Abstract
Recent knowledge in chemistry has enabled the material utilization of greenhouse gas (CO2) for the production of organic carbonates using mild reaction conditions. Organic carbonates, especially cyclic carbonates, are applicable as green solvents, electrolytes in batteries, feedstock for fine chemicals and monomers for polycarbonate production. This review summarizes new developments in the ring opening of epoxides with subsequent CO2-based formation of cyclic carbonates. The review highlights recent and major developments for sustainable CO2 conversion from 2000 to the end of 2021 abstracted by Web of Science. The syntheses of epoxides, especially from bio-based raw materials, will be summarized, such as the types of raw material (vegetable oils or their esters) and the reaction conditions. The aim of this review is also to summarize and to compare the types of homogeneous non-metallic catalysts. The three reaction mechanisms for cyclic carbonate formation are presented, namely activation of the epoxide ring, CO2 activation and dual activation. Usually most effective catalysts described in the literature consist of powerful sources of nucleophile such as onium salt, of hydrogen bond donors and of tertiary amines used to combine epoxide activation for facile epoxide ring opening and CO2 activation for the subsequent smooth addition reaction and ring closure. The most active catalytic systems are capable of activating even internal epoxides such as epoxidized unsaturated fatty acid derivatives for the cycloaddition of CO2 under relatively mild conditions. In case of terminal epoxides such as epichlorohydrin, the effective utilization of diluted sources of CO2 such as flue gas is possible using the most active organocatalysts even at ambient pressure.
1. Introduction
Carbon dioxide capture and utilization (CCU technologies) has been recognized as a possible and cost-effective way to reduce worldwide greenhouse gas emissions [1,2,3,4,5,6,7,8,9,10]. The use of CO2 as a raw material in chemical synthesis is a research area of great scientific, economic and ecological interest [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. The abundance and benignity of carbon dioxide, which is cheap, nontoxic and nonflammable, makes it a very attractive low-cost C1-synthon in organic chemistry. Moreover, the mitigation of CO2 emission from industrial processes in order to reduce CO2 causing the greenhouse effect encourages chemists to carry out research in this area.
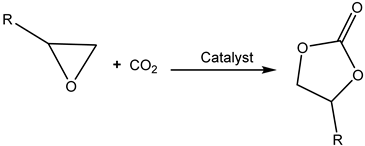
CO2 is thermodynamically stable (ΔG0 = −394.228 kJ/mol) [10], however, and needs catalytic activation and a corresponding reactive agent for its possible fixation into the organic molecules. Thermodynamically non-stable three-membered heterocyclic rings such as epoxides serve as ideal reactants for CO2 fixation. The reaction of epoxides and carbon dioxide to produce cyclic carbonates is attractive because CO2 can be incorporated in the epoxide molecule without the formation of any side products (with 100% atom-economy) (Scheme 1) [1,2].
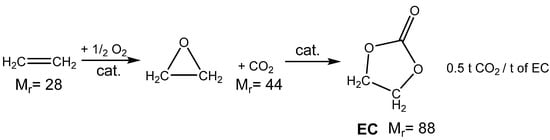
Scheme 1.
CO2 consumption for ethylene carbonate (EC) production applying the carbonation of oxirane produced from ethylene [2].
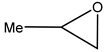
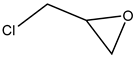
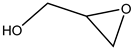
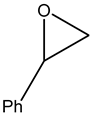
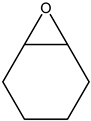
The epoxides available for carbonation can be classified according to their bound substituents as terminal epoxides (containing at least one unsubstituted CH2 group in the oxirane ring) and internal epoxides (containing substituents on both carbon atoms of the oxirane ring, Figure 1).
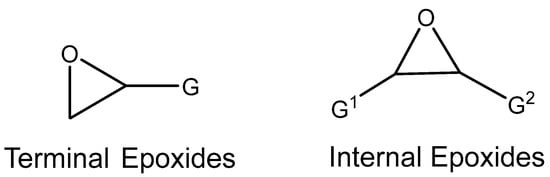
Figure 1.
Epoxides available for cycloaddition reactions.
The reaction conditions for the efficient carbonation of epoxides differ significantly, utilizing terminal (for example, glycidyl derivatives such as epichlorohydrin or glycidol and corresponding glycidyl ethers) [1,2,3,5,6,7,8,9,10,11,12,13,14]) or sterically, much more hindered internal epoxides (for example, epoxidized fatty acids and their derivatives [4,8,9,10,11,12,13,14]). From the sustainability point of view, bio-based epoxides ideally serve as suitable reactants for the production of cyclic carbonates utilizing waste CO2.
In particular, the connection of direct air oxidation and carbonation of ethylene oxide serves as a simple and effective technology that is useful for the subsequent efficacious utilization of anthropogenic CO2 (Scheme 1). In case of ethylene carbonate (EC) produced from ethylene, 44 g of CO2 is utilized per mol of produced EC (approximately 2/3 of EC molecular weight creates CO2) according to the stoichiometry of this reaction. On the other hand, CO2 is emitted during the production of ethylene using conventional technologies such as steam cracking (discussed in Section 2). According to the life cycle assessment (LCA) methodology, technology based on the carboxylation of petrochemical ethylene via catalytic air oxidation emits only 0.92 t CO2/t EC [2].
The catalytic carboxylation of epoxides may afford either cyclic carbonates (Scheme 2, Path a) or eventually polycarbonates (Scheme 2, Path b) [4,7,9], depending on the used catalyst and the reaction conditions.
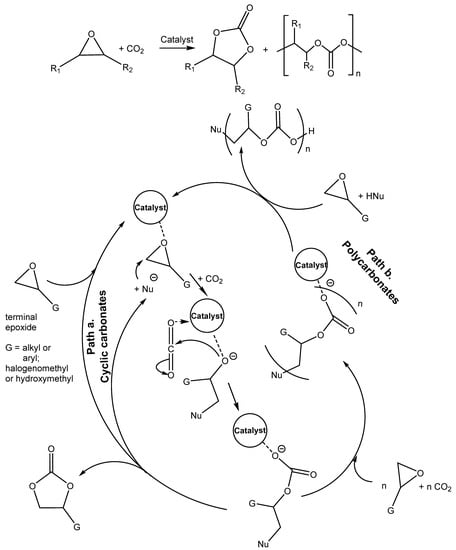
Scheme 2.
Reaction of CO2 with epoxides producing cyclic carbonates and/or polycarbonates [11].
The nucleophile-based ring opening of oxirane activated by the catalyst with the subsequent addition of CO2 and five-membered ring closure accompanied by the release of nucleophile is described in Scheme 1, Path a. The catalyst that activates oxirane (H-bond donor or Lewis acid) decreases the highest reaction barrier of the ring opening (usually the rate-determining step) or/and stabilizes the alkoxide produced by the ring-opening, thus promoting the cycloaddition reaction. Different Lewis bases act as nucleophiles, preferentially bromide or iodide.
Path b. describes the insertion of another oxirane molecule followed by alternate copolymerization with carbon oxide and epoxide. The polymerization occurs in the case of insertion of another epoxide molecule competes with the ring-closure process. Generally, the utilization of metal-based catalysts is necessary for the direct formation of polycarbonates starting from CO2 and epoxides via ring-opening polymerization.
Utilizing CO2 via cycloaddition to epoxides is the exothermic process that can be carried out under mild reaction conditions using a broad spectrum of catalysts [11,12].
Cyclic carbonates are generally stable liquids, which enables the long-term sequestration of CO2, especially when cyclic carbonates are subsequently used for polymerization. Although the production of cyclic carbonates from CO2 and epoxides has been industrialized since 1958 and uses inexpensive catalysts such as KI, the current production processes still suffer from major disadvantages, such as high reaction temperatures (180–200 °C), high pressure (5–8 MPa) and stoichiometric amounts of activating reagents [13,14].
It is known that the increasing of the reaction rate with the increase in CO2 pressure is not only counterproductive due to the high energy consumption, but is even limited. The high increase in CO2 pressure (and the overrunning at a concentration of 0.47 g CO2/mL) is accompanied by sudden decrease in reaction rate due to the dilution effect causing the reduction in epoxide and catalyst concentrations in the reaction mixture [11].
Cyclic carbonates are used for polymer production, as electrolytes in lithium-ion batteries, polar aprotic (ethylene or propylene carbonate) or protic solvents (glycerol carbonate, etc.) and as chemical intermediates in organic synthesis [1,2,3,4,5,6,7,8,9,10,11,12,13,14] (Scheme 3).
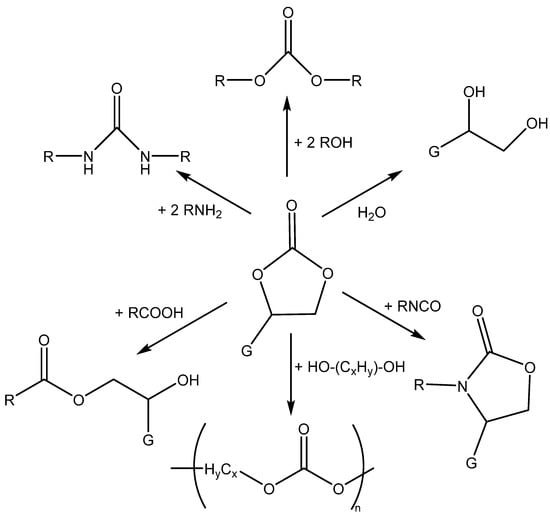
Scheme 3.
Possibilities for the chemical transformation of cyclic carbonates to linear (poly)carbonates, esters, ureas, ethyleneglycols and urethanes (carbamates) [1,2,5,13].
Alluding to cyclic carbonates used as solvents, a grade deal of attention has been given to the application of glycerol carbonate as it possesses low toxicity and good biodegradability, and has a high boiling point and simple availability from lipids and CO2, giving it many applications [10]. Generally, the direct fixation of CO2 in cyclic carbonates and their products is regarded as a greener approach than the existing practices. As North et al. have mentioned, only two reactions of CO2, the dry reforming of methane (for fuel production) and cyclic carbonate chemical production, could consume up to 25% of the anthropogenic CO2 produced annually [12].
The cyclic carbonate formation based on the cycloaddition of CO2 requires oxirane ring opening in the first reaction step, which is followed by insertion of CO2 in the second reaction step and ring closure to form cyclic carbonate. The general mechanisms of this reaction are illustrated in Scheme 4 [3,12].
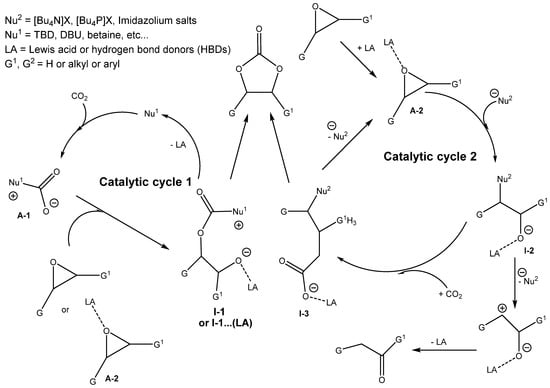
Scheme 4.
Generally accepted mechanisms of cyclic carbonate formation via the cycloaddition of epoxide on CO2 [3,12].
According to the computations, the epoxide ring-opening has the highest energetic barrier. Due to this reason, the applicable catalysts decrease the reaction barrier, acting as Lewis bases (nucleophile, Nu) that are capable of nucleophilic attack and opening the oxirane ring and/or stabilizing intermediates. In addition, the oxirane can be activated by hydrogen bond formation interacting with a Lewis acid or a hydrogen bond donor (HBD), thereby decreasing the energetic barrier of the epoxide ring opening. In order to catalyze cyclic carbonate formation most effectively, the best catalysts often contain a combination of both Lewis base and Lewis acid components or Lewis base/HBD parts. If the catalysis influencing the ring opening is very effective, then the ring closure step can be the rate-determining step with a higher energetic barrier [15].
The action of some described effective catalysts is based even on the activation of CO2, as is assumed. The activation of CO2 comprises formation of carbamate or carbonate, which may also take part in the epoxide ring-opening step [3,5,10,11,12].
Catalytic cycle 1 in Scheme 4 (CO2 activation mechanism) describes the nucleophilic activation of CO2 using nucleophiles such as tertiary amines, amidines, guanidines or carbenes (Nu1) to form an intermediate A-1 (carbamate or carboxylate), which subsequently promotes oxirane ring opening, leading to the second intermediate (I-1). A-1 attacks oxirane or oxirane activated by Lewis acid (A-2). Cyclization then occurs to produce a cyclic carbonate while the nucleophile (Nu1) is recycled for a new catalytic cycle. The CO2 activation mechanism requires a catalyst nucleophilic towards CO2, but not for epoxide [4,12].
In addition, catalytic cycle 2 demonstrates a more common carbonation pathway through the activation of epoxide. The hydrogen bond donor(s) (HBDs) coordinate(s) to the epoxide ring (formation of intermediate A-2). Subsequently, the Lewis base, for instance, onium halide salts, acting as nucleophile (Nu2) attacks the oxirane to enable the ring opening, producing an intermediate (I-2). Subsequently, upon ring opening, the CO2 insertion occurs in the O-H bond of the intermediate (I-2), producing a new intermediate (I-3). The cyclization of the intermediate (I-3) accompanied by cleavage of the leaving group (Nu2), produces cyclic carbonate as the final product, and nucleophile is recycled for a further reaction. Alternatively, cleavage of nucleophile Nu2 from I-2 before CO2 addition causes the formation of alternative products such as corresponding ketones via a Meinwald rearrangement.
2. Sources of Epoxides
Epoxides are generally accessible either via the oxidation of the C=C double bond in alkenes (using peroxides or O2/Ag-based reaction) or via the dehydrohalogenation of geminal halogenoethanols [5].
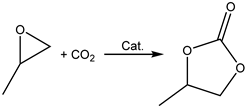
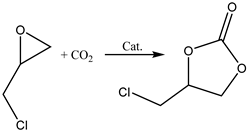
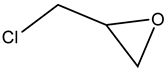
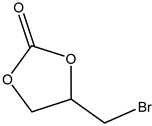
Terminal epoxides such as epichlorohydrin (EPIC), glycidol (GL), methyloxirane (propylene oxide, PO) and oxirane (ethylene oxide, EO), and their functional derivatives, are the most studied epoxides for the CO2/epoxide coupling reaction.
EPIC and GL are chemicals that were recently produced from bio-based glycerol obtained as a by-product from the chemical utilization of lipids (Scheme 5). Transesterification or hydrolysis of lipids is the main pathway for the production of bio-based glycerol and fatty acid esters (for biodiesel) and soap (sodium or potassium salts of fatty acids are used as surfactants) [5] (Scheme 6).
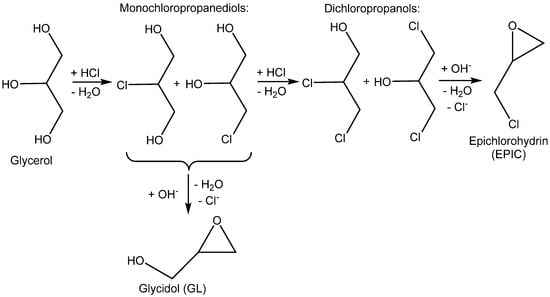
Scheme 5.
Production of glycidol and epichlorohydrin starting from bioglycerol [16,17,18,19,20].
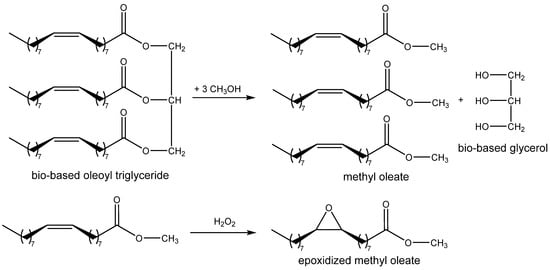
Scheme 6.
Synthesis of glycerol and epoxidized methyl oleate from triglyceride of oleic acid (vegetable oil).
The nucleophilic substitution of OH groups in the glycerol structure with Cl via the action of gaseous HCl enables the formation of monochloropropanediols or dichloropropanols, in the presence of a catalyst, usually a carboxylic acid [2,5,16,17,18], as the crucial feedstock for production.
The subsequent ring closure induced by an inorganic base such as calcium or sodium hydroxide enables epoxide formation via a dehydrochlorination reaction with inorganic chloride (NaCl or CaCl2) being obtained as a co-product [19,20] (Scheme 5).
Similarly, starting from chloropropanediols, glycidol is produced by basic hydrodechlorination [20].
EO and PO are still mainly derived from ethylene and propylene produced by the cracking of petrochemical feedstock with subsequent silver-catalyzed direct air oxidation [2].
The development of appropriate sustainable alkenes production is based on the utilization of bio-based syngas (CO + H2) obtained by the gasification of waste biomass [21]. The most promising pathway for alkene production exploits methanol as a crucial intermediate simply available from syngas with its subsequent dehydrogenation/coupling catalyzed by zeolites (methanol-to-olefins technology, MTO) [21]. Apart from the above-mentioned, the direct catalytic cracking of vegetable oils (lipids) may produce propylene [21].
The other sustainable pathway for ethylene or butylene production is based on the dehydrogenation of bio-based alcohols (bioethanol and biobutanol) [22] accessible by fermentation of oligosaccharides. Brasco Co. produces bio-ethylene, for example, through the dehydration of bioethanol for polyethylene production in Brazil [23].
In addition, other types of epoxide can be produced from waste biomass, such as limonene oxide and limonene dioxide, which can be synthesized via the promising epoxidation of bio-based limonene obtained from citrus peels, oak and pine tree, under solvent-free conditions with hydrogen peroxide and a tungsten-based catalyst [24].
In addition, vegetable oil-derived triglycerides and fatty acids contain double bond(s) (Scheme 6), which can undergo epoxidation with peroxides [2]. The epoxidized fatty acid derivatives can be subsequently exploited as the starting materials for cycloaddition or even a subsequent one-pot polymerization reaction with CO2 [25,26]. Scheme 6 shows the transesterification of natural triglycidyl oleate producing methyl oleate and bioglycerol, and the subsequent epoxidation of methyl oleate using hydrogen peroxide.
It is worth noting, however, that epoxidation is a highly exothermic reaction (as ∆H = −55 kcal/mol for each double bond); thus, H2O2 is slowly added or added in a stepwise manner in semi-batch operations, and requires a long reaction time. In order to avoid problems with heat and mass transfer, process intensification using a microreactor was proposed [27]. The microreactor could significantly decrease the reaction time with higher epoxide selectivity.
3. Homogeneous Metal-Free Catalysts
This review focuses on the recent development of homogeneous organocatalysts for the cycloaddition reaction of CO2 and epoxides (insertion of CO2 into the oxirane ring) with the aim of producing cyclic carbonates. Homogeneous catalytic systems (catalyst and reagent dissolved in the same phase) usually display the highest conversion and selectivity and are usually significantly cheaper compared with heterogeneous ones. Metal-free organocatalysts are usually readily available even from renewable sources, non-recalcitrant, biodegradable, affordable, and inert towards air and moisture. They may, however, be more difficult to separate and recycle from the produced cyclic carbonates. On the other hand, after the discovery of a new effective homogeneous catalysts, subsequent attempts to immobilize it on the insoluble surface have followed in order to solve problems with catalyst separation and recycling [12].
3.1. Catalytically Active Amines and Their Salts
Generally, the effective absorption of CO2 into the liquid phase and its subsequent activation of chemisorbed CO2 are important steps for the subsequent cycloaddition reaction of CO2 with epoxides.
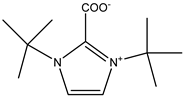
The alkaline absorbents described in the literature as being capable of effectively absorbing CO2 are different amines [28], including amidines such as 1,8-diazabicyclo [5.4.0]undec-7-ene (DBU) [28,29,30], guanidines such as 1,5,7-triazabicyclo[4.4.0]dec-5-enium (TBD) [28,29], and azaheterocycles such as pyridines and imidazoles [31,32], which are efficient for the chemisorption and activation of CO2.
The correlation between the structures of different organic amines and their catalytic activity in the cycloaddition of CO2 was studied by Yu et al. [28]. Their article compares the catalytic activity of a variety of nucleophilic aliphatic amines (ethanolamine, bis-(3-aminopropyl)-amine, oleylamine), basic aminoacid arginine, nucleophilic aromatic amines (1,2-phenylenediamine, 2-aminobenzylamine) and non-nucleophilic cyclic amines such as TBD and DBU for the cycloaddition of CO2 to methyloxirane (propylene oxide, PO) [28].
It is well known that the chemisorption ability of CO2 (formation of carbamate) increases with the increasing basicity of amine [33,34]. Yu et al. observed no apparent relationship of the pKa value of conjugated acids of the tested amines with respect to their catalytic activity in the case of propylene carbonate (PC) formation (Figure 2, Table 1). The authors observed the lowest propylene carbonate conversion when applying aliphatic amines.
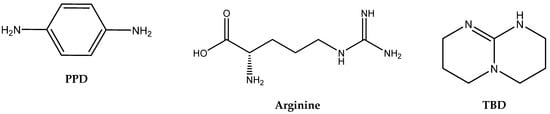
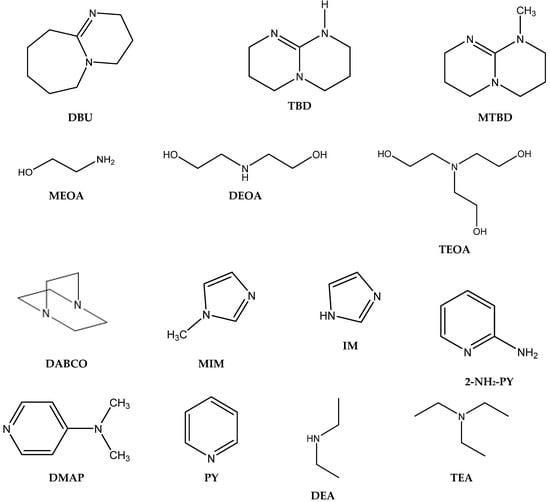
Figure 2.
Structures and abbreviations of the tested amines.

Table 1.
Effect of amine structure on the cycloaddition of CO2 to propylene oxide (PO) producing propylene carbonate (PC).
Low conversion was even observed in the case of amine forming intramolecular hydrogen bonds (1,2-phenylenediamine and 2-aminobenzylamine).
In contrast, aromatic amines (such as 1,4-phenylenediamine) and amidines or guanidines involving conjugated “N=C–N” structures (arginine, TBD) were found to be the most active ones [28].
According to the published information [35,36], tertiary amines are often higher in activity than primary or secondary ones (Table 2, Entries 5, 10–12). It has been proven that amidines containing the “N=C–N” bond in their structures are particularly favorable for the cycloaddition of CO2 with epoxides (Table 1, Entries 6–8, 17 and Table 2, Entries 3 and 12) [2,3,4,6,32]. The above-mentioned observations are in agreement with the statement of North et al. that for the efficient activation of CO2, compounds that nucleophilically attack CO2 but not the epoxide ring are sought out [12].

Table 2.
The effect of organic amines on the conversion of PO [36].
Azzouz et al. demonstrated the effective utilization of 2-aminopyridine (2-NH2-PY) as a catalyst (using 10 molar % of 2-NH2-PY) for the carbonation of different terminal epoxides at 60–85 °C and 1 MPa of CO2 [35]. This carbonation was performed even at pilot scale.
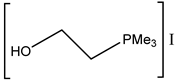
Interestingly, corresponding salts with protic (Bronsted) acids (base.HA) of the above-mentioned non-nucleophilic amines (DBU.HA, N-methylimidazole (MIM.HA), N,N-dimethylaminopyridine (DMAP.HA)) and, alongside these, even triethanolamine, pyridine and caffeine (Figure 3), are quite catalytically active in the cycloaddition of CO2 with epoxides (Table 3, Entries 3–12, 15–24). Hydrogen halides in particular are the most active in comparison with corresponding free bases [29,30,32,37,38,39] (Table 3, Entries 3–6, 8–12). The most active seems to be hydroiodides of the corresponding amines (Table 3, Entries 10, 18–24, 26–27). For the above-mentioned catalytic action of amine salts, the reaction mechanism based on the activation of epoxide via the protonation with amine salt in the role of Bronsted acid (HA) is proposed with subsequent anion-based epoxide ring opening.
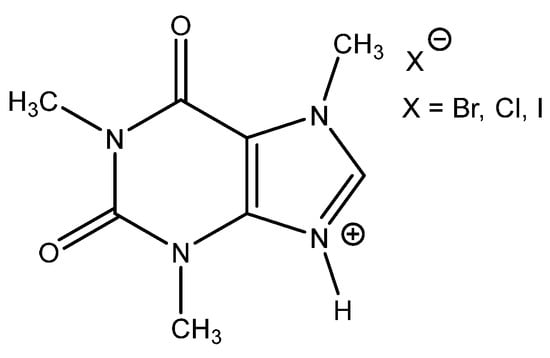
Figure 3.
Structure of catalytically active caffeine hydrohalides [37].

Table 3.
Comparison of the catalytic effects of amines and their salts on the carbonation of PO.
Sun et al. reported very effective carbonation using triethanolamine hydroiodide, including the simple recyclability of this catalyst without loss of activity even after four recycling steps [38].
The published results indicate that the synergetic effect of hydroxyl groups from protonated aminoalcohol in the role of HBD, together with naked bromide or iodide, significantly influences the cycloaddition of CO2 to the studied epoxides and makes possible the application of this reaction even at ambient conditions (Table 3, Entry 17–18).
Catalytically active ammonium halide activates not only the epoxide ring for opening through the H-bond with hydroxyl groups of triethanolammonium cation and the subsequent addition of intermediate 2-halogenoalkoxide to CO2, but even the next ring closure caused by the facile withdrawal of halide from the produced 2-halogenocarbonate [38] (Scheme 3 and Scheme 4).
Apart from the above-mentioned, in the case of caffeine hydrobromide, potassium halides added to the reaction mixture as an additional source of nucleophiles were successfully tested. With the same reaction conditions, the enhanced efficiency of cyclic carbonate formation was observed utilizing equimolar quantities of KX and caffeine.HBr or even 2: 1 KX: caffeine.HBr using DMSO as the reaction solvent at 70 °C and 0.7 MPa CO2 pressure [29,37]. The yield of cyclic carbonate increased following the trend KF < KCl < KBr < KI, which was in good agreement with nucleophilicity and nucleofugacity of the corresponding halide anions (Table 4, Entries 17–25).

Table 4.
Catalyst screening for the cycloaddition of EPIC.
In Table 5, the increase in carbonate yields using CAFH.Br/KI in the case of carbonation of terminal epoxides is documented. In the case of internal epoxide (limonene oxide), however, no carbonation was observed using caffeine hydrobromide or even its mixture with KI (Table 5, Entry 5).

Table 5.
Reaction of various epoxides with CO2 [37].
Roshan et al. came to the conclusion that even the addition of a low quantity (a catalytic amount) of H2O significantly enhances CO2-based formation of PC over tertiary heterocyclic amines such as IM, PY and DMAP, giving over 98% selectivity of PC formation (at 120 °C, 1.2 MPa, 3 h). The observed results were evaluated by a DFT study comparing energy profiles for free amine in comparison with corresponding amine hydrogencarbonate-mediated cycloadditions of CO2 to propylene oxide. Ammonium hydrogencarbonates produced in situ from heterocyclic amine, H2O and CO2 works similarly to the above-mentioned amine salts as activators of the epoxide ring. As was argued, the HCO3− anion generated in the water-CO2-base reaction was the key active species that gave the higher activity of the base-water systems rather than the carbamate salt (produced by a reaction of R3N with CO2) [32].
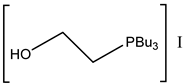
It should be mentioned that low-melting salts (melting point below 100 °C) obtained by the neutralization of organic bases with organic or inorganic acids embodies are called protic ionic liquids (PILs). The melting of PILs could enhance the miscibility of catalytically active PILs with reacting epoxide and CO2 compared with solid catalysts, as was published by Zhang et al. in the case of DMAP hydroiodide [42] or by Kumatabara et al. using triethylamine hydroiodide [41] at ambient pressure (Table 3, Entry 10 and Table 4 Entry 26).
Zhang et al. published results obtained even by means of the capture and utilization of CO2 for the cycloaddition into SO using PIL (DMAP hydrobromide) at ambient pressure and 120 °C [42]. This PIL has superior catalytic effect compared with other hydrogenhalides of tertiary bases such as DBU, MIM, DABCO or tetramethylguanidine (Table 6). DMAP.HBr is well reusable with no drop in activity after five recycling steps. DMAP.HBr is able to carbonate even internal epoxides such as ChO, although this cycloaddition is quite sluggish.

Table 6.
Synthesis of SC catalyzed by different PILs [42].
3.2. Two Components Catalysts Based on a Combination of Organic Base and Hydrogen Bond Donor
As was mentioned in Section 3.1, the combined action of protonated amine with nucleophilic anion positively influences the efficiency of cycloaddition. This synergic action between the Lewis base, such as the amine, and hydrogen bond donors (HBDs) was reported in the literature [2,4,6,32,36,43].
The possible synergic effects of alcohols (glycerol, glycidol, 1,2-propylene glycol (PG), poly(ethylene glycol)-600 (PEG600), poly(ethylene glycol)-400 (PEG400), cellulose, chitosan and β-cyclodextrin (β-CD)) known as HBDs was explored in CO2-based cyclic carbonates synthesis catalyzed by amines, as mentioned in Section 3.1 [36]. For this purpose, the most catalytically active DBU and DMAP were tested in relation to the co-action of the chosen HBDs (Table 7).

Table 7.
Catalyst screening for the base catalyzed cycloaddition reaction of PO; effects of different HBDs [36].
Out of the set of experiments, cellulose was recognized as the most effective HBD in the addition of CO2 to propylene oxide [36].
The effective quantity of cellulose used as HBD in the case of the DBU catalysis of the chemical fixation of CO2 into propylene carbonate is very low with respect to the optimal quantity of DBU (15 mg of cellulose + 300 mg of DBU per mL of PO). The effect of the DBU excess on the yield of PC in the DBU-cellulose reaction system was studied. Generally, the yield rises with the increasing of the DBU: cellulose ratio with the maximum conversion and selectivity reached at a mass ratio of 25–30:1 [36]. The high catalytical activity of cellulose was, in all probability, explained by Khiari et al. and Gunnarson et al. [44,45]. Cellulose reacts in co-action with a significant excess of non-nucleophilic DBU, with CO2 producing carbonate by means of a reaction similar to that of cellulose xanthate formation during the production of viscose utilizing a sulfur analogue of CO2, carbon disulfide. The produced carbonate should be a nucleophilic agent that attacks and opens the epoxide ring rather than the known non-nucleophilic DBU.
Aoyragi et al. described the markedly increased formation of cyclic carbonates in isopropylalcohol using triphenylphosphine hydroiodide as a catalyst. 1H NMR spectra documented the formation of H-bonds between the used isopropylalcohol and the starting epoxide [46]. The high activity of the above-mentioned hydroiodide (compared with other HXs) was explained by both the high nucleophilicity and even the high leaving ability (nucleofugality) of iodide ion.
Section 3.1 mentioned the significant catalytic activity of triethanolamine [36], which could be explained by the synergy of HBDs (bound alcoholic OH groups) and the Lewis basicity of the tertiary amine.
More advanced catalysts such as 2-hydroxymethylpyridine (2-PY-CH2OH) and 2,6-bis(hydroxymethyl)pyridine (2,6-PY-CH2OH)2 were developed for the high-efficiency cycloaddition of CO2 with epichlorohydrin (EPIC) under a slightly elevated temperature and ambient pressure (T = 25–60 °C, 0.1 MPa of CO2; see Table 4, Entry 4) [40]. The high catalytic effect was demonstrated by 1H NMR spectroscope observing the formation of a stable H-bond between the PY-CH2OH and oxygen of epichlorohydrin. The authors demonstrated that the tested compounds with either heterocyclic nitrogen (benzylalcohol PhCH2OH) or hydroxymethyl (CH2OH) groups (PY) catalyzed EC formation only sparingly (PY) or not at all (PhCH2OH) (Table 4, Entries 1–5).
The catalytic activity of nitrogen-doped charcoal for CO2 cycloaddition reactions could be explained by the co-operation of OH groups working as HBDs and tertiary amines bound in the graphitic structure of specially prepared N-doped carbons together with the ability of active carbon to adsorb CO2 [47].
3.3. Aminoacids (AAs) as Catalysts
AAs contain amino and carboxylic groups in their structures. Amino groups can react with CO2 to form N-COO− (carbamate) products with low binding energy, which can catalyze the transfer of CO2 to the 2-halogenoalcoholate produced by the halide-based opening of the epoxide ring. The carboxylate group (–COOH) can catalyze the oxirane ring opening as effective HBD analogously to the amino group (–NHR) and hydroxyl (–OH). Some AA salts have been successfully tested in the capture of CO2 from flue gas. In addition, the amino groups can also be utilized in quaternization with the aim of introducing halide as a nucleophile into the engineered catalytically active molecules (Table 8).

Table 8.
PO catalyzed by AAs.
The yield of PO is dependent on the AA structure; basic AAs such as L-histidine (L-His) and proline (Pro) containing basic additional groups (imidazolium and amino, respectively) provided higher yields than acidic aspartic or glutamic acids (Figure 4).
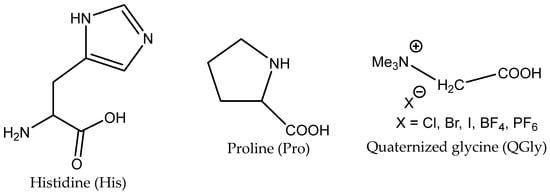
Figure 4.
Structures of highly catalytically active AAs.
In addition, the combination of an amino acid with an HBD results in a higher catalytic activity under milder reaction conditions than in the absence of an HBD. The binary catalytic systems formed with the amino acid and H2O produced, for example, more active systems for the synthesis of PO than in the absence of H2O [53]. In the case of L-His, the time taken for the nearly total conversion was reduced from 48 h to 3 h by adding H2O as an HBD (L-His:H2O ratio = 1:29) under similar conditions (Table 8, Entries 1 and 2). The low H2O concentration was used to avoid the hydrolysis of the produced PC [49,53].
Roshan et al. showed that the combination of halide ions as nucleophiles (added in the form of KI, for example) with L-histidine produced highly active catalytic systems for the cycloaddition of CO2 to epoxides [51] (Table 8, Entry 4).
The most effective binary catalytic systems contained KI with basic AAs such as His/KI (Table 8, Entry 4). In a related work, Yang et al. proved the high stability of AA/KI catalytic systems, namely L-Trp/KI, for the cycloaddition of CO2 to PO to form propylene carbonate. After carbonate separation conducted by means of distillation, the catalytic system was reused five times without loss of activity [54].
The catalytic activity of the different KX salts followed the order Cl < Br < I corresponding to the increasing nucleophilicity and leaving group ability [54]. This confirmed the role of these anions in the opening of the epoxide ring [54].
3.4. Onium Salts as Catalysts
Quaternary ammonium (most often tetrabutylammonium bromide, TBAB, and iodide (TBAI), phosphonium and sometimes even sulfonium salts [55]) are common catalysts for the formation of cyclic carbonates from epoxides and CO2 [5,6,12] (Figure 5).
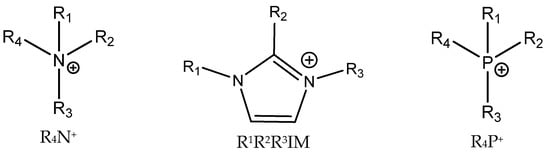
Figure 5.
Structures of cations of commonly used ILs.
The low-melting (below 100 °C) quaternary ammonium salts are called ionic liquids (ILs). In addition, ILs show good solvating ability including CO2, variable polarity and negligible vapor pressure [56]. ILs are considered to be sustainable (“green”) solvents because of their properties such as relatively high thermal stability and negligible vapor pressure, high chemical stability and simple separability, and the modularity of their properties changing the structure of anions and cations.
The first published article that mentioned the formation of cyclic carbonates via the cycloaddition of CO2 in epoxides catalyzed solely by ILs was published by Deng and Peng [57]. The authors studied different 1-butyl-3-methylimidazolium (BMIM) and N-butylpyridinium (BPY) salts varying in anions (chloride and tetrafluoroborate (BF4−, hexafluorophosphate (PF6−)) and observed the highest activity in the case of BMIMBF4 salt. The catalytic activity increase was in the order: BMIMPF6 < BPYBF4 < BMIMCl < BMIMBF4. This observation is in agreement with the solubility of CO2 in these Ils; the highest solubility of CO2 was determined in BMIMBF4 [58].
Dyson et al. and Wang et al. studied the abilities of different ILs that differed in terms of the cations (alkylated imidazolium and tetraalkylammonium) and anions (halides) used [59,60]. Interestingly, cheap Bu4NCl was found to be a very active catalyst compared with the much more expensive alkylated imidazolium halides. Based on the experimental results obtained at ambient pressure and 50 °C, they hypothesized that the balance between the nucleophilicity of anions and the acidity of hydrogens bound in the imidazolium ring of used IL is most important.
Yang et al. published an even higher catalytic activity of corresponding bromides when comparing them with tetrafluoroborates (Table 9, Entries 10 and 11) [29].

Table 9.
Effect of the ionic liquid structure on the cycloaddition reaction of PO.
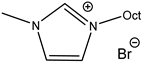
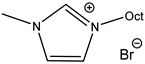
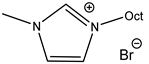
An increase in catalytical activity was observed with the increasing of the lipophilic alkyl chain length in RMIMXs when comparing 1-butyl-3-methylimidazolium and 1-octyl-3-methylimidazolium bromide [29].
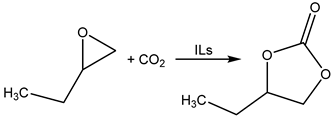
Based on this idea, Akhdar et al. successfully tested the carbonation of internal epoxide produced via the epoxidation of methyl oleate [61,62,63]. The catalysts were prepared by means of the alkylation of N-alkylimidazoles with oligoethylene iodide and modified by ion-exchange to the corresponding bromide. N′-Oligoethylene-N-butylimidazolium bromide was recognized as the most active catalyst enabling the carbonation of epoxidized methyl oleate in 96% yield even at 100 °C and 2 MPa of CO2.
The catalytic activity of ILs cannot be known based on a comparison of the anion effects using one type of cation. In the case of N-benzyl-N′-methylimidazolium salts, the most active one is N-(o-methyl)benzyl)-N′-methylimidazolium chloride o-MeBzMIMCl; the catalytic activity of the corresponding o-MeBzMIMBF4− and o-MeBzMIMPF6− salts is lower (Table 9, Entries 14–16) [60].
Anthofer et al. studied the relationship between the structure, affinity to CO2 and catalytic activity of different low-melting N,N′-dialkylimidazoles in detail [64] (Figure 6).
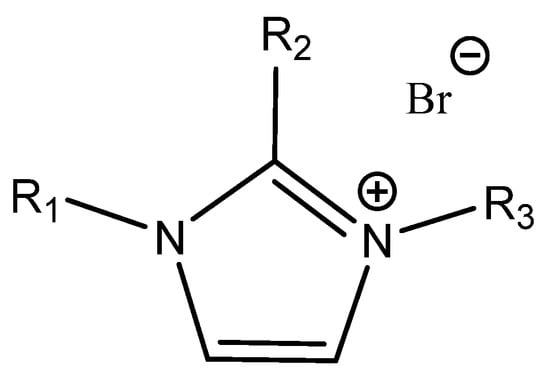
Figure 6.
Structures of tested N,N′-dialkyl IM-based ILs in the carbonation of PO [64]. R1 = Me; R3 = Bu; R2 = H = 1-methyl-3-butyl imidazolium bromide [BMIm]Br; R1 = Me; R3 = Bu; R2 = Me = 1,2-dimethyl-3-butylimidazolium bromide [BM2Im]Br; R1 = Me; R3 = Bu; R2 = Et = 1-methyl-2-ethyl-3-butylimidazolium bromide [BEMIm]Br; R1 = Me; R3 = Oct; R2 = H = 1-methyl-3-octylimidazolium bromide [OMIm]Br; R1 = Me; R3 = Oct; R2 = Me = 1,2-dimethyl-3-octylimidazolium bromide [OM2Im]Br; R1 = Me; R3 = Oct; R2 = Et = 1-methyl-2-ethyl-3-octylimidazolium bromide [OEMIm]Br; R1 = Bz; R2 = H; R3 = Bu = 1-benzyl-3-butylimidazolium bromide [BBzIm]Br; R1 = Bz; R2 = H; R3 = Oct = 1-benzyl-3-octylimidazolium bromide [OBzIm]Br; R1 = CH2C6F5; R2 = H; R3 = Bu = 1-(2,3,4,5,6-pentafluoro)benzyl-3-butylimidazolium bromide [BBzF5Im]Br; R1 = CH2C6F5; R2 = H; R3 = Oct = 1-(2,3,4,5,6-pentafluoro)benzyl-3-octylimidazolium bromide [OBzF5Im]Br.
As could be seen, the most active ILs were those that contained bulk lipophilic substituents (N-octyl-N′-pentafluorophenyl-, N-butyl-N′-pentafluorophenyl- or N-methyl-N′-octyl-imidazole). The observed catalytic activity correlates well with the measured sorption of CO2 in the studied ILs under the reaction conditions of this study. The substitution of the 2-position also significantly reduced the activity of the tested ILs [64].
The authors detected an interaction (hydrogen bond formation) between the acidic hydrogen of the used imidazole bromides (bound in position 2) and the oxygen atom of PO using FT-IR spectroscopy [64]. The mentioned catalyst was very effective even for the carbonation of internal epoxides such as cyclohexene oxide (ChO).
As could be seen, the most active ILs were those that contained bulk lipophilic substituents (N-octyl-N′-pentafluorophenyl-, N-butyl-N′-pentafluorophenyl- or N-methyl-N′-octyl-imidazole). The observed catalytic activity correlates well with the measured sorption of CO2 in the studied ILs under the reaction conditions of this study. The substitution of the 2-position also significantly reduced the activity of the tested ILs [64] (Table 10).

Table 10.
Synthesis of PC from CO2 and PO using IL catalysts [64].
The authors detected an interaction (hydrogen bond formation) between the acidic hydrogen of the used imidazole bromides (bound in position 2) and the oxygen atom of PO using FT-IR spectroscopy [64]. The mentioned catalyst was very effective even for the carbonation of internal epoxides such as ChO (Table 11).

Table 11.
Cycloaddition of different epoxides catalyzed by [OBzF5Im]Br; effect of epoxide structure [64].
As was published by Yang et al., in the case of butylated DABCO (BuDABCO), the corresponding bromides, chlorides and hydroxides were recognized as the most effective cycloaddition catalysts [29] (Table 12, Entries 1–4). In contrast, non-nucleophilic anions such as NTf2−, PF6− or BF4− caused the loss of the catalytical activity of the studied BuDABCO salts.

Table 12.
PC synthesis catalyzed by DABCO-based Lewis basic ionic liquids [29].
Surprisingly, when ILs containing activated CO2 in their structures in N,N′-di(alkyl)imidazolium-2-carboxylates were tested as CO2 cycloaddition catalysts by Kayaki et al. [65], the observed activity was quite low and a high CO2 pressure was required to obtain satisfactory conversion (Table 13). The hydrogen in position 2 on the imidazolium ring is, in all probability, important as an HBD for epoxide ring activation and substitution with -COO− causes a decrease in catalytic activity (Table 13).

Table 13.
Cycloaddition of CO2 to epoxides catalyzed by 1,3-di-tert-butylimidazolium-2-carboxylate [65].
Interestingly, some of the attempts to boost the catalytic activity of onium salts constructing di- or tricationic ILs (Scheme 7) often fall flat (Figure 7) [66,67,68].
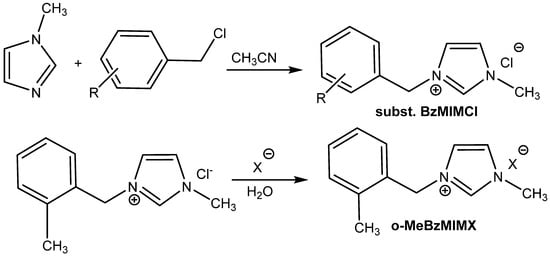
Scheme 7.
Preparation of tested ILs (substituted BzMIMs) described by Yang et al. [60]. R = H = [BzMIM]Cl; R = 4-CH3 = [p-MBzMIM]Cl; R = 2-CH3 = [o-MeBzMIM]Cl; R = 4-NO2 = [p-NBzMIM]Cl; R = 2-Cl = [o-ClBzMIM]Cl; R = 4-Cl = [p-ClBzMIM]Cl; X = PF6 = o-MeBzMIMBF4−; X = BF4 = o-MeBzMIMPF6.
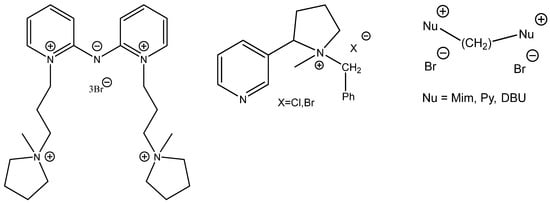
Figure 7.
Structures of studied task-specific dicationic ILs (amino-pyridinium-pyrrolidinium bromide [66], Quaternized nicotine based ammonium ILs [67] and CH2-bridged tertiary amines [68].
Isothiouronium salts (Scheme 8) were also chosen for the testing of catalytic activity for CO2 addition, providing encouraging results (over 90% yield with selectivity over 99%) at 2 MPa pressure of CO2 and 140 °C after 2 h of action using 1 molar % of catalyst. The corresponding thiourea was practically nonactive [55].
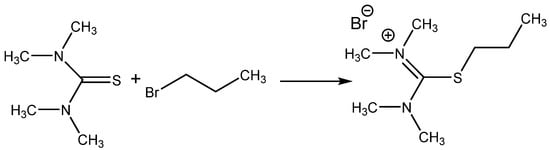
Scheme 8.
Preparation of catalytically active S-alkylisothiouronium salt [55].
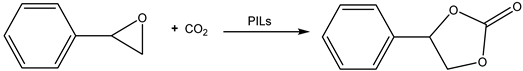
Apart from ammonium and sulfonium salts, the methyl-trioctylphosphonium-based ILs with organic anions were studied as cycloaddition catalysts. Their catalytic activity was remarkable even for cycloaddition reaction of less reactive styrene oxide (SO) with CO2 at ambient pressure [46].
Wilhelm et al. compared the action of different aromatic or heterocyclic alcoholates (phenolates or anions of hydroxypyridine regioisomers, Figure 8) used as anions in combination with tetrabutylphosphonium, tetrabutylammonium and N-ethyl-DBU-based cations [69] (Table 14). The authors discovered the cooperative effect of the alcoholate anion of 2-hydroxypyridine with the tetrabutylphosphonium cation in the case of a reaction of CO2 with epichlorohydrin. The catalytic activity, however, of other ILs containing Bu4N+ or Et-DBU+ cations was slender (Table 14).
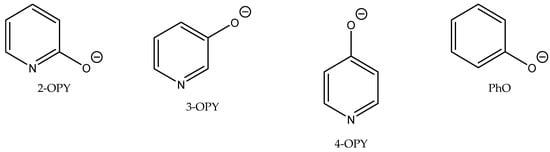
Figure 8.
Structures of hydroxypyridine anion OPYs [69].

Table 14.
Cycloaddition of CO2 with EPIC catalyzed ILs [69].
The authors suggested the mechanism of this reaction based on activation of the epoxide ring with the tetrabutylphosphonium cation as the Lewis acid with the simultaneous activation of CO2 and phenolate [69]. The most active [Bu4P] 2-OPY was tested at ambient pressure for the carbonation of different terminal epoxides with satisfactory results, using 50 molar % quantity of [Bu4P] 2-OPY to an appropriate epoxide (Table 15).

Table 15.
Carbonation of different epoxides catalyzed by Bu4P.2-OPY at 0.1 MPa of CO2 and at 30 °C [69].
Wang et al. prepared ammonium salts in situ by alkylating tertiary amides (N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMAc), N-formylmorpholine, N-methylpyrrolidone, tetramethylurea and N-formylpiperidine) with benzyl halogenides. The prepared ammonium salts enabled the formation of cyclic carbonates even at an ambient pressure, especially those prepared from DMF using benzylbromide [70] (Table 16).

Table 16.
The effect of organic bases on cycloaddition (compared with DBU in co-action with alkyl halides) [71].
Wang et al. attributed the high activity of the DMF + BnBr mixture in particular to the activation of the oxirane ring by benzyl cations and the contemporary nucleophilic activation of CO2 by DMF [70] (Scheme 9).
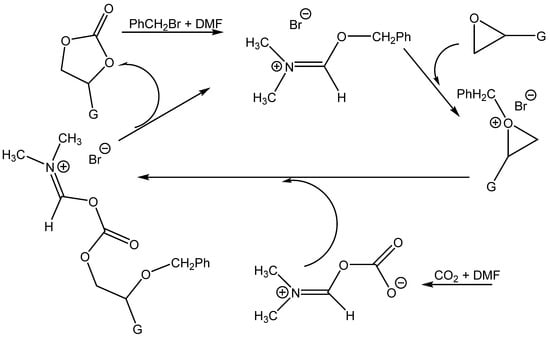
Scheme 9.
Proposed reaction pathway for the formation of cyclic carbonates catalyzed by benzylbromide/DMF [71].
Similarly, the effectiveness tertiary amines described earlier as active catalysts (see Section 3.1) was satisfactorily proven for the reaction of epoxides with CO2 at an ambient pressure after in situ quaternization via benzylation [71] (Table 16).
For a comparison of the action with the ammonium salts formed using arylmethylbromide derivatives, Bu4NBr was employed as the bromide source using the same reaction conditions as the model reaction (Table 16, Entry 15).
As could be seen, the yield was lower than that using benzyl bromide as the bromide anion source, which was presumably due to the electrostatic interaction between the bromide anion and the ammonium center decreasing with the bulkiness of the cation. The authors stated, based on above-mentioned results, that the nucleophilicity of the bromide anion is weaker for Bu4NBr than for the salts (Bn-DBU+.Br−).
The successful utilization of tetrabutylammonium halides, especially bromide and iodide, in CO2 cycloaddition reactions was reported by Calo [72] (Table 17). The higher reactivity of Bu4NBr/Bu4NI in comparison with RMIMBr or RPYBr salts was explained by less coordination of halide with the bulkier Bu4N+ cation [72]. In addition, the catalytic activity of cheap and commercially available Bu4NXs is high and quite comparable with much more expensive PPNXs salts (Figure 9).

Table 17.
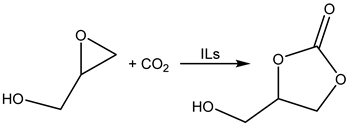
Cycloaddition of CO2 to glycidol producing hydroxymethyl ethylene carbonate (HMEC) [74].
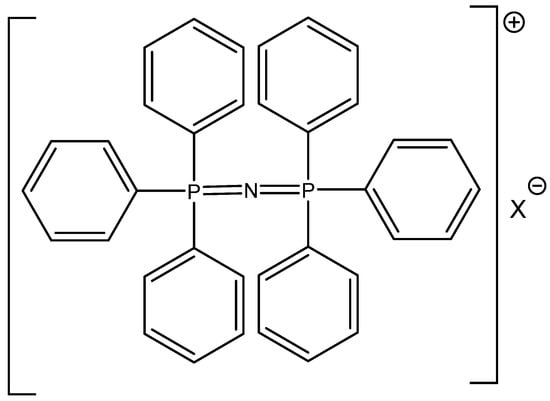
Figure 9.
Structure of bis(triphenylphosphoranylidene)ammonium halide (PPNX) [73].
3.5. Two Component Catalysts Containing HDBs and Onium Salts
The cheap and easily available quaternary ammonium halides TBAB and TBAI are often combined with different HDBs with the aim of boosting catalytic activity for the insertion of CO2 in the oxirane ring.
It was observed that even the addition of glycidol to the Bu4NX significantly increased the yield of PO compared with Bu4NX used alone [74] (Table 18, Entries 4, 7–9).

Table 18.
Comparison of the catalytic activity of various PILs and DESs for the carbonation of SO.
Some mixtures of onium salts with HBDs produce low-melting eutectic solvents (DESs) that readily dissolve both CO2 and epoxide, enabling cycloaddition even at ambient pressure and low temperature [75]. DES is defined as a mixture of two or more compounds that are typically solid at room temperature, but when combined at a particular molar ratio, changes into liquid at room temperature [76].
They not only possessed comparable physicochemical properties to traditional ILs (designability, non-volatility and high thermal stability), but also had advantages such as low cost and a simple preparation process (mixing and melting) without the need for purification.
DESs prepared via the mixing of tertiary amines hydrogen halides (R3N.HX) and ethylene diamine or different aminoethanols were compared in the carbonation of SO at an ambient pressure, obtaining intriguing yields and selectivities of SC even in the case of DES prepared from hydrobromide of cheap triethyl amine and diethanolamine [75] (Table 18, Entries 10–13). DBU hydrobromide mixed with diethanolamine at a molar ratio of 2:1 was recognized as the most catalytically active. Testing this most effective DES, high yields of different carbonates were determined by GC-MS even at an ambient pressure and room temperature of CO2 after 48 h using 20 molar % of this DES. Testing the carbonation of internal ChO, the yield of CC was 43%. Applying a mixture of 15% CO2 with nitrogen (simulated flue gas) drops, however, the yield decreased from 92% (100% CO2 at an ambient pressure after 48 h at room temperature) to 10% (using 15%CO2 in nitrogen under the same reaction conditions, Table 18, Entries 14–21).
Comparing the catalytic activity of different DESs with various protic ILs, the DES (2 DBU.HBr + 1 DEA) is much more active than PIL DBU hydroiodide (DBU.HI) alone. The observed high catalytic activity was explained by the synergistic action of DEA (as HBD) and DBU.HBr as a source of highly nucleophilic naked bromide [75] (Table 18, Entries 16–21 and 24).
Similarly, high activity was observed using DES prepared from Bu4PBr with 2-aminophenol for the carbonating of terminal epoxides. The carbonation of internal epoxide CO to CC was, however, very slow [79] (Table 18, Entry 28).
Pentaerythritol as an aliphatic polyol-based HDB was recognized as effective for the carbonation of PO at elevated pressure [80]. Although completely inactive used alone or with KI, in a mixture with Bu4N+ bromide or iodide, it is very active, obtaining a 97% yield of PC at 70 °C after 22 h of CO2 (0.4 MPa) action (Table 19).

Table 19.
Comparison of catalytic activities of DESs based on pentaerythritol (PETT) and Bu4NXs for the preparation of PC [80].
Choline iodide together with N-hydroxysuccinimide forms DES, enabling the high-yield carbonation of PO to SC at 30–80 °C and 1 MPa pressure of CO2. Instead of choline iodide, Bu4NX in a mixture with N-hydroxysuccinimide is applicable [81]. Using a 2 MPa pressure of CO2, a high yield of CC from internal ChO was obtained at 70 °C after 10 h of reaction (Table 18, Entry 29).
A broad set of aliphatic and aromatic alcohols in terms of their role as potential HDBs was studied by Alves et al. [82] in the co-action of Bu4NBr using pressurized CO2 (2 MPa) for PO carbonation. The authors observed that the most active HDBs were low-polar polyfluorinated secondary alcohols such as tertiary alcohols HFTI or 1,3-bis-HFAB (Figure 10).
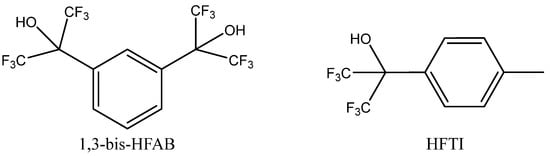
Figure 10.
Structures of the most effective HBDs in co-action with Bu4NBr for the carbonation of PO [81].
Aromatic polyols such as pyrocatechol, pyrogallol and gallic acid were less catalytically active. Aliphatic alcohols exhibited low cooperative activity in the case of the tested Bu4NBr, which was practically comparable with the catalytic effect of sole Bu4NBr. Interestingly, some of tested alcohols even exhibited inhibition effects.
The high catalytical activity of RMIMs/phenols-based DESs was published by Liu et al. even at an ambient pressure of CO2 and at room temperature for SO [83]. Especially N-ethyl-N′-methylimidazolium iodide (EMIMI) was recognized as a very suitable part of DES in co-action with phenols substituted with electron-donating groups such as –NH2, –C(CH3)3 and –Cl, –OH. The most effective DES contained EMIMI (2 mol) and resorcinol (1 mol). The authors explained its high catalytic activity as multifunctional HBD-based activation by acidic hydrogen bound in position 2 of EMIM salt together with hydrogen from the –OH group of resorcinol (Figure 11) and the subsequent action of iodide as a nucleophile. Interestingly, comparing the activity of (2 EMIMI + 1 resorcinol) DES with the much cheaper (2 Bu4NI + 1 resorcinol) binary system for SO carbonation, the obtained yields of PEC were very similar [83]. SO was the single epoxide studied, however, in this article. Another catalytically very effective DESs containing mixture of choline chloride and malic acid or choline iodide and glycerol published Vagnoni et al. [84].
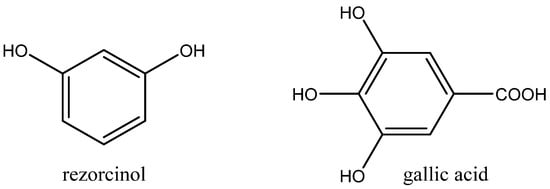
Figure 11.
Structures of resorcinol and gallic acid.
Additional very effective DESs were obtained as catalysts by mixing 2-hydroxymethylpyridine or 2,6-hydroxymethylpyridine with Bu4NI [40]. These DESs were able to catalyze the carbonation of EPIC to chloromethyl-ethylenecarbonate (CMEC) even at room temperature and ambient CO2 pressure. The carbonation of internal epoxide ChO was very slow, however, under ambient conditions even after 20 h using 8 molar % of catalyst [40].
Gallic acid (Figure 11), as a green, biobased and biodegradable HDB, was discovered by Sopena et al. as a more effective alternative of resorcinol in a binary Bu4NI + gallic acid catalytic system dissolved in 2-butanone [85]. Even internal epoxide was carbonated with a high yield at 80 °C and 1 MPa pressure of CO2 after 18 h [85].
Polycarboxylic acids such as citric acid were effectively applied as the HDB part of DES together with choline iodide [86]. The other tested carboxylic acids were less active HDBs compared with citric acid. Additionally, it was observed that the molar ratio of the used HBA and HDB is crucial. For DES obtained by the melting of choline iodide, citric acid at a molar ratio of 2:1 (excess of iodide source) is highly active. Changing the molar ratios significantly decreased the reaction yield (but not selectivity). ChO tested as an internal epoxide at 70 °C and 0.5 MPa of CO2 produced only 36% CC after 6 h [86].
The attempts to substitute ILs-based iodides or bromides as key parts of DESs were described by Wang et al. [87]. Applying boric and glutaric acids, together with BMIMCl, the authors described significant catalytic activity even without the presence of bromide or iodide ions for the carbonation of terminal epoxides at 0.8 MPa of CO2 and 70 °C. [87]. The carbonation of internal ChO was below 40% after 7 h of CO2 action.
The most active HDB described until this time for the catalysis of epoxides’ carbonation is ascorbic acid in co-action with Bu4NI [15] (Table 20). This mixture was effective even for the carbonation of internal epoxides, even at an elevated temperature (100 °C) and 2 MPa CO2 pressure [15] (Table 21).

Table 20.
Comparison of various HBD/Bu4NI catalytic systems for the carbonation of EPIC.

Table 21.
Carbonation of methyl oleate using ascorbic acid (HBD) and different sources of nucleophile (Bu4NX) [91].
Encouraged by the robustness of this catalytic system, Elia et al. tested the Bu4NI/ascorbic acid system for the cycloaddition of CO2 in epoxidized fatty acid esters [91]. Cyclic carbonates based on fatty acid esters seemed to be potential plasticizers for polyvinyl chloride instead of harmful phtalates, for example [92].
As can be seen in Table 21, the most effective catalytic mixture found contains Bu4NCl/ascorbic acid. Bu4NCl is superior because overly nucleophilic Bu4NI causes undesirable Meinwald rearrangement producing ketones instead of cyclic carbonates, probably due to the sterical hindrance in the case of epoxidized oleic acid methyl ester [91]. In the case of epoxidized polyunsaturated fatty acid esters, allylic alcohols are produced as by-products using Bu4NI [91].
3.6. Application of Bifunctional (or Multifunctional) Onium Salts
The functionalization of ILs involves an increase in catalytic activity owing the synergistic effect between the bound functional groups (nucleophilic iodide or bromide anions together with –NH2, –COOH or –OH groups in the role of HBDs). The reached synergism enables a decrease in the quantity of the multifunctional catalyst and simpler separation in an optimal case [93] (Table 22, Figure 12). On the other hand, multifunctional ILs are more difficult to prepare and more expensive due to this reason.

Table 22.
Carbonation of butylene oxide (BO) using bifunctional phosphonium salts and onium salts [93].
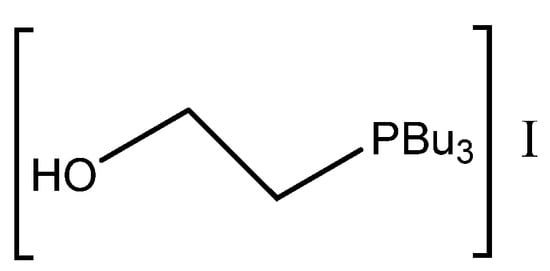
Figure 12.
Structure of the most active bifunctional phosphonium salt tri-n-butyl-(2-hydroxyethyl) phosphonium iodide [93].
Bifunctional catalysis based on aromatic OH-functionalized onium salt was described by Tsutsumi et al. [94]. This research work demonstrates that it still possible to discover a very active and quite cost-effective bifunctional catalyst such as m-trimethylammonium phenolate, which is much more effective than more structurally complicated ones [94]. This catalyst was only tested, however, for the carbonation of terminal epoxides and still requires higher pressure of CO2 at an elevated temperature [94] (Scheme 10, Table 23).
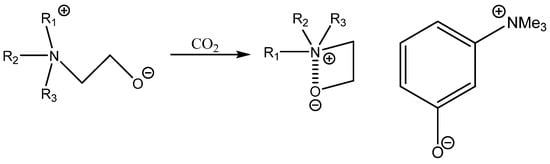
Scheme 10.
CO2 activation by ammonium betaine organocatalyst 3-trimethylammonium phenolate [94].

Table 23.
Comparison of catalytic action of different substituted phenols on carbonation of hexylene oxide [94].
Meng et al. published very promising results obtained by testing a series of OH-functionalized DBU-based ILs (Figure 13) [95]. They discovered not only a recyclable organocatalyst with excellent activity for carbonation of EPIC at 30 °C and an ambient pressure of CO2, but also a compound suitable for the utilization of CO2 from a simulated flue gas (Scheme 11). Its high activity is explained by the cooperative activation of the epoxide ring by protonated DBU in the role of HDB and the activation of CO2 via carbonate formation utilizing alcoholate on a bridge-functionalized bis-DBU anion.
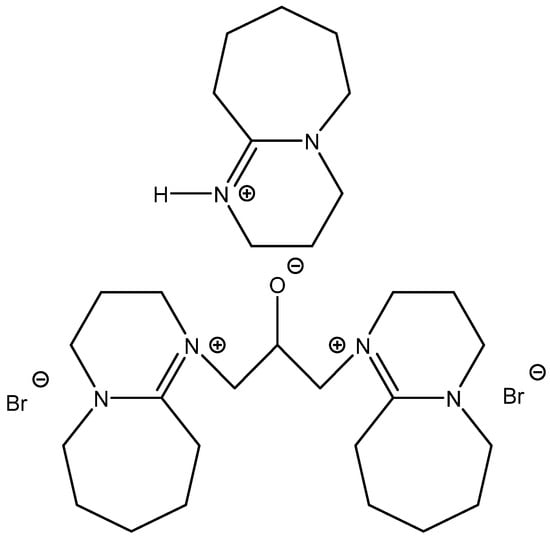
Figure 13.
Structure of most effective OH-functionalized DBU-based IL [95].
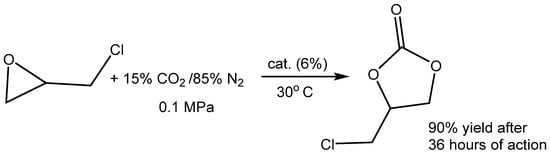
Scheme 11.
Carbonation of EPIC using simulated flue gas (15% CO2 in nitrogen) catalyzed by OH-functionalized DBU-based IL at ambient pressure and at 30 °C [95].
Another described group of catalytically active bifunctional catalysts consists of 1-alkyl-2-hydroxyalkyl pyrazolium salts [96]. The most active one from the broad group of tested derivatives was 1-ethyl-2-(2-hydroxy)ethylpyrazolium bromide (Figure 14). It was demonstrated that using 1 MPa pressure of CO2 at 110 °C, even internal epoxide ChO was carbonated to CC with a considerable yield of over 60% after 4 h of action [96].
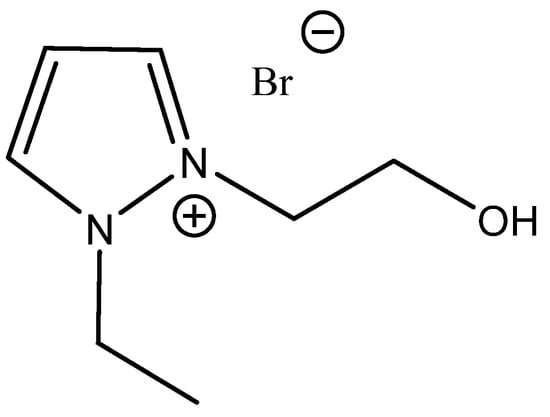
Figure 14.
Structure of 1-ethyl-2-(2-hydroxy)ethylpyrazolium bromide, the most effective carbonation catalyst based on 1-alkyl-2-hydroxyalkyl pyrazolium salts [96].
Zhou et al. compared the catalytic activity of quaternized aminoacid glycine (betaine) and quaternized aminoethanol salts (choline salts, Scheme 12) [97].
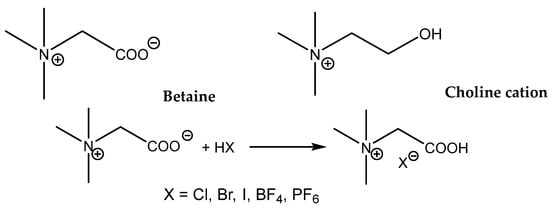
Scheme 12.
Structures of tested bifunctional onium salts and scheme of preparation of betaine hydrohalides [97].
The main difference in the structures of these onium salts is the presence of a more acidic carboxylic group (a stronger HBD) in the betaine structure compared with the choline hydroxyl group. In addition, the effects of different anions in the case of protonated betaine were compared, and it was observed that the most active was the corresponding iodide salt. The catalytic activity of different betaine and choline salts decreased in the range: betaine.HI > betaine.HCl > choline.HCl > betaine.HBr > betaine.BF4−. The authors interpret the low betaine.HBr activity as the effect of its low solubility in the reaction mixture. The tested choline.HCl possesses an activity comparable with Bu4NBr, which supports the positive effect of the hydroxyl group in the activation of the oxirane ring of PO. This positive effect could be both based on HBD action and/or the activation of CO2 on account of carbonate formation. The considerable HDB effect of the carboxylic group of protonated betaines exceeds, however, the effect of the hydroxyl group in choline. The reaction conditions for effective carbonation even of terminal epoxides are, however, harsh (8 MPa CO2, 140 °C) [97]. Due to the above-mentioned reasons, the research that focused on ILs functionalized with the carboxylic group(s) provided fruitful results.
Xiao et al. suggested that the influence of suitable acidity, even with the flexibility of the bound -(CH2)n-COOH chain in the IL structure, is crucial for the carbonation of epoxides due to the cooperation function of the ring-opening of oxiranes [98]. When 1-(2-carboxyethyl)-3-methylimidazolium bromide was used as bifunctional IL, the PC from PO was obtained with ca. 96% yield using pressurized CO2 (1.5 MPa) at 110 °C after 2 h. The IL showed high thermal stability and could be recycled with a slight loss in activity, while the selectivity of the cyclic carbonates remained at over 98%. The catalytic activity of the described functionalized IL-based carboxylic acids is still not unique enough, however, and these types of catalysts still require elevated pressure of CO2 to maintain a high conversion of epoxides to cyclic carbonates. In addition, the carbonation of internal epoxide is still quite slow even at the above-mentioned high pressure and elevated temperature [98].
The construction of bridge-functionalized bisimidazolium-based ILs improves the catalytic activity of acidic ILs, as was discovered by Kuhn et al. [99]. The most active catalyst is the most branched one, bis(imidazoyl)isobutyric acid derivative, N-arylated with hydrophobic mesitylene (Figure 15). It is well recyclable and active even using 0.4 MPa CO2 at 70 °C. It is ineffective, however, in the case of internal epoxides’ carbonation [99].
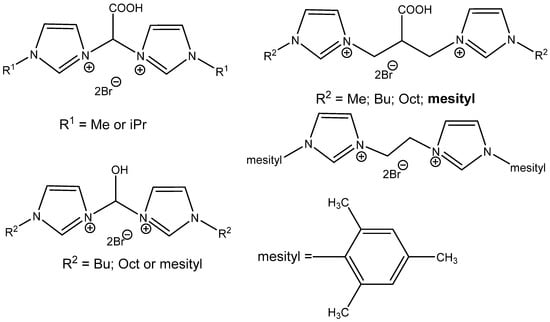
Figure 15.
Multifunctional bisimidazolium bromides tested for carbonation of epoxides [99].
An additional direction of research related to the significant increasing of catalytic activity was discovered by Han et al. [100] and Wang et al. [101]. The Han and Wang research groups recognized the crucial role of ion pairs produced by a combination of acidic ILs with guanidinium cations. Using the same acidic ILs, neutralized by alkylated guanidines, enables an increase in activity, probably due to the distinctive activation of reacting CO2. This catalytic system is active at 0.1 MPa CO2 and 30 °C for the carbonation of EPIC, but fails even in the case of PO (Table 24). Additionally, the used ion pairs are simply separable from the produced cyclic carbonates by means of extraction with ethyl acetate, enabling simple recycling without a significant drop in conversion.

Table 24.
Catalytic activity of multifunctional IM-based ILs on the synthesis of CPC by carbonation of EPIC [100,101].
Bridged methylene(bis)imidazolium salts substituted on both N′-nitrogens by carboxymethyl groups are more active after neutralization with tetramethylguanidine [101] (Figure 16, Table 24). Even this catalytic system is active at 0.1 MPa CO2 and 50 °C but the carbonation of internal ChO seems to be sluggish using the above-mentioned reaction conditions. Additionally, the used catalyst is simply separable from the produced cyclic carbonates and enables simple recycling without a significant drop in conversion.
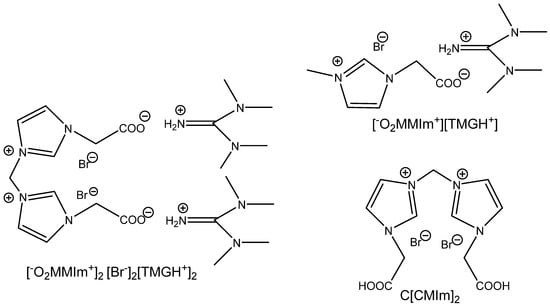
Figure 16.
Structures of multifunctional IM-based ILs tested for the carbonation of EPIC [100,101].
The reverse activation of dual amino-functionalized ILs neutralized with acidic aminoacids, such as glutamic or aspartic acids, is possible, as was documented by Yue et al. [102]. This ion-pair-based catalytic system produces, however, high yields at 0.5 MPa and a temperature of 105 °C after 13 h of CO2 action. It is recyclable without loss of activity and works well in the case of terminal epoxides [102] (Figure 17).
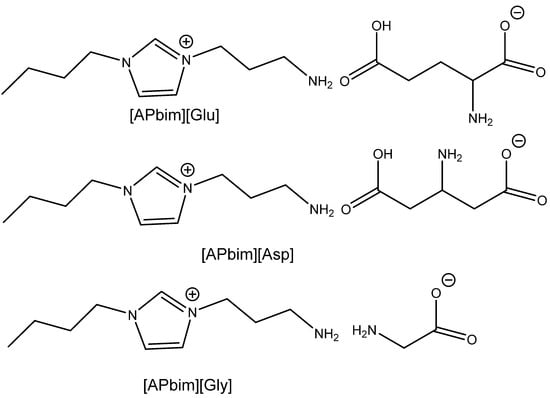
Figure 17.
Structures of dual amino-functionalized IM-based ILs [102].
A very attractive alternative approach was published by Kumar et al. [103]. Their research was focused on the utilization of CO2 from model flue gas (5–15% CO2 in N2 stream at atmospheric pressure) at 80 °C using task specific AA-based ILs (Scheme 13). The authors verified that tetrabutylammonium salt with histidine dissolved in dialkyl carbonate enables the capture and usage of CO2 for the carbonation of terminal epoxides at the above-mentioned reaction conditions, with a high yield. This research work is one of the very infrequent examples of the direct capture and subsequent utilization of CO2 from (model) flue gas. The authors documented that this catalyst is recyclable with no drop in efficiency after six recycling steps. This catalytic system was proved, however, only on terminal epoxides [103].
Scheme 13.
Carbonation of terminal epoxides using model flue gas (5–15% CO2 in nitrogen) [103].
A different approach was used for the preparation of highly catalytically active bifunctional ILs using allylation by Hui et al. [104] (Figure 18). They discovered tetramethylguanidine-based allylated IL with superior activity for the capture and utilization of CO2 from simulated flue gas at 120 °C, ambient pressure and solventless conditions (Table 25). This catalyst is effective even for carbonation of ChO and reusable with low loss of activity after five recycling steps [104]. This type of catalyst seems to be very attractive even for the carbonation of other internal epoxides including eventually epoxidized fatty acid esters.
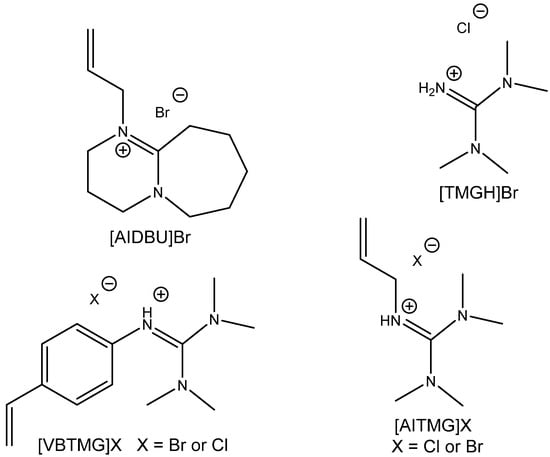
Figure 18.
Structures of functionalized ILs tested as highly active CO2 cycloaddition catalysts [104].

Table 25.
Effect of different PILs and ILs on the carbonation of SO [71].
4. Conclusions
The carboxylation of epoxides is a sustainable pathway for the fixation of CO2 into valuable chemicals, considering the industrial utilization of cyclic and polymeric carbonates. The effect of homogeneous organocatalysts published in recent literature is presented. We hope that this review affords insights into the recent research and development of efficient metal-free homogeneous catalysts.
Hopefully, the next development of homogeneous catalysts, including organocatalysts (e.g., organic salt, ILs and DESs), will facilitate the expanding of the spectrum of available metal-free organocatalysts applicable for the reaction of CO2 with terminal epoxides even at CO2 pressures of 1 bar and reaction temperatures of less than around 50 °C. Except high catalytic activity, the simple catalyst separability should be profitable because of the necessity of high catalytic loading for the effective course of cycloaddition reaction. The bulky tetrabutylammonium or tetrabutylphosphonium cations in Bu4NX or Bu4PX enable the high nucleophilic activity of the appropriate naked anions of X−, such as bromide or iodide, in most cases. Onium salts are widely applied as part of multicomponent catalytic systems in the research and development of epoxides’ carbonation processes. Most of all, deep eutectic solvents constitute an important group of multicomponent low-cost homogeneous organocatalysts. In particular, DESs containing choline chloride and urea exhibit high catalytic activity [84,85]. Besides the above-mentioned onium halides, some ion pairs produced by the mixing of DBU with amidine-based alcohols are highly active [95]. The most effective halide free IL-based homogeneous catalyst was recognized to be the Bu4N salt of histidine [103]. Several ion pairs based on N′-carboxymethylated MIM bromides neutralized with tetraalkylguanidines enable CO2 cycloaddition at ambient pressure [100,101,102]. Searching for simple and cheap catalytic systems that are active at mild reaction conditions is attractive not only due to the environmental point of view (lower energy consumption) but even due to the thermodynamic reasons. As the formation the cyclic carbonate is exothermic, the lower reaction temperature affects the shifting of the reaction equilibrium in the products.
As we illustrate in this review, many simple molecules are known to act as effective mediators and/or catalysts, including Lewis and Bronsted acids such as water, ascorbic acid, cellulose, etc. Based on generally accepted mechanisms of carboxylation of epoxides, research focused on utilization of other HBDs such as bidentate nucleophiles could be profitable. The promising groups of simply available catalytically active compounds such as polyalkyl guanidines [100,101,104], enaminones [105], N-hydroxylamines [106] and amidoximes [107] should, in our opinion, be investigated in more detail.
The utilization of tandem reactions such as the one-pot production of cyclic carbonates starting directly from biobased unsaturated fatty acids esters [8], the one-pot production of ethylene carbonate from ethylene produced by low-energy-demanding methods [2,108] or the production of HMEC from chlorinated bio-based glycerol [109] seems to be very promising for effective CO2 fixation.
It is evident from the recently published results that both the possible utilization of CO2 from flue gas and the carbonation of internal bio-based epoxides such as epoxidized fatty acid esters are the main developing areas of research focused on CO2 capture and utilization. However, the mild reaction conditions and lower catalytic loading are still challenging for both the carboxylation of internal epoxide substrates such as epoxidized fatty acid esters as well as for the direct utilization of waste CO2 from power plant flue gas.
Author Contributions
T.W. conceived, designed and wrote the paper. B.K. provided technical support. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Faculty of Chemical Technology, University of Pardubice, with the support of excellent research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Artz, J.; Müller, T.E.; Thenert, K. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic Strategies for the Cycloaddition of Pure, Diluted, and Waste CO2 to Epoxides under Ambient Conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Cui, S.; Borgemenke, J.; Liu, Z.; Li, Y. Recent advances of “soft” bio-polycarbonate plastics from carbon dioxide and renewable bio-feedstocks via straightforward and innovative routes. J. CO2 Util. 2019, 34, 40–52. [Google Scholar] [CrossRef]
- Kiatkittipong, K. Green Pathway in Utilizing CO2 via Cycloadditon Reaction with Epoxide-A Mini Review. Processes 2020, 8, 548. [Google Scholar] [CrossRef]
- Cahugule, A.A.; Tamboli, A.H.; Kim, H. Ionic liquid as a catalyst for utilization of carbon dioxide to production of linear and cyclic carbonate. Fuel 2017, 200, 316–332. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef]
- Calmanti, R.; Sargentoni, N.; Selva, M.; Perosa, A. One-Pot Tandem Catalytic Epoxidation—CO2 Insertion of Monounsaturated Methyl Oleate to the Corresponding Cyclic Organic Carbonate Catalysts. J. CO2 Util. 2021, 11, 1477. [Google Scholar] [CrossRef]
- Coates, G.W.; Moore, D.R. Discrete Metal-Based Catalysts for the Copolymerization of CO2 and Epoxides: Discovery, Reactivity, Optimization, and Mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618. [Google Scholar] [CrossRef]
- Gorbunov, D.N.; Nenasheva, M.V.; Terenina, M.V.; Kardasheva, Y.S.; Kardashev, S.V.; Naranov, E.R.; Bugaev, A.L.; Soldatov, A.V.; Maximov, A.L.; Karakhanova, E.A. Transformations of Carbon Dioxide under Homogeneous Catalysis Conditions (A Review). Petroleum Chem. 2022, 62, 1–39. [Google Scholar] [CrossRef]
- Rehman, A.; Saleem, F.; Javed, F.; Ikhlaq, A.; Ahmad, S.W.; Harvey, A. Recent advances in the synthesis of cyclic carbonates via CO2 cycloaddition to epoxides. J. Environ. Chem. Eng. 2021, 9, 105113. [Google Scholar] [CrossRef]
- Guo, L.; Lamb, K.J.; North, M. Recent developments in organocatalyzed transformations of epoxides and carbon dioxide into cyclic carbonates. Green Chem. 2021, 23, 77–118. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365. [Google Scholar] [CrossRef]
- You, H.; Wang, E.; Cao, H.; Zhuo, C.; Liu, S.; Wang, X.; Wang, F. From Impossible to Possible: Atom-Economic Polymerization of Low Strain Five-Membered Carbonates. Angew. Chem. Int. Ed. 2022, 61, e202113152. [Google Scholar] [CrossRef]
- Arayachukiat, S.; Kongtes, C.; Barthel, A.; Vummaleti, S.V.C.; Poater, A.; Wannakao, S.; Cavallo, L.; D’Elia, V. Ascorbic acid as a bifunctional hydrogen bond donor for the synthesis of cyclic carbonates from CO2 under ambient conditions. ACS Sustain. Chem. Eng. 2017, 5, 6392–6397. [Google Scholar] [CrossRef]
- Lari, G.M.; Pastore, G.; Mondelli, C.; Pérez-Ramírez, J. Towards sustainable manufacture of epichlorohydrin from glycerol using hydrotalcite-derived basic oxides. Green Chem. 2018, 20, 148–159. [Google Scholar] [CrossRef]
- Vitiello, R.; Tesser, R.; Santacesaria, E.; Di Serio, M. New Production Processes of Dichlorohydrins from Glycerol Using Acyl Chlorides as Catalysts or Reactants. Ind. Eng. Chem. Res. 2016, 55, 1484–1490. [Google Scholar] [CrossRef]
- Morodo, R.; Gerardy, R.; Petit, G.; Monbaliu, J.M. Continuous flow upgrading of glycerol toward oxiranes and active pharmaceutical ingredients thereof. Green Chem. 2019, 21, 4422–4433. [Google Scholar] [CrossRef]
- Kubicek, P.; Sladek, P. Method of Preparing Dichloropropanols from Glycerine. U.S. Patent EP 1663924 B1, 23 August 2004. [Google Scholar]
- Cespi, D.; Cucciniello, R.; Ricciardi, M.; Capacchione, C.; Vassura, I.; Passarini, F.; Proto, A. A simplified early stage assessment of process intensification: Glycidol as a value-added product from epichlorohydrin industry wastes. Green Chem. 2016, 18, 4559–4570. [Google Scholar] [CrossRef]
- Phung, T.K.; Pham, T.L.M.; Vu, K.B.; Busca, G. (Bio)Propylene production processes: A critical review. J. Environ. Chem. Eng. 2021, 9, 105673. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Y. Dehydration of ethanol to ethylene. Ind. Eng. Chem. Res. 2013, 52, 9505–9514. [Google Scholar] [CrossRef]
- Broeren, M. Production of Bio-Ethylene; IEA-ETSAP: Paris, France; IRENA: Abu Dhabi, United Arab Emirates, 2013; pp. 1–20. [Google Scholar]
- Resul, M.F.M.G.; López Fernández, A.M.; Rehman, A.; Harvey, A.P. Development of a selective, solvent-free epoxidation of limonene using hydrogen peroxide and a tungsten-based catalyst. React. Chem. Eng. 2018, 3, 747–756. [Google Scholar] [CrossRef]
- Shaarani, F.W.; Bou, J.J. Synthesis of vegetable-oil based polymer by terpolymerization of epoxidized soybean oil, propylene oxide and carbon dioxide. Sci. Total Environ. 2017, 598, 931–936. [Google Scholar] [CrossRef]
- Cui, S.; Qin, Y.; Li, Y. Sustainable Approach for the Synthesis of Biopolycarbonates from Carbon Dioxide and Soybean Oil. ACS Sustain. Chem. Eng. 2017, 5, 9014–9022. [Google Scholar] [CrossRef]
- Phimsen, S.; Yamada, H.; Tagawa, T.; Kiatkittipong, W.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Epoxidation of methyl oleate in a TiO2 coated-wall capillary microreactor. Chem. Eng. J. 2017, 314, 594–599. [Google Scholar] [CrossRef]
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Morganstewart, H.; Tsang, S.C. Catalytic Coupling of CO2 with Epoxide Over Supported and Unsupported Amines. J. Phys. Chem. A 2010, 114, 3863–3872. [Google Scholar] [CrossRef]
- Yang, Z.Z.; He, L.N.; Miao, C.X.; Chanfreau, S. Lewis basic ionic liquids-catalyzed conversion of carbon dioxide to cyclic carbonates. Adv. Synth. Catal. 2010, 352, 2233–2240. [Google Scholar] [CrossRef]
- Fanjul-Mosteirin, N.; Jehanno, C.; Ruiperez, F.; Sardon, H.; Dove, A. Rational study of DBU salts for the CO2 insertion into epoxides for the synthesis of cyclic carbonates. ACS Sustain. Chem. Eng. 2019, 7, 10633–10640. [Google Scholar] [CrossRef]
- Aoyagi, N.; Furusho, Y.; Endo, T. Cyclic amidine hydroiodide for the synthesis of cyclic carbonates and cyclic dithiocarbonates from carbon dioxide or carbon disulfide under mild conditions. Tetrahedron 2019, 75, 130781. [Google Scholar] [CrossRef]
- Roshan, K.R.; Palissery, R.A.; Kathalikkattil, A.C.; Babu, R.; Mathai, G.; Lee, H.S.; Park, D.W. A computational study of the mechanistic insights into base catalysed synthesis of cyclic carbonates from CO2: Bicarbonate anion as an active species. Catal. Sci. Technol. 2016, 6, 3997–4004. [Google Scholar] [CrossRef]
- Lee, C.S.; Ong, Y.L.; Aroua, M.K.; Daud, W.M.A.W. Impregnation of palm shell based activated carbon with sterically hindered amines for CO2 adsorption. Chem. Eng. J. 2013, 219, 558–564. [Google Scholar] [CrossRef]
- Zelenak, V.; Halamova, D.; Gaberova, L.; Bloch, E.; Llewellyn, P. Amine-modified SBA-12 mesoporous silica for carbon dioxide capture: Effect of amine basicity on sorption properties. Microporous Mesoporous Mater. 2008, 116, 358–364. [Google Scholar] [CrossRef]
- Azzouz, R.; Moreno, V.C.; Herasme-Grullon, C.; Levacher, V.; Estel, L.; Ledoux, A.; Bischoff, L. Efficient Conversion of Epoxides into Carbonates with CO2 and a Single Organocatalyst: Laboratory and Kilogram-Scale Experiments. Synlett 2020, 31, 183–188. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, W.; Yang, Z.; Wang, J.; Xu, T.; Xin, J.; Zhang, S. Superbase/cellulose: An environmentally benign catalyst for chemical fixation of carbon dioxide into cyclic carbonates. Green Chem. 2014, 16, 3071–3078. [Google Scholar] [CrossRef]
- Qaroush, A.K.; Hasan, A.K.; Hammad, S.B.; Feda’a, M.; Assaf, K.I.; Alsoubani, F.; Ala’a, F.E. Mechanistic insights on CO2 utilization using sustainable catalysis. New J. Chem. 2021, 45, 22280–22288. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Liang, L.; Sun, J. Protonated triethanolamine as multi-hydrogen bond donors catalyst for efficient cycloaddition of CO2 to epoxides under mild and cocatalyst-free conditions. J. CO2 Util. 2016, 16, 384–390. [Google Scholar] [CrossRef]
- Lan, D.H.; Fan, N.; Wang, Y.; Gao, X.; Zhang, P.; Chen, L.; Yin, S.F. Recent advances in metal-free catalysts for the synthesis of cyclic carbonates from CO2 and epoxides. Chin. J. Catal. 2016, 37, 826–845. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.Y.; Kodama, K.; Hirose, T. An efficient metal- and solvent-free organocatalytic system for chemical fixation of CO2 into cyclic carbonates under mild conditions. Green Chem. 2016, 18, 1229–1233. [Google Scholar] [CrossRef]
- Kumatabara, Y.; Okada, M.; Shirakawa, S. Triethylamine hydroiodide as a simple yet effective bifunctional catalyst for CO2 fixation reactions with epoxides under mild conditions. ACS Sustain. Chem. Eng. 2017, 5, 7295–7301. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Fan, F.J.; Xing, H.B.; Yang, Q.W.; Bao, Z.B.; Ren, Q.L. Efficient synthesis of cyclic carbonates from atmospheric CO2 using a positive charge delocalized ionic liquid catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2841–2846. [Google Scholar] [CrossRef]
- Shen, Y.M.; Duan, W.L.; Shi, M. Phenol and organic bases co-catalyzed chemical fixation of carbon dioxide with terminal epoxides to form cyclic carbonates. Adv. Synth. Catal. 2003, 345, 337–340. [Google Scholar] [CrossRef]
- Khiari, R.; Salon, M.C.B.; Mhenni, M.F.; Mauret, E.; Belgacem, M.N. Synthesis and characterization of cellulose carbonate using greenchemistry: Surface modification of Avicel. Carbohydr. Polym. 2017, 163, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, M.; Bernin, D.; Hasani, M.; Lund, M.; Bialik, E. Direct evidence for reaction between cellulose and CO2 from nuclear magnetic resonance. ACS Sustain. Chem. Eng. 2021, 9, 14006–14011. [Google Scholar] [CrossRef]
- Aoyagi, N.; Furusho, Y.; Endo, T. Effective synthesis of cyclic carbonates from carbon dioxide and epoxides by phosphonium iodides as catalysts in alcoholic solvents. Tetrahedron Lett. 2013, 54, 7031–7034. [Google Scholar] [CrossRef]
- Samikannua, A.; Konwara, L.J.; Mäki-Arvelab, P.; Mikkola, J.P. Renewable N-doped active carbons as efficient catalysts for direct synthesis of cyclic carbonates from epoxides and CO2. Appl. Catal. B Environ. 2019, 241, 41–51. [Google Scholar] [CrossRef]
- Qi, C.; Jiang, H. Histidine-catalyzed synthesis of cyclic carbonates in supercritical carbon dioxide. Sci. China Chem. 2010, 53, 1566–1570. [Google Scholar] [CrossRef]
- Tharun, J.; Roshan, K.R.; Kathalikkattil, A.C.; Kang, D.H.; Ryu, H.M.; Park, D.W. Natural amino acids/H2O as a metal- and halide-free catalyst system for the synthesis of propylene carbonate from propylene oxide and CO2 under moderate conditions. RSC Adv. 2014, 4, 41266–41270. [Google Scholar] [CrossRef]
- Tharun, J.; Mathai, G.; Roshan, R.; Kathalikkattil, A.C.; Bomi, K.; Park, D.W. Simple and efficient synthesis of cyclic carbonates using quaternized glycine as a green catalyst. Phys. Chem. Chem. Phys. 2013, 15, 9029–9033. [Google Scholar] [CrossRef]
- Roshan, K.R.; Kathalikkattil, A.C.; Tharun, J.; Kim, D.W.; Won, Y.S.; Park, D.W. Amino acid/KI as multi-functional synergistic catalysts for cyclic carbonate synthesis from CO2 under mild reaction conditions: A DFT corroborated study. Dalton Trans. 2014, 43, 2023–2031. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, W.; Xu, F.; Chen, S.; Zhang, S. Amino acids/superbases as eco-friendly catalyst system for the synthesis of cyclic carbonates under metal-free and halide-free conditions. Synth. Commun. 2018, 48, 876–886. [Google Scholar] [CrossRef]
- Claver, C.; Yeamin, M.B.; Reguero, M.; Masdeu-Bultó, A.M. Recent advances in the use of catalysts based on natural products for the conversion of CO2 into cyclic carbonates. Green Chem. 2020, 22, 7665–7706. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, J.; Cheng, W.; Wang, J.; Li, Q.; Zhang, S. Biocompatible and recyclable amino acid binary catalyst for efficient chemical fixation of CO2. Catal. Commun. 2014, 44, 6–9. [Google Scholar] [CrossRef]
- Dai, W.; Yang, W.; Zhang, Y.; Wang, D.; Luo, X.; Tu, X. Novel isothiouronium ionic liquid as efficient catalysts for the synthesis of cyclic carbonates from CO2 and epoxides. J. CO2 Util. 2017, 17, 256. [Google Scholar] [CrossRef]
- Orhan, O.Y. Effects of various anions and cations in ionic liquids on CO2 capture. J. Mol. Liq. 2021, 333, 115981. [Google Scholar] [CrossRef]
- Peng, J.; Deng, Y. Cycloaddition of carbon dioxide to propylene oxide catalyzed by ionic liquids. New J. Chem. 2001, 25, 639–641. [Google Scholar] [CrossRef]
- Neumann, J.G.; Stassen, H. Anion Effect on Gas Absorption in Imidazolium-Based Ionic Liquids. J. Chem. Inf. Model. 2020, 60, 661–666. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Vasilyev, D.; Hulla, M.; Chamam, S.; Menoud, F.; Laurenczy, G.; Katsyuba, S.; Dyson, P.J. Intricacies of Cation−Anion Combinations in Imidazolium SaltCatalyzed Cycloaddition of CO2 Into Epoxides. ACS Catal. 2018, 8, 2589–2594. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, D.; Ma, Y.; Guo, J.; He, Z.; Ma, B.; Zhang, J. Benzyl substituted imidazolium ionic liquids as efficient solvent-free catalysts for the cycloaddition of CO2 with epoxides: Experimental and Theoretic study. J. CO2 Util. 2017, 22, 44–52. [Google Scholar] [CrossRef]
- Akhdar, A.; Onida, K.; Vu, N.D.; Grollier, K.; Norsic, S.; Boisson, C.; Duguet, N. Thermomorphic Polyethylene-Supported Organocatalysts for the Valorization of Vegetable Oils and CO2. Adv. Sustain. Syst. 2021, 5, 2000218. [Google Scholar] [CrossRef]
- Buettner, H.; Grimmer, C.; Steinbauer, J.; Werner, T. Iron-based binary catalytic system for the valorization of CO2 into biobased cyclic carbonates. ACS Sustain. Chem. Eng. 2016, 4, 4805–4814. [Google Scholar] [CrossRef]
- Carrodeguas, L.P.; Cristòfol, À.; Fraile, J.M.; Mayoral, J.A.; Dorado, V.; Herrerías, C.I.; Kleij, A.W. Fatty acid based biocarbonates: Al-mediated stereoselective preparation of mono-, di-and tricarbonates under mild and solvent-less conditions. Green Chem. 2017, 19, 3535–3541. [Google Scholar] [CrossRef]
- Anthofer, M.H.; Wilhelm, M.E.; Cokoja, M.; Markovits, I.I.; Pöthig, A.; Mink, J.; Kühn, F.E. Cycloaddition of CO2 and epoxides catalyzed by imidazolium bromides under mild conditions: Influence of the cation on catalyst activity. Catal. Sci. Technol. 2014, 4, 1749–1758. [Google Scholar] [CrossRef]
- Yoshihito, K.; Masafumi, Y.; Takao, I. N-Heterocyclic Carbenes as Efficient Organocatalysts for CO2 Fixation Reactions. Angew. Chem. Int. Ed. 2009, 48, 4194–4197. [Google Scholar] [CrossRef]
- Wong, W.L.; Chan, P.H.; Zhou, Z.Y.; Lee, K.H.; Cheung, K.C.; Wong, K.Y. A robust ionic liquid as reaction medium and efficient organocatalyst for carbon dioxide fixation. ChemSusChem 2008, 1, 67–70. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Heidari, Y.; Kozehgary, G. Nicotine-derived ammonium salts as highly efficient catalysts for chemical fixation of carbon dioxide into cyclic carbonates under solvent-free conditions. RSC Adv. 2015, 5, 61179–61183. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Liang, T.; Lin, X.; Shi, L.; Wang, F. Cycloaddition of CO2 and epoxides catalyzed by dicationic ionic liquids mediated metal halide: Influence of the dication on catalytic activity. J. Mol. Catal. A Chem. 2015, 408, 242. [Google Scholar] [CrossRef]
- Yuan, G.; Zhao, Y.; Wu, Y.; Li, R.; Chen, Y.; Xu, D.; Liu, Z. Cooperative effect from cation and anion of pyridine-containing anion-based ionic liquids for catalysing CO2 transformation at ambient conditions. Sci. China Chem. 2017, 60, 958–963. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.; Zhang, G.; Kodama, K.; Yasutake, M.; Hirose, T. Synthesis of cyclic carbonates from CO2 and epoxides catalyzed by low loadings of benzyl bromide/DMF at ambient pressure. Chem. Commun. 2014, 50, 14813–14816. [Google Scholar] [CrossRef]
- Lin, W.; Koichi, K.; Takuji, H. DBU/benzyl bromide: An efficient catalytic system for the chemical fixation of CO2 into cyclic carbonates under metal- and solvent- free conditions. Catal. Sci. Technol. 2016, 6, 3872–3877. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Fanizzi, A. Cyclic carbonate formation from carbon dioxide and oxiranes in tetrabutylammonium halides as solvents and catalysts. Org. Lett. 2002, 4, 2561–2563. [Google Scholar] [CrossRef]
- Sit, W.N.; Ng, S.M.; Kwong, K.Y.; Lau, C.P. Coupling reactions of CO2 with neat epoxides catalyzed by PPN salts to yield cyclic carbonates. J. Org. Chem. 2005, 70, 8583–8586. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, F.; Buonerba, A.; Grassi, A.; Capacchione, C.; Milione, S. Glycidol: An hydroxyl-containing epoxide playing the double role of substrate and catalyst for CO2 cycloaddition reactions. ChemSusChem 2016, 9, 3457–3464. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zou, Q.; Zhao, T.; Chen, P.; Liu, Z.; Liu, F.; Lin, Q. Deep Eutectic Solvents as Efficient Catalysts for Fixation of CO2 to Cyclic Carbonates at Ambient Temperature and Pressure through Synergetic Catalysis. ACS Sustain. Chem. Eng. 2021, 9, 10437–10443. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Ionic Liquids and Deep Eutectic Solvents in Natural Products Research: Mixtures of Solids as Extraction Solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef]
- Xiao, L.; Su, D.; Yue, C.; Wu, W. Protic ionic liquids: A highly efficient catalyst for synthesis of cyclic carbonate from carbon dioxide and epoxides. J. CO2 Util. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Wu, K.; Su, T.; Hao, D.; Liao, W.; Zhao, Y.; Ren, W.; Lü, H. (Choline chloride-based deep eutectic solvents for efficient cycloaddition of CO2 with propylene oxide. Chem. Commun. 2018, 54, 9579–9582. [Google Scholar] [CrossRef]
- Liu, F.; Gu, Y.; Xin, H.; Zhao, P.; Gao, J.; Liu, M. Multifunctional phosphonium-based deep eutectic ionic liquids: Insights into simultaneous activation of CO2 and epoxide and their subsequent cycloaddition. ACS Sustain. Chem. Eng. 2019, 7, 16674–16681. [Google Scholar] [CrossRef]
- Wilhelm, M.E.; Anthofer, M.H.; Cokoja, M.; Markovits, I.I.E.; Herrmann, W.A.; Kuhn, F.E. Cycloaddition of Carbon Dioxide and Epoxides using Pentaerythritol and Halides as Dual Catalyst System. ChemSusChem 2014, 7, 1357–1360. [Google Scholar] [CrossRef]
- Liu, F.; Gu, Y.; Zhao, P.; Xin, H.; Gao, J.; Liu, M. N-hydroxysuccinimide based deep eutectic catalysts as a promising platform for conversion of CO2 into cyclic carbonates at ambient temperature. J. CO2 Util. 2019, 33, 419–426. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Gennen, S.; Méreau, R.; Detrembleur, C.; Jérôme, C.; Tassaing, T. Organocatalytic promoted coupling of carbon dioxide with epoxides: A rational investigation of the cocatalytic activity of various hydrogen bond donors. Catal. Sci. Technol. 2015, 5, 4636–4643. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Z.; Zhou, Z.; Zhou, A. Imidazolium-based deep eutectic solvents as multifunctional catalysts for multisite synergistic activation of epoxides and ambient synthesis of cyclic carbonates. J. CO2 Util. 2021, 53, 101717. [Google Scholar] [CrossRef]
- Vagnoni, M.; Samori, C.; Galletti, P. Choline-based eutectic mixtures as catalysts for effective synthesis of cyclic carbonates from epoxides and CO2. J. CO2 Util. 2020, 42, 101302. [Google Scholar] [CrossRef]
- Sopena, S.; Fiorani, G.; Martin, C.; Kleij, A.W. Highly Efficient Organocatalyzed Conversion of Oxiranes and CO2 into Organic Carbonates. ChemSusChem 2015, 8, 3248–3254. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, W.; Yang, Y.; Ma, J.; Liu, F.; Liu, M. Novel biomass-derived deep eutectic solvents promoted cycloaddition of CO2 with epoxides under mild and additive-free conditions. J. CO2 Util. 2021, 54, 101750. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.; Hao, D.; Su, T.; Len, C.; Ren, W.; Lü, H. Synthesis cyclic carbonates with BmimCl-based ternary deep eutectic solvents system. J. CO2 Util. 2020, 40, 101250. [Google Scholar] [CrossRef]
- Hardman-Baldwin, A.M.; Mattson, A.E. Silanediol-catalyzed carbon dioxide fixation. ChemSusChem 2014, 7, 3275–3278. [Google Scholar] [CrossRef]
- Kaneko, S.; Shirakawa, S. Potassium iodide–tetraethylene glycol complex as a practical catalyst for CO2 fixation reactions with epoxides under mild conditions. ACS Sustain. Chem. Eng. 2017, 5, 2836–2840. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, G.X.; Zhang, W.Z.; Lu, X.B. CO2 adducts of phosphorus ylides: Highly active organocatalysts for carbon dioxide transformation. ACS Catal. 2015, 5, 6773–6779. [Google Scholar] [CrossRef]
- Natongchai, W.; Pornpraprom, S.; D’Elia, V. Synthesis of Bio-Based Cyclic Carbonates Using a Bio-Based Hydrogen Bond Donor: Application of Ascorbic Acid to the Cycloaddition of CO2 to Oleochemicals. Asian J. Org. Chem. 2020, 9, 801–810. [Google Scholar] [CrossRef]
- Schäffner, B.; Blug, M.; Kruse, D.; Polyakov, M.; Köckritz, A.; Martin, A.; Rajagopalan, P.; Bentrup, U.; Brückner, A.; Jung, S.; et al. Synthesis and Application of Carbonated Fatty Acid Esters from Carbon Dioxide Including a Life Cycle Analysis. ChemSusChem 2014, 7, 1133–1139. [Google Scholar] [CrossRef]
- Werner, T.; Buttner, H. Phosphorus-based Bifunctional Organocatalysts for the Addition of Carbon Dioxide and Epoxides. ChemSusChem 2014, 7, 3268–3271. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.; Yamakawa, K.; Yoshida, M.; Ema, T.; Sakai, T. Bifunctional Organocatalyst for Activation of Carbon Dioxide and Epoxide To Produce Cyclic Carbonate: Betaine as a New Catalytic Motif. Org. Lett. 2010, 12, 5728–5731. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ju, Z.; Zhang, S.; Liang, X.; von Solms, N.; Zhang, X.; Zhang, X. Efficient transformation of CO2 to cyclic carbonates using bifunctional protic ionic liquids under mild conditions. Green Chem. 2019, 21, 3456–3463. [Google Scholar] [CrossRef]
- Wang, T.; Ma, Y.; Jiang, J.; Zhu, X.; Fan, B.; Yu, G.; Zhang, J. Hydroxyl-functionalized pyrazolium ionic liquids to catalyze Chemical fixation of CO2: Further benign reaction condition for the singlecomponent catalyst. J. Mol. Liq. 2019, 293, 111479. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, S.; Ma, X.; Liang, S.; Jiang, T.; Han, B. Synthesis of cyclic carbonates from carbon dioxide and epoxides over betaine-based catalysts. J. Mol. Catal. A Chem. 2008, 284, 52–57. [Google Scholar] [CrossRef]
- Xiao, L.F.; Lv, D.W.; Su, D.; Wu, W.; Li, H.F. Influence of acidic strength on the catalytic activity of Brønsted acidic ionic liquids on synthesizing cyclic carbonate from carbon dioxide and epoxide. J. Clean. Prod. 2014, 67, 285–290. [Google Scholar] [CrossRef]
- Li, Y.; Dominelli, B.; Reich, R.M.; Liu, B.; Kühn, F.E. Bridge-functionalized bisimidazolium bromides as catalysts for the conversion of epoxides to cyclic carbonates with CO2. Catal. Commun. 2019, 124, 118–122. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Liu, H.; Qian, Q.; Xie, C.; Han, B. Dual-ionic liquid system: An efficient catalyst for chemical fixation of CO2 to cyclic carbonates under mild conditions. Green Chem. 2018, 20, 2990–2994. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, D.; Hu, Y.; Zhou, J.; Liu, Y.; Zhang, J.; Wang, L. Efficient responsive ionic liquids with multiple active centers for the transformation of CO2 under mild conditions: Integrated experimental and theoretical study. J. CO2 Util. 2021, 49, 101573. [Google Scholar] [CrossRef]
- Yue, S.; Wang, P.; Hao, X.; Zang, S. Dual amino-functionalized ionic liquids as efficient catalysts for carbonate synthesis from carbon dioxide and epoxide under solvent and cocatalyst-free conditions. J. CO2 Util. 2017, 21, 238–246. [Google Scholar] [CrossRef]
- Kumar, P.; Varyani, M.; Khatri, P.K.; Paul, S.; Jain, S.L. Post combustion capture and conversion of carbon dioxide using histidine derived ionic liquid at ambient conditions. J. Ind. Eng. Chem. 2017, 49, 152–157. [Google Scholar] [CrossRef]
- Hui, W.; Wang, X.; Li, X.-N.; Wang, H.-J.; He, X.-M.; Xu, X.-Y. Protic ionic liquids tailored by different cationic structures for efficient chemical fixation of diluted and waste CO2 into cyclic carbonates. New J. Chem. 2021, 45, 10741–10748. [Google Scholar] [CrossRef]
- Yue, Z.X.; Pudukudy, M.; Chen, S.; Liu, Y.; Zhao, W.; Wang, J.; Jia, Q. A non-metal Acen-H catalyst for the chemical fixation of CO2 into cyclic carbonates under solvent-and halide-free mild reaction conditions. Appl. Catal. A Gen. 2020, 601, 117646. [Google Scholar] [CrossRef]
- Ravi, S.; Puthiaraj, P.; Ahn, W.S. Hydroxylamine-Anchored Covalent Aromatic Polymer for CO2 Adsorption and Fixation into Cyclic Carbonates. ACS Sustain. Chem. Eng. 2018, 6, 9324–9332. [Google Scholar] [CrossRef]
- Isik, M.; Ruiperez, F.; Sardon, H.; Gonzalez, A.; Zulfiqar, S.; Mecerreyes, D. Innovative poly (ionic liquid) s by the polymerization of deep eutectic monomers. Macromol. Rapid Commun. 2016, 37, 1135–1142. [Google Scholar] [CrossRef]
- Fairuzov, D.; Gerzeliev, I.; Maximov, A.; Naranov, E. Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology. Catalysts 2021, 11, 833. [Google Scholar] [CrossRef]
- Khokarale, S.; Shelke, G.; Mikkola, J.P. Integrated and Metal Free Synthesis of Dimethyl Carbonate and Glycidol from Glycerol Derived 1,3-Dichloro-2-propanol via CO2 Capture. Clean Technol. 2021, 3, 685–698. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).