Abstract
The presence of hazardous chemical compounds in foods is a growing concern in almost every country. Although some toxins come from microbial contamination, a major part comes from residues of pesticides used for plant health and food preservation. Despite plans to decrease their use, the concentration of hazardous residues encountered in food is growing. The societal solution to this issue is to find alternatives to chemicals and replace the most hazardous by biodegradable, fewer toxic compounds. However, as this greener transition takes some time, any transitory solution to decrease the risks of contamination is welcome. Among them, the stimulation of microbial pesticide degradation in food in a similar way to bioremediation in the environment would be very positive. In this review, we present the problem of food contamination, focusing on organophosphates and organochlorines, and the various possibilities of microbial decontamination. We discuss the possible use of microbial biocatalysts as a biopreservation tool. We conclude that, although this process is very promising, it lacks research taking into account the various degradation products and the elaboration of screening procedures able to choose some rare, efficient biopreservation strains.
1. Introduction
The consumption of plant products has been, for many years, a way to improve health and increase life expectancy all over the world. Therefore, health agencies in most countries have tried to stimulate vegetable and fruit consumption through various nutritional programs that recommend amounts of fruits and vegetables in the daily diet [1]. Although the risks related to the presence of phytosanitary chemical residues were known, some nutritionists were surprised to see that the nutritional benefits of increasing plant consumption were higher than the correlated detrimental effects of ingesting more toxic agrochemicals. This time may be at an end, as the results of some studies have now suggested that eating more vegetables decreases life expectancy [2]. These results have been obtained in an American study, taking only conventional agriculture without any labels of low or no use of agrochemicals into account, with this negative effect being especially significant for the consumption of some categories of vegetables. However, this problem of agrochemical residues is acute everywhere, and several national or regional programs exist to decrease the use of agrochemicals (e.g., Ecophyto in France), despite their popularity for pest control and in genetically modified organism (GMO) culture models. Due to the strong implantation of agrochemical companies in agriculture, their lobbying actions towards governments, the insufficient taking into account of the health and environmental costs as well as the pest resistance emergence of these practices, and of course, the lack of validated alternatives, these programs are far below their targets. This has been shown in reports from the Pesticide Action Network (PAN), which point out the increase in hazardous compounds encountered in European fruits and vegetables [3]. There is a societal necessity to decrease the risks due to the use of agrochemicals by decreasing the use of hazardous compounds, improving management, changing agricultural practices, and finding new alternatives to pesticides. However, there is no other solution in the short term than stimulating remediation of agrochemical contaminants in food, and one solution is the use of microorganisms in food just as they are used in fermented products, biopreservation, or clean label strategies. This review analyzes the positive results and discusses the limits obtained in the degradation of pesticides by microorganisms commonly active in fermentation. A particular focus will be on organochlorine and organophosphate pesticides since they are very persistent and cause a public health problem.
2. Pesticides
The global population growth, which will reach over 10 billion individuals by 2050, will result in a corresponding increase in the demand for food. The Food and Agricultural Organization of the United Nations has reported a 70% increase in global food production [4]. In order to address the increasing demand for food, it is common practice to use pesticides [4]. Pesticides can be classified into various categories based on:
- -
- Application: In agriculture, pesticides are used to safeguard crops against pests, in-sects, and weeds; in public health, pesticides are employed to eradicate vectors that cause diseases (e.g., malaria); and domestic pesticides are used to eliminate insects such as cockroaches, bacteria, protozoa, and mice [5].
- -
- Target organisms: Insecticides, which are chemicals designed to eliminate insects; fungicides, which are applied to inhibit or eradicate fungi; herbicides, which are used to control or eliminate weeds; rodenticides, which are pesticides intended to eliminate rodents; fumigants, which are gaseous pesticides used to control or eliminate pests such as bedbugs; and insect repellents, which are applied to the skin or clothing to deter insects from approaching [5].
- -
- Chemical composition: Including organochlorines (OC), organophosphates (OP), carbamates, pyrethroids, phenyl amides (carbanilates, acylanalides, toluidines, and acetamides), phenoxyalkonates, trazines, benzoic acid derivatives, benzonitriles, phtalimide derivatives, and dipyrids [5]. The main categories of pesticides are reported in Figure 1.
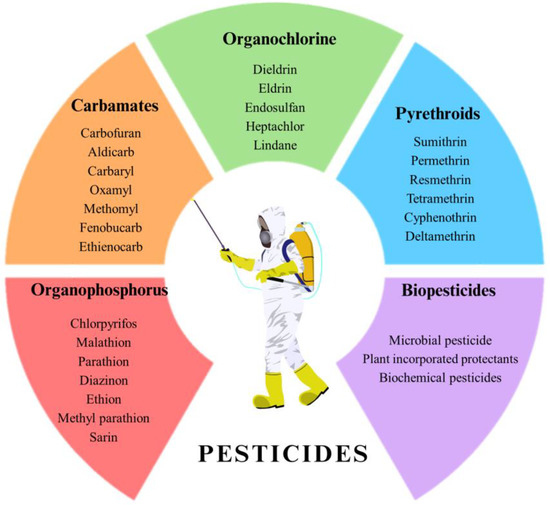 Figure 1. Main categories of pesticides.
Figure 1. Main categories of pesticides.
The majority of pesticides are considered dangerous due to their toxicity towards non-target organisms, tendency to bioaccumulate, and lack of ecological sustainability [6]. A mere 0.1% of pesticides exhibit selectivity toward their intended targets [7]. The use of pesticides has been found to have detrimental effects on the air, water, and soil of the environment. Bioaccumulation has facilitated the integration of pesticides into the food chain.
According to Mostafalou et al. [8], exposure to pesticides can lead to acute and chronic health issues for humans, animals, and aquatic organisms. Human exposure to pesticides can occur through various means, including the inhalation of polluted air, consumption of contaminated food and water, and application of pesticide-containing products such as cosmetics [9]. The primary route of human exposure to pesticides is through the consumption of food contaminated with pesticides [9]. Moreover, it should be noted that maternal transmission to the fetus can occur through breast milk or the placenta [9]. In Europe, the maximum residue limits (MRLs) for pesticides are set by the European Union (EU) and enforced by the European Food Safety Authority (EFSA). MRLs are the maximum amounts of pesticide residues that are allowed to remain in or on food products sold in the EU. The EU sets MRLs for over 1000 different pesticides, and these limits are regularly reviewed and updated based on the latest scientific research. The MRLs take into account factors such as the toxicity of the pesticide, the type of exposure, and the potential risk to human health. In addition to MRLs, the EU also sets maximum levels for certain pesticides in drinking water and establishes guidelines for the safe use of pesticides in agriculture. The use of some pesticides in the EU is restricted due to their harmful effects on human health and the environment. For example, the use of neonicotinoid insecticides, which have been linked to declines in bee populations, has been severely restricted in the EU since 2013 [10].
Organophosphates and organochlorines are two classes of pesticides that have been widely used as pesticides and insecticides. It is important to note that the use of both OP and OC has declined in many countries due to their associated health and environmental risks. The development and use of alternative pesticides with a lower toxicity and environmental impact have become more prevalent in recent years. Organophosphate pesticides are a group of synthetic chemicals that was developed as insecticides in the mid-20th century. Organophosphates are a prevalent and extensively utilized category of pesticides on a global scale, constituting 45% of the overall world market for pesticides [11]. Organophosphates are a class of chemical compounds that consist of esters of phosphoric acids. These compounds exhibit complex chemical structures that may include aliphatic, phenyl, or heterocyclic derivatives [11]. Organophosphates are commonly classified into four distinct subcategories on the basis of their molecular structures, namely: (1) phosphates; (2) phosphothioates; (3) phosphorodithioates; and (4) phosphorothilates. They work by disrupting the nervous systems of insects, causing paralysis and death. In fact, OP pesticides form a covalent bond with the enzyme acetylcholinesterase (AChE) found in the nervous system. The primary function of AChE is to catalyze the hydrolysis of acetylcholine into choline and acetic acid. Acetylcholine, a crucial neurotransmitter, plays a vital role in the transmission of nerve impulses within the brain, muscular, and skeletal systems [12] (Figure 2). Examples of OP pesticides include parathion, malathion, diazinon, and glyphosate [12]. OP pesticides are known to have negative effects on human health and the environment. They have been linked to a range of health problems, including neurological effects and cancer. In addition, OP pesticides can persist in the environment and contaminate water and soil, posing a risk to wildlife and humans who come into contact with them [11]. The toxic effects of OP on humans can be broadly classified into three categories: (i) acute effects or short-term effects—they are the result of acetylcholine accumulation, which causes acute effects in the central nervous system due to muscarinic and nicotinic effects; (ii) delayed or intermediate effects—muscle weakness involving the neck, limb, and respiratory muscles; and iii) long-term effects—headache, fatigue, anxiety, confusion, impaired concentration, sleep disorders, muscle spasms, muscular pains, incoordination, irritability, depression, intolerance to alcohol and other chemicals, memory loss, nightmares, nausea, and respiratory disease [11].

Figure 2.
OP have an anti-esterase activity. They phosphorylate acetylcholine, thereby reducing the ability of the enzyme to break down the neurotransmitter, causing an accumulation of acetylcholine in the central and peripheral nervous systems, resulting in an acute cholinergic syndrome via continuous neurotransmission.
Due to their harmful effects, the use of some OP pesticides has been banned or restricted in many countries. For example, in the United States, the use of chlorpyrifos has been banned for household use, and its use in agriculture is being phased out. Malathion is still used in some agricultural applications, but its use has been restricted in residential areas [11].
Organochlorines (OC) are a class of chlorinated compounds that exhibits high environmental persistence and is extensively employed globally as potent pesticides. The compounds exhibit varying degrees of solubility in organic solvents. These pesticides exhibit lipophilic characteristics and possess a significantly low rate of biodegradation [13]. OC pesticides are divided into four groups:
- -
- Dichlorodiphenylethane or diphenyl aliphatics (DDT (dichlorodiphenyltrichloro-ethane), DDD (dichlorodiphenyldichloroethane), dicofol, ethylan, chlorobenzilate, and methoxychlor);
- -
- Cyclodienes (CHL (chlordane), aldrin, dieldrin, heptachlor, endrin, dodecachloro-pentacyclodecane (mirex), and endosulfan (cyclic ester of sulfuric acid));
- -
- Cylohexanes;
- -
- Chlorinated camphenes (toxaphene and chlordecone).
The overuse or misuse of OC pesticides has been rising continuously, posing potential hazards to environmental and human health. The level of toxicity exhibited by OC is contingent upon human doses and periods of exposure. According to Al Antary et al. [14], when individuals are exposed to doses of approximately 280 mg/kg, they may experience symptoms such as nausea, vomiting, convulsions, fatigue, and flu-like symptoms. On the other hand, chronic exposure can impact multiple organ systems, including the hepatic, renal, nervous, and immune systems, which can lead to the development of cancer, neurologic symptoms, infertility, and other disorders. In the 1960s, OC pesticides were banned in most developed countries. However, they are still widely used in most third-world countries since they are cheap and represent an effective option for disease management, thereby leading to an increase in agricultural production [13].
3. Pesticides and Public Health
According to the World Health Organization, there are over 3 million cases of pesticide poisoning each year and up to 220,000 fatalities, mostly in developing nations. The harmful effect of pesticide exposure was mostly influenced by the amount of pesticide and the persistence time. Some pesticides are so hazardous to people that even a few drops in the mouth or on the skin can have harmful effects. Other less dangerous pesticides can also have negative consequences in cases of long-term exposure [15]. Several studies found a close relationship between pesticides and a variety of diseases and cancers, including leukemia, Parkinson’s disease, and problems of the reproductive and endocrine systems [15]. Moreover, these compounds interfere with endocrine systems and have detrimental effects on physiological processes such as growth, development, and reproduction [16,17,18]. According to Yan et al. [16], chronic exposure to dieldrin, paraquat, organophosphates, and organochlorine causes ROS-driven neurotoxicity, which is then linked to Alzheimer’s disease.
Numerous epidemiological and clinical studies have shown a strong association between asthma and bronchial hyperreactivity symptoms and pesticide exposure. A significant asthma exacerbation may result from exposure to pesticides due to inflammation, irritation, endocrine disruption, or immunosuppression [17]. According to Mehrpour et al. [18], exposure to OP pesticides poses a risk to the male reproductive system through mechanisms such as a decline in sperm counts, density, viability, and motility, inhibition of sperm formation, abnormal sperm morphology, and decrease in testis weights (Figure 3).
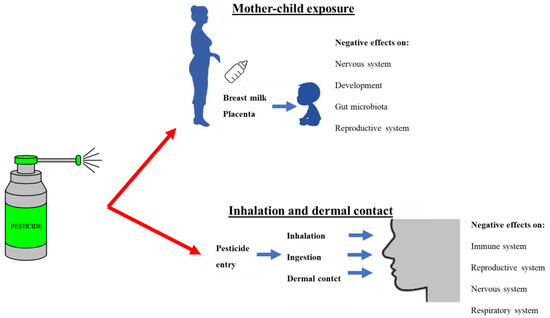
Figure 3.
Exposure route of pesticides and their effects on human health.
4. Bioremediation
The integration of industrial practices in agriculture, as well as the utilization of both organic and inorganic compounds in farming, has resulted in the degradation of soil quality [19]. Pesticides were developed and produced for application in agriculture with the purpose of controlling fungi, insects, nematodes, weeds, mollusks, rodents, and plant growth. The presence of pesticides was not limited solely to soil and agricultural products, as evidenced by their migration into various other systems [19]. According to Sun et al. [4], a significant proportion of applied pesticides, approximately 80–90%, remain as residues in the soil. This residue has the potential to harm non-targeted vegetation and organisms, which can have severe implications for the agricultural ecosystem [4].
Furthermore, pesticides, which are essential chemical compounds extensively employed in agriculture to enhance the productivity of crucial crops for human sustenance and to manage pests, leave considerable residual quantities in plant- and animal-derived food products. In addition to conventional physical detoxification techniques, an efficacious and encouraging strategy involves the process of fermentation through the microorganisms present in foods or through intentionally incorporated strains (Figure 4). The utilization of microorganisms to facilitate the degradation of pesticides, which is commonly known as “bioremediation”, represents a feasible, ecologically responsible, and economical approach for eradicating pollutants from agricultural soil [20]. Microorganisms have the capacity to break down complex and recalcitrant compounds that are harmful, using a series of enzymatic processes, into simpler inorganic molecules such as CO2, oxides, mineral salts of elements, and water, which are harmless. The process of the complete degradation of pesticides is referred to as “biomineralization” [21]. Microorganisms and plants utilize the resultant simpler inorganic compounds either to fulfill their nutritional needs or as electron acceptors in the respiratory chain [22].

Figure 4.
Bioremediation mechanisms. Biosorption is a passive, metabolically independent process covering all sorbate–biological matrix contact features; biomineralization is a general term for the processes by which living organisms form minerals, and this can result in pesticide removal; biodegradation can be mediated by intra- and extra-cellular enzymes.
The process of detoxification encompasses the enzymatic hydrolysis or oxidation of pesticides, leading to the formation of less toxic substances. Several scientific publications have reported the presence of intermediate metabolites through the use of diverse analytical techniques. In the majority of instances, the precise metabolic pathway of degradation by the strains present in food remains unclear and necessitates additional investigations [23].
5. Microbial Degradation of Pesticides in Fermented Foods
Human beings can come into contact with pesticides through the ingestion of contaminated foods [24,25].
Over the course of 18 years, from 2002 to 2020, a total of 5211 notifications regarding pesticide residues in food were submitted to the Rapid Alert System for Food and Feed (RASFF) [26]. These notifications pertained to 5232 distinct products, e.g., vegetables (31.6%), legumes (8.1%), edible flowers (8.1%), berries and small fruits (6.6%), miscellaneous fruits (6.6%), such as pomegranates, and citrus fruits (5.9%) [26]. Numerous investigations have revealed varying degrees of contamination from OC and OP pesticides in food items, including grains, vegetables, and fruits. Adeniyi and Osibanjo [27] detected OC pesticide residues in various vegetables, uncooked fruits, and tubers in Nigeria. Ibotom and Mohammade [28] conducted a study to detect the existence of OP and OC pesticide residues in various fruits (lime, apple, and orange) and vegetables (cucumber, cabbage, and lettuce) obtained from selected markets in Kaduna. Residues of OC and OP pesticides were identified. Omwenga et al. [29] evaluated the presence of OP and carbamate pesticides in vegetables from Kenya. A proportion of 21% of the samples contained detectable levels of profenofos, omethoate, acephate, methamidophos, and chlorpyrifos, with concentrations exceeding the MRL allowed in Europe. In order to reduce the presence of pesticides in foods, a sustainable strategy could be the selection of microbes able to degrade them. The first studies about the degradation of pesticide residues by microorganisms began in the 1940s, coinciding with an increased focus on safeguarding the environment [30]. Microorganisms have the ability to metabolically degrade pesticides. Microorganisms utilize pesticides as their primary source of energy, serving as a carbon, nitrogen, or phosphorus source during catabolic degradation [31]. Various studies have investigated the decrease in pesticide residues that occurs during the process of fermentation in different food items [31,32,33,34,35,36]. The genera Bacillus, Micrococcus, Arthrobacter, Corynebacterium, Flavobacterium, Pseudomonas, and Rhodococcus, as well as fungi from the genera Penicillium, Aspergillus, Fusarium, and Trichoderma, exhibit the highest levels of pesticide degradation activity [37,38,39]. Some examples are reported in Table 1. The subject of microbial degradation in fermented food has been recently reviewed [23], and we will thus only present the various studies and give illustrative examples of decontamination. As will be discussed later, a big bias of most studies on bacterial pesticide degradation is that they only deal with the disappearance of the pesticide molecule, but for some compounds, pesticides are actually propesticide compounds that are bioactivated in a 10- to 100-fold more active molecule. As even non-propesticide molecules can be degraded into active metabolites, the simple degradation of a pesticide residue, without knowledge of the impact on toxicity, provides little information.

Table 1.
Main microbes used for the bioremediation of foods.
6. Lactic Acid Bacteria as Pesticide-Degraders
Lactic acid bacteria (LAB) are, in the meantime, very active in many fermented foods and constitute a family containing a lot of probiotic and biopreservative strains. They can interfere in the world of pesticides in several ways. In agriculture and food production, they can act by avoiding the development of pathogenic fungi without the use of chemical fungicides [40]. In addition, they even promote plant growth and fulfill the various roles of agrochemicals [41]. At the other end of the pesticide contamination chain, they can exhibit a protective effect on animals with pesticide-induced diseases [42,43]. In between, they can decrease intestinal absorption of pesticides by adsorbing the compounds on their surface [44,45], and of course, they can participate in pesticide degradation [23]. Lactic acid bacteria are involved in both silage and food fermentation. In both cases, they can provoke a decrease in pesticide residues [46], although this activity is usually very partial and LAB are not as efficient as other microorganisms. A major problem resulting from research based on only monitoring pesticide degradation without analyses of metabolites and of their bioactivity, which is the most common research strategy in this field, is that it does not inform whether this degradation is positive or results in more active products. However, some studies have gone deeper into the mechanisms of pesticide degradation, identifying some enzymes responsible for pesticide hydrolysis and their encoding genes [47]. Pesticide degradation by LAB has been recently reviewed by Armenova et al. [23], and we will therefore not describe this in this part, but we will discuss the LAB potential in the perspective part of this article.
7. Filamentous Fungi as Pesticide-Degraders
Fungi are widely distributed chemoheterotrophic organisms. They are commonly found in both terrestrial and aquatic ecosystems [48]. Most fungi are aerobic and prefer oxygen-rich environments. However, anaerobic fungi have been discovered in areas with low oxygen levels, such as the ocean and the digestive tracts of animals [49]. Fungi are recognized for their ability to degrade complex organic matter and participate in decay processes. They are capable of breaking down wood, including lignin and cellulose, as well as other plant-based materials that are frequently generated as agricultural waste products [50]. Fungi possess the capability to metabolize diverse compound classes, including pesticides, owing to their intra- and extra-cellular enzymatic machinery [51]. In particular, fungi are capable of degrading a diverse array of pesticides, including OC and OP compounds [5,52,53]. The degradation of pesticides by fungi involves three stages. During the initial stage, the pesticide is transformed into soluble and non-toxic compounds through processes such as reduction, hydrolysis, or oxidation. The subsequent stage involves the conjugation process, which enhances the aqueous solubility properties of the intermediary substances. In the end, the conversion of intermediate metabolites into non-toxic compounds is carried out by enzymes such as peroxidases and oxygenases [30].
However, some studies revealed that the fungal population converts pesticides into non-toxic products, releasing them into the soil, and then they are further degraded by the bacterial population through processes such as cometabolism and mineralization. Therefore, it is possible that the fungal contribution did not involve the direct degradation of the compounds but rather facilitated a bacterial dispersal through hyphae or the translocation of the compounds to other microbial communities [5,54].
The main fungal genera able to degrade pesticides are Trametes, Ganoderma, Aspergillus, Fusarium, Pleurotus, Cladosporium, Rhizopus, Penicillium, Phlebia, and Mortiella [54]. A detailed list of the fungal species involved in the degradation of pesticides is reported in Table 2.

Table 2.
Main filamentous fungi involved in the degradation of pesticides. Modified from Vaksmaa et al. [54] and Bose et al. [5].
Some studies have demonstrated that a collective of diverse fungal species exhibits superior pesticide degradation capabilities compared to individual strains. As reported by Nyakundi et al. [55], a group of five fungal isolates, consisting of three from the Pleurotus genus, one from the Coriolopsis genus, and one unknown, exhibited superior capabilities in breaking down diazinon and methomyl pesticides compared to individual isolates tested. Ellegaard-Jensen et al. [56] reported a comparable advantage in the degradation of diuron by consortia of fungi and bacteria. Knudsen et al. [57] reported that the consortium of Mortierella spp. LEJ702 and Aminobacter spp. MSH1 exhibited an improved ability to degrade the herbicide 2,6-dichlorobenzamide.
Fungal Enzymes Involved in the Degradation of Pesticides
The low substrate specificity of fungal enzymes has been noted by El-Gendi et al. [58]. According to Harms et al. [59], this phenomenon confers an advantage since it allows fungi to utilize a broad spectrum of compounds as carbon, nitrogen, and energy sources. The ability of fungi to degrade biodegradable substances is commonly associated with enzymatic reactions that involve oxidases and peroxidases [60]. Therefore, the functioning of fungi generally necessitates an environment with sufficient oxygen.
The taxonomic abilities of fungi with regard to enzymes have been effectively categorized into mechanisms that are either intra- or extra-cellular [58] (Table 3). While extra-cellular ligninolytic enzymes facilitate the breakdown of water-insoluble contaminants, intra-cellular enzymes such as cytochrome P450 play a crucial role in this process as well [61]. Filamentous fungi exhibit a diverse range of oxidative and extra-cellular ligninolytic enzymes, including, but not limited to, lignin peroxidase (LiP), manganese peroxidase (MnP), versatile peroxidase (VP), and laccase (Lac) [62]. Trametes versicolor was employed for the degradation of three distinct hydrophobic pesticides, namely chlorpyriphos, dicofol, and cypermethrin [63]. The degradation rates of chlorpyriphos, dicofol, and cypermethrin at 25 °C were observed to be 95%, 88%, and 93%, respectively, within a duration of 14 days [63]. A study conducted by Coelho-Moreira et al. [64] demonstrated the degradation of diuron by Ganoderma lucidum. The researchers observed an increase in laccase activity in the presence of diuron.

Table 3.
Enzymes involved in pesticide degradation.
Trametes versicolor has the capability to degrade Fipronil. The intra-cellular metabolism of this substance was confirmed through the detection of hydroxylated and glycosylated transformation products, which were produced by cytochrome P450 [65]. According to Hu et al. [66], it has been demonstrated that this particular enzyme is capable of breaking down Acetamiprid and Imidacloprid, which are highly polar pesticides. P. chrysosporium has been found to possess approximately 150 P450 monooxygenase genes in its genome [67]. This organism has demonstrated the ability to degrade four neonicotinoids through the utilization of two cytochrome P450 isozymes [67]. Mori et al. [68] identified four neonicotinoid insecticides, namely Acetamiprid, Clothianidin, Imidacloprid, and Thiacloprid. Moreover, it has been observed that the breakdown of Endosulfan can occur through either a hydrolytic or oxidative mechanism in P. chrysosporium [69]. The utilization of a cytochrome P450 inhibitor resulted in the partial suppression of oxidative metabolism, leading to a shift towards hydrolytic pathways.
The main pesticides degraded by fungi and the involved metabolic pathways are reported in the following paragraphs and in Table 3.
8. Limits of Pesticide Bioremediation Biocatalysts
8.1. Are Degradation Products Worse Than the Pesticide Itself?
For all compounds exhibiting toxicity, degradation of the initial compound does not necessarily mean the acquisition of a safe product. Such a question can apply to food for several families of products like pesticides, mycotoxins, or antinutritional factors. For these later, the example of vicine or convicine is of interest. These pyrimidine glycosides can indeed be hydrolyzed into the aglycones divicine or isouramil, but these degradation products are the actual factors of favism, and degradation does not mean remediation [70]. A related question concerns the level of degradation of the chemical. A complete degradation would involve the breaking down of elementary building blocks that can be reintroduced into the microbial metabolism. However, in some cases, some blocks cannot be hydrolyzed or transformed, and this leads to residues. Coming back to our example of vicine and convicine, although these compounds can be stable in food, the cited study shows that it is easy to find bacterial glycosidase activities to obtain the aglycone forms and that, despite a lack of knowledge of the metabolic pathway involved, these compounds disappeared from the fermented food after some time. The glycoside hydrolysis, maybe in addition to the acidic conditions after LAB fermentation, was sufficient, at least in some cases, to get rid of the product. Although most of the studies published on pesticide microbial degradation in food seem to monitor only the presence of the initial pesticide and not its degradation products [71], which is often a tough task, these metabolites can be important. Some studies have, for instance, investigated the degradation of chlorpyrifos. Many organisms can degrade this organophosphate compound. The main initial reaction is a hydrolysis giving rise to 3,5,6-trichloro-2-pyrinidol, but another possibility is the desulfurization into chlorpyrifos oxon (Figure 5). This second reaction may be seen as a bioactivation of the insecticide, as the oxon is considered the active form of the proinsecticide chlorpyrifos [72]. It is usually catalyzed by cytochrome P450 in insects, and the reduction in the expression of the corresponding genes may be a strategy of resistance to insecticides. Interestingly, for some insects not exhibiting this activity, enzymes from the microbiota can give rise to an oxon and, as a result, to toxicity [73]. More interestingly for our subject, different strains of Lactobacilli species that can be present in food exhibited different metabolic activity, as Lactiplantibacillus plantarum from the insect microbiota produced an oxon, while the probiotic Lactobacillus rhamnosus GG could bind chlorpyrifos but not transform it, thus decreasing toxicity. The hydrolysis giving rise to the pyrinidol compound (Figure 2), although this compound exhibits environmental persistence, can then follow several catabolic pathways detected in aerobic Bacillus subtilis, Micrococcus luteus, and Pseudomonas sp. [5].
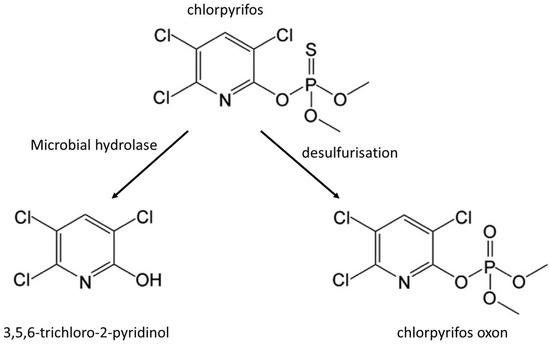
Figure 5.
Products of the initial degradation of chlorpyrifos.
8.2. Applying Bioremediation Biocatalysts in Food
The question of using a microorganism to fulfill a metabolic function in food is always an issue. If it is part of a fermentation process, other metabolic reactions exhibiting an organoleptic impact are acceptable. But there will be competition between microorganisms and the success of the desired reaction is not guaranteed. If it is not part of any fermentation process, the biocatalyst has to be active without modifying any sensory properties of the food product. This question is the classical one in all the clean label strategies based on microorganisms [74] and molds that will be visible on foods, aerobic strains able to develop only on surfaces, or strains producing alcohol, acids, or gas that will be difficult to use. However, from what has been seen above, fungi and aerobic bacteria are the most efficient organisms for bioremediation. We will see in this part how the main species can be used.
8.2.1. Lactic Acid Bacteria and Their Enzymes
Lactic acid bacteria constitute a microbial family of species that is often targeted in studies looking for starters, biopreservation, or clean-label catalysts as there is a long and positive shared history between humans and these bacteria. As a result, many species are Generally Recognized as Safe (GRAS) and have a qualified presumption of safety (QPS) status, and they can fulfill regulation criteria [75]. Unfortunately, as shown above, these bacteria are not very good at degrading chemicals other than carbohydrates or proteins, and it appears, for instance, that degradation is much more developed for in situ biodegradation by soil microbes. The latter are a mixture of Gram-negative and Gram-positive microorganisms, among which some possess an aerobic metabolism, providing them with an oxidative degradation capability [24]. The adaptation of the mechanisms described so far to LAB, which has a fermentative and therefore anaerobic metabolism, represents a real challenge for researchers. To allow the complete oxidation of metabolites, a final oxygen acceptor is required. A study was conducted to assess the effectiveness of inducing aerobic metabolism in LAB through the addition of a heme group [76]. Interestingly, some LABs show predispositions as they are genetically equipped with aerobic respiration and therefore require only heme for effective respiration as they already carry cytochrome oxidase and menaquinone biosynthesis.
Pesticide degradation also involves several classes of enzymes, including esterases, mixed-function oxidases (MFOs), and hydrolases. It is possible for LAB to produce enzymes belonging to this last class of enzymes. The presence of the opdA and opdE genes has been shown to be responsible for the production of hydrolases by a strain of Leuconostoc mesenteroides isolated during the fermentation of kimchi [47]. As we will see in the next part on the use of single enzymes, LAB could be used together with mold enzymes to complete the metabolic pathway.
Another important characteristic that can be applied for bioremediation is linked to the surface properties of LAB. Their diversity can contribute to the adhesion of many different chemicals [77], and this property can be used to bind, separate, and inhibit the adsorption of some compounds like pesticides, heavy metals, or mycotoxins [44,73,78,79]. This property has proven efficacy against the toxicity of these hazardous compounds in different organisms. Interestingly, not only the bacterial surface but also exopolysaccharide-derived exopolysaccharides can exhibit such a behavior on heavy metals [80] and, for sediment microbes, on chlorpyrifos [81].
8.2.2. Fungi and Their Enzymes
There are a few limitations that prevent the widespread use of fungal strains to remove pesticides. For instance, it has typically been found that the biodegradation of organic compounds by fungi, including pesticides, is a comparatively longer process and occasionally does not result in the entire elimination of contaminants [15]. The fact that different fungal strains have been found to only partially degrade pesticides is another disadvantage of fungal biodegradation [82]. It has occasionally been discovered that these secondary metabolites are significantly more toxic than the original chemicals [83]. In comparison to the microorganisms from which they can be isolated, enzymes are significantly more efficient. To lower the levels of toxicity, they remove the parent compound’s functional group [5]. The use of these enzymes in bioremediation has drawn a lot of attention as a sustainable technique to eliminate pesticides. However, due to the complexity and chemical diversity of pesticides, the detailed biochemistry of enzyme-based degradation needs a variety of catalytic processes. For instance, laccase only directly degrades phenolic compounds with a slow redox potential because of its low redox potential [84]. However, mediators can act as an electron shuttle between the laccase and the target molecules, expanding the substrate range of this enzyme [85]. Therefore, laccase is also capable of oxidizing non-phenolic compounds in the presence of an appropriate redox mediator. Many different pesticides have been degraded using laccase-mediator systems (LMS), which also use artificial and natural mediators. Utilizing T. versicolor laccase, Zeng et al. [86] studied the breakdown of the herbicide isoproturon using six mediators, including acetosyringone, 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS), 1-hydroxybenzotriazole (HBT), syringaldehyde (SA), violuric acid (VA), and vanillin (VAN). Without the addition of HBT, there was a slow degradation of isoproturon, whereas LMS in conjugation with HBT resulted in its complete degradation within 24 h. Kupski et al. [87] tested different LMS to remove various pesticides from aqueous samples. The LMS studied included carbofuran, diuron, bentazone, tebuconazole, and pyraclostrobin. The laccase–vanillin system was the most effective, removing 77% of pesticides. The use of immobilized enzymes on various support materials is a different method to increase the effectiveness of pesticide breakdown. In order to maintain a high stability in terms of the pH, temperature, packing, reuse, and separation, enzymes are immobilized into solid, stable supports. In fact, a number of variables, including the pH and temperatures, can affect enzyme activity, and their immobilization appears to increase enzyme stability (Table 4). According to Vidal-Limon et al. [88], immobilized laccase from Coriollopsis gallica on mesoporous nanostructured silicon foam effectively oxidized the dichlorophen insecticide and reduced the related apoptotic and genotoxic effects. Magnetic iron nanoparticles with a size range of 10 to 15 nm were used to study the degradation of the pesticide chlorpyrifos. Results of the study showed that laccase immobilized on this support was effective in degrading more than 99% of the chlorpyrifos in 12 h at pH 7 and 60 °C [89].

Table 4.
Main factors influencing microbial enzyme activity.
In recent times, there have been notable developments in the immobilization of laccases on innovative support materials aimed at enhancing the efficacy of pesticide degradation [15]. Metal–organic frameworks (MOFs) represent a novel category of porous materials that possess a distinct pore and crystal structure, exhibit a favorable biocompatibility, have a high surface area, and have a good chemo- and mechanical stability. These properties make MOFs an ideal host matrix for the development of enzyme–MOF composites. The majority of composites formed by enzymes and metal–organic frameworks (MOFs) demonstrate an exceptional catalytic efficacy and enhanced stability [90]. In a recent study conducted by Ladole et al. [91], the degradation of methylene blue and crystal violet dyes was investigated using laccase-immobilized peroxidase-mimicking MOFs in both batch and continuous modes. A significant reduction in the range of 90–95% was observed in both batch and continuous operations. The enzyme activity of laccase-immobilized MMOF was sustained at a rate of 89% throughout the 10th cycle for a duration of 25 days. The utilization of zeolitic imidazolate frameworks (ZIFs), a type of metal–organic frameworks (MOFs), has recently gained attention as a viable option for the encapsulation of enzymes due to their substantial surface area and straightforward synthesis conditions [92]. Numerous sources have documented the utilization of ZIF materials for the immobilization of enzymes. However, the encapsulation of enzymes may result in partial or complete inactivity due to the confinement effect and competing coordination. Additionally, the protonation effect of 2-methylimidazole (2-MeIM) may create an alkaline environment that can damage enzyme molecules [92]. The adoption of glutathione as a ligand candidate for constructing hybrid ZIF crystals has been found to result in enzymes exhibiting higher activity and a superior stability after immobilization compared to pure ZIFs that utilize 2-MeIM as the sole organic ligand [92]. The present techniques employed for the identification and measurement of pesticides, such as liquid chromatography or immunoassay-based methods, are constrained by their protracted duration, unsuitability for on-site determination, and incapability for real-time monitoring. In recent times, biosensors have become prominent analytical methods due to the reduction in the size of transduction mechanisms, advancements in microelectronic circuitry, and utilization of bio-recognition units for interfacing. The development of cost-effective and miniaturized analytical tools has facilitated the transformation of complex instruments into point-of-care devices. Enzymes are frequently employed as biological recognition entities due to their practicality and translatability [93]. The investigation of OP can be effectively conducted through the utilization of organophosphorus hydrolase (OPH) or organophosphorus acid anhydrolase (OPAA) as biological recognition units. This can be achieved by converting the catalytic reaction into a measurable signal on a biosensor. The wide-ranging substrate specificity exhibited by these enzymes renders them highly desirable enzymes for scientific investigators and practitioners who are concerned with the surveillance and elimination of OP that are extant within the natural milieu. For instance, the hydrolysis reaction of organophosphates (OP) catalyzed by OPH results in the production of an alcohol and two protons [94]. An innovative biosensor that combines nanozymes and natural enzymes has been developed to achieve the highly sensitive detection of glutamate. A MIL-88B(Fe)-NH2 substance, which exhibits exceptional peroxidase mimic activity and stability, was employed as a nanozyme and carrier for the immobilization of glutamate oxidase (GLOX) via a Schiff base reaction, resulting in the development of a chem-enzyme cascade detector (MIL-88B(Fe)-NH2@GLOX). The MIL-88B(Fe)-NH2@GLOX composite demonstrated a broad linear range spanning from 1 to 100 μM, and a low limit of detection of 2.5 μM for the purpose of detecting glutamate. In addition, the MIL-88B(Fe)-NH2@GLOX exhibited a remarkable capacity for repeated use and preservation over time. Following seven successive cycles, the MIL-88B(Fe)-NH2-GLOX, where GLOX was adsorbed onto MIL-88B(Fe)-NH2, exhibited a significant decline in its activity. Conversely, the MIL-88B(Fe)-NH2@GLOX demonstrated a retention of 69% of its original activity. During a storage period of 90 days, the MIL-88B(Fe)-NH2@GLOX exhibited a retention of 60% of its initial activity, whereas the free GLOX only retained 30% of its initial activity [95]. However, these studies are still in their infancy. By applying cutting-edge omics-based methodologies to discover the genes and intermediate metabolites connected to pesticide mineralization, future studies should be conducted to define the genetic basis of a pesticide’s biodegradation. Understanding the full potential and sustainability of immobilized enzyme technology requires a thorough understanding of the long-term ecological and financial effects of using enzymes for the remediation of pesticides. Further investigations should be conducted to improve the detection limits and sensitivity of biosensors. One potential strategy for accomplishing this objective is to select recombinant enzymes with an enhanced sensitivity, specificity, and stability in high-efficiency matrices. Additionally, these can be combined with techniques that provide an improved ease of quantification, resulting in an electrical or optical signal.
9. Conclusions
As for pesticide degradation in the environment, microorganisms can have a big impact on agrochemical degradation in animal feed or human food. Using them for this functionality of cleaning food would be a possibility to respond to the issue of pesticide poisoning through food consumption. It appears that finding bioremediation strains usable in food without transforming the product’s organoleptic properties is still a medium- or long-term objective. Indeed, the most active strains belong to the fungi kingdom, and their presence in food is usually detectable by consumers. Lactic acid bacteria that are often good candidates for clean-label and biopreservation strategies are mostly less efficient, or their degradation capability is still not well characterized. Anyway, some strains have already been identified for the degradation of some families of pesticides, and their application in food could be envisaged. Biotechnological approaches, such as modifying the fermentation conditions or the genetic material, are difficult to use in food processing. The main actions that can be used in food fermentation are the modification of the temperature, gas, or changes in the recipe with natural compounds that bring additional chemical properties. Techniques based on the genetic modification of metabolism and system biology are also a way to improve the control of pesticide catabolism. In this sense, it should be noted that secondary metabolites released after pesticide degradation can be more toxic than their parental compound. This approach has been recently reviewed in bioremediation in the environment [96]. Synthetic biology could represent a valuable tool to find solutions for the degradation of pesticide residues. Synthetic biologists may develop various modules, and design a multi-module integration genetic circuit that integrates numerous degradation systems. These approaches offer efficacious tools and methodologies for pesticide remediation. Researchers have the option to select various tools to engineer bacteria for the purpose of pesticide degradation with an optimal efficiency and safety, depending on the specific application scenarios. However, this technique raises the problem of gene or GMO dissemination in food and human organisms, and, except in some rare applications where GMOs are completely put away before human consumption, this technical solution efficient for bioremediation can also address other issues. The use of biocatalysts for bioremediation is thus a good perspective for human health, but strains will have to be tailor-chosen for each pesticide and food.
Author Contributions
Conceptualization, Y.W. and G.P.; figure and table preparation, O.N.; P.A.N.F., S.K. and A.P.; writing—original draft preparation, G.P., R.T. and Y.W.; writing—review and editing, G.P., R.T. and Y.W.; supervision, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful to European Union—Next Generation EU. Project Code: ECS00000041; Project CUP: C43C22000380007; Project Title: Innovation, digitalization and sustainability for the diffused economy in Central Italy—VITALITY.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, S.; Moore, L.; Park, S.; Harris, D.; Blanck, H. Adults Meeting Fruit and Vegetable Intake Recommendations—United States, 2019. Morbid. Mortal. Wkly. Rep. 2022, 71, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Insausti, H.; Chiu, Y.; Wang, Y.; Hart, J.; Bhupathiraju, S.; Mínguez-Alarcón, L.; Ding, M.; Willett, W.; Laden, F.; Chavarro, J. Intake of fruits and vegetables according to pesticide residue status in relation to all-cause and disease-specific mortality: Results from three prospective cohort studies. Environ. Int. 2022, 159, 107024. [Google Scholar] [CrossRef] [PubMed]
- Pesticide Action Network Europe. The dramatic rise in the most toxic pesticides found on fruits and vegetables sold in Europe and evidence that governments are failing their leagal obligations. In Forbidden Fruit; PAN Europe: Brussels, Belgium, 2022. [Google Scholar]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide pollution in agricultural soils and sustainable remediation methods: A review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.V.N.; Rajamohan, N.; Saravanan, R. Microbial degradation of recalcitrant pesticides: A review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Carriger, J.F.; Rand, G.M.; Gardinali, P.R.; Perry, W.B.; Tompkins, M.S.; Fernandez, A.M. Pesticides of potential ecological concern in sediment from South Florida canals: An ecological risk prioritization for aquatic arthropods. Soil Sediment Contam. 2006, 15, 21–45. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Concerns of environmental persistence of pesticides and human chronic diseases. Clin. Exp. Pharmacol. 2012, S5, e002. [Google Scholar] [CrossRef]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure routes and health risks associated with pesticide application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef]
- Wahab, S.; Muzammil, K.; Nasir, N.; Khan, M.S.; Ahmad, M.F.; Khalid, M.; Ahmad, W.; Dawria, A.; Reddy, L.K.V.; Busayli, A.M. Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review. Plants 2022, 11, 1106. [Google Scholar] [CrossRef]
- Mali, H.; Shah, C.; Raghunandan, B.H.; Prajapati, A.S.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Organophosphate pesticides an emerging environmental contaminant: Pollution, toxicity, bioremediation progress, and remaining challenges. J. Environ. Sci. 2022, 127, 234–250. [Google Scholar] [CrossRef]
- Farnoosh, G.; Latifi, A.M. A review on engineering of organophosphorus hydrolase (OPH) Enzyme. J. Appl. Biotechnol. Rep. 2014, 1, 1–10. [Google Scholar]
- Keswani, C.; Dilnashin, H.; Birla, H.; Roy, P.; Tyagi, R.K.; Singh, D.; Rajput, V.D.; Minkina, T.; Singh, S.P. Global footprints of organochlorine pesticides: A pan-global survey. Environ. Geochem. Health 2022, 44, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Al Antary, T.M.; Alawi, M.A.; Estityah, H.; Haddad, N.; Al-Antary, E.T. Chlorinated pesticide residues in human breast milk collected from southern Jordan in 2012/2013. Toxin Rev. 2015, 34, 190–194. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N.; Barceló, D. Persistence of pesticides-based contaminants in the environment and their effective degradation using laccase-assisted biocatalytic systems. Sci. Total Environ. 2019, 695, 133896. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, Y.; Liu, L.; Yan, H. Pesticide exposure and risk of Alzheimer’s disease: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32222. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Nicell, J.A. Impact of reaction conditions on the laccase-catalyzedconversion of bisphenol. Bioresour. Technol. 2006, 97, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Mehrpour, O.; Karrari, P.; Zamani, N.; Tsatsakis, A.M.; Abdollahi, M. Occupational exposure to pesticides and consequences on male semen and fertility: A review. Toxicol. Lett. 2014, 230, 146–156. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide pesticide usage and its impacts on ecosystem. Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Verma, J.P.; Jaiswal, D.K.; Sagar, R. Pesticide relevance and their microbial degradation: A-state-of-art. Rev. Environ. Sci. Biotechnol. 2014, 13, 429–466. [Google Scholar] [CrossRef]
- Odukkathil, G.; Vasudevan, N. Enhanced biodegradation of endosulfan and its major metabolite endosulfate by a biosurfactant producing bacterium. J. Environ. Sci. Health Part B 2013, 48, 462–469. [Google Scholar] [CrossRef]
- Bajaj, A.; Pathak, A.; Mudiam, M.R.; Mayilraj, S.; Manickam, N. Isolation and characterization of a Pseudomonas sp. strain IITR01 capable of degrading α-endosulfan and endosulfan sulfate. J. Appl. Microbiol. 2010, 109, 2135–2143. [Google Scholar] [CrossRef]
- Armenova, N.; Tsigoriyna, L.; Arsov, A.; Petrov, K.; Petrova, P. Microbial detoxification of residual pesticides in fermented foods: Current status and prospects. Foods 2023, 12, 1163. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Ghosh, P.; Malyan, S.K.; Sharma, J.; Kumar, V. A comprehensive review on enzymatic degradation of the organophosphate pesticide malathion in the environment. J. Environ. Sci. Health 2019, 37, 288–329. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.U.; Parveen, S. Pesticides pollution and risk assessment of river ganga: A review. Heliyon 2021, 7, e07726. [Google Scholar] [CrossRef] [PubMed]
- Pigłowski, M. Notifications on Pesticide Residues in the Rapid Alert System for Food and Feed (RASFF). Int. J. Environ. Res. Public Health 2022, 19, 8525. [Google Scholar] [CrossRef]
- Adeyeye, A.; Osibanjo, O. Residues of organochlorine pesticides in fruits, vegetables and tubers from Nigerian markets. Sci. Total Environ. 1999, 231, 227–233. [Google Scholar] [CrossRef]
- Ibotom, M.O.; Mohammade, F. Determination of Pesticides Residues in Fruits and Vegetables in Kaduna Metropolis, Nigeria. Int. J. Environ. Sci. Toxicol. Res. 2016, 4, 185–189. [Google Scholar]
- Omwenga, I.; Kanja, L.; Zomer, P.; Louisse, J.; Rietjens, I.M.C.M.; Mol, H. Organophosphate and carbamate pesticide residues and accompanying risks in commonly consumed vegetables in Kenya. Food Addit. Contam. Part B Surv. 2021, 14, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial Degradation of Pesticide Residues and an Emphasis on the Degradation of Cypermethrin and 3-phenoxy Benzoic Acid: A Review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef]
- Abou-Arab, A. Effect of Ras cheese manufacturing on the stability of DDT and its metabolites. Food Chem. 1997, 59, 115–119. [Google Scholar] [CrossRef]
- Navarro, S.; Pérez, G.; Navarro, G.; Vela, N. Decline of pesticide residues from barley to malt. Food Addit. Contam. 2007, 24, 851–859. [Google Scholar] [CrossRef]
- Rajashekar, K.; Kondal, K.; Narasimha, K.; Sudhakar, K. Effect of processing of milk into products on the residue levels of certain pesticides. J. Food. Sci. Technol. 2007, 44, 551–552. [Google Scholar]
- Jung, K.; Park, Y.; Kim, H.; Kang, M.; Yang, Y.; Kang, A.; Chun, K.; Park, Y. Removal effects of Bifenthrin and Metalaxyl pesticides during preparation and fermentation of Baechu Kimchi. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1258–1264. [Google Scholar] [CrossRef]
- Bo, L.-Y.; Zhang, Y.-H.; Zhao, X.-H. Degradation kinetics of seven organophosphorus pesticides in milk during yoghurt processing. J. Serb. Chem. Soc. 2011, 76, 353–362. [Google Scholar] [CrossRef]
- Cuš, F.; Cesnik, B.; Bolta, V.; Gregorcic, A. Pesticide residues in grapes and during vinification process. Food Control 2010, 21, 1512–1518. [Google Scholar] [CrossRef]
- Ksiazek-Trela, P.; Szpyrka, E. The effect of natural and biological pesticides. Plant Prot. Sci. 2022, 58, 273–291. [Google Scholar] [CrossRef]
- Nandhini, A.; Harshiny, M.; Gummadi, S. Chlorpyrifos in environment and foods: A critical review of detection methods and degradation pathways. Environ. Sci. Process. Impacts 2021, 23, 1255–1277. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Zaborowska, M.; Kucharski, J. The Impact of Permethrin and Cypermethrin on Plants, Soil Enzyme Activity, and Microbial Communities. Int. J. Mol. Sci. 2023, 24, 2892. [Google Scholar]
- Hirozawa, M.; Ono, M.; Suguiura, I.; Bordini, J.; Ono, E. Lactic acid bacteria and Bacillus spp. as fungal biological control agents. J. Appl. Microbiol. 2023, 34, lxac083. [Google Scholar] [CrossRef]
- Jaffar, N.; Jawan, R.; Chong, K. The potential of lactic acid bacteria in mediating the control of plant diseases and plant growth stimulation in crop production—A mini review. Front. Plant. Sci. 2023, 13, 1047945. [Google Scholar] [CrossRef]
- Chu, C.; Yu, L.; Li, Y.; Guo, H.; Zhai, Q.; Chen, W.; Tian, F. Lactobacillus plantarum CCFM405 against Rotenone-Induced Parkinson’s Disease Mice via Regulating Gut Microbiota and Branched-Chain Amino Acids Biosynthesis. Nutrients 2023, 15, 1737. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Cheng, S.; Chang, M.; Lin, Y.; Wu, C.; Tsai, Y. Neuroprotective Effects of Lactobacillus plantarum PS128 in a Mouse Model of Parkinson’s Disease: The Role of Gut Microbiota and MicroRNAs. Int. J. Mol. Sci. 2023, 24, 6794. [Google Scholar] [CrossRef] [PubMed]
- Trinder, M.; Bisanz, J.; Burton, J.; Reid, G. Probiotic lactobacilli: A potential prophylactic treatment for reducing pesticide absorption in humans and wildlife. Benef. Microb. 2015, 6, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Leska, A.; Nowak, A.; Miśkiewicz, K.; Rosicka-Kaczmarek, J. Binding and Detoxification of Insecticides by Potentially Probiotic Lactic Acid Bacteria Isolated from Honeybee (Apis mellifera L.) Environment-An In Vitro Study. Cells 2022, 11, 3743. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Li, F.; Bai, J.; Su, R. Current approaches on the roles of lactic acid bacteria in crop silage. Microb. Biotechnol. 2023, 16, 67–87. [Google Scholar] [CrossRef]
- Haque, M.A.; Hong, S.Y.; Hwang, C.E.; Kim, S.C.; Cho, K.M. Cloning of an organophosphorus hydrolase (opdD) gene of Lactobacillus sakei WCP904 isolated from chlorpyrifos impregnated kimchi and hydrolysis activities of its gene product for organophosphorus pesticides. Appl. Biol. Chem. 2018, 61, 643–651. [Google Scholar] [CrossRef]
- Zeghal, E.; Vaksmaa, A.; Vielfaure, H.; Boekhout, T.; Niemann, H. The potential role of marine fungi in plastic degradation–A review. Front. Mar. Sci. 2021, 8, 738877. [Google Scholar] [CrossRef]
- Stief, P.; Fuchs-Ocklenburg, S.; Kamp, A.; Manohar, C.-S.; Houbraken, J.; Boekhout, T.; de Beer, D.; Stoeck, T. Dissimilatory nitrate reduction by Aspergillus terreus isolated from the seasonal oxygen minimum zone in the Arabian Sea. BMC Microbiol. 2014, 14, 35. [Google Scholar] [CrossRef]
- Goodell, B.; Winandy, J.E.; Morrell, J.J. Fungal degradation of wood: Emerging data, new insights and changing perceptions. Coatings 2020, 10, 1210. [Google Scholar] [CrossRef]
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H.J. Diverse metabolic capacities of fungi for bioremediation. Indian J. Microbiol. 2016, 56, 247–264. [Google Scholar] [CrossRef]
- Maqbool, Z.; Hussain, S.; Imran, M.; Mahmood, F.; Shahzad, T.; Ahmed, Z.; Azeem, F.; Muzammil, S. Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: A critical review. Environ. Sci. Pollut. Res. Int. 2016, 23, 16904–16925. [Google Scholar] [CrossRef] [PubMed]
- Bokade, P.; Purohit, H.J.; Bajaj, A. Myco-remediation of Chlorinated Pesticides: Insights into Fungal Metabolic System. Indian J. Microbiol. 2021, 61, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Nyakundi, W.; Magoma, G.; Ochora, J.; Nyende, A. Biodegradation of diazinon and methomyl pesticides by white rot fungi from selected horticultural farms in rift valley and central Kenya. J. Appl. Technol. Environ. Sanit. 2011, 1, 107–124. [Google Scholar]
- Ellegaard-Jensen, L.; Knudsen, B.E.; Johansen, A.; Albers, C.N.; Aamand, J.; Rosendahl, S. Fungal-bacterial consortia increase diuron degradation in water-unsaturated systems. Sci. Total Environ. 2014, 1, 466–705. [Google Scholar] [CrossRef]
- Knudsen, B.E.; Ellegaard-Jensen, L.; Albers, C.N.; Rosendahl, S.; Aamand, J. Fungal hyphae stimulate bacterial degradation of 2,6-dichlorobenzamide (BAM). Environ. Pollut. 2013, 181, 122–127. [Google Scholar] [CrossRef]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A comprehensive insight into fungal enzymes: Structure, classification, and their role in mankind’s challenges. J. Fungi 2021, 8, 23. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Hofrichter, M. Review: Lignin conversion by manganese peroxidase (MnP). Enzyme Microb. Technol. 2022, 30, 454–466. [Google Scholar] [CrossRef]
- Magan, N.; Fragoeiro, S.; Bastos, C. Environmental factors and bioremediation of xenobiotics using white rot fungi. Mycobiology 2010, 38, 238–248. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Peris, A.; Torán, J.; Eljarrat, E.; Sarrà, M.; Blánquez, P.; Caminal, G. Exploring the degradation capability of trametes versicolor on selected hydrophobic pesticides through setting sights simultaneously on culture broth and biological matrix. Chemosphere 2020, 250, 126293. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Moreira, J.; Brugnari, T.; Sá-Nakanishi, A.; Castoldi, R.; Souza, C.; Bracht, A.; Peralta, R.M. Evaluation of diuron tolerance and biotransformation by the white-rot fungus Ganoderma lucidum. Fungal Biol. 2017, 122, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Wolfand, J.M.; Lefevre, G.H.; Luthy, R.G. Metabolization and degradation kinetics of the urban-use pesticide fipronil by white rot fungus Trametes versicolor. Environ. Sci. Process. Impacts 2016, 18, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Barbieri, M.V.; López-García, E.; Postigo, C.; López De Alda, M.; Caminal, G.; Sarrà, M. Fungal degradation of selected medium to highly polar pesticides by Trametes versicolor: Kinetics, biodegradation pathways, and ecotoxicity of treated waters. Anal. Bioanal. Chem. 2022, 414, 439–449. [Google Scholar] [CrossRef]
- Doddapaneni, H.; Chakraborty, R.; Yadav, J.S. Genome-wide structural and evolutionary analysis of the P450 monooxygenase genes (P450ome) in the white rot fungus Phanerochaete chrysosporium: Evidence for gene duplications and extensive gene clustering. BMC Genom. 2005, 6, 92. [Google Scholar] [CrossRef]
- Mori, T.; Ohno, H.; Ichinose, H.; Kawagishi, H.; Hirai, H. White-rot fungus Phanerochaete chrysosporium metabolizes chloropyridinyl-type neonicotinoid insecticides by an n-dealkylation reaction catalyzed by two cytochrome P450s. J. Hazard. Mater. 2021, 402, 123831. [Google Scholar] [CrossRef]
- Kullman, S.W.; Matsumura, F. Metabolic pathways utilized by Phanerochaete chrysosporium for degradation of the cyclodiene pesticide endosulfan. Appl. Environ. Microbiol. 1996, 62, 593–600. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Losito, I.; Facchini, L.; Katina, K.; Palmisano, F.; Gobbetti, M.; Coda, R. Degradation of vicine, convicine and their aglycones during fermentation of faba bean flour. Sci. Rep. 2016, 6, 32452. [Google Scholar] [CrossRef]
- Đorđević, T.; Đurović-Pejčev, R. The potency of Saccharomyces cerevisiae and Lactobacillus plantarum to dissipate organophosphorus pesticides in wheat during fermentation. J. Food Sci. Technol. 2016, 53, 4205–4215. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, B.; Yang, Z.; Kai, L.; Liu, Z. Alternative splicing and expression reduction of p450 genes mediating the oxidation of chlorpyrifos revealed a novel resistance mechanism in Nilaparvata lugens. J. Agric. Food Chem. 2023, 71, 4036–4042. [Google Scholar] [CrossRef]
- Daisley, B.; Trinder, M.; McDowell, T.; Collins, S.; Sumarah, M.; Reid, G. Microbiota-Mediated Modulation of Organophosphate Insecticide Toxicity by Species-Dependent Interactions with Lactobacilli in a Drosophila melanogaster Insect Model. Appl. Environ. Microbiol. 2018, 84, e02820-17. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, G.; Chuenchomrat, P.; Pereyron, V.; Haure, M.; Lorn, D.; Quan, L.-H.; Ho, P.-H.; Nguyen, T.-T.; Do, T.-Y.; Phi, Q.-T.; et al. Microorganisms, the Ultimate Tool for Clean Label Foods? Inventions 2021, 6, 31. [Google Scholar] [CrossRef]
- Laulund, S.; Wind, A.; Derkx, P.M.F.; Zuliani, V. Regulatory and safety requirements for food cultures. Microorganisms 2017, 5, 28. [Google Scholar] [CrossRef]
- Lechardeur, D.; Cesselin, B.; Fernandez, A.; Lamberet, G.; Garrigues, C.; Pedersen, M.; Gaudu, P.; Gruss, A. Using heme as an energy boost for lactic acid bacteria. Curr. Opin. Biotechnol. 2011, 22, 143–149. [Google Scholar] [CrossRef]
- Ly, M.H.; Vo, N.H.; Le, T.M.; Belin, J.-M.; Waché, Y. Diversity of the surface properties of Lactococci and consequences on adhesion to food components. Colloids Surf. B 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Adunphatcharaphon, S.; Petchkongkaew, A.; Visessanguan, W. In Vitro Mechanism Assessment of Zearalenone Removal by Plant-Derived Lactobacillus plantarum BCC 47723. Toxins 2021, 13, 286. [Google Scholar] [CrossRef]
- Trinder, M.; McDowell, T.; Daisley, B.; Ali, S.; Leong, H.; Sumarah, M.; Reid, G. Probiotic Lactobacillus rhamnosus reduces organophosphate pesticide absorption and toxicity to Drosophila melanogaster. Appl. Environ. Microbiol. 2016, 82, 6204–6213. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Szwajgier, D.; Waśko, A. Biosorption of Al+3 and Cd+2 by an exopolysaccharide from Lactobacillus rhamnosus. J. Food Sci. 2014, 79, T2404–T2408. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, A.; Bertilsson, S.; Goedkoop, W.E. Effects of extracellular polymeric and humic substances on chlorpyrifos bioavailability to Chironomus riparius. Ecotoxicology 2010, 19, 614–622. [Google Scholar] [CrossRef]
- López-Cabeza, R.; Francioso, A. Chiral pesticides with asymmetric sulfur: Extraction, separation, and determination in different environmental matrices. Separations 2022, 9, 29. [Google Scholar] [CrossRef]
- Boopathy, R. Factors limiting bioremediation technologies. Bioresour. Technol. 2000, 74, 63–67. [Google Scholar] [CrossRef]
- Xu, F.; Shin, W.; Brown, S.H.; Wahleithner, J.A.; Sundaram, U.M.; Solomon, E.I. A study of a series of recombinant fungal laccases and bilirubin oxidase thatexhibit significant differences in redox potential substrate specificity, and stability. Biochim. Biophys. Acta 1996, 1292, 303–311. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Qin, X.; Xia, L. Degradation of the herbicide isoproturon by laccase mediator systems. Biochem. Eng. J. 2017, 119, 92–100. [Google Scholar] [CrossRef]
- Kupski, L.; Salcedo, G.M.; Caldas, S.S.; de Souza, T.D.; Furlong, E.B.; Primel, E.G. Optimization of a laccase-mediator system with natural redox-mediating compounds for pesticide removal. Environ. Sci. Pollut. Res. 2019, 26, 5131–5139. [Google Scholar] [CrossRef]
- Vidal-Limon, A.; García Suárez, P.C.; Arellano-García, E.; Contreras, O.E.; Aguila, S.A. Enhanced degradation of pesticide dichlorophen by laccase immobilized on nanoporous materials: A cytotoxic and molecular simulation investigation. Bioconjug. Chem. 2018, 29, 1073–1080. [Google Scholar] [CrossRef]
- Das, A.; Singh, J.; Yogalakshmi, K.N. Laccase immobilized magnetic iron nanoparticles: Fabrication and its performance evaluation in chlorpyrifos degradation. Int. Biodeter. Biodegr. 2017, 117, 183–189. [Google Scholar] [CrossRef]
- Du, Y.; Jia, X.; Zhong, L.; Jiao, Y.; Zhang, Z.; Wang, Z.; Feng, Y.; Bila, M.; Cui, J.; Jia, S. Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@MOF composites. Coord. Chem. Rev. 2022, 454, 214327. [Google Scholar] [CrossRef]
- Ladole, M.R.; Pokale, P.B.; Patil, S.S.; Belokar, P.G.; Pandit, A.B. Laccase immobilized peroxidase mimicking magnetic metal organic frameworks for industrial dye degradation. Bioresour Technol. 2020, 317, 124035. [Google Scholar] [CrossRef]
- Shen, X.; Du, Y.; Du, Z.; Tang, X.; Li, P.; Cheng, J.; Yan, R.; Cui, J. Construction of enzyme@glutathione hybrid metal-organicframeworks: Glutathione-boosted microenvironment fine-tuning of biomimetic immobilization for improving catalytic performance. Mater. Today Chem. 2023, 27, 101326. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, P.; Joshi, A.; Kodgire, P. Advances in detection of hazardous organophosphorus compounds using organophosphorus hydrolase based biosensors. Crit. Rev. Toxicol. 2019, 49, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Vyas, T.; Singh, V.; Kodgire, P.; Joshi, A. Insights in detection and analysis of organophosphates using organophosphorus acid anhydrolases (OPAA) enzyme-based biosensors. Crit. Rev. Biotechnol. 2023, 43, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, P.; Wang, Z.; Du, Y.; Kuang, G.; Feng, Y.; Jia, S.; Cui, J. Glutamate oxidase-integrated biomimetic metal–organic framework hybrids as cascade nanozymes for ultrasensitive glutamate detection. J. Agric. Food Chem. 2022, 70, 3785–3794. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Ganai, B. Deciphering the recent trends in pesticide bioremediation using genome editing and multi-omics approaches: A review. World J. Microbiol. Biotechnol. 2023, 39, 151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).