Halloysite@Polydopamine Nanoplatform for Ultrasmall Pd and Cu Nanoparticles: Suitable Catalysts for Hydrogenation and Reduction Reactions
Abstract
1. Introduction
2. Results and Discussion
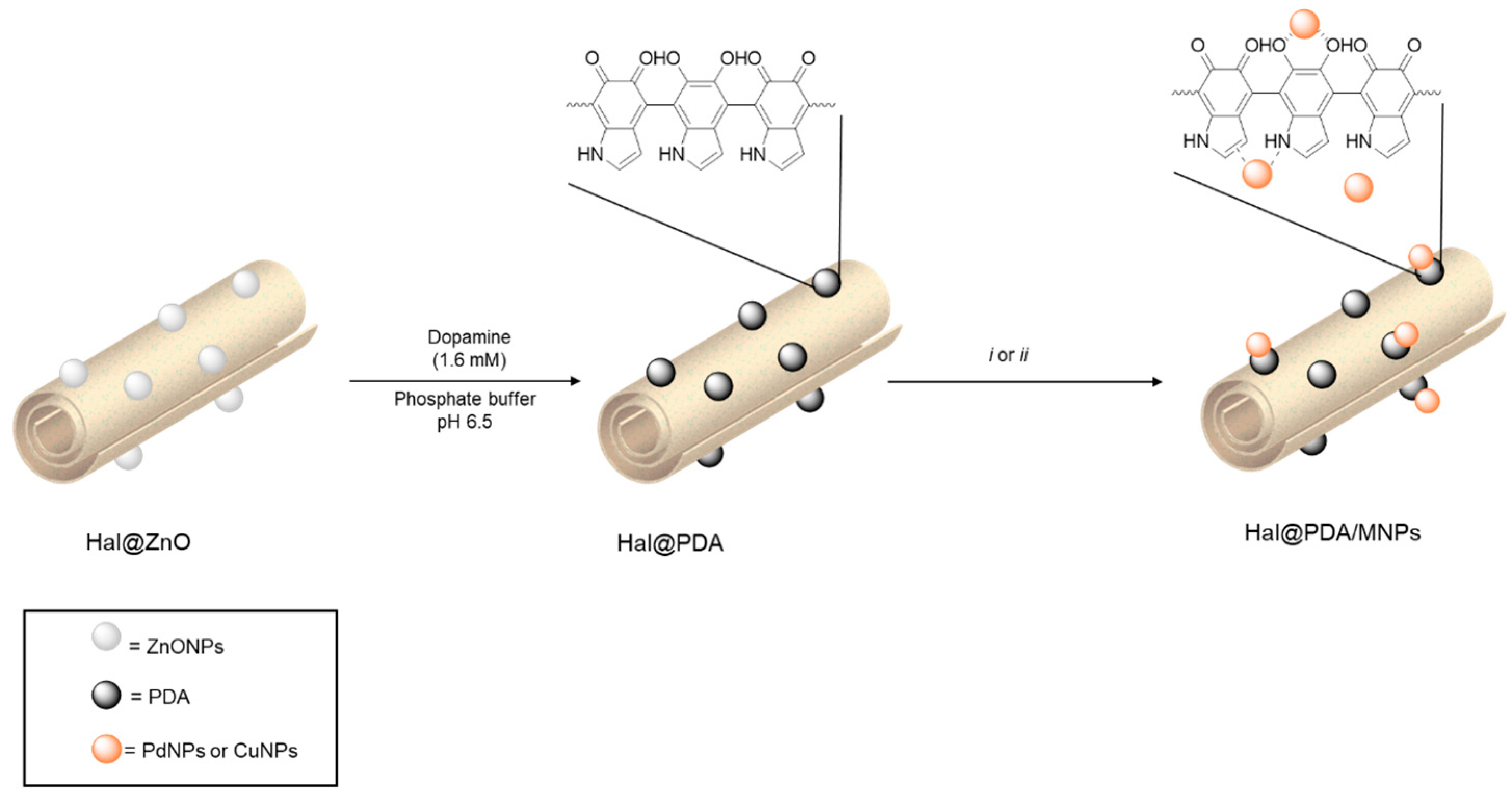
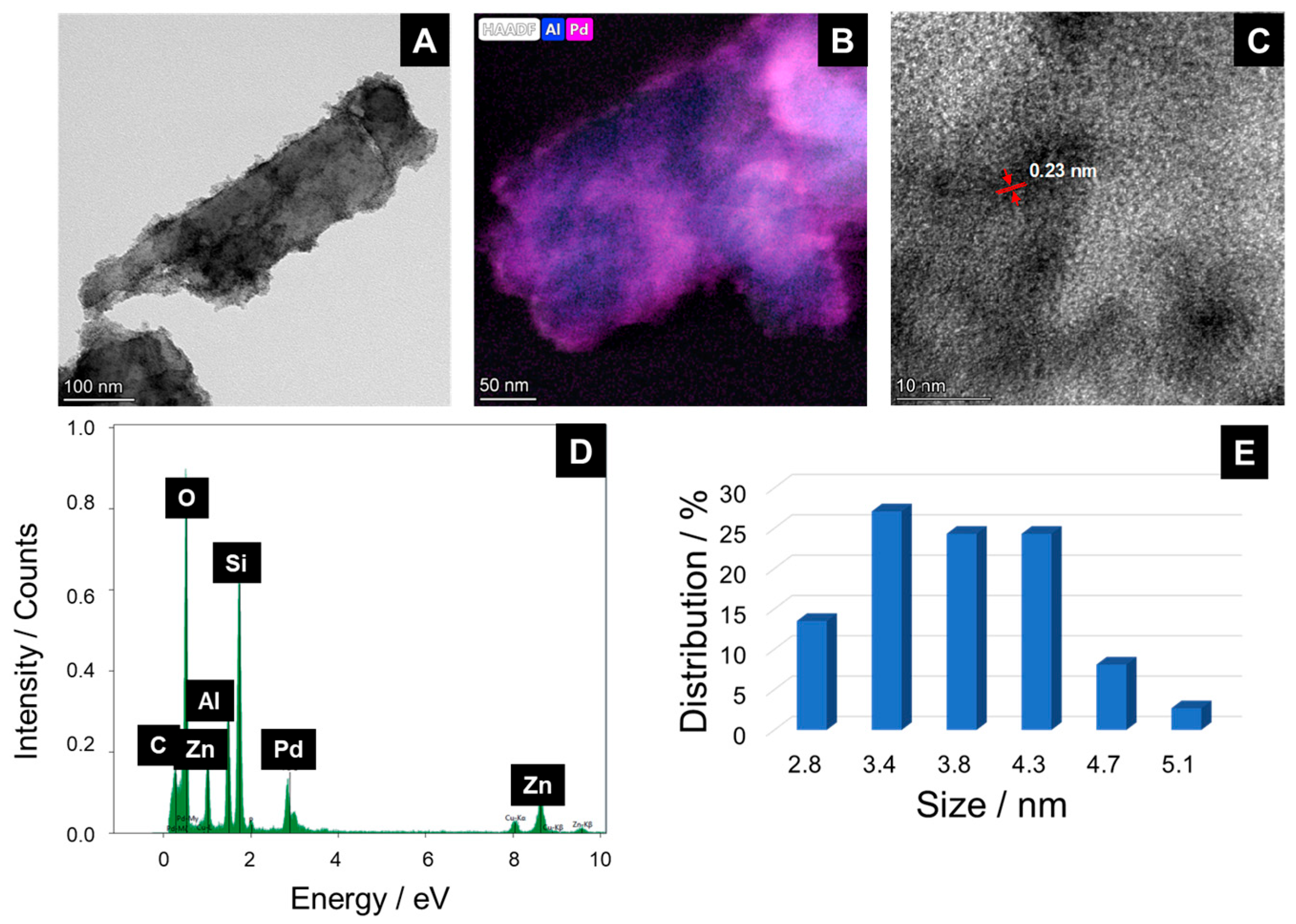
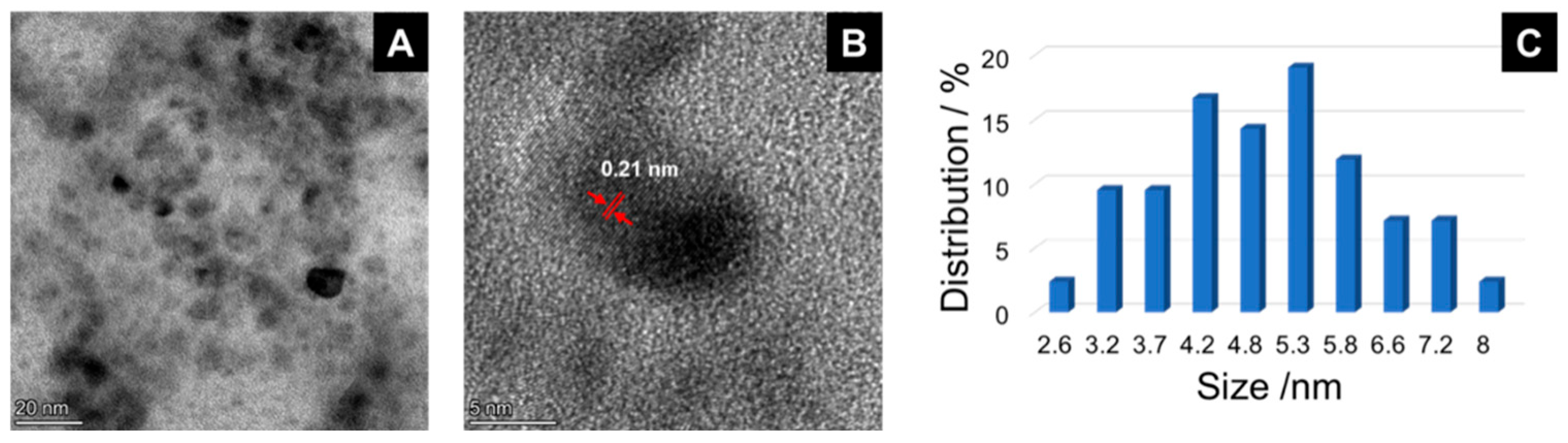
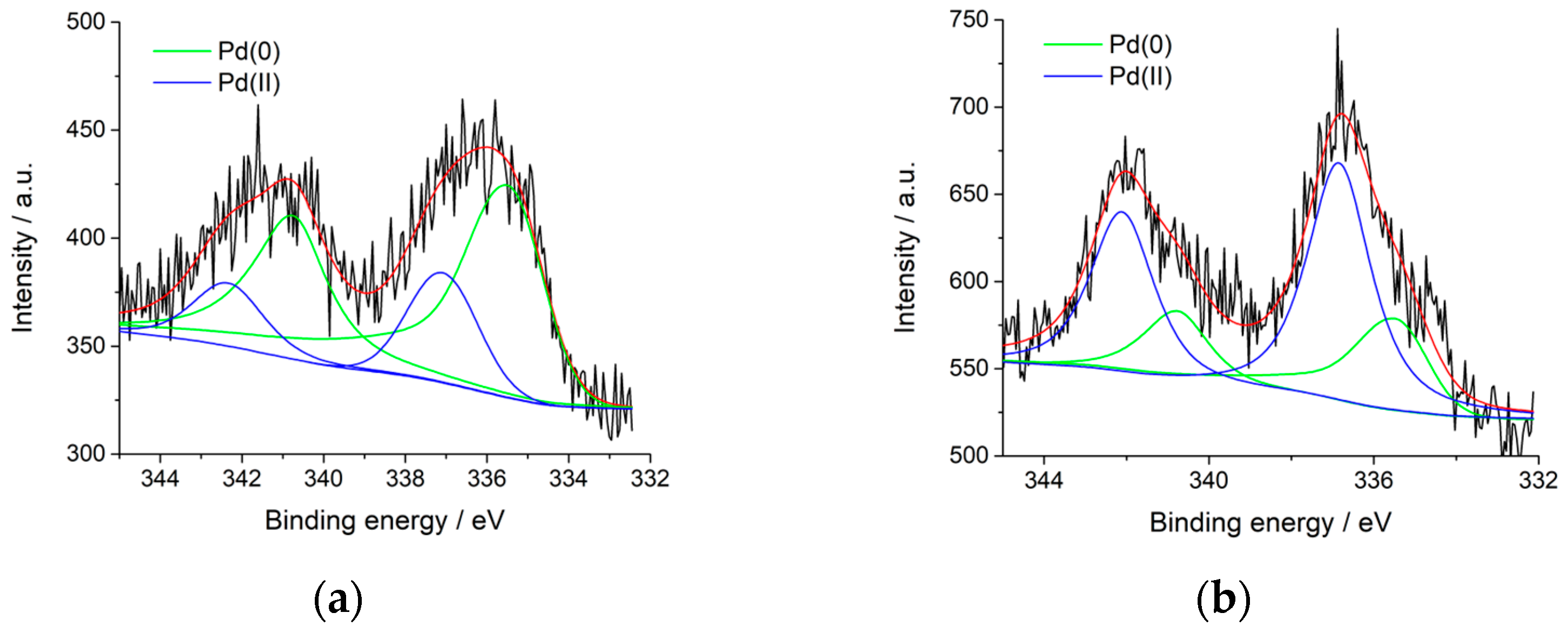
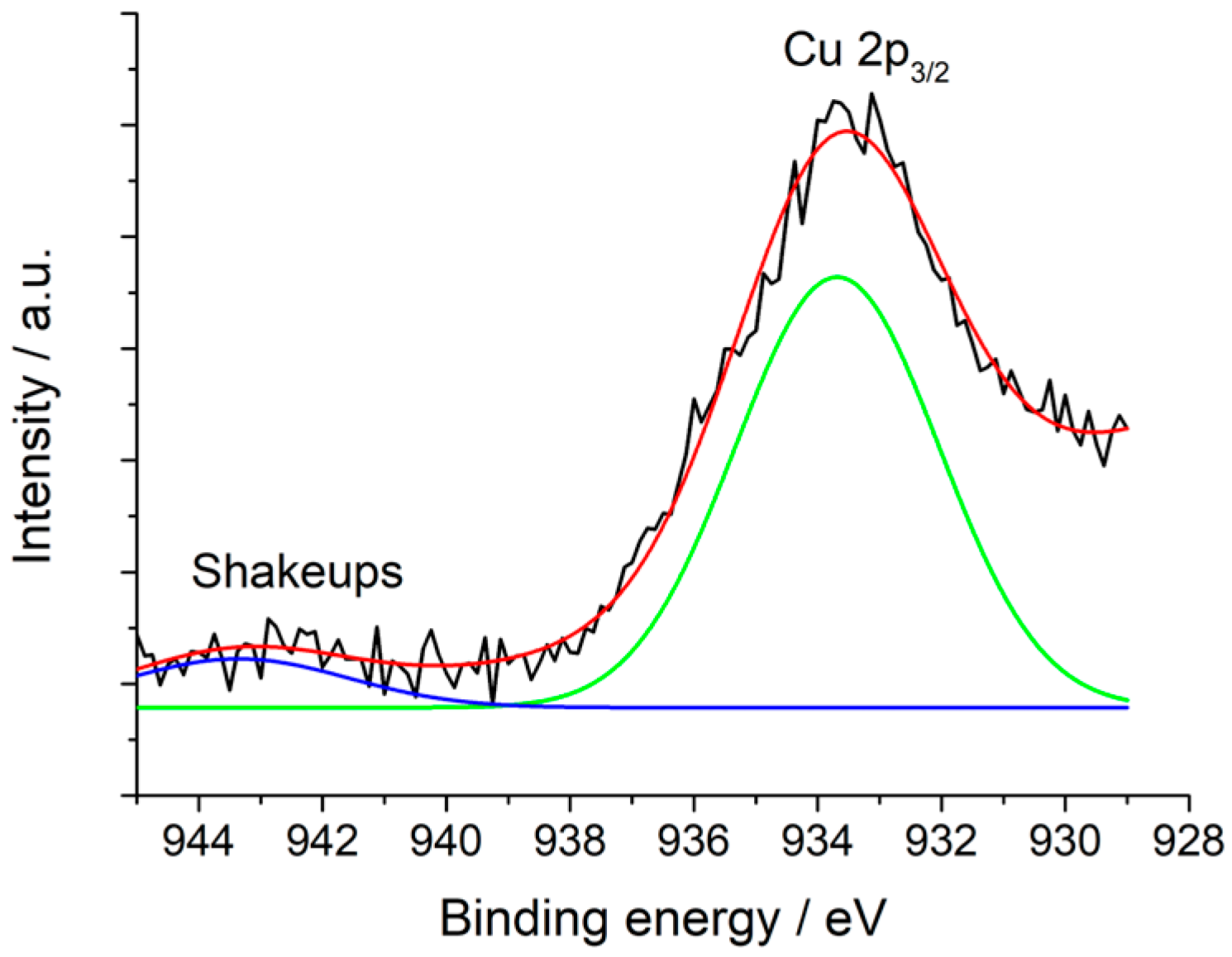
2.1. Synthesis and Characterization of the Hal@PDA/Supported Metal Nanoparticles
2.2. Catalytic Experiments
2.2.1. Cinnamaldehyde Hydrogenation
2.2.2. 4-Nitrophenol Reduction
3. Materials and Methods
3.1. General Procedure for the Synthesis of the Hal@PDA/PdNP Nanomaterial
3.2. General Procedure for the Synthesis of Hal@PDA/CuNP Nanomaterials
3.3. General Procedure for the Hydrogenation of Cinnamaldehyde (I)
3.4. 4-NP Reduction Reaction
3.5. Recyclability of Hal@PDA/CuNP Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Structured clay minerals-based nanomaterials for sustainable photo/thermal carbon dioxide conversion to cleaner fuels: A critical review. Sci. Total Environ. 2022, 845, 157206. [Google Scholar] [CrossRef] [PubMed]
- Calvino, M.M.; Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Yadav, R.P.; Dolgan, K.; Lvov, Y.M. The Emerging Role of Halloysite Clay Nanotube Formulations in Cosmetics and Topical Drug Delivery. ACS Appl. Bio Mater. 2025, 8, 2674–2690. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Jana, S. A tubular nanoreactor directing the formation of in situ iron oxide nanorods with superior photocatalytic activity. Environ. Sci. Nano 2017, 4, 596–603. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Rubtsova, M.; Glotov, A.; Vinokurov, V.; Vutolkina, A.; Fakhrullin, R.; Lvov, Y. Architectural design of core–shell nanotube systems based on aluminosilicate clay. Nanoscale Adv. 2022, 4, 2823–2835. [Google Scholar] [CrossRef] [PubMed]
- Stavitskaya, A.; Glotov, A.; Pouresmaeil, F.; Potapenko, K.; Sitmukhanova, E.; Mazurova, K.; Ivanov, E.; Kozlova, E.; Vinokurov, V.; Lvov, Y. CdS Quantum Dots in Hierarchical Mesoporous Silica Templated on Clay Nanotubes: Implications for Photocatalytic Hydrogen Production. ACS Appl. Nano Mater. 2022, 5, 605–614. [Google Scholar] [CrossRef]
- Yaghoubi, S.; Sadjadi, S.; Zhong, X.; Yuan, P.; Heravi, M.M. Clay-supported bio-based Lewis acid ionic liquid as a potent catalyst for the dehydration of fructose to 5-hydroxymthylfurfural. Sci. Rep. 2024, 14, 82. [Google Scholar] [CrossRef]
- Casiello, M.; Savino, S.; Massaro, M.; Liotta, L.F.; Nicotra, G.; Pastore, C.; Fusco, C.; Monopoli, A.; D’Accolti, L.; Nacci, A.; et al. Multifunctional halloysite and hectorite catalysts for effective transformation of biomass to biodiesel. Appl. Clay Sci. 2023, 242, 107048. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Y.; Hu, C.; Deng, X.; Zhang, L.; Chen, S.; Zhang, H. Catalytic Nano-Gold Immobilized on the Inner Surfaces of Halloysite Nanotubes for Selective Reduction of Nitroaromatics. ACS Appl. Nano Mater. 2024, 7, 16669–16678. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, Z.; Kim, K.; Yu, T. Natural halloysite nanotubes enclosing PdAg alloy nanoparticles as nanoreactors with enhanced catalytic performance. J. Colloid Interface Sci. 2025, 700, 138531. [Google Scholar] [CrossRef]
- Glotov, A.; Vutolkina, A.; Pimerzin, A.; Vinokurov, V.; Lvov, Y. Clay nanotube-metal core/shell catalysts for hydroprocesses. Chem. Soc. Rev. 2021, 50, 9240–9277. [Google Scholar] [CrossRef]
- Lan, Y.; Ma, Y.; Hou, Q.; Luo, Z.; Wang, L.; Ran, M.; Dai, T. Immobilization of palladium nanoparticles on polydopamine spheres with superior activity and reusability in Heck reaction. J. Catal. 2024, 430, 115333. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, Y.; Yang, G.; Liu, M.; Zhu, G.; Sheng, X.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. Mussel-inspired fabrication of halloysite nanotube-based magnetic composites as catalysts for highly efficient degradation of organic dyes. Appl. Clay Sci. 2020, 198, 105835. [Google Scholar] [CrossRef]
- Liu, J. Catalysis by Supported Single Metal Atoms. ACS Catal. 2017, 7, 34–59. [Google Scholar] [CrossRef]
- Massaro, M.; Casiello, M.; D’Accolti, L.; Lazzara, G.; Nacci, A.; Nicotra, G.; Noto, R.; Pettignano, A.; Spinella, C.; Riela, S. One-pot synthesis of ZnO nanoparticles supported on halloysite nanotubes for catalytic applications. Appl. Clay Sci. 2020, 189, 105527. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Massaro, M.; d’Ischia, M.; D’Errico, G.; Gallucci, N.; Gruttadauria, M.; Licciardi, M.; Liotta, L.F.; Nicotra, G.; Sfuncia, G.; et al. Site-specific halloysite functionalization by polydopamine: A new synthetic route for potential near infrared-activated delivery system. J. Colloid Interface Sci. 2022, 606, 1779–1791. [Google Scholar] [CrossRef]
- Falanga, A.P.; Massaro, M.; Borbone, N.; Notarbartolo, M.; Piccialli, G.; Liotta, L.F.; Sanchez-Espejo, R.; Viseras Iborra, C.; Raymo, F.M.; Oliviero, G.; et al. Carrier capability of halloysite nanotubes for the intracellular delivery of antisense PNA targeting mRNA of neuroglobin gene. J. Colloid Interface Sci. 2024, 663, 9–20. [Google Scholar] [CrossRef]
- Yi, F.; DeLisio, J.B.; Nguyen, N.; Zachariah, M.R.; LaVan, D.A. High heating rate decomposition dynamics of copper oxide by nanocalorimetry-coupled time-of-flight mass spectrometry. Chem. Phys. Lett. 2017, 689, 26–29. [Google Scholar] [CrossRef]
- Zheng, C.; Cao, J.; Zhang, Y.; Zhao, H. Insight into the Oxidation Mechanism of a Cu-Based Oxygen Carrier (Cu → Cu2O → CuO) in Chemical Looping Combustion. Energy Fuels 2020, 34, 8718–8725. [Google Scholar] [CrossRef]
- Gil, S.; Garcia-Vargas, J.M.; Liotta, L.F.; Pantaleo, G.; Ousmane, M.; Retailleau, L.; Giroir-Fendler, A. Catalytic Oxidation of Propene over Pd Catalysts Supported on CeO2, TiO2, Al2O3 and M/Al2O3 Oxides (M = Ce, Ti, Fe, Mn). Catalysts 2015, 5, 671–689. [Google Scholar] [CrossRef]
- Massaro, M.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; Viseras Iborra, C.; Cavallaro, G.; García-Villén, F.; Guernelli, S.; Lazzara, G.; Miele, D.; Sainz-Díaz, C.I.; et al. Ciprofloxacin carrier systems based on hectorite/halloysite hybrid hydrogels for potential wound healing applications. Appl. Clay Sci. 2021, 215, 106310. [Google Scholar] [CrossRef]
- Al Abdullah, K.; Awad, S.; Zaraket, J.; Salame, C. Synthesis of ZnO Nanopowders By Using Sol-Gel and Studying Their Structural and Electrical Properties at Different Temperature. Energy Procedia 2017, 119, 565–570. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Genet, M.J.; Eloy, P.; Ruiz, P.; Gaigneaux, E.M. Determination of the Size of Supported Pd Nanoparticles by X-ray Photoelectron Spectroscopy. Comparison with X-ray Diffraction, Transmission Electron Microscopy, and H2 Chemisorption Methods. J. Phys. Chem. C 2010, 114, 16677–16684. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Sun, H.; Shi, L.; Wei, J.; Xu, D.; Zhang, S.; Zhang, J.; Wang, S.; Sun, H. Uncovering catalytic activity of Cu species on boron/nitrogen co–doped carbon nanotubes for efficient hydrogenation of nitroaromatics: Beyond the size of metal active center. Compos. Part B Eng. 2025, 293, 112112. [Google Scholar] [CrossRef]
- Sanakal, S.I.; Das, A.; Babu, A.; Kar, P.; Nutalapati, V.; Datta, K.K.R.; Banerjee, S.; Maji, S. Alginate-aminoclay/CuO nanocomposite beads: A sustainable and green approach for catalytic reduction of toxic nitroaromatic compounds. J. Environ. Chem. Eng. 2025, 13, 115398. [Google Scholar] [CrossRef]
- Xu, X.; Ma, J.; Kui, B.; Zhu, G.; Jia, G.; Wu, F.; Gao, P.; Ye, W. Effect of Crystal Planes of Pd and the Structure of Interfacial Water on the Electrocatalytic Hydrogenation of Alkynes to Alkenes. ACS Appl. Nano Mater. 2023, 6, 5357–5364. [Google Scholar] [CrossRef]
- Sahiner, M.; Demirci, S.; Sahiner, N. Enhanced Bioactive Properties of Halloysite Nanotubes via Polydopamine Coating. Polymers 2022, 14, 4346. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Geng, P.; Li, Q. Recent Advances in Selective Hydrogenation of Cinnamaldehyde over Supported Metal-Based Catalysts. ACS Catal. 2020, 10, 2395–2412. [Google Scholar] [CrossRef]
- Patil, K.N.; Manikanta, P.; Srinivasappa, P.M.; Jadhav, A.H.; Nagaraja, B.M. State-of-the-art and perspectives in transition metal-based heterogeneous catalysis for selective hydrogenation of cinnamaldehyde. J. Environ. Chem. Eng. 2023, 11, 109168. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, Z.; Wang, Y.; Zhang, P.; Wang, Y.; Qu, J. Ruthenium nanoparticles loaded on multiwalled carbon nanotubes for liquid-phase hydrogenation of fine chemicals: An exploration of confinement effect. J. Catal. 2015, 329, 95–106. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, Z.; Wang, Y.; Qu, J. Ruthenium nanoparticles loaded on functionalized graphene for liquid-phase hydrogenation of fine chemicals: Comparison with carbon nanotube. J. Catal. 2016, 333, 8–16. [Google Scholar] [CrossRef]
- Li, L.; Gao, G.; Zheng, J.; Shi, X.; Liu, Z. Three-dimensional graphene aerogel supported Ir nanocomposite as a highly efficient catalyst for chemoselective cinnamaldehyde hydrogenation. Diam. Relat. Mater. 2019, 91, 272–282. [Google Scholar] [CrossRef]
- Zhong, R.-Y.; Sun, K.-Q.; Hong, Y.-C.; Xu, B.-Q. Impacts of Organic Stabilizers on Catalysis of Au Nanoparticles from Colloidal Preparation. ACS Catal. 2014, 4, 3982–3993. [Google Scholar] [CrossRef]
- Fujiwara, S.; Takanashi, N.; Nishiyabu, R.; Kubo, Y. Boronate microparticle-supported nano-palladium and nano-gold catalysts for chemoselective hydrogenation of cinnamaldehyde in environmentally preferable solvents. Green Chem. 2014, 16, 3230–3236. [Google Scholar] [CrossRef]
- Patil, K.N.; Manikanta, P.; Srinivasappa, P.M.; Jadhav, A.H.; Nagaraja, B.M. Exploring the confined space and active sites of Ni@OCNTs catalyst for chemoselective hydrogenation of cinnamaldehyde to hydrocinnamaldehyde. J. Environ. Chem. Eng. 2022, 10, 108208. [Google Scholar] [CrossRef]
- Wang, S.; Wu, B.; Zhang, Q.; Li, Y.; Zhu, L.; Yu, H.; Yin, H. Design of Pt@Sn core–shell nanocatalysts for highly selective hydrogenation of cinnamaldehyde to prepare cinnamyl alcohol. Chem. Eng. J. 2024, 488, 151019. [Google Scholar] [CrossRef]


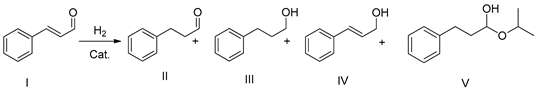 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | t (h) | Conv. (%) a | II Yield (%) a | III Yield (%) a | V Yield (%) a | TOF (s−1) c | |
| 1 b | 0.5 | 16 | 100 | 68 | 32 | / | 0.02 |
| 1 b* | 0.5 | 16 | 100 | 69 | 31 | / | |
| 2 | 0.5 | 5 | 100 | 77 | 17 | 6 | 0.06 |
| 2 * | 0.5 | 5 | 100 | 86 | 14 | / | |
| 2 ** | 0.5 | 5 | 100 | 86 | 14 | / | |
| 2 *** | 0.5 | 5 | 100 | 80 | 13 | 7 | |
| 2 **** | 0.5 | 5 | 100 | 83 | 9 | 8 | |
| 2 ***** | 0.5 | 5 | 99 | 83 | 10 | 6 | |
| 3 | 0.1 | 5 | 100 | 89 | 9 | 2 | 0.06 |
| 3 * | 0.1 | 5 | 100 | 95 | 4 | 1 | |
| 4 | 0.1 | 3 | 69 | 54 | 7 | 8 | 0.06 |
| 4 * | 0.1 | 3 | 100 | 91 | 3 | 6 | 0.1 |
| 4 ** | 0.1 | 3 | 100 | 96 | 3 | 1 | |
| 4 *** | 0.1 | 3 | 100 | 91 | 6 | 3 | |
| Catalyst | TOF/s−1 | Ref. |
|---|---|---|
| Ru/CNTs | 0.17 | [31] |
| Ru/TEGO | 0.033 | [32] |
| Ir/GA | 0.005 | [33] |
| Au-PVP/SiO2-12UVO | 0.028 | [34] |
| Pd/BP | 0.014 | [35] |
| Ni-CNT | 0.0002 | [36] |
| Pt/SiO2 | 0.013 | [37] |
| Pt-SnO2/SiO2 | 0.03 | [37] |
| Hal@PDA/PdNPs | 0.1 | This work |
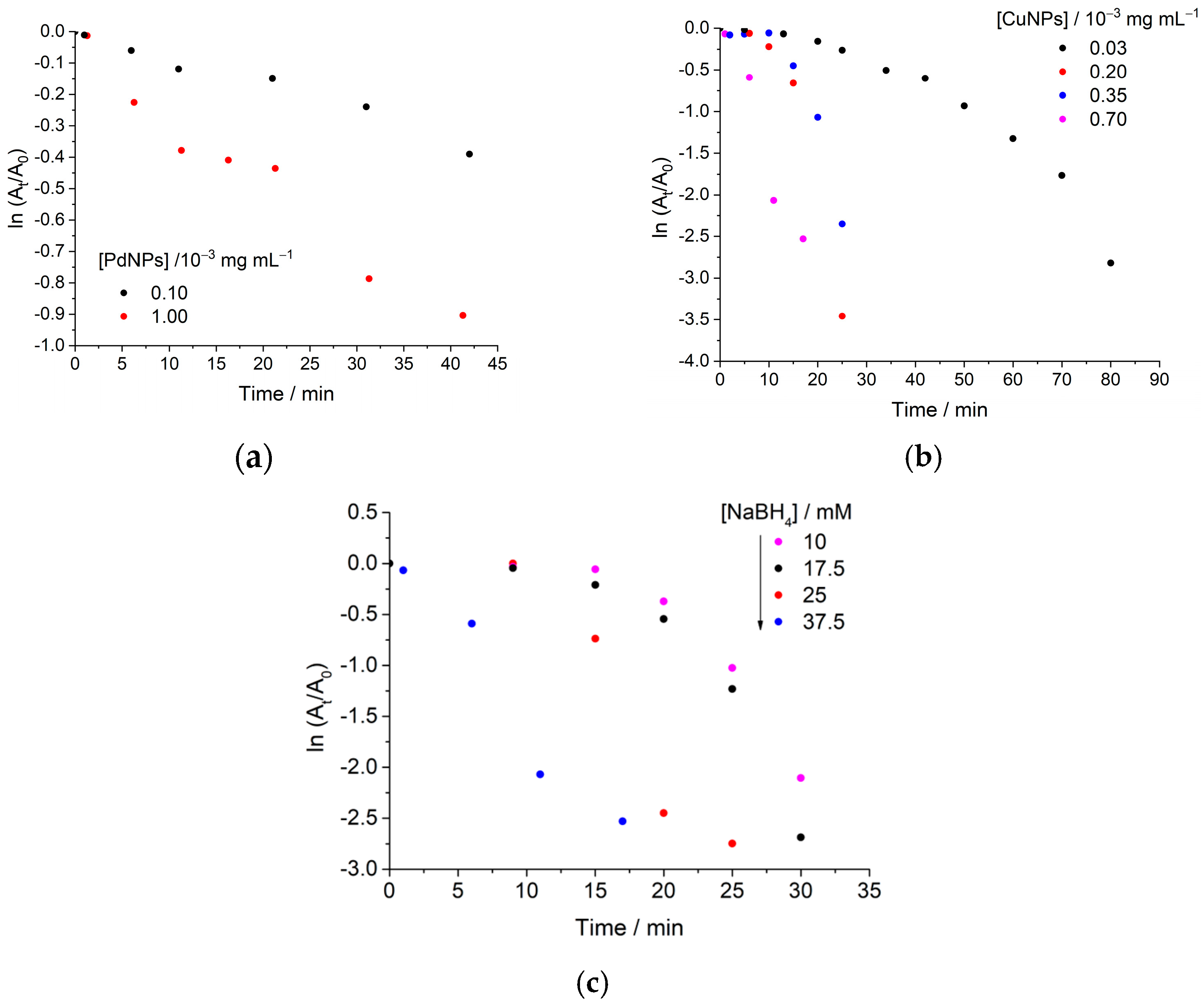
| Entry | MNP Concentration/ ×10−3 mg mL−1 | kapp/×10−3 min−1 |
|---|---|---|
| Hal@PDA/PdNPs | ||
| 1 | 1.00 | 22 |
| 2 | 0.10 | 8 |
| Hal@PDA/CuNPs | ||
| 4 | 0.70 | 160 |
| 5 | 0.35 | 80 |
| 6 | 0.20 | 70 |
| 7 | 0.03 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaro, M.; D’Acunzi, C.; Paganelli, S.; Alfieri, M.L.; Liotta, L.F.; Lopez-Galindo, A.; de Melo Barbosa, R.; Piccolo, O.; Sánchez-Espejo, R.; Viseras, C.; et al. Halloysite@Polydopamine Nanoplatform for Ultrasmall Pd and Cu Nanoparticles: Suitable Catalysts for Hydrogenation and Reduction Reactions. Catalysts 2025, 15, 1029. https://doi.org/10.3390/catal15111029
Massaro M, D’Acunzi C, Paganelli S, Alfieri ML, Liotta LF, Lopez-Galindo A, de Melo Barbosa R, Piccolo O, Sánchez-Espejo R, Viseras C, et al. Halloysite@Polydopamine Nanoplatform for Ultrasmall Pd and Cu Nanoparticles: Suitable Catalysts for Hydrogenation and Reduction Reactions. Catalysts. 2025; 15(11):1029. https://doi.org/10.3390/catal15111029
Chicago/Turabian StyleMassaro, Marina, Chiara D’Acunzi, Stefano Paganelli, Maria Laura Alfieri, Leonarda F. Liotta, Alberto Lopez-Galindo, Raquel de Melo Barbosa, Oreste Piccolo, Rita Sánchez-Espejo, César Viseras, and et al. 2025. "Halloysite@Polydopamine Nanoplatform for Ultrasmall Pd and Cu Nanoparticles: Suitable Catalysts for Hydrogenation and Reduction Reactions" Catalysts 15, no. 11: 1029. https://doi.org/10.3390/catal15111029
APA StyleMassaro, M., D’Acunzi, C., Paganelli, S., Alfieri, M. L., Liotta, L. F., Lopez-Galindo, A., de Melo Barbosa, R., Piccolo, O., Sánchez-Espejo, R., Viseras, C., & Riela, S. (2025). Halloysite@Polydopamine Nanoplatform for Ultrasmall Pd and Cu Nanoparticles: Suitable Catalysts for Hydrogenation and Reduction Reactions. Catalysts, 15(11), 1029. https://doi.org/10.3390/catal15111029












