PtM/CNT (M = Mo, Ni, CoCr) Electrocatalysts with Reduced Platinum Content for Anodic Hydrogen Oxidation and Cathodic Oxygen Reduction in Alkaline Electrolytes
Abstract
1. Introduction
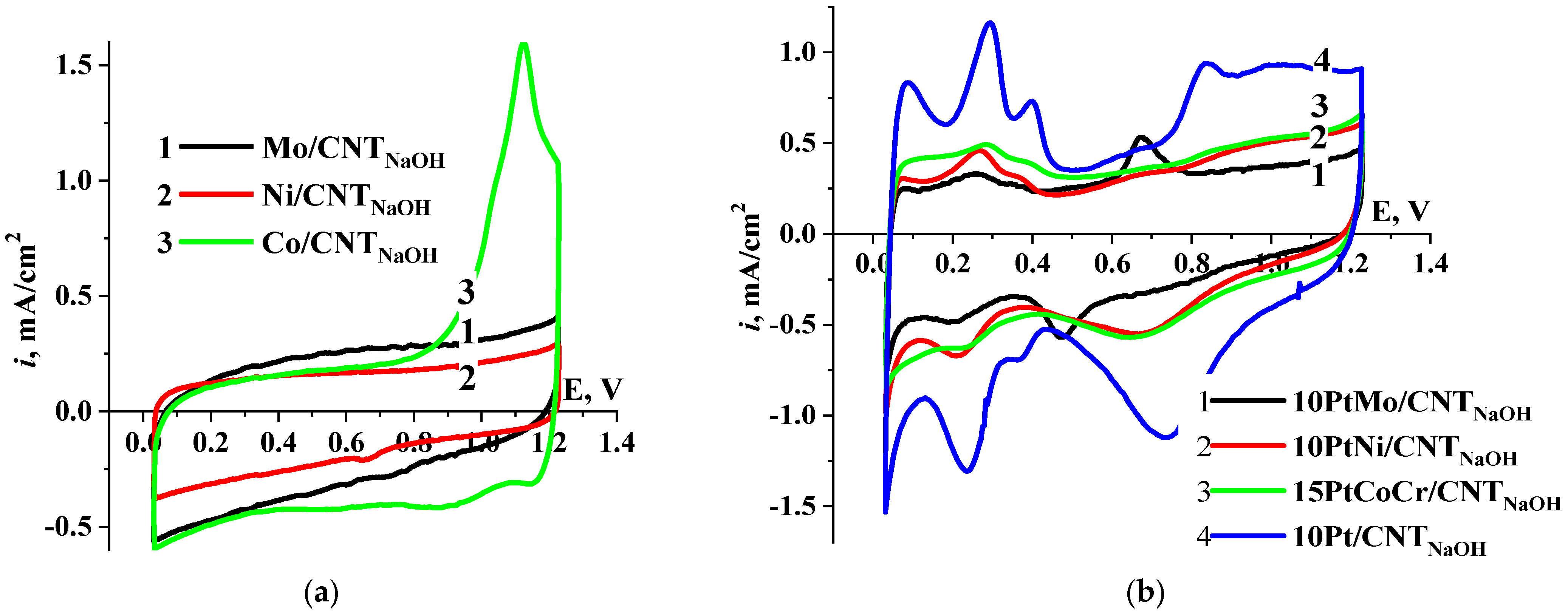
2. Results and Discussion
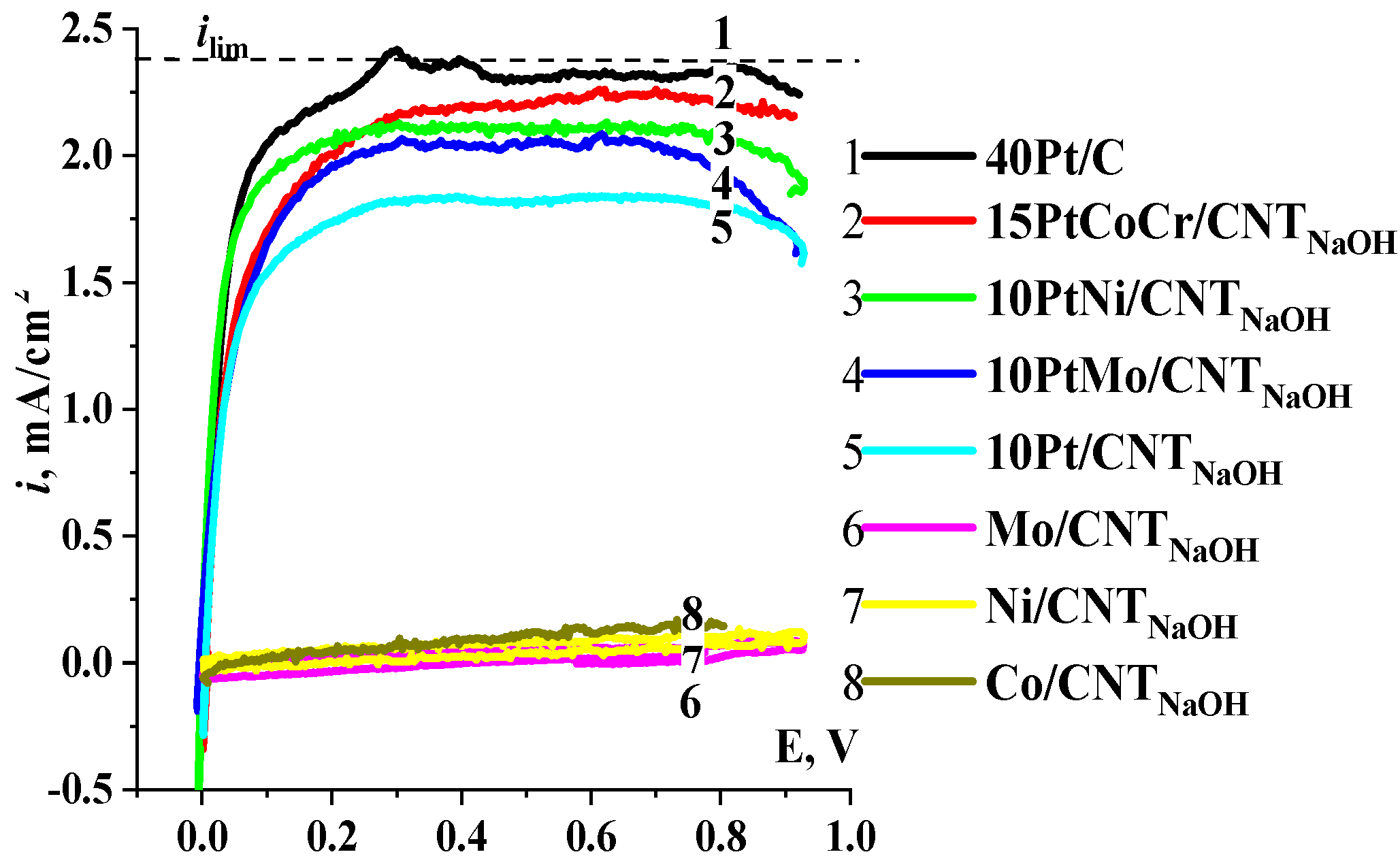
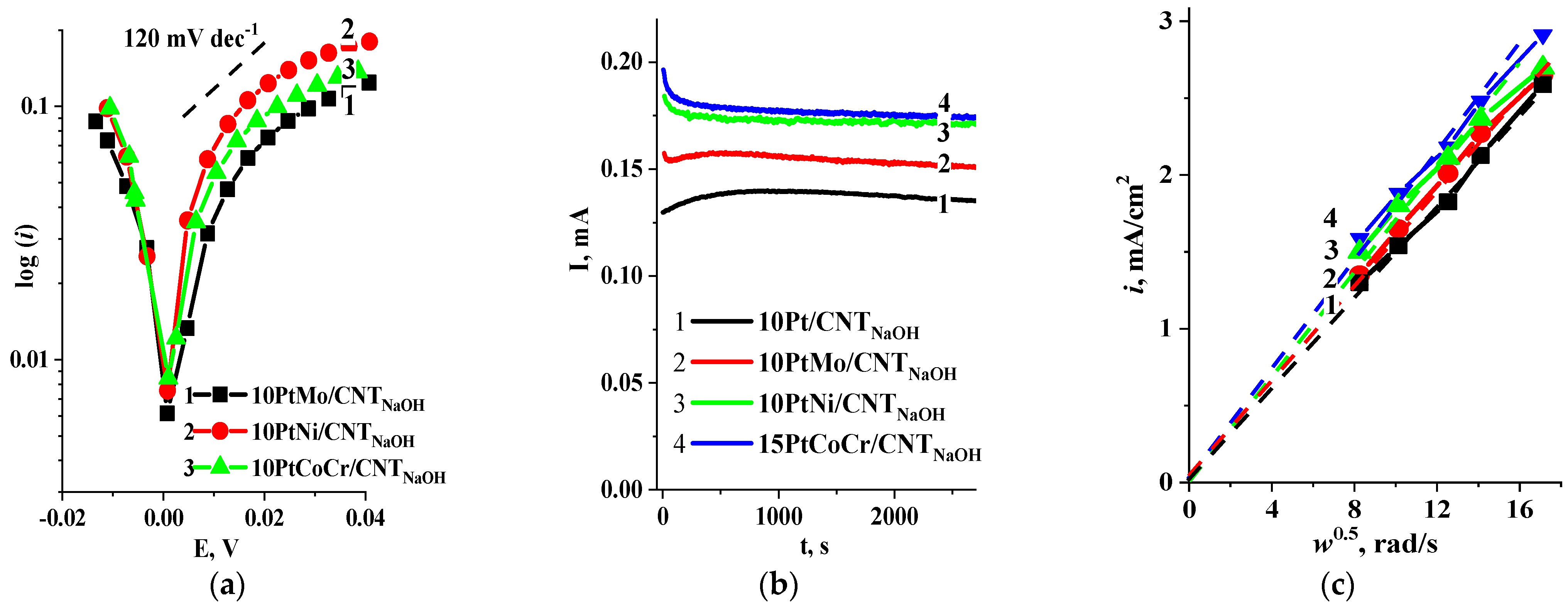
2.1. Hydrogen Electrooxidation Reaction
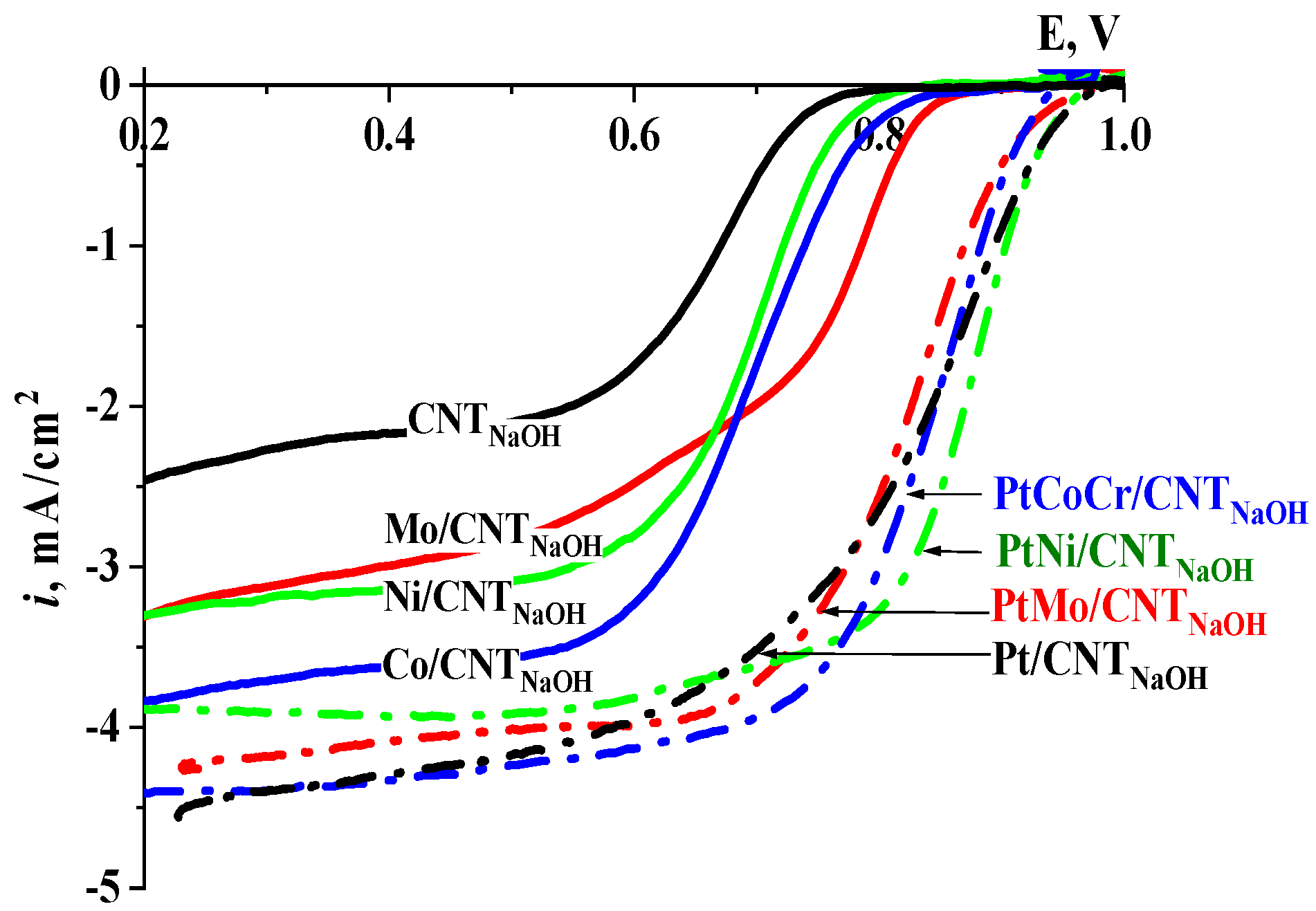
2.2. Oxygen Reduction Reaction
2.3. Corrosion Stability Study
3. Materials and Methods
3.1. Method of Processing CNTs in an Alkaline Solution
3.2. Synthesis of Mo/CNTNaOH, PtMo/CNTNaOH, Ni/CNTNaOH, PtNi/CNTNaOH, and Co/CNTNaOH Catalysts
3.3. Synthesis of PtCoCr/CNTNaOH
3.4. Modification of CNT with Platinum Using Polyol Method
3.5. Electrochemical Measurements
3.6. Determination of Stability
3.7. Determination of Platinum Content in Synthesized Catalysts
3.8. X-ray Photoelectron Spectroscopy (XPS)
3.9. Scanning Electron Spectroscopy and EDX Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barbir, F. PEM Fuel Cells: Theory and Practice, 2nd ed.; Elsevier, Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Colella, W.G.; Golden, D.M. Cleaning the air and improving health with hydrogen fuel-cell vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent Progresses in Oxygen Reduction Reaction Electrocatalysts for Electrochemical Energy Applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef]
- Talukder, N.; Wang, Y.; Nunna, B.B.; Lee, E.S. An In-Depth Exploration of the Electrochemical Oxygen Reduction Reaction (ORR) Phenomenon on Carbon-Based Catalysts in Alkaline and Acidic Mediums. Catalysts 2022, 12, 791. [Google Scholar] [CrossRef]
- Qian, Z.; Sun, B.; Du, L.; Lou, S.; Du, C.; Zuo, P.; Ma, Y.; Cheng, X.; Gao, Y.; Yin, G. Insights into the role of oxygen functional groups and defects in the rechargeable nonaqueous Li–O2 batteries. ElectrochimicaActa 2018, 292, 838–845. [Google Scholar] [CrossRef]
- Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Sobolev, V.; Andreev, V.; Nikolskaya, N. Modified Carbon Nanotubes: Surface Properties and Activity in Oxygen Reduction Reaction. Catalysts 2021, 11, 1354. [Google Scholar] [CrossRef]
- Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Andreev, V.; Korchagin, O.; Novikov, V. Carbon Nanotube Modified by (O, N, P) Atoms as Effective Catalysts for Electroreduction of Oxygen in Alkaline Media. Catalysts 2020, 10, 892. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Doping of carbon materials for metal-free electrocatalysis. Adv. Mater. 2019, 31, 1804672. [Google Scholar]
- Zhang, X.; Zhang, X.; Zhao, S.; Wang, Y.Q.; Lin, X.; Tian, Z.Q.; Shen, P.K.; Jiang, S.P. Precursor modulated active sites of nitrogen doped graphene-based carbon catalysts via one-step pyrolysis method for the enhanced oxygen reduction reaction. Electrochimica Acta 2021, 370, 137712. [Google Scholar] [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasch’e, F.; Herranz, J.; Gasteiger, H.A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255. [Google Scholar] [CrossRef]
- Cong, Y.; Yi, B.; Song, Y. Hydrogen oxidation reaction in alkaline media: From mechanism to recent electrocatalysts. Nano Energy 2018, 44, 288–303. [Google Scholar] [CrossRef]
- Campos-Roldán, C.A.; Alonso-Vante, N. The Hydrogen Oxidation Reaction in Alkaline Medium: An Overview. Electrochem. Energy Rev. 2019, 2, 312–331. [Google Scholar] [CrossRef]
- Zheng, J.; Nash, J.; Xu, B.; Yan, Y. Towards establishing apparent hydrogen binding energy as the descriptor for hydrogen oxidation/evolution reactions. J. Electrochem. Soc. 2018, 165, H27–H29. [Google Scholar] [CrossRef]
- Campos-Roldán, C.A.; Alonso-Vante, N. Understanding the oxophilic effect on the hydrogen electrode reaction through PtM nanostructures. J. Solid State Electrochem. 2021, 25, 187–194. [Google Scholar] [CrossRef]
- Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; van der Vliet, D.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 2013, 5, 300–306. [Google Scholar] [CrossRef]
- Hassan, A.; Carreras, A.; Trincavelli, J.; Ticianelli, E.A. Effect of heat treatment on the activity and stability of carbon supported PtMo alloy electrocatalysts for hydrogen oxidation in proton exchange membrane fuel cells. J. Power Sources 2014, 247, 712–720. [Google Scholar] [CrossRef]
- Gao, J.; Zou, J.; Zeng, X.; Ding, W. Carbon supported nano Pt–Mo alloy catalysts for oxygen reduction in magnesium–air batteries. RSC Adv. 2016, 6, 83025. [Google Scholar] [CrossRef]
- Torres-Santillan, E.; Capula-Colindres, S.; Teran, G.; German, C.M.R.; Flores, M.E.; Valencia, O.G.R. Synthesis of Pt-Mo/WMCNTs nanostructures reduced by the green chemical route and its electrocatalytic activity in the ORR. In Carbon Nanotubes—Recent Advances, New Perspectives and Potential Applications; Rahman, M.M., Asiri, A.M.A., Chowdhury, M.A., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Do, C.L.; Pham, T.S.; Nguyen, N.P.; Tran, V.Q.; Pham, H.H. Synthesis and characterization of alloy catalyst nanoparticles PtNi/C for oxygen reduction reaction in proton exchange membrane fuel cell. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 025009. [Google Scholar]
- Kong, F.; Ren, Z.; Banis, N.M. Active and Stable Pt–Ni Alloy Octahedra Catalyst for Oxygen Reduction via Near-Surface Atomical Engineering. ACS Catal. 2020, 10, 4205–4214. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, G.-M.; Yao, R.; Yu, T.; Han, M.-F.; Huang, R.-S. High Oxygen Reduction Activity of Pt-Ni Alloy Catalyst for Proton Exchange Membrane Fuel Cells. Catalysts 2022, 12, 250. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, Y.; Lu, Y.; Zheng, L.; Sun, S.; Li, H.; Chen, G.; Zhang, J. Single-atom alloy with Pt-Co dual sites as an efficient electrocatalyst for oxygen reduction reaction. Appl. Catal. B Environ. 2022, 306, 121112. [Google Scholar] [CrossRef]
- Choi, D.S.; Robertson, A.W.; Warner, J.H.; Kim, S.O.; Kim, H. Low-Temperature Chemical Vapor Deposition Synthesis of Pt–Co Alloyed Nanoparticles with Enhanced Oxygen Reduction Reaction. Catal. Adv. Mater. 2016, 28, 7115–7122. [Google Scholar] [CrossRef]
- Wang, X.X.; Hwang, S.; Pan, Y.-T.; Chen, K.; He, Y.; Karakalos, S.; Zhang, H.; Spendelow, J.S.; Su, D.; Wu, G. Ordered Pt3Co Intermetallic Nanoparticles Derived from Metal–Organic Frameworks for Oxygen Reduction. Nano Lett. 2018, 18, 4163–4171. [Google Scholar] [CrossRef]
- Zaman, S.; Su, Y.-Q.; Dong, C.-L.; Qi, R.; Huang, L.; Qin, Y.; Huang, Y.-C.; Li, F.-M.; You, B.; Guo, W.; et al. Scalable Molten Salt Synthesis of Platinum Alloys Planted in Metal–Nitrogen–Graphene for Efficient Oxygen Reduction. Angew. Chem. Int. Ed. 2022, 61, e202115835. [Google Scholar] [CrossRef]
- Zaman, S.; Tian, X.; Su, Y.-Q.; Cai, W.; Yan, Y.; Qi, R.; Douka, A.I.; Chen, S.; You, B.; Liu, H.; et al. Direct integration of ultralow-platinum alloy into nanocarbon architectures for efficient oxygen reduction in fuel cells. Sci. Bulletin. 2021, 66, 2207–2216. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Fundamental Mechanistic Understanding of Electrocatalysis of Oxygen Reduction on Pt and Non-Pt Surfaces: Acid versus Alkaline Media. Adv. Phys. Chem. 2012, 2012, 491604. [Google Scholar] [CrossRef]
- Bogdanovskaya, V.A.; Kuzov, A.V.; Radina, M.V.; Filimonov, V.Y.; Sudarev, G.M.; Osina, M.A. Stability against Degradation and Activity of Catalysts with Different Platinum Load Synthesized at Carbon Nanotubes. Russ. J. Electrochem. 2020, 56, 969. [Google Scholar] [CrossRef]
- Rheinländer, P.J.; Herranz, J.; Durst, J.; Gasteiger, H.A. Kinetics of the Hydrogen Oxidation/Evolution Reaction on Polycrystalline Platinum in Alkaline Electrolyte Reaction Order with Respect to Hydrogen Pressure. J. Electrochem. Soc. 2014, 161, 1448. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Du, C.; Tan, Q.; Yin, G.; Zhang, J. 5—Rotating disk electrode method. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Xing, W., Yin, G., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 171–198. [Google Scholar] [CrossRef]
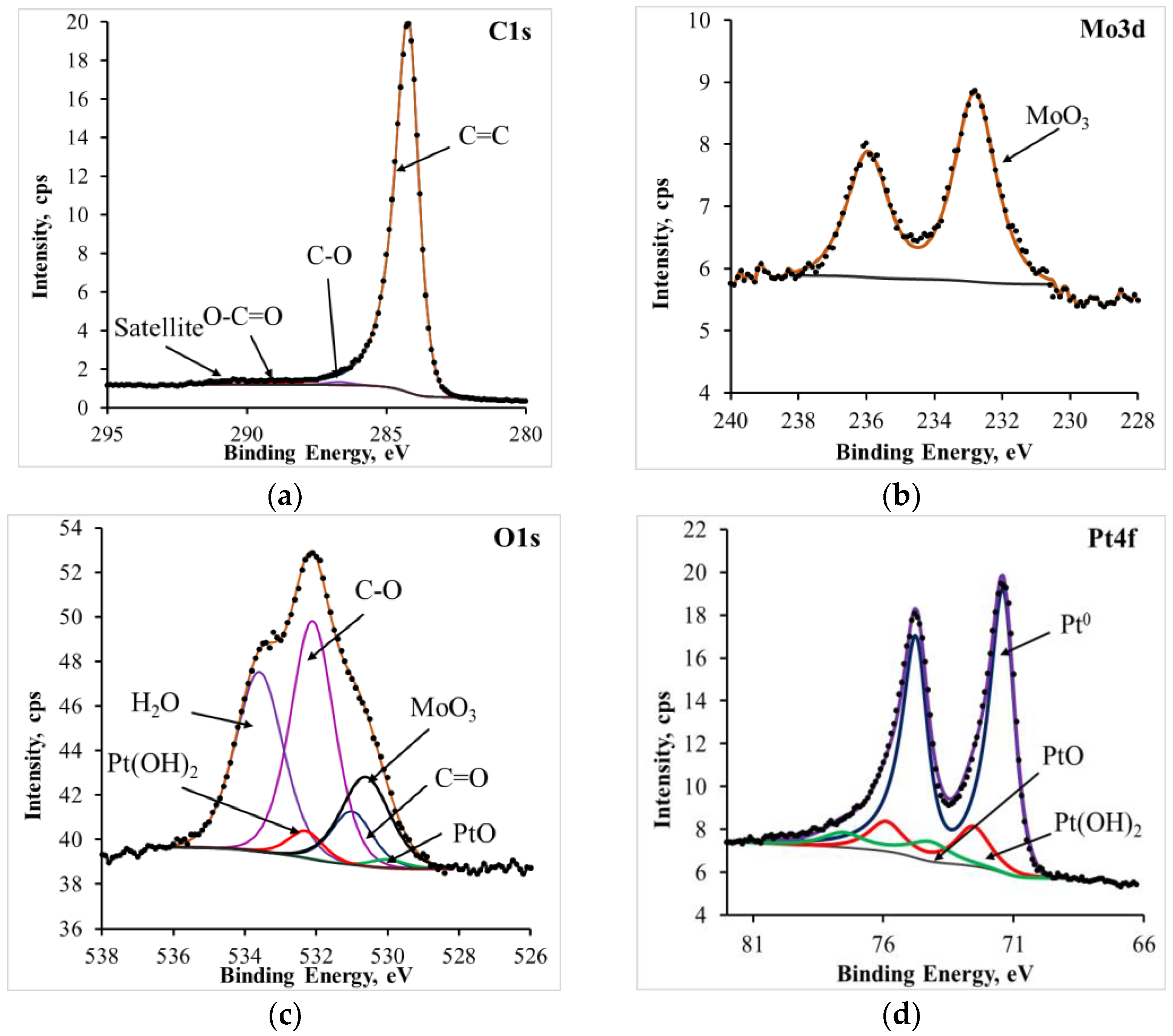

| Catalyst | C1s | O1s | Pt4f | Ni2p | Mo3d | Co3d | Cr2p |
|---|---|---|---|---|---|---|---|
| at% | |||||||
| 10Pt/CNTNaOH | 94.69 | 2.46 | 2.18 | ||||
| 10PtNi/CNTNaOH | 96.8 | 2.21 | 0.63 | 0.36 | |||
| 10PtMo/CNTNaOH | 96.66 | 2.81 | 0.36 | 0.16 | |||
| 15PtCoCr/CNTNaOH | 89.67 | 6.69 | 1.63 | 0.74 | 1.27 | ||
| Catalyst | idif, mA/cm2 | jmass × 103, mA/mg Pt | η, V |
|---|---|---|---|
| at 0.4 V | at 1.5 mA/cm2 (1500 rpm) | ||
| 40Pt/C | 2.31 | 36.2 | 0.037 |
| 10Pt/CNTNaOH | 1.82 | 114.6 | 0.088 |
| 10PtMo/CNTNaOH | 2.05 | 128.8 | 0.066 |
| 10PtNi/CNTNaOH | 2.11 | 132.9 | 0.035 |
| 15PtCoCr/CNTNaOH | 2.18 | 91.6 | 0.076 |
| Catalyst | E 1/2, V | ikin, mA/cm2 at 0.9 V | n//% HO2− at 0.5 V |
|---|---|---|---|
| CNTNaOH | 0.66 | - | - |
| Mo/CNTNaOH | 0.76 | - | - |
| Ni/CNTNaOH | 0.70 | - | - |
| Co/CNTNaOH | 0.71 | - | - |
| 10Pt/CNTNaOH | 0.84 | 0.88 | 3.42//28.9 |
| 10PtMo/CNTNaOH | 0.83 | 0.49 | 3.59//20.0 |
| 10PtNi/CNTNaOH | 0.87 | 1.07 | 3.78//10.8 |
| 15PtCoCr/CNTNaOH | 0.84 | 0.61 | 3.68//15.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernigor, I.; Bogdanovskaya, V.; Radina, M.; Andreev, V.; Grafov, O. PtM/CNT (M = Mo, Ni, CoCr) Electrocatalysts with Reduced Platinum Content for Anodic Hydrogen Oxidation and Cathodic Oxygen Reduction in Alkaline Electrolytes. Catalysts 2023, 13, 161. https://doi.org/10.3390/catal13010161
Vernigor I, Bogdanovskaya V, Radina M, Andreev V, Grafov O. PtM/CNT (M = Mo, Ni, CoCr) Electrocatalysts with Reduced Platinum Content for Anodic Hydrogen Oxidation and Cathodic Oxygen Reduction in Alkaline Electrolytes. Catalysts. 2023; 13(1):161. https://doi.org/10.3390/catal13010161
Chicago/Turabian StyleVernigor, Inna, Vera Bogdanovskaya, Marina Radina, Vladimir Andreev, and Oleg Grafov. 2023. "PtM/CNT (M = Mo, Ni, CoCr) Electrocatalysts with Reduced Platinum Content for Anodic Hydrogen Oxidation and Cathodic Oxygen Reduction in Alkaline Electrolytes" Catalysts 13, no. 1: 161. https://doi.org/10.3390/catal13010161
APA StyleVernigor, I., Bogdanovskaya, V., Radina, M., Andreev, V., & Grafov, O. (2023). PtM/CNT (M = Mo, Ni, CoCr) Electrocatalysts with Reduced Platinum Content for Anodic Hydrogen Oxidation and Cathodic Oxygen Reduction in Alkaline Electrolytes. Catalysts, 13(1), 161. https://doi.org/10.3390/catal13010161









