Abstract
Layered double hydroxides (LDHs) have emerged as promising catalysts for various acid–base catalytic reactions. Due to their unique structure and regulatable dual acid–base properties, they offer more environmentally friendly and sustainable alternatives to traditional liquid acid and base catalysts. This study introduces the structural composition, preparation methods, and acid–base catalytic properties of LDH-based catalysts. Recent application progress in LDHs and rehydrated LDHs, LDH-based metal nanocatalysts, and LDH-based mixed metal oxide catalysts used as solid acid–base catalysts in acid–base green catalytic conversion is reviewed. The challenges and prospects of LDH-based catalysts as green and sustainable catalysts are summarized and proposed.
1. Introduction
Catalytic chemical processes are essential to many industries, from pharmaceuticals and materials to energy production. However, the environmental impact and sustainability challenges associated with these processes have become increasingly apparent. Conventional liquid acids and bases, commonly used as catalysts, raise concerns because they are toxic, generate hazardous waste, and consume large amounts of energy. For example, in traditional ester exchange and esterification reactions, strong acids or bases are often added, but they pose serious environmental pollution and equipment corrosion problems, requiring good separation and purification steps, which will result in additional expensive costs [1,2]. Thus, the demand for sustainable and environmentally friendly chemical processes has propelled the exploration of effective and green alternatives to conventional liquid acids and bases in catalytic transformation [3,4,5,6]. To tackle these challenges, there has been a significant amount of focus on the green catalytic transformation process. Green catalysis aims to develop clean and sustainable chemical processes that replace or minimize the use of hazardous liquid acids and bases with eco-friendlier alternatives. These alternatives ought to have comparable or superior catalytic performance while reducing their adverse impact on the environment. Crucial for promoting sustainable and environmentally friendly chemical processes, green acid–base catalytic transformation significantly enhances the chemical industry’s sustainability, minimizing environmental impact, improving energy efficiency, and utilizing renewable resources for a more efficient and eco-friendly approach [7,8,9,10].
As heterogeneous catalysts, solid acid–base catalysts offer a promising solution for eco-friendly industrial catalysis. Compared to liquid catalysts, solid acid–base catalysts offer stronger stability, easy separation from reaction mixtures, and recyclability. Solid acid–base catalysts, unlike conventional homogeneous ones, provide localized acid and base sites for catalytic reactions that enhance reaction rates and selectivity, making them essential for eco-friendly processes. In addition, using solid acid–base catalysts promotes sustainable and efficient chemical catalytic transformations, leading to a greener chemical industry [11,12]. Among various solid acid–base catalysts, layered double hydroxides (LDHs) have distinctive properties, adjustable acid–base properties, and elevated thermal stability, making them promising for solid acid–base catalysis. LDHs have a unique structure consisting of positively charged metal hydroxide nanosheets, typically with divalent metal cations, like Mg2+, Zn2+, or Ni2+, with anions and water molecules between the sheets. Due to this structure, LDHs exhibit dual acid–base properties, which enable LDHs to catalyze a wide range of acid–base catalytic reactions, such as esterification, transesterification, aldol condensation, and biomass conversion. The catalytic performance, selectivity, and stability of LDHs can be enhanced by adjusting their composition, structure, and interlayer anions to manipulate their acid–base properties, which makes them a promising option for environmentally friendly acid–base catalytic transformation [13,14].
This review aims to review the application of LDHs and their derivatives as solid acid–base catalysts in green catalytic transformation (Scheme 1). Introducing the structural composition, preparation methods, and acid–base catalytic properties of LDH-based catalysts aims to clarify their unique properties and application potential for green catalytic transformation. This contribution reviews the application of LDHs and their derivatives as solid acid–base catalysts in acid–base green catalytic conversion, particularly the research progress in LDHs and rehydrated LDHs, LDH-based metal nanocatalysts, and LDH-based mixed metal oxide catalysts. The challenges and prospects of LDH-based catalysts as green and sustainable catalysts are summarized and proposed.
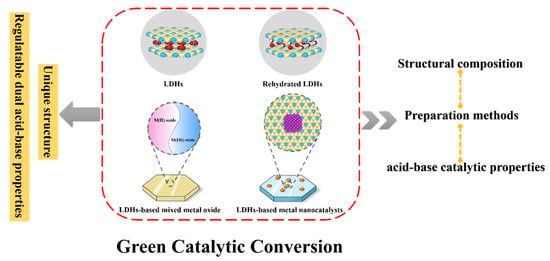
Scheme 1.
Schematic illustration of LDHs as solid acid–base catalysts for green catalytic transformations.
2. Chemical Composition, Preparation, and Acid–Base Properties of LDHs
2.1. Chemical Composition
Layered double hydroxides (LDHs) are inorganic materials with positively charged layers of brucite Mg(OH)2 and interlayer anions to balance the charge. The chemical composition of LDHs can be described as [M(II)1−xM(III)x(OH)2]x+ (An−)x/n·mH2O. M(II) and M(III) could be divalent and trivalent metal cations, such as Cu2+, Mg2+, Co2+, Zn2+, Al3+, Fe3+, and Mn3+. X represents the molar ratio of M2+/(M2+ + M3+), and its value greatly influences the composition and structure of hydrotalcite materials. An− could be organic or inorganic anions, such as Cl−, NO3−, CO32−, and CH3COO−. The LDHs comprise a positively charged metal hydroxide layer with intercalated anions and water molecules. The metal cations are octahedrally coordinated by six hydroxide ions, which are tightly packed to form a positively charged metal hydroxide layer. The metal hydroxide layer is typically balanced by the presence of exchangeable interlayer anions [15,16,17]. Figure 1 shows the layered structure of MgAl (2:1) LDHs. In LDHs, –OH groups on lamellae provide Brønsted acid–base sites, and metal cations in the lattice create Lewis acid–base sites. LDHs’ catalytic activity is due to these acid–base sites, which enable them to perform well in green catalytic reactions, such as condensation, hydrogenation, and biomass conversion [18,19]. In addition, doping LDHs with transition metal cations introduces additional Lewis acid–base sites that enhance their acid–base and catalytic activities, thus improving their catalytic performance for redox reactions during acid–base catalytic transformations [20].
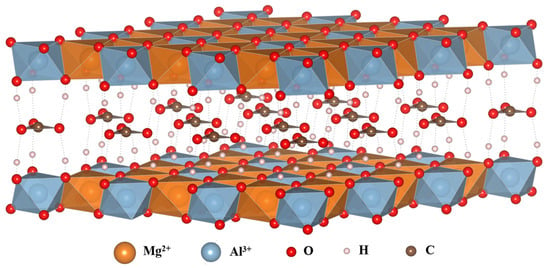
Figure 1.
Schematic diagram of the layered structure of MgAl (2:1) LDHs.
2.2. Preparation Methods
Four commonly used methods for preparing LDHs are illustrated schematically in Figure 2: 1. The coprecipitation method involves the simultaneous precipitation of aqueous solutions containing metal salts under controlled pH conditions. 2. The ion exchange method replaces the initial interlayer anions and intercalates the desired anions into the pre-synthesized LDHs. 3. The hydrothermal method synthesizes LDHs under high temperature and pressure conditions in an aqueous medium. 4. The rehydration method is the structural reconstruction after calcination.
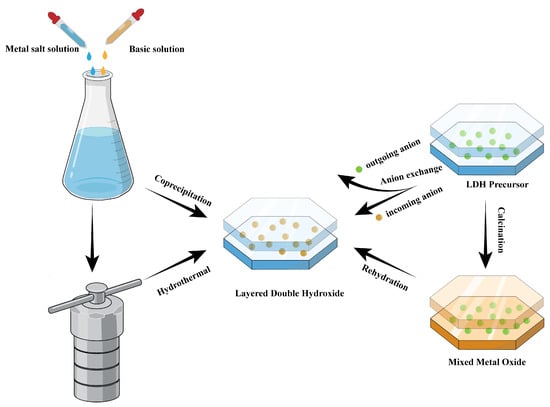
Figure 2.
Schematic diagram of the four development and modification methods of LDHs.
The coprecipitation method is commonly used to synthesize LDHs due to its simplicity and easy scalability. Conventional coprecipitation synthesis involves the simultaneous precipitation of metal salt solutions with suitable divalent and trivalent metal cation proportions under saturated conditions by adjusting the pH of the solutions [21]. When the hexahydrate metal complexes condense in an aqueous solution, they form uniformly distributed metal cations and interlayer anions, resulting in a hydromagnesite-like layer [22]. This method enables a more efficient and straightforward synthesis of LDHs and their composites. The particle size, composition, and incorporation of interlayer anions can be controlled by adjusting pH, reactant concentration, and reaction temperature. Using the traditional coprecipitation method, Panda and colleagues synthesized multiple MgAl LDHs by systematically varying the molar concentration of cations, aging time, and pH. Mixing magnesium and aluminum nitrate solutions with sodium carbonate solution and maintaining the pH at different values using sodium hydroxide resulted in MgAl LDHs with different growth rates [23]. Seftel et al. used a coprecipitation method to prepare various ZnAl LDHs with different Zn/Al ratios, and the photocatalytic activity was evaluated for the degradation of the methyl-orange dye. The photocatalytic activity increases with the increase in the cationic ratio and the calcination temperature [24].
The anion exchange method is also referred to as indirect synthesis. Typically, the precursor of LDHs is immersed in a solution with the desired anion in an inert environment. The ion exchange process takes place, leading to the formation of new LDHs that possess different interlayer anions. The ion exchange procedure relies on the electrostatic force, as the hydroxide layer carries a positive charge, and the interlayer area contains an abundance of anions [25]. This method provides precise control over interlayer anion composition and offers an alternative synthetic route for difficult-to-synthesize LDHs due to large anion size. Zou et al. exchanged CO32− with OH− in an alkaline solution to produce modified NiCo–OH LDHs, which was used as electrode nanoarrays and showed improved cycling stability and rate capacity [26]. Das et al. used an indirect intercalation method to intercalate heteropoly acids (molybdophosphoric acid and tungstophosphoric acid) into Zn–Al hydrotalcite-like compounds to increase the acid sites on the catalysts’ surface. The intercalated catalysts showed improved catalytic activity when used in the liquid phase esterification of acetic acid and n-butanol [27].
The hydrothermal method involves mixing metal salt solution, alkali solution, and interlayer anions in a stainless steel closed reactor. This mixture is then heated under self-generated pressure, which promotes the nucleation and growth of LDH crystals. Urea is usually added in hydrothermal processes to improve crystallinity and form stable ultra-thin structures [28]. This method necessitates specialized equipment and a longer reaction time than other methods. However, this process enables the creation of well-crystallized LDHs with controllable particle size and interlayer spacing and enhanced catalytic activity. Ultrathin Ni–Al LDH nanosheets were prepared by a simple hydrothermal method using urea as an auxiliary agent. Ni–Al LDH nanosheets exhibited an ultrathin structure, high surface area, and excellent electrochemical properties as supercapacitor electrodes [29]. Jiang et al. fabricated NiCo LDHs loaded on carbon fiber under hydrothermal conditions using carbon fibers as the substrate material and hexamethylenetetramine (HMT) as the hydrolyzing agent [30].
The reconstruction and rehydration method is also known as the memory effect method. Calcination can effectively destroy the structure of LDHs. Typically, LDHs are calcined at 400–500 °C and the water molecules between the layers, the hydroxyl groups of the LDHs, and the embedded anions will be completely removed to obtain the corresponding composite metal oxides [31]. Then, composite metal oxides are regenerated by mixing with water or other anionic solutions and rehydrating under an inert atmosphere to restore the original structure of the LDHs. The selection of calcination temperature is crucial, as high temperatures can affect the structural recovery of LDHs, and if the calcination temperature is too high, a structurally stable spinel will be formed, greatly affecting the reconstruction of LDHs [32,33]. Not only that, taking advantage of the topological transformation of LDHs by the calcination method, a series of mixed metal oxide (MMO) catalysts and supported catalysts can be obtained, which all show excellent catalytic activity, selectivity, and stability [34]. Dubnova et al. converted ZnAl LDHs into composite metal oxides by heat treatment at 400 °C and then structurally reconstructed them in distilled water under an N2 atmosphere. The reconstructed LDH catalysts exhibit high crystallinity and numerous base sites, resulting in remarkable catalytic activity toward furfural (FF) aldol condensation reactions [35].
The coprecipitation method, widely used and yielding higher quantities, is preferred for general applications, while the anion exchange method is suitable for synthesizing LDHs with specific and large-sized anions. The choice of synthesis method depends on the catalyst’s requirements and the intended application, with the hydrothermal method favoring higher-quality LDH crystals and the rehydration method leading to lower-quality crystallization.
2.3. Acid–Base Properties
Solid acid–base catalysts are widely used in many green acid–base catalytic reactions, and their high acid–base activity is essential for achieving superior selectivity and conversion rates. Therefore, it is necessary to approach rational design from the perspective of acid–base properties. LDHs exhibit Lewis and Brønsted acid–base properties due to their unique structures and compositions. LDHs and their derived catalysts can be applied in various green catalytic reactions by adjusting the intensity and concentration of their acid and base sites on the catalysts’ surface.
Differences in acidity and alkalinity of LDHs arise from different parts of their structure. Lewis acidity and alkalinity of LDHs are attributed to divalent metal cations located in their metal hydroxide layers. These metal cations, including Mg2+, Zn2+, and Ni2+, have partially filled d orbitals that can accept electron pairs. Lewis acid–base sites in LDHs facilitate acid–base catalyzed reactions by interacting with electron-rich species, such as lone-pair electrons or π-bonding of reactant molecules. Lewis acid–base sites enhance the catalytic activity of LDHs by facilitating bond cleavage, isomerization, and other transformations. The selectivity of catalytic reactions can be influenced by the Lewis acidity and basicity of LDHs, which provide specific binding sites and control reaction pathways. Therefore, the Lewis acidity and alkalinity of LDHs can be adjusted to enhance selectivity for the desired product. Maria et al. discovered that the amount of acid–base sites in MgAl LDHs can be tailored by adjusting the Al3+ content. The decrease in Al3+ content and the corresponding increase in Mg2+ led to the increase in the base sites and the simultaneous decrease in acid sites [36].
The Brønsted acidity and alkalinity of LDHs arise from the hydroxide ions (OH−) in the metal hydroxide layer and interlayer anions. The catalytic activity of LDHs in proton transfer reactions is enhanced by Brønsted acid–base sites, which are crucial for hydrolysis, esterification, and dehydration. The Brønsted acidity and alkalinity of LDHs can control reaction selectivity by affecting the protonation of specific functional groups and by the reaction kinetics relying on acidity and alkalinity.
Carolina et al. replaced the carbonate anions in the interlayer of ZnAl LDHs and MgAl LDHs by tungstate anion (WO42−) and molybdate anion (MoO42−) as intercalation anions. The resulting modified LDH catalysts were used to catalyze the oxidation reaction of cyclohexanes and exhibited higher catalytic performances than the initial LDHs. The intercalation of WO42− and MoO42− enhanced the alkalinity of the original LDHs, resulting in a substantial improvement in the selectivity of cyclohexanone and cyclohexanol production [37].
The coexistence of both Lewis and Brønsted acid–base sites in LDHs exhibits complementary acid–base properties, enabling them to catalyze various green acid–base catalytic reactions with different mechanisms. The Brønsted acid–base properties of LDHs can also be adjusted by changing the metal ion composition. MgAl LDHs were modified by introducing a rare-earth element, Y, and subsequently calcined to produce MgAl mixed oxides, which exhibited relatively well-developed small-flake morphology with high surface area and pore volume, exposing more base sites on the catalyst surface and improving the catalytic activity for the aldol condensation reaction [38].
3. Application of LDH-Based Catalysts in the Green Acid–Base Catalytic Transformation
LDHs along with rehydrated LDHs, LDH-based metal nanocatalysts, and LDH-based mixed metal oxide catalysts are considered promising catalysts due to their unique structures, excellent stability, and tunable acid–base catalytic properties. Table 1 summarizes some of the LDH-based acid–base catalysts outlined in this paper, along with their corresponding green acid–base catalytic process conditions.

Table 1.
LDH-based catalysts for green acid–base catalysis.
3.1. LDH Catalysts
3.1.1. Original LDH Catalysts
LDHs are a class of two-dimensional inorganic nanomaterials that are widely applied as catalysts in various acid–base catalytic reactions. Phenol hydroxylation is an essential industrial reaction. Using traditional homogeneous catalysts like Fe2+ and Co2+ usually requires harsh reaction conditions, making separation and recovery difficult [60,61].
Therefore, as solid acid–base catalysts, LDHs are environmentally friendly and used under moderate reaction conditions, showing significant advantages. Jiang et al. prepared a series of CuZnFeAl-LDH catalysts by a coprecipitation method for phenol hydroxylation. The electron transfer from oxygen vacancies to Cu2+ on the LDHs surface generates Cu+, promoting the formation of hydroxyl radicals and enhancing catalytic activity. CuZnFeAl-LDH with 15% copper content (15/CuZnFeAl-LDH) exhibited the optimal conversion of phenol (66.9%) and selectivity of benzene diol (71.3%), which is mainly attributed to the synergistic effect between Cu+ and oxygen vacancies promoted by the acid–base ratio. Moreover, 15/CuZnFeAl-LDH presented stable recyclability under mild conditions (60 °C, 1.0 MPa) while also being eco-friendly and energy efficient [39].
In addition, LDHs can be used directly as solid acid–base catalysts for the catalytic conversion of biomass resources, that is, transformation to high-value-added chemicals and biofuels from sugar, lignocellulosic materials, and other biomass feedstocks. For instance, efficient hydrodeoxygenation of lignin-derived vanillin to 2-methoxy-4-methylphenol was achieved by calcining and reducing NiZnAl LDHs to prepare flower-like Ni-based catalysis [62]. In recent years, there has been significant interest in the electrocatalytic oxidation of aldehydes derived from biomass into valuable acids using LDHs as catalysts. LDHs, with a thin nano-lamellar structure, provide a large number of catalytic active sites. Meanwhile, the metal valence state of the cations in LDHs varies by changing the electron layer, which exhibits excellent acid–base catalytic performance. For example, on ultra-thin NiCoMn layered double-hydroxide nanosheets rich in oxygen vacancies, the redox cycle between Mn2+ and Mn3+ was utilized to promote the electro oxidation of 5-hydroxymethylfurfural and furfural to 2,5-furan-dicarboxylic acid (FDCA) and furoic acid (FurAC) [63]. Liu and colleagues studied the electrocatalytic oxidation of biomass-derived aldehydes using ultrathin NiV LDHs for efficient catalysis [40]. They discovered that ultrathin NiV LDHs with a size of 2.6 nm can expose a larger surface area with rich metal active sites, which facilitates the adsorption of aldehydes on the catalyst surface and the generation of hydroxyl radicals, resulting in superior electrocatalytic performance. The generated hydroxyl radicals can favorably promote the conversion of aldehyde to carboxyl groups and generally apply to different biomass-derived benzylic aldehydes and furan aldehydes.
Zhang et al. synthesized trimetallic NiCoFe LDHs with a one-step controllable synthesis for both an efficient oxygen evolution reaction (OER) and the highly selective oxidation of biomass-derived 5-hydroxymethylfurfural (HMF) into value-added FDCA, which is shown in Figure 3 [41]. In the OER study, the NiCoFe LDHs showed superior performance compared to previously reported LDH catalysts. They exhibited the lowest necessary overpotential (288 mV), a much smaller charge transfer resistance (1.0 Ω), and a larger Cdl value of 2.62 mF/cm2, indicating low charge transfer resistance and fast catalytic kinetics of oxygen evolution. It was discovered that the introduction of Fe3+ led to increased active sites and enhanced OER activity of NiCoFe LDHs compared to NiCo LDHs. NiCoFe LDHs exhibited excellent performance, with up to 95% HMF conversion and 84.9% FDCA selectivity in 1 h during the oxidation reaction of HMF. The HMF oxidation is more likely to occur at lower potentials due to the rich active metal sites on the thin layer of NiCoFe LDHs. Additionally, the introduced Fe3+ leads to electronic synergistic effects with Ni2+ and Co2+, enhancing the electrocatalytic oxidation of HMF. This study provides an effective method for converting biomass-derived chemicals into value-added products without using noble metal-containing catalysts.
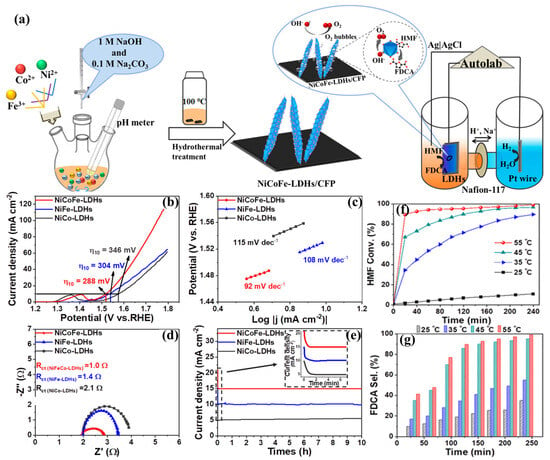
Figure 3.
(a) Schematic illustration of NiCo-LDH, NiFe-LDH, and NiCoFe-LDH materials and the electrochemical system of HMFOR. Polarization curves of NiCo-, NiFe-, and NiCoFe-LDHs (b) and corresponding Tafel plots (c). Nyquist plots (d) of impedances of the as-synthesized three materials in the frequency range from 10 mHz to 100 kHz. Chronoamperometric test (e) at a potential of 1.54 VRHE for 10 h. Conversion of HMF (f) and selectivity of FDCA product (g) over the reaction time at different temperature influences on the surface of NiCoFe-LDHs/CFP [41].Copyright © 2019, adapted with permission from the American Chemical Society.
Furthermore, LDH catalysts are widely used in thermal catalysis to convert biomass derivatives into value-added products. Wang et al. investigated the catalytic transfer hydrogenation of FF to furfuryl alcohol by NiFe LDHs using 2-propanol as a hydrogen donor [42]. LDHs with a Ni/Fe molar ratio of 3:1 demonstrated the best catalytic activity, which was attributable to the synergistic effect of acidic and base sites. At 140 °C for 5 h, it converted 97.0% of FF and yielded 90.2% furfuryl alcohol. First, 2-propanol was absorbed on NiFe LDHs and dissociated into alkoxide and protons by interacting with acid sites (attributing to Ni2+ and Fe3+) and base sites (attributing to OH−). Then, the acidic site may activate the carbonyl group of FF and form a transitional state with six links, leading to the production of free furfuryl alcohol and acetone through hydrogen transfer. Moreover, this catalyst applies to catalytic hydrogenation reactions of various aldehydes and ketones.
In addition to catalyzing the conversion of biomass-derived aldehydes, LDHs are also widely utilized as solid acid–base catalysts for transforming biomass-derived sugars. A series of MgAl LDH catalysts were synthesized by Ye et al. and applied to the conversion of glucose/food waste to methyl lactate (MLA) without homogeneous alkalines [48]. MgAl LDHs with Mg/Al (5:1) showed the largest interlayer distance and the highest density of basic site, resulting in superior catalytic activity. Due to the in situ reconstruction, the reused MgAl LDHs exhibited higher specific surface area and larger interlayer space, gradually increasing catalytic activity during the recycling test.
The alkalinity of LDHs can be regulated by changing the ratio of metal ions and modifying the interlayer anions. Therefore, LDHs can be designed for the cascade conversion of glucose. However, LDH catalysts are not commonly used to directly convert glucose to lactic acid because the acidic environment can deactivate the catalyst. Most current research on LDH catalysts has focused on glucose isomerization for fructose production [49,64,65]. Yu et al. investigated the performance of LDHs as solid-base catalysts for the thermochemical isomerization of biomass-derived glucose to fructose [49]. The MgAl LDHs were synthesized via conventional urea hydrolysis and coprecipitation methods, followed by aqueous miscible organic solvent treatment. It was found that the treated LDHs exposed more active sites due to their high porosity, and the fructose yield increased with the increasing crystallite size of LDHs. This study presents a methodology to establish the relationship between the structure and catalytic performance of LDHs in thermochemistry. It proposes a practical approach for the tunable structural design of LDHs as solid-base catalysts.This work provides a methodology for establishing the structure–catalytic performance relationship of LDHs in thermochemistry. It elucidates a practical idea for the tunable structural design of LDHs as solid base catalysts.
Photocatalysis is widely acknowledged as a clean and sustainable technology with the potential to transform the environment and regenerate energy. Biomass and its derivatives can be photocatalytically to produce numerous high-value-added products such as organic acids and furan compounds. Layered double hydroxides can effectively promote electron transfer and significantly improve the reaction rate and selectivity due to their unique structure and abundant surface hydroxyl groups [66,67]. Ding et al. synthesized NTCN/LDH composite materials composed of two main catalytic active species, which can efficiently produce lactic acid from various carbohydrates during the light/heat alternating catalytic reaction process [68]. The Lewis acidic sites derived from LDH provide active sites for isomerization, while photocatalysis achieves selective cleavage of C–C bonds by regulating the generation of reactive oxygen species. Wang et al. prepared Pd/NiTi·LDH nanocomposites by loading small Pd nanoparticles onto NiTi·LDH nanosheets for efficient one pot synthesis of secondary amines under visible light [69]. In previous studies, NiTi LDHs have been shown to exhibit excellent photocatalytic performance due to their abundant surface oxygen defects. The deposition of Pd metal nanoparticles has successfully achieved visible light triggered multi-step cascade reactions. This study demonstrates the potential application of metal/LDH nanocomposites in photocatalysis.
3.1.2. Rehydrated LDH Catalysts
The composite metal oxides obtained by calcination LDHs at a specific temperature possess a sound structure and memory effect. The original structure of LDHs can be restored by doping the corresponding anions (such as OH−) between the layers of the composite metal oxides. The rehydrated LDHs with numerous Brønsted acid–base sites allow for effective regulation of the density and intensity of base sites, resulting in a notable enhancement of the catalytic performance of the original LDH catalysts in alkali-catalyzed reactions [70]. Due to the distinct structural defects generated in the reconstruction process, rehydrated LDHs can act as carriers by loading metal atoms or clusters onto the cationic vacancies on the surface. The catalytic performance of rehydrated LDHs can be enhanced by modifying their surface structure and creating strong metal-carrier connections through the local electron-transfer effect [71,72]. The aldehyde-alcohol condensation reaction is a classic and essential organic synthesis reaction that can produce numerous value-added chemicals for industrial use. One example of such reactions is the condensation of isobutyraldehyde (IBD) and formaldehyde (FA), an industrially significant alkali-catalyzed reaction. Rehydrated LDHs have been widely discussed as solid-base catalysts for aldehyde-alcohol condensation reactions due to environmental pollution and equipment corrosion caused by homogeneous alkali catalysts such as NaOH and KOH [73,74].
Bing et al. synthesized two rehydrated LDH catalysts (re-CaxAl-LDH and re-MgxAl-LDH) and used them for aldol condensation reactions of IBD and FA [52]. The relevant schematic is presented in Figure 4. The re-CaxAl-LDH host layer was found to contain a distorted Ca(OH)2 octahedron and has additional Ca−OH coordination, which provides weak Brønsted base sites. On the contrary, re-MgxAl-LDH consists of an octahedral structure formed by Mg(OH)2 and surface-adsorbed hydroxyl group via non-covalent interaction, which provides medium Brønsted base sites. The Ca/Al molar ratio in the LDH precursor can be adjusted to increase the concentration of weak Brønsted base sites for re-CaxAl-LDH. The optimized re-Ca4Al-LDH showed excellent catalytic performance in producing hydroxypivaldehyde (HPA) through the condensation of IBD and FA, with a 61.5% HPA yield. This result is significantly higher than that of re-MgxAl-LDH and other solid base catalysts and is comparable to the catalytic results of liquid bases.
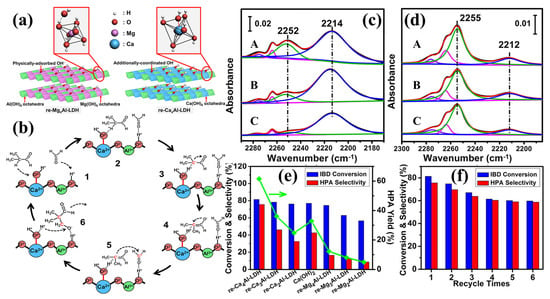
Figure 4.
(a) Schematic illustration for the structure of re-MgxAl-LDH and re-CaxAl-LDH; (b) schematic reaction mechanism for the aldol condensation of IBD and FA over re-CaAl-LDH catalyst; (c) FTIR transmission spectra of (A) re-Mg2Al-LDH, (B) re-Mg3Al-LDH, and (C) re-Mg4Al-LDH recorded in 2170–2300 cm–1 after CDCl3 chemiadsorption at 25 °C; (d) FTIR transmission spectra of (A) re-Ca2Al-LDH, (B) re-Ca3Al-LDH, and (C) re-Ca4Al-LDH recorded in 2185–2300 cm–1 after CDCl3 chemiadsorption at 25 °C; (e) IBD conversion, HPA selectivity, and HPA yield over pure Ca(OH)2, re-MgxAl-LDH, and re-CaxAl-LDH catalysts, respectively; (f) catalytic behavior of re-Ca4Al-LDH for six consecutive cycles [52]. Copyright © 2017, adapted with permission from the American Chemical Society.
To further improve the fine structure of weak Brønsted alkaline sites, Bing et al. prepared rehydrated CaMnAl-LDH (re-Ca4MnxAl-LDH) as a solid base catalyst utilizing Mn as a promoter [53]. The catalytic performance of the aldol condensation reaction was enhanced by modulating the activity of weak Brønsted base sites by altering the electronic structure of Ca–O–Al with Mn. Compared to re-Ca4Al-LDH, re-Ca4MnxAl-LDH displayed highly exposed Ca2+ s-orbital and strengthened coordination between Ca2+ with sevenfold OH−, providing weakened Brønsted base sites. The optimized re-Ca4MnxAl-LDH displayed high catalytic performance in the IBD and FA condensation, resulting in the formation of HPA with a yield of 70.3%.
Regarding the isomerization of biomass-derived sugars, the process typically requires catalysis from base sites as well. Kwon et al. investigated the alkaline and catalytic properties of MgAl LDHs prepared by calcination and rehydration as solid base catalysts for the isomerization of glucose to fructose [50]. It was found that the hydroxyl groups were released from the interlayers of the LDH precursors under calcination at a specific temperature, giving composite metal oxides. The alkaline properties of the catalysts rely on the calcination temperature. Weak base sites were generally obtained when the LDH precursors were calcinated at low temperatures. In contrast, strong base sites were obtained when the LDH precursors were calcinated at relatively high temperatures. Remarkably, the rehydrated MgAl LDHs exhibit both weak and strong base sites, and their base properties could be systematically tuned by heat treatment and rehydration. Therefore, the rehydrated MgAl LDHs can effectively isomerize glucose to fructose because of their appropriate base and structure properties.
Yabushita et al. studied the effective isomerization of glucose to fructose in ethanol solvent catalyzed by rehydrated MgAl LDHs, giving up to 56% yield with a high selectivity of 80% [51]. The reaction process and mechanism are shown in Figure 5. The rehydrated MgAl LDHs were obtained by calcination and subsequent reconstruction of the original layered structure via the memory effect. The mechanistic study demonstrates that the base sites are responsible for the catalysis of glucose-to-fructose isomerization. The rehydrated MgAl LDHs exhibit the highest catalytic performance due to their high density of base sites. An active and selective catalyst for large-scale fructose production as a renewable feedstock could be developed by precisely controlling the strength and density of base sites.
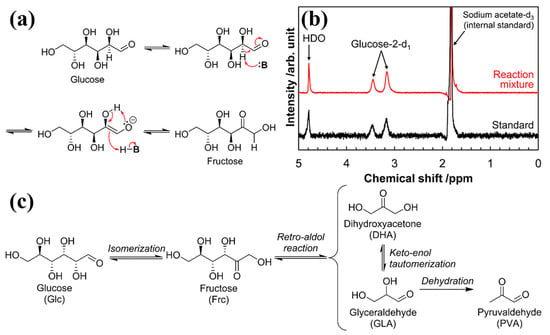
Figure 5.
(a) Glucose-to-fructose isomerization via the LdB–AvE mechanism catalyzed by bases (:B) and (b) 2H NMR spectrum of reaction mixture synthesized from glucose-2-d1, along with the spectrum for glucose-2-d1 dissolved in water (i.e., standard). (c) Isomerization of glucose to fructose, along with side reactions typically observed in the presence of base catalysts [51]. Copyright © 2019, adapted with permission from the American Chemical Society.
3.2. LDH-Based Metal Nanocatalysts
It is well known that LDHs can be converted into highly dispersed active metal nanoparticles by in situ reduction in the H2 atmosphere and anchored to a mixed metal oxide matrix, giving supported metal catalysts. The most attractive characteristic of this structure topotactic transformation process is the facile tuning over metal–support interactions, such as electronic metal–support interactions and metal–support acid–base interactions. The quantity and strength of the acid–base structure of supports can be efficiently modified by precisely controlling the topotactic transformation parameters of LDH precursors, such as reduction atmosphere, temperature, and heating rate. Moreover, the reduction temperature has a significant impact on the size and catalytic performance of the metal nanoparticles. Increasing the reduction temperature leads to more metal nanoparticles and larger particle sizes while also altering their catalytic performance [75].
Cui et al. reported the synthesis of Cu-based nanoparticle catalysts by calcining CuMgAl LDH precursors to obtain mixed metal oxides, followed by the reduction treatment process [54]. The resulting Cu-based nanoparticle catalysts (Cu/MMO) exhibited high dispersibility of 6–9 nm Cu nanoparticles and configurable acid–base sites on the support (MgO and Al2O3). The Cu-based nanoparticle catalysts were applied for low-temperature hydrogenation of dimethyl oxalate (DMO) to ethylene glycol, and the optimal catalyst Cu/MMO-S3 resulted in an excellent catalytic performance of 98.2% conversion and selectivity of 96.1%. The results indicated that the Lewis acid sites (Al3+) and medium/strong base sites (Mg2+−O2− pair) of supports act as active sites for the adsorption of polarized C=O/C−O group in the DMO molecule, while H2 undergoes dissociation adsorption on the Cu0 site. The unique ternary synergistic catalysis of Cu and acid–base sites makes a predominant contribution to the remarkable catalytic performance toward DMO hydrogenation to ethylene glycol.
This LDH-derived metal nanocatalyst is also widely used in hydrogenolysis and hydrogenation reactions of biomass-derived aldehydes. As a building block, FF offers a promising and rich platform for lignocellulosic biofuels and value-added chemicals. It stands out as a bridge connecting biomass resources and the chemical industry. It is well known that the C=O bond in FF molecules can interact with the base sites on the catalyst surface to promote the catalytic conversion performance [76,77]. The LDH-based metal nanocatalysts possess abundant acid sites, base sites, and metal active sites on the surface, which provide high catalytic performance for FF hydrogenation. Yang et al. reported three non-precious intermetallic compounds (Ni3Sn1, Ni3Sn2, and Ni3Sn4) derived from LDH precursors [43]. These compounds showed a highly uniform dispersion of metal nanoparticles, and they exhibited surprisingly improved catalytic performance toward FF selective hydrogenation (C=O) to furfuryl alcohol. Notably, Ni3Sn2 nanocatalysts showed the best catalytic performance with 100% conversion of FF and 99% selectivity of furfuryl alcohol at 100 °C under 2 MPa H2. The research results verify that the electron transfer from Sn to Ni promoted the activation adsorption of C=O bonds on Ni top site while inhibiting the adsorption of C=C bonds.
Zhu and colleagues synthesized nano-twin Cu particles with rich defects via topotactic transformation of LDH precursors. These particles effectively enhanced the target activation of C–O and C=O bonds, facilitating the conversion of FF to cyclopentanone [44]. Compared with regular spherical Cu nanoparticles, nano-twin Cu particles showed higher conversion kinetics with close to 100% FF conversion and up to 92% selectivity of cyclopentanone. Producing cyclopentanone (CPO) starts with the hydrogenation of C=O on metal sites, which results in furfuryl alcohol (FA). FA then undergoes a rearrangement on acid sites, forming 4-hydroxy-2-cyclopentenone (HCP). The C–OH in HCP is then selectively hydro-deoxygenated and hydrogenated to C=C on metal sites, resulting in CPO. Therefore, the multi-stepped surface defects of nano-twin Cu particles provided abundant acid sites and metal sites, which promoted the selective hydrogenation conversion of FF to CPO.
5-hydroxymethylfurfural (HMF) is also a particularly promising biomass-based intermediate due to its wide resource of fructose, glucose, and cellulose. Also, because of its versatile reactive functional groups to convert into value-added chemicals by selective hydrogenation, notably 2,5-dimethylfuran (DMF) and 2,5-bis(hydroxymethyl)furan (DHMF) [78,79]. Similar to the catalytic hydrogenation of furfuryl, the LDH-based metal catalysts can effectively catalyze the hydrodeoxygenation of HMF.
Wang et al. constructed a highly efficient and selective Cu/ZnO–Al2O3 catalyst via in situ reduction of a CuZnAl LDH precursor [45]. They studied the impact of Cu0/Cu+ species on the formation of various intermediates and their synergistic promoting effect on HMF hydrodeoxygenation. The formation of different intermediates depends on the Cu+/Cu0 ratio. Cu/ZnO–Al2O3 with a high Cu+/Cu0 ratio showed higher DMF selectivity (90.1%) at 100% HMF conversion than Cu/MgO–Al2O3. During the conversion process, Cu0 was preferable to adsorb the C=O bond and the hydrogen molecule, while the Cu+ site selectively absorbed and activated the C−O bond in the hydroxymethyl group. With the synergy of dual active sites, the Cu/ZnO–Al2O3 catalyst showed high activity and selectivity to DMF.
Wang et al. fabricated different Cu-based catalysts with multiple interfaces derived from LDHs to control the reaction pathway and product selectivity in the hydrogenation of HMF [46]. It was found that the Cu/Co ratio at the metal nanoparticle interface significantly affected the hydrogenation of C=O bonds in HMF. The reaction pathways for hydrogenation of HMF on different catalysts are illustrated in Figure 6a. When Cu/MgAlOx with no Co elements is used as a catalyst, a hydrogenation reaction is only carried out on the C=O bond, mainly producing 2,5-bis (hydroxymethyl) furan (DHMF). Co@Cu/3CoAlOx catalyzes both C=O bond hydrogenation and C–OH bond hydrogenolysis, mainly producing 2,5-dimethylfuran (DMF), whereas Co@Cu/5CoAlOx produces DMF together with the over-hydrogenated product (DMTHF). The possible mechanism of HMF hydrogenation is illustrated in Figure 6b. The C=O bond in HMF interacts with the Co metal site in Co@Cu/CoAlOx, and the resulting intermediate is then hydrogenated by Cu nanoparticles to form a C–OH group. Subsequently, the saturated C–OH groups undergo cleavage over the same sites, producing 5-methylfurfuryl alcohol (MFA), ultimately yielding DMF. For Cu/MgAlOx, the C=O bond interacts with the Cu metal site, leading to exclusive DHMF production.
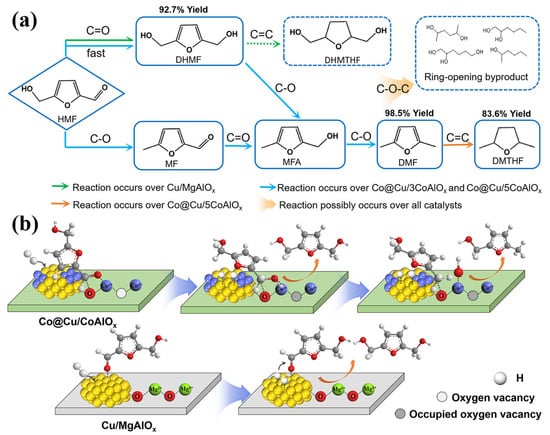
Figure 6.
(a) Reaction pathway for hydrogenation of HMF on different catalysts; (b) possible mechanism of HMF hydrogenation on Co@Cu/CoAlOx and Cu/MgAlOx catalysts inferred from IR [46]. Copyright © 2019, adapted with permission from the American Chemical Society.
Zhe et al. developed well-distributed Co-based catalysts through the in situ H2 reduction of LDHs and applied them for efficient selective hydrogenation of HMF to DMF [47]. The results showed that the Co metal nanoparticles uniformly distributed on the metal oxide matrix and demonstrated strong synergistic interaction with the metal oxides, therefore exhibiting superior reactivity under mild conditions. The well-distributed Co–metal active sites on the composite metal oxide support promoted hydrogenation in this reaction. The Co-based catalysts show high activity on the hydrogenation of C−O and C=O, making them promising for biomass utilization.
3.3. LDH-Based Mixed Metal Oxide Catalysts
LDH-derived mixed metal oxides are obtained from calcination at various temperatures of an LDH precursor, possessing high surface area, phase purity, basic surface properties, and structural stability [80]. Moreover, LDH-derived mixed metal oxides containing various metal cations exhibit their original acid–base properties of the MgAl system and reduction–oxidation properties as catalysts depending on the other metal species incorporated [81]. LDH-based mixed metal oxides are rich in Lewis acid–base sites, making them commonly used in acid–base catalysis due to their high metal dispersion and porosity. It is possible to manipulate the crystal structure of mixed metal oxides by adjusting the calcination conditions of the LDH precursors. This regulation affects the density and intensity of their acid–base sites and, ultimately, their catalytic properties [82,83]. LDH-based mixed metal oxides can act as carriers for depositing metal nanoparticles onto laminates via calcination, which produces metal-based catalysts derived from LDHs, exhibiting better catalytic properties [84].
Yan et al. synthesized acid–base mixed metal oxides (MgZrAl-MMO) via the calcination transformation of MgZrAl LDH precursors. The obtained MgZrAl-MMO catalysts are evaluated by the ethanolysis of urea reaction to produce diethyl carbonate (DEC) [58]. It has been discovered that both the weak acid sites and medium-strength base sites contribute to the overall yield of DEC, indicating an acid–base synergistic catalysis in this reaction. Specifically, the medium-strength Lewis base sites (Zr4+–O2−, Mg2+–O2−) activate ethanol, while weak Lewis acid sites (mostly ZrO2) activate urea and intermediate ethyl carbamate.
The Mg/Al mixed metal oxide catalysts were prepared by J. Kuljiraseth et al. and used as acid–base catalysts for the esterification of benzoic acid with 2-ethylhexanol. After being calcinated at 500 °C, the Mg/Al mixed metal oxides retained their clay structure and possess both acid and base sites. The total density of acid and base sites decreased with the increase in the Mg/Al ratio. Furthermore, the strength of the acid and base sites varied depending on their phase compositions and coordination number. The activity of calcined LDH catalysts was tested for the esterification of benzoic acid with 2-ethylhexanol to produce 2-ethylhexyl benzoate. The acid–base sites of the catalysts enhanced significantly the conversion of benzoic acid [59].
Recently, there has been much interest in converting biomass-derived glycerol to value-added oligo glycerol through catalytic etherification using acid and base sites of catalysts [85]. Elena et al. examined the calcined MgAl and CaAl LDHs with different acid–base properties used as catalysts for the one-pot etherification reaction of glycerol towards short-chain polyglycerols [55]. The results reveal that MgAl mixed metal oxides possess robustly base sites, whereas CaAl mixed metal oxides exhibit relatively high acidity. The acidity and basicity of the mixed metal oxide catalysts affect the catalytic results. Catalysts with higher acidity showed higher conversion, whereas catalysts with lower acidity resulted in lower conversion. Moreover, stronger base sites and lower acidity favored short-chain polyglycerols, and higher calcination temperature favored a higher degree of polymerization.
Glycerol can also obtain high-value products with industrial potential through the ester exchange reaction with dimethyl carbonate [86]. Zhou et al. synthesized CaAl LDH precursors using two preparation methods and calcined them to investigate the effects of different preparation methods on the structure and catalytic performance of CaAl composite metal oxides in the reaction of dimethyl carbonate and glycerol [87]. Due to the different structures obtained by different preparation methods, CaAl composite metal oxides with higher specific surface area and surface alkalinity exhibit higher catalytic activity.
Pyrolysis is an efficient method of using low-value resources to create high-value products and fuels. Acid–base synergistic catalysis is especially effective for pyrolysis production of desirable chemicals [88]. In recent years, acid–base bifunctional catalysts derived from LDHs have garnered significant interest due to their distinct structures and acid–base properties. Pyrolysis reactions with acid–base bifunctional catalysts produce high-value products while minimizing waste. The researchers, Yang et al., prepared a series of MgAl mixed metal oxide catalysts with varying acidity and alkalinity [57]. They achieved this by adjusting the Mg/Al ratio in the precursor of MgAl LDHs. Then, they studied the catalytic ability to upgrade low-rank coal resource pyrolysis through an acid–base synergistic effect. The results showed that as the Mg/Al ratio increased in the MgAl mixed metal oxides, the base sites increased, while the quantity of acid sites decreased. The acid sites in the MgAl mixed metal oxides facilitated the cracking of heavy components and decreased the production of heavy tar. The introduction of basicity sites in the MgAl mixed metal oxides enhanced the resistance to carbon deposition. After upgrading, the CO2 yield and the content of light aromatic hydrocarbons in tar increased, which can be attributed to the acid–base synergistic effect of the MgAl mixed metal oxides.
In biofuels, biodiesel has become an important alternative to traditional diesel due to its advantages in renewable resources. LDH-based derivative catalysts are widely used in biodiesel production reactions due to their multifunctional structure, which can achieve high yields [89]. Emine et al. prepared a series of CaMgAl composite metal oxides with different alkalinity. The alkalinity range of active metal oxides was adjusted by varying the content of Ca, and their catalytic activity for producing biodiesel from microalgal methanol was evaluated. Research has found that the content and structure of Ca have a significant impact on the production of biodiesel.
LDH-based mixed metal oxides have also been applied as acid–base bifunctional catalysts in hydrogen production via auto-thermal reforming of biomass-derived acetic acid [56]. Ni-based catalysts (Ni/CaO–Al2O3) originating from CaAl LDHs were synthesized by the direct calcination of CaAl LDHs, which are prepared by the coprecipitation method. Ni nanoparticles were anchored in the framework of CaO–Al2O3 support, resulting in a strong interaction between Ni metal active sites and the acid–base sites on the support. The interaction among Ni, CaO, and Ca12Al14O33 effectively enhanced the catalytic performance of the reaction and was responsible for thermal stability, resistance to oxidation of Ni0 species, and inhibiting coke deposition. Ni species are mainly used as active sites in ethanol steam reforming reactions due to their ability to cleave C–C bonds in ethanol and exhibit excellent catalytic performance [90]. Liu et al. synthesized Ni-based catalysts based on CaAl LDHs by coprecipitation method using Mg as promoter. The change in Mg content will have an impact on strong alkaline sites, and the formation of MgNiO2 mixed oxides will lead to a decrease in NiO crystal size, enhance the reducibility of Ni species, and greatly improve catalytic performance. The results of this work indicate that the catalytic performance of the ethanol steam reforming reaction is mainly related to Ni grain size, reducibility, and alkalinity.
4. Conclusions and Perspectives
LDHs and their derivatives are widely used as solid acid–base catalysts in green catalytic transformations due to their unique structure and properties. LDH-based catalysts exhibit both Lewis and Brønsted acid–base sites, allowing for a wide range of acid–base catalytic reactions in biomass conversion. The dual acid–base nature enables substrate activation and conversion, increasing catalytic activity and selectivity. LDH-based acid–base catalysts also possess adjustable acid–base properties, which can be modified through ion doping, anion exchange, and structural alterations. There are opportunities available to improve the catalytic performance of specific substrates and achieve desired target products. LDH-based acid–base catalysts typically demonstrate high surface area and porosity, providing adequate active sites for catalytic reactions and facilitating the efficient diffusion of reactants and products. In addition, LDH-based acid–base catalysts exhibit thermal and chemical stability, ensuring stable and sustained catalytic performance under the harsh reaction conditions encountered during the catalytic conversion process.
Despite their effectiveness, LDH-based acid–base catalysts still encounter several challenges during the transformation process. For the full potential of LDH-based catalysts in acid–base green conversion applications to be realized, further research and development are necessary.
The primary issue is catalyst deactivation. During the conversion of biomass-derived substrates, tar and coke residues can accumulate on the catalysts’ surface, causing deactivation. These residues obstruct the active sites, preventing reactant contact with catalytic active sites and inhibiting catalytic activity. Researchers are currently exploring strategies, such as regeneration and modification treatments, to improve the fouling resistance of LDH-based catalysts.
It is essential to pay attention to the adaptability of specific substrates. Different substrates have unique chemical compositions and structural complexities. The catalytic performance of LDH-based catalysts may vary depending on the specific substrate. Therefore, further optimization of the catalyst composition and acid–base properties is necessary to accommodate the unique properties of different substrates. It is crucial to optimize LDH-based catalysts for specific substrates to achieve superior selectivity and conversion. The optimization involves tailoring the LDH-based catalysts to match the unique characteristics of the substrates in terms of composition, morphology, and acid–base properties. In addition, it is important to understand the interactions between LDH-based catalysts and substrates to design catalysts for specific green catalytic conversion reactions.
Maximizing the catalytic activity and selectivity of LDH-based catalysts in acid–base green catalytic conversion reactions requires optimizing reaction conditions, such as temperature, pressure, and substrate-to-catalyst ratio. Changes in reaction conditions can significantly impact catalytic performance, conversion efficiency, and product selectivity. Further studies are needed to determine the optimal reaction parameters for different acid–base catalytic conversions using LDH-based catalysts considering the specific characteristics of LDH-based catalysts.
The precise control of composition, morphology, and acid–base properties of LDH-based catalysts presents another challenge. Further optimization and development of synthesis methods are required to ensure reproducibility, scalability, and cost-effectiveness. Developing efficient synthetic routes and understanding the structure–property relationship are crucial for the practical application of LDH-based catalysts in green catalytic conversion processes.
An in-depth understanding of the reaction mechanisms that occur during green acid–base catalytic conversion over LDH-based catalysts is essential for the rational design and optimization of catalysts. Mechanistic studies can lead to the development of more efficient and selective LDH-based catalysts by providing insights into active sites, reaction pathways, and intermediate species. Elucidating the underlying mechanisms and guiding catalyst design strategies can be accomplished using techniques such as in situ spectroscopy, kinetic modeling, and computational simulation.
Collaborative efforts among researchers in catalysis, materials science, and acid–base catalytic conversion can overcome these challenges and limitations. LDH-based solid acid–base catalysts can be optimized and tailored for specific substrates and target products, enabling efficient and sustainable chemical production from renewable resources.
Author Contributions
Conceptualization, X.Y. and S.H.; formal analysis, X.Y. and L.C.; investigation, X.Y. and L.C.; writing—original draft preparation, X.Y. and L.C.; resources, S.H.; writing—review and editing, S.H. and G.Z.; supervision, S.H. and G.Z.; project administration, S.H. and G.Z.; funding acquisition, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the R&D Program of Beijing Municipal Education Commission (KZ202210011015).
Data Availability Statement
The corresponding author can provide the data from this study upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Su, F.; Guo, Y.H. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Liu, F.J.; Zheng, A.M.; Noshadi, I.; Xiao, F.S. Design and synthesis of hydrophobic and stable mesoporous polymeric solid acid with ultra strong acid strength and excellent catalytic activities for biomass transformation. Appl. Catal. B Environ. 2013, 136, 193–201. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Alinezhad, H. Brønsted acidic ionic liquids: Green catalysts for essential organic reactions. J. Mol. Liq. 2016, 218, 95–105. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Fattahi, A. A study on the catalytic activity and theoretical modeling of a novel dual acidic mesoporous silica. RSC Adv. 2014, 4, 16647–16654. [Google Scholar] [CrossRef]
- Manríquez-Ramírez, M.; Gómez, R.; Hernández-Cortez, J.G.; Zúñiga-Moreno, A.; Reza-San Germán, C.M.; Flores-Valle, S.O. Advances in the transesterification of triglycerides to biodiesel using MgO–NaOH, MgO–KOH and MgO–CeO2 as solid basic catalysts. Catal. Today 2013, 212, 23–30. [Google Scholar] [CrossRef]
- Suwannakarn, K.; Lotero, E.; Goodwin, J.G. Solid bronsted acid catalysis in the gas-phase esterification of acetic acid. Ind. Eng. Chem. Res. 2007, 46, 7050–7056. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.; Dai, W.-L. Recent advances in silver-based heterogeneous catalysts for green chemistry processes. Appl. Catal. B Environ. 2014, 160–161, 730–741. [Google Scholar] [CrossRef]
- Patial, S.; Raizada, P.; Aslam Parwaz Khan, A.; Singh, A.; Van Le, Q.; Huy Nguyen, V.; Selvasembian, R.; Mustansar Hussain, C.; Singh, P. Emerging new-generation covalent organic frameworks composites as green catalysts: Design, synthesis and solar to fuel production. Chem. Eng. J. 2022, 433, 134594. [Google Scholar] [CrossRef]
- Liu, K.-G.; Sharifzadeh, Z.; Rouhani, F.; Ghorbanloo, M.; Morsali, A. Metal-organic framework composites as green/sustainable catalysts. Coord. Chem. Rev. 2021, 436, 213827. [Google Scholar] [CrossRef]
- Yadav, G.; Yadav, N.; Ahmaruzzaman, M. Microwave-assisted synthesis of biodiesel by a green carbon-based heterogeneous catalyst derived from areca nut husk by one-pot hydrothermal carbonization. Sci. Rep. 2022, 12, 21455. [Google Scholar] [CrossRef]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Synthesis and acid catalysis of cellulose-derived carbon-based solid acid. Solid State Sci. 2010, 12, 1029–1034. [Google Scholar] [CrossRef]
- Elmekawy, A.A.; Shiju, N.R.; Rothenberg, G.; Brown, D.R. Environmentally Benign Bifunctional Solid Acid and Base Catalysts. Ind. Eng. Chem. Res. 2014, 53, 18722–18728. [Google Scholar] [CrossRef][Green Version]
- Yan, Q.; Hou, X.; Liu, G.; Li, Y.; Zhu, T.; Xin, Y.; Wang, Q. Recent advances in layered double hydroxides (LDHs) derived catalysts for selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2020, 400, 123260. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-L.; Liang, S.; Mo, L.-Z.; Su, H.-H.; Huang, J.-S.; Zhang, P.-J.; Qin, J.-N. Conversion of biomass-derived monosaccharide into furfural over Cr–Mg-LDO@bagasse catalysts. Sustain. Chem. Pharm. 2023, 32, 101013. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, K.; Li, B.; Shim, H.; Huang, Y. Key Considerations on the Industrial Application of Lignocellulosic Biomass Pyrolysis toward Carbon Neutrality. Engineering 2023, 29, 35–38. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, S.; Ke, L.; Wu, Q.; Zhang, Q.; Cui, X.; Dai, A.; Xu, C.; Cobb, K.; Liu, Y.; et al. Research progress on pyrolysis of nitrogen-containing biomass for fuels, materials, and chemicals production. Sci. Total Environ. 2023, 872, 162214. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M.; Khan, N. Recent Developments in Titanium Carbide (Ti3C2)-Based Layered Double Hydroxide (LDH) Nanocomposites for Energy Storage and Conversion Applications: A Minireview and Perspectives. Energy Fuels 2022, 36, 9821–9843. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Li, C.; Zhang, C. Recent Progress of Hydrogenation and Hydrogenolysis Catalysts Derived from Layered Double Hydroxides. Catalysts 2022, 12, 1484. [Google Scholar] [CrossRef]
- Zhu, J.; Li, T.; Wang, S.; Chen, Y.; Ge, F.; Xu, Y. Lattice-distortion active sites of Ni-doped CuMgFe LDH for benzotraizole degradation. J. Environ. Chem. Eng. 2022, 10, 107903. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Panda, H.S.; Srivastava, R.; Bahadur, D. Synthesis and in situ mechanism of nuclei growth of layered double hydroxides. Bull. Mater. Sci. 2011, 34, 1599–1604. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Mertens, M.; Witte, K.; Tendeloo, G.; Cool, P.; Vansant, E.F. Zn–Al layered double hydroxides: Synthesis, characterization and photocatalytic application. Microporous Mesoporous Mater. 2008, 113, 296–304. [Google Scholar] [CrossRef]
- Morel-Desrosiers, N.; Pisson, J.; Israëli, Y.; Taviot-Guého, C.; Besse, J.-P.; Morel, J.-P. Intercalation of dicarboxylate anions into a Zn–Al–Cl layered double hydroxide: Microcalorimetric determination of the enthalpies of anion exchange. J. Mater. Chem. 2003, 13, 2582–2585. [Google Scholar] [CrossRef]
- Zou, W.; Guo, W.; Liu, X.; Luo, Y.; Ye, Q.; Xu, X.; Wang, F. Anion Exchange of Ni-Co Layered Double Hydroxide (LDH) Nanoarrays for a High-Capacitance Supercapacitor Electrode: A Comparison of Alkali Anion Exchange and Sulfuration. Chem. Eur. J 2018, 24, 19309–19316. [Google Scholar] [CrossRef]
- Das, J.; Parida, K.M. Heteropoly acid intercalated Zn/Al HTlc as efficient catalyst for esterification of acetic acid using n-butanol. J. Mol. Catal. A Chem. 2007, 264, 248–254. [Google Scholar] [CrossRef]
- Wei, Y.; Li, F.; Liu, L. Liquid exfoliation of Zn–Al layered double hydroxide using NaOH/urea aqueous solution at low temperature. RSC Adv. 2014, 4, 18044–18051. [Google Scholar] [CrossRef]
- Liu, H.; Yu, T.; Su, D.; Tang, Z.; Zhang, J.; Liu, Y.; Yuan, A.; Kong, Q. Ultrathin Ni-Al layered double hydroxide nanosheets with enhanced supercapacitor performance. Ceram. Int. 2017, 43, 14395–14400. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, X. Carbon fiber/Ni-Co layered double hydroxide@NiMoO4/graphene oxide sandwich structure flexible electrode materials: Facile synthesis and high supercapacitor performance. J. Alloys Compd. 2019, 794, 13–20. [Google Scholar] [CrossRef]
- Erickson, K.L.; Bostrom, T.E.; Frost, R.L. A study of structural memory effects in synthetic hydrotalcites using environmental SEM. Mater. Lett. 2005, 59, 226–229. [Google Scholar] [CrossRef]
- Gao, Z.; Sasaki, K.; Qiu, X. Structural Memory Effect of Mg–Al and Zn–Al layered Double Hydroxides in the Presence of Different Natural Humic Acids: Process and Mechanism. Langmuir 2018, 34, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Z.; Shi, E.W.; Li, W.J.; Zheng, Y.Q.; Wu, N.C.; Zhong, W.Z. Particle size comparison of hydrothermally synthesized cobalt and zinc aluminate spinels. J. Am. Ceram. Soc. 2002, 85, 2949–2955. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Jia, X.D.; Chen, G.B.; Shang, L.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T.R. Ultrafine NiO Nanosheets Stabilized by TiO2 from Monolayer NiTi-LDH Precursors: An Active Water Oxidation Electrocatalyst. J. Am. Chem. Soc. 2016, 138, 6517–6524. [Google Scholar] [CrossRef]
- Dubnova, L.; Danhel, R.; Meinhardova, V.; Korolova, V.; Smolakova, L.; Kondratowicz, T.; Kikhtyanin, O.; Capek, L. Reconstruction of the ZnAl Mixed Oxides Into the Layered Double Hydroxide Catalysts Active in the Aldol Condensation of Furfural: The Role of ZnO Particles. Front. Chem. 2021, 9, 803764. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, M.N.; Kapustin, A.E.; Panchenko, V.N.; Butenko, E.O.; Krupskaya, V.V.; Gil, A.; Vicente, M.A. Synthetic and natural materials with the brucite-like layers as high active catalyst for synthesis of 1-methoxy-2-propanol from methanol and propylene oxide. J. Mol. Catal. A Chem. 2016, 423, 22–30. [Google Scholar] [CrossRef]
- Terzi, C.M.; dos Santos, E.H.; Carvalho, C.; Prevot, V.; Wypych, F.; Forano, C.; Nakagaki, S. MgAl and ZnAl layered double hydroxides modified with molybdate and tungstate anions as catalysts for oxidation of cyclohexane. Catal. Today 2023, 422, 114221. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, G.; Guo, Y.; Guo, Y.; Gong, X.-Q. Effect of One-Pot Rehydration Process on Surface Basicity and Catalytic Activity of MgyAl1-aREEaOx Catalyst for Aldol Condensation of Citral and Acetone. ACS Sustain. Chem. Eng. 2016, 4, 1591–1601. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, W.; Liang, J.; Shen, J.; Fu, X.; He, H.; Yan, S.; Ren, X. Enhanced catalytic phenol hydroxylation by CuZnFeAl layered double hydroxides: Synergistic effects of Cu+ and oxygen vacancies. New J. Chem. 2021, 45, 179–188. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Z.; Liu, Y.; Zhang, M.; Wang, Y.; Wan, Y.; Yan, K. Efficient electrooxidation of biomass-derived aldehydes over ultrathin NiV-layered double hydroxides films. J. Energy Chem. 2023, 78, 412–421. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Liu, B.; Chen, Z.; Xu, H.; Yan, K. Trimetallic NiCoFe-Layered Double Hydroxides Nanosheets Efficient for Oxygen Evolution and Highly Selective Oxidation of Biomass-Derived 5-Hydroxymethylfurfural. ACS Catal. 2020, 10, 5179–5189. [Google Scholar] [CrossRef]
- Wang, T.; Hu, A.; Wang, H.; Xia, Y. Catalytic transfer hydrogenation of furfural into furfuryl alcohol over Ni–Fe-layered double hydroxide catalysts. J. Chin. Chem. Soc. 2019, 66, 1610–1618. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Chen, Y.; Liu, W.; Feng, H.; Wang, B.; Zhang, X.; Wei, M. The selective hydrogenation of furfural over intermetallic compounds with outstanding catalytic performance. Green Chem. 2019, 21, 5352–5362. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Ma, X.; An, Z.; Guo, S.; Shu, X.; Song, H.; Xiang, X.; He, J. A gradient reduction strategy to produce defects-rich nano-twin Cu particles for targeting activation of carbon-carbon or carbon-oxygen in furfural conversion. J. Catal. 2020, 389, 78–86. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Z.; Feng, J.; Fornasiero, P.; He, Y.; Li, D. Insight into the Effect of Dual Active Cu0/Cu+ Sites in a Cu/ZnO–Al2O3 Catalyst on 5-Hydroxylmethylfurfural Hydrodeoxygenation. ACS Sustain. Chem. Eng. 2020, 8, 15288–15298. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, J.; Zheng, L.; Wang, B.; Bi, R.; He, Y.; Liu, H.; Li, D. Interfacial Structure-Determined Reaction Pathway and Selectivity for 5-(Hydroxymethyl)furfural Hydrogenation over Cu-Based Catalysts. ACS Catal. 2019, 10, 1353–1365. [Google Scholar] [CrossRef]
- An, Z.; Wang, W.; Dong, S.; He, J. Well-distributed cobalt-based catalysts derived from layered double hydroxides for efficient selective hydrogenation of 5-hydroxymethyfurfural to 2,5-methylfuran. Catal. Today 2019, 319, 128–138. [Google Scholar] [CrossRef]
- Ye, X.; Shi, X.; Xu, H.; Feng, Y.; Jin, B.; Duan, P. Enhanced catalytic activity of layered double hydroxides via in-situ reconstruction for conversion of glucose/food waste to methyl lactate in biorefinery. Sci. Total Environ. 2022, 829, 154540. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Hanif, A.; Tsang, D.C.W.; Shang, J.; Su, Z.; Song, H.; Ok, Y.S.; Poon, C.S. Tuneable functionalities in layered double hydroxide catalysts for thermochemical conversion of biomass-derived glucose to fructose. Chem. Eng. J. 2020, 383, 122914. [Google Scholar] [CrossRef]
- Kwon, D.; Kang, J.Y.; An, S.; Yang, I.; Jung, J.C. Tuning the base properties of Mg–Al hydrotalcite catalysts using their memory effect. J. Energy Chem. 2020, 46, 229–236. [Google Scholar] [CrossRef]
- Yabushita, M.; Shibayama, N.; Nakajima, K.; Fukuoka, A. Selective Glucose-to-Fructose Isomerization in Ethanol Catalyzed by Hydrotalcites. ACS Catal. 2019, 9, 2101–2109. [Google Scholar] [CrossRef]
- Bing, W.; Zheng, L.; He, S.; Rao, D.; Xu, M.; Zheng, L.; Wang, B.; Wang, Y.; Wei, M. Insights on Active Sites of CaAl-Hydrotalcite as a High-Performance Solid Base Catalyst toward Aldol Condensation. ACS Catal. 2017, 8, 656–664. [Google Scholar] [CrossRef]
- Bing, W.; Wang, H.; Zheng, L.; Rao, D.; Yang, Y.; Zheng, L.; Wang, B.; Wang, Y.; Wei, M. A CaMnAl-hydrotalcite solid basic catalyst toward the aldol condensation reaction with a comparable level to liquid alkali catalysts. Green Chem. 2018, 20, 3071–3080. [Google Scholar] [CrossRef]
- Cui, G.; Meng, X.; Zhang, X.; Wang, W.; Xu, S.; Ye, Y.; Tang, K.; Wang, W.; Zhu, J.; Wei, M.; et al. Low-temperature hydrogenation of dimethyl oxalate to ethylene glycol via ternary synergistic catalysis of Cu and acid−base sites. Appl. Catal. B Environ. 2019, 248, 394–404. [Google Scholar] [CrossRef]
- Pérez-Barrado, E.; Pujol, M.C.; Aguiló, M.; Llorca, J.; Cesteros, Y.; Díaz, F.; Pallarès, J.; Marsal, L.F.; Salagre, P. Influence of acid–base properties of calcined MgAl and CaAl layered double hydroxides on the catalytic glycerol etherification to short-chain polyglycerols. Chem. Eng. J. 2015, 264, 547–556. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, W.; Jia, X.; Chen, B.; An, S.; Xie, X.; Huang, L. Ca–Al layered double hydroxides-derived Ni-based catalysts for hydrogen production via auto-thermal reforming of acetic acid. Int. J. Hydrog. Energy 2019, 44, 20007–20016. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Li, C.; Chen, Z.; Wang, D.; Wang, D.; Ibrahim Sharif, M.; Li, J.; Yu, J.; Gao, S. The effect of acid-base synergistic catalysis on upgrading of volatiles from coal pyrolysis over Mg-Al composite oxides. Fuel 2022, 321, 124030. [Google Scholar] [CrossRef]
- Yan, T.; Bing, W.; Xu, M.; Li, Y.; Yang, Y.; Cui, G.; Yang, L.; Wei, M. Acid-base sites synergistic catalysis over Mg–Zr–Al mixed metal oxide toward synthesis of diethyl carbonate. RSC Adv. 2018, 8, 4695–4702. [Google Scholar] [CrossRef]
- Kuljiraseth, J.; Wangriya, A.; Malones, J.M.C.; Klysubun, W.; Jitkarnka, S. Synthesis and characterization of AMO LDH-derived mixed oxides with various Mg/Al ratios as acid–basic catalysts for esterification of benzoic acid with 2-ethylhexanol. Appl. Catal. B Environ. 2019, 243, 415–427. [Google Scholar] [CrossRef]
- Pithakratanayothin, S.; Tongsri, R.; Chaisuwan, T.; Wongkasemjit, S. Influences of M–Sn intermetallics (M = Ni, Cu) prepared by mechanical alloying on phenol hydroxylation. Catal. Sci. Technol. 2017, 7, 5413–5421. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Konnerth, H.; Yeh, J.-Y.; Prechtl, M.H.G.; Wen, C.-Y.; Wu, K.C.W. De novo synthesis of Cr-embedded MOF-199 and derived porous CuO/CuCr2O4 composites for enhanced phenol hydroxylation. Green Chem. 2019, 21, 1889–1894. [Google Scholar] [CrossRef]
- Yue, X.K.; Zhang, L.H.; Sun, L.X.; Gao, S.T.; Gao, W.; Cheng, X.; Shang, N.Z.; Gao, Y.J.; Wang, C. Highly efficient hydrodeoxygenation of lignin-derivatives over Ni-based catalyst. Appl. Catal. B Environ. 2021, 293, 120243. [Google Scholar] [CrossRef]
- Liu, B.Y.; Xu, S.J.; Zhang, M.; Li, X.; Decarolis, D.; Liu, Y.Q.; Wang, Y.C.; Gibson, E.K.; Catlow, C.R.A.; Yan, K. Electrochemical upgrading of biomass-derived 5-hydroxymethylfurfural and furfural over oxygen vacancy-rich NiCoMn-layered double hydroxides nanosheets. Green Chem. 2021, 23, 4034–4043. [Google Scholar] [CrossRef]
- Upare, P.P.; Chamas, A.; Lee, J.H.; Kim, J.C.; Kwak, S.K.; Hwang, Y.K.; Hwang, D.W. Highly Efficient Hydrotalcite/1-Butanol Catalytic System for the Production of the High-Yield Fructose Crystal from Glucose. ACS Catal. 2019, 10, 1388–1396. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic Isomerization of Biomass-Derived Aldoses: A Review. ChemSusChem 2016, 9, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Huang, H.W.; Zhao, Q.; Ma, T.Y.; Wang, L.Z. Thin-Layered Photocatalysts. Adv. Funct. Mater. 2020, 30, 1910005. [Google Scholar] [CrossRef]
- Kong, W.F.; Xing, Z.P.; Fang, B.; Cui, Y.Q.; Li, Z.Z.; Zhou, W. Plasmon Ag/Na-doped defective graphite carbon nitride/NiFe layered double hydroxides Z-scheme heterojunctions toward optimized photothermal-photocatalytic-Fenton performance. Appl. Catal. B Environ. 2022, 304, 120969. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Y.Y.; Chen, D.D.; Li, J.; Wu, H.G.; Meng, Y.; Huang, J.S.; Yuan, J.F.; Su, Y.Q.; Wang, J.Q.; et al. Relay photo/thermal catalysis enables efficient cascade upgrading of sugars to lactic acid: Mechanism study and life cycle assessment. Chem. Eng. J. 2023, 452, 139687. [Google Scholar] [CrossRef]
- Wang, J.Q.; Jiang, J.Q.; Li, Z.H. Efficient one-pot syntheses of secondary amines from nitro aromatics and benzyl alcohols over Pd/NiTi-LDH under visible light. Dalton Trans. 2023, 52, 16935–16942. [Google Scholar] [CrossRef]
- Liu, W.; Sun, J.; Zhang, X.; Wei, M. Supported Ag Catalysts on Mg–Al Oxides toward Oxidant-Free Dehydrogenation Reaction of Benzyl Alcohol. Ind. Eng. Chem. Res. 2018, 57, 15606–15612. [Google Scholar] [CrossRef]
- Valente, J.S.; Pfeiffer, H.; Lima, E.; Prince, J.; Flores, J. Cyanoethylation of alcohols by activated Mg–Al layered double hydroxides: Influence of rehydration conditions and Mg/Al molar ratio on Brönsted basicity. J. Catal. 2011, 279, 196–204. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Tišler, Z.; Velvarská, R.; Kubička, D. Reconstructed Mg–Al hydrotalcites prepared by using different rehydration and drying time: Physico-chemical properties and catalytic performance in aldol condensation. Appl. Catal. A Gen. 2017, 536, 85–96. [Google Scholar] [CrossRef]
- Liang, G.; Wang, A.; Zhao, X.; Lei, N.; Zhang, T. Selective aldol condensation of biomass-derived levulinic acid and furfural in aqueous-phase over MgO and ZnO. Green Chem. 2016, 18, 3430–3438. [Google Scholar] [CrossRef]
- Lee, R.; Vanderveen, J.R.; Champagne, P.; Jessop, P.G. CO2-Catalysed aldol condensation of 5-hydroxymethylfurfural and acetone to a jet fuel precursor. Green Chem. 2016, 18, 5118–5121. [Google Scholar] [CrossRef]
- Zhan, Y.; Song, K.; Shi, Z.; Wan, C.; Pan, J.; Li, D.; Au, C.; Jiang, L. Influence of reduction temperature on Ni particle size and catalytic performance of Ni/Mg(Al)O catalyst for CO2 reforming of CH4. Int. J. Hydrog. Energy 2020, 45, 2794–2807. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Kazi, F.K.; Patel, A.D.; Serrano-Ruiz, J.C.; Dumesic, J.A.; Anex, R.P. Techno-economic analysis of dimethylfuran (DMF) and hydroxymethylfurfural (HMF) production from pure fructose in catalytic processes. Chem. Eng. J. 2011, 169, 329–338. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
- Gao, D.L.; Lin, W.W.; Lin, Q.J.; Dai, F.F.; Xue, Y.X.; Chen, J.H.; Liu, Y.X.; Huang, Y.; Yang, Q. Remarkable adsorption capacity of Cu2+-doped ZnAl layered double hydroxides and the calcined materials toward phosphate. J. Environ. Chem. Eng. 2023, 11, 109472. [Google Scholar] [CrossRef]
- Takehira, K. Recent development of layered double hydroxide-derived catalysts—Rehydration, reconstitution, and supporting, aiming at commercial application. Appl. Clay Sci. 2017, 136, 112–141. [Google Scholar] [CrossRef]
- Teruel, L.; Bouizi, Y.; Atienzar, P.; Fornes, V.; Garcia, H. Hydrotalcites of zinc and titanium as precursors of finely dispersed mixed oxide semiconductors for dye-sensitized solar cells. Energy Environ. Sci. 2010, 3, 154–159. [Google Scholar] [CrossRef]
- Xia, S.; Shao, M.; Zhou, X.; Pan, G.; Ni, Z. Theoretical and experimental investigation into photoelectrocatalytic oxidation and reduction property of ZnFeTi mixed metal oxides. J. Mol. Catal. A Chem. 2015, 406, 127–136. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; He, Y.; Feng, J.; Wu, T.; Li, D. Highly efficient PdAg catalyst using a reducible Mg-Ti mixed oxide for selective hydrogenation of acetylene: Role of acidic and basic sites. J. Catal. 2017, 348, 135–145. [Google Scholar] [CrossRef]
- Salehpour, S.; Dubé, M.A. Towards the Sustainable Production of Higher-Molecular-Weight Polyglycerol. Macromol. Chem. Phys. 2011, 212, 1284–1293. [Google Scholar] [CrossRef]
- Szabados, M.; Adám, A.A.; Traj, P.; Muráth, S.; Baán, K.; Bélteky, P.; Kónya, Z.; Kukovecz, A.; Sipos, P.; Pálinkó, I. Mechanochemical and wet chemical syntheses of CaIn-layered double hydroxide and its performance in a transesterification reaction compared to those of other Ca2M(III) hydrocalumites (M: Al, Sc, V, Cr, Fe, Ga) and Mg(II)-, Ni(II)-, Co(II)- or Zn(II)-based hydrotalcites. J. Catal. 2020, 391, 282–297. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C. Effect of Preparation Method on the Catalytic Property of Calcined Ca–Al Hydrotalcite for the Synthesis of Ethyl Methyl Carbonate. ACS Omega 2021, 6, 5056–5060. [Google Scholar] [CrossRef]
- Wei, B.; Jin, L.; Wang, D.; Shi, H.; Hu, H. Catalytic upgrading of lignite pyrolysis volatiles over modified HY zeolites. Fuel 2020, 259, 116234. [Google Scholar] [CrossRef]
- Çakirca, E.E.; Akin, A.N. Study on heterogeneous catalysts from calcined Ca riched hydrotalcite like compounds for biodiesel production. Sustain. Chem. Pharm. 2021, 20, 100378. [Google Scholar] [CrossRef]
- Liu, H.R.; Ding, R.; Zhang, Y.G.; Li, H.S.; Li, S.Z. Magnesium promoted hydrocalumite derived nickel catalysts for ethanol steam reforming. Int. J. Hydrog. Energy 2023, 48, 13804–13813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).