Abstract
The truncated octagonal cuprous oxide photocatalysts were synthesized in the presence of polyvinylpyrrolidone. Zn-doped Cu2O photocatalysts were successfully synthesized with different ZnSO4/CuSO4 ratios. The effects of Zn doping on the light absorption, morphology, separation of photogenerated charge carriers, and hydrogen production performance of the photocatalyst were investigated. The size and morphology of the Zn-doped Cu2O-based nanomaterials change with increasing dosages of zinc sulfate dopant. Zn doping resulted in a reduction in crystallite size, a change in morphology, and a decrease in the size of the nanomaterial. The hydrogen production activity of the Zn-Cu2O photocatalyst Zn-Cu2O-2 with optimized dopant content can reach 9690 μmol h−1g−1. The enhanced photocatalytic activity of Zn-doped Cu2O photocatalyst is achieved through significantly improved electron-hole separation, which is maximized at an optimal Zn dopant concentration.
1. Introduction
Growing concerns about climate change have accelerated the search for clean and sustainable energy alternatives to fossil fuels. Hydrogen, with its high energy density and zero carbon emissions, is a promising candidate for use in energy applications. Among various production methods, photocatalytic water splitting offers a direct route to convert solar energy into hydrogen fuel [1]. Photocatalytic H2 production is attractive due to its mild operating conditions and use of diverse sacrificial agents. Advancing this technology requires the development of efficient, stable, and scalable photocatalysts, supported by a deep understanding of light-induced redox reactions, charge separation, and hydrogen evolution mechanisms [2]. Despite notable progress in nanostructured and composite photocatalysts, there remains an urgent need to improve photocatalytic activity [3].
The development of effective photocatalysts for hydrogen evolution remains a significant challenge in the field of solar energy conversion. Cu2O, CuO, Cu2S, and CuS-based nanomaterials are promising candidates for the photocatalytic hydrogen evolution application [4,5,6,7,8,9,10,11,12]. Among them, Cu2O serves as a p-type semiconductor material, characterized by a forbidden bandgap ranging from 1.8 to 2.0 eV, and it exhibits notable light absorption efficiency [13,14]. Cu2O, characterized by its narrow band gap and significant absorption of visible light, is a noteworthy candidate. Nonetheless, its practical utilization is impeded by the rapid recombination of photogenerated electron-hole pairs.
Several techniques have been proposed as potential solutions to the issues associated with photocatalysis applications, including the incorporation of a cocatalyst [15], the formation of a heterojunction [16,17,18,19], and doping [20,21]. Recent research has demonstrated that doping specific elements into semiconductor functional materials can significantly enhance the light absorption, electron-hole pair separation, carrier transport, and photocatalytic performance of photocatalysts [22,23,24,25]. Doping can modify the surface and interfacial properties of photocatalysts, alter their electronic band structure, and reduce the recombination of electron-hole pairs. Akhirudin et al. [26] reported that Zn-doped Cu2O was synthesized via electrodeposition and evaluated its performance in degrading methylene blue (MB). The synthesized materials exhibited morphology-dependent properties, forming hexagonal and pentagonal particle structures under different deposition conditions. The resulting variation in phase composition and surface characteristics significantly influenced the photocatalytic performance, with a degradation efficiency of 63.23% being achieved. Zerouali et al. [27] observed that the incorporation of zinc in CuO film substantially improves the efficiency of dye degradation, underscoring the catalytic capabilities of zinc as a vital element in photocatalytic processes. Based on the type of dopant ions, doping can be classified into non-metal doping, metal doping, and co-doping [28]. Yu et al. [29] successfully synthesized Zn-doped Cu2O via a hydrothermal method for the degradation of ciprofloxacin, increasing the degradation efficiency of ciprofloxacin from 76.1% to 94.6%. Their findings indicated that the morphology of Cu2O changed as the concentration of Zn ions increased. Santhosh et al. [30] deposited Zn-doped CuO thin films on glass substrates using an ultrasonic spray pyrolysis method. They found that Zn doping led to increased crystal size and surface roughness.
In this study, the photocatalytic H2 production activity of truncated octagonal Cu2O-based photocatalysts was studied for the first time. Various amounts of the zinc dopant were introduced to control the morphology of Cu2O-based nanomaterials and suppress electron-hole recombination. The effects of Zn doping on the surface chemical composition, light absorption, photocurrent response, electrochemical impedance, and hydrogen production performance of the photocatalyst were investigated.
2. Results and Discussion
2.1. Crystal Structure Analysis
Figure 1 presents the XRD patterns of undoped Cu2O and Zn-Cu2O photocatalysts doped with different Zn concentrations. The diffraction peaks observed at 2θ positions of 29.6°, 36.4°, 42.3°, 61.3°, 73.5°, and 77.3° correspond to the (110), (111), (200), (220), (311), and (222) crystal planes of cuprous oxide (Cu2O), respectively, matching well with the standard JCPDS card no. 78-2076 [31]. Compared to undoped Cu2O, the peak positions of Zn-doped Cu2O photocatalysts exhibited no significant shift. This is attributed to the similar ionic radii of Cu+ (77 pm) and Zn2+ (74 pm), which allows Zn2+ ions to be uniformly incorporated into the Cu2O lattice by substituting for Cu+ ions [32]. The lack of additional ZnO impurity peaks in the diffraction spectra of the Zn-doped Cu2O photocatalysts provides further evidence for the successful incorporation of Zn2+ into the Cu2O lattice. The results demonstrate that the substitution occurred without the formation of any secondary phases [33].
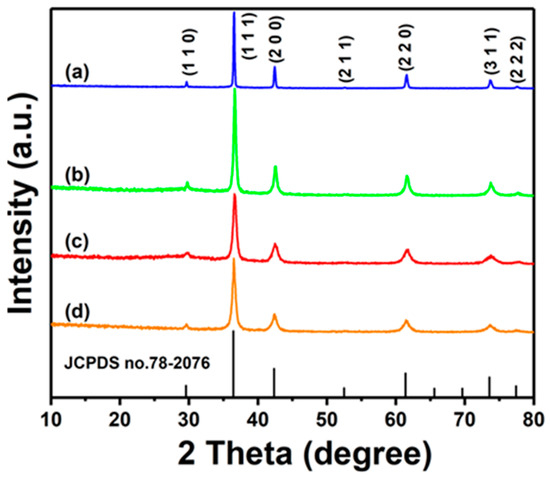
Figure 1.
XRD patterns of (a) Cu2O, (b) Zn-Cu2O-2, (c) Zn-Cu2O-6, and (d) Zn-Cu2O-20.
The average crystallite size of the photocatalysts was calculated using the Scherrer equation:
where D is the average crystallite size;
D = 0.9λ/βcosθ,
θ is the diffraction angle;
β is the FWHM (full width at half maximum) of the characteristic peak;
λ is the wavelength of the incident X-ray.
The average crystallite size of the photocatalysts decreased with increasing Zn doping concentration. The undoped Cu2O exhibited an average crystallite size of approximately 33.9 nm, while the crystallite sizes of Zn-Cu2O-2, Zn-Cu2O-6, and Zn-Cu2O-20 were 13.4 nm, 10.2 nm, and 9.2 nm, respectively. Similar results were reported by Jiang et al. [34], who investigated Zn-doped CuO with pine-needle-like structures for enhanced photocatalytic performance, where the average crystallite size of CuO decreased with increasing Zn doping concentration.
2.2. Surface Morphology
The surface morphology of the photocatalysts was analyzed through FE-SEM. Figure 2a–d displays the FE-SEM images of Cu2O photocatalysts with different Zn doping concentrations. Figure 2a shows that the undoped Cu2O photocatalyst exhibited a truncated octahedral nanostructure with a smooth surface. Figure 2b reveals that Zn-Cu2O-2 retained the truncated octahedral nanostructure after Zn doping. However, as the Zn doping concentration increased, the morphology gradually became distorted. As shown in Figure 2c,d, Zn-Cu2O-6 transformed from a truncated octahedral structure into spherical aggregates composed of irregular nanoparticles, while Zn-Cu2O-20 also exhibited an agglomerated spherical morphology. Additionally, the particle size of Zn-Cu2O-20 was larger than that of Zn-Cu2O-6. Similar observations were reported by Goyal et al. [35], who studied Zn-doped Cu2O for enhanced photocatalytic degradation activity. They found that pure Cu2O particles exhibited a cubic morphology, whereas Zn doping caused the Cu2O particles to transform into distorted spherical shapes with surface defects. The morphological changes observed in this study may be attributed to the effects of Zn doping. The introduction of Zn alters the Gibbs free energy of the system, restricting the mobility of adsorbed atoms. Consequently, the kinetic energy of adsorbed atoms (adatoms) decreases, leading to the formation of agglomerated spherical structures of Zn-doped Cu2O. This mechanism provides a possible explanation for the shape evolution observed in Zn-doped Cu2O in this study.
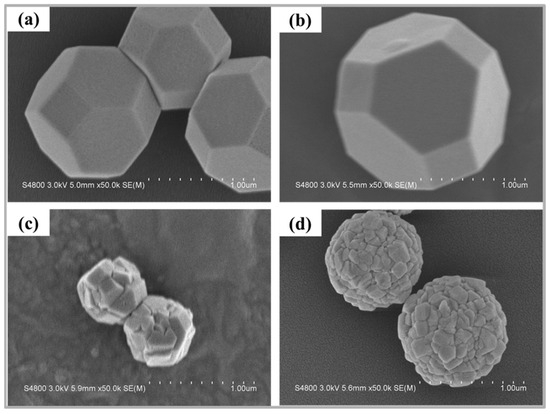
Figure 2.
FE-SEM images of (a) Cu2O (b) Zn-Cu2O-2 (c) Zn-Cu2O-6 (d) Zn-Cu2O-20.
2.3. Nanostructure Characterizations
Figure 3a–d display the high-resolution TEM (HRTEM) images, revealing a lattice spacing of 0.30 nm, which corresponds to the (110) crystal plane of Cu2O, in agreement with the XRD pattern (JCPDS no. 78-2076) of cuprous oxide [31]. Selected area electron diffraction (SAED) patterns also confirm the presence of diffraction spots corresponding to the (110), (220), and (211) planes of Cu2O, further supporting the XRD results.
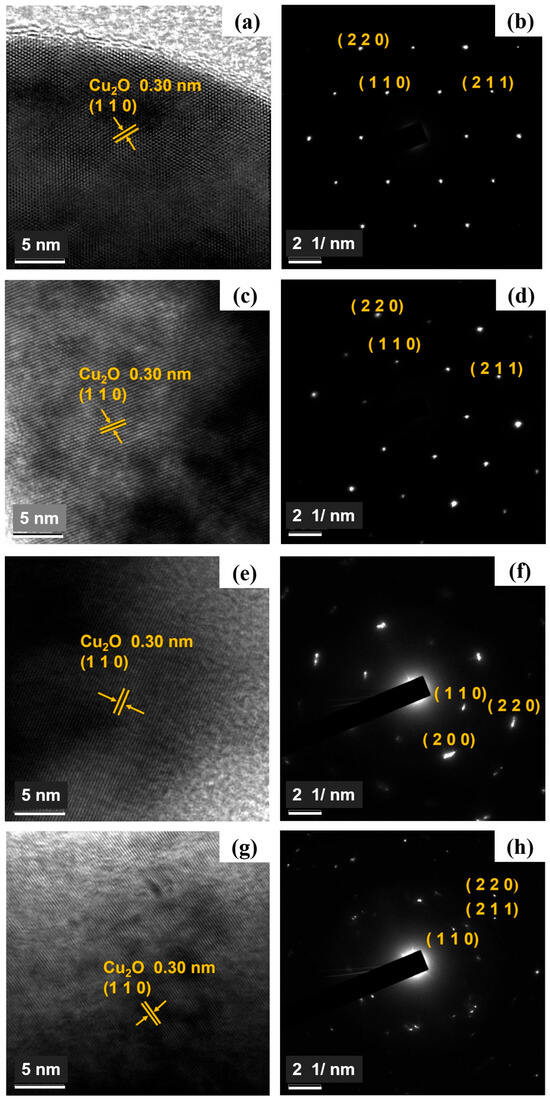
Figure 3.
(a) HRTEM (b) SAED images of Cu2O, (c) HRTEM (d) SAED images of Zn-Cu2O-2, (e) HRTEM (f) SAED images of Zn-Cu2O-6, (g) HRTEM (h) SAED images of Zn-Cu2O-20.
Figure 4 shows the elemental mapping of Cu2O, Zn-Cu2O-2, and Zn-Cu2O-20 photocatalysts. The Cu and O signals originate from Cu2O. Figure 4b–d reveal weak Zn signals, indicating the successful incorporation of Zn dopant into the Zn-Cu2O-2 and Zn-Cu2O-20 photocatalysts. The Zn content of Zn-doped Cu2O samples was monitored by EDS analysis. The Zn contents of Cu2O, Zn-Cu2O-2, Zn-Cu2O-6, Zn-Cu2O-20 photocatalysts are 0, 0.22%, 1.12%, and 4.12%, respectively. The measured Zn content of Zn-doped Cu2O increases with increasing Zn/Cu feeding ratio. The KSP of Cu(OH)2 (2.2 × 10−20) [36] is lower than that of Zn(OH)2 (22.5 × 10−17) [37]. In this study, the measured Zn content of Zn-doped Cu2O is lower than the feed ratio used in the synthesis. Hence, the discrepancy may likely result from limitations in solubility and reaction kinetics. The lower Zn content of Zn-doped Cu2O (compared with the feed ratio used in the synthesis) may be the reason why Zn doping resulted in a decrease in crystallite size without altering the crystal structure, even at a higher Zn/Cu feeding ratio (zinc sulfate/copper(II) sulfate) (Figure 1).
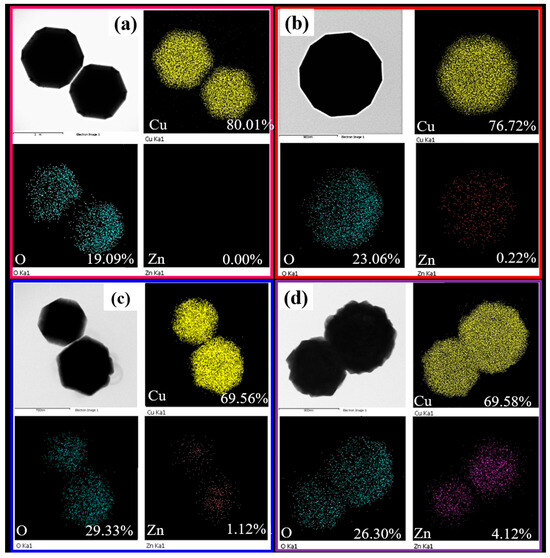
Figure 4.
Elemental mapping images of (a)Cu2O (b) Zn-Cu2O-2 (c) Zn-Cu2O-6 (d) Zn-Cu2O-20 photocatalysts.
2.4. Surface Chemical Properties
XPS was utilized to investigate the surface chemistry and oxidation states of the elements present in the photocatalysts. Figure 5a shows the XPS Cu spectra of Cu2O, Zn-Cu2O-2, and Zn-Cu2O-20 photocatalysts. After peak deconvolution, the peaks at 932.2 eV and 952 eV correspond to Cu+ in Cu2O, specifically the Cu 2p3/2 and Cu 2p1/2 peaks, respectively. The Cu 2p3/2 and Cu 2p1/2 peaks of Zn-Cu2O-20 exhibited a slight shift to lower binding energy, indicating that Cu functions as the electron acceptor in the Zn-doped Cu2O samples. Additionally, the Auger parameters (ά) determined for Cu2O, Zn-Cu2O-2, and Zn-Cu2O-20 are 1849.20 eV, 1849.10 eV, and 1848.61 eV, respectively. The values are consistent with the reported range ~1848.5–1849.5 eV [38,39,40,41] for copper in the Cu+ oxidation state. Consequently, all three samples demonstrate the presence of Cu in the Cu(I) state. Suggesting that the Cu+ was not reduced after Zn doping.
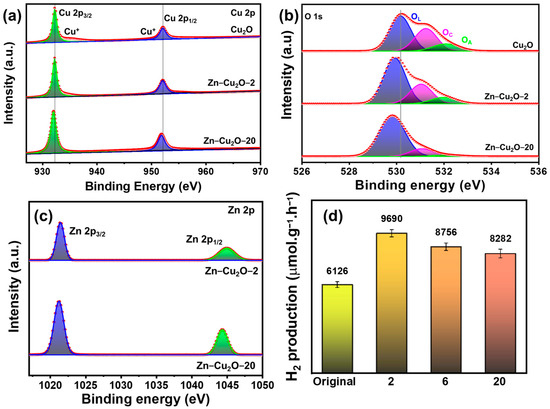
Figure 5.
XPS (a) Cu 2p (b) O1s spectra of Cu2O, Zn-Cu2O-2, and Zn-Cu2O-20 (c) Zn 2p spectra of Zn-Cu2O-2 and Zn-Cu2O-20 photocatalysts (d) Hydrogen evolution rates of truncated octahedral Zn-Cu2O-2, Zn-Cu2O-6, Zn-Cu2O-20 photocatalysts.
Figure 5b displays the O1s XPS for Cu2O, Zn–Cu2O-2, and Zn–Cu2O-20 photocatalysts. The peaks identified at approximately 530.1, 531.5, and 532.1 eV are associated with lattice oxygen (OL), chemisorbed oxygen (OC), and adsorbed oxygen (OA), respectively. The relative intensity of the OL peak varied with Zn doping, resulting in a shift to lower binding energy (Figure 5b). The relative intensity and binding energy (531.28 eV for Zn–Cu2O-2 and 531.08 eV for Zn–Cu2O-20) of the OC peak decreased as the introduction of Zn doping increased. These observed trends align with findings in the literature regarding similar oxide systems, where dopants effectively diminish surface defects or chemisorbed oxygen species, thereby altering the local electronic environment and resulting in shifts in binding energy [42,43]. For example, Li et al. [44] observed that K doping of CuFe2O4 resulted in a shift in lattice oxygen to lower BE, accompanied by changes in the relative intensities of OC and OA. Additionally, Meena et al. [45] indicated that following C/N doping, the OL of C/N-co-doped ZnO BE shifted to a lower binding energy. These results show that Zn doping alters the concentration and chemical environment of chemisorbed or surface oxygen species.
Figure 5c presents the XPS Zn 2p spectra of Zn-Cu2O-2 and Zn-Cu2O-20 photocatalysts. The Zn 2p3/2 and Zn 2p1/2 peaks correspond to Zn2+, suggesting that Zn is incorporated into the Cu2O lattice via Zn–O–Cu bonding [46], confirming the successful synthesis of Zn-doped Cu2O. The intensity of the Zn peaks in Zn-Cu2O-2 is weaker than that in Zn-Cu2O-20, indicating a lower Zn2+ ion content in Zn-Cu2O-2.
2.5. Hydrogen Production Efficiency
Figure 5d illustrates the hydrogen evolution rates of truncated octahedral Cu2O and Zn-doped Cu2O photocatalysts (Zn-Cu2O-2, Zn-Cu2O-6, Zn-Cu2O-20) with different Zn doping concentrations. This analysis evaluates the effect of Zn doping concentration on the photocatalytic hydrogen production rate.
The undoped Cu2O photocatalyst exhibited a hydrogen production rate of approximately 6126 μmol h−1g−1. After Zn doping, the hydrogen evolution efficiency of truncated octahedral Cu2O improved. As depicted in Figure 5d, Zn-Cu2O-2 reached the highest hydrogen production rate of 9690 mol h−1g−1 among other investigated photocatalysts. The performance of photocatalytic activity suggests that introducing a specific impurity energy level within the Cu2O energy band significantly enhances the lifetime of excited electrons, consequently improving hydrogen production activity [47,48]. However, as the Zn-doping amount is higher than the optimized value, there is a decline in hydrogen production. The hydrogen production rates of Zn-Cu2O-6, and Zn-Cu2O-20 were 8756, and 8282 μmol h−1g−1, respectively. The observed decrease in hydrogen production efficiency as the Zn doping concentration rises might be due to increased electron–hole recombination [48]. As a result, the overall photocatalytic efficiency declines with increased levels of Zn doping compared to the Zn-Cu2O-2. The Zn-Cu2O-2 activity is higher than that of the other tested photocatalysts, suggesting that an optimized Zn doping concentration is necessary to achieve better photogenerated charge carrier production, charge separation, and electron transfer. The apparent quantum yield (AQY) for the H2 evolution was measured in a 60 mL quartz container using a similar experimental setup, with a band pass filter (λ = 340 nm with a photon flux of 1.91 mW cm−2). The AQY of the Zn-Cu2O-2 photocatalyst at 340 nm reaches 1.14%.
Table S1 presents the morphology, key findings, and applications of Zn-doped Cu2O materials. Zn-doped Cu2O films, nanomaterials with hollow microcubes, and truncated octahedral structures were synthesized. These materials were used for different applications, such as photodetectors, photocatalysts for H2 production, and generating photocurrent. Compared with the above-mentioned literature, this study provides insights into the doping-induced shape changes from truncated octahedral to spherical structures, the morphology-performance relationship, and the high H2 production activity of Zn-doped Cu2O photocatalysts.
Table 1 presents the composition, morphology, light source, sacrificial agent, and hydrogen generation activity of various Cu2O-based photocatalysts. Cu2O-based photocatalysts with various morphologies, including dendrites, cubes, particles, and hollow spheres, were synthesized using different methods. Lactic acid and methanol are added as the sacrificial agents, as reported in the published literature [49,50,51,52,53]. In this study, the photocatalytic H2 production activity of truncated octagonal Cu2O-based photocatalysts was studied for the first time. The truncated octagonal Zn-doped Cu2O photocatalyst achieved high hydrogen generation activity.

Table 1.
The composition, morphology, light source, sacrificial agent, and hydrogen (H2) production activity of various Cu2O-based photocatalysts.
2.6. Optical and Electrochemical Characteristics
Figure 6a displays the UV-Vis DRS spectra of undoped Cu2O and Zn-doped Cu2O photocatalysts with different Zn concentrations. The undoped Cu2O photocatalyst appeared as a red powder. Its primary absorption range was between 300 and 650 nm, with an absorption edge around 650 nm, beyond which slight absorption was observed (Figure 6a). By comparing the DRS spectra of Zn-Cu2O-2, Zn-Cu2O-6, and Zn-Cu2O-20, it was found that increasing the Zn doping concentration resulted in a gradual hypsochromic (blue) shift in the absorption edge. Similar results were reported by Yu et al. [29], who observed a comparable trend in their study on Zn-doped Cu2O for photocatalytic degradation of ciprofloxacin.
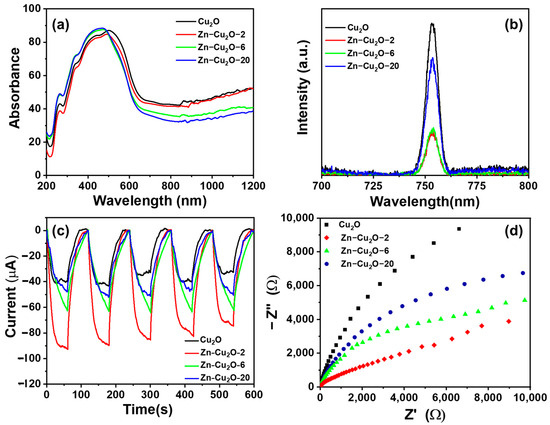
Figure 6.
(a) UV-Vis DRS spectra, (b) fluorescence spectra, (c) photocurrent response curves, and (d) Nyquist plots of undoped Cu2O and Zn-doped Cu2O photocatalysts.
The electron-hole separation efficiency of the photocatalysts was evaluated using steady-state fluorescence spectroscopy. Figure 6b presents the fluorescence spectra of truncated octahedral Cu2O and Zn-doped Cu2O photocatalysts with different Zn concentrations. Under excitation with a 500 nm light source at room temperature, all samples exhibit the fluorescence peak at a wavelength of 753 nm, as they exhibit approximately the same band-edge emission characteristics [54]. The undoped Cu2O photocatalyst showed the highest fluorescence intensity, indicating a higher electron-hole recombination rate [55]. In contrast, the fluorescence intensity of Zn-doped photocatalysts (Zn-Cu2O-2, Zn-Cu2O-6, and Zn-Cu2O-20) significantly decreased, suggesting that the Zn dopants exert a measurable influence on the Cu2O nanostructure. The fluorescence intensity of Zn-Cu2O diminishes compared to Cu2O as a result of the reduction in particle size and the lattice distortion, which arises from the substitution of Cu+ ions by Zn2+ ions within the Cu2O lattice. Ibrahim et al. [54] reported a similar finding for Ni-doped Cu2O photoelectrode for water splitting. Kim et al. [56] have reported similar findings regarding the influence of lattice distortion and particle size on fluorescence emission intensity of ZnO. These results suggest that Zn doping effectively reduced the recombination of photogenerated electron-hole pairs.
Compared to undoped Cu2O, the fluorescence intensity of Zn-Cu2O-2 and Zn-Cu2O-6 decreased. Zn dopant can act as shallow traps that reduce the recombination of photoexcited electrons and holes. However, further increasing the Zn doping concentration in Zn-Cu2O-20 resulted in a slightly higher fluorescence intensity than Zn-Cu2O-6. This suggests that an optimal Zn doping level can act as an electron or hole trapping site, reducing electron-hole recombination and enhancing photocatalytic activity. However, excessive Zn doping may introduce recombination centers, leading to an increased electron-hole recombination rate in Zn-Cu2O-20 [57].
Photocurrent measurements were conducted to gain insights into the efficiency of photoinduced electron-hole separation and the operational stability of photocatalysts under repeated light irradiation [58]. Figure 6c illustrates the photocurrent intensity for the following samples: pure Cu2O, Zn-doped Zn-Cu2O-2, Zn-Cu2O-6, and Zn-Cu2O-20, all of which are subjected to Xenon lamp irradiation. The pure Cu2O exhibited a reduced photocurrent intensity. All Zn-doped samples demonstrated a greater photocurrent intensity compared to pure Cu2O. Nonetheless, an increased amount of Zn doping resulted in a diminished photocurrent intensity for both Zn-Cu2O-6 and Zn-Cu2O-20. The reduced photocurrent intensity is likely due to the rapid occurrence of radiative charge recombination. In Zn-Cu2O-2, enhanced charge separation and transfer mechanisms result in an increased photocurrent. The findings validate that the truncated octahedral nanostructure of Zn-Cu2O-2, containing an optimized amount of Zn dopant, significantly enhances the separation and transfer of photogenerated electron-hole pairs [59].
EIS was utilized to evaluate the charge transfer resistance of different photocatalysts through the use of Nyquist plots. A smaller semicircle in the Nyquist plot typically signifies reduced charge transfer resistance, which suggests improved mobility of photogenerated charge carriers and more effective charge separation and migration within the material [60]. The measurements were conducted at frequencies ranging from 0.01 Hz to 100,000 Hz with an amplitude of 0.01 V. Figure 6d illustrates the Nyquist plots for the photocatalysts Cu2O, Zn-Cu2O-2, Zn-Cu2O-6, and Zn-Cu2O-20. Among these, pristine Cu2O displays the largest diameter of the arc, indicating the most significant charge transfer resistance. In contrast, the introduction of Zn dopant leads to a notable reduction in the radius, indicating a substantial decrease in charge transfer resistance. Zn-Cu2O-2 exhibits the smallest semicircle, indicating superior charge transport properties. However, at higher Zn doping concentrations, the available substitution sites for Zn2+ within the Cu2O lattice decreased, leading to the formation of Zn clusters. The increased probability of Zn cluster formation resulted in higher charge transfer resistance for Zn-Cu2O-6 and Zn-Cu2O-20. These findings are consistent with trends reported in previous studies [61,62].
2.7. Band Structure—Mott-Schottky Plot
Figure 7a presents the Tauc plots of truncated octahedral Cu2O and Zn-doped Cu2O photocatalysts. The estimated band gaps of Cu2O, Zn-Cu2O-2, Zn-Cu2O-6, and Zn-Cu2O-20 were 1.89 eV, 1.90 eV, 1.91 eV, and 1.93 eV, respectively. These results suggest that Zn doping results in an increased band gap of Cu2O compared to its undoped version, as demonstrated by the observed shift towards the higher energy (blueshift) in the absorption edge. The enhancement of the band gap has been mainly attributed to a decrease in particle size resulting from the incorporation of Zn. The increased band gap in Zn-doped Cu2O effectively reduces the recombination of photogenerated electron–hole pairs, which enhances its photocatalytic performance.
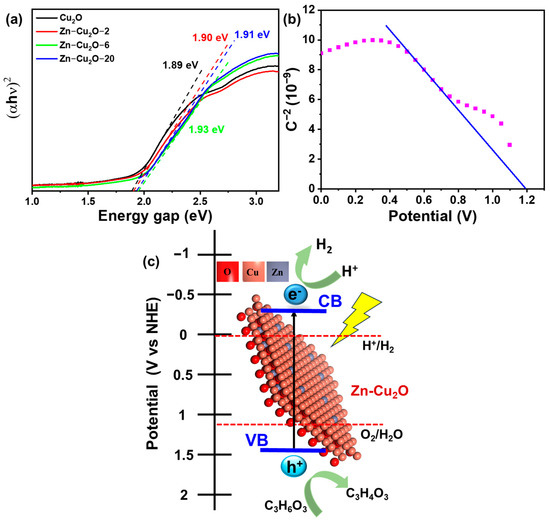
Figure 7.
(a) Tauc plots of Cu2O and Zn-doped Cu2O photocatalysts, (b) Mott-Schottky plot of Zn-Cu2O-2 photocatalyst, (c) proposed photocatalytic hydrogen evolution mechanism of Zn-doped Cu2O.
Figure 7b presents the Mott-Schottky plot of Zn-Cu2O. The flat band potential (Efb) of Zn-Cu2O was determined to be 1.2 V vs. Hg/HgO (1 M NaOH) using Mott-Schottky analysis. Since the potential of Hg/HgO (1 M NaOH) is +0.14 V relative to the standard hydrogen electrode (SHE), the corrected flat band potential for Zn-Cu2O was calculated as 1.34 V vs. SHE. For a p-type semiconductor, the valence band (VB) is typically 0.1–0.3 eV more positive than the flat band potential. Based on this, the valence band of Zn-Cu2O was estimated to be 1.44 V vs. SHE. Given that the bandgap energy (Eg) of Zn-Cu2O was 1.9 eV, the conduction band (CB) position was calculated as −0.46 V vs. SHE.
2.8. Reaction Mechanism
The energy band structure of Zn-doped Cu2O was determined using the flat band potential (Efb) from Mott-Schottky analysis and the bandgap (Eg) from Tauc plots. The resulting band alignment diagram is shown in Figure 7c, illustrating the hydrogen evolution mechanism of Zn-doped Cu2O. The diagram confirms that the valence and conduction bands of Zn-doped Cu2O straddle the oxidation and reduction potentials of water, enabling photocatalytic water splitting for hydrogen production. The possible reaction mechanisms are as follows [63]:
When subjected to light, Zn-doped Zn-Cu2O-2 demonstrates photon absorption attributed to its narrow bandgap, which is approximately 1.9 eV. This mechanism facilitates the excitation of electrons from the VB (valence band) to the CB (conduction band), resulting in the formation of electron–hole pairs (Equation (1)). The VB and CB of Zn-doped Cu2O are located at +1.44 V and –0.46 V versus NHE, respectively, enabling thermodynamically favorable proton reduction. The electrons produced in the CB effectively facilitate the conversion of H+ to H2 (Equation (4)). In addition, the holes in the VB have sufficient capacity to oxidize sacrificial material (lactic acid). The •OH forms when the hole oxidizes the water molecule (Equation (2)). The further oxidation of lactic acid by •OH and hole produces pyruvic acid and water (Equation (3)). When Zn2+ ions are incorporated into the Cu2O lattice, Cu+ is partially substituted, causing the appearance of shallow trapping states. The existence of Zn improves charge separation and migration. According to the experimental results, Zn-Cu2O-2 photocatalysts exhibit enhanced charge separation and migration, resulting in the highest photocatalytic H2 generation.
3. Materials and Methods
3.1. Synthesis of Truncated Octahedral Zn-Doped Cu2O Photocatalyst
To prepare the Zn-doped Cu2O photocatalyst with a truncated octahedral morphology, 3.6 g of polyvinylpyrrolidone (PVP) and varying amounts of zinc sulfate were added to 50 mL of deionized water. Then, 3 mL of a 0.68 M aqueous solution of copper(II) sulfate pentahydrate ( SHOWA) was added, and the mixture was stirred for 20 min. Subsequently, 3 mL of 0.74 M sodium citrate (J.T.Baker, Center Valley, PA, USA) aqueous solution and 3 mL of 1.2 M sodium carbonate (Thermo Fisher Scientific, Ward Hill, MA, USA) aqueous solution were added dropwise, and the mixture was stirred for 10 min. Afterward, 3 mL of 1.4 M glucose (Sigma, St. Louis, MO, USA) aqueous solution was slowly added dropwise. The solution was stirred thoroughly and heated in a water bath at 80 °C for 2 h. After allowing the reaction mixture to stand for 1 h, the precipitate was collected by centrifugation, washed several times with deionized water and ethanol, and dried to obtain the Zn-doped Cu2O photocatalyst with a truncated octahedral structure. The undoped Cu2O was prepared by the same method without the addition of zinc sulfate.
Zinc-doped cuprous oxide samples, denoted as Zn-Cu2O-x, were synthesized using varying molar ratios of Zn to Cu precursors, where x = 2, 6, or 20. These correspond to Zn/Cu molar ratios of 2:100, 6:100, and 20:100, respectively.
3.2. Hydrogen Production Experiment Using the Photocatalyst
Different concentrations of 30% lactic acid (TEDIA, Fairfield, CA, USA) aqueous solution and 0.01 g of the photocatalyst were placed into a quartz tube. A magnetic stir bar was added, and the solution was thoroughly mixed. The quartz tube was then sealed with a rubber stopper. The sealed tube was immersed in a thermostatic water bath, and the solution was continuously stirred while being irradiated with a 300 W xenon (Xe) lamp for 3 h. The light intensity is 0.17 W/cm2. The irradiated area is 41.4 cm2. The generated gas was extracted using a syringe and analyzed by gas chromatography (GC) (Shimadzu, Kyoto, Japan) to determine the amount of hydrogen produced.
3.3. Characterization
The morphology of the photocatalyst was observed by a scanning electron microscope (S-4800, Hitachi, Tokyo, Japan). XRD patterns were measured by X-ray diffraction analyzer (D8 SSS, Bruker, Karlsruhe, Germany). X-ray diffraction patterns were obtained using Cu Kα as the emission source and a scanning range of 10° to 80°. X-ray photoelectron spectra (XPS) were obtained by an ULVAC PHI/PHI 5000 VersaProbe Ⅲ instrument (ULVAC, Chigasaki, Japan). Photoluminescence (PL) spectra were monitored using a fluorescence spectrometer (F-7000, HITACHI, Hitachi, Tokyo, Japan) at an excitation wavelength of 500 nm. A diffuse reflectance spectrophotometer (JASCO/V-700, JASCO, Tokyo, Japan) was used to obtain the light absorption of the samples.
3.4. Electrochemical Property
Electrochemical impedance spectroscopy (EIS) was obtained by a CHI 920D workstation (CH Instruments, Austin, Texas, USA) and a three-electrode system in an electrolyte containing Na2SO4 (0.1 M). The platinum plate and Ag/AgCl were the counter electrode and reference electrode, respectively. The photocatalyst was coated on an ITO glass to make the working electrode. The photocurrent response is investigated under light (300 W Xenon lamp, Prosper OptoElectronic, Ilan, Taiwan) in the three-electrode system with the electrolyte containing Na2SO3 (0.25 M) and Na2S (0.35 M).
4. Conclusions
Zn-doped Cu2O photocatalysts were successfully synthesized with different ZnSO4/CuSO4 ratios. The morphology of Zn-doped Cu2O photocatalysts changes from truncated octahedron to spherical aggregates as the ZnSO4/CuSO4 ratio increases. Meanwhile, the size of the nanomaterial decreases with increasing zinc sulfate precursor content. Zn doping resulted in a reduction in crystalline properties (reduction in crystallite size) without altering the crystal structure. Zn dopant can act as shallow traps that reduce the recombination of photoexcited electrons and holes. Excessive Zn dopant content distorts the crystal lattice, leading to reduced crystallinity and charge separation property. Photoluminescence and photocurrent response measurements indicated that an optimal amount of Zn doping effectively suppressed electron-hole recombination, thereby improving the photocatalytic hydrogen evolution activity. Zn-Cu2O-2 with an optimal Zn concentration unveiled the highest H2 production efficiency of 9690 μmol h−1g−1, which is 1.58 times higher than that of undoped Cu2O, thereby highlighting the advantage of Zn doping.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15111030/s1: Figure S1. Full survey spectrum of Cu2O, Zn-Cu2O-2, and Zn-Cu2O-20 photocatalysts; Table S1. Morphology, key findings, and application of Zn-doped Cu2O materials. References [30,38,39,47,48,60] are cited in the Supplementary Materials.
Author Contributions
A.P.: writing—original draft, data curation, formal analysis. C.-W.K.: data curation, writing—original draft, formal analysis; C.-J.C.: conceptualization, methodology, writing—original draft, supervision, writing—review and editing; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science and Technology Council, grant number MOST 111-2221-E-035-002-MY3.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank the financial support from the National Science and Technology Council under the contract of MOST 111-2221-E-035-002-MY3.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Colón, G. Towards the hydrogen production by photocatalysis. Appl. Catal. A. 2016, 518, 48–59. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chiao, Y.C.; Fun, Y.X. Cu2O/CuS/ZnS nanocomposite boosts blue LED-light-driven photocatalytic hydrogen evolution. Catalysts 2022, 12, 1035. [Google Scholar] [CrossRef]
- Chang, C.J.; Wei, Y.H.; Kuo, W.S. Free-standing CuS–ZnS decorated carbon nanotube films as immobilized photocatalysts for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 30553–30562. [Google Scholar] [CrossRef]
- Chiu, M.H.; Kuo, C.C.; Huang, C.W.; Yang, W.D. Preparation of CuS/PbS/ZnO heterojunction photocatalyst for application in hydrogen production. Catalysts 2022, 12, 1677. [Google Scholar] [CrossRef]
- Son, N.; Heo, J.N.; Youn, Y.S.; Kim, Y.; Do, J.Y.; Kang, M. Enhancement of hydrogen productions by accelerating electron-transfers of sulfur defects in the CuS@ CuGaS2 heterojunction photocatalysts. Catalysts 2019, 9, 41. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, Y.G.; Chen, J.; Huang, C.Y.; Hsieh, S.C.; Wu, S.Y. Ionic liquid/surfactant-hydrothermal synthesis of dendritic PbS@CuS core-shell photocatalysts with improved photocatalytic performance. Appl. Surf. Sci. 2021, 546, 149106. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Mohamed, S.H.; Hadia, N.M.A.; Rabia, M.; Awad, M.A. Some characteristics of Cu/Cu2O/CuO nanostructure heterojunctions and their applications in hydrogen generation from seawater: Effect of surface roughening. Phys. Scripta. 2024, 99, 045939. [Google Scholar] [CrossRef]
- Chang, C.J.; Kao, Y.C.; Lin, K.S.; Chen, C.Y.; Kang, C.W.; Yang, T.H. Carbon fiber cloth@BiOBr/CuO as immobilized membrane-shaped photocatalysts with enhanced photocatalytic H2 production activity. J. Taiwan Inst. Chem. Eng. 2023, 149, 104998. [Google Scholar] [CrossRef]
- Ma, J.; Hua, Y.; Cao, Y.; Jia, C.; Li, J. Anchoring Cu2O nanoparticles on g-C3N4 nanosheets for enhanced photocatalytic performance. Fuel 2024, 364, 131139. [Google Scholar] [CrossRef]
- Abdullahi, A.G.; Hafeez, H.Y.; Mohammed, J.; Bala, A.A.; Suleiman, C.E.N.A. Current trends and strategies on the development of Cu2O-based photocatalysts for efficient solar fuel hydrogen production via photocatalytic water splitting. J. Alloys Compd. Commun. 2025, 6, 100061. [Google Scholar] [CrossRef]
- Yu, X.; Kou, S.; Zhang, J.; Tang, X.; Yang, Q.; Yao, B. Preparation and characterization of Cu2O nano-particles and their photocatalytic degradation of fluroxypyr. Environ. Technol. 2018, 39, 2967–2976. [Google Scholar] [CrossRef]
- Yu, X.; Chen, H.; Ji, Q.; Chen, Y.; Wei, Y.; Zhao, N.; Yao, B. p-Cu2O/n-ZnO heterojunction thin films with enhanced photoelectrochemical properties and photocatalytic activities for norfloxacin. Chemosphere 2021, 267, 129285. [Google Scholar] [CrossRef]
- Men, X.; Liang, H.; Fan, X.; Bai, J. Synergistic incorporation of MoS2 cocatalyst into ZnIn2S4 nanoflower architectures for efficient photocatalytic H2 production and selective benzyl alcohol oxidation. Opt. Mater. 2025, 166, 117208. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, J.; Wei, L.; Liu, Y.; Yang, C. Z-scheme heterostructure of Fe-doped SnO2 decorated layered g-C3N4 with enhanced photocatalytic activity under simulated solar light irradiation. Opt. Mater. 2020, 101, 109769. [Google Scholar] [CrossRef]
- Kamalakannan, S.; Balasubramaniyan, N.; Neppolian, B. Z-scheme based fabrication of Cu2CdSnS4/Au/g-C3N4 ternary heterojunction with enhanced photocatalytic hydrogen production. Opt. Mater. 2025, 164, 117051. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, J.; Li, J. Reinforced photocatalytic H2 generation behavior of S-scheme NiO/g-C3N4 heterojunction photocatalysts with enriched nitrogen vacancies. Opt. Mater. 2023, 135, 113296. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, S.; Ma, C.; Zhou, Y.; Ye, Z.; Dai, X.; Cao, X. Synthesis of Z-type heterojunction bifunctional composites with Mn-doped CdS nanoparticles supported on NH2-MIL-125 (Ti) for hydrogen evolution and antibiotic degradation under visible light. Opt. Mater. 2023, 135, 113087. [Google Scholar] [CrossRef]
- Sahoo, U.; Pattnayak, S.; Choudhury, S.; Aparajita, P.; Das, S.; Hota, G. B, S co-doped g-C3N4 hollow nanotubes/MIL-53 heterostructure: A MOF derived high performance Z scheme photocatalyst for bisphenol A degradation and H2 evolution. Opt. Mater. 2025, 160, 116778. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Lin, K.S.; Chang, C.J.; Lin, Y.S.; Hassan, S.F. Adsorption and photocatalytic degradation of dye contaminants in wastewater over W-doped titania nanotubes. J. Taiwan Inst. Chem. Eng. 2023, 146, 104863. [Google Scholar] [CrossRef]
- Nie, J.; Yu, X.; Liu, Z.; Zhang, J.; Ma, Y.; Chen, Y.; Chang, Z. Energy band reconstruction mechanism of Cl-doped Cu2O and photocatalytic degradation pathway for levofloxacin. J. Clean. Prod. 2022, 363, 132593. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Yang, N.C.; Lin, K.S.; Chang, C.J.; Dinh, K.T.; Lin, Y.G. Formulation and characterization of W-doped titania nanotubes for adsorption/photodegradation of methylene blue and basic violet 3 dyes. Catal. Today 2022, 388, 36–46. [Google Scholar] [CrossRef]
- Patil, A.B.; Jadhav, B.D.; Bhoir, P. Optical band gap modification of Ce/ZnO for visible light photocatalytic H2 production from aqueous methanol solution. Opt. Mater. 2021, 121, 111503. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Chung, C.Y.; Chang, C.J.; Chang, Y.C.; Chen, C.Y.; Wu, S.Y. Fe-doped g-C3N4/Bi2MoO6 heterostructured composition with improved visible photocatalytic activity for rhodamine B degradation. Molecules 2024, 29, 2631. [Google Scholar] [CrossRef]
- Akhirudin, I.; Budi, S. Electrodeposition of Zn-doped Cu2O for the Photodegradation of Methylene Blue. J. Phys. Conf. Ser. 2020, 1428, 012064. [Google Scholar] [CrossRef]
- Zerouali, M.; Bouras, D.; Daïra, R.; Fellah, M.; Boudjema, B.; Barille, R.; El-Hiti, G.A. Effect of Zn-doped CuO thin films on structural, morphological, optical, and electrical properties for photocatalysis application. Opt. Mater. 2024, 152, 115495. [Google Scholar] [CrossRef]
- Mohtar, S.S.; Aziz, F.; Ismail, A.F.; Sambudi, N.S.; Abdullah, H.; Rosli, A.N.; Ohtani, B. Impact of doping and additive applications on photocatalyst textural properties in removing organic pollutants: A review. Catalysts 2021, 11, 1160. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Zhang, J.; Niu, J.; Zhao, J.; Wei, Y.; Yao, B. Photocatalytic degradation of ciprofloxacin using Zn-doped Cu2O particles: Analysis of degradation pathways and intermediates. Chem. Eng. J. 2019, 374, 316–327. [Google Scholar] [CrossRef]
- Kumar Jacob, S.S.; Kulandaisamy, I.; Poul Raj, I.L.; Abdeltawab, A.A.; Mohammady, S.Z.; Ubaidullah, M. Improved optoelectronic properties of spray pyrolysis coated Zn doped Cu2O thin films for photodetector applications. Opt. Mater. 2021, 116, 111086. [Google Scholar] [CrossRef]
- Borik, M.A.; Diab, M.A.; El-Sabban, H.A.; El-Adasy, A.B.A.; El-Gaby, M.S. Designed construction of boosted visible-light Z-scheme TiO2/PANI/Cu2O heterojunction with elaborated photocatalytic degradation of organic dyes. Synth. Met. 2024, 306, 117642. [Google Scholar] [CrossRef]
- Nesa, M.; Sharmin, M.; Hossain, K.S.; Bhuiyan, A.H. Structural, morphological, optical and electrical properties of spray deposited zinc doped copper oxide thin films. J. Mater. Sci. Mater. Electron. 2017, 28, 12523–12534. [Google Scholar] [CrossRef]
- Yuan, B.; Liu, X.; Cai, X.; Fang, X.; Liu, J.; Wu, M.; Zhu, Q. Preparation of zinc and cerium or both doped Cu2O photoelectric material via hydrothermal method. Sol. Energy 2020, 196, 74–79. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Y.; Meng, D.; Wang, D. One-step hydrothermal synthesis and enhanced photocatalytic performance of pine-needle-like Zn-doped CuO nanostructures. J. Mater. Sci. Mater. Electron. 2016, 27, 12884–12890. [Google Scholar] [CrossRef]
- Goyal, C.P.; Goyal, D.; Ganesh, V.; Ramgir, N.S.; Navaneethan, M.; Hayakawa, Y.; Muthamizhchelvan, C.; Ikeda, H.; Ponnusamy, S. Improvement of Photocatalytic Activity by Zn Doping in Cu2O. Phys. Solid State 2020, 62, 1796–1802. [Google Scholar] [CrossRef]
- Poulson, S.R.; Drever, J.I. Aqueous complexing of nickel and zinc with 3-(N-morpholino) propanesulfonic acid and the solubility products of nickel and zinc hydroxides. Talanta 1996, 43, 1975–1981. [Google Scholar] [CrossRef]
- Shimizu, H.; Sasano, J.; Khoo, P.L.; Izaki, M. Chemical Preparation of Metallic Cu Layer on Glass Substrate Using Intermediate Cu(OH)2/Cu(O,S) Bilayer. J. Electrochem. Soc. 2022, 169, 122506. [Google Scholar] [CrossRef]
- Cocco, F.; Elsener, B.; Fantauzzi, M.; Atzei, D.; Rossi, A. Nanosized surface films on brass alloys by XPS and XAES. RSC Adv. 2016, 6, 31277–31289. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Azenha, C.; Mateos-Pedrero, C.; Lagarteira, T.; Mendes, A.M. Tuning the selectivity of Cu2O/ZnO catalyst for CO2 electrochemical reduction. J. CO2 Util 2023, 68, 102368. [Google Scholar] [CrossRef]
- Moretti, G.; Beck, H.P. On the Auger parameter of Cu (II) compounds. Surf. Interface Anal. 2022, 54, 803–812. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo-and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Lu, C. Chemisorbed oxygen on the surface of catalyst-improved cataluminescence selectivity. Anal. Chem. 2016, 88, 4987–4994. [Google Scholar] [CrossRef]
- Li, T.; Abuelgasim, S.; Wang, W.; Xiao, Y.; Liu, C.; Ying, Y.; Liu, D. Enhanced soot oxidation by oxygen vacancies via K+ doped CuFe2O4 spinel catalysts. Int. J. Energy Res. 2022, 46, 15376–15386. [Google Scholar] [CrossRef]
- Meena, P.L.; Surela, A.K.; Chhachhia, L.K.; Meena, J.; Meena, R. Investigation of the photocatalytic potential of C/N-co-doped ZnO nanorods produced via a mechano-thermal process. Nanoscale Adv. 2025, 7, 1335–1352. [Google Scholar] [CrossRef]
- Zhu, C.; Panzer, M.J. Synthesis of Zn:Cu2O Thin Films Using a Single Step Electrodeposition for Photovoltaic Applications. ACS Appl. Mater. Interfaces 2015, 7, 5624–5628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jing, D.; Guo, L.; Yao, X. In situ photochemical synthesis of Zn-doped Cu2O hollow microcubes for high efficient photocatalytic H2 production. ACS Sustain. Chem. Eng. 2014, 2, 1446–1452. [Google Scholar] [CrossRef]
- Hu, F.; Zou, Y.; Wang, L.; Wen, Y.; Xiong, Y. Photostable Cu2O photoelectrodes fabricated by facile Zn-doping electrodeposition. Int. J. Hydrogen Energy 2016, 41, 15172–15180. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, M.M.; Chen, J.L.; Xie, K.F.; Fang, S.M. Dendritic branching Z-scheme Cu2O/TiO2 heterostructure photo-catalysts for boosting H2 production. J. Phys. Chem. Solids 2021, 152, 109948. [Google Scholar] [CrossRef]
- Park, B.H.; Park, H.; Kim, T.; Yoon, S.J.; Kim, Y.; Son, N.; Kang, M. S-scheme assisted Cu2O/ZnO flower-shaped heterojunction catalyst for breakthrough hydrogen evolution by water splitting. Int. J. Hydrogen Energy 2021, 46, 38319–38335. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, Y.L.; Jiu, B.B.; Gong, F.L.; Chen, J.L.; Fang, S.M.; Zhang, H.L. Highly enhanced photocatalytic H2 evolution of Cu2O microcube by coupling with TiO2 nanoparticles. Nanotechnology 2019, 30, 145401. [Google Scholar] [CrossRef]
- Lou, Y.; Zhang, Y.; Cheng, L.; Chen, J.; Zhao, Y. A Stable Plasmonic Cu@Cu2O/ZnO Heterojunction for Enhanced Photocatalytic Hydrogen Generation. ChemSusChem 2018, 11, 1505–1511. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Cai, X.L.; Li, Y.L.; Liu, M.M.; Ding, C.L.; Chen, J.L.; Fang, S.M. Facile synthesis of hollow p-Cu2O/n-ZnO mi-crospheres with enhanced photocatalytic H2 production. Chem. Phys. Lett. 2019, 734, 136748. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Abdel-wahab, M.S.; Elfayoumi, M.A.K.; Tawfik, W.Z. Highly efficient sputtered Ni-doped Cu2O photoelec-trodes for solar hydrogen generation from water-splitting. Int. J. Hydrogen Energy 2023, 48, 1863–1876. [Google Scholar] [CrossRef]
- Chang, C.J.; Tsai, Z.T.; Lin, K.S.; Nian, Y.H. Enhanced photocatalytic H2 production of flower-like MoS2@Ag2S photocatalysts with matched band structures. Photochem. Photobiol. A Chem. 2023, 445, 115027. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, S. Effect of particle size on photoluminescence emission intensity in ZnO. Acta Mater. 2011, 59, 3024–3031. [Google Scholar] [CrossRef]
- Karazmoudeh, N.J.; Soltanieh, M.; Hasheminiasari, M. Structural and photocatalytic properties of undoped and Zn-doped CuO thin films deposited by reactive magnetron sputtering. J. Alloys Compd. 2023, 947, 169564. [Google Scholar] [CrossRef]
- Chang, C.J.; Lee, Z.; Wang, C.F. Photocatalytic hydrogen production by stainless steel@ZnS core–shell wire mesh photocatalyst from saltwater. Int. J. Hydrogen Energy 2014, 39, 20754–20763. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Cui, M.; Li, X.; Niu, L.; Wu, X.; Shao, C. Z-type ZnIn2S4 homojunction for high performance photocatalytic hydrogen evolution. Chem. Eng. J. 2025, 507, 160370. [Google Scholar] [CrossRef]
- Wang, M.; Wang, K.; Jin, Z. Fabrication of Zn-doped Cu2O for auxiliary graphdiyne to enhance photocatalytic H2 evolution performance. J. Environ. Chem. Eng. 2024, 12, 113530. [Google Scholar] [CrossRef]
- Jafari, H.; Sadeghzadeh, S.; Rabbani, M.; Rahimi, R. Effect of Nb on the structural, optical and photocatalytic properties of Al-doped ZnO thin films fabricated by the sol-gel method. Ceram. Int. 2018, 44, 20170–20177. [Google Scholar] [CrossRef]
- Han, C.; Duan, L.; Zhao, X.; Hu, Z.; Niu, Y.; Geng, W. Effect of Fe doping on structural and optical properties of ZnO films and nanorods. J. Alloys Compd. 2019, 770, 854–863. [Google Scholar] [CrossRef]
- Cai, W.; Liu, J.; Luo, Y.; Liao, Z.; Li, B.; Xiang, X.; Fang, Y. Bifunctional CdS-MoO2 catalysts for selective oxidation of lactic acid coupled with photocatalytic H2 production. J. Colloid Interface Sci. 2024, 675, 836–847. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).