Three-Dimensionally Ordered Macroporous La2O3-Supported Ni Catalyst for Methane Dry Reforming
Abstract
1. Introduction
2. Results and Discussion
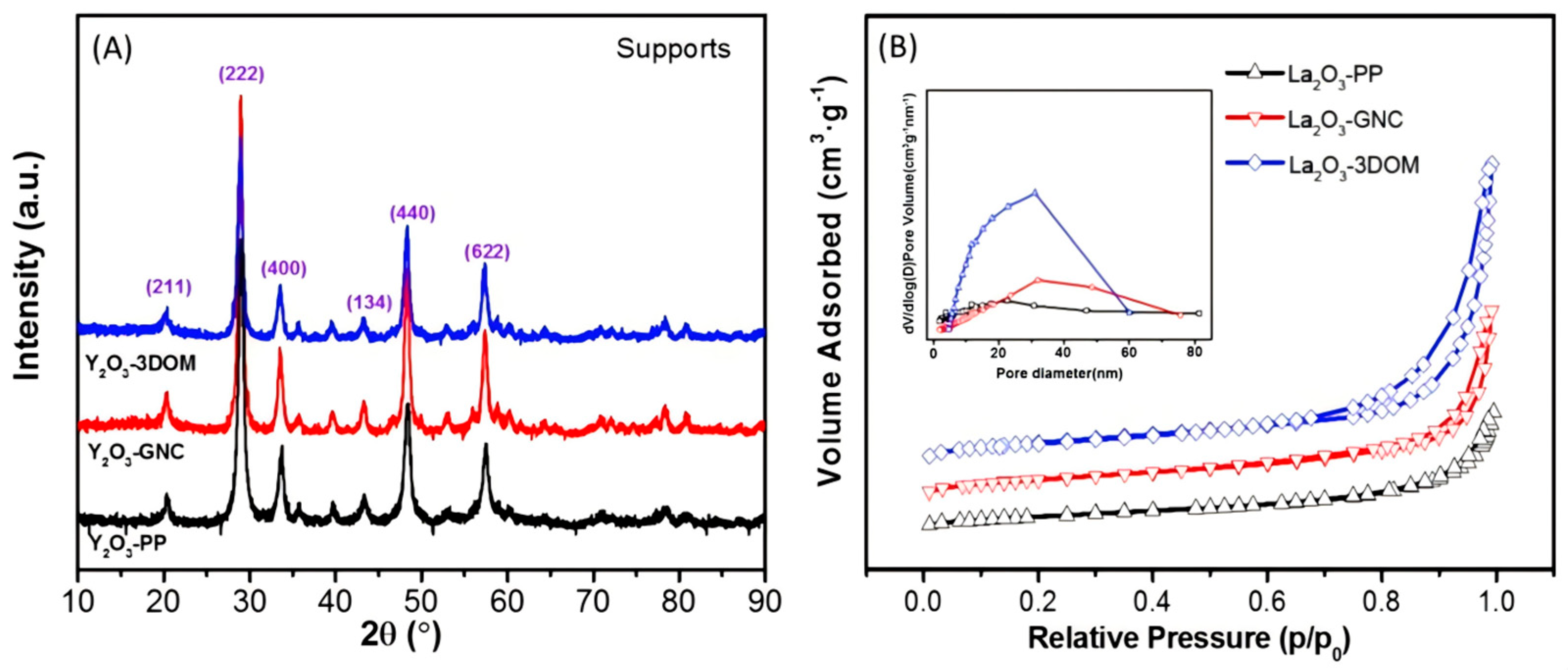
2.1. Structure Characterization of Supports
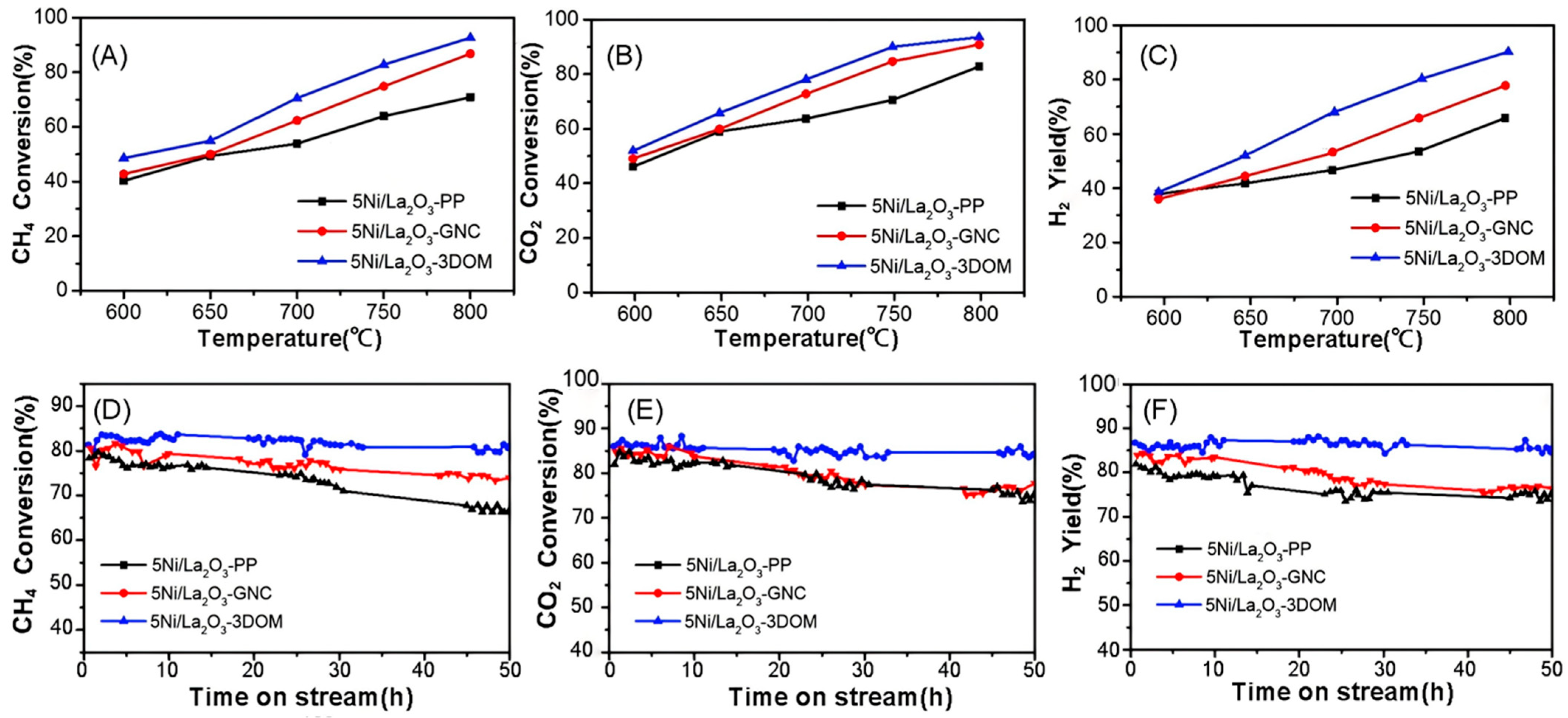
2.2. Activity and Stability Evaluation
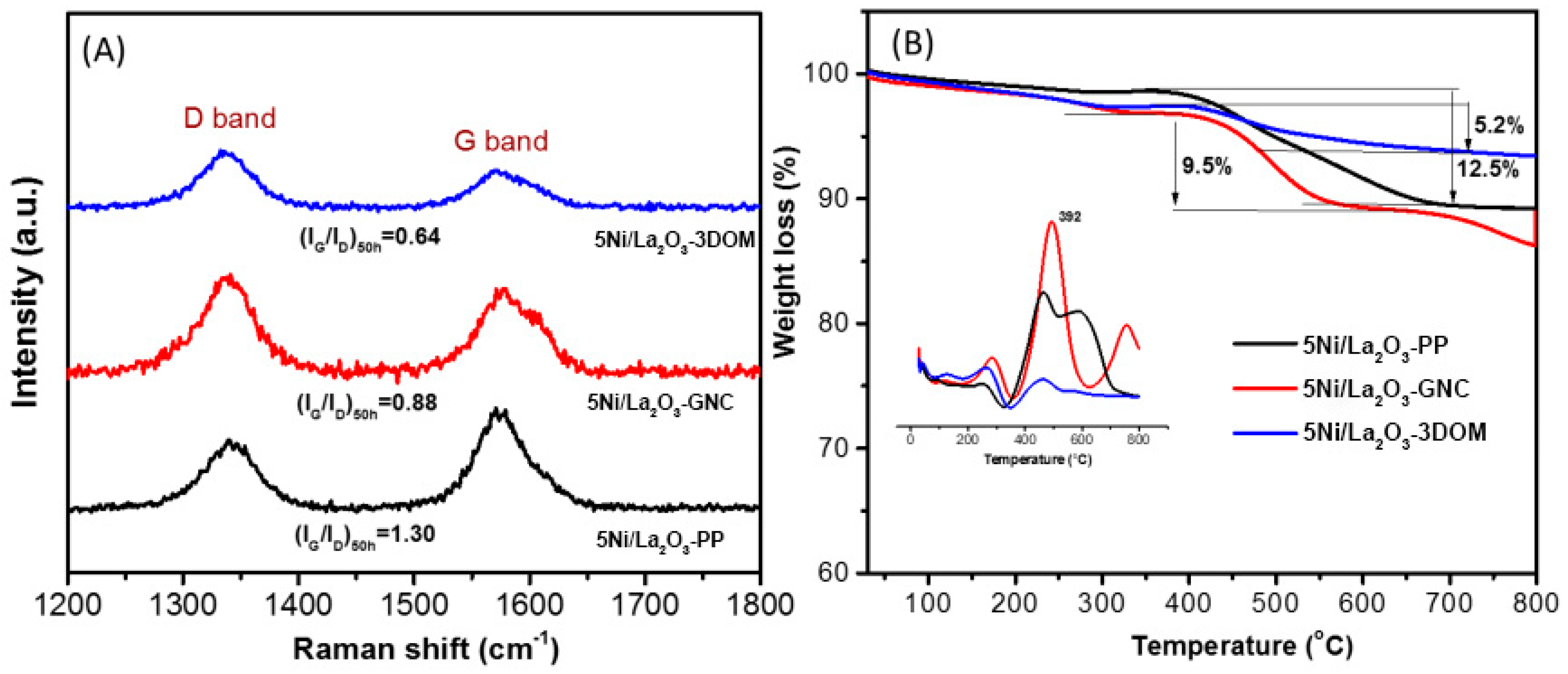
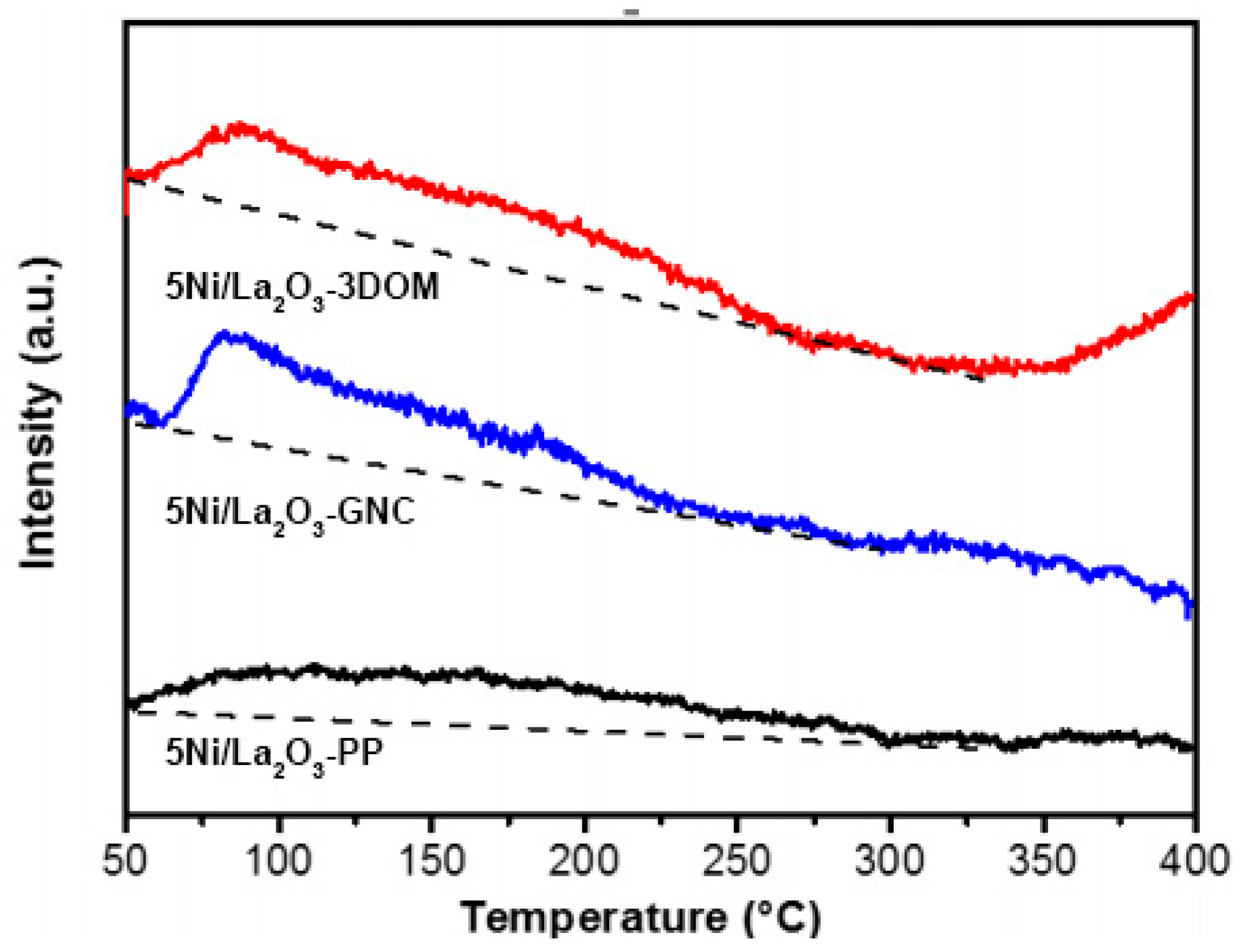
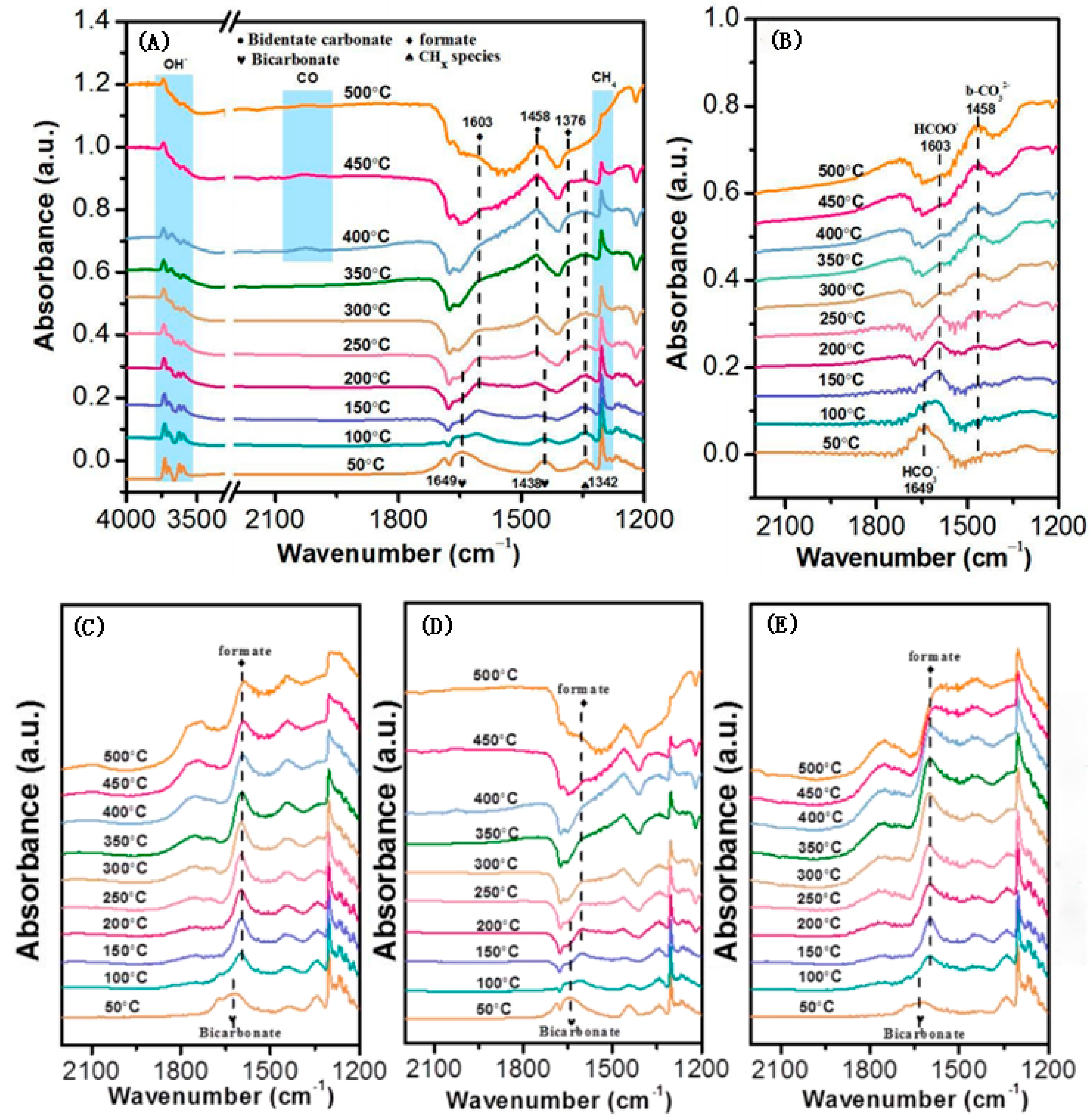
2.3. Carbon Deposition and Resistance Mechanism
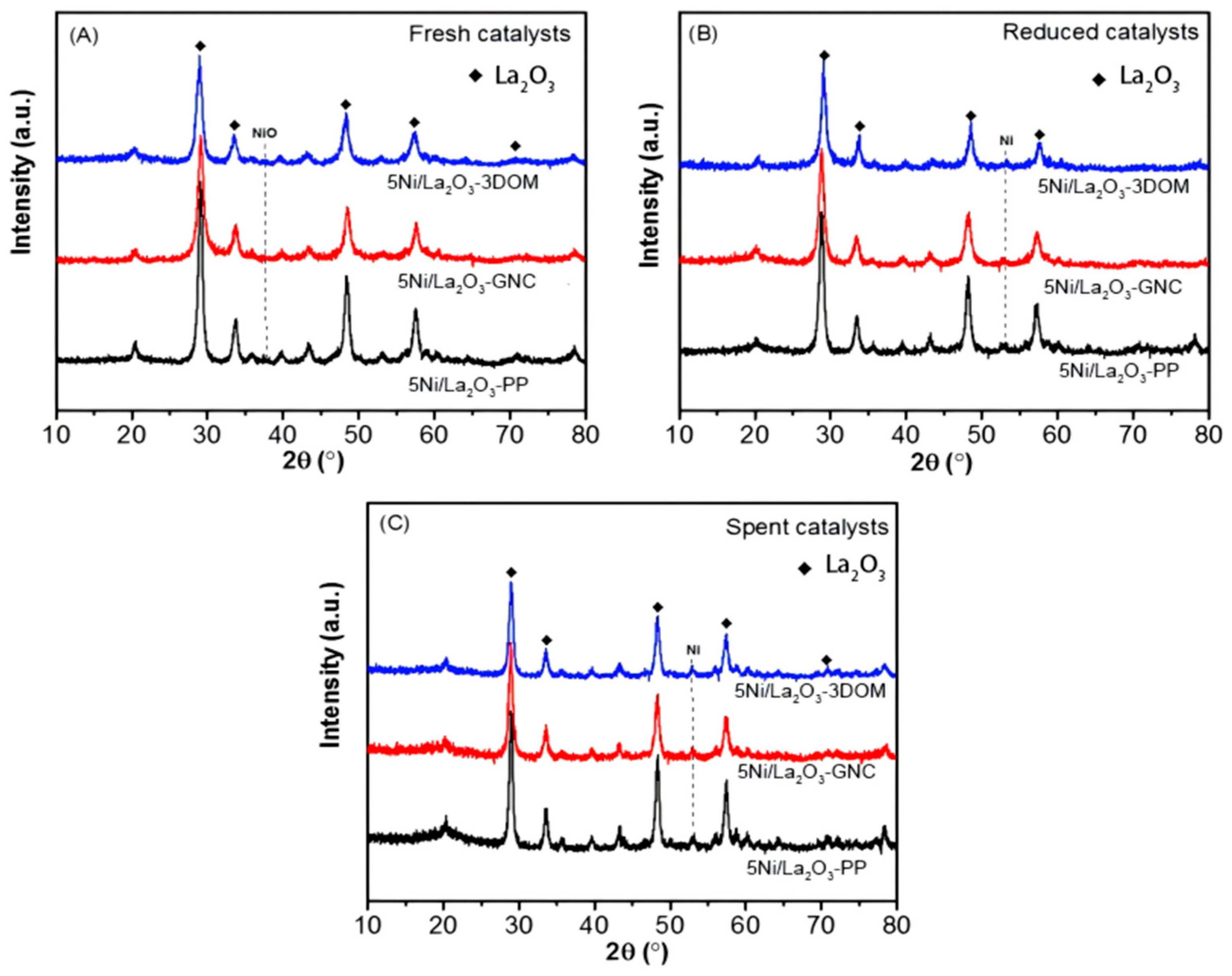
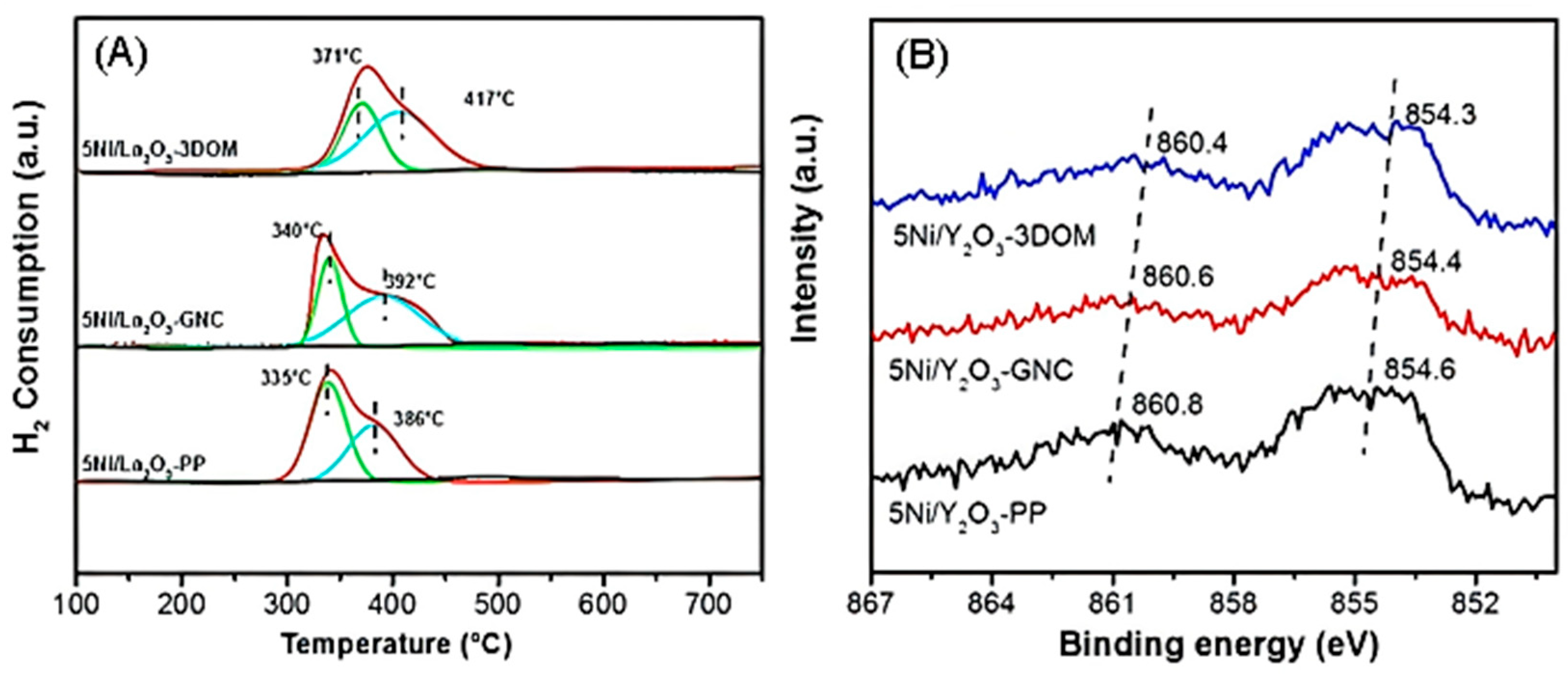
2.4. Metal–Support Interaction
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Activity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, Z.; Weng, W.; Zhou, J.; Gu, D.; Xiao, W. Catalytic decomposition of methane to produce hydrogen: A review. J. Energy Chem. 2021, 58, 415–430. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 22, 7813–7837. [Google Scholar] [CrossRef]
- Laan, J. Negative-emissions hydrogen energy. Nat. Clim. Change 2018, 8, 560–561. [Google Scholar]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-Hydrogen Energy Conversion Based on Water Splitting. Adv. Energy Mater. 2017, 8, 1701620. [Google Scholar] [CrossRef]
- Meiliefiana, M.; Nakayashiki, T.; Yamamoto, E.; Hayashi, K.; Ohtani, M.; Kobiro, K. One-Step Solvothermal Synthesis of Ni Nanoparticle Catalysts Embedded in ZrO2 Porous Spheres to Suppress Carbon Deposition in Low-Temperature Dry Reforming of Methane. Nanoscale Res. Lett. 2022, 17, 47. [Google Scholar] [CrossRef]
- Bahari, M.B.; Mamat, C.R.; Jalil, A.A.; Hassan, N.S.; Hatta, A.H.; Alhassan, M.; Aziz, M.A.; Le, V.G.; Siang, T.J.; Timmiati, S.N. Mitigating deactivation in dry methane reforming by lanthanum catalysts for enhanced hydrogen production: A review. Int. J. Hydrogen Energy 2025, 104, 426–443. [Google Scholar] [CrossRef]
- Liu, H.; Swirk, K.; Galvez, M.E.; da Costa, P. Nickel Supported Modified Ceria Zirconia Lanthanum/Praseodymium/Yttrium Oxides Catalysts for Syngas Production through Dry Methane Reforming. In Proceedings of the 10th International Conference on Processing and Manufacturing of Advanced Materials Processing, Fabrication, Properties, Applications (THERMEC), Cite Sci Paris, Paris, France, 9–13 July 2018; Volume 941, pp. 2214–2219. [Google Scholar]
- Zhang, J.-C.; Ge, B.-H.; Liu, T.-F.; Yang, Y.-Z.; Li, B.; Li, W.-Z. Robust Ruthenium-Saving Catalyst for HighTemperature Carbon Dioxide Reforming of Methane. ACS Catal. 2020, 10, 783–791. [Google Scholar] [CrossRef]
- Su, B.; Wang, Y.; Xu, Z.; Han, W.; Jin, H.; Wang, H. Novel ways for hydrogen production based on methane steam and dry reforming integrated with carbon capture. Energy Convers. Manag. 2022, 270, 116199. [Google Scholar] [CrossRef]
- Alhassan, M.; Jalil, A.A.; Nabgan, W.; Hamid, M.Y.S.; Bahari, M.B.; Ikram, M. Bibliometric studies and impediments to valorization of dry reforming of methane for hydrogen pro-duction. Fuel 2022, 328, 125240. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. Overview of hydrogen production from biogas reforming: Technological advancement. Int. J. Hydrogen Energy 2022, 47, 34831–34855. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B Environ. 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Ou, Z.; Zhang, Z.; Qin, C.; Xia, H.; Deng, T.; Niu, J.; Ran, J.; Wu, C. Highly active and stable Ni/perovskite catalysts in steam methane reforming for hydrogen production. Sustain. Energy Fuels 2021, 5, 1845–1856. [Google Scholar] [CrossRef]
- Choudhary, V.; Rajput, A.; Prabhakar, B.J.C. Energy efficient methane-to-syngas conversion with low H2/CO ratio by simultaneous catalytic reactions of methane with carbon dioxide and oxygen. Catal. Lett. 1995, 32, 391–396. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Zhang, Q.; Yang, Z.; Sun, Y.; Zou, G. Methane dry reforming over activated carbon supported Ni catalysts prepared by solid phase synthesis. J. Clean. Prod. 2020, 274, 122256. [Google Scholar] [CrossRef]
- Fan, M.-S.; Abdullah, A.Z.; Bhatia, S. Catalytic Technology for Carbon Dioxide Reforming of Methane to Synthesis Gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Li, M.; van Veen, A.C. Coupled reforming of methane to syngas (2H2-CO) over Mg-Al oxide supported Ni catalyst. Appl. Catal. A Gen. 2017, 550, 176–183. [Google Scholar] [CrossRef]
- Tavasoli, A.; Gouda, A.; Zähringer, T.; Li, Y.F.; Quaid, H.; Perez, C.J.V.; Song, R.; Sain, M.; Ozin, G. Enhanced hybrid photocatalytic dry reforming using a phosphated Ni-CeO2 nanorod heterostructure. Nat. Commun. 2023, 14, 1435. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, E.; Botana, K.J.; Garcia, R.; Ramirez, F.; Rodriguez-Izquierdo, J. Preparation of some rare earth oxide supported rhodium catalysts: Study of the supports. Mater. Chem. Phys. 1987, 17, 433–443. [Google Scholar] [CrossRef]
- Zhang, Y.; Jung, I.-H. Critical evaluation of thermodynamic properties of rare earth sesquioxides (RE = La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Sc and Y). Calphad 2017, 58, 169–203. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni-La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrogen Energy 2011, 36, 14435–14446. [Google Scholar] [CrossRef]
- Li, Y.H.; Zeng, D.M.; Huang, K.L. Preparation and Applications of Ordered Macroporous Materials. Prog. Chem. 2008, 20, 245–252. [Google Scholar]
- Yu, J.Y.; Li, Q.; Ma, X.H.; Sun, X.D. Preparation and Applications of Three-Dimensionally Ordered Macroporous Materials. Mater. Rev. 2008, 20, 21–24. [Google Scholar]
- Zhang, C.; Zhao, P.; Liu, S.; Yu, K. Three-dimensionally ordered macroporous perovskite materials for environmental applications. Chin. J. Catal. 2019, 40, 1324–1338. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, T.; Gao, W.; Ge, H.; Yang, Z.; Lin, R.; Wang, X. Three-dimensionally ordered macroporous Ce-W-Nb oxide catalysts for selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2022, 433, 134576. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Z.; Li, J.; Lu, S. A new route to three-dimensionally well-ordered macroporous rare-earth oxides. New J. Chem. 2001, 25, 1118–1120. [Google Scholar] [CrossRef]
- Silva, R.S.; Cunha, F.; Barrozo, P. Raman spectroscopy of the Al-doping induced structural phase transition in LaCrO3 perovskite. Solid State Commun. 2021, 333, 114346. [Google Scholar] [CrossRef]
- Triyono, D.; Hanifah, U.; Laysandra, H. Structural and optical properties of Mg-substituted LaFeO3 nanoparticles prepared by a sol-gel method. Results Phys. 2020, 16, 102995. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, R.; Hao, X.; Bao, Z.; Wu, T.; Wang, B.; Yu, F. Sandwiched SiO2@Ni@ZrO2 as a coke resistant nanocatalyst for dry reforming of methane. Appl. Catal. B Environ. 2019, 254, 612–623. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Oemar, U.; Saw, E.T.; Li, Z.; Kawi, S. Kinetic and mechanistic aspects for CO2 reforming of methane over Ni based catalysts. Chem. Eng. J. 2015, 278, 62–78. [Google Scholar] [CrossRef]
- Qian, L.; Ma, Z.; Ren, Y.; Shi, H.; Yue, B.; Feng, S.; Shen, J.; Xie, S. Investigation of La promotion mechanism on Ni/SBA-15 catalysts in CH4 reforming with CO2. Fuel 2014, 122, 47–53. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, G.; Xue, Q.; Chen, L.; Lu, Y. High carbon-resistance Ni/CeAlO3-Al2O3 catalyst for CH4/CO2 reforming. Appl. Catal. B Environ. 2013, 136–137, 260–268. [Google Scholar] [CrossRef]
- Egawa, C. Methane dry reforming reaction on Ru(001) surfaces. J. Catal. 2018, 358, 35–42. [Google Scholar] [CrossRef]
- Hou, W.; Wang, Y.; Bai, Y.; Sun, W.; Yuan, W.; Zheng, L.; Han, X.; Zhou, L. Carbon dioxide reforming of methane over Ni/Mg0.4Al0.4- La0.1Zr0.1(O) catalyst prepared by recombination sol-gel method. Int. J. Hydrogen Energy 2017, 42, 16459–16475. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Wang, Y.; Li, K.; Wang, Y.; Jiang, L.; Zhu, X.; Wei, Y.; Wang, H. Syngas production modified by oxygen vacancies over CeO2-ZrO2-CuO oxygen carrier via chemical looping reforming of methane. Appl. Surf. Sci. 2019, 481, 151–160. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Xu, J.; Xi, R.; Zhang, Z.; Xu, X.; Fang, X.; Wang, X. Promoting the surface active sites of defect BaSnO3 perovskite with BaBr2 for the oxidative coupling of methane. Catal. Today 2020, 374, 29–37. [Google Scholar] [CrossRef]
- Long, R.Q.; Wan, H.L. In situ confocal microprobe Ramanspectroscopy study of CeO2/BaF2 catalyst for theoxidative coupling of methane. J. Chem. Soc. Faraday Trans. 1997, 93, 355–358. [Google Scholar] [CrossRef]
- Abd Ghani, N.A.; Azapour, A.; Syed Muhammad, A.F.; Ramli, N.M.; Vo, D.-V.N.; Abdullah, B. Dry reforming of methane for hydrogen production over NiCo catalysts: Effect of NbZr promoters. Int. J. Hydrogen Energy 2018, 44, 20881–20888. [Google Scholar] [CrossRef]
- Zhang, S.; Ying, M.; Yu, J.; Zhan, W.; Wang, L.; Guo, Y.; Guo, Y. NixAl1O2-δ mesoporous catalysts for dry reforming of 75 methane: The special role of NiAl2O4 spinel phase and its reaction mechanism. Appl. Catal. B Environ. 2021, 291, 120047. [Google Scholar] [CrossRef]
- Cao, T.; You, R.; Zhang, X.; Chen, S.; Li, D.; Zhang, Z.; Huang, W. An in situ DRIFTS mechanistic study of CeO2-catalyzed acetylene semihydrogenation reaction. Phys. Chem. Chem. Phys. 2018, 20, 9659–9670. [Google Scholar] [CrossRef]
- Lavoie, J.-M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed]


| Catalysts | Surface Area (m2/g) [a] | Crystallite Size (nm) [b] | Mesopore (cm3/g) | Macropore (cm3/g) | Total (cm3/g) |
|---|---|---|---|---|---|
| La2O3-PP | 41 | 13.3 | 0.08 | 0.02 | 0.10 |
| La2O3-GNC | 50 | 12.8 | 0.13 | 0.03 | 0.16 |
| La2O3-3DOM | 62 | 10.9 | 0.19 | 0.10 | 0.29 |
| Catalysts | Fresh Catalysts | Reduced Catalysts | Used Catalysts | |||
|---|---|---|---|---|---|---|
| Surface Area (m2/g) [b] | NiO Crystallite Size (nm) [a] | Surface Area (m2/g) | Ni Crystallite Size (nm) | Surface Area (m2/g) | Ni Crystallite Size (nm) | |
| 5Ni/La2O3-PP | 38 | 12.8 | 36 | 13.8 | 31 | 23.5 |
| 5Ni/La2O3-GNC | 46 | 11.5 | 43 | 11.9 | 40 | 19.5 |
| 5Ni/La2O3-3DOM | 61 | 10.9 | 58 | 11.2 | 57 | 14.0 |
| Catalysts | Carbon Deposition Amount (%) | Coking Rate (mgC·g−1·h−1) [a] | IG/ID | Total Weight Loss (300–800 °C, %) |
|---|---|---|---|---|
| 5Ni/La2O3-PP | 12.5 | 2.5 | 1.30 | 15.2 |
| 5Ni/La2O3-GNC | 9.5 | 2.1 | 0.88 | 12.1 |
| 5Ni/La2O3-3DOM | 5.2 | 1.1 | 0.64 | 7.8 |
| Catalysts | H2 Consumption (mmol·g−1) | |||
|---|---|---|---|---|
| α Peak | β Peak | Total | H/Ni | |
| 5Ni/La2O3-PP | 0.47 | 0.39 | 0.86 | 1.02 |
| 5Ni/La2O3-GNC | 0.39 | 0.48 | 0.87 | 1.05 |
| 5Ni/La2O3-3DOM | 0.33 | 0.56 | 0.89 | 1.06 |
| Catalysts | H2 Desorption Amount (μmol·g−1) | Metallic Ni Surface Area (m2·g−1·cat−1) | Metallic Ni Surface Area (m2·g−1) | Ni Dispersion (%) |
|---|---|---|---|---|
| 5Ni/La2O3-PP | 29.4 ± 1.5 | 1.4 ± 0.1 | 29.2 ± 1.5 | 3.4 ± 0.3 |
| 5Ni/La2O3-GNC | 34.7 ± 1.7 | 1.7 ± 0.1 | 34.3 ± 1.7 | 4.0 ± 0.3 |
| 5Ni/La2O3-3DOM | 42.5 ± 2.1 | 2.0 ± 0.2 | 42.1 ± 2.1 | 4.9 ± 0.4 |
| Catalysts | CO2 Desorption (μmol/g) | Total (μmol/g) | O2 Desorption (μmol·g−1) | Total (μmol/g) | ||||
|---|---|---|---|---|---|---|---|---|
| Weak | Moderate | Strong | α Peak | β Peak | Olatt | |||
| 5Ni/La2O3-PP | 9.3 | 21.8 | 5.2 | 36.3 | 11.2 | 2.3 | 4.6 | 18.1 |
| 5Ni/La2O3-GNC | 11.1 | 23.3 | 4.8 | 39.2 | 14.8 | 8.9 | -- | 23.7 |
| 5Ni/La2O3-3DOM | 12.1 | 26.5 | 4.6 | 43.2 | 12.7 | 18.9 | 6.4 | 38.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Ding, A.; Zhang, W.; Xie, Z.; Papini, M.P.; Xuan, Y.; Zheng, H. Three-Dimensionally Ordered Macroporous La2O3-Supported Ni Catalyst for Methane Dry Reforming. Catalysts 2025, 15, 992. https://doi.org/10.3390/catal15100992
Li S, Ding A, Zhang W, Xie Z, Papini MP, Xuan Y, Zheng H. Three-Dimensionally Ordered Macroporous La2O3-Supported Ni Catalyst for Methane Dry Reforming. Catalysts. 2025; 15(10):992. https://doi.org/10.3390/catal15100992
Chicago/Turabian StyleLi, Shoufu, Aizhong Ding, Wenchuan Zhang, Zhongdong Xie, Marco Petrangeli Papini, Yuanyan Xuan, and Hongguang Zheng. 2025. "Three-Dimensionally Ordered Macroporous La2O3-Supported Ni Catalyst for Methane Dry Reforming" Catalysts 15, no. 10: 992. https://doi.org/10.3390/catal15100992
APA StyleLi, S., Ding, A., Zhang, W., Xie, Z., Papini, M. P., Xuan, Y., & Zheng, H. (2025). Three-Dimensionally Ordered Macroporous La2O3-Supported Ni Catalyst for Methane Dry Reforming. Catalysts, 15(10), 992. https://doi.org/10.3390/catal15100992








