Abstract
The increasing discharge of organic pollutants such as dyes and antibiotics poses severe threats to aquatic ecosystems and human health. Conventional wastewater treatment methods are often limited by high energy consumption, secondary pollution, or low efficiency under visible light. It is crucial to design novel photocatalysts that can simultaneously utilize visible photons and enable swift transport of photoinduced charge carriers to drive contaminant decomposition. Herein, novel BiOCl/MIL-121 composites were synthesized via a straightforward hydrothermal route. A suite of complementary microscopic and spectroscopic analyses, including SEM, TEM, XRD and XPS, were employed to elucidate the material’s composition. Furthermore, collective evidence from spectroscopic and electrochemical analyses confirms markedly improved light absorption and charge separation efficiency within the BiOCl/MIL-121 photocatalyst. The 5% BiOCl/MIL-121 composite achieved 93.7% removal of Rhodamine B in 60 min, exhibiting a high photocatalytic degradation rate. Similarly, 5% BiOCl/MIL-121 photodegraded 80.4% of tetracyclin, which was much better than that of BiOCl. A plausible interfacial charge-transfer mechanism was deduced from the band structure of the 5% BiOCl/MIL-121 composite and experimental evidence from radical scavenger studies. This study provides an effective strategy for constructing a composite photocatalyst and offers a green way for the efficient degradation of organic pollutants.
1. Introduction
Along with the sustained growth of industrial output and the escalating density of human settlements, the discharge of massive hazardous organic pollutants into natural aquatic systems has given rise to a deteriorating water pollution scenario that continues to intensify [1,2]. The intrinsic chemical stability and low biodegradability of specific organic pollutants, particularly synthetic dyes and antibiotics, have aroused considerable concern. This is because such characteristics significantly impede their removal by traditional water treatment methodologies. Rhodamine B (RhB), a representative triphenylmethane synthetic dye, finds extensive application in industries like textile dyeing, papermaking, and plastics. The compound’s chemically stable structure leads to its environmental durability, prompting significant concerns given its well-documented harmful impacts on aquatic organisms, genotoxicity potential, and potential human carcinogenic risk [3]. Tetracycline (TC), a broad-spectrum antibiotic frequently employed in clinical settings, undergoes metabolic excretion and subsequently enters water bodies in substantial amounts. Its potential to disrupt aquatic ecosystems thereby poses a tangible risk to human health, given the documented interference with ecological balance [4].
A wide range of treatment technologies have been developed for pollutant elimination, embracing approaches like physical adsorption and membrane filtration [5,6], advanced oxidation processes such as ozonation and Fenton reactions [7,8], and biological treatments utilizing specialized microbes [9]. While demonstrating practical applicability in specific scenarios, traditional treatment approaches face enduring obstacles, including high operational expenses, insufficient removal efficiencies, potential secondary pollution, and poor specificity toward particular contaminants [10]. As an alternative strategy, photocatalysis facilitates effective water treatment by directly harnessing light energy, emerging as a purification technique with negligible ecological footprint [11]. As an alternative approach, photocatalysis enables efficient water purification by directly exploiting light energy, standing out as a treatment technology with minimal environmental impact [12]. These advantages make it especially promising for the degradation of contaminants like RhB and TC [13].
Among various photocatalysts, BiOCl, a representative V-VI-VII ternary semiconductor compound, exhibits distinctive properties including adjustable band gaps and remarkable photocatalytic activity [14,15,16], which endow BiOCl with promising potential for water splitting [17], indoor-gas purification [18], CO2 reduction [19] and, in particular, the photodegradation of organic pollutants [20,21,22,23]. The practical application of pristine BiOCl in photocatalysis is constrained by inherent limitations. These encompass its properties, including a limited specific surface area and propensity for agglomeration, as well as its electronic structure, which features a positive conduction band potential, inferior reduction capability and fast charge carrier recombination [24]. Thus, strategies such as microstructure modulation [11], facet engineering and defect regulation [25], material composition optimization [24] and elemental doping [26], have been put forward to address the aforementioned limitations. Composite formation stands as a highly effective tactic, Harnessing synergistic effects to not only inhibit the recombination of electron-hole pairs but also markedly enhance the semiconductor’s ability to utilize visible light. Thus, a variety of BiOCl-based composite materials have been engineered through the introduction of g-C3N4 [27], CeO2 [28], Bi2S3 [29], Metal–Organic Framework (MOFs) [30] and so on.
The highly ordered, porous architecture of metal–organic frameworks (MOFs), consisting of periodic coordination frameworks of metal clusters and organic ligands, makes them inherently conducive as catalysts or co-catalysts in photocatalytic degradation processes [31]. The material possesses a substantial specific surface area, a tunable pore architecture and remarkable adsorption capacity, all of which are pivotal for enhancing photocatalytic efficiency. MIL-121 features a rod-like morphology and an aluminum-based framework. Its structure consists of unbroken chains of AlO4(OH)2 octahedra linked by pyromellitic acid ligands [32]. In the MIL-121 structure, each pyromellitic acid ligand leverages only two of its carboxyl groups for metal coordination. This deliberate bonding pattern leaves a high density of uncoordinated carboxyls exposed, which can function as versatile chemical handles for engaging with other components or reactants [33]. Yao et al. fabricated a Bi2MoO6/MIL-121 composite via a two-step solvothermal and thermal-reduction approach. Benefiting from the strong interfacial interaction and the synergistic effect between Bi2MoO6 and MIL-121, the composite exhibited significantly enhanced photocatalytic activity toward the reduction of Cr(VI) compared with pristine Bi2MoO6 [34].
This study designed and fabricated novel BiOCl/MIL-121 heterostructures via a facile hydrothermal method, assessing their photocatalytic activity against TC and RhB. The composites exhibited significantly improved degradation efficiency for both contaminants when compared to pristine BiOCl under visible-light illumination. A series of experiments were performed to assess the photocatalytic efficiency, identify the active species involved in TC and RhB decomposition via quenching tests, and determine the material’s reusability through repeated trials. The obtained results provide fundamental knowledge of the degradation process and facilitate the development of optimized MOF-semiconductor composites as advanced visible-light photocatalysts.
2. Results and Discussion
2.1. Characterization of Catalysts
To examine the nanostructural features of the synthesized specimens, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were employed. Pure BiOCl crystals exhibit a uniform square-shaped three-dimensional nanosheet morphology, the measured lateral dimensions were on the order of 150 nm, with a typical thickness measuring about 20 nm (Figure 1a). As shown in Figure 1b, MIL-121 presents a plate-like structure with a size of about 40 μm. The SEM image of the 5% BiOCl/MIL-121 composite in Figure 1c clearly demonstrates that the incorporation of MIL-121 significantly alters the morphology of BiOCl. The original 3D cuboidal structure of BiOCl transforms into a flattened 2D nanosheet-like morphology, with the lateral dimension extending from 150 nm to 500 nm. Moreover, the previously uniform distribution becomes disrupted and more irregular. These observations suggest that the presence of MIL-121 influences the growth behavior of BiOCl, indirectly confirming the successful formation of the BiOCl/MIL-121 composite. In Figure 1d, HRTEM images clearly reveal that BiOCl grows in situ on MIL-121, A distinct interfacial region between the two components can be observed, indicating the intimate contact achieved during the synthesis process. The lattice fringes with an interplanar spacing of 0.273 nm are assigned to the (110) crystal plane of tetragonal BiOCl (JCPDS No. 06-0249). In contrast, no obvious lattice fringes are detected for MIL-121, which can be attributed to its relatively low crystallinity and the organic–inorganic hybrid framework nature of MOFs [22].
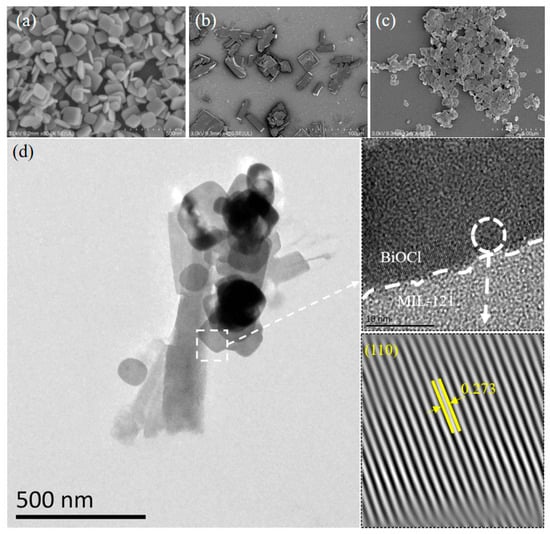
Figure 1.
SEM images of (a) BiOCl, (b) MIL-121, and (c) 5% BiOCl/MIL-121. HRTEM image of 5% BiOCl/MIL-121 (d).
XRD is employed to investigate the crystal phases of MIL-121, BiOCl, and 5% BiOCl/MIL-121 composites, with the results depicted in Figure 2a, The XRD pattern of the pure BiOCl sample revealed multiple diffraction peaks, which were indexed to the (001), (101), (110), (102), (112), (200), and (220) crystallographic planes of its tetragonal phase (JCPDS No. 6-249). These peaks were located at 2θ values of 12.16°, 25.81°, 32.74°, 33.44°, 40.88°, 46.55°and 68.55°, respectively. Notably, the (001) value in the BiOCl/MIL-121 composite series is higher than that in standard BiOCl. The incorporation of MIL-121 promotes the preferential growth of BiOCl crystals along the (001) crystal plane, indicating that the presence of MIL-121 in the designed BiOCl/MIL-121 composite photocatalyst has a significant influence on the crystal structure of BiOCl [35]. This may alter the nucleation and growth mechanisms of the BiOCl crystals. This structural engineering is expected to facilitate the migration of photoinduced charge carriers and optimize the distribution of surface reactive sites, thereby enhancing the overall photocatalytic performance [36].
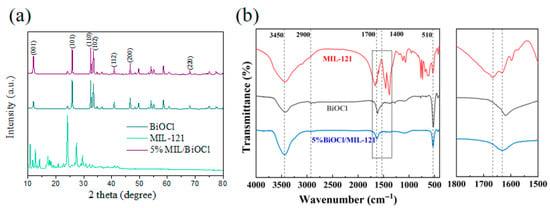
Figure 2.
XRD and (a) FT-IR spectra (b) of MIL-121, BiOCl and 5% BiOCl/MIL-121.
The FTIR spectra of MIL-121, BiOCl and the 5% BiOCl/MIL-121 composite are presented in Figure 2b. The FTIR spectra further corroborate the structural insights obtained from XRD. Pristine MIL-121 exhibits discernible FTIR signals, including the O-H (3450 cm−1) and C-H (2900 cm−1) stretching vibrations, along with prominent bands from 1700 to 1400 cm−1 corresponding to carboxylate moieties [35]. While BiOCl shows a distinct Bi-O vibration at 510 cm−1 [37]. The 5% BiOCl/MIL-121 composite retains the characteristic features of both components, yet the carboxyl-related bands undergo slight shifts relative to MIL-121 alone, indicating the formation of Bi3+-COOH coordination at the interface [38]. Such interfacial interactions not only confirm the intimate coupling of BiOCl with MIL-121 but also rationalize the enhanced (001) diffraction intensity observed in XRD.
To gain insight into the interfacial interactions within the BiOCl/MIL-121 composite, an XPS analysis was conducted on the 5% BiOCl/MIL-121 sample to determine its surface chemistry. As shown in Figure 3a, 5% BiOCl/MIL-121 contains four elements: Bi, O, Cl, C and Al, which match their chemical compositions. In the Cl 2p spectrum of 5% BiOCl/MIL-121 (Figure 3b), the Cl 2p 3/2 and Cl 2p 1/2 orbitals are identified at binding energies of 198.4 eV and 200.0 eV, respectively. verifying the existence of Cl− in BiOCl. The 5% BiOCl/MIL-121 composite contains O-H bonds and Bi-O bonds within its ligand structure. These chemical species account for the two distinct signals in the O 1s spectrum (Figure 3c), located at 530.1 eV and 532.1 eV, which correspond to lattice oxygen (OL) and OOH, respectively [39]. In Figure 3d, the Bi 4f spectrum of BiOCl displays two distinct peaks centered at 159.7 and 165.0 eV [40], which are assigned to Bi 4f 7/2 and Bi 4f 5/2, respectively [41,42]. In Figure 3e, Deconvolution of the C 1s spectrum reveals three individual components with binding energies of 284.8 eV and 286.2 eV. The main peak corresponds to C=C (sp2), while the other peak could be assigned to C-C (sp3) [39,43]. The latter arises from the abundant carboxylate groups of MIL-121, confirming the preservation of organic linkers upon composite formation. The Al 2p region (Figure 3f) exhibits a pronounced peak at 74.2 eV, characteristic of Al-O coordination, further verifying the structural integrity of MIL-121 [44]. Through the Bi3+-COOH interfacial linkage, an electron-accepting effect is induced, which establishes a direct charge transport channel between BiOCl and MIL-121. This configuration effectively facilitates the dissociation of photogenerated electron-hole pairs in the composite material [45].
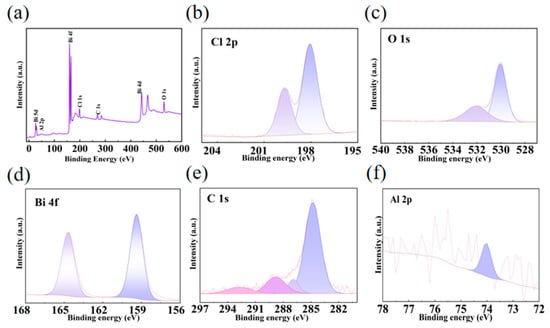
Figure 3.
XPS spectra of 5% BiOCl/MIL-121: (a) Full survey, (b) Cl 2p, (c) Bi 4f, (d) O 1s, (e) C 1s and (f) Al 2p.
2.2. Photocatalytic Properties
2.2.1. Optical Characteristics and Band Structure Configurations
UV-Vis diffuse reflectance spectroscopy was utilized to examine the optical properties of BiOCl, MIL-121, and their composite (Figure 4a). MIL-121 exhibits a pronounced absorption edge in the ultraviolet region (250–350 nm), whereas BiOCl extends slightly into the near-visible range. Notably, the 5% MIL/BiOCl composite displays an elevated baseline absorption throughout 400–800 nm, suggesting the presence of defect- or interface-induced states that broaden the optical response. To estimate the optical band gaps, Tauc analysis was performed by extrapolating the linear region of (F(R)hv)2 versus photon energy, assuming a direct electronic transition, yielded optical band gaps of approximately 3.2 eV for MIL-121, 3.6 eV for BiOCl and 3.4 eV for the 5% BiOCl/MIL-121 composite (Figure 4b). The narrowed band gap exhibited by the composite points to a substantial reconstruction of the electronic configuration resulting from the formation of the hybrid. The aforementioned finding verifies the successful fabrication of the composite, thereby potentially enabling superior separation and migration of photogenerated charge carriers. It is noted that while the composite presents a moderate band gap value, it demonstrates improved baseline absorption in the visible-light spectrum. Such interfacial adjustments are anticipated to exert a pivotal function in regulating charge separation and transport, as further supported by the spectroscopic and photoelectrochemical analyses presented below. We employed the Mott–Schottky plot of MIL-121 to determine its HOMO and LUMO energy levels, thereby clarifying the charge carrier transfer mechanism. The determined flat-band potential of BiOCl provides an estimate of its conduction band position, as this potential typically aligns with the CB edge in n-type semiconductors. Using the formula E (vs. RHE) = E (vs. Ag/AgCl) + 0.197, the CB potential of BiOCl was calculated as −1.2 V. Considering its optical band gap of 3.6 eV, the corresponding valence band (VB) potential was calculated to be +2.4 V (Figure 4c). Similarly, the band structure of MIL-121 was determined by combining Mott–Schottky analysis and UV-Vis diffuse reflectance spectroscopy (Figure 4d). The CB potential was −1.4 V, and the band gap was 3.2 eV, giving a calculated VB potential of +1.8 V [46]. Consequently, the band structures of BiOCl and MIL-121 were systematically established, furnishing a robust basis for interpreting their optical absorption behaviors and photocatalytic characteristics.
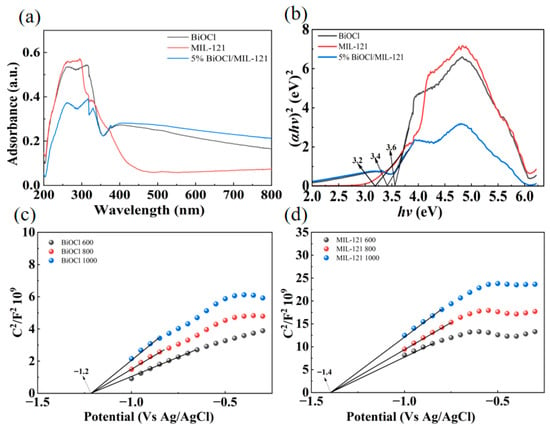
Figure 4.
UV–vis DRS (a), bandgap values (b), Mott–Schottky plots of BiOCl (c) and MIL-121 (d).
2.2.2. Characterization of Charge Separation
Depicted in Figure 5a, the transient photocurrent response reveals significant differences among MIL-121, BiOCl, and the 5% BiOCl/MIL-121, 3% BiOCl/MIL-121, 7% BiOCl/MIL-121 and 10% BiOCl/MIL-121 composites. Bare MIL-121 exhibits negligible photocurrent intensity due to its poor charge separation efficiency, while pristine BiOCl generates a moderate response under visible-light illumination. Strikingly, the 5% BiOCl/MIL-121 composite delivers the highest photocurrent density, nearly doubling that of BiOCl and All BiOCl/MIL-121 composites displayed stronger photocurrent responses than the individual components. Electrochemical impedance spectroscopy further validates the improved carrier dynamics. The Nyquist plots clearly show that the composite exhibits the smaller semicircle radius compared with pristine MIL-121 and BiOCl (Figure 5b), indicating the lower interfacial charge-transfer resistance. Such reduced resistance facilitates more rapid electron transport across the interface. In summary, these findings demonstrate that the internal electric field at the BiOCl/MIL-121 interface serves as the driving force for photogenerated charge separation, endowing the composite with stronger photocatalytic activity compared to the single components.
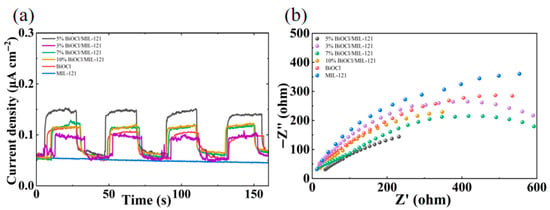
Figure 5.
Photocurrent responses curves (a) and EIS (b) of MIL-121, BiOCl, 5% BiOCl/MIL-121, 3% BiOCl/MIL-121, 7% BiOCl/MIL-121 and 10% BiOCl/MIL-121.
2.3. Photocatalytic Activity
2.3.1. Photocatalytic Degradation Rhodamine B (RhB)
The photocatalytic degradation performances of BiOCl and 3% BiOCl/MIL-121, 5% BiOCl/MIL-121, 7% BiOCl/MIL-121 and 10% BiOCl/MIL-121 composites were systematically evaluated via RhB dye degradation under visible-light irradiation. In this work, the degradation efficiency achieved at 60 min of reaction served as the metric to evaluate and compare the catalytic performance. Following 60 min of catalytic reaction, the degradation efficiency of BiOCl, 3% BiOCl/MIL-121, 5% BiOCl/MIL-121, 7% BiOCl/MIL-121, 10% BiOCl/MIL-121, are 48.2%, 79.8%, 93.7%, 86.9% and 82.7%, respectively (Figure 6a). Notably, the catalytic efficiency of 5% BiOCl/MIL-121 surpasses that of other samples. The k values of BiOCl, 3% BiOCl/MIL-121, 5% BiOCl/MIL-121, 7% BiOCl/MIL-121 and 10% BiOCl/MIL-121 are 0.011 min−1, 0.027 min−1, 0.044 min−1, 0.032 min−1 and 0.031 min−1 (Figure 6b). The maximum k value was observed in the case of 5% BiOCl/MIL-121 (Figure 6c). However, an excessive amount of MIL-121 adversely affects the composite’s light-harvesting ability within the visible spectrum, consequently diminishing its photocatalytic efficiency. As shown in Figure 6d, the catalyst showed a minor efficiency decline after four testing cycles, stemming from progressive material loss that occurs throughout the photocatalytic process. Notably, 5% BiOCl/MIL-121 retained significant catalytic activity, illustrating its comparatively stable catalytic performance. After four cyclic tests, 5% BiOCl/MIL-121 retained 84.3% of its initial catalytic performance, which well demonstrates the good chemical stability of the photocatalyst.
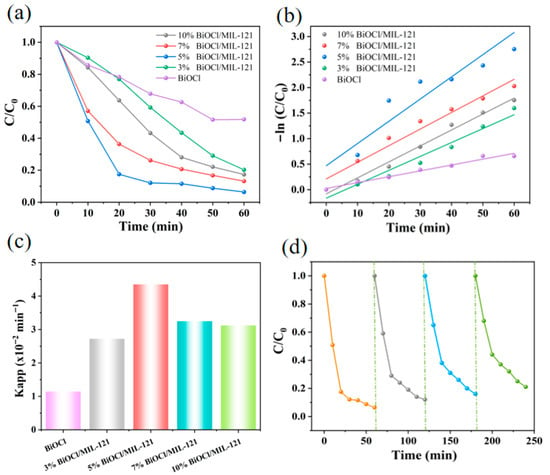
Figure 6.
(a) Photocatalytic degradation profiles, (b) time-dependent reaction kinetics, and (c) pseudo-first-order rate constants. (d) Reusability of 5% BiOCl/MIL-121 for RhB degradation.
2.3.2. Photocatalytic Degradation Tetracycline (TC)
A consistent observation from Figure 7 is that the degradation behavior of TC mirrors that of RhB, and all BiOCl/MIL-121 hybrid materials show a marked improvement in activity compared to the single-component BiOCl. Among these samples, the 5% BiOCl/MIL-121 composite exhibited the strongest photocatalytic performance, It achieved a degradation efficiency of 80.4% for TC under 120 min of visible-light irradiation. The corresponding kinetic data are presented in Figure 7b. Compared with pure BiOCl, all composite samples exhibited enhanced reaction rates. Notably, the 5% BiOCl/MIL-121 composite achieved a reaction rate constant (k) of 0.012 min−1, a value 2.57 times greater than that of the pure BiOCl sample (Figure 7c). For the purpose of further assessing photocatalytic stability, the 5% BiOCl/MIL-121 composite was subjected to four consecutive degradation cycles, as shown in Figure 7d. The composite demonstrated satisfactory stability, maintaining a TC removal efficiency exceeding 66% throughout four consecutive catalytic cycles. Nevertheless, the composite retained significant degradation capacity, thus confirming its robust operational stability in the tetracycline photocatalytic process. 5% BiOCl/MIL-121 also exhibits superior degradation performance compared to other photocatalysts reported in previous studies (Table S1) [47,48,49,50,51,52,53,54,55,56].
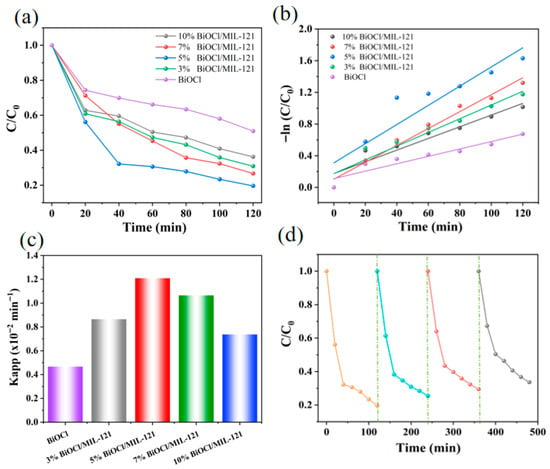
Figure 7.
(a) Photocatalytic degradation profiles, (b) time-dependent reaction kinetics, and (c) pseudo-first-order rate constants. (d) Reusability of 5% BiOCl/MIL-121 for TC degradation.
2.3.3. Photocatalysis Mechanism
To elucidate the underlying photocatalytic mechanism, a series of radical scavenging tests were conducted using the 5% BiOCl/MIL-121 catalyst for the degradation of RhB (Figure 8a) and TC (Figure 8b). Specifically, 1,4-benzoquinone (BQ), iso-propyl alcohol (IPA) and ethylene diamine tetraacetic acid (EDTA) were chosen as the quenchers for O2•−, •OH and h+, respectively. The introduction of BQ and EDTA caused distinct inhibition of the degradation process, indicating that superoxide radicals and holes function as the dominant active species in the TC photodegradation mediated by BiOCl/MIL-121.
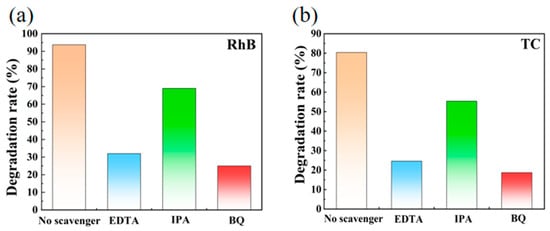
Figure 8.
Photodegradation profiles of RhB (a) and TC (b) over 5% BiOCl/MIL-121 with various trapping agents under visible-light irradiation.
The detailed photocatalytic mechanism is illustrated in Figure 9. Under white-light illumination, MIL-121 serves as a photoactive antenna unit by efficiently harvesting visible photons and enabling the generation of electron-hole pairs. Notably, the holes in the valence band (VB) of MIL-121 exhibit insufficient oxidizing capacity to generate radicals •OH (E(H2O/•OH) = +2.38 eV), as experimentally observed [57]. Because MIL-121 possesses a conduction band edge that is more positive than that of BiOCl, it drives the photogenerated electron flow toward BiOCl as a favored direction. The migration of electrons leads to a marked increase in reactive oxygen species (ROS) yield. Electron migration to the BiOCl surface facilitates the reduction of O2, generating O2•− species (E(O2/O2•−) = −0.33 eV) [58]. The generated O2•− radical actively takes part in the degradation of RhB and TC. Simultaneously, the holes produced in the valence band of MIL-121 retain sufficient potential to directly decompose RhB and TC molecules. The cooperative effect between the components provides a markedly improved separation efficiency for electron-hole pairs, with a consequential enhancement in O2•− radical production. These combined effects allow the 5% BiOCl/MIL-121 catalyst to maintain continuous decomposition of RhB and TC molecules. The underlying reaction pathway can be summarized by the following processes:
BiOCl/MIL-121 + hυ→h+ + e−
O2 + e−→ O2•−
OH− + h+ → •OH
TC/RhB + 1O2•−/•OH/h+→CO2 + H2O
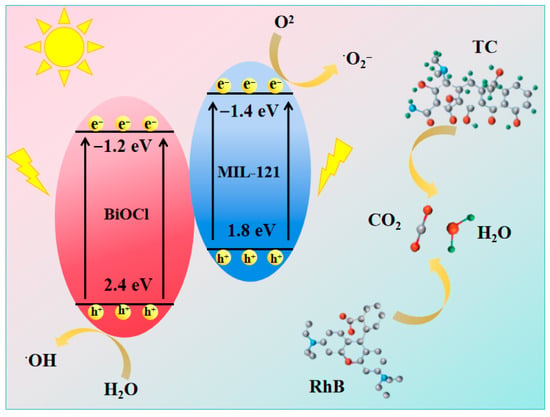
Figure 9.
A mechanism illustration for the photocatalytic removal of TC and RhB using the BiOCl/MIL-121 composite.
3. Materials and Methods
3.1. Chemicals and Reagents
The chemicals used in the experiment can be used directly without further purification. Bi(NO3)3•5H2O, Aluminum nitrate nonahydrate (Al (NO3)3•9H2O),1, 2, 4, 5-Benzenetetracarboxylic acid (H4BTC), Ethylene glycol (C2H6O2), Iodine monochloride (KCl), Ethanol (C2H6O), Hydrochloric acid (HCl) and p-benzoquinone (C6H4O2, BQ) were bought from Macklin Biochemical Co., Ltd. (Shanghai, China). TC and RhB was purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Ethylenediaminetetraacetic acid disodium salt (EDTA) and isopropanol (IPA) were bought in Sinopharm Chemical Reagent Co. Ltd. (Beijing, China). Thanol (C2H5OH) was bought in Titan Scientific Co. Ltd. (Shanghai, China).
3.2. Synthesis of Catalysts
3.2.1. Synthesis of BiOCl Nanosheets
The synthesis of BiOCl nanosheets employed a solvothermal process. Initially, 0.97 g Bi(NO3)3·5H2O was stirred in 15 mL ethylene glycol for one hour to form a transparent solution (Solution A). Concurrently, 0.1491 g KCl was dissolved in 15 mL deionized water (Solution B). Solutions A and B were then blended under continuous agitation to produce a uniform white suspension. The mixture was transferred to a 50 mL Teflon-lined autoclave, which was then sealed and heated at 160 °C for 12 h. Upon natural cooling to room temperature, the precipitate was collected Via centrifugation and alternately rinsed with deionized water and absolute ethanol three times. The sample was then dried under vacuum at 60 °C for 12 h, yielding the final BiOCl powder.
3.2.2. Synthesis of MIL-121
The preparation of MIL-121 employed a hydrothermal method. Specifically, 2.4 g Al(NO3)3·9H2O (6.4 mmol) and 0.80 g H4BTEC (3.2 mmol) were dissolved in 10 mL deionized water and stirred for 2 h at room temperature. To this mixture, 0.2 mL of HCl (36–38%) was added dropwise under agitation. The homogeneous suspension formed was charged into a 50 mL Teflon-lined autoclave and maintained at 180 °C for 48 h. The collected precipitate after natural cooling was centrifuged, washed alternately with deionized water and ethanol three times and then dried at 80 °C for 24 h to obtain the final MIL-121.
3.2.3. Synthesis of BiOCl/MIL-121 Composites
During the synthesis of BiOCl nanosheets, MIL-121 was introduced into the white mixed suspension at various mass ratios, followed by thorough stirring to ensure uniform dispersion. The subsequent synthesis procedure was identical to that of pure BiOCl. Composite photocatalysts with different MIL-121 loadings were obtained, with mass ratios of MIL-121 to BiOCl set at 0.03, 0.05, 0.07 and 0.10. The corresponding composites were denoted as 3% BiOCl/MIL-121, 5% BiOCl/MIL-121, 7% BiOCl/MIL-121 and 10% BiOCl/MIL-121, respectively.
3.3. Characterization Techniques and Degradation Testing
3.3.1. Assessment of Photocatalytic Performance
In a typical photocatalytic test, 30 mg of photocatalyst was dispersed in 50 mL of RhB solution (10 mg/L) and ultrasonicated for 20 min. The suspension was stirred in the dark for 30 min to reach adsorption–desorption equilibrium, followed by visible-light irradiation using a 250 W Xenon lamp with water cooling. During the reaction, 3 mL aliquots were taken every 10 min, centrifuged to remove the catalyst, and the RhB concentration was determined by UV-Vis spectroscopy at 554 nm.
For the photocatalytic degradation of TC, 50 mg of photocatalyst was dispersed in 50 mL of TC solution (10 mg/L) and ultrasonicated for 20 min. The suspension was stirred in the dark for 30 min to reach adsorption–desorption equilibrium, followed by visible-light irradiation using a 250 W Xenon lamp with water cooling for 2 h. During the reaction, 3 mL aliquots were taken every 20 min, centrifuged to remove the catalyst, and the TC concentration was determined by UV-Vis spectroscopy at 357 nm. The degradation rate is defined as below in Equation (1):
where C0 is the concentration of the initial target pollutant solution at the maximum characteristic peak wavelength and C is the concentration of the pollutant solution at the maximum characteristic peak wavelength at time t.
The kinetic process of the photocatalytic degradation of target pollutants generally follows the quasi-primary kinetic model. The kinetic process of the photocatalytic degradation reaction of the target pollutants generally follows the quasi-primary kinetic model and its Equation (2) is expressed as:
where C0 is the concentration of the initial target pollutant solution at the wavelength of the largest characteristic peak, C is the concentration of the post-reaction solution at the wavelength of the largest characteristic peak, and kapp is the apparent rate constant for the first-stage reaction and t is time.
−ln(C/C0) = kappt
The main active species in the system were deduced by free radical trapping experiments. To each photocatalytically active optimal system in the above scheme, BQ, EDTA and IPA were added according to the needs and the concentration of the regulated system was 1 mmol/L. The rest of the experimental procedure and the calculation of degradation rate were consistent with the degradation experiments.
3.3.2. Characterization
X-ray diffraction (XRD, Rigaku Ultima IV, Tokyo, Japan) was used to determine the crystal structure and composition of the samples. The microstructure and internal morphology of the nanomaterials were obtained by scanning electron microscopy (SEM, S-4800, Hitachi company, Kyoto, Japan) and transmission electron microscopy (TEM, UV-2450, Shimadzu Company, Kyoto, Japan), which were used to ensure the correct synthesis of the materials. The UV-vis diffuse reflectance spectra (DRS) were tested on a UV-vis spectrophotometer (PG, UH-4150) at room temperature. X-ray photoelectron spectroscopy (XPS) analysis was recorded on an American electronics physical HI5700ESCA system with X-ray photoelectron spectroscope using Al K (1486.6 eV) monochromatic X-ray radiation. The separation rate and photoelectric activity of the photocatalysts were investigated by Mott Schottky test and electrochemical impedance spectroscopy (CHI-760E, Shanghai, China).
4. Conclusions
In this work, the construction of a BiOCl/MIL-121 composite fundamentally reshapes the charge transfer pathway, thereby unlocking superior photocatalytic activity. The 5% BiOCl/MIL-121 composite achieves a rate constant of 0.044 min−1 for RhB degradation and 0.012 min−1 for TC degradation-both nearly 4.00 and 2.57-fold higher than those of pristine BiOCl. Moreover, the composite retains high catalytic activity over successive cycles, attesting to its excellent chemical stability. Overall, BiOCl/MIL-121 acts as a representative photocatalytic composite, presenting a transferable strategy for integrating MOFs with oxide semiconductors to address the critical challenge of organic pollutant elimination in aquatic systems.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15100995/s1, Table S1: The degradation time and degradation rate in this experiment compared with previous papers.
Author Contributions
Conceptualization, methodology, formal analysis and writing—original draft preparation were performed by T.X.; Material preparation and characterization were performed by J.C., Y.M., Y.P. and H.H.; Manuscript review, supervision and funding acquisition were performed by G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Opening Project of Guangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology (2023K004), Natural Science Foundation of Jiangsu Province in China (BK20230410), Natural Science Research of Jiangsu Higher Education Institution of China (23KJB610010). Thanks Scientific Compass (https://www.shiyanjia.com, accessed on 30 September 2025) for help with testing.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lu, Y.; Hao, C.; Gao, S.; Zhao, X.; An, X.; Hu, G. Piezoelectric catalysts for water pollutant remediation: Achievements, challenges, and perspectives. Mater. Today 2025, 88, 415–440. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Liu, Y.; Han, J.; Duan, G.; Fu, Q.; Han, X.; Zhang, C.; He, S.; Jiang, S. Structure modifications of wood-based materials for water treatment applications: A review. Mater. Today 2025, 87, 252–286. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B Environ. Energy 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Mengting, Z.; Kurniawan, T.A.; Avtar, R.; Othman, M.H.D.; Ouyang, T.; Yujia, H.; Xueting, Z.; Setiadi, T.; Iswanto, I. Applicability of TiO2(B) nanosheets@hydrochar composites for adsorption of tetracycline (TC) from contaminated water. J. Hazard. Mater. 2021, 405, 123999. [Google Scholar] [CrossRef]
- Secondes, M.F.N.; Naddeo, V.; Belgiorno, V.; Ballesteros, F. Removal of emerging contaminants by simultaneous application of membrane ultrafiltration, activated carbon adsorption, and ultrasound irradiation. J. Hazard. Mater. 2014, 264, 342–349. [Google Scholar] [CrossRef]
- Li, P.; Zhao, T.; Zhao, Z.; Tang, H.; Feng, W.; Zhang, Z. Biochar derived from chinese herb medicine residues for Rhodamine B dye adsorption. ACS Omega 2023, 8, 4813–4825. [Google Scholar] [CrossRef]
- Tariq, M.; Muhammad, M.; Khan, J.; Raziq, A.; Uddin, M.K.; Niaz, A.; Ahmed, S.S.; Rahim, A. Removal of Rhodamine B dye from aqueous solutions using photo-Fenton processes and novel Ni-Cu@MWCNTs photocatalyst. J. Mol. Liq. 2020, 312, 113399. [Google Scholar] [CrossRef]
- Yang, X.; Lu, J.; Zhou, L.; Wang, Q.; Wu, F.; Pan, Y.; Zhang, M.; Wu, G. Morphological regulation of Bi5O7I for enhanced efficiency of Rhodamine B degradation under Visible-Light. Catalysts 2025, 15, 714. [Google Scholar] [CrossRef]
- Çelik, M.S.; Çetinus, Ş.A.; Yenidünya, A.F.; Çetinkaya, S.; Tüzün, B. Biosorption of Rhodamine B dye from aqueous solution by Rhus coriaria L. plant: Equilibrium, kinetic, thermodynamic and DFT calculations. J. Mol. Struct. 2023, 1272, 134158. [Google Scholar] [CrossRef]
- Wu, L.; Yang, X.-J.; Ma, K.; Qu, X.; Zhang, Y.; Xia, H.; Wu, J.; Lu, S.; Liu, D. 2D/2D ultrathin (Zn/Ti)LDH/BiOCl Z-scheme heterostructure nanosheets boosted visible-light-catalytic degradation of dyes and antibiotics. Compos. Part B Eng. 2025, 293, 112144. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, L.; Liang, F.; An, D.; Chen, Z.; Feng, D.; Xian, M. Water-assisted synthesis of shape-specific BiOCl nanoflowers with enhanced adsorption and photosensitized degradation of rhodamine B. Environ. Chem. Lett. 2020, 18, 243–249. [Google Scholar] [CrossRef]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef]
- Noël, T.; Zysman-Colman, E. The promise and pitfalls of photocatalysis for organic synthesis. Chem. Catal. 2022, 2, 468–476. [Google Scholar] [CrossRef]
- Alhokbany, N.S.; Mousa, R.; Naushad, M.; Alshehri, S.M.; Ahamad, T. Fabrication of Z-scheme photocatalysts g-C3N4/Ag3PO4/chitosan for the photocatalytic degradation of ciprofloxacin. Int. J. Biol. Macromol. 2020, 164, 3864–3872. [Google Scholar] [CrossRef] [PubMed]
- Bhachu, D.S.; Moniz, S.J.A.; Sathasivam, S.; Scanlon, D.O.; Walsh, A.; Bawaked, S.M.; Mokhtar, M.; Obaid, A.Y.; Parkin, I.P.; Tang, J.; et al. Bismuth oxyhalides: Synthesis, structure and photoelectrochemical activity. Chem. Sci. 2016, 7, 4832–4841. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, Y.; Yang, J.; Liu, X.; Li, W.; Zhang, L.; Wu, Y.; Sun, J.; Feng, J.; Zhu, Y. Visible-light responsive PDI/rGO composite film for the photothermal catalytic degradation of antibiotic wastewater and interfacial water evaporation. Appl. Catal. B Environ. Energy 2021, 291, 120127. [Google Scholar] [CrossRef]
- Du, F.; Sun, L.; Huang, Z.; Chen, Z.; Xu, Z.; Ruan, G.; Zhao, C. Electrospun reduced graphene oxide/TiO2/poly(acrylonitrile-co-maleic acid) composite nanofibers for efficient adsorption and photocatalytic removal of malachite green and leucomalachite green. Chemosphere 2020, 239, 124764. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, Q.; Tang, G.; Zhu, W.; Yang, X.; Luo, Y. TBAOH assisted synthesis of ultrathin BiOCl nanosheets with enhanced charge separation efficiency for superior photocatalytic activity in carbamazepine degradation. J. Colloid Interface Sci. 2020, 570, 242–250. [Google Scholar] [CrossRef]
- Gao, X.; Gong, C.; Wang, X.; Zhu, W.; Luo, Y. Facile synthesis of cobalt doped BiOCl ultrathin nanosheets as superior photocatalyst for degradation of carbamazepine under visible light. J. Solid State Chem. 2021, 298, 122131. [Google Scholar] [CrossRef]
- Wu, F.; Tang, Y.; Pan, Y.; Han, J.; Xing, W.; Zhang, J.; Wu, G.; Huang, Y. Interfacial linkage engineering inducted directional electron transfer over ZnIn2S4@BiOCl S-Scheme heterojunctions for CO2 photoreduction and tetracycline decomposition. Small 2025, 21, 2500670. [Google Scholar] [CrossRef]
- Wu, F.; Wu, G.; Tang, Y.; Pan, Y.; Han, J.; Zhang, J.; Xing, W.; Huang, Y. Dual electron transfer path and LSPR photothermal enhancement in BiOCl@ZnIn2S4 heterojunction for enhanced photocatalytic H2 evolution, H2O2 production and tetracycline removal. Inorg. Chem. Front. 2025, 12, 1200–1213. [Google Scholar] [CrossRef]
- Liu, G.; Guo, Y.; Chen, C.; Lu, Y.; Chen, G.; Liu, G.; Han, Y.; Jin, W.; Xu, N. Eliminating lattice defects in metal-organic framework molecular-sieving membranes. Nat. Mater. 2023, 22, 769–776. [Google Scholar]
- He, R.; Xue, K.; Wang, J.; Yan, Y.; Peng, Y.; Yang, T.; Hu, Y.; Wang, W. Nitrogen-deficient g-C3Nx/POMs porous nanosheets with P-N heterojunctions capable of the efficient photocatalytic degradation of ciprofloxacin. Chemosphere 2020, 259, 127465. [Google Scholar] [PubMed]
- Xu, K.; Shen, J.; Zhang, S.; Xu, D.; Chen, X. Efficient interfacial charge transfer of BiOCl-In2O3 step-scheme heterojunction for boosted photocatalytic degradation of ciprofloxacin. J. Mater. Sci. Technol. 2022, 121, 236–244. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, T.; Hou, J.; Zhang, T.; Zhang, G.; Zhang, Y.; Wang, X. Oxygen vacancies induced narrow band gap of BiOCl for efficient visible-light catalytic performance from double radicals. J. Mater. Sci. Technol. 2022, 114, 240–248. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Ran, W.; Wang, Y.; Tian, F.; Zhang, F.; Xu, M.; Zhang, D.; Li, N.; Yan, T. Spin polarization regulates photocatalytic CO2 into hydrocarbons by Co doped BiOCl. Appl. Catal. B Environ. Energy 2024, 351, 123978. [Google Scholar]
- Song, T.; Yu, X.; Tian, N.; Huang, H.W. Preparation, structure and application of g-C3N4/BiOX composite photocatalyst. Int. J. Hydrogen Energy 2021, 46, 1857–1878. [Google Scholar]
- Yang, J.; Liang, Y.; Li, K.; Yang, G.; Yin, S. One-step low-temperature synthesis of 0D CeO2 quantum dots/2D BiOX (X = Cl, Br) nanoplates heterojunctions for highly boosting photo-oxidation and reduction ability. Appl. Catal. B Environ. Energy 2019, 250, 17–30. [Google Scholar] [CrossRef]
- Long, Z.; Zhang, G.; Du, H.; Zhu, J.; Li, J. Preparation and application of BiOBr-Bi2S3 heterojunctions for efficient photocatalytic removal of Cr(VI). J. Hazard. Mater. 2021, 407, 124394. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, F.; Ling, M.; Zheng, H.; Wu, Y.; Li, L. In-situ constructed indirect Z-type heterojunction by plasma Bi and BiO2-X-Bi2O2CO3 co-modified with BiOCl@Bi-MOF for enhanced photocatalytic efficiency toward antibiotics. Chem. Eng. J. 2023, 464, 142762. [Google Scholar]
- Aloni, P.; Venkatesan, P.; Roy, D.; Nguyen, M.D.; Doong, R.A. Synergistic effect of g-C3N4/NH2-functionalized MIL-125(Ti) heterojunction and Schottky Ti3C2 MXene for the visible-light-driven photodegradation of diclofenac. Sep. Purif. Technol. 2025, 377, 134201. [Google Scholar] [CrossRef]
- Volkringer, C.; Loiseau, T.; Guillou, N.; Férey, G.; Haouas, M.; Taulelle, F.; Elkaim, E.; Stock, N. High-throughput aided synthesis of the porous metal−organic framework-type aluminum pyromellitate, MIL-121, with extra carboxylic acid functionalization. Inorg. Chem. 2010, 49, 9852–9862. [Google Scholar]
- Qiu, J.; Li, M.; Yang, L.; Yao, J. Facile construction of three-dimensional netted ZnIn2S4 by cellulose nanofibrils for efficiently photocatalytic reduction of Cr(VI). Chem. Eng. J. 2019, 375, 121990. [Google Scholar]
- Qiu, J.; Dai, D.; Zhang, L.; Xia, G.; Yao, J. Oxygen vacancy-rich Bi2MoO6 anchored on cuboid metal-organic frameworks for photocatalytic elimination of Cr(VI)/2-nitrophenol mixed pollutants. Sep. Purif. Technol. 2022, 301, 121990. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J.; Chen, X.; Wang, Z.; Ji, H.; Chen, L.; Liu, W.; Wang, C.C. Bifunctional Bi12O17Cl2/MIL-100(Fe) composites toward photocatalytic Cr(VI) sequestration and activation of persulfate for bisphenol A degradation. Sci. Total Environ. 2021, 752, 141901. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, S.; Zhang, F.; Xu, D.; Wang, Q.; Wang, K.; Zhang, L.; Liang, L.; Ren, Z.; Wang, P. A novel lateral epitaxial Bi2O3@BiOCl heterostructure for photocatalytic antibiotic degradation in an internal circulation fluidized bed reactor. Chem. Eng. J. 2023, 478, 147540. [Google Scholar]
- Wang, S.; Song, D.; Liao, L.; Li, M.; Li, Z.; Zhou, W. Surface and interface engineering of BiOCl nanomaterials and their photocatalytic applications. Adv. Colloid Interface Sci. 2024, 324, 103088. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, J.; Li, M.; Xu, J.; Zhang, L.; Yao, J. Construction of two-dimensional BiOI on carboxyl-rich MIL-121 for visible-light photocatalytic degradation of tetracycline. J. Alloys Compd. 2021, 872, 159711. [Google Scholar]
- Ahmed, S.; Shin, J.; Zubair, M.; Shim, J.; Park, G. Carbon wrapped cobalt as bifunctional catalysts in rechargeable zinc-air batteries. Inorg. Chem. Commun. 2025, 182, 115390. [Google Scholar] [CrossRef]
- Song, H.; Wu, R.; Yang, J.; Dong, J.; Ji, G. Fabrication of CeO2 nanoparticles decorated three-dimensional flower-like BiOI composites to build p-n heterojunction with highly enhanced visible-light photocatalytic performance. J. Colloid Interface Sci. 2018, 512, 325–334. [Google Scholar] [PubMed]
- Chen, S.; Mukherjee, S.; Lucier, B.E.G.; Guo, Y.; Wong, Y.T.A.; Terskikh, V.V.; Zaworotko, M.J.; Huang, Y. Cleaving carboxyls: Understanding thermally triggered hierarchical pores in the metal-organic framework MIL-121. J. Am. Chem. Soc. 2019, 141, 14257–14271. [Google Scholar]
- Liu, Y.; Guo, H.; Zhang, Y.; Cheng, X.; Zhou, P.; Zhang, G.; Wang, J.; Tang, P.; Ke, T.; Li, W. Heterogeneous activation of persulfate for Rhodamine B degradation with 3D flower sphere-like BiOI/Fe3O4 microspheres under visible light irradiation. Sep. Purif. Technol. 2018, 192, 88–98. [Google Scholar]
- Tang, J.; Gao, G.; Luo, W.; Dai, Q.; Wang, Y.; Elzilal, H.A.; Abo-Dief, H.M.; Algadi, H.; Zhang, J. Z-scheme metal organic framework@graphene oxide composite photocatalysts with enhanced photocatalytic degradation of tetracycline. Adv. Compos. Hybrid Mater. 2023, 6, 190. [Google Scholar]
- Greczynski, G.; Pshyk, O.; Hultman, L. Toward an increased reliability of chemical bonding assignment in insulating samples by X-ray photoelectron spectroscopy. Sci. Adv. 2023, 9, 3192. [Google Scholar] [CrossRef]
- Zhu, M.; Kim, S.; Mao, L.; Fujitsuka, M.; Zhang, J.; Wang, X.; Majima, T. Metal-free photocatalyst for H2 evolution in visible to near-infrared region: Black phosphorus/graphitic carbon nitride. J. Am. Chem. Soc. 2017, 139, 13234–13242. [Google Scholar] [PubMed]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Construction of hierarchical hollow Co9S8/ZnIn2S4 tubular heterostructures for highly efficient solar energy conversion and environmental remediation. Angew. Chem. Int. Ed. 2020, 59, 8255–8261. [Google Scholar] [CrossRef]
- Chandrapal, R.R.; Bharathi, K.; Bakiyaraj, G.; Bharathkumar, S.; Priyajanani, Y.; Manivannan, S.; Archana, J.; Navaneethan, M. Harnessing ZnCr2O4/g-C3N4 nanosheet heterojunction for enhanced photocatalytic degradation of rhodamine B and ciprofloxacin. Chemosphere 2024, 350, 141094. [Google Scholar]
- Osanloo, M.; Khorasheh, F.; Larimi, A. Fabrication of nano-dandelion magnetic TiO2/CuFe2O4 doped with silver as a highly visible-light-responsive photocatalyst for degradation of Naproxen and Rhodamine B. J. Mol. Liq. 2024, 407, 125242. [Google Scholar]
- Xi, H.; Wang, H.; Liu, D.; Mu, Q.; Xu, X.; Kang, Q.; Yang, Y.; Yang, Z.; Lei, Z. Boosting the photocatalytic benzylamine oxidation and Rhodamine B degradation using Z-scheme heterojunction of NiFe2O4/rGO/Bi2WO6. J. Alloys Compd. 2025, 1010, 177818. [Google Scholar]
- Abd-Rabboh, H.S.M.; Benaissa, M.; Hamdy, M.S.; Ahmed, M.A.; Glal, M. Synthesis of an efficient, and recyclable mesoporous BiVO4/TiO2 direct Z-scheme heterojunction by sonochemical route for photocatalytic hydrogen production and photodegradation of rhodamine B dye in the visible region. Opt. Mater. 2021, 114, 110761. [Google Scholar]
- Shi, K.; Li, X.; Tian, Z.; Luo, Y.; Ding, R.; Zhu, Y.; Yao, H. Synergistic and efficient photocatalytic degradation of rhodamine B and tetracycline in wastewater based on novel S-scheme heterojunction phosphotungstic Acid@MIL-101(Cr). J. Environ. Manag. 2025, 373, 123716. [Google Scholar]
- Goyal, J.; Sharma, S.; Basu, S. Solar light-induced photocatalytic response of BiOCl/PANI composite towards the degradation of tetracycline. Catalysts 2023, 13, 795. [Google Scholar] [CrossRef]
- Shkir, M.; Aldirham, S.H.; AlFaify, S.; Ali, A.M. A novel BiOBr/rGO photocatalysts for degradation of organic and antibiotic pollutants under visible light irradiation: Tetracycline degradation pathways, kinetics, and mechanism insight. Chemosphere 2024, 357, 141934. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, C.; Hao, H.; Li, L.; Zhu, B.; Chen, X.; Tao, H. Photocatalytic degradation of tetracycline antibiotic over a flower-like S-doped BiOBr: Performance, mechanism insight and toxicity assessment. Front. Nanotechnol. 2022, 4, 1023489. [Google Scholar] [CrossRef]
- Ding, S.; Dong, T.; Peppel, T.; Steinfeldt, N.; Hu, J.; Strunk, J. Construction of amorphous SiO2 modified β-Bi2O3 porous hierarchical microspheres for photocatalytic antibiotics degradation. J. Colloid Interface Sci. 2022, 607, 1717–1729. [Google Scholar]
- Guo, F.; Chen, Z.; Huang, X.; Cao, L.; Cheng, X.; Shi, W.; Chen, L. Ternary Ni2P/Bi2MoO6/g-C3N4 composite with Z-scheme electron transfer path for enhanced removal broad-spectrum antibiotics by the synergistic effect of adsorption and photocatalysis. Chin. J. Chem. Eng. 2022, 44, 157–168. [Google Scholar] [CrossRef]
- Zhan, H.; Zhou, R.; Wang, P.; Zhou, Q. Selective hydroxyl generation for efficient pollutant degradation by electronic structure modulation at Fe sites. Proc. Natl. Acad. Sci. USA 2023, 120, 2305378120. [Google Scholar] [CrossRef]
- Qin, C.; Yang, Y.; Wu, X.; Chen, L.; Liu, Z.; Tang, L.; Lyu, L.; Huang, D.; Wang, D.; Zhang, C.; et al. Twistedly hydrophobic basis with suitable aromatic metrics in covalent organic networks govern micropollutant decontamination. Nat. Commun. 2023, 14, 6740. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).