Progress & Prospect of Enzyme-Mediated Structured Phospholipids Preparation
Abstract
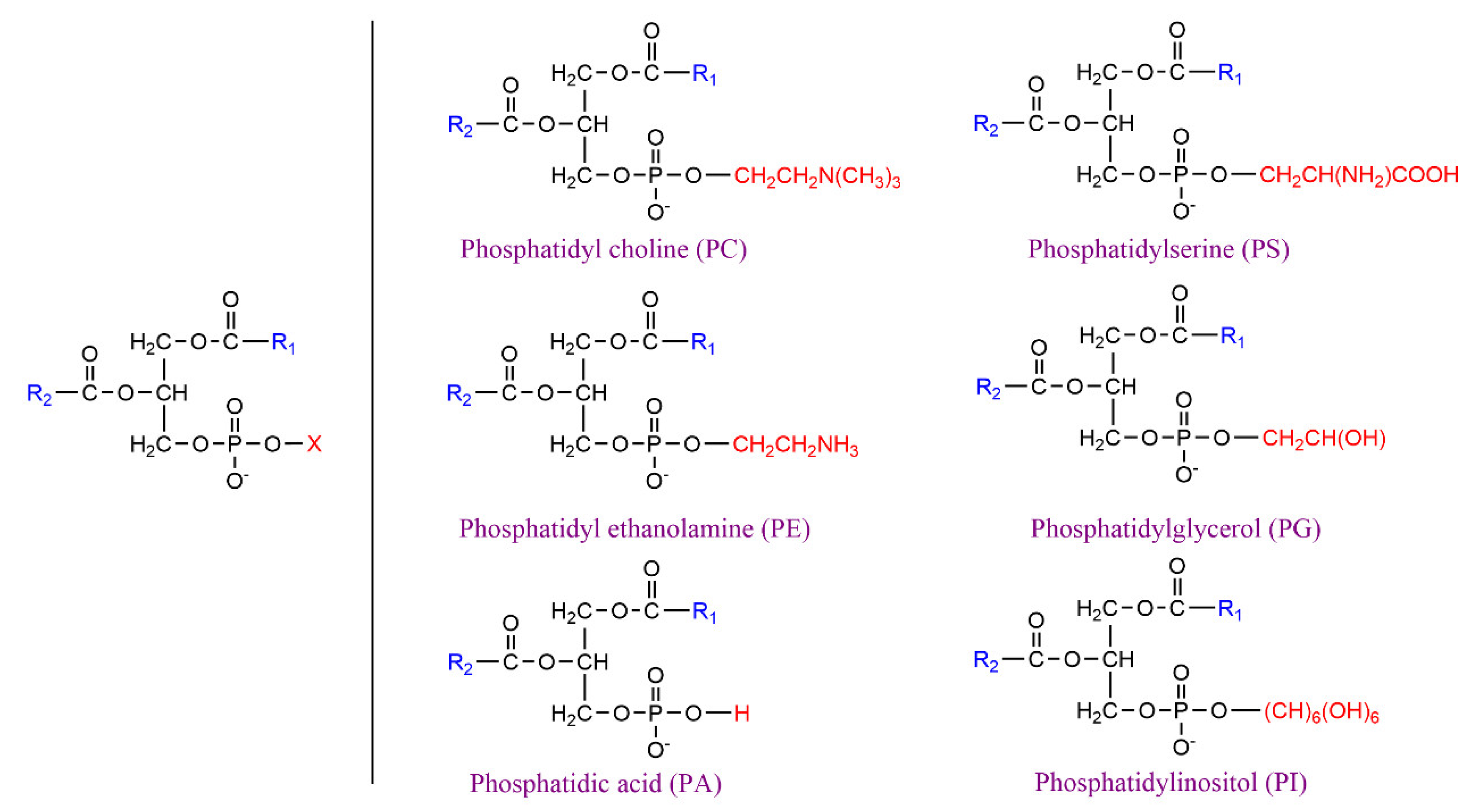
:1. Introduction
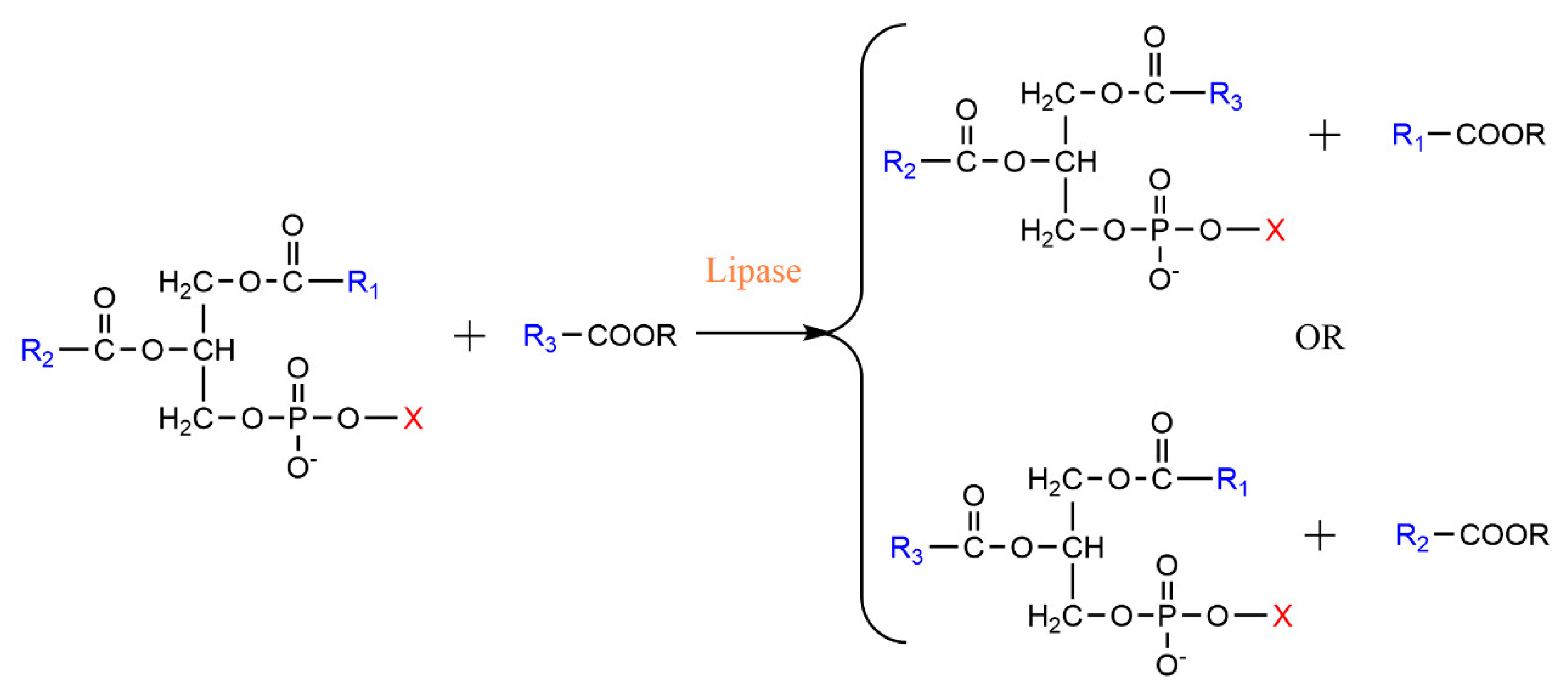
2. Lipase-Catalyzed Structured Phospholipids
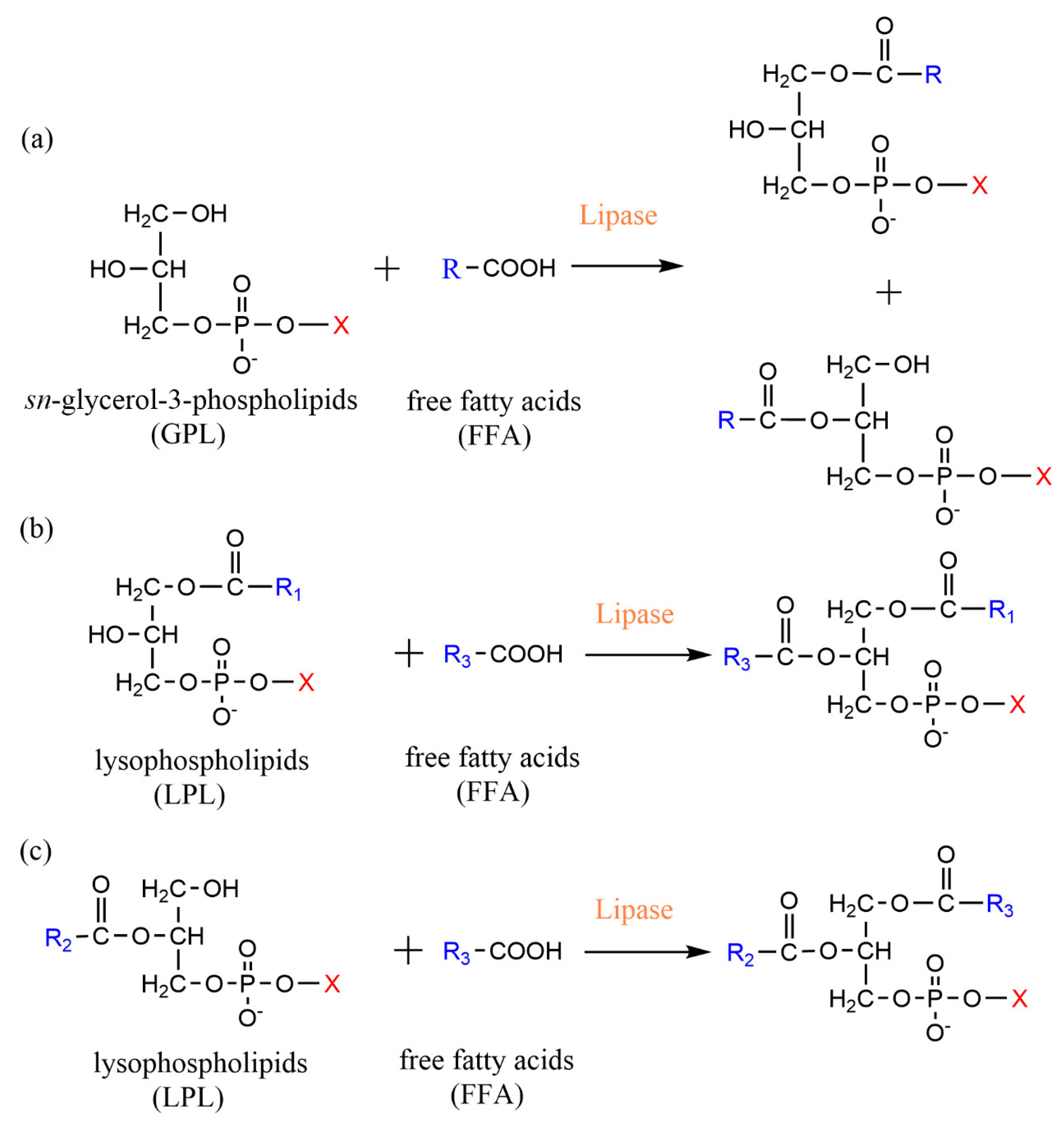
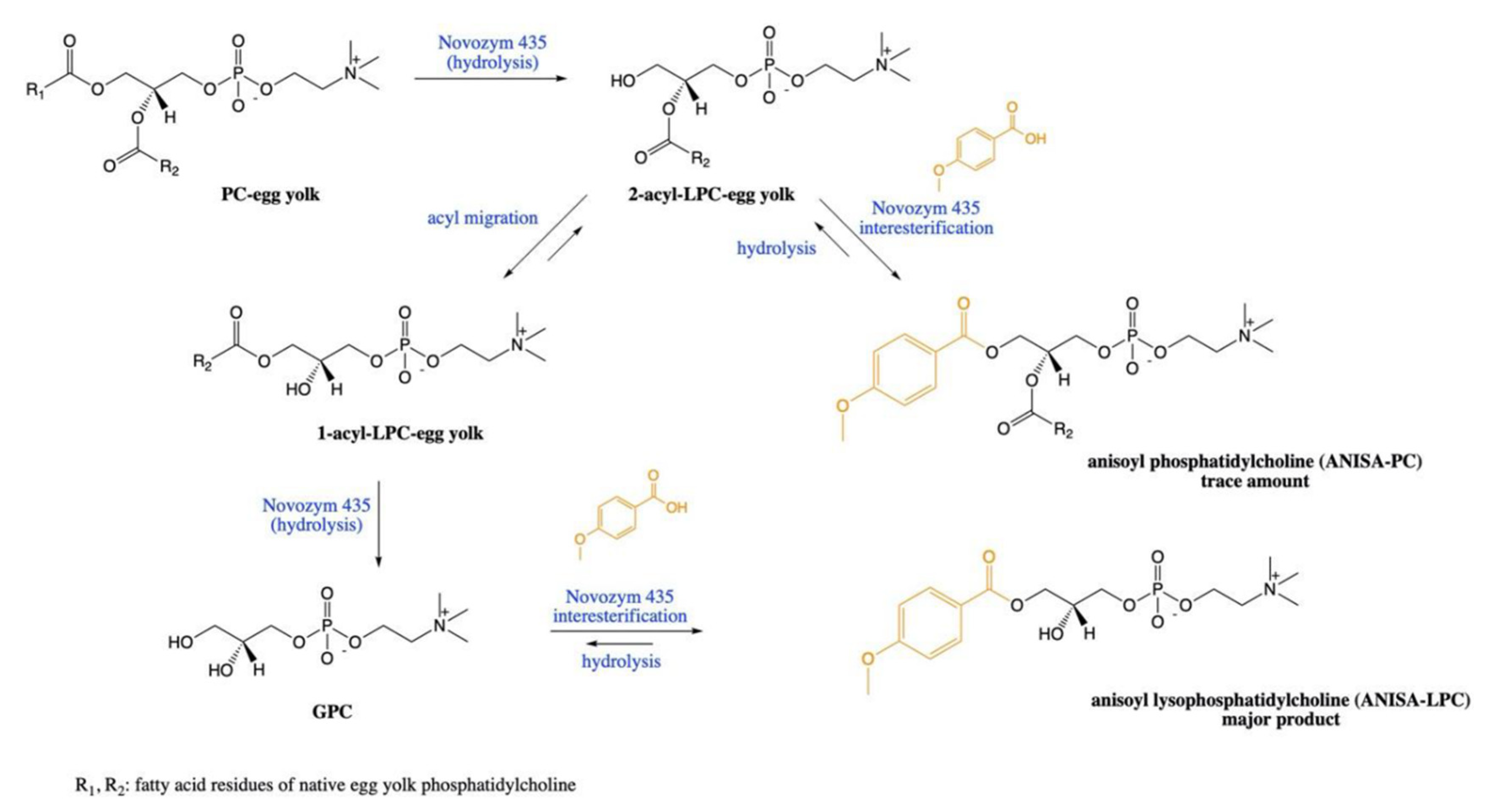
2.1. Esterification
2.2. Acidolysis
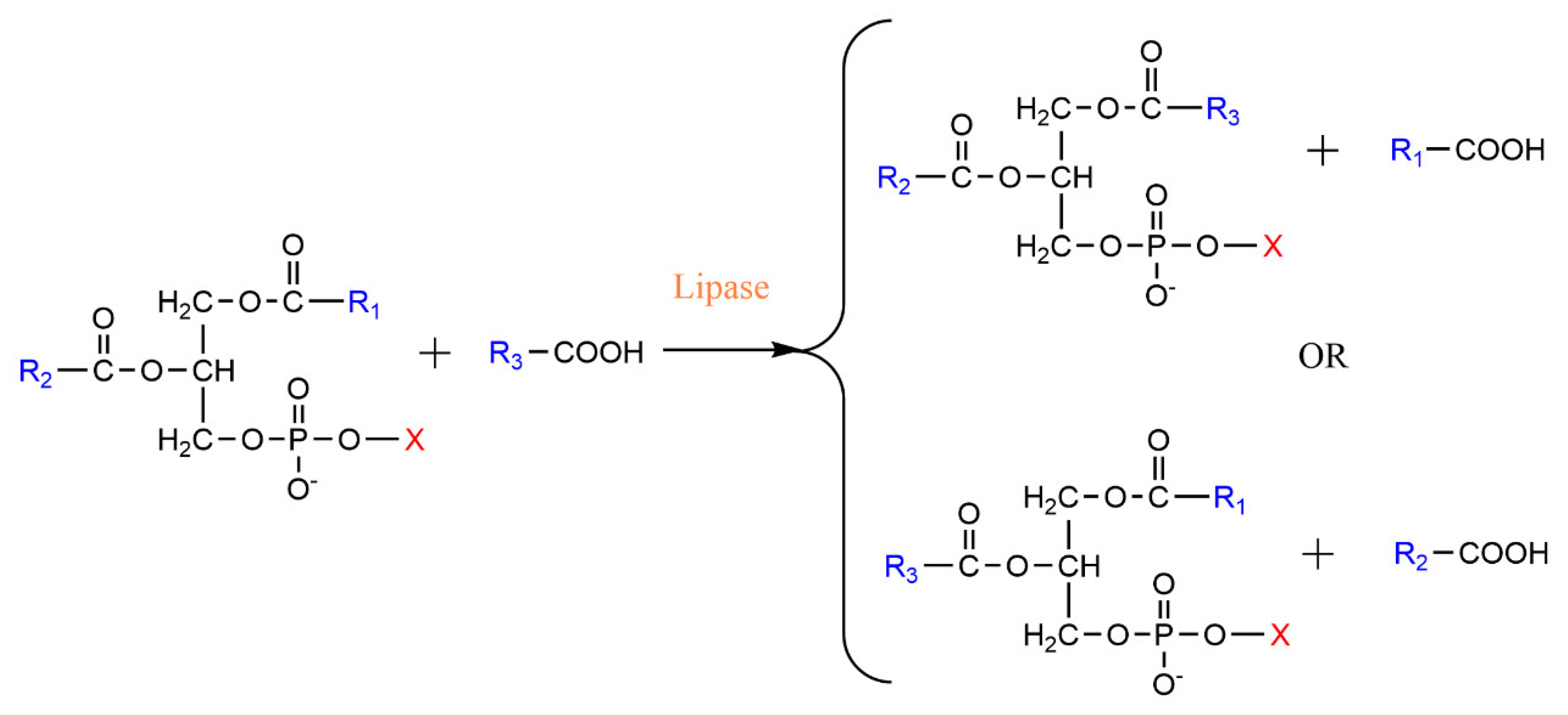
2.3. Transesterification
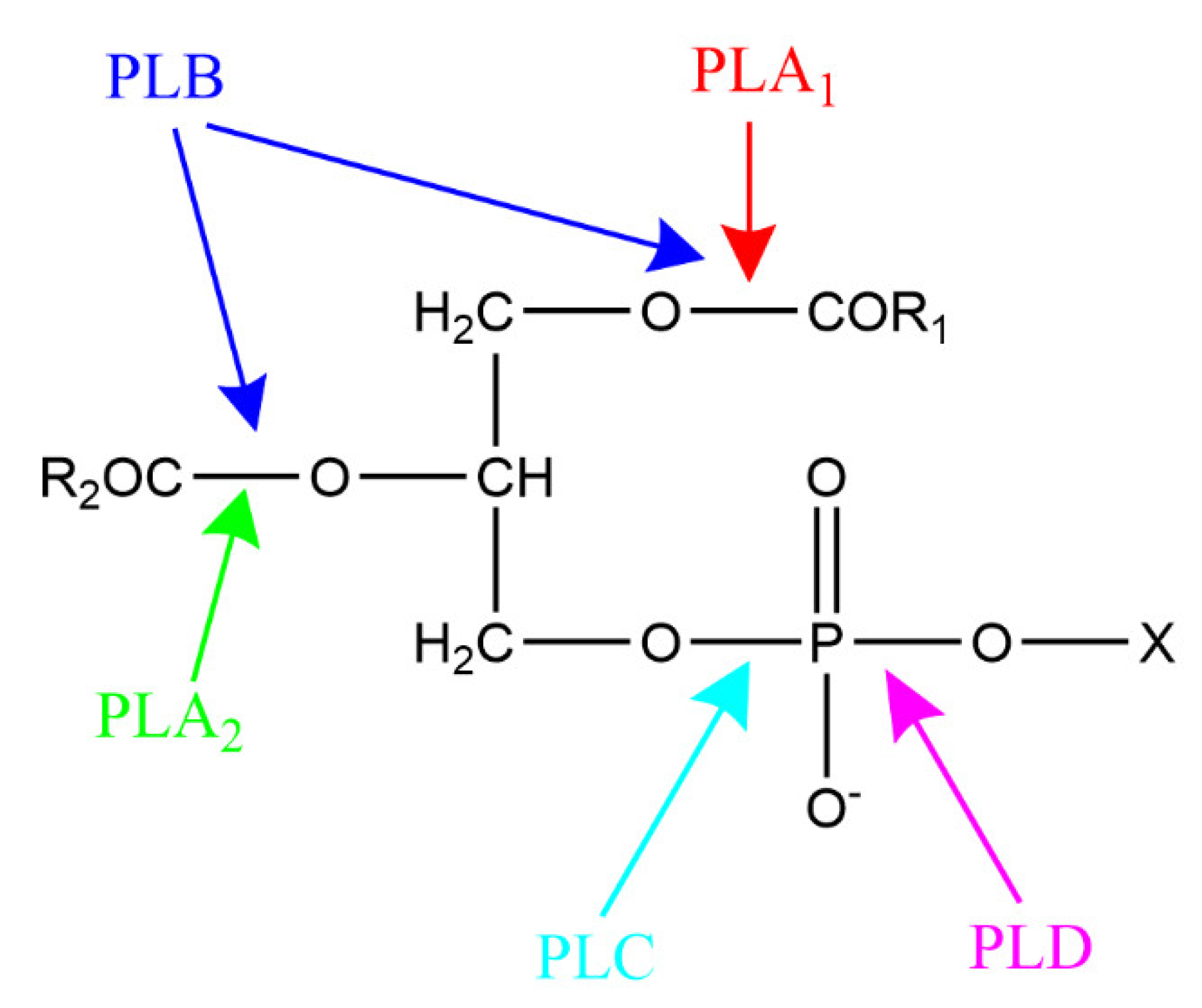
3. Phospholipase-Catalyzed Structured Phospholipids
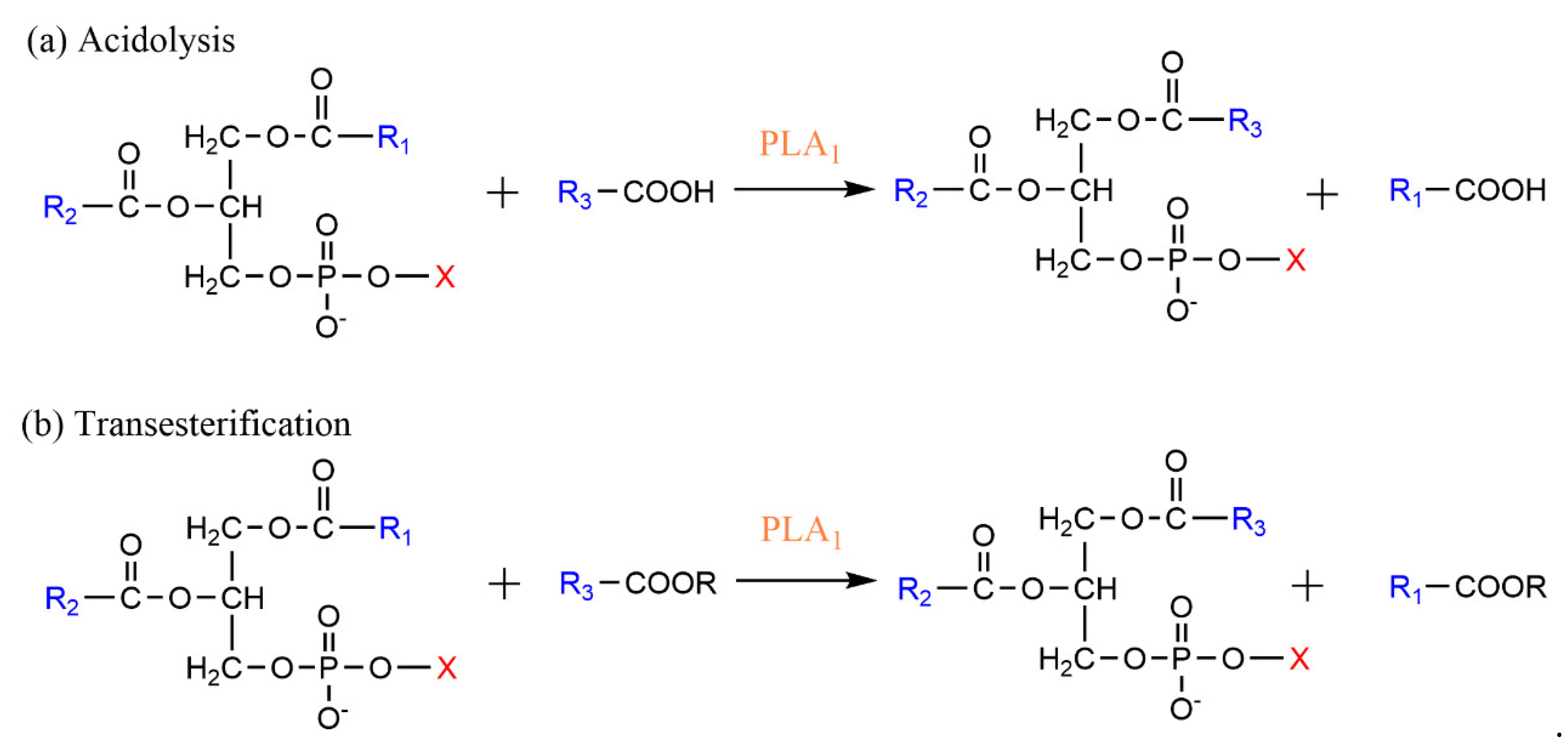
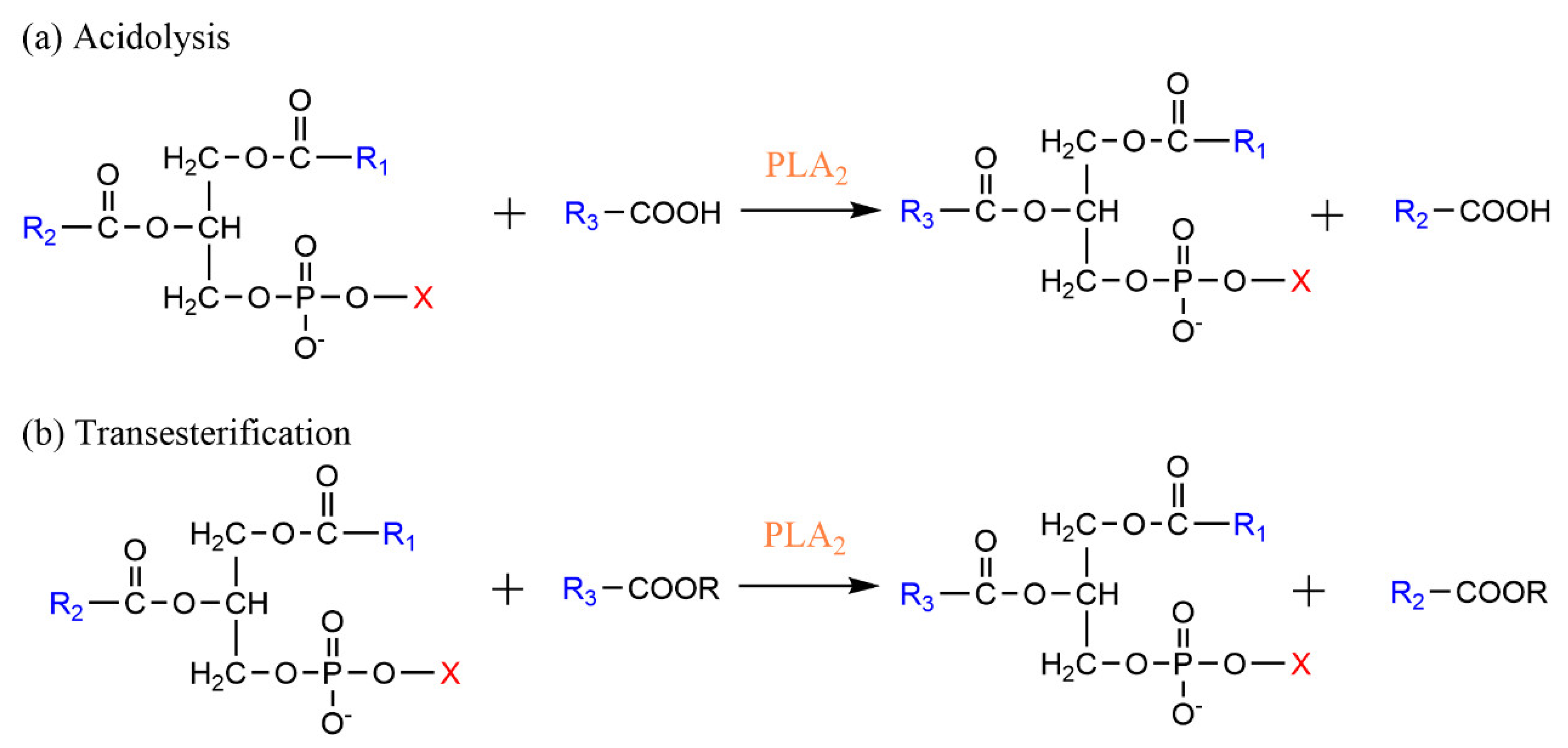
3.1. Phospholipase A-Mediated the Preparation of Structured Phospholipid
3.2. Phospholipase D-Mediated the Preparation of Structured Phospholipid
4. Factors Influencing Enzyme-Mediated Structured Phospholipids and Related Mechanism
4.1. Reaction Medium/Solvent
4.2. Acyl Migration
4.3. Water Content/Activity
4.4. Oher Conditions
5. Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ang, X.; Chen, H.; Xiang, J.Q.; Wei, F.; Quek, S.Y. Preparation and functionality of lipase-catalysed structured phospholipid—A review. Trends Food Sci. Technol. 2019, 88, 373–383. [Google Scholar] [CrossRef]
- Sun, N.; Chen, J.; Wang, D.; Lin, S.Y. Advance in food-derived phospholipids: Sources, molecular species and structure as well as their biological activities. Trends Food Sci. Technol. 2018, 80, 199–211. [Google Scholar] [CrossRef]
- Song, F.; Zhang, S.; Tian, S. Effect evaluation and analysis of phospholipids on liposomes properties. China Oils Fats 2020, 45, 32. [Google Scholar]
- Chandran, M.P.S.; Prasobh, G.R.; Jaghatha, T.; Aswathy, B.S.; Remya, S.B. An Overview on Liposomal Drug Delivery System. Int. J. Pharm. Phytopharm. Res. 2019, 9, 61–68. [Google Scholar]
- Hama, S.; Ogino, C.; Kondo, A. Enzymatic synthesis and modification of structured phospholipids: Recent advances in enzyme preparation and biocatalytic processes. Appl. Microbiol. Biotechnol. 2015, 99, 7879–7891. [Google Scholar] [CrossRef]
- Balakrishna, M.; Kaki, S.S.; Karuna, M.S.L.; Sarada, S.; Kumar, C.G.; Prasad, R.B.N. Synthesis and in vitro antioxidant and antimicrobial studies of novel structured phosphatidylcholines with phenolic acids. Food Chem. 2017, 221, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Petelska, A.D.; Kazimierska-Drobny, K.; Janicka, K.; Majewski, T.; Urbaniak, W. Understanding the Unique Role of Phospholipids in the Lubrication of Natural Joints: An Interfacial Tension Study. Coatings 2019, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.W.; Han, J.Z.; Ye, A.Q.; Liu, W.L.; Tian, M.M.; Lu, Y.J.; Wu, K.R.; Liu, J.; Lou, M.P.Z. Influence of Phospholipids Structure on the Physicochemical Properties and In Vitro Digestibility of Lactoferrin-Loaded Liposomes. Food Biophys. 2019, 14, 287–299. [Google Scholar] [CrossRef]
- Cavazos-Garduno, A.; Flores, A.A.O.; Serrano-Nino, J.C.; Martinez-Sanchez, C.E.; Beristain, C.I.; Garcia, H.S. Preparation of betulinic acid nanoemulsions stabilized by omega-3 enriched phosphatidylcholine. Ultrason. Sonochem. 2015, 24, 204–213. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Wu, X.; Guo, Y.; Song, T.; Li, Z.; Song, D.; Guan, H. Preparation and Characterization of Usnic acid Phospholipid Complex. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 20–25. [Google Scholar]
- Qian, J.J.; Cheng, W.Y.; Zhang, C.Y.; Hong, L.F.; Chen, W.D.; Li, G.X. Preparation, physicochemical characterization and pharmacokinetics of paeoniflorin-phospholipid complex. Bio-Med. Mater. Eng. 2019, 30, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Palko-Labuz, A.; Gliszczynska, A.; Skonieczna, M.; Pola, A.; Wesolowska, O.; Sroda-Pomianek, K. Conjugation with Phospholipids as a Modification Increasing Anticancer Activity of Phenolic Acids in Metastatic Melanoma-In Vitro and In Silico Studies. Int. J. Mol. Sci. 2021, 22, 8397. [Google Scholar] [CrossRef] [PubMed]
- Hachem, M.; Nacir, H.; Picq, M.; Belkouch, M.; Bernoud-Hubac, N.; Windust, A.; Meiller, L.; Sauvinet, V.; Feugier, N.; Lambert-Porcheron, S.; et al. Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC((R)). Nutrients 2020, 12, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachem, M.; Belkouch, M.; Lo Van, A.; Picq, M.; Bernoud-Hubac, N.; Lagarde, M. Brain targeting with docosahexaenoic acid as a prospective therapy for neurodegenerative diseases and its passage across blood brain barrier. Biochimie 2020, 170, 203–211. [Google Scholar] [CrossRef]
- Zhang, J.H.; Cheng, K.; Li, H.Y.; Yin, F.W.; Wang, Q.E.; Cui, L.; Yang, S.S.; Nie, J.G.; Zhou, D.Y.; Zhu, B.W. Efficient Synthesis of Structured Phospholipids Containing Short-Chain Fatty Acids over a Sulfonated Zn-SBA-15 Catalyst. J. Agric. Food Chem. 2020, 68, 12444–12453. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Chen, M.; Xu, W.; Zhang, W.L.; Zhang, T.; Guang, C.E.; Mu, W.M. Microbial phospholipase D: Identification, modification and application. Trends Food Sci. Technol. 2020, 96, 145–156. [Google Scholar] [CrossRef]
- Goncalves, D.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, immobilization methods, and industrial applications. Appl. Microbiol. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef]
- Arhab, Y.; Abousalham, A.; Noiriel, A. Plant phospholipase D mining unravels new conserved residues important for catalytic activity. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2019, 1864, 688–703. [Google Scholar] [CrossRef]
- Mahima, G.; Sumati, H.; Krishnan, H. Bacterial Lipases: An Overview of Sources and Production. Res. J. Biotechnol. 2020, 15, 158–171. [Google Scholar]
- Wang, X.J.; Du, H.; Wang, Z.; Mu, W.; Han, X.J. Versatile Phospholipid Assemblies for Functional Synthetic Cells and Artificial Tissues. Adv. Mater. 2021, 33. [Google Scholar] [CrossRef]
- Sandoval, G. Methods in Molecular Biology. In Lipases and Phospholipases; Springer: New York, NY, USA, 2018; Volume 1835, pp. 3–38. [Google Scholar] [CrossRef]
- Baeza-Jimenez, R.; Lopez-Martinez, L.X.; Garcia, H.S. Biocatalytic Modification of Food Lipids: Reactions and Applications. Rev. Mex. Ing. Quim. 2014, 13, 29–47. [Google Scholar]
- Wang, X.M.; Qin, X.L.; Li, X.T.; Zhao, Z.X.; Yang, B.; Wang, Y.H. An Efficient Synthesis of Lysophosphatidylcholine Enriched with n-3 Polyunsaturated Fatty Acids by Immobilized MAS1 Lipase. J. Agric. Food Chem. 2020, 68, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.I.; Kim, Y.; Kim, C.T.; Kim, I.H. Enzymatic synthesis of lysophosphatidylcholine containing CLA from sn-glycero-3-phosphatidylcholine (GPC) under vacuum. Food Chem. 2011, 129, 1–6. [Google Scholar] [CrossRef]
- Okulus, M.; Gliszczynska, A. Enzymatic Synthesis of O-Methylated Phenophospholipids by Lipase-Catalyzed Acidolysis of Egg-Yolk Phosphatidylcholine with Anisic and Veratric Acids. Catalysts 2020, 10, 538. [Google Scholar] [CrossRef]
- Rychlicka, M.; Niezgoda, N.; Gliszczynska, A. Lipase-Catalyzed Acidolysis of Egg-Yolk Phosphatidylcholine with Citronellic Acid. New Insight into Synthesis of Isoprenoid-Phospholipids. Molecules 2018, 23, 314. [Google Scholar] [CrossRef] [Green Version]
- Estiasih, T.; Marianty, R.; Ahmadi, K. Characteristics and emulsifying properties of structured phospholipids from palm pressed fiber and omega-3 fatty acid concentrates from by-products of fish processing by enzymatic acidolysis. J. Food Sci. Technol.-Mysore 2021, 58, 3689–3700. [Google Scholar] [CrossRef]
- Niezgoda, N.; Gliszczynska, A.; Gladkowski, W.; Chojnacka, A.; Kielbowicz, G.; Wawrzenczyk, C. Lipase-catalyzed enrichment of egg yolk phosphatidylcholine with conjugated linoleic acid. J. Mol. Catal. B-Enzym. 2016, 123, 14–22. [Google Scholar] [CrossRef]
- Balcao, V.M.; Malcata, F.X. Lipase catalyzed modification of milkfat. Biotechnol. Adv. 1998, 16, 309–341. [Google Scholar] [CrossRef]
- Niezgoda, N.; Gliszczynska, A. Lipase Catalyzed Acidolysis for Efficient Synthesis of Phospholipids Enriched with Isomerically Pure cis-9,trans-11 and trans-10,cis-12 Conjugated Linoleic Acid. Catalysts 2019, 9, 1012. [Google Scholar] [CrossRef] [Green Version]
- Niezgoda, N.; Gliszczynska, A.; Gladkowski, W.; Chojnacka, A.; Kielbowicz, G.; Wawrzenczyk, C. Production of concentrates of CLA obtained from sunflower and safflower and their application to the lipase-catalyzed acidolysis of egg yolk phosphatidylcholine. Eur. J. Lipid Sci. Technol. 2016, 118, 1566–1578. [Google Scholar] [CrossRef]
- Rychlicka, M.; Gliszczynska, A. Interesterification of Egg-Yolk Phosphatidylcholine with p-Methoxycinnamic Acid Catalyzed by Immobilized Lipase B from Candida Antarctica. Catalysts 2020, 10, 1181. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.; Xu, X. Parameters affecting incorporation and by-product formation during the production of structured phospholipids by lipase-catalyzed acidolysis in solvent-free system. J. Mol. Catal. B-Enzym. 2005, 36, 14–21. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.L.; Xu, X.B. Lipase-catalyzed acyl exchange of soybean phosphatidylcholine in n-hexane: A critical evaluation of both acyl incorporation and product recovery. Biotechnol. Prog. 2005, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Vikbjerg, A.F.; Peng, L.F.; Mu, H.L.; Xu, X.B. Continuous production of structured phospholipids in a packed red reactor with lipase from Thermomyces lanuginosa. J. Am. Oil Chem. Soc. 2005, 82, 237–242. [Google Scholar] [CrossRef]
- Peng, L.F.; Xu, X.B.; Mu, H.L.; Hoy, C.E.; Adler-Nissen, J. Production of structured phospholipids by lipase-catalyzed acidolysis: Optimization using response surface methodology. Enzym. Microb. Technol. 2002, 31, 523–532. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.L.; Xu, X.B. Synthesis of structured phospholipids by immobilized phospholipase A(2) catalyzed acidolysis. J. Biotechnol. 2007, 128, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rychlicka, M.; Niezgoda, N.; Gliszczynska, A. Development and Optimization of Lipase-Catalyzed Synthesis of Phospholipids Containing 3,4-Dimethoxycinnamic Acid by Response Surface Methodology. Catalysts 2020, 10, 588. [Google Scholar] [CrossRef]
- Marsaoui, N.; Naghmouchi, K.; Baah, J.; Raies, A.; Laplante, S. Incorportation of Ethyl Esters of EPA and DHA in Soybean Lecithin Using Rhizomucor miehei Lipase: Effect of Additives and Solvent-Free Conditions. Appl. Biochem. Biotechnol. 2015, 176, 938–946. [Google Scholar] [CrossRef]
- Wang, F.H.; Chen, W.C.; Abousalham, A.; Yang, B.; Wang, Y.H. Exploring the influence of phospholipid monolayer conformation and environmental conditions on the interfacial binding of Gibberella Zeae lipase. Int. J. Biol. Macromol. 2019, 132, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Chojnacka, A.; Adkowski, W.; Bowicz, G.; Wawrzeńczyk, C. Enzymatic enrichment of egg-yolk phosphatidylcholine with α-linolenic acid. Biotechnol. Lett. 2009, 31, 705–709. [Google Scholar] [CrossRef]
- Chojnacka, A.; Gladkowski, W.; Gliszczynska, A.; Niezgoda, N.; Kielbowicz, G.; Wawrzenczyk, C. Synthesis of structured phosphatidylcholine containing punicic acid by the lipase-catalyzed transesterification with pomegranate seed oil. Catal. Commun. 2016, 75, 60–64. [Google Scholar] [CrossRef]
- Yang, G.L.; Yang, L.H. Increase of Oleic Acid Content in Phosphatidylcholine through Lipase-catalyzed Interesterification: Optimization by Response Surface Methodology. J. Oleo Sci. 2015, 64, 673–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacka, A.; Gladkowski, W. Production of Structured Phosphatidylcholine with High Content of Myristic Acid by Lipase-Catalyzed Acidolysis and Interesterification. Catalysts 2018, 8, 281. [Google Scholar] [CrossRef] [Green Version]
- Adlercreutz, P.; Lyberg, A.M.; Adlercreutz, D. Enzymatic fatty acid exchange in glycerophospholipids. Eur. J. Lipid Sci. Technol. 2003, 105, 638–645. [Google Scholar] [CrossRef]
- Samantha, A.; Damnjanovic, J.; Iwasaki, Y.; Nakano, H.; Vrielink, A. Structures of an engineered phospholipase D with specificity for secondary alcohol transphosphatidylation: Insights into plasticity of substrate binding and activation. Biochem. J. 2021, 478, 1749–1767. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Chu, W.Q.; Sun, J.N.; Liu, Z.; Huang, W.C.; Xue, C.H.; Mao, X.Z. Combining Cell Surface Display and DNA-Shuffling Technology for Directed Evolution of Streptomyces Phospholipase D and Synthesis of Phosphatidylserine. J. Agric. Food Chem. 2019, 67, 13119–13126. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Chu, W.Q.; Sun, J.A.; Liu, Z.; Huang, W.C.; Xue, C.H.; Mao, X.Z. A novel autolysis system for extracellular production and direct immobilization of a phospholipase D fused with cellulose binding domain. Bmc Biotechnol. 2019, 19, 29. [Google Scholar] [CrossRef]
- Damnjanovic, J.; Nakano, H.; Iwasaki, Y. Acyl chain that matters: Introducing sn-2 acyl chain preference to a phospholipase D by protein engineering. Protein Eng. Des. Sel. 2019, 32, 1–11. [Google Scholar] [CrossRef]
- Durrani, R.; Khan, F.I.; Ali, S.; Wang, Y.H.; Yang, B. A Thermolabile Phospholipase B from Talaromyces marneffei GD-0079: Biochemical Characterization and Structure Dynamics Study. Biomolecules 2020, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Wang, X.T.; Li, G.L.; Zeng, J.; Li, J.; Liu, J.W. SS-mPEG chemical modification of recombinant phospholipase C for enhanced thermal stability and catalytic efficiency. Int. J. Biol. Macromol. 2018, 111, 1032–1039. [Google Scholar] [CrossRef]
- Mitula, P.; Wawrzenczyk, C.; Gladkowski, W. Comparative Studies on the Susceptibility of (R)-2,3-Dipalmitoyloxypropylphosphonocholine (DPPnC) and Its Phospholipid Analogues to the Hydrolysis or Ethanolysis Catalyzed by Selected Lipases and Phospholipases. Catalysts 2021, 11, 129. [Google Scholar] [CrossRef]
- Verdasco-Martin, C.M.; Corchado-Lopo, C.; Fernandez-Lafuente, R.; Otero, C. Prolongation of secondary drying step of phospholipid lyophilization greatly improves acidolysis reactions catalyzed by immobilized lecitase ultra. Enzym. Microb. Technol. 2020, 132, 109388. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Cui, Y.; Shen, Q.; Zheng, Z.; Dai, Z. Enzymatic Preparation of EPA/DHA-Rich Phospholipids and Structure Characterization. J. Nucl. Agric. Sci. 2020, 34, 2780–2792. [Google Scholar]
- Chen, W.X.; Guo, W.; Gao, F.; Chen, L.M.; Chen, S.L.; Li, D.M. Phospholipase A1-Catalysed Synthesis of Docosahexaenoic Acid-Enriched Phosphatidylcholine in Reverse Micelles System. Appl. Biochem. Biotechnol. 2017, 182, 1037–1052. [Google Scholar] [CrossRef]
- Ochoa-Flores, A.A.; Hernandez-Becerra, J.A.; Cavazos-Garduno, A.; Vernon-Carters, E.J.; Garcia, H.S. Optimization of the Synthesis of Structured Phosphatidylcholine with Medium Chain Fatty Acid. J. Oleo Sci. 2017, 66, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Li, D.M.; Qin, X.L.; Wang, W.F.; Li, Z.G.; Yang, B.; Wang, Y.H. Synthesis of DHA/EPA-rich phosphatidylcholine by immobilized phospholipase A(1): Effect of water addition and vacuum condition. Bioprocess Biosyst. Eng. 2016, 39, 1305–1314. [Google Scholar] [CrossRef]
- Gan, L.J.; Wang, X.Y.; Yang, D.; Zhang, H.; Shin, J.A.; Hong, S.T.; Park, S.H.; Lee, K.T. Emulsifying Properties of Lecithin Containing Different Fatty Acids Obtained by Immobilized Lecitase Ultra-Catalyzed Reaction. J. Am. Oil Chem. Soc. 2014, 91, 579–590. [Google Scholar] [CrossRef]
- Zhao, T.; No, D.S.; Kim, B.H.; Garcia, H.S.; Kim, Y.; Kim, I.H. Immobilized phospholipase A1-catalyzed modification of phosphatidylcholine with n-3 polyunsaturated fatty acid. Food Chem. 2014, 157, 132–140. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.F.; Yang, B.; Li, D.M.; Wang, Y.H.; Wang, W.F. Production of Structured Phosphatidylcholine with High Content of DHA/EPA by Immobilized Phospholipase A(1)-Catalyzed Transesterification. Int. J. Mol. Sci. 2014, 15, 15244–15258. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, A.A.; Hernandez-Becerra, J.A.; Cavazos-Gardun, A.; Garcia, H.S.; Vernon-Carter, E.J. Phosphatidylcholine enrichment with medium chain fatty acids by immobilized phospholipase A(1)-catalyzed acidolysis. Biotechnol. Prog. 2013, 29, 230–236. [Google Scholar] [CrossRef]
- Baeza-Jimenez, R.; Gonzalez-Rodriguez, J.; Kim, I.H.; Garcia, H.S.; Otero, C. Use of immobilized phospholipase A(1)catalyzed acidolysis for the production of structured phosphatidylcholine with an elevated conjugated linoleic acid content. Grasas Y Aceites 2012, 63, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.H.; Garcia, H.S.; Hill, C.G. Synthesis of Structured Phosphatidylcholine Containing n-3 PUFA Residues via Acidolysis Mediated by Immobilized Phospholipase A(1). J. Am. Oil Chem. Soc. 2010, 87, 1293–1299. [Google Scholar] [CrossRef]
- Garcia, H.S.; Kim, I.H.; Lopez-Hernandez, A.; Hill, C.G. Enrichment of lecithin with n-3 fatty acids by acidolysis using immobilized phospholipase A1. Grasas Y Aceites 2008, 59, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yoon, S.H. Effects of organic solvents on transesterification of phospholipids using phospholipase A(2) and lipase. Food Sci. Biotechnol. 2014, 23, 1207–1211. [Google Scholar] [CrossRef]
- More, H.T.; Pandit, A.B. Enzymatic acyl modification of phosphatidylcholine using immobilized lipase and phospholipase A(2). Eur. J. Lipid Sci. Technol. 2010, 112, 428–433. [Google Scholar] [CrossRef]
- Baeza-Jimenez, R.; Noriega-Rodriguez, J.A.; Garcia, H.S.; Otero, C. Structured phosphatidylcholine with elevated content of conjugated linoleic acid: Optimization by response surface methodology. Eur. J. Lipid Sci. Technol. 2012, 114, 1261–1267. [Google Scholar] [CrossRef]
- Hu, R.K.; Cui, R.G.; Lan, D.M.; Wang, F.H.; Wang, Y.H. Acyl Chain Specificity of Marine Streptomyces klenkii PhosPholipase D and Its Application in Enzymatic Preparation of Phosphatidylserine. Int. J. Mol. Sci. 2021, 22, 10580. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.Y.; Zhang, X.L.; Zhang, T.T.; Wang, H.; Shu, W.F.; Liu, L.; Zhao, B.X.; Li, B.L. Two-step modification of silica for efficient adsorption of phosphatidylcholine in water and application in phospholipase D-catalyzed transphosphatidylation. Biotechnol. Prog. 2020, 36, e2949. [Google Scholar] [CrossRef]
- Casado, V.; Reglero, G.; Torres, C.F. Production and Scale-up of phosphatidyl-tyrosol catalyzed by a food grade phospholipase D. Food Bioprod. Process. 2013, 91, 599–608. [Google Scholar] [CrossRef]
- Song, S.; Cheong, L.Z.; Guo, Z.; Kristensen, K.; Glasius, M.; Jensen, H.M.; Bertelsen, K.; Tan, T.; Xu, X. Phospholipase D (PLD) catalyzed synthesis of phosphatidyl-glucose in biphasic reaction system. Food Chem. 2012, 135, 373–379. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kurihara, H.; Miyashita, K.; Hosokawa, M. Synthesis of novel phospholipids that bind phenylalkanols and hydroquinone via phospholipase D-catalyzed transphosphatidylation. New Biotechnol. 2011, 28, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vikbjerg, A.F.; Rusig, J.Y.; Jonsson, G.; Mu, H.L.; Xu, X.B. Strategies for-lipase-catalyzed production and the purification of structured phospholipids. Eur. J. Lipid Sci. Technol. 2006, 108, 802–811. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.L.; Xu, X.B. Elucidation of acyl migration during lipase-catalyzed production of structured phospholipids. J. Am. Oil Chem. Soc. 2006, 83, 609–614. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Zhang, L.X.; Qu, L.B.; Wang, X.G.; Liu, Y.F. Mechanism and Effective Factors of Acyl Migration of Lysophospholipid. Chin. J. Org. Chem. 2014, 34, 2529–2536. [Google Scholar] [CrossRef] [Green Version]
- Poisson, L.; Devos, M.; Godet, S.; Ergan, F.; Pencreac’h, G. Acyl migration during deacylation of phospholipids rich in docosahexaenoic acid (DHA): An enzymatic approach for evidence and study. Biotechnol. Lett. 2009, 31, 743–749. [Google Scholar] [CrossRef]
- Pacheco, C.; Crapiste, G.H.; Carrin, M.E. Study of acyl migration during enzymatic interesterification of liquid and fully hydrogenated soybean oil. J. Mol. Catal. B-Enzym. 2015, 122, 117–124. [Google Scholar] [CrossRef]
- Svensson, I.; Adlercreutz, P.; Mattiasson, B. Interesterification of Phosphatidylcholine with Lipases in Organic Media. Appl. Microbiol. Biotechnol. 1990, 33, 255–258. [Google Scholar] [CrossRef]
- Vikbjerg, A.F.; Mu, H.L.; Xu, X.B. Monitoring of monooctanoylphosphatidylcholine synthesis by enzymatic acidolysis between soybean phosphatidylcholine and caprylic acid by thin-layer chromatography with a flame ionization detector. J. Agric. Food Chem. 2005, 53, 3937–3942. [Google Scholar] [CrossRef]
- Lim, C.W.; Kim, B.H.; Kim, I.H.; Lee, M.W. Modeling and Optimization of Phospholipase A(1)-Catalyzed Hydrolysis of Phosphatidylcholine Using Response Surface Methodology for Lysophosphatidylcholine Production. Biotechnol. Prog. 2015, 31, 35–41. [Google Scholar] [CrossRef]
- Wang, X.M.; Qin, X.L.; Li, X.T.; Zhao, Z.X.; Yang, B.; Wang, Y.H. Insight into the Modification of Phosphatidylcholine with n-3 Polyunsaturated Fatty Acids-Rich Ethyl Esters by Immobilized MAS1 Lipase. Molecules 2019, 24, 3528. [Google Scholar] [CrossRef] [Green Version]
- Egger, D.; Wehtje, E.; Adlercreutz, P. Characterization and optimization of phospholipase A(2) catalyzed synthesis of phosphatidylcholine. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 1997, 1343, 76–84. [Google Scholar] [CrossRef]
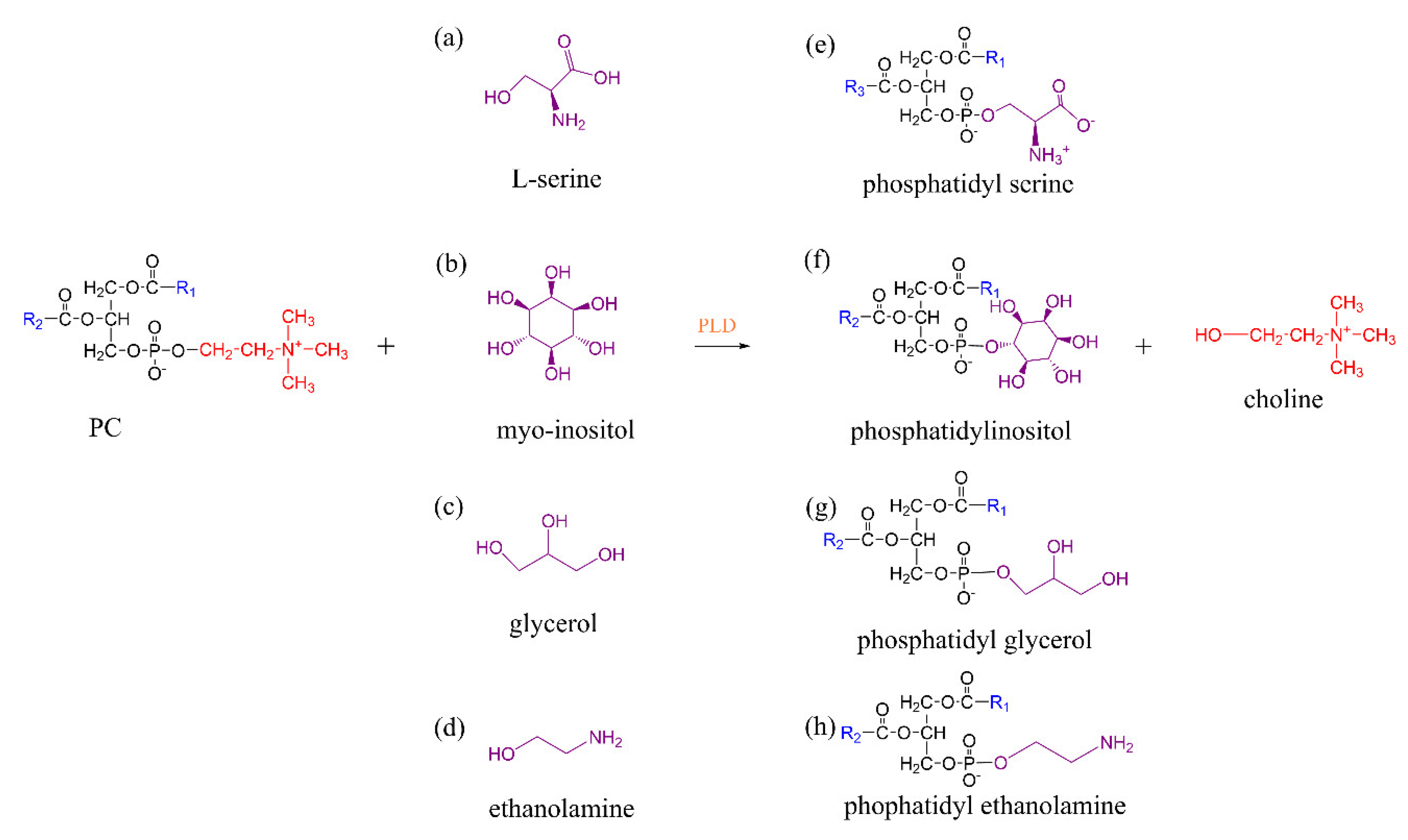
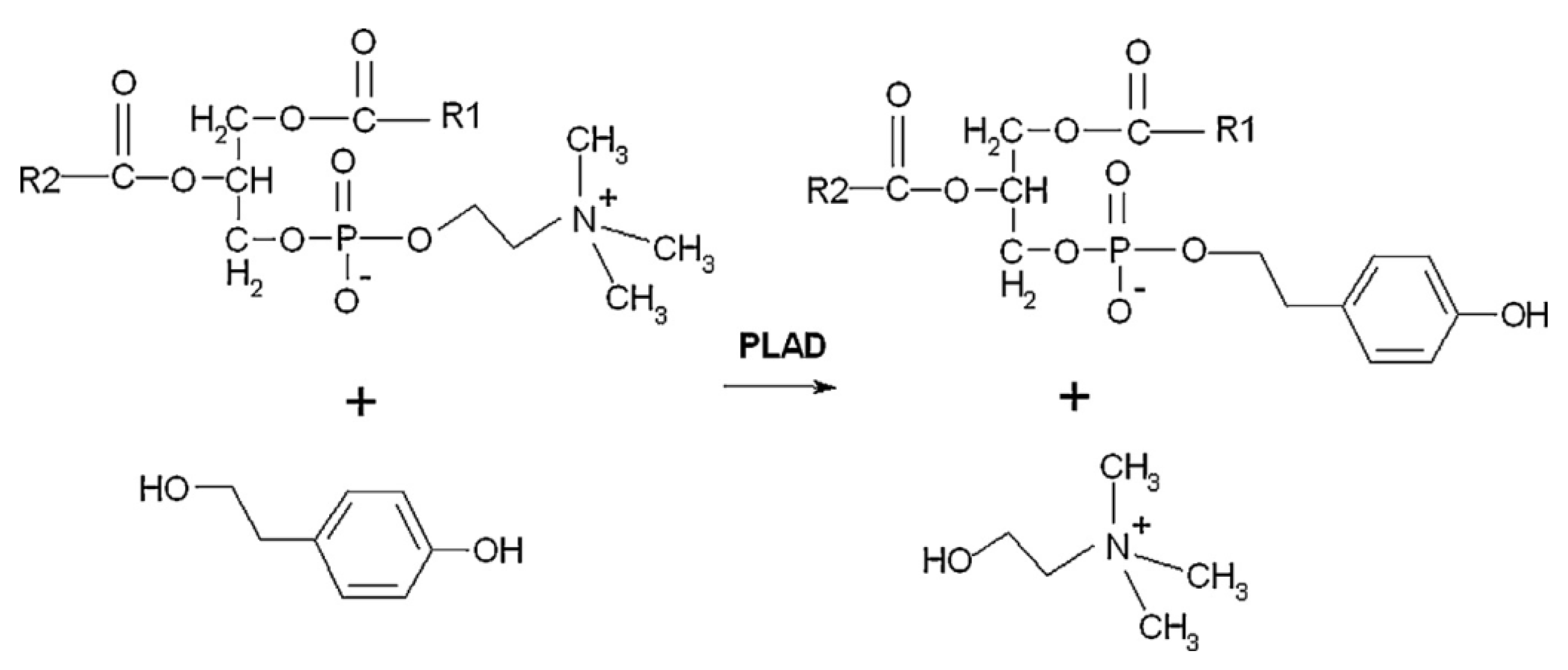

| FFA Type | Lipase | Reaction Conditions | Yield (%) | Reference |
|---|---|---|---|---|
| DHA and EPA from four different fish oil sources | RML (Lipase from Rhizomucor miehei) | 40 °C, 24 h around, 1/3.6 around substrate molar ratio SPLs/ω-3 fatty acids, 20% (w/w) enzyme load in organic solvent (hexane) | 70% ω-3 fatty acids-SPLs | [27] |
| Conjugated linoleic acid (CLA) | RML (Lipase from Rhizomucor miehei) | 45 °C, 48 h, 1/6 substrate molar ratio PC/CLA, 24% (w/w) enzyme load in organic solvent (heptane and 5 v/v% DMF) | 50% incorporation of CLA isomers into PC | [30] |
| Conjugated linoleic acid (CLA) | RML (Lipase from Rhizomucor miehei) | 45 °C, 36 h, 1/8 substrate molar ratio PC/CLA, 24% (w/w) enzyme load in organic solvent (heptane) | 33.8% and 50.1% incorporation of CLA into PC and LPC, respectively | [28] |
| Conjugated linoleic acid (CLA) obtained from sunflower and safflower | RML (Lipase from Rhizomucor miehei) | 45 °C, 36 h, 1/8 substrate molar ratio PC/CLA, 24% (w/w) enzyme load in organic solvent (heptane) | 42% incorporation of CLA into PC | [31] |
| Anisic (ANISA) and veratric (VERA) | Novozyme 435 (Lipase B from Candida Antarctica) | 50 °C, 72 h, 1/15 substrate molar ratio PC/ANISA, 30% (w/w) enzyme load in the selected binary solvent system | 28.5% (w/w) ANISA-LPC and 2.5% (w/w) ANISA-PC | [25] |
| p-Methoxcinnamic acid (p-MCA) | Novozyme 435 (Lipase B from Candida Antarctica) | 50 °C, 72 h, 1/10 substrate molar ratio PC/Ep-MCA, 30% (w/w) enzyme load in a binary solvent system of toluene/chloroform 9/1 (v/v) | 32% (w/w) p-MCA-LPC and 3% (w/w) p-MCA-PC | [32] |
| Citronellic Acid | Novozyme 435 (Lipase B from Candida Antarctica) | 30 °C, 48 h, 1/60 substrate molar ratio PC/CA, 30% enzyme load in organic solvent (toluene) | 33% CA-PC | [26] |
| Caprylic acid | Lipozyme TL IM (Lipase from Thermomyces lanuginose) | 55 °C, 70 h, 1/6 substrate molar ratio PC/caprylic acid, 40% enzyme load in a solvent-free system | 46% incorporation of caprylic acid into PC | [33] |
| Caprylic acid | Lipozyme TL IM (Lipase from Thermomyces lanuginose) | 54 °C, 50 h, 1/15 substrate molar ratio PC/caprylic acid, 29% enzyme load in organic solvent (hexane) | 46% incorporation of caprylic acid into PC | [34] |
| Caprylic acid | Lipozyme TL IM (Lipase from Thermomyces lanuginose) | Continuous production in a packed bed reactor | 25% incorporation of caprylic acid into PC | [35] |
| Caprylic acid | Lipozyme TL IM (Lipase from Thermomyces lanuginose) | 57 °C, 70 h, 1/5.5 substrate molar ratio PLs/caprylic acid, 30% (w/w) enzyme load in a solvent-free system | 39% incorporation of caprylic acid into PLs | [36] |
| PL Source | Acyl Donor | Lipase | Reaction Conditions | Yield (%) | Reference |
|---|---|---|---|---|---|
| Palm pressed fiber PLs | DHA and EPA from four different fish oil sources | RML (Lipase from Rhizomucor miehei) | 40 °C, 24 h around, 1/3.6 around substrate molar ratio SPLs/ω-3 fatty acids, 20% (w/w) enzyme load in organic solvent (hexane) | 70% ω-3 fatty acids-SPLs | [27] |
| Egg yolk PC | Conjugated linoleic acid (CLA) | RML (Lipase from Rhizomucor miehei) | 45 °C, 48 h, 1/6 substrate molar ratio PC/CLA, 24% (w/w) enzyme load in organic solvent (heptane and 5 v/v% DMF) | 50% incorporation of CLA isomers into PC | [30] |
| Egg yolk PC | Conjugated linoleic acid (CLA) | RML (Lipase from Rhizomucor miehei) | 45 °C, 36 h, 1/8 substrate molar ratio PC/CLA, 24% (w/w) enzyme load in organic solvent (heptane) | 33.8% and 50.1% incorporation of CLA into PC and LPC, respectively | [28] |
| Egg yolk PC | Conjugated linoleic acid (CLA) obtained from sunflower and safflower | RML (Lipase from Rhizomucor miehei) | 45 °C, 36 h, 1/8 substrate molar ratio PC/CLA, 24% (w/w) enzyme load in organic solvent (heptane) | 42% incorporation of CLA into PC | [31] |
| Egg yolk PC | Anisic (ANISA) and veratric (VERA) | Novozyme 435 (Lipase B from Candida Antarctica) | 50 °C, 72 h, 1/15 substrate molar ratio PC/ANISA, 30% (w/w) enzyme load in the selected binary solvent system | 28.5% (v/v) ANISA-LPC and 2.5% (v/v) ANISA-PC | [25] |
| Egg yolk PC | p-Methoxcinnamic acid (p-MCA) | Novozyme 435 (Lipase B from Candida Antarctica) | 50 °C, 72 h, 1/10 substrate molar ratio PC/Ep-MCA, 30% (w/w) enzyme load in a binary solvent system of toluene/chloroform 9/1 (v/v) | 32% (w/w) p-MCA-LPC and 3% (w/w) p-MCA-PC | [32] |
| Egg yolk PC | Citronellic Acid | Novozyme 435 (Lipase B from Candida Antarctica) | 30 °C, 48 h, 1/60 substrate molar ratio PC/CA, 30% enzyme load in organic solvent (toluene) | 33% CA-PC | [26] |
| Soy PC | Caprylic acid | Lipozyme TL IM (Lipase from Thermomyces lanuginose) | 55 °C, 70 h, 1/6 substrate molar ratio PC/caprylic acid, 40% enzyme load in a solvent-free system | 46% incorporation of caprylic acid into PC | [33] |
| Soy PC | Caprylic acid | Lipozyme TL IM (Lipase from Thermomyces lanuginose) | 54 °C, 50 h, 1/15 substrate molar ratio PC/caprylic acid, 29% enzyme load in organic solvent (hexane) | 46% incorporation of caprylic acid into PC | [34] |
| Soy PC | Caprylic acid | Lipozyme TL IM (Lipase from Thermomyces lanuginose) | Continuous production in a packed bed reactor | 25% around the incorporation of caprylic acid into PC | [35] |
| Soy PLs | Caprylic acid | Lipozyme TL IM (Lipase from Thermomyces lanuginose) | 57 °C, 70 h, 1/5.5 substrate molar ratio PLs/caprylic acid, 30% (w/w) enzyme load in a solvent-free system | 39% incorporation of caprylic acid into PLs | [36] |
| PL Source | Acyl Donor | Enzyme | Reaction Conditions | Yield (%) | Reference |
|---|---|---|---|---|---|
| Soy PC | - | PLA1 | 60 °C, 9 h, 200 U/g enzyme load in water system | over 100% hydrolysis rate | [52] |
| Soy PC | Conjugated linolenic acid (CLA) | PLA1 immobilized on Duolite A658 | 50 °C, at least 24 h, 1/4 substrate molar ratio of PC/CLA, and 15% (w/w) enzyme load | Maximal (>99%) molar yield of structured PC with 72.3% CLA content | [53] |
| Antarctic krill PLs | EPA and DHA | PLA1 | 55.22 °C, 24 h, 1/5.15 substrate molar ratio of PLs/FFA, 20% (w/w) enzyme load in organic solvent (hexane) | 64.35% incorporation of EPA/DHA into PLs | [54] |
| Soy PC | DHA-enriched fatty acids | PLA1 | 45 °C, 24 h, 1/2.13 substrate molar ratio of PC/fatty acids, 40% (w/w) enzyme load in the selected binary solvent system | 20.90% incorporation of DHA into PC | [55] |
| Soy PC | Free medium-chain fatty acid | PLA1 immobilized on Duolite A568 | 45 °C, 24 h, 1/15 substrate molar ratio of PC/free medium-chain fatty acid, 12% (w/w) enzyme load in solvent-free system | 52.98% modified PC | [56] |
| Soy PC | DHA/EPA-rich ethyl esters | PLA1 immobilized on macroporous resin | 55.7 °C, 24 h, 1/6.8 substrate molar ratio of PC/ethyl ester, and 15% (w/w) enzyme load | 19.09% incorporation of DPA/EPA into PC | [57] |
| Soy PC | Capric acid | PLA1 immobilized on Amberlite XAD 7HP | 45 °C, 72 h, 1/10 substrate molar ratio of PC/capric acid, 10% (w/w) enzyme load in organic solvent (hexane) | 51.0 mol% incorporation of capric acid into PC | [58] |
| Soy PC | Fatty acid enriched with n-3 PUFA from fish oil | PLA1 immobilized on VP OC 1600 | 55 °C, 24 h, 1/8 substrate molar ratio of PC/fatty acids, 20% (w/w) enzyme load in a solvent-free system | 57.4 mol% incorporation of n-3 PUFA into PC | [59] |
| Soy PC | DHA/EPA-rich ethyl esters | PLA1 immobilized on resin D380 | 55 °C, 24 h, 1/6 substrate molar ratio of PC/ethyl esters, 15% (w/w) enzyme load in solvent-free system | 16.5% PC, 26.3% 1-LPC, 31.4% 2-LPC and 25.8% GPC | [56,60] |
| Soy PC | Medium-chain fatty acids (MCFAs) | PLA1 immobilized on Duolite A568 | 50 °C, 72 h, 1/16 substrate molar ratio of PC/MCFAs, 16% (w/w) enzyme load in solvent-free system | 41% incorporation of free MCFAs into PC | [61] |
| Soy PC | Conjugated linolenic acid (CLA) | PLA1 immobilized on Duolite A568 | 50 °C, 24 h, 1/4 substrate molar ratio of PC/CLA, and 15% (w/w) enzyme load | 90% incorporation of CLA into PC | [62] |
| Granulated PC | Free fatty acids enriched in EPA and DHA from a fish oil concentrate | PLA1 from Thermomyces lanuginosus/Fusarium oxysporum immobilized on Duolite A568 | 50 °C, 48 h, 1/8 substrate molar ratio of PC/FFA, 10% (w/w) enzyme load in solvent-free system | 50 mol% around n-3 PUFA content of total LPC residues | [63] |
| Soy PC | Concentrated fish oil enriched in n-3 fatty acids | PLA1 immobilized on Duolite A568 | 50 °C, 12 h, 1/8 substrate molar ratio of PC/free fatty acids, 10% (w/w) enzyme load in solvent-free system | Nearly 35% of the total esterified fatty acid residues were n-3 species (EPA, DPA, or DHA) | [64] |
| Soy PC | Medium-chain fatty acids (MCFAs) | PLA2 | 40 °C, 70 h, 1/6 substrate molar ratio of PC/caprylic acid, 40% (w/w) enzyme load in organic solvent (hexane) | 87.7% incorporation of caprylic acid into PC | [65] |
| Soy PC | Caprylic acid | PLA2 immobilized on the hydrophobic resin Diaion HP-20 | 50 °C, 48 h, 1/12 substrate molar ratio of PC/caprylic acid, 50% (w/w) enzyme load in organic solvent (hexane) | 45.29 mol% the ML-type PC (M: medium-chain fatty acid; L: long-chain fatty acid) | [66] |
| Epikuron 200 (PC, 93%) | Caprylic acid | PLA2 immobilized on Amberlite XAD7 | 45 °C, 48 h, 1/9 substrate molar ratio of PC/caprylic acid, 30% (w/w) enzyme load in solvent-free system | 36% incorporation of caprylic acid | [37] |
| PL Source | Acceptor Alcohol | Enzyme | Reaction Conditions | Yield (%) | Reference |
|---|---|---|---|---|---|
| Soy PC | L-serine | PLD from marine Streptomyces klenkii (SkPLD) | 40 °C, 12 h, 1/5 substrate mass ratio of PC/L-serine, 25% (w/w) enzyme load in organic solvent (hexane) | 26.18% yield of PS | [68] |
| Soy PC | L-serine | PLD from Streptomyces sp. | 30 °C, 70 min, alcohol to PL ratio of 100/1, and 25% (v/w) enzyme load | 98.3% yield of PS | [69] |
| Soy PC | Tyrosol | PLD from Actinamadure sp. | 30 °C, 8 h, 1/20 substrate mass ratio of PC/Tyrosol, 1% (w/w) enzyme load in the selected binary solvent system | 94% PC conversion | [70] |
| Soy PC | Glucose | PLD from Streptomyces sp. | 60 °C, 1.5 h, 1/20 substrate mass ratio of PC/Glucose, 30 U/mL enzyme load in the biphasic reaction system | 95 mol% PL-Glu | [71] |
| Soy PC | Phenylalkanols | PLD from Streptomyces sp. | 37 °C, 24 h, 1/10 substrate mass ratio of PC/phenylalkanols, 1.6 U enzyme in the biphasic reaction system | 87% yield of phosphatidyl-tyrosol | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Dai, L.; Liu, D.; Du, W. Progress & Prospect of Enzyme-Mediated Structured Phospholipids Preparation. Catalysts 2022, 12, 795. https://doi.org/10.3390/catal12070795
Li Y, Dai L, Liu D, Du W. Progress & Prospect of Enzyme-Mediated Structured Phospholipids Preparation. Catalysts. 2022; 12(7):795. https://doi.org/10.3390/catal12070795
Chicago/Turabian StyleLi, Yuhan, Lingmei Dai, Dehua Liu, and Wei Du. 2022. "Progress & Prospect of Enzyme-Mediated Structured Phospholipids Preparation" Catalysts 12, no. 7: 795. https://doi.org/10.3390/catal12070795
APA StyleLi, Y., Dai, L., Liu, D., & Du, W. (2022). Progress & Prospect of Enzyme-Mediated Structured Phospholipids Preparation. Catalysts, 12(7), 795. https://doi.org/10.3390/catal12070795








