Abstract
Herein, new substituted ligands based on pyrazole (L1–L4) were synthesized via a one-step by condensing (1H-pyrazole-1-yl) methanol with different primary amine compounds. The present work utilized the catalytic properties of the in situ complexes formed by these ligands with various copper (II) salts viz. Cu(CH3COO)2, CuSO4, CuCl2, and Cu(NO3)2 for the oxidation of catechol to o-quinone. The studies showed that the catalytic activities depend on the nature and concentration of the ligand, the nature of the counterion, and the solvent. It was observed that the complex formed by L2 and Cu(CH3COO)2 exhibited good catalytic activity in methanol with Vmax of 41.67 µmol L−1 min−1 and Km of 0.02 mol L−1.
1. Introduction
Pyrazole-based compounds have gathered tremendous advancement in the last decade [1,2,3,4,5,6,7,8,9,10] toward numerous versatile applications in the field of bioinorganic and medical sciences. These compounds have been utilized as precursors in the synthesis of many compounds, including artificial metalloenzymes with important biological activities [11,12,13]. The pyrazole-based ligands have been employed in a wide range of applications, such as electronics [14], catalysis [15,16,17,18,19,20,21,22,23,24,25,26,27,28], and pharmacology [29]; they also exhibit anticorrosion [30], anticancer, antifungal, antiviral, and antibacterial behavior [31,32]. Additionally, they exhibit cytotoxic activities [33,34] and are used for the extraction of lithium and cesium cations [35,36].
Furthermore, the complexation chemistry of these ligands with transition metal ions shows great potential in catalysis, especially in mimicking enzyme activity [37]. One of the important metal ions is copper, which has the ability to combine with various organic ligands to catalyze diverse biological processes. In this regard, a variety of copper-based complexes are employed to study the oxidation reaction of catechol to quinone [19,32,35]. It is remarkable how these complexes can bind reversibly to oxygen under ambient conditions, thus, aiding the reaction [24,25,26,27,28]. Several studies have explored the catalytic activity of copper-based complexes, either in situ [28,32] or through isolated complexes [15,16,17,18,35]. Numerous studies have also investigated the catalytic activity of catechol oxidation using a variety of metal complexes, such as iron, cobalt, manganese-based complexes, etc. [22,23,24]. However, it has been recently reported that copper-based complexes exhibit excellent catalytic activity toward the oxidation reaction [35].
Thus, the present work aims to study the catalytic activity of in situ-prepared pyrazole-based ligands and copper complexes for the conversion of catechol to o-quinone in the presence of atmospheric oxygen (O2). Four different pyrazole-based ligands (L1–L4) were synthesized and characterized using 1H NMR, 13C NMR, elemental analysis (EA (%)), and Fourier transform infrared (FT-IR) spectroscopy (please see supplementary information, SI Figures S1–S15).
2. Experimental Section
2.1. Materials and Characterization Techniques
Catechol, Cu(NO3)2, Cu(CH3COO)2, CuSO4, CuCl2, and solvents, such as methanol, tetrahydrofuran, acetonitrile, and chloroform, were purchased from Aldrich and used as received without further purification.
All compounds were characterized using the 1H NMR, 13C NMR, and IR spectroscopy techniques. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded in CDCl3 at room temperature (Varian Unity-Plus (400 MHz) spectrometer for NMR, and the elemental analyses were retrieved with the help of 2400 Series II CHNS/O elemental analyzer). Chemical shifts (δ) were recorded in parts-per-million (ppm) using tetramethylsilane (TMS) as the internal standard. FT-IR spectra were recorded on a Schimadzu 8201 PC FT-IR spectrophotometer (νmax in cm−1) supported by pressed KBr pellets. The UV-vis absorbance was recorded on a JENWAY 7315-spectrophotometer, and the experiments concerning the oxidation of catechol to o-quinone were carried out at the Higher Institute of Nursing and Health Professions Techniques Laboratory, Oujda.
2.2. Synthesis and Characterization
2.2.1. Preparation of Compounds L1–L4
The four pyrazole-based ligands (L1–L4) were synthesized via a one-step process by the condensation of primary amines and pyrazole derivatives reported in our previous work [38]. Briefly, (1H-pyrazol-1-yl) methanol derivatives (1.1eq.) were added to the solution of one equivalent of a primary amine in 10 mL of acetonitrile at room temperature (RT). The reaction was carried out under continuous stirring for 6 h at 60 °C. As the reaction progressed, the solid product separated from the clear and homogenous mixture of amine and (pyrazolyl-1-yl) methanol derivatives in acetonitrile. Thereafter, the unreacted starting material and solvent were removed from the mixture by the filtration process. Subsequently, the isolated product was washed with acetonitrile and ethyl acetate and dried at reduced pressure to remove all the volatile organic compounds. The synthesized pathway is depicted in Scheme 1.
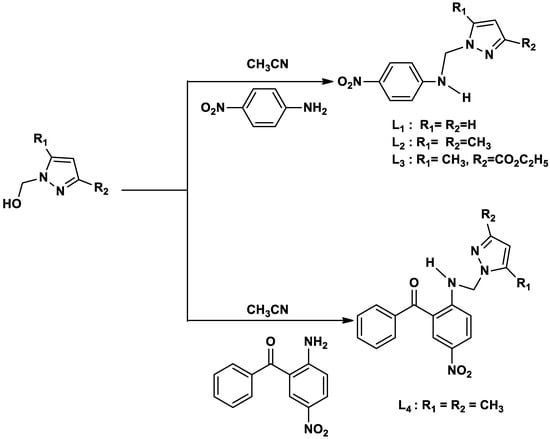
Scheme 1.
Synthesis pathway for preparing ligands L1–L4.
2.2.2. Characterization
N-((1H-pyrazol-1-yl)methyl)-4-nitroaniline L1: This compound was obtained as a yellow solid, yield 85%, MP: 118–120 °C, FT-IR: (KBr, ν(cm−1): 3256, 3165, 3114, 3023, 2985, 1722, 1605, 1545, 1384, 1214, 1106, 1020, 766; 1H NMR (400 MHz, CDCl3): δH = 5.58 (d, 2H, CH2), 6.26 (dd, 1H, CHPy), 6.94 (dd, 1H, CHAr), 7.48 (dd, 1H, CHPy), 7.87 (dd, 1H, CHPy), 8.03 (dd, H, CHAr, 8.24 (t, H, NH);13C NMR (100 MHz, CDCl3): δC = 58.3 (CH2), 106.5 (CHPy), 113.8 (CHAr), 126.1 (CHAr), 130.4 (CHPy), 139.7 (CHPy), 138.1 (CAr), 153.2 (CAr). EA(%): Calcd: (55.04)C; (4.62)H; (25.68)N; Obsd: (55.10)C; (4.57)H; (25.63)N.
N-((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-4-nitroaniline L2: This compound was obtained as a yellow solid, yield: 90%, MP: 128–130 °C, FT-IR: (KBr, ν(cm−1): 3224, 3316, 3230, 3135, 2993, 2920, 1591, 1580, 1487, 1340, 1246, 1230, 1087, 1048, 750; 1H NMR (400 MHz, CDCl3): δH = 2.07 (s, 3H, CH3), 2.29 (s, 3H, CH3), 5.41 (d, 2H, CH2), 5.81 (s, 1H, CHPy), 6.94 (dd, 1H, CHAr), 8.03 (dd, H, CHAr), 8.12 (t, H, NH); 13C NMR (100 MHz, CDCl3): δC = 11.2 (CH3), 14.5 (CH3), 56.7 (CH2), 106.1 (CHPy), 112.6 (CHAr), 126.4 (CHAr), 138.5 (CPy), 139.1 (CAr), 146.2 (CPy), 153.3 (CAr). EA(%): Calcd: (58.53)C; (5.73)H; (22.75)N. Obsd: (58.62)C; (5.71)H; (22.65)N.
Ethyl 5-methyl-1-(((4-nitrophenyl)amino)methyl)-1H-pyrazole-3-carboxylate L3: This compound was obtained as a yellow solid, yield: 90%, MP: 125–127 °C, FT-IR: (KBr, ν(cm−1): 3379, 3280, 3129, 2983, 2952, 2836, 1635, 1553, 1456, 1424, 1385, 1310, 1226, 1068, 1040, 805, 765, 702; 1H NMR (400 MHz, CDCl3): δH = 1.26 (t, 3H, CH3), 2.39 (s, 3H, CH3), 4.24 (q, 2H, CH2), 5.64 (d, 2H, CH2), 6.53 (s, 1H, CHPy), 6.95 (dd, H, CHAr), 8.04 (dd, H, CHAr), 8.27 (t, H, NH); 13C NMR (100 MHz, CDCl3): δC = 11.7 (CH3), 15.1(CH3), 58.4(CH2), 60.0 (CH2), 109.4 (CHPy), 112.7 (CHAr), 126.8 (CHAr), 138.5 (CPy), 141.2(CAr), 142.3(CPy), 153.5 (CAr), 162.6 (CO). EA(%): Calcd: (55.26)C; (5.30)H; (18.41N). Obsd: (56.01)C; (5.21)H; (18.56)N.
(2-(((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amino)-nitrophenyl)(phenyl)methanone L4: This compound was obtained as a yellow solid, yield: 84%, MP: 94–96 °C, FT-IR: (KBr, ν(cm−1): 3462, 3338, 3264, 3127, 2955, 2847, 1642, 1603, 1479, 1278, 1085, 1055, 766; 1H NMR (400 MHz, CDCl3): δH = 2.08 (s,3H,CH3), 2.23 (s, 3H, CH3), 5.22 (d, 2H, CH2), 5.81 (s, 1H, CHPy), 7.31–8.32 (m, 8H, CHAr), 8.11 (t,1H, NH); 13C NMR (100 MHz, CDCl3): δC = 11.3 (CH3), 14.1 (CH3), 71.5(CH2), 106.2 (CHPy), 115.5 (CHAr), 117.5 (CHAr), 129.3(CHPy), 129.2 (CHAr), 129.1 (CHAr), 129.7 (CHAr), 130.3(CHAr), 130.5(CHAr), 132.3(CHPy), 132.1 (CHAr), 135.8 (C), 139.1 (C), 139.4 (C), 157.6(C), 197.7 (CO). EA(%): Calcd: (62.75)C; (7.12)H; (30.13)N. Found: (62.81)C; (7.08)H; (30.21)N.
3. Study of Conversion Reaction of Catechol to o-Quinone
The in situ complexes were formed by mixing the organic pyrazole-based ligands with the different copper (II) salts, which were utilized for the oxidation reaction by adding to the catechol solution under ambient conditions. The oxidation of catechol to o-quinone was carried out following the reaction shown in Scheme 2. The oxidation activity was determined directly by recording the absorbance of the o-quinones at 390 nm (maximum absorption of o-quinone) using UV-Visible absorbance spectroscopy. Firstly, the catechol oxidation reactions were performed without the in situ catalysts in order to validate their effect on the catechol to o-quinone conversion reaction under the experimental conditions. It was observed that the absorbance vs. time was almost unchanged, suggesting the role of the in situ catalysts in the oxidation reaction rates.
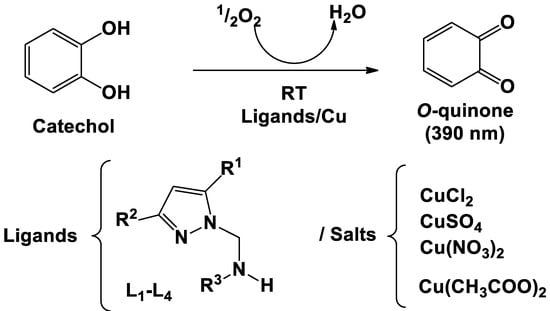
Scheme 2.
General reaction for the oxidation of catechol to o-quinone.
3.1. Effect of Concentration
3.1.1. Catalytic Studies Using Ligand/Metal Ratio as 1L/1M in MeOH
The experiments were conducted at room temperature (RT) in methanol (99.99%, MeOH). The complexes were synthesized in situ by successively mixing 0.15 mL of copper (II) salt solution (2 × 10−3 mol L−1; CuCl2, Cu(CH3COO)2, CuSO4, and Cu(NO3)2) with 0.15 mL of the ligand solution (2 × 10−3 mol L−1). This step was followed by the addition of 2 mL of a catechol solution (0.1 mol L−1) to the mixture. Finally, the oxidation reaction kinetics was studied by observing the changes in the absorbance of o-quinone at 390 nm as a function of time. The results obtained using the ligands (L1–L4) and various copper (II) salts are presented in Figures S16–S19 (see SI).
It is important to study the changes in the absorbance of o-quinone when various combinations of the ligands (L1–L4) with the copper (II) salts (CuCl2, Cu(CH3COO)2, CuSO4, and Cu(NO3)2) were used as the catalysts. It was observed that the changes in the absorbance during the oxidation of catechol in the presence of the complex formed by the ligand L1 and CuSO4 were greater than those with other copper (II) salts. In the case of L2 as the ligand with CuCl2, very low absorbance was recorded for the oxidation reaction. On the other hand, the absorbance of the o-quinone exceeded 1.5 times for all the complexes formed between ligand L3 and four copper (II) salts, Cu(NO3)2, Cu(CH3CO2)2, CuSO4, and CuCl2, respectively. While in the case of the complexes formed between ligand L4 and all four copper (II) salts, the absorbance exceeded 0.8 times (Figure S16). It should be mentioned here that the oxidation rates for the transformation in the presence of in situ complexes formed by the combination of ligands(L1–L4) and copper (II) salts in MeOH in the ratio (1L/1M) are tabulated in Table 1 and calculated by the following expression
where A is the absorbance of o-quinone measured by UV-vis spectrophotometer at 390 nm, ε is the linear molar extinction coefficient expressed in L/mol/cm, L is the optical distance of the cuvette, generally equal to 1 cm, and t is time in min.

Table 1.
The reaction rate of catechol oxidation in methanol (µmol L−1 min−1) (1L/1M).
The results presented in Table 1 revealed that all the copper complexes formed in situ with the ligands L1–L4 showed catalytic activity toward the oxidation reaction of catechol to o-quinone. However, the oxidation rates differ drastically from 0.1458 µmol L−1 min−1 to 14.115 µmol L−1 min−1 for the complexes formed between ligand L1 and CuCl2 (weak catalyst) and L2 and CuSO4 (strong catalyst), respectively.
3.1.2. Catalytic Studies Using Ligand/Metal Ratio as (2L/1M) in Methanol
These complexes were synthesized in situ by successively mixing one equivalent (0.15 mL, 10−3 mol L−1) of copper (II) salt solution (CuCl2, Cu(CH3COO)2, CuSO4, and Cu(NO3)2) with two equivalents of a ligand solution (0.15 mL, 2 × 10−3 mol L−1). The resultant mixture was added with 2 mL of catechol solution (0.1 mol L−1). The evolution of absorbance of o-quinone at 390 nm with respect to time is shown in Figure 1 and Figure 2, while the oxidation rates are summarized in Table 2.
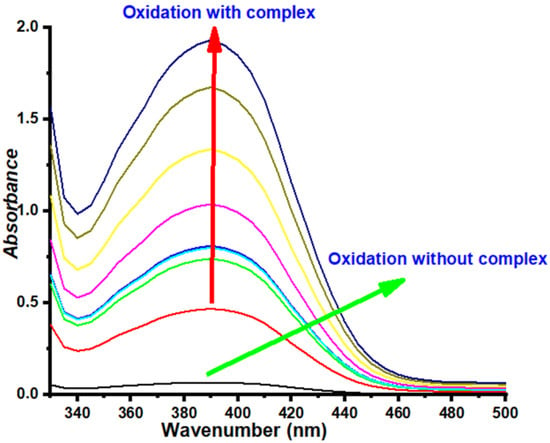
Figure 1.
Absorbance spectrum of o-quinone as a function of time for the combination L2/Cu(CH3COO)2.
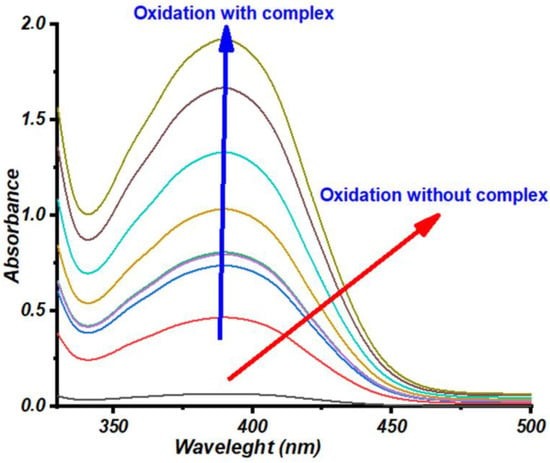
Figure 2.
Absorbance spectrum of o-quinone as a function of time for the combination 2L2/Cu(NO3)2.

Table 2.
The rate of oxidation of catechol in methanol (μmol·L−1·min−1) (2L/1M).
As shown in Figure 2, the absorbance of o-quinone is more significant when the catechol oxidation reaction was catalyzed by the complexes formed by the ligand L1 and Cu(NO3)2 and CuSO4, while the other two copper (II) salts led to precipitation, thereby gave the unstable absorbance values. As observed from Figure 3, the absorbance evolution of o-quinone was significant when the copper complexes formed by the ligand L2 and Cu(NO3)2, Cu(CH3COO)2, and CuSO4 were used as the catalyst. In contrast, the absorbance remained constant when CuCl2 was used as the metal salt for the complex formation with ligand L2. Furthermore, the absorbance value of o-quinone for the complex formed by ligand L3 and Cu(NO3)2 and Cu(CH3COO)2 was significant, as shown in Figure S21. In contrast, unstable absorbance values were observed in the case of the other two copper salts (CuSO4 and CuCl2) due to the precipitation in the reaction. Additionally, the absorbance value for the complex formed by the ligand L4 with CuSO4 was greater than those for Cu(CH3CO2)2 and Cu(NO3)2, as shown in Figure S20 (see SI). It was noted that the presence of CuCl2 led to precipitation in the reaction system, which gave unstable absorbance values. Thus, according to the results summarized in Table 2, it was noted that all copper complexes formed in situ with the ligands L1–L4 showed catalytic activity toward the oxidation reaction of catechol to o-quinone. However, the oxidation rates differ from 0.0937 µmol L−1 min−1 to 32.2917 µmol L−1 min−1 for the complexes formed by the ligand L4 and CuSO4 (weak catalyst), and L2 and Cu(CH3CO2)2 (strong catalyst), respectively.
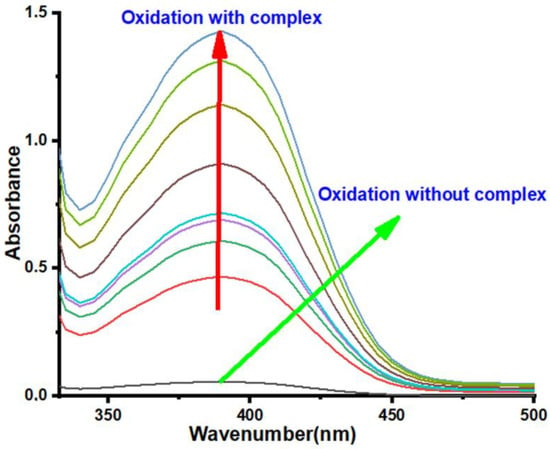
Figure 3.
Absorbance spectrum of o-quinone as a function of time for the 2L2/CuSO4 combination.
A comparison of the results presented in Table 1 and Table 2 revealed the difference in the oxidation rates of catechol to o-quinone. This difference may be related to the effect of ligand and its concentration which can be explained by the nature of the coordination environment due to the electronic effect of the group. The geometry imposed by the ligand on the metal ion and the characteristics of the steric effect of the ligand may have impacted the coordination and, thus, the activity. Therefore, the effect of the counter anion on the catalytic activity was noted, and the best results were obtained with CH3COO− as the anion in the reaction mixture.
3.2. Solvent Effect
To study the effect of the solvent on the rate and efficiency of the catechol oxidation reaction, the experiments were performed under the same reaction conditions with ligand L2, using methanol (MeOH), tetrahydrofuran (THF), acetonitrile (CH3CN), and chloroform (CHCl3) as the solvents. The oxidation reaction was carried out using the complexes formed in situ by the combinations of two equivalents of ligand L2 and one equivalent of various copper (II) salts solution in four different solvents. The results summarized in Table 3 showed that a significant change in the absorbance of o-quinone was observed when MeOH was used as a solvent to conduct the oxidation reactions using the complexes formed between 2L2 and Cu(NO3)2 and CuSO4. However, the maximum change in the absorbance was recorded when 2L2 was complexed with Cu(CH3COO)2 in MeOH with the oxidation rate of 32.2917 µmol L−1 min−1. In contrast, the absorbance showed lower values when THF, CH3CN, and CHCl3 were used as the solvents, as shown in Figures S22–S25 (see SI).

Table 3.
Catechol oxidation rates for combinations (2L/1M) in different solvents.
The above-mentioned results indicated the notable effect of solvent on the catalytic activity of the in situ catalysts. Methanol, a polar protic solvent, was considered the best solvent among all, as better catalytic activity was shown by the complexes in this solvent. Moreover, it showed improved results when compared to THF, which is a polar aprotic solvent. Assuming that the general physical parameters of the solvents, including dielectric constant, dipole moment, and polarity, have no significant effect on the activity of the complexes in oxidizing catechol, as a consequence, it can be hypothesized that the coordination power or the protic nature of the solvents are the most important factors that can alter the catalytic activity of the system [39,40,41]. It has been widely reported that polar protic solvents can strongly solvate X anions (X = Cl−, CH3COO−, SO42−, and NO3−) by forming hydrogen bonding with them. Hence, the hydrogen bonding keeps the anions isolated, resulting in the least reactivity in this solvent system, whereas the counterion also solvated the cationic copper ions. However, due to the absence of hydrogen bonding in the case of cations, the solvation is comparatively less strong, leaving the cations to be more reactive. This reactivity, in turn, facilitated the formation of the complexes between the ligands and cations, which overall increased the rate of oxidation of catechol to o-quinone. To validate the effect of solvent on the catalytic activity, the ligand combination (2L/1M) was tested using 2L2/Cu(CH3COO)2, 2L2/Cu(NO3)2, and 2L2/CuSO4 in MeOH. The kinetic experiments were performed at room temperature, and the absorbance evolution of o-quinone was recorded every 5 min, as shown in Figure 1, Figure 2 and Figure 3. The appearance of an intense band at 390 nm for the three combinations, 2L2/Cu(CH3COO)2, 2L2Cu(NO3)2, and 2L2CuSO4 in MeOH confirmed that the catechol could be successfully oxidized to o-quinone with these combinations of catalysts.
3.3. Kinetic Study
The kinetics of the oxidation reaction was studied to get more information on the catalytic efficiency of the complexes. This study was conducted to determine the kinetics parameters, such as Vmax (maximum reaction rate) and Km (reaction constant), using the initial rate method. The experiments were conducted in MeOH using the ligand-metal combinations as 2L2/Cu(NO3)2, 2L2/Cu(CH3COO)2, and 2L2/CuSO4 under ambient conditions. This was treated with various concentrations of catechol substrate ranging from 4 × 10−2 mol L−1 to 4 × 10−1 mol L−1. The evolution of the absorbance of o-quinone at 390 nm was observed and recorded as a function of time, and then the relationship between initial rates, Vi (µmol L1 min−1), and the substrate (catechol) concentration was established (mol L−1).
As shown in Figures S26–S28 (see SI), a linear relationship between the initial velocities and substrate concentration was obtained; thus, the Michaelis–Menten model was applied to obtain the kinetic parameters in the reaction, summarized in Table 4. The results showed that the reactions rates, Vmax, varied from 41.67 µmol L−1 min−1 to 33.56 µmol L−1 min−1 to 32.86 µmol L−1 min−1 when the combination 2L2/Cu(CH3COO)2, 2L2/CuSO4, and 2L2/Cu(NO3)2 was used for the catalysis, respectively. Furthermore, a low value of Km was obtained for the combination 2L2/Cu(CH3COO)2, demonstrating that MeOH is the suitable solvent for this catalytic study. It should be mentioned here that the smaller value of Km results in the greater affinity of the catalyst toward the catechol substrate [42]. Thus, the best results were obtained in the case of 2L2/Cu(CH3COO)2, which achieved the highest catalytic efficiency.

Table 4.
Values of the Vmax and Km constants.
4. Conclusions
In summary, the catalytic activity of complexes formed by pyrazole-based ligands and different copper (II) salts were studied for the oxidation reaction of catechol. It was found that all combinations could catalyze the oxidation of catechol to o-quinone; however, their reaction rates varied under ambient conditions in the presence of atmospheric oxygen as the oxidant. It was also demonstrated that the reaction rates and catalytic efficiency of the particular combination were influenced by the nature and concentration of ligands with the combination (2L/1M) as an excellent catalyst. In addition, the nature of the solvent significantly affected the catalytic activity of the in situ complexes. Furthermore, the combinations of Cu(CH3COO)2 with the pyrazole-based ligands in methanol were found to be more effective in catalyzing the oxidation reaction, suggesting the significant role of the counterion on the activity. The kinetics of the oxidation reaction was also studied using the Michaelis–Menten model and demonstrated that the results were in agreement with this model. It was reported that the combinations 2L2/Cu(CH3COO)2, 2L2/CuSO4, and 2L2/Cu(NO3)2 in MeOH were considered the best catalysts for the oxidation reaction of catechol.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13010162/s1, Figure S1: 1H NMR spectrum of L1 in DMSO (400 MHz); Figure S2: 13C NMR spectrum of L1 in DMSO (400 MHz); Figure S3: FT-IR spectrum of L1; Figure S4: 1H NMR spectrum of L2 in DMSO (400 MHz); Figure S5: 13C NMR spectrum of L2 in DMSO (400 MHz); Figure S6: DEPT- 135 NMR spectrum of L2 in DMSO (400 MHz); Figure S7: FT-IR spectrum of L2; Figure S8: 1H NMR spectrum of L3 in DMSO (400 MHz); Figure S9: 13C NMR spectrum of L3 in DMSO (400 MHz); Figure S10: DEPT- 135 NMR spectrum of L3 in DMSO (400 MHz); Figure S11: FT-IR spectrum of L3; Figure S12: 1H NMR spectrum of L4 in DMSO (400 MHz); Figure S13: 13C NMR spectrum of L4 in DMSO (400 MHz); Figure S14: 13C NMR spectrum of L4 in DMSO (400 MHz); Figure S15: FT-IR spectrum of L4; Figure S16: Absorbance evolution of o-quinone in presence of complexes formed by L1 and different copper salts in MeOH; Figure S17: Absorbance evolution of o-quinone in presence of complexes formed by L2 and different copper salts in MeOH; Figure S18: Absorbance evolution of o-quinone in presence of complexes formed by L3 and different copper salts in MeOH; Figure S19: Absorbance evolution of o-quinone in presence of complexes formed by L4 and different copper salts in MeOH; Figure S20: Absorbance evolution of o-quinone in presence of complexes formed by L1 and L4 with different copper salts in MeOH; Figure S21: Absorbance evolution of o-quinone in presence of complexes formed by L2 and L3 with different copper salts in MeOH; Figure S22: Absorbance evolution of o-quinone in presence of complexes formed by 2L2/Cu(NO3)2 in different solvents; Figure S23: Absorbance evolution of o-quinone in presence of complexes formed by 2L2/Cu(CH3COO)2 in different solvents; Figure S24: Absorbance evolution of o-quinone in presence of complexes formed by 2L2/CuCuSO4 in different solvents; Figure S25: Absorbance evolution of o-quinone in presence of complexes formed by 2L2/CuCl2 in different solvents; Figure S26: Reaction dependence on the concentration of catechol using 2L2/Cu(NO3)2; Figure S27: Reaction dependence on the concentration of catechol using 2L2/Cu(CH3COO)2; Figure S28: Reaction dependence on the concentration of catechol using 2L2/CuSO4.
Author Contributions
Conceptualization, R.T. and B.H.; methodology, K.Z. and A.T. software, A.T.; validation, B.H. and K.Z.; formal analysis, E.B.Y. and M.E.K.; investigation, R.T.; data curation, A.T. and K.Z.; writing—original draft preparation, A.T., K.Z. and M.E.K.; writing—review and editing, A.Y.A.A. and A.M.; supervision, R.T., M.E.K. and E.B.Y.; project administration, A.Y.A.A. and A.M.; funding acquisition, supervision & validation; A.Y.A.A. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
King Khalid University, project number RGP.2/226/43.
Data Availability Statement
Please feel free to contact the authors.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Groups Project under grant number RGP.2/226/43.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bouroumane, N.; El Kodadi, M.; Touzani, R.; El Boutaybi, M.; Oussaid, A.; Hammouti, B.; Nandiyanto, A.B.D. New Pyrazole-Based Ligands: Synthesis, Characterization, and Catalytic Activity of Their Copper Complexes. Arab. J. Sci. Eng. 2022, 47, 269–279. [Google Scholar] [CrossRef]
- Titi, A.; Almutairi, S.M.; Touzani, R.; Messali, M.; Tillard, M.; Hammouti, B.; El Kodadi, M.; Eddike, D.; Zarrouk, A.; Warad, I. A new mixed pyrazole-diamine/Ni(II) complex, Crystal structure, physicochemical, thermal and antibacterial investigation. J. Mol. Struct. 2021, 1236, 130304. [Google Scholar] [CrossRef]
- Kaddouri, Y.; Abrigach, F.; Ouahhoud, S.; Benabbes, R.; El Kodadi, M.; Alsalme, A.; Al-Zaqri, N.; Warad, I.; Touzani, R. Synthesis, characterization, reaction mechanism prediction and biological study of mono, bis and tetrakis pyrazole derivatives against Fusarium oxysporum f. sp. Albedinis with conceptual DFT and ligand-protein docking studies. Bioorg. Chem. 2021, 110, 104696. [Google Scholar] [CrossRef]
- Ouadi, Y.E.; Lamsayah, M.; Bendaif, H.; Benhiba, F.; Touzani, R.; Warad, I.; Zarrouk, A. Electrochemical and theoretical considerations for interfacial adsorption of novel long chain acid pyrazole for mild steel conservation in 1 M HCl medium. Chem. Data Collec. 2021, 31, 100638. [Google Scholar] [CrossRef]
- Kaddouri, Y.; Bouchal, B.; Abrigach, F.; El Kodadi, M.; Bellaoui, M.; Touzani, R. Synthesis, Molecular Docking, MEP and SAR Analysis, ADME-Tox Predictions, and Antimicrobial Evaluation of Novel Mono-and Tetra-Alkylated Pyrazole and Triazole Ligands. J. Chem. 2021, 2021, 6663245. [Google Scholar] [CrossRef]
- Titi, A.; Messali, M.; Alqurashy, B.A.; Touzani, R.; Shiga, T.; Oshio, H.; Fettouhi, M.; Rajabi, M.; Almalki, F.A.; Ben Hadda, T. Synthesis, characterization, X-Ray crystal study and bioctivities of pyrazole derivatives: Identification of antitumor, antifungal and antibacterial pharmacophore sites. J. Mol. Struct. 2020, 1205, 127625. [Google Scholar] [CrossRef]
- Kaddouri, Y.; Abrigach, F.; Yousfi, E.B.; El Kodadi, M.; Touzani, R. New thiazole, pyridine and pyrazole derivatives as antioxidant candidates: Synthesis, DFT calculations and molecular docking study. Heliyon 2020, 6, e03185. [Google Scholar] [CrossRef]
- Khoutoul, M.; Djedouani, A.; Lamsayah, M.; Abrigach, F.; Touzani, R. Liquid-liquid extraction of metal ions, DFT and TD-DFT analysis for some pyrane derivatives with high selectivity for Fe(II) and Pb(II). Sep. Sci. Technol. 2016, 51, 1112–1123. [Google Scholar] [CrossRef]
- Abrigach, F.; Bouchal, B.; Riant, O.; Macé, Y.; Takfaoui, A.; Radi, S.; Oussaid, A.; Bellaoui, M.; Touzani, R. New N,N,N′,N′-tetradentate pyrazoly agents: Synthesis and evaluation of their antifungal and antibacterial activities. Med. Chem. 2016, 12, 83–89. [Google Scholar] [CrossRef]
- Takfaoui, A.; Zhao, L.; Touzani, R.; Dixneuf, P.H.; Doucet, H. Palladium-catalysed direct diarylations of pyrazoles with aryl bromides: A one step access to 4,5-diarylpyrazoles. Tetrahedron Lett. 2015, 55, 1697–1701. [Google Scholar] [CrossRef]
- Penning, T.D.; Talley, J.J.; Bertenshaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Miyashiro, J.M.; Rogers, R.S.; et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: Identification of 4-[5-(4-methylphenyl)- 3(trifluoromethyl)-1h-pyrazol-1-yl]benzenesulfonamide (sc-58635, celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef]
- Lv, P.-C.; Li, H.-Q.; Sun, J.; Zhou, Y.; Zhu, H.-L. Synthesis and biological evaluation of pyrazole derivatives containing thiourea skeleton as anticancer agents. Bioorg. Med. Chem. 2010, 18, 4606–4614. [Google Scholar] [CrossRef]
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; dos Santos, M.S.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. [Google Scholar] [CrossRef]
- Zavozin, A.G.; Ignat’ev, N.V.; Schulte, M.; Zlotin, S.G. Synthesis of novel tridentate pyrazole–bipyridine ligands for Co-complexes as redox-couples in dye-sensitized solar cells. Tetrahedron 2015, 71, 8551–8556. [Google Scholar] [CrossRef]
- Megyes, T.; May, Z.; Schubert, G.; Grósz, T.; Simándi, L.I.; Radnai, T. Synthesis and structure study of some catecholase-mimetic iron complexes. Inorg. Chim. Acta 2006, 359, 2329–2336. [Google Scholar] [CrossRef]
- Ayad, M.I. Synthesis, characterization and catechol oxidase biomimetic catalytic activity of cobalt (II) and copper (II) complexes containing N2O2 donor sets of imine ligands. Arab. J. Chem. 2016, 9, S1297–S1306. [Google Scholar] [CrossRef]
- Dey, S.K.; Mukherjee, A. The synthesis, characterization and catecholase activity of dinuclear cobalt (II/III) complexes of an O-donor rich Schiff base ligand. New J. Chem. 2014, 38, 4985–4995. [Google Scholar] [CrossRef]
- Saddik, R.; Abrigach, F.; Benchat, N.; El Kadiri, S.; Hammouti, B.; Touzani, R. Catecholase activity investigation for pyridazinone-and thiopyridazinone-based ligands. Res. Chem. Inter. 2012, 38, 1987–1998. [Google Scholar] [CrossRef]
- Mouadili, A.; Attayibat, A.; El Kadiri, S.; Radi, S.; Touzani, R. Catecholase activity investigations using in situ copper complexes with pyrazole and pyridine based ligands. Appl. Cat. A Gen. 2013, 454, 93–99. [Google Scholar] [CrossRef]
- Marion, R.; Saleh, N.M.; Le Poul, N.; Lavastre, D.; Geneste, F. Rate enhancement of the catechol oxidase activity of a series of biomimetic monocopper (II) complexes by introduction of non-coordinating groups in N-tripodal ligands. New J. Chem. 2012, 36, 1828–1835. [Google Scholar] [CrossRef]
- Sarkar, S.; Sim, A.; Kim, S.; Lee, H.I. Catecholase activity of a self-assembling dimeric Cu (II) complex with distant Cu (II) centers. J. Mol. Cat. A Chem. 2015, 410, 149–159. [Google Scholar] [CrossRef]
- Allam, A.; Dechamps-Olivier, I.; Behr, J.B.; Dupont, L.; Plantier-Royon, R. Thermodynamic, spectroscopic studies and catechol oxidase activity of copper (II) complexes with amphiphilic d-galacturonic acid derived ligands. Inorg. Chim. Acta 2011, 366, 310–319. [Google Scholar] [CrossRef]
- Titi, A.; Al-Noaimi, M.; Kaddouri, Y.; El Ati, R.; Yousfi, E.B.; El Kodadi, M.; Touzani, R. Study of the catecholase catalytic properties of copper (II) complexes prepared in-situ with monodentate ligands. Mater. Today Proc. 2019, 13, 1134–1142. [Google Scholar] [CrossRef]
- El Boutaybi, M.; Titi, A.; Alzahrani, A.Y.A.; Bahari, Z.; Tillard, M.; Hammouti, B.; Touzani, R. Aerial Oxidation of Phenol/Catechol in the Presence of Catalytic Amounts of [(Cl)2Mn(RCOOET)], RCOOET=Ethyl-5-Methyl-1-(((6-methyl-3-nitropyridin-2-yl)amino)methyl)-1Hpyrazole-3-carboxylate. Catalysts 2022, 12, 1642. [Google Scholar] [CrossRef]
- Titi, A.; Rachid Touzani, R.; Moliterni, A.; Giacobbe, C.; Baldassarre, F.; Taleb, M.; Al-Zaqri, N.; Zarrouk, A.; Warad, I. Ultrasonic Clusterization Process to Prepare [(NNCO)6Co4Cl2] as a Novel Double-Open-Co4O6 Cubane Cluster: SXRD Interactions, DFT, Physicochemical, Thermal Behaviors, and Biomimicking of Catecholase Activity. ACS Omega 2022, 7, 32949. [Google Scholar] [CrossRef]
- Titi, A.; Shiga, T.; Oshio, H.; Touzani, R. Synthesis of novel Cl2Co4L6 cluster using 1-hydroxymethyl-3, 5-dimethylpyrazole (LH) ligand: Crystal structure, spectral, thermal, Hirschfeld surface analysis and catalytic oxidation evaluation. J. Mol. Struct. 2020, 1199, 126995. [Google Scholar] [CrossRef]
- Titi, A.; Warad, I.; Tillard, M.; Messali, M.; Touzani, R. Inermolecular interaction in [C6H10N3]2[CoCl4] complex: Synthesis, XRD/HSA relation, spectral and catecholase catalytic analysis. J. Mol. Struct. 2020, 1217, 128422. [Google Scholar] [CrossRef]
- Titi, A.; Oshio, H.; Messali, M.; Touzani, R.; Warad, I. Synthesis and XRD of Novel Ni 4 (µ 3-O) 4 Twist Cubane Cluster Using Three NNO Mixed Ligands: Hirshfeld, Spectral, Thermal and Oxidation Properties. J. Cluster Sci. 2020, 32, 227–234. [Google Scholar] [CrossRef]
- El Kodadi, M.; Malek, F.; Touzani, R.; Ramdani, A. Synthesis of new tripodal ligand 5-(bis (3, 5-dimethyl-1H-pyrazol-1-ylmethyl) amino) pentan-1-ol, catecholase activities studies of three functional tripodal pyrazolyl N-donor ligands, with different copper (II) salts. Cat. Commun. 2008, 9, 966–969. [Google Scholar] [CrossRef]
- Elmsellem, H.; Harit, T.; Aouniti, A.; Malek, F.; Riahi, A.; Chetouani, A.; Hammouti, B. Adsorption properties and inhibition of mild steel corrosion in 1 M HCl solution by some bipyrazolic derivatives: Experimental and theoretical investigations. Prot. Met. Phys. Chem. Sur. 2015, 51, 873–884. [Google Scholar] [CrossRef]
- Touzani, R.; Ramdani, A.; Ben-Hadda, T.; El Kadiri, S.; Maury, O.; Le Bozec, H.; Dixneuf, P.H. Efficient synthesis of new nitrogen donor containing tripods under microwave irradiation and without solvent. Synth. Commun. 2001, 31, 1315–1321. [Google Scholar] [CrossRef]
- Bouabdallah, I.; Touzani, R.; Zidane, I.; Ramdani, A. Synthesis of new tripodal ligand: N,N-bis [(1, 5-dimethylpyrazol-3-yl) methyl] benzylamine.: Catecholase activity of two series of tripodal ligands with some copper (II) salts. Catal. Commun. 2007, 8, 707–712. [Google Scholar] [CrossRef]
- Harit, T.; Malek, F.; El Bali, B.; Khan, A.; Dalvandi, K.; Marasini, B.P.; Noreen, S.; Malik, R.; Khan, S.; Choudhary, M.I. Synthesis and enzyme inhibitory activities of some new pyrazole-based heterocyclic compounds. Med. Chem. Res. 2012, 21, 2772–2778. [Google Scholar] [CrossRef]
- El Kodadi, M.; Benamar, M.; Bouabdallah, I.; Zyad, A.; Malek, F.; Touzani, R.; Ramdani, A.; Melhaoui, A. New synthesis of two tridentate bipyrazolic compounds and their cytotoxic activity tumor cell lines. Nat. Prod. Res. 2007, 21, 947–952. [Google Scholar] [CrossRef]
- Kaddouri, Y.; Haddari, H.; Titi, A.; Yousfi, E.B.; Chetouani, A.; Touzani, R. Catecholase catalytic properties of copper (II) complexes prepared in-situ with heterocyclic ligands: Experimental and DFT study. Mor. J. Chem. 2020, 8, 8-1. [Google Scholar]
- Harit, T.; Isaad, J.; Malek, F. Novel efficient functionalized tetrapyrazolic macrocycle for the selective extraction of lithium cations. Tetrahedron 2016, 72, 2227–2232. [Google Scholar] [CrossRef]
- Ding, H.Y.; Cheng, H.J.; Wang, F.; Liu, D.X.; Li, H.X.; Fang, Y.Y.; Zhao, W.; Lang, J.P. [(bmppy) Cu (μ-I)]2 (bmppy = 2,6-bis (1-methyl-1H-pyrazol-3-yl) pyridine): Synthesis, crystal structure and its catalytic performance for MMA polymerization. J. Organomet. Chem. 2013, 741, 1–6. [Google Scholar] [CrossRef]
- Titi, A.; Touzani, R.; Moliterni, A.; Ben Hadda, T.; Messali, M.; Benabbes, R.; Berredjem, M.; Bouzina, A.; Al-Zaqri, N.; Taleb, M.; et al. Synthesis, Structural, Biocomputational Modeling and Antifungal Activity of Novel Armed pyrazoles. J. Mol. Struct. 2022, 1264, 133156. [Google Scholar] [CrossRef]
- Zerrouki, A.; Touzani, R.; Bouabdallah, I.; El Kadiri, S.; Ghalem, S. Synthesis of new derivatized pyrazole based ligands and their catecholase activity studies. Arab. J. Chem. 2011, 4, 459–464. [Google Scholar] [CrossRef]
- Banu, K.S.; Mukherjee, M.; Guha, A.; Bhattacharya, S.; Zangrando, E.; Das, D. Dinuclear copper(2) complexes:solvent dependent catecholase activity. Polyhedron 2012, 45, 245–254. [Google Scholar] [CrossRef]
- Boussalah, N.; Touzani, R.; Bouabdallah, I.; Ghalem, S.; El Kadiri, S. Oxidation catalytic properties of new amino acid based on pyrazole tripodal ligands. Inter. J. Acad. Res. 2009, 1, 137–143. [Google Scholar]
- Takfaoui, A.; Lamsayah, M.; El Ouafi, A.; Oussaid, A.; Kabouche, Z.; Touzani, R. N,N′-bipyrazole compounds: Effect of concentration, solvent, ligand and metal anions on the catecholase properties. J. Mater. Environ. Sci. 2015, 6, 2129–2136. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).