1. Introduction
CO
2, as a major component of greenhouse gases, primarily originates from the burning of fossil fuels and has caused serious climate and environmental problems [
1]. In this context, controlling excessive CO
2 emissions through carbon capture, storage, and utilization (CCUS) has attracted great attention from countries around the world [
2,
3]. Converting the well-known greenhouse gas CO
2 into chemical fuels is a very promising approach, as it not only helps mitigate global warming but also serves as an alternative to chemical fuels [
4]. In CO
2 conversion technology, catalytic hydrogenation of CO
2 is considered one of the most commercially feasible methods [
5], especially by converting CO
2 into CO through the endothermic RWGS reaction. Since CO is an important chemical raw material for the synthesis of fine chemicals and fuels, converting CO
2 into CO through the RWGS reaction can obviously bring considerable economic benefits and provide a reliable solution to energy crises and environmental challenges [
6,
7].
However, CO
2 is a molecule with high kinetic and thermodynamic stability, as the cleavage of its C=O bonds requires a high activation barrier under ambient conditions. Under normal conditions, the cleavage of the C=O bond in CO
2 molecules requires a very high activation barrier, making the development of efficient catalysts urgently needed. A good catalyst should have a low hydrogenation activation barrier, good C=O bond cleavage ability, and the ability to dissociate hydrogen [
8]. In order to obtain high-performance RWGS reaction catalysts, researchers have conducted extensive studies. Research on noble metal catalysts (such as Pt [
9], Au [
10], and Pd [
11]) has found that they all possess suitable hydrogenation capabilities and good RWGS catalytic activity. However, noble metals are prone to sintering at high temperatures, resulting in poor stability of noble metal catalysts, high costs, and low natural abundance, which is unfavorable for their widespread application in industrial production. To reduce costs and improve the high-temperature stability of the corresponding catalysts, researchers have started studying transition metal catalysts [
12]. Transition metal catalysts, especially nickel, are widely used in CO
2 hydrogenation reactions due to their advantages such as low cost and high activity at low temperatures, including CO
2 methanation, CO
2 hydrogenation to methanol or formic acid, and water–gas shift reactions [
13,
14]. Although nickel catalysts exhibit high hydrogenation activity, they face issues such as carbon deposition and sintering. To address these problems, the oxide supports of many RWGS catalysts have been studied, including CeO
2, Al
2O
3, SiO
2, TiO
2, and ZrO
2 [
15]. CeO
2 is known for its unique redox properties because it can rapidly interconvert between Ce
4+ and Ce
3+ [
16]. At the same time, Ce
3+ ions can enhance the stability of the CeO
2 lattice, helping to prevent carbon deposition on the catalyst surface [
17]. In addition, the high oxygen mobility on the surface of CeO
2 also promotes the activation and transfer of oxygen molecules, which is crucial for the catalytic process. The presence of a large number of oxygen vacancies on nickel catalysts supported on reducible oxides originates from the metal-support interaction, which facilitates the adsorption and activation of CO
2 [
18,
19]. Nickel catalysts supported on CeO
2 exhibit excellent catalytic performance in terms of activity, selectivity, and stability in the RWGS reaction [
20]. However, most nickel catalysts exhibit high CH
4 selectivity in the hydrogenation of CO
2. Therefore, developing catalysts for the RWGS reaction that are highly active, highly selective, and low-cost remains a challenge.
Numerous studies have shown that particle sizes significantly affect the selectivity of the RWGS reaction [
21]. For the CO
2 hydrogenation reaction, larger nickel particles are conducive to promoting the methanation of CO
2, whereas smaller nickel particles are more favorable for the production of CO [
22,
23]. Improving the dispersion of nickel by reducing its loading, introducing a second metal additive to form bimetallic alloys, and activating metal-support interactions will be an effective strategy to enhance CO selectivity [
24,
25,
26]. Wu et al. [
27] studied nickel metals supported on silica with mass fractions ranging from 0.5% to 10%. Their findings indicate that different nickel loadings result in varying coverages of H species on the catalyst surface, thereby affecting the selectivity of CO and CH
4. A similar trend has also been observed in Ni/Al
2O
3, Ni/MgO, and Ni/CeO
2 catalysts, where a low Ni loading is associated with an increase in RWGS activity [
28]. Christopher and others found that atomically dispersed isolated metal centers are favorable for the RWGS reaction, whereas metal clusters serve as active centers for carbon dioxide methanation [
29]. DFT has become an important tool for studying the surface structure, active sites, and microscopic mechanisms of catalysts, providing a powerful method for elucidating the microscopic characteristics of catalysts [
30]. Hu et al. [
31] used DFT to simulate single Pt atoms loaded on TiO
2(110) and TiO
2(101), respectively, and found that single Pt loaded on TiO
2(101) exhibited a lower energy barrier for COOH intermediate formation and a more favorable redox process, thereby enhancing the CO
2 conversion rate. Yang et al. [
32] found through experiments and DFT simulations that the RWGS reaction catalyzed by single-atom Re
1 loaded on TiO
2 follows the formate pathway, while the RWGS reaction catalyzed by Re
7 clusters loaded on TiO
2 follows the redox pathway. Li et al. [
33] demonstrated through experiments and DFT calculations that atomically dispersed Co-N
4 centers follow a hydrogen-assisted pathway, where the intermediate COOH* desorbs and dissociates into CO, while Co nanoparticle catalysts mainly follow a direct dissociation pathway. Although some progress has been made in highly efficient RWGS reaction catalysts, the rate-determining steps and mechanisms followed in the RWGS reaction catalyzed by CeO
2−x and Ni/CeO
2 have not been fully clarified, which limits further optimization and application of the catalysts.
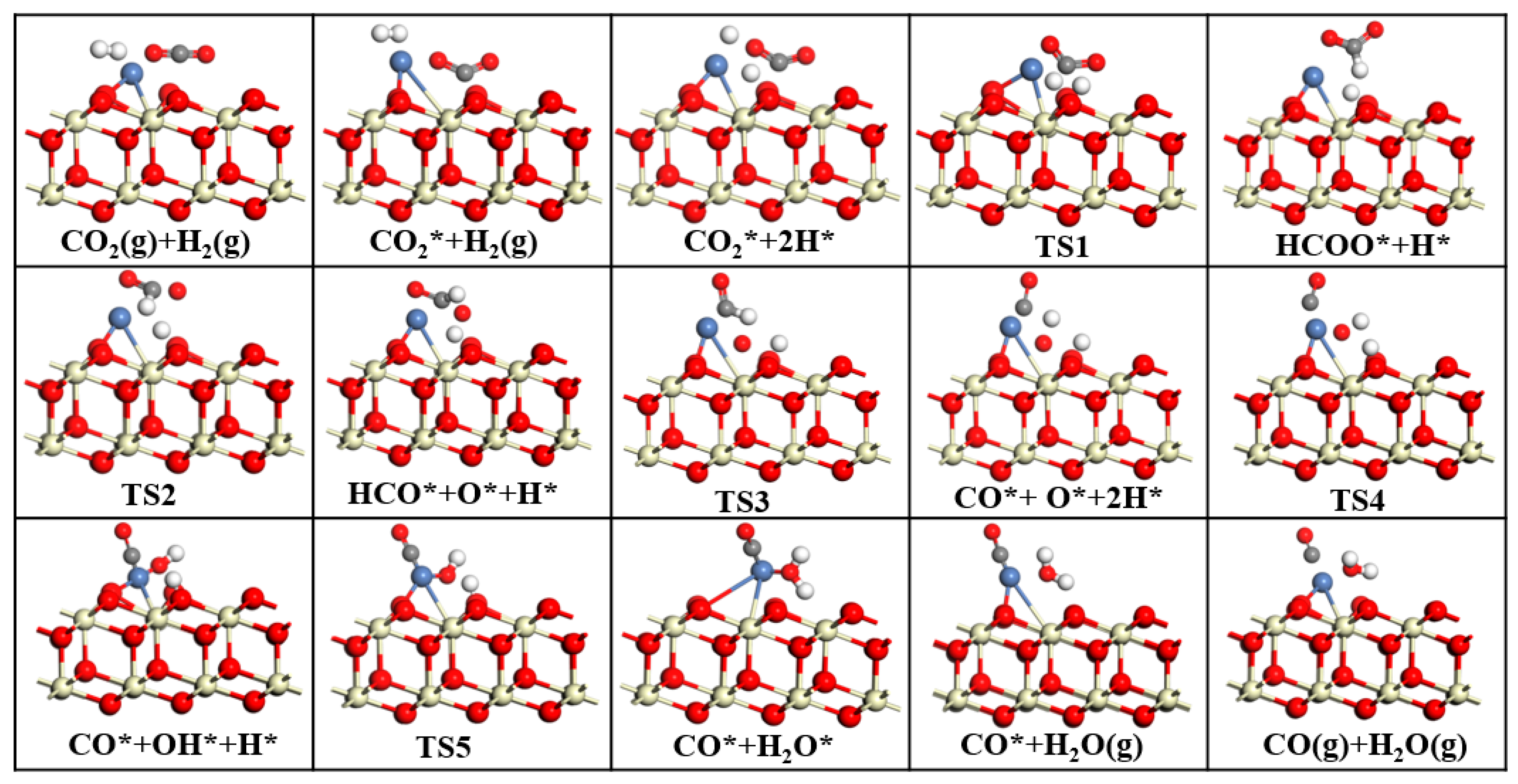
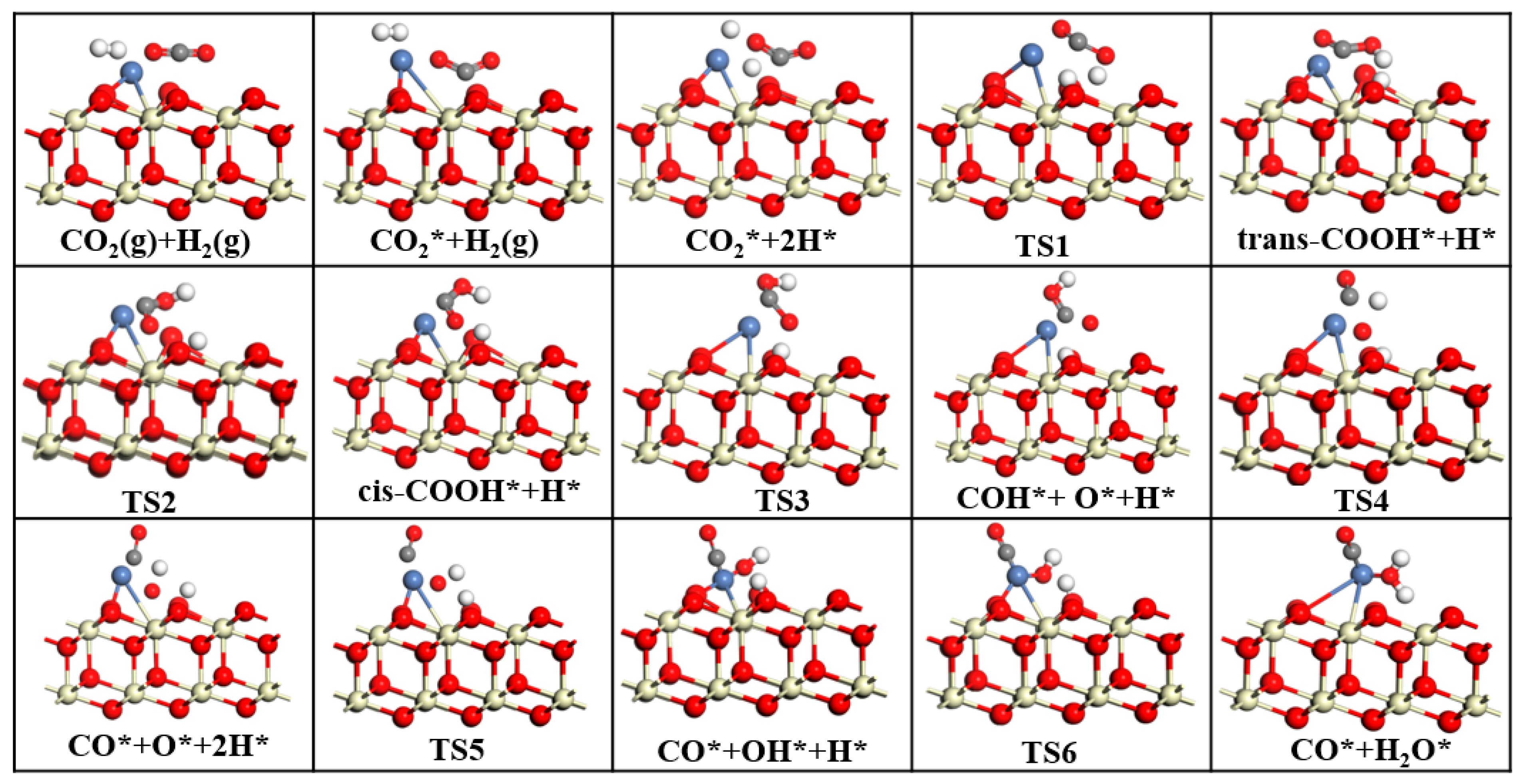
The purpose of this study is to develop catalysts with high activity, high selectivity, and high stability. In this study, we investigated the effects of CeO2, CeO2−x, and Ni/CeO2 on CO2 catalysis using Mulliken charges and band gaps in DFT. By analyzing the adsorption energies and geometric parameters of CO2 on different CeO2 surfaces, we identified the optimal adsorption sites for CO2 on each surface. Next, to explore the influence of different CeO2 on CO2 orbitals, we analyzed the density of states of CO2 on CeO2, CeO2−x, and Ni/CeO2. Finally, to investigate the rate-determining steps and reaction mechanisms of CeO2−x and Ni/CeO2 catalyzed RWGS reactions, we conducted transition state searches. Through DFT calculations, this study provides theoretical guidance for designing efficient CeO2-based RWGS reaction catalysts.
3. Calculation Details
All density functional calculations were performed using the DMol3 module of Materials Studio [
43]. The exchange-correlation function used the generalized gradient approximation (GGA) and the Perdew-Burke-Ernzerhof (PBE) equation [
44]. The core is treated with DFT semi-core pseudopotentials (DSPP) for self-consistent field calculations. The atomic orbital basis set is based on the double numerical plus polarization (DNP) basis set. The integrations of the Brillouin zone were described using a 2 × 2 × 1 k-point grid according to the Monkhorst-Pack method. The convergence tolerance of geometric optimization and property calculation were 1 × 10
−5 Ha for the tolerance of energy, 0.002 Ha/Å for maximum force, 0.005 Å for maximum displacement and 1.0 × 10
−6 Ha for self-consistent field (SCF) tolerance. Studies have shown that the PBE functional can be used to obtain accurate structural information in calculations involving transition metals and transition metal oxides [
45,
46]. The transition state of the reaction was determined using the full LST/QST method [
47].
The definition of adsorption energy in this article is shown in Equation (1):
Eadsorbate-catalyst represents the total energy of the adsorbate and the catalyst when the adsorbate is stable on the catalyst surface, while Eadsorbate and Ecatalyst represent the total energy of the adsorbate and the catalyst, respectively.
The energy required to move from reactant to transition state on a catalyst in this paper is shown in Equation (2):
where ΔE represents the energy required for the reactants to the transition state, and E
R represents the energy of the reactants adsorbed on the catalyst. E
TS represents the energy of the transition state adsorbed on the catalyst.
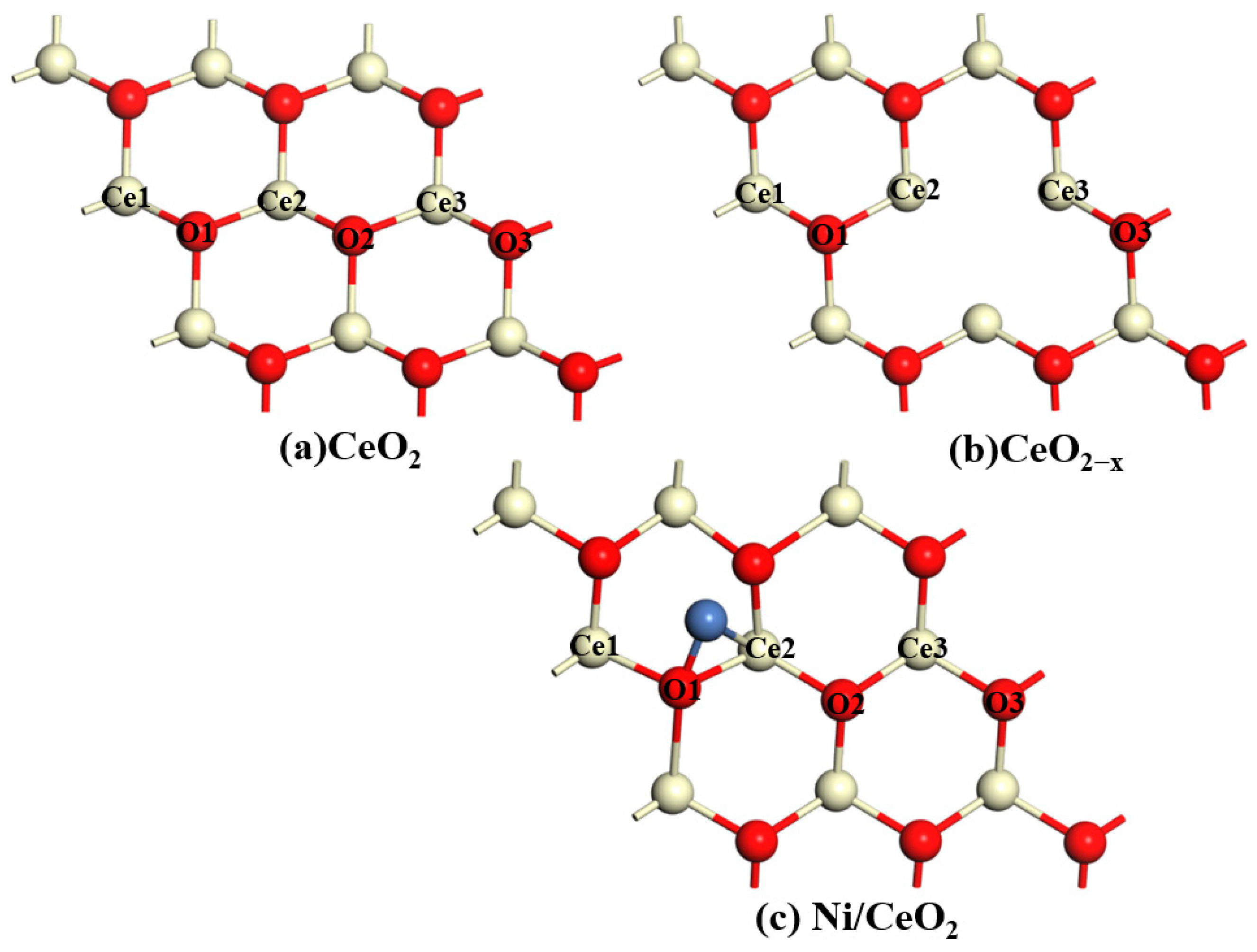
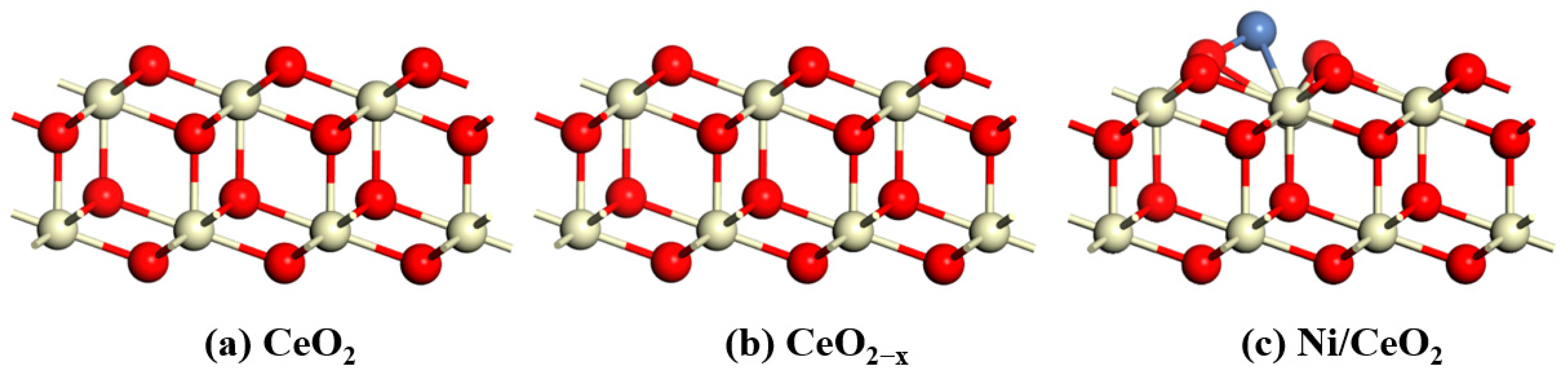
All catalyst models use a periodic 3 × 3 supercell representation with six layers and 20 Å of vacuum to avoid interactions between periodic images. The bottom four atomic layers are fixed, while the top two layers and the adsorbates are fully relaxed without any constraints. The CeO
2(111) surface is the first stable surface of CeO
2, while the CeO
2(110) surface is its second stable surface. Therefore, we chose the CeO
2(111) surface for our study. An oxygen atom is removed from the outermost CeO
2 layer to form an oxygen vacancy CeO
2−x(111). A Ni atom is loaded onto the CeO
2, forming Ni/CeO
2. The front and top views of the structural models of CeO
2, CeO
2−x, and Ni/CeO
2 are shown in
Figure 13 and
Figure 14.
4. Conclusions
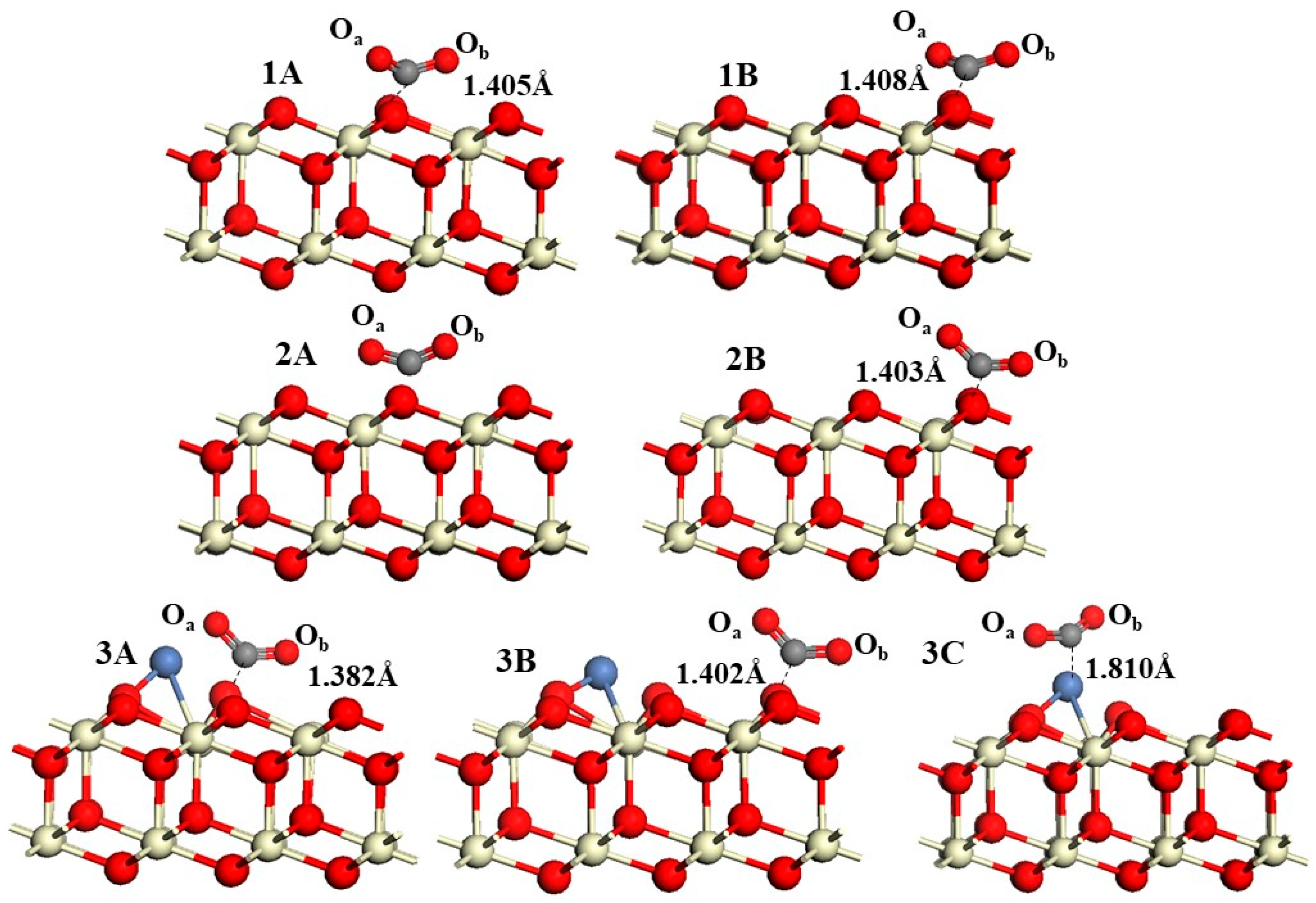
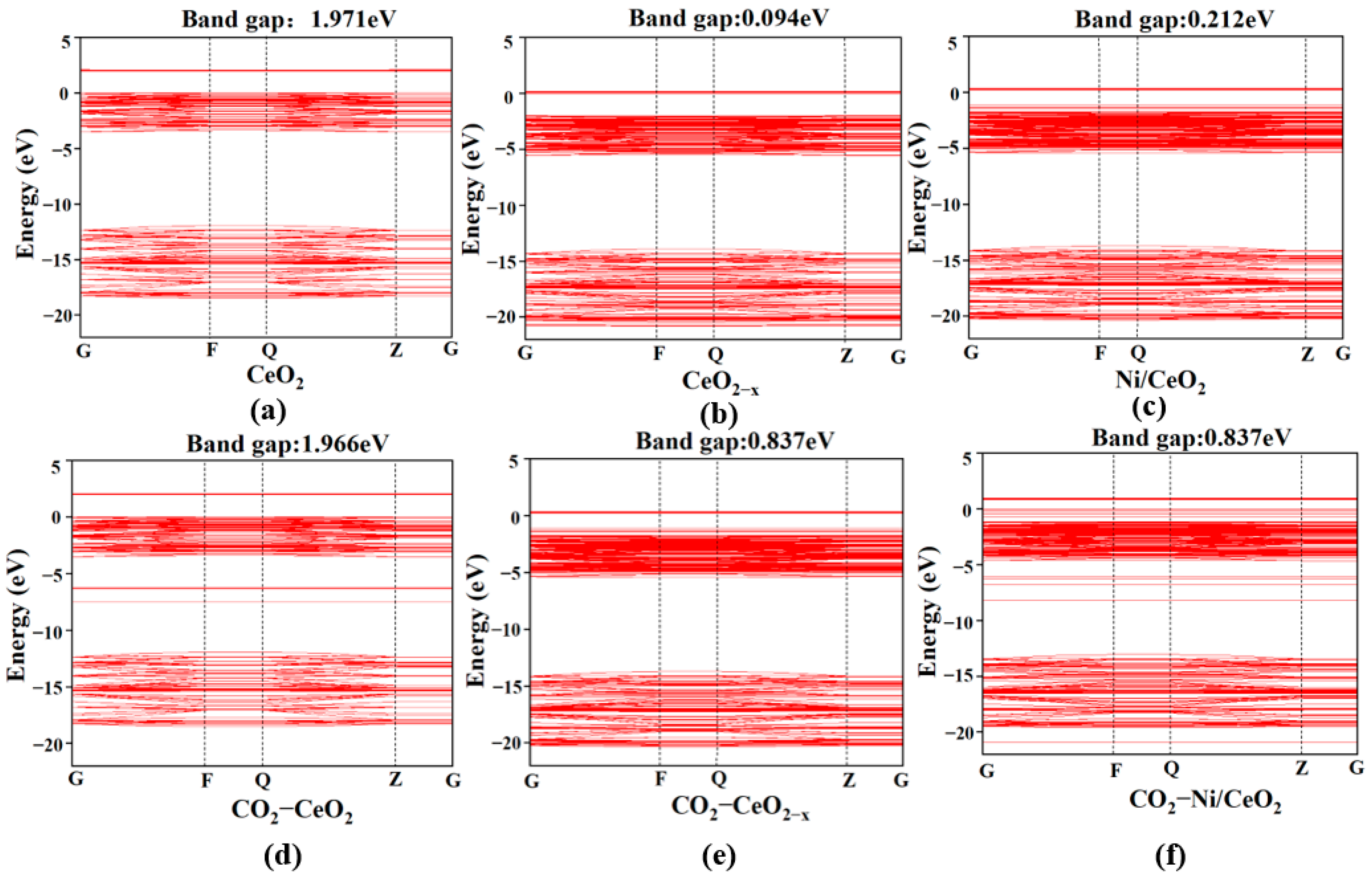
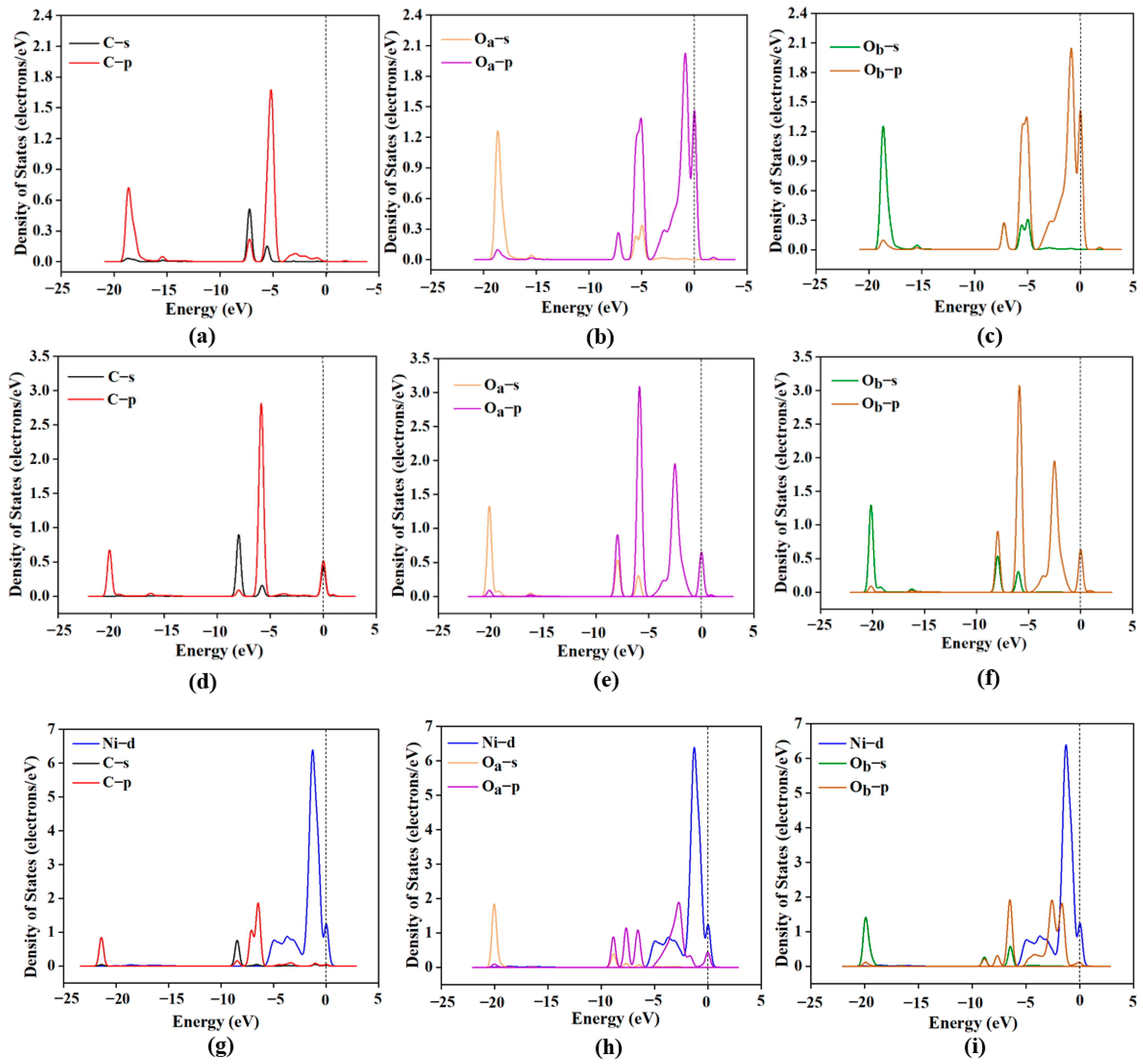
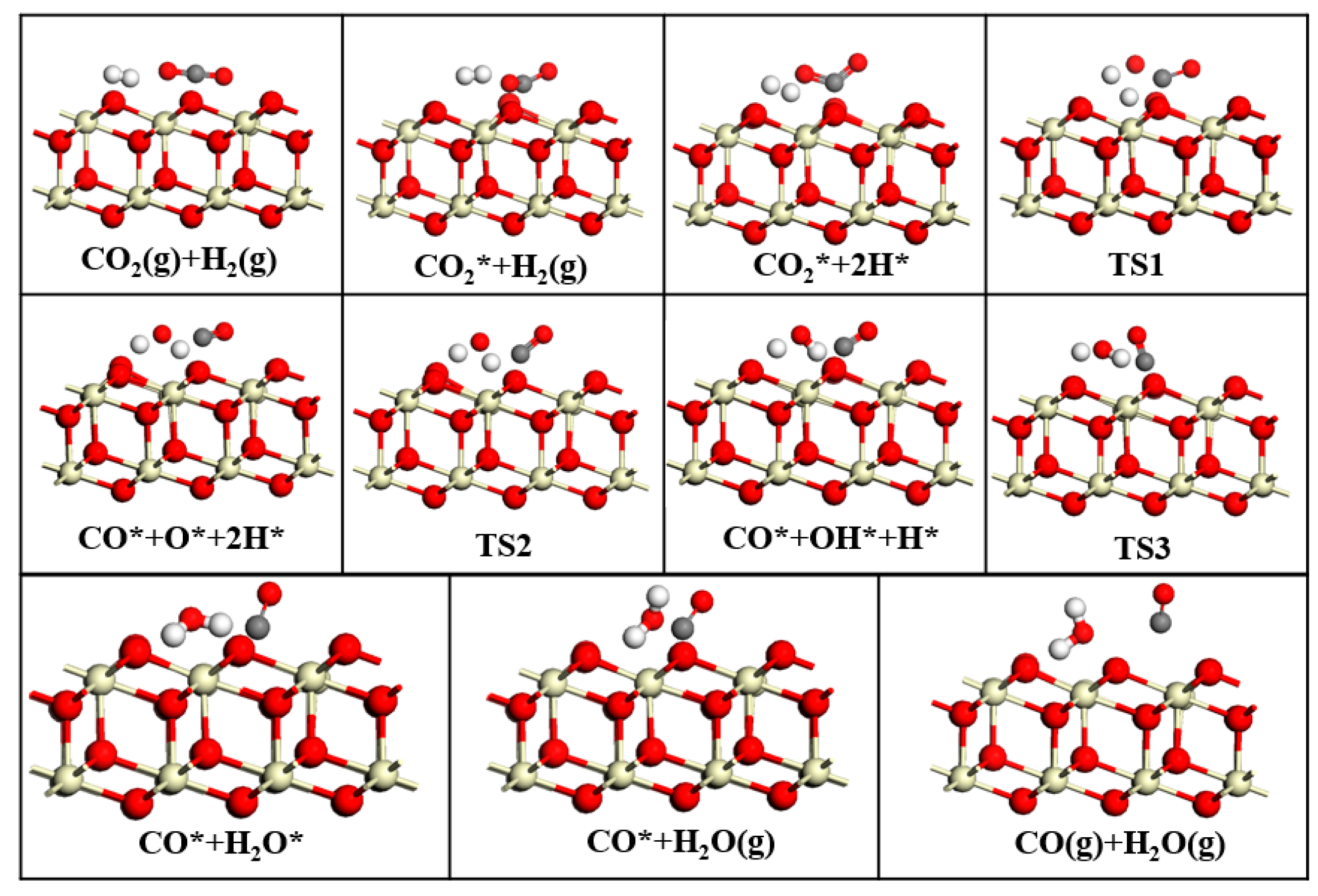
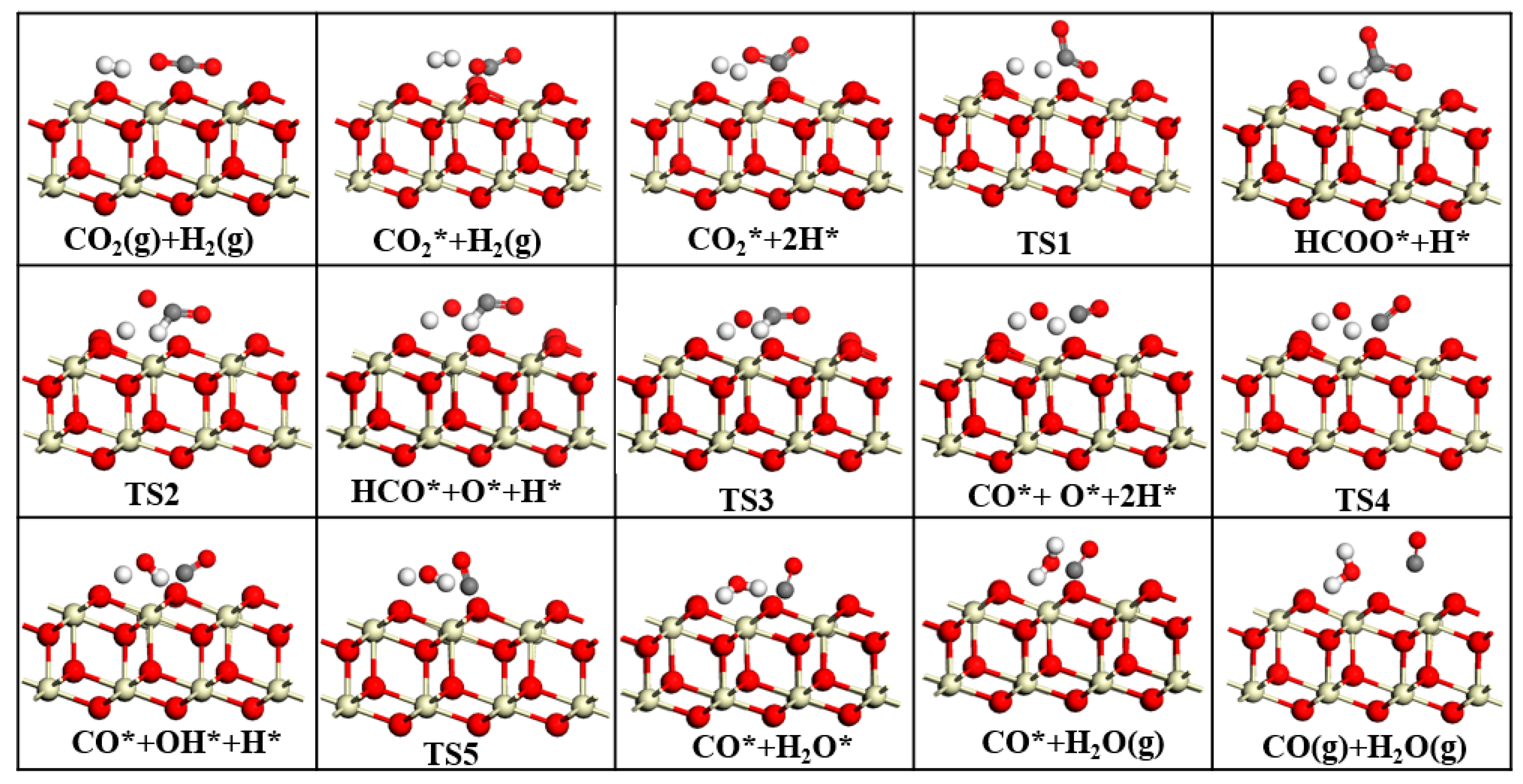
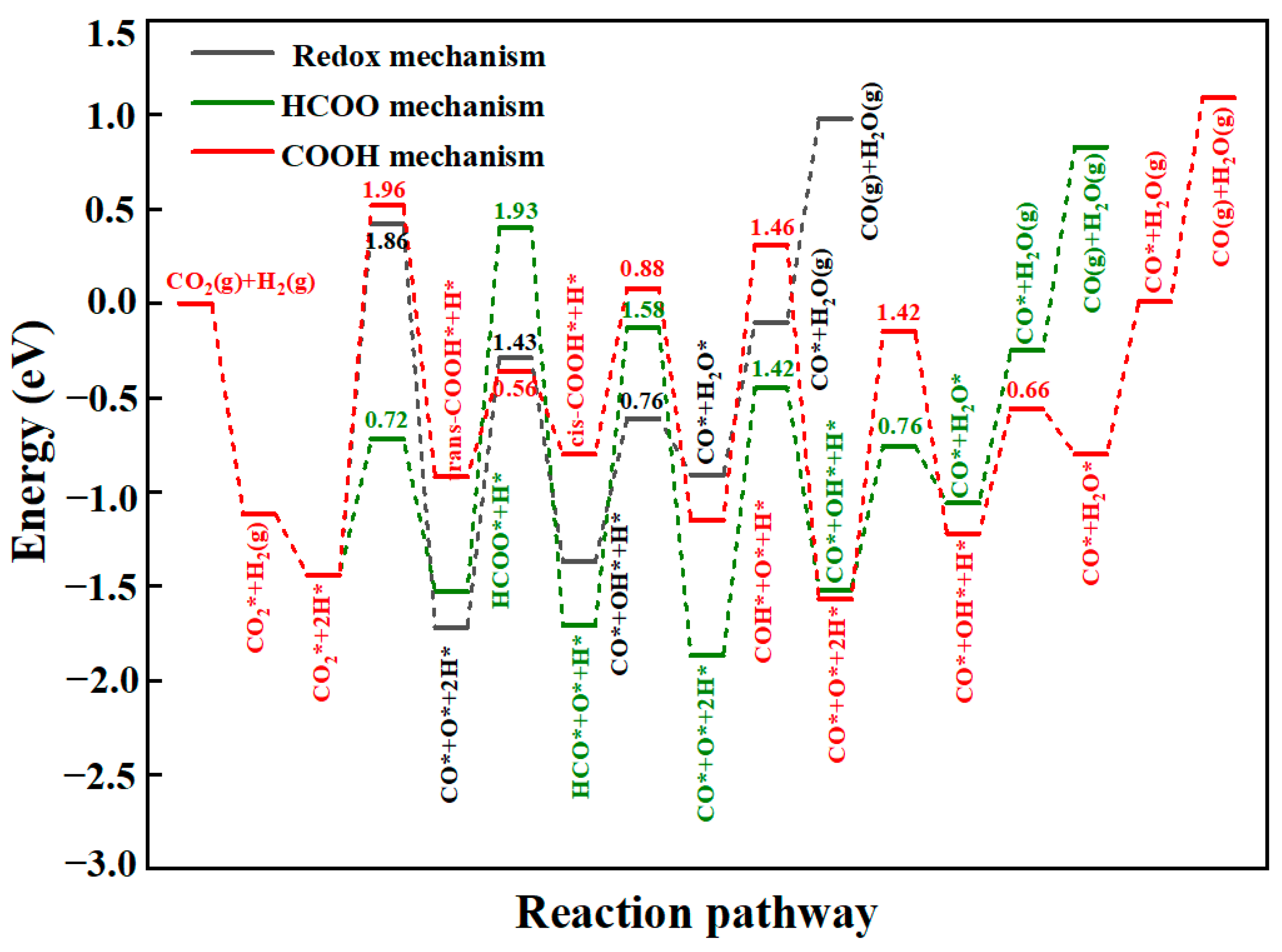
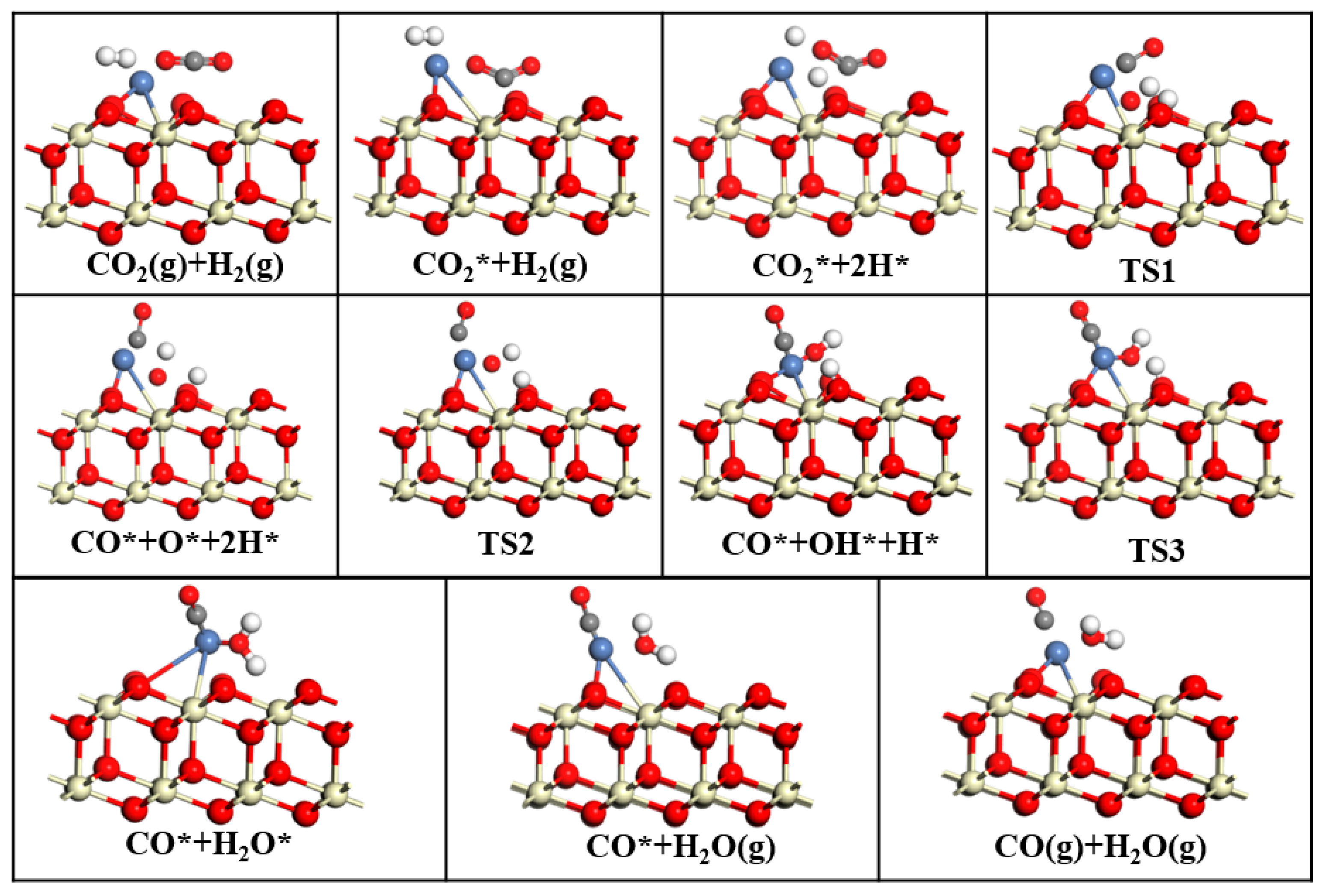
This study employed DFT to systematically investigate the RWGS reaction over three CeO2-based catalysts: pure CeO2, CeO2−x, and Ni/CeO2, with key findings summarized as follows: First, regarding CO2 adsorption sites: DFT calculations revealed that CO2 is more easily adsorbed at the intermediate oxygen sites of CeO2 and Ni/CeO2, while it preferentially binds to the oxygen vacancies of CeO2−x. Second, on catalytic activity: Through Mulliken charge analysis and band gap calculations, the catalytic activities of the three catalysts were compared. The results indicated that CeO2−x and Ni/CeO2 exhibit significantly higher activity than pure CeO2. For Ni/CeO2, the enhanced activity is closely related to the electronic interaction between Ni and the CeO2 support, which modulates the electronic structure of active sites and promotes CO2 activation. Third, regarding the CO2 activation mechanism: the activation mechanisms of different CeO2-based catalysts were revealed using density of states analysis. The study found that all three catalysts can activate CO2 to varying degrees; notably, there is strong hybridization between the d orbitals of Ni and the p orbitals of oxygen in CO2 in Ni/CeO2, which serves as the core electronic driving force for the high activation efficiency of Ni/CeO2. Fourth, regarding the RWGS reaction mechanism and rate-determining steps: the study further explored the reaction mechanisms of CeO2−x and Ni/CeO2 in catalytic RWGS reactions, as well as the rate-determining steps of each mechanism. The results show that for RWGS reactions catalyzed by these two catalysts: the rate-determining step of the redox mechanism is the breaking of the C=O bond; the rate-determining step of the formate mechanism is the decomposition of HCOO*; and the rate-determining step of the carboxylate mechanism is the formation of COOH*. Finally, regarding the dominant mechanism of the catalyst: key studies on the mechanisms of CeO2−x and Ni/CeO2 confirmed that CeO2−x catalyzes the RWGS reaction through the redox mechanism, while Ni/CeO2 follows a mechanism related to formate. This clear understanding of the dominant mechanisms provides a precise theoretical basis for the design and optimization of CeO2−x and Ni/CeO2 catalysts in RWGS applications.
Although this study conducted an in-depth investigation of the RWGS reaction on CeO2, CeO2−x, and Ni/CeO2 using DFT calculations, certain challenges still exist. Traditional DFT methods have obvious limitations when dealing with strongly correlated systems, and they cannot fully account for defect sites commonly present in actual catalytic systems (such as multiple oxygen vacancies and surface hydroxyl groups) as well as dynamic structural evolution processes (such as catalyst surface reconstruction). These limitations may lead to discrepancies between theoretical predictions and experimental results. To address these issues, it is recommended that future research focus on the following directions: constructing a closed-loop research paradigm of computational design–machine learning optimization–experimental validation, integrating theoretical predictions with experimental verification to improve the reliability of catalyst design, and establishing standardized multi-source databases, integrating experimental data (such as catalytic activity and structural characterization) with simulation data (such as DFT-calculated adsorption energies and electronic structures), to facilitate data sharing and cross-validation.