Agric. 2013, 93, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Song, C.; Cui, W.; Han, L.; Zhou, Z. Counteraction of stability-activity trade-off of nattokinase through flexible region shifting. Food Chem. 2023, 423, 136241. [Google Scholar] [CrossRef]
- Pinto, G.P.; Corbella, M.; Demkiv, A.O.; Kamerlin, S.C.L. Exploiting enzyme evolution for computational protein design. Trends Biochem. Sci. 2022, 47, 375–389. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Q.; Qu, G.; Feng, Y.; Reetz, M.T. Utility of b-factors in protein science: Interpreting rigidity, flexibility, and internal motion and engineering thermostability. Chem. Rev. 2019, 119, 1626–1665. [Google Scholar] [CrossRef]
- Li, T.; Wang, R.; Hua, B.; Cao, L.; Zhang, Q.; Zhai, Y.; Ling, S.; Wang, M.; Li, E. Improving the Thermal Stability of GH11 Xylanase XynASP through Cord Region Engineering. J. Agric. Food Chem. 2025, 73, 1516–1528. [Google Scholar] [CrossRef]
- Dotsenko, A.S.; Denisenko, Y.A.; Rozhkova, A.M.; Zorov, I.N.; Korotkova, O.G.; Sinitsyn, A.P. Enhancement of thermostability of gh10 xylanase e penicillium canescens directed by δδg calculations and structure analysis. Enzyme Microb. Technol. 2021, 152, 109938. [Google Scholar] [CrossRef]
- Bu, Y.; Cui, Y.; Peng, Y.; Hu, M.; Tian, Y.E.; Tao, Y.; Wu, B. Engineering improved thermostability of the gh11 xylanase from neocallimastix patriciarum via computational library design. Appl. Microbiol. Biotechnol. 2018, 102, 3675–3685. [Google Scholar] [CrossRef]
- Lai, Z.; Zhou, C.; Ma, X.; Xue, Y.; Ma, Y. Enzymatic characterization of a novel thermostable and alkaline tolerant GH10 xylanase and activity improvement by multiple rational mutagenesis strategies. Int. J. Biol. Macromol. 2021, 170, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Crean, R.M.; Biler, M.; van der Kamp, M.W.; Hengge, A.C.; Kamerlin, S.C.L. Loop Dynamics and Enzyme Catalysis in Protein Tyrosine Phosphatases. J. Am. Chem. Soc. 2021, 143, 3830–3845. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, J.; Wu, Y.; Wang, Z.; Zeng, M.; He, Z.; Chen, J.; Mu, W. Rational Design of Loop Dynamics for a Barrel-Shaped Enzyme by Introducing Disulfide Bonds. J. Agric. Food Chem. 2024, 72, 16541–16542. [Google Scholar] [CrossRef]
- Zheng, F.; Vermaas, J.V.; Zheng, J.; Wang, Y.; Tu, T.; Wang, X.; Xie, X.; Yao, B.; Beckham, G.T.; Luo, H. Activity and thermostability of gh5 endoglucanase chimeras from mesophilic and thermophilic parents. Appl. Environ. Microbiol. 2019, 85, e02079-18. [Google Scholar] [CrossRef]
- Liu, W.; Tu, T.; Gu, Y.; Wang, Y.; Zheng, F.; Zheng, J.; Wang, Y.; Su, X.; Yao, B.; Luo, H. Insight into the Thermophilic Mechanism of a Glycoside Hydrolase Family 5 β-Mannanase. J. Agric. Food Chem. 2019, 67, 473–483. [Google Scholar] [CrossRef]
- Li, T.; Zhu, X.; Ye, M.; Wang, M.; Zhu, J.; Shen, F.; Li, E. Rapid improvement in thermostability of gh11 xylanase (xynasp) from aspergillus saccharolyticus jop 1030-1 by tryptophan residue substitution. J. Biotech Res. 2022, 13, 26–39. [Google Scholar]
- Li, T.; Yang, S.; Wang, X.; Cai, H.; Wang, Y.; Li, C.; Li, E. Improving thermostability of gh11 xylanase xynasp by the design of loop region. Crystals 2022, 12, 1228. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, C.; Dong, W.; Lu, H.; Yang, Y.; Li, W.; Xu, Y.; Li, X. Simultaneously enhanced thermostability and catalytic activity of xylanase from streptomyces rameus l2001 by rigidifying flexible regions in loop regions of the n-terminus. J. Agric. Food. Chem. 2023, 71, 12785–12796. [Google Scholar] [CrossRef]
- Vucinic, J.; Novikov, G.; Montanier, C.Y.; Dumon, C.; Schiex, T.; Barbe, S. A Comparative Study to Decipher the Structural and Dynamics Determinants Underlying the Activity and Thermal Stability of GH-11 Xylanases. Int. J. Mol. Sci. 2021, 22, 5961. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Lu, H.; Wu, Q.; Wu, Y.; Li, W.; Li, X. Improvement of thermostability and catalytic efficiency of xylanase from myceliophthora thermophilar by n-terminal and c-terminal truncation. Front. Microbiol. 2024, 15, 1385329. [Google Scholar] [CrossRef]
- Ajeje, S.B.; Hu, Y.; Song, G.; Peter, S.B.; Afful, R.G.; Sun, F.; Asadollahi, M.A.; Amiri, H.; Abdulkhani, A.; Sun, H. Thermostable Cellulases/Xylanases from Thermophilic and Hyperthermophilic Microorganisms: Current Perspective. Front. Bioeng. Biotechnol. 2021, 9, 794304. [Google Scholar] [CrossRef] [PubMed]
- Marneth, K.; van den Elst, H.; Cramer-Blok, A.; Codee, J.; Overkleeft, H.S.; Aerts, J.M.F.G.; Ubbink, M.; Ben Bdira, F. Tuning the Transglycosylation Reaction of a GH11 Xylanase by a Delicate Enhancement of its Thumb Flexibility. Chembiochem 2021, 22, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Dai, S.; Chen, X.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. Alteration of chain-length selectivity and thermostability ofrhizopus oryzae lipase via virtual saturation mutagenesis coupled with disulfide bond design. Appl. Environ. Microbiol. 2023, 89, e01878-22. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhu, W.; Wu, Q.; Li, W.; Li, X. Improvement the thermostability and specific activity of acidic xylanase PjxA from Penicillium janthinellum via rigid flexible sites. Int. J. Biol. Macromol. 2024, 279, 135399. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.Y.; Chen, Z.L.; Li, Y.H. Enzymatic characterization and thermostability improvement of an acidophilic endoxylanase PphXyn11 from Paenibacillus physcomitrellae XB. Protein Expr. Purif. 2024, 219, 106482. [Google Scholar] [CrossRef]
- Meyer, M.M.; Hochrein, L.; Arnold, F.H. Structure-guided schema recombination of distantly related β-lactamases. Protein Eng. Des. Sel. 2006, 19, 563–570. [Google Scholar] [CrossRef]
- Heinzelman, P.; Snow, C.D.; Wu, I.; Nguyen, C.; Villalobos, A.; Govindarajan, S.; Minshull, J.; Arnold, F.H. A family of thermostable fungal cellulases created by structure-guided recombination. Proc. Natl. Acad. Sci. USA 2009, 106, 5610–5615. [Google Scholar] [CrossRef]
- Song, L.; Dumon, C.; Siguier, B.; André, I.; Eneyskaya, E.; Kulminskaya, A.; Bozonnet, S.; O Donohue, M.J. Impact of an n-terminal extension on the stability and activity of the gh11 xylanase from thermobacillus xylanilyticus. J. Biotechnol. 2014, 174, 64–72. [Google Scholar] [CrossRef]
- Corbella, M.; Pinto, G.P.; Kamerlin, S.C.L. Loop dynamics and the evolution of enzyme activity. Nat. Rev. Chem. 2023, 7, 536–547. [Google Scholar] [CrossRef]
- Yasuda, S.; Kazama, K.; Akiyama, T.; Kinoshita, M.; Murata, T. Elucidation of cosolvent effects thermostabilizing water-soluble and membrane proteins. J. Mol. Liq. 2020, 301, 112403. [Google Scholar] [CrossRef]
- Briganti, L.; Capetti, C.; Pellegrini, V.O.A.; Ghio, S.; Campos, E.; Nascimento, A.S.; Polikarpov, I. Structural and molecular dynamics investigations of ligand stabilization via secondary binding site interactions in Paenibacillus xylanivorans GH11 xylanase. Comput. Struct. Biotechnol. J. 2021, 19, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.; Zhang, Z.; Li, Z.; Meng, S.; Nian, B.; Hu, Y. Hook loop dynamics engineering transcended the barrier of activity-stability trade-off and boosted the thermostability of enzymes. Int. J. Biol. Macromol. 2024, 278, 134953. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Huang, H.; Rao, S.; Zhang, Q.; Zhou, J.; Li, J.; Du, G.; Liu, S. Significantly enhanced thermostability of aspergillus niger xylanase by modifying its highly flexible regions. J. Agric. Food Chem. 2022, 70, 4620–4630. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Xing, J.; Li, S.; Zhang, L.; Shen, C.; Hong, J.; Huang, S.; Li, T.; Wei, L.; Ding, R. Enhancing the catalytic performance of xylanase XynASP through semi-rational design in the cord region to promote its application in juice clarification. Int. J. Biol. Macromol. 2025, 305, 141138. [Google Scholar] [CrossRef]
- Wang, L.; Cao, K.; Pedroso, M.M.; Wu, B.; Gao, Z.; He, B.; Schenk, G. Sequence- and structure-guided improvement of the catalytic performance of a gh11 family xylanase from bacillus subtilis. J. Biol. Chem. 2021, 297, 101262. [Google Scholar] [CrossRef]
- Dong, R.; Liu, X.; Wang, Y.; Qin, X.; Wang, X.; Zhang, H.; Wang, Y.; Luo, H.; Yao, B.; Bai, Y.; et al. Fusion of a proline-rich oligopeptide to the C-terminus of a ruminal xylanase improves catalytic efficiency. Bioengineered 2022, 13, 10482–10492. [Google Scholar] [CrossRef]
- Sinha, R.; Shukla, P. Current trends in protein engineering: Updates and progress. Curr. Protein Pept. Sci. 2019, 20, 398–407. [Google Scholar] [CrossRef]
- Tsou, C.L. Conformational flexibility of enzyme active sites. Science 1993, 262, 380–381. [Google Scholar] [CrossRef]





| Enzyme | Segment | Original Sequence | Substituted Sequence |
|---|---|---|---|
| Z1 | β1 | 1APVASLEEEEERASNFSSALAARSTASSTGWSN33 | 21DTTITQNQTGYDN33 |
| Z2 | β3 | 46GDVEYTNG53 | 46GTVSMTLH53 |
| Z3 | β6 | 80TITYSGSWTS90 | 80TVTYNASFNP90 |
| Z4 | β7 | 94SNSYLSVYGWTTS106 | 94GNAYLTLYGWYRN106 |
| Z5 | α1 | 175TTANHFNAWAAL186 | 175TIGNHFDAWARA186 |
| Z6 | β14 | 206SGSASITVS214 | 206SGSSTVSIS214 |
| Enzyme | Optimal Temperature (°C) | Specific Activity (U/mg) | t1/245 °C (min) | t1/250 °C (min) | t1/255 °C (min) | Tm (°C) |
|---|---|---|---|---|---|---|
| Wild type | 45 | 51.0 ± 2.4 | 22.0 | 5.3 | 4.3 | 46.0 |
| Z2 | 60 | 222.1 ± 1.3 | 339.8 | 45.1 | 11.9 | |
| A144T/V198M | 55 | 32.8 ± 1.9 | 44.7 | 22.7 | 8.2 | |
| ZM | 60 | 197.4 ± 1.4 | 343.5 | 140.3 | 37.6 | 64.5 |
| Enzyme | Vmax (µmol·min−1 mg−1) | Km (mg/mL) | kcat (s−1) | kcat/Km (mL·mg−1 s−1) |
|---|---|---|---|---|
| Wild type | 75.7 ± 3.5 | 0.18 ± 0.02 | 11.5 ± 0.5 | 63.8 ± 7.7 |
| Z2 | 361.6 ± 16.8 | 0.34 ± 0.03 | 131.0 ± 6.1 | 385.3 ± 38.5 |
| A144T/V198M | 91.4 ± 9.6 | 0.84 ± 0.15 | 55.6 ± 0.1 | 66.1 ± 14.9 |
| ZM | 399.1 ± 23.3 | 0.48 ± 0.06 | 167.4 ± 9.8 | 349.2 ± 46.3 |
| Enzyme | SASA (Å2) | Active Pocket Region Characteristics | |
|---|---|---|---|
| Surface Area (Å2) | Volume (Å3) | ||
| Wild type | 8370.2 | 643.0 | 794.3 |
| ZM | 8297.7 | 643.5 | 793.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhang, Q.; Zhao, J.; Fu, X.; Wang, M.; Liu, Y.; Wang, H.; Xi, H.; Li, T. Improving the Thermal Stability of Xylanase XynASP from Aspergillus Saccharolyticus JOP 1030-1 Through Modular Assembly. Catalysts 2025, 15, 563. https://doi.org/10.3390/catal15060563
Zhu J, Zhang Q, Zhao J, Fu X, Wang M, Liu Y, Wang H, Xi H, Li T. Improving the Thermal Stability of Xylanase XynASP from Aspergillus Saccharolyticus JOP 1030-1 Through Modular Assembly. Catalysts. 2025; 15(6):563. https://doi.org/10.3390/catal15060563
Chicago/Turabian StyleZhu, Jinjin, Qing Zhang, Jiaxin Zhao, Xueting Fu, Mingzhu Wang, Yan Liu, Hui Wang, Hongli Xi, and Tongbiao Li. 2025. "Improving the Thermal Stability of Xylanase XynASP from Aspergillus Saccharolyticus JOP 1030-1 Through Modular Assembly" Catalysts 15, no. 6: 563. https://doi.org/10.3390/catal15060563
APA StyleZhu, J., Zhang, Q., Zhao, J., Fu, X., Wang, M., Liu, Y., Wang, H., Xi, H., & Li, T. (2025). Improving the Thermal Stability of Xylanase XynASP from Aspergillus Saccharolyticus JOP 1030-1 Through Modular Assembly. Catalysts, 15(6), 563. https://doi.org/10.3390/catal15060563







