Abstract
Reactions of VCl3 with 1,2-Bis[(4-methylphenyl)imino]acenaphthene (4-Me-C6H4-bian) or 1,2-Bis[(2-methylphenyl)imino]acenaphthene (2-Me-C6H4-bian) in air lead to the formation of [VOCl2(R-bian)(H2O)] (R = 4-Me-C6H4 (1), 2-Me-C6H4 (2)). Thes complexes were characterized by IR and EPR spectroscopy as well as elemental analysis. Complexes 1 and 2 have high catalytic activity in the oxidation of hydrocarbons with hydrogen peroxide and alcohols with tert-butyl hydroperoxide in acetonitrile at 50 °С. The product yields are up to 40% for cyclohexane. Of particular importance is the addition of 2-pyrazinecarboxylic acid (PCA) as a co-catalyst. Oxidation proceeds mainly with the participation of free hydroxyl radicals, as evidenced by taking into account the regio- and bond-selectivity in the oxidation of n-heptane and methylcyclohexane, as well as the dependence of the reaction rate on the initial concentration of cyclohexane.
1. Introduction
In recent decades, some metal complexes have been described as catalysts in alkane oxygenation with dioxygen or peroxides [1,2,3,4]. In most oxidation reactions with peroxides, the key oxidizing species is the hydroxyl radical. The first such system that performs oxidation with the participation of hydroxyl radicals is the combination of H2O2 with an iron salt. In the case of Fe(II) it is called “Fenton’s reagent” [5,6]. Vanadium coordination compounds have attracted increasing interest due to their structural features [7,8,9,10,11,12,13,14,15,16,17,18]. Shulpin et al. discovered in 1993 a new, very efficient catalytic system using vanadium complexes in the presence of pyrazinecarboxylic acid with hydrogen peroxide as an oxidizing agent [19,20,21,22]. Oxidation mechanisms were later proposed for this system [23,24]. Further studies of various vanadium complexes in the oxidative catalysis of alkanes and alcohols also turned out to be very fruitful [25,26]. These complexes showed high yields of alkane oxidation products. In this case, an important factor is the presence of redox-active ligands in the composition of the complexes. Bis(imino)-acenaphthenes (BIANs) belong to the class of α-diimines, which combine 1,4-diazabutadiene and naphthalene fragments [27,28,29]. Due to this combination, BIANs have strong σ-donor and π-acceptor properties, providing stabilization of both high and low oxidation states of the metal upon coordination. BIANs form complexes with almost all main group elements [30,31,32,33,34] and transition metals [35,36,37,38,39,40,41,42,43,44]. The key feature of BIANs is their pronounced redox activity, and this property is widely exploited by scientists to implement various catalytic transformations [27]. Historically, the first BIAN-based catalysts were Brookhart’s catalysts for the polymerization of olefins [45,46]. The various stereoelectronic properties of BIAN ligands, including their oxidation states, allowed for the modulation of catalyst properties, polyethylene branching, and polymer microstructure [47,48,49]. Much less attention has been paid to the study of other catalytic processes involving metal/BIAN complexes. The most striking examples are reduction processes, hydrogenation [39,50,51,52,53,54,55], reduction of nitroarenes [56,57,58], and hydroamination [33,59,60,61]. Examples of oxidative transformations catalyzed by metal/BIAN complexes are even rarer, possibly due to the electron-withdrawing properties of ligands [62,63,64,65,66,67]. There are several examples of vanadium-BIAN complexes that have been tested as catalysts in oxidation reactions. In particular, square-pyramidal V(IV) complexes [VO(acac)(R-bian)]Cl efficiently catalyze the epoxidation of terminal and internal olefins with tert-butyl hydroperoxide or hydrogen peroxide [68] and the related complexes [VOCl2(dpp-bian)] or [VOCl2(dpp-mian)(CH3CN)] provide easy CH-oxidation of alkanes with hydrogen peroxide [62,64]. In this work, we synthesized two new oxidovanadium(IV) complexes with redox-active BIAN ligands [VOCl2(R-bian)(H2O)] (R = 4-Me-C6H4 (1) and 2-Me-C6H4 (2)) and studied their catalytic properties in the oxidation of cyclohexane with hydrogen peroxide in the presence of 2-pyrazinecarboxylic acid (PCA).
2. Results and Discussion
2.1. Synthesis of Complexes 1 and 2
For the synthesis of complexes 1 and 2, an approach was applied that included the use of vanadium trichloride as a starting compound. During the reaction, vanadium(III) was oxidized in air to form the {VO}2+ fragment. Previously, we successfully used this approach to obtain a series of oxidovanadium(IV) complexes with redox-active ligands [62,67,69]. Complexes 1 and 2 were synthesized by a similar method (Scheme 1), by refluxing vanadium trichloride with R-bian (R = 4-Me-C6H4-bian or 2-Me-C6H4) in acetonitrile for 10 h. Fine crystalline powders of complexes 1 and 2 were obtained by recrystallization from a mixture of methylene chloride and hexane in 57% to 49% yields, respectively. Complex 1 is more soluble in most organic solvents than complex 2.
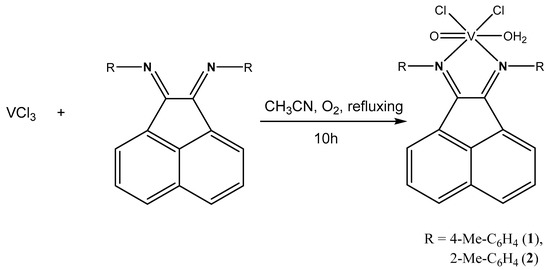
Scheme 1.
Сomplex synthesis reaction.
Our attempts to obtain single crystals suitable for X-ray structural analysis for both complexes failed. Therefore, indirect methods were used to determine the composition and structure: elemental analysis, and IR, UV-vis, and EPR spectroscopies. Elemental analysis data are in good agreement with the proposed formula. The IR spectra of these complexes showed broad vibration bands of the OH group from the coordinated H2O in the range of 3600–3100 cm−1. CH vibrations of the methyl group at 3057–2870 cm−1 for 1 and 3055–2869 cm−1 for 2 were found. Vibration bands of СС and СN groups of the R-bian ligand appeared in the region of 1661–1018 cm−1 for 1 and 1662–1045 cm−1 for 2. Very strong bands at 983 cm−1 for 1 and 989 cm−1 for 2 were assigned to the VO group [70,71]. The vibrations at 890–818 cm−1 for 1 and 870–831 cm−1 for 2 can be attributed to the linear chain V = O … V = O [69].
The electronic absorption spectra of solutions 1 and 2 in acetonitrile revealed strong absorption in the region of 260–410 nm, which can be attributed to charge transfer bands (involving ligand and metal), as well as a low-intensity band at 497 nm for 1 and 489 nm for 2, which is typical for d-d transitions.
2.2. EPR Spectroscopy Studies
The EPR spectra of 1 and 2 in dichloromethane were recorded at 77 K (Figure 1 and Figure 2). In both cases spectra in solution revealed an eight-line isotropic signal characteristic of VIV (d1) complexes. The spectrum of 1 turned out to be a superposition of two spectra with very close parameters, related to different forms. The ratio between these species was 10:1. The simulation analysis for the strong spectrum (spectrum b) showed the following EPR parameters: g1 = 1.96, g2 = g3 = 1.98, A1 = 17.15 mT, A2 = A3 = 6.1 mT, and for the weak EPR spectrum (spectrum c): g1 = 1.952, g2 = g3 = 1.982, A1 = 17.45 mT, A2 = A3 = 7.5 mT.
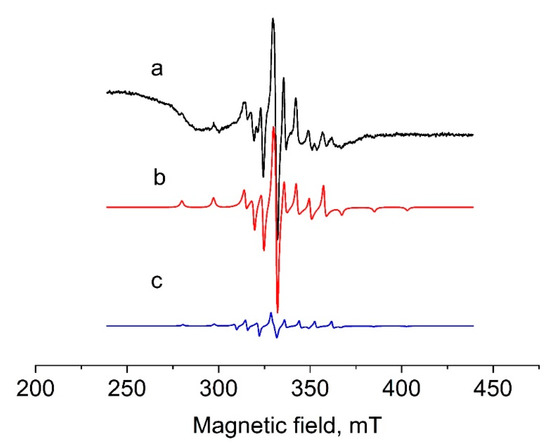
Figure 1.
EPR spectra of solution of 1 in dichloromethane at 77 K. Black—experimental, red and blue—simulated.
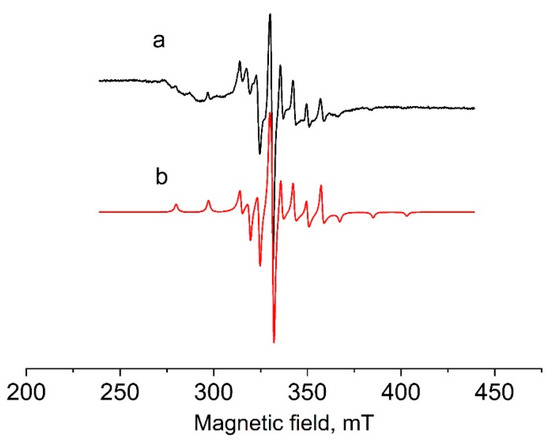
Figure 2.
EPR spectra of solution of 2 in dichloromethane at 77 K. Black—experimental, red—simulated.
The simulation analysis for the spectrum of 2 gave the following EPR parameters: g1 = 1.96, g2 = g3 = 1.98, A1 = 17.15 mT, A2 = A3 = 6.1 mT. These parameters coincided with those for the strong signal in spectrum of 1 (spectrum b), which indicated an identical coordination environment around vanadium. The weak EPR signal with different parameters found in the spectrum of 1 probably belongs to a complex in which vanadium has a different coordination environment. This could be a complex with coordinated acetonitrile [VOCl2(CH3CN)(4-Me-C6H4-bian)], which was formed as a by-product at the stage of synthesis in acetonitrile. In general, the EPR parameters for 1 and 2 are typical for oxidovanadium(IV) complexes [62,72].
To confirm the composition and structure of the complexes, DFT calculations were carried out. The proposed geometries of the complexes were optimized (Supplementary Tables S1 and S2), and the g-factors and hyperfine interaction (HFI) A tensors were calculated for both complexes.
The calculated EPR parameters were (see Table 1):

Table 1.
EPR parameters.
The calculated values of the EPR parameters are in good agreement with the experimental data, which indicates the legitimacy of the attributed composition [VIVOCl2(R-bian)(H2O)] (R = 4-Me-C6H4 (1), 2-Me-C6H4 (2)). Previously, we obtained a similar complex [VIVOCl2(H2O)(dbbpy)] having a 4,4’-di-tert-butyl-2,2’-dipyridine ligand instead of BIAN, for which very similar EPR parameters were found: gxx = gyy = 1.978, gzz = 1.945, Axx = Ayy = 6.5 mT, Azz = 17.86 mT [69].
2.3. Oxidation of Alkanes
We have found that compounds 1 and 2 catalyze the oxidation of alkanes with H2O2 in acetonitrile in the presence of 2-pyrazinecarboxylic acid (PCA). Accumulation of cyclohexanol and cyclohexanone in oxidation of cyclohexane with hydrogen peroxide catalyzed by compound 1 and 2 is demonstrated by Figure 3, Figure 4, Figure 5 and Figure 6. The data obtained in the oxidation of cyclohexane for both complexes showed that in the presence of 2-pyrazinecarboxylic acid, the reactions proceeded much faster, which is consistent with our previous studies of oxidative processes using vanadium complexes as the catalysts [25]. A co-catalyst in these reactions was 2-pyrazinecarboxylic acid.
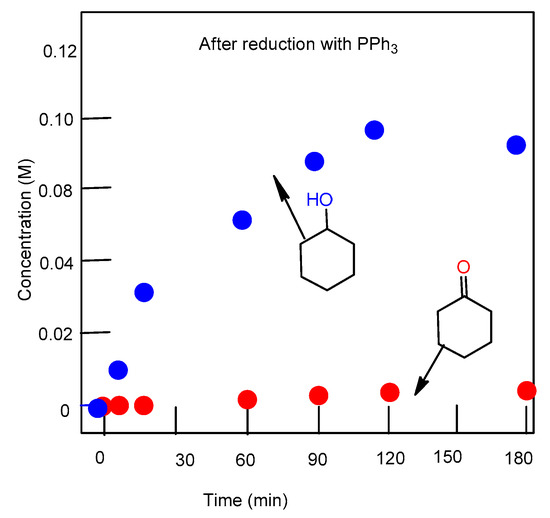
Figure 3.
Accumulation of cyclohexanol and cyclohexanone in the oxidation of cyclohexane (0.46 M) with H2O2 (2.0 M) catalyzed by complex 1 (5 × 10−4 M) in the absence of PCA, at 50 °C in acetonitrile. Concentrations of products were measured by GC after the reduction of the reaction samples with solid PPh3.
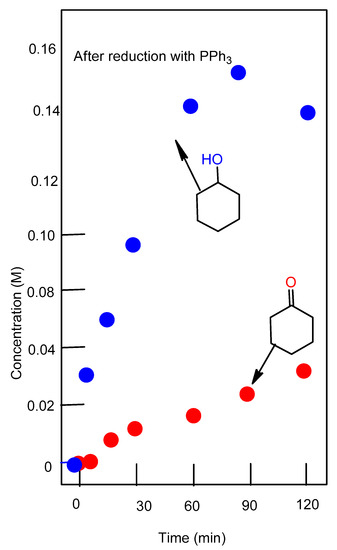
Figure 4.
Accumulation of cyclohexanol and cyclohexanone in the oxidation of cyclohexane (0.46 M) with H2O2 (2.0 M) catalyzed by complex 1 (5 × 10−4 M) in the presence of PCA, at 50 °C in acetonitrile. Concentrations of products were measured by GC after the reduction of the reaction samples with solid PPh3.
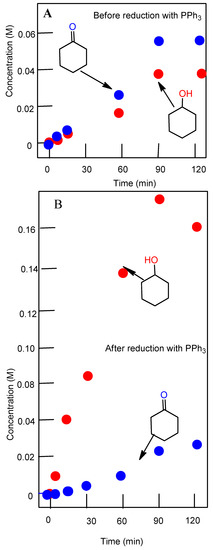
Figure 5.
Accumulation of cyclohexanol and cyclohexanone in the oxidation of cyclohexane (0.46 M) with H2O2 (2.0 M) catalyzed by complex 2 (5 × 10−4 M) in the presence of PCA (2 × 10−3 M), at 50 °C in acetonitrile. Concentrations of products were measured by GC before (A) and after (B) the reduction of the reaction samples with solid PPh3.
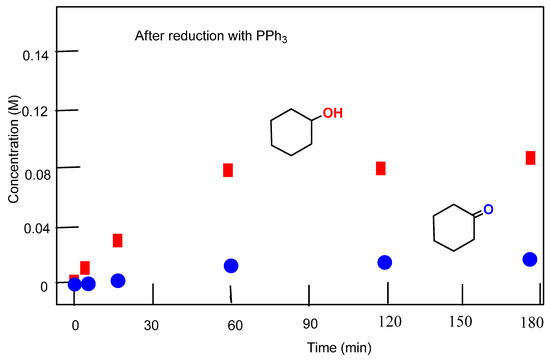
Figure 6.
Accumulation of cyclohexanol and cyclohexanone in the oxidation of cyclohexane (0.46 M) with H2O2 (2.0 M) catalyzed by complex 2 (5 × 10−4 M) in the absence of PCA, at 50 °C in acetonitrile. Concentrations of products were measured by GC after the reduction of the reaction samples with solid PPh3.
Figure 3 shows the accumulation of products of cyclohexane oxidation with hydrogen peroxide using complex 1 as a catalyst in the absence of 2-pyrazinecarboxylic acid (PCA). Figure 4 shows the accumulation of cyclohexane oxidation products when PCA was added.
Complex 2 was studied in more detail. The reduction of the reaction solution with PPh3 gave rise to a higher concentration of cyclohexanol and a decrease in cyclohexanone concentration (Figure 4) (compare Graphs A and B). These changes indicate (the so-called Shul’pin method [24,62,73]), that alkyl hydroperoxide is formed in the course of the oxidation. Alkyl hydroperoxides are transformed in the GC injector into a mixture of the corresponding ketone and alcohol. Due to this, we quantitatively reduced the reaction samples with PPh3 to obtain the corresponding alcohol. Shul’pin’s method allows us to calculate the real concentrations not only of the hydroperoxide but of the alcohols and ketones present in the solution at a given moment.
The curve of dependence of the initial oxidation rate by complex 2 in the case of catalysis approaches a plateau at a cyclohexane concentration of >0.4 M (Figure 7 and Figure 8). The rate at [CyH]0 = 0.1 M is approximately equal to half of the maximum rate.
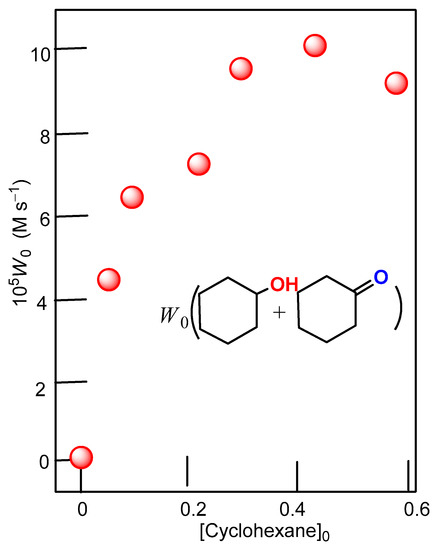
Figure 7.
Dependence of the initial rate of oxygenate formation W0 on initial concentration of cyclohexane (0.46 M) for the oxidation of cyclohexane with system: complex 2 (5 × 10−4 M)/H2O2 (2.0 M)/PCA (2 × 10−3 M), at 50 °C in acetonitrile.
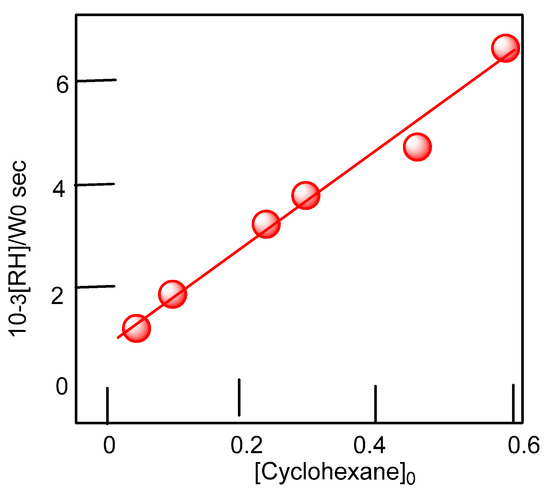
Figure 8.
Linear anamorphosis of the dependence of the reaction rate on the concentration of cyclohexane (data in Figure 7) in accordance with Equation (11).
We studied the parameters of selectivity in the oxidation of n-heptane (regioselectivity) and methylcyclohexane (bond selectivity) catalyzed by complexes 1 and 2. The regio-selectivity parameters for the oxidation of n-heptane were obtained for complex 1:C (1):C (2):C (3):C (4) = 1.0:5.6:5.7:5.4 (Complex-1 (5 × 10−4 M); n-heptane (0.5 M); H2O2 (2.0 M); PCA (2 × 10−3 M), at 50 °C in acetonitrile (yield of reaction products—16% in 4 h; TON = 160)) and for complex 2:C (1):C (2):C (3):C (4) = 1.0:5.0:5.2:4.7 (Complex-2 (5 × 10−4 M); n-heptane (0.5 M); H2O2 (2.0 M); PCA (2 × 10−3 M), at 50 °C in acetonitrile (yield of reaction products—18% in 3 h; TON = 180)).
The bond-selectivity parameters for the oxidation of methylcyclohexane were obtained: 1°:2°:3° = 1.0:5.5:17.7 for complex 1 (Complex-1 (5 × 10−4 M); n-heptane (0.5 M); H2O2 (2.0 M); PCA (2 × 10−3 M), at 50 °C in acetonitrile (yield of reaction products—16% in 4 h; TON = 160)); and 1°:2°:3° = 1.0:6.6:18.5 for complex 2 (Complex-2 (5 × 10−4 M); methylcyclohexane (0.5 M); H2O2 (2.0 M); PCA (2 × 10−3 M), at 50 °C in acetonitrile (yield of reaction products—15% in 3 h; TON = 150)).
Data on the selectivity of oxidation (see above) show that the oxidizing species in the studied catalytic system are hydroxyl radicals. This is also confirmed by the data presented below on the reactivity of the oxidizing species. In the system under study, the decomposition of hydroxyl radicals can occur when they interact with the ligand (L) bound to vanadium
with 2-pyrazinecarboxylic acid present in the system
or with acetonitrile
L + OH•→decomposition
PCA + OH•→decomposition
CH3CN + OH•→decomposition
The interaction of the hydroxyl radical with hydrogen peroxide leads to the formation of the HO2• radical, which most likely reduces the vanadium ion
H2O2 + OH•→H2O + HO2•
V(5+) + HO2•→V(4+) + H+ + O2
The reduced vanadium ion is oxidized by peroxide and the hydroxyl radical is regenerated
V(4+) + H2O2→V(5+) + OH− + OH•
Thus, the interaction of OH• with H2O2 does not in fact lead to the destruction of the hydroxyl radical. Taking the maximum possible value of 1010 M−1 s−1 for the rate constants of the interaction of OH• with L and PCA, we obtained for the rate constants of the pseudo-first order of the decomposition of OH• with L and PCA under our experimental conditions the values k1[L] = 5 × 106 s−1, k2[PCA] = 2 × 107 s−1. A similar characteristic for OH• decomposition with acetonitrile is k3[CH3CN] no less than 5 × 107 s−1. Thus, the latter reaction is the main one in the absence of a substrate; the proportion of the reaction involving PCA does not exceed 30% in the total hydroxyl decomposition channel, and the contribution of the reaction involving L is negligibly small. Therefore, the dependence of the initial rate of formation of oxygenation products on the concentration of cyclohexane (Figure 7) reflects the competition of acetonitrile and PCA (reactions (2) and (3)) with the introduced cyclohexane (reaction (7)) for the hydroxyl radical
С6Н12 + ОН• →
Let us assume that the rate of generation of hydroxyl radicals by the catalytic system under study under the conditions of Figure 7 is Wi. Let us further assume that steps (2), (3), and (7) are limiting the decomposition of OH• with PCA, acetonitrile, and cyclohexane, and the OH• concentration is quasi-stationary. Then we can write
Wi = (k2[PCA] + k3[CH3CN] + k7[C6H12]) [OH•]
It follows from (7) that the quasi-stationary OH• concentration is determined by the relation
[OH•] = Wi/(k2[PCA] + k3 [CH3CN] + k7[C6H12])
Assuming that reaction (7) is the limiting one in the sequence of transformations leading to the formation of cyclohexyl hydroperoxide, we obtain the following equation for the initial rate of its formation
(d[ROOH]/dt)0 = k7[C6H12]0Wi/(k2[PCA]0 + k3 [CH3CN]0 + k7[C6H12]0).
To analyze the data in Figure 5, we transform the Equation (9)
[C6H12]0/(d[ROOH]/dt)0 = Wi−1{((k2[PCA]0 + k3 [CH3CN]0)/k7) + [C7H12]0)}
In accordance with (11), a linear dependence should be observed:
[C6H12]0/(d[ROOH]/dt)0 from [C6H12]0. The experimental data satisfy the expected linear dependence, from the analysis of which it follows that
(k2[PCA]0 + k3[CH3CN]0)/k7 = 0.11М, а Wi = 1.1 × 10−4 M с−1
From the above estimates for k2[PCA]0 and k3[CH3CN]0 under the experimental conditions presented in Figure 7, and relation (12), it follows that
0.08 < k3[CH3CN]0)/k7 < 0.11
The estimated value of k3[CH3CN]0)/k7 is close to the values obtained earlier for other catalytic systems in which the formation of hydroxyl radicals has been established [74,75]. Thus, data on the reactivity of an intermediate species of an oxidizing nature that arises during the catalytic decomposition of hydrogen peroxide in the presence of the catalyst under study, as well as data on the regioselectivity of the oxidation of linear alkanes, indicate that the detected intermediate species is a hydroxyl radical.
The dependence of the initial rate of ROOH formation on temperature is shown in Figure 9. It follows from the presented data that the effective activation energy of the process leading to the formation of ROOH equals 18 ± 2 kcal/mol. This value is close to the values obtained for the activation energy of alkane oxidation reactions in other previously published works [24,76,77].
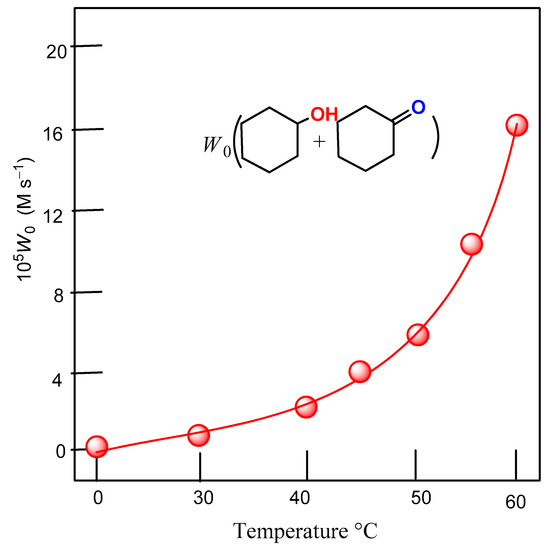
Figure 9.
Dependence of the initial rate of oxygenate formation W0 on temperature of reaction (conditions of reaction: cyclohexane (0.46 M), complex-2 (5 × 10−4 M)/H2O2(2.0 M)/PCA (2 × 10−3 M) in acetonitrile).
These data as well as the character of dependence of the initial cyclohexane oxidation rate on the initial hydrocarbon concentration indicate that the reaction occurs with the participation of hydroxyl radicals, and that alkyl hydroperoxides are formed as the primary products.
Comparative parameters of the oxidation of cyclohexane catalyzed by complexes 1 and 2 and other previously published vanadium complexes are presented in Table 2, and the differences among them are noticeable—the maximum yields of oxidation products are obtained in less time, since the reaction rate is higher, apparently due to the influence of ligands.

Table 2.
Comparative parameters of the oxidation of cyclohexane catalyzed by complexes 1 and 2 and other previously published vanadium complexes.
2.4. Oxidation of Alcohols
The complexes show moderate activity in the oxidation of alcohols with tert-butyl hydroperoxide. The yields for the oxidation of phenylethanol (0.5 M) to acetophenone with tert-butyl (1.5 M) hydroperoxide under catalysis with complexes 1 (5 × 10−4 M) and 2 (5 × 10−4 M) were 28% (TON = 280) and 56% (TON = 560), respectively, at a temperature of 50 °C, in acetonitrile for 5 h. In analogous reactions of oxidation of cyclohexanol (0.5 M) to cyclohexanone, corresponding yields were 15% (TON = 150) and 20% (TON = 200) after 5 h. In reactions of oxidation of 2-heptanol (0.5 M) to 2-heptanone, corresponding yields were 36% (TON = 360) and 46% (TON = 460) after 5 h. Hydrogen peroxide was much less productive in these reactions.
3. Experimental Section
3.1. General Procedures
All manipulations were carried out in air. VCl3 was commercially available. 1,2-Bis[(2-methylphenyl)imino]acenaphthene (2-Me-C6H4-bian) and 1,2-Bis[(4-methylphenyl)imino]acenaphthene (4-Me-C6H4-bian) were prepared as reported [78]. Organic solvents (CH2Cl2, MeCN, glacial acetic acid, and hexane) were dried by standard methods before use. All solvents were distilled by standard methods before use.
3.2. Physical Measurements
Elemental C, H, and N analyses were performed with a EuroEA3000 Eurovector analyzer. The IR spectra were recorded in the 4000–400 cm–1 range with a Perkin–Elmer System 2000 FTIR spectrometer, with samples in KBr pellets and Nujol. EPR spectra were recorded in the X band at 77 and 300 K on an E-109 Varian spectrometer equipped with an analog-to-digital signal converter. To analyze and simulate EPR spectra, EasySpin (Matlab software package) was used [79]. A UV-2501 PC spectrometer was used for UV-vis spectroscopic study. The UV-vis spectra were recorded in a quartz cuvette of 2 mm optical layer at room temperature.
3.3. DFT Calculations
The spin-unrestricted DFT calculations were performed using the ADF 2021 program package [80,81]. The optimized geometries were obtained with the generalized gradient approximation (GGA) functional BP86 [82,83] and the triple-zeta basis sets (with one polarization function) TZP [84]. The g- and A-tensors were calculated with hybrid PBE0 functional (25% HF exchange) [85,86] and TZP basis sets for all atoms except V, for which the larger basis set TZ2P-J (triple-zeta with two polarization and several extra tight, mainly 1s, functions) was used [84]. The EPR parameters were derived as second derivative properties with spin-orbit coupling and external magnetic field taken as perturbation [87,88]. In all calculations, the scalar relativistic effects were accounted for by the zeroth-order regular approximation (ZORA) formalism [89,90]. Solvent effects (CH2Cl2) were considered using the conductor-like screening model (COSMO) of solvation [91] as implemented on ADF 2021 [80,81].
Synthesis of [VOCl2(4-Me-C6H4-bian)(H2O)] (1). A mixture of VCl3 (43.6 mg, 277 µmol) and 4-Me-C6H4-bian (100 mg, 277 µmol) was dissolved in 10 mL of acetonitrile. The mixture was refluxed for 10 h. The resulting bright brown solution was evaporated to dryness. Crude product of 1 was redissolved in methylene chloride followed by a layering of hexane. Brown-green crystalline precipitate of 1 formed after 1 day. Yield: 81 mg (57%). Anal. Calc. for C26H22Cl2N2O2V*H2O: C 58.4, H 4.5, N 5.2; Found C 58.6, H 4.8, N 4.9. IR (KBr) ν/cm−1: 3600–3070 (br. s), 3057 (w), 3034 (w), 2970 (w), 2925 (w), 2870 (w), 1964 (w), 1899 (w), 1772 (w), 1726 (w), 1661 (m), 1623 (s), 1586 (vs), 1507 (vs), 1489 (m), 1435 (w), 1419 (m), 1377 (w), 1357 (w), 1312 (w), 1292 (m), 1252 (m), 1226 (w), 1212 (w), 1187 (w), 1150 (w), 1132 (w), 1108 (m), 1064 (w), 1050 (w), 1039 (w), 1018 (w), 983 (vs), 890 (w), 858 (w), 830 (s), 818 (s), 776 (vs), 711 (w), 658 (w), 637 (w), 624 (w), 605 (w), 554 (w), 528 (w), 514 (w), 489 (m), 458 (w), 424 (m). UV-Vis (MeCN): λ(ε) = 271 nm (15254 M−1 cm−1), 316 (6899 M−1 cm−1), 410 (2247 M−1 cm−1), 497 (536 M−1 cm−1) nm.
Synthesis of [VOCl2(2-Me-C6H4-bian)(H2O)] (2). A mixture of VCl3 (43.6 mg, 277 µmol) and 2-Me-C6H4-bian (100 mg, 277 µmol) was dissolved in 10 mL of acetonitrile. The mixture was refluxed for 10 h. The resulting bright brown solution and brown precipitate were formed. The solution was filtered and evaporated to dryness. Crude product of 2 was redissolved in methylene chloride, filtered again, and followed by a layering of hexane. Brown-green crystalline precipitate of 2 formed after 1 day. Yield: 70 mg (49%). Anal. Calc. for C26H22Cl2N2O2V*0.2CH2Cl2: C 59.7, H 4.3, N 5.3; Found C 59.4, H 4.5, N 5.4. IR (KBr) ν/cm−1: 3600–3120 (br.s), 3055 (w), 3018 (w), 2976 (w), 2924 (w), 2869 (w), 2579 (w), 1955 (w), 1909 (w), 1843 (w), 1708 (w), 1662 (m), 1619 (vs), 1596 (s), 1586 (vs), 1486 (vs), 1457 (w), 1445 (w), 1435 (m), 1418 (m), 1384 (w), 1357 (w), 1318 (w), 1294 (m), 1243 (m), 1228 (m), 1192 (m), 1155 (w), 1123 (m), 1094 (w), 1045 (m), 989 (vs), 931 (w), 870 (w), 853 (w), 831 (s), 777 (vs), 758 (s), 719 (m), 699 (w), 668 (w), 650 (w), 631 (w), 612 (w), 555 (w), 543 (w), 531 (w), 511 (w), 500 (w), 475 (w), 488 (m), 412 (w). UV-Vis (MeCN): λ(ε) = 268 nm (ε = 9584 M−1 cm−1), 316 nm (ε = 6112 M−1 cm−1), 404 nm (ε = 1136 M−1 cm−1), 489 nm (ε = 520 M−1 cm−1) nm.
3.4. Catalytic Studies
Total volume of the reaction solution was 5 mL. (CAUTION: the combination of air or molecular oxygen and H2O2 with organic compounds at elevated temperatures may be explosive!) Cylindrical glass vessels with vigorous stirring of the reaction mixture were used for the oxidation of alkanes with hydrogen peroxide or tert-butyl hydroperoxide (for alcohols), typically carried out in air in thermostated solution. Initially, a portion of 50% aqueous solution of hydrogen peroxide was added to the solution of the catalyst, co-catalyst (PCA), and substrate in acetonitrile. The aliquots of the reaction solution were analyzed by GC (3700, fused silica capillary column FFAP/OV-101 20/80 w/w, 30 m × 0.2 mm × 0.3 µm; argon as a carrier gas. Attribution of peaks was made by comparison with chromatograms of authentic samples). Usually samples were analyzed twice, i.e., before and after the addition portion by portion of the excess of solid PPh3.This method was proposed and used previously by one of us [92,93].
4. Conclusions
New oxidovanadium(IV) complexes 1 and 2 were synthesized by reacting vanadium trichloride with BIAN-type ligands (4-Me-C6H4-bian and 2-Me-C6H4-bian) in 57% and 49% yields, respectively. These compounds were characterized by elemental analysis and IR and EPR spectroscopy. Compounds 1 and 2 are a powerful catalyst for the efficient oxidation of alkanes with peroxides. Data on the selectivity of oxidation and the nature of the dependence of the initial rate of cyclohexane oxidation on the initial concentration of hydrocarbon, as well as kinetic studies, indicate that the reaction proceeds with the participation of hydroxyl radicals and alkyl hydroperoxides are formed as the primary products.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/article/10.3390/catal12101168/s1, Figure S1: UV-spectrum of 1 in the CH3CN, Figure S2: UV-spectrum of 2 in the CH3CN, Table S1: Optimized coordinates for complex 1, Table S2: Optimized coordinates for complex 2.
Author Contributions
I.S.F.: Investigation, Writing-original draft. Synthesis and description of vanadium complex compounds; M.I.G.: Investigation. Synthesis of ovanadium complex compounds; L.S.S.: Investigation, Writing-original draft. Сonducting and describing catalytic experiments on oxidation catalyzed by vanadium compounds; N.S.I.: Investigation. Сonducting catalytic experiments; A.Y.K.: Investigation. DFT calculations. Conducting DFT calculations for EPR; V.A.N.: Investigation. Recording and description of EPR spectra; Y.N.K.: Investigation. Proposal and discussion of the mechanism for vanadium catalysed alkane oxidation.; A.L.G.: Conceptualization, Writing-review & editing. Writing and editing the first draft of the article; G.B.S.: Conceptualization, Writing-review & editing. Writing and editing the first draft of the article. Communication with the editor of the journal. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from the Russian Science Foundation (grant No. 22-23-20123) and the government of the Novosibirsk region (contract r-39) is acknowledged. This work was also performed within the framework of the Program for Fundamental Research of the Russian Federation, Reg. No. 122040500068-0.; GC analysis was performed with the financial support from the Ministry of Education and Science of the Russian Federation using the equipment of the Center for Molecular Composition Studies of the A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences (INEOS RAS). The authors thank the Ministry of Science and Higher Education of the Russian Federation for access to the equipment of the Centre of Collective Usage, NIIC SB RAS (grant No. 121031700315-2).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429131646. [Google Scholar]
- Shul’pin, G.B. Selectivity in CH Functionalizations. In Comprehensive Inorganic Chemistry II; Elsevier: Amsterdam, The Netherlands, 2013; pp. 79–104. [Google Scholar]
- Wójtowicz-Młochowska, H. Synthetic utility of metal catalyzed hydrogen peroxide oxidation of C–H, C–C and C=C bonds in alkanes, arenes and alkenes: Recent advances. Arkivoc 2016, 2017, 12–58. [Google Scholar] [CrossRef]
- Shul’pin, G. New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Rachmilovich-Calis, S.; Masarwa, A.; Meyerstein, N.; Meyerstein, D.; van Eldik, R. New Mechanistic Aspects of the Fenton Reaction. Chem.–Eur. J. 2009, 15, 8303–8309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Goldsmith, C.R. Kinetic Analysis of the Formation and Decay of a Non-Heme Ferric Hydroperoxide Species Susceptible to O–O Bond Homolysis. Inorg. Chem. 2014, 53, 5206–5211. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.C.; Zubieta, J. Hydrothermal synthesis and structure of [VO2(terpy)][(VO2)2(PO4)], a novel network material (terpy=2,2′:6′,2″ terpyridine). Solid State Sci. 2002, 4, 845–849. [Google Scholar] [CrossRef]
- Correia, I.; Costa Pessoa, J.; Duarte, M.T.; Henriques, R.T.; Piedade, M.F.M.; Veiros, L.F.; Jakusch, T.; Kiss, T.; Dörnyei, Á.; Castro, M.M.C.A.; et al. N,N′-Ethylenebis(pyridoxylideneiminato) and N,N′-Ethylenebis(pyridoxylaminato): Synthesis, Characterization, Potentiometric, Spectroscopic, and DFT Studies of Their Vanadium(IV) and Vanadium(V) Complexes. Chem.–Eur. J. 2004, 10, 2301–2317. [Google Scholar] [CrossRef]
- Wang, J.-B.; Lu, L.-P.; Liu, J.-Y.; Li, Y.-S. [ONN]-type amine pyridine(s) phenolate-based oxovanadium(v) catalysts for ethylene homo- and copolymerization. Dalton Trans. 2014, 43, 12926. [Google Scholar] [CrossRef]
- Chieregato, A.; López Nieto, J.M.; Cavani, F. Mixed-oxide catalysts with vanadium as the key element for gas-phase reactions. Coord. Chem. Rev. 2015, 301–302, 3–23. [Google Scholar] [CrossRef]
- Yucesan, G.; Armatas, N.G.; Zubieta, J. Hydrothermal synthesis of molecular oxovanadium compounds. The crystal and molecular structures of [VO2(terpy)]NO3, [VO(terpy)(OH3PC6H5)2], [{Cu(H2O)(terpy)}V2O6], [{Cu(ttbterpy)}V2O6] and [{Cu(ttbterpy)}VO2(HO3PCH2PO3)]·H2O (terpy=2,2′:6′,2″-terpyridine. Inorg. Chim. Acta 2006, 359, 4557–4564. [Google Scholar] [CrossRef]
- Tutusaus, O.; Ni, C.; Szymczak, N.K. A Transition Metal Lewis Acid/Base Triad System for Cooperative Substrate Binding. J. Am. Chem. Soc. 2013, 135, 3403–3406. [Google Scholar] [CrossRef]
- Sheppard, B.J.H.; Shaver, M.P.; Pearson, J.K. Assessment and Application of Density Functional Theory for the Prediction of Structure and Reactivity of Vanadium Complexes. J. Phys. Chem. A 2015, 119, 8537–8546. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Pombeiro, A.J.L. Coordination chemistry of non-oxido, oxido and dioxidovanadium(IV/V) complexes with azine fragment ligands. Coord. Chem. Rev. 2014, 265, 89–124. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Hanson, S.K.; Baker, R.T.; Gordon, J.C.; Scott, B.L.; Silks, L.A.; Thorn, D.L. Mechanism of Alcohol Oxidation by Dipicolinate Vanadium(V): Unexpected Role of Pyridine. J. Am. Chem. Soc. 2010, 132, 17804–17816. [Google Scholar] [CrossRef]
- Barroso, S.; Adão, P.; Madeira, F.; Duarte, M.T.; Pessoa, J.C.; Martins, A.M. Vanadium Diaminebis(phenolate) Complexes: Syntheses, Structures, and Reactivity in Sulfoxidation Catalysis. Inorg. Chem. 2010, 49, 7452–7463. [Google Scholar] [CrossRef]
- Zhang, G.; Scott, B.L.; Wu, R.; Silks, L.A.; Hanson, S.K. Aerobic Oxidation Reactions Catalyzed by Vanadium Complexes of Bis(Phenolate) Ligands. Inorg. Chem. 2012, 51, 7354–7361. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Attanasio, D.; Suber, L. Oxidations by a H2O2-VO3 −-pyrazine-2-carboxylic acid reagent. Russ. Chem. Bull. 1993, 42, 55–59. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Druzhinina, A.N.; Nizova, G.V. Oxidation with the H2O2/VO3 −-pyrazine-2-carboxylic acid reagent. Russ. Chem. Bull. 1993, 42, 1326–1329. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Nizova, G.V.; Stanislas, S.; Shul’pin, G.B. Oxidations by the reagent ‘O2–H2O2–vanadate anion–pyrazine-2-carboxylic acid’. J. Mol. Catal. A Chem. 1998, 130, 163–170. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Stanislas, S.; Shul’pin, G.B.; Nizova, G.V.; Stoeckli-Evans, H.; Neels, A.; Bobillier, C.; Claude, S. Oxidative functionalisation of alkanes: Synthesis, molecular structure and catalytic implications of anionic vanadium(V) oxo and peroxo complexes containing bidentate N,O ligands. J. Chem. Soc. Dalton Trans. 1999, 3169–3175. [Google Scholar] [CrossRef]
- Khaliullin, R.Z.; Bell, A.T.; Head-Gordon, M. A Density Functional Theory Study of the Mechanism of Free Radical Generation in the System Vanadate/PCA/H2O2. J. Phys. Chem. B 2005, 109, 17984–17992. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Kuznetsov, M.L.; Romakh, V.B.; Shul’pina, L.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of oxidations with H2O2 catalyzed by vanadate anion or oxovanadium(V) triethanolaminate (vanadatrane) in combination with pyrazine-2-carboxylic acid (PCA): Kinetic and DFT studies. J. Catal. 2009, 267, 140–157. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Shul’pin, G.B. Pyrazinecarboxylic acid and analogs: Highly efficient co-catalysts in the metal-complex-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2013, 257, 732–754. [Google Scholar] [CrossRef]
- Sutradhar, M.; Pombeiro, A.J.L.; da Silva, J.A.L. (Eds.) Vanadium Catalysis; Catalysis Series; Royal Society of Chemistry: Cambridge, UK, 2020; ISBN 978-1-78801-857-9. [Google Scholar]
- Bernauer, J.; Pölker, J.; Jacobi von Wangelin, A. Redox-active BIAN-based Diimine Ligands in Metal-Catalyzed Small Molecule Syntheses. ChemCatChem 2022, 14, e202101182. [Google Scholar] [CrossRef] [PubMed]
- Abakumov, G.A.; Piskunov, A.V.; Cherkasov, V.K.; Fedushkin, I.L.; Ananikov, V.P.; Eremin, D.B.; Gordeev, E.G.; Beletskaya, I.P.; Averin, A.D.; Bochkarev, M.N.; et al. Organoelement chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2018, 87, 393–507. [Google Scholar] [CrossRef]
- Kaim, W. Chelate rings of different sizes with non-innocent ligands. Dalton Trans. 2019, 48, 8521–8529. [Google Scholar] [CrossRef]
- Hill, N.J.; Vargas-Baca, I.; Cowley, A.H. Recent developments in the coordination chemistry of bis(imino)acenaphthene (BIAN) ligands with s- and p-block elements. Dalton Trans. 2009, 240–253. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Moskalev, M.V.; Lukoyanov, A.N.; Tishkina, A.N.; Baranov, E.V.; Abakumov, G.A. Dialane with a Redox-Active Bis-amido Ligand: Unique Reactivity towards Alkynes. Chem. Eur. J. 2012, 18, 11264–11276. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Skatova, A.A.; Bazyakina, N.L.; Chudakova, V.A.; Khvoinova, N.M.; Nikipelov, A.S.; Eremenko, O.V.; Piskunov, A.V.; Fukin, G.K.; Lyssenko, K.A. Syntheses and structures of magnesium, calcium, europium, gallium, and zinc complexes with bis(imino)acenaphthene ligands. Russ. Chem. Bull. 2013, 62, 1815–1828. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Hill, M.S.; Kociok-Köhn, G. Dearomatized BIAN Alkaline-Earth Alkyl Catalysts for the Intramolecular Hydroamination of Hindered Aminoalkenes. Organometallics 2014, 33, 206–216. [Google Scholar] [CrossRef]
- Wang, J.; Ganguly, R.; Yongxin, L.; Díaz, J.; Soo, H.S.; García, F. Synthesis and the Optical and Electrochemical Properties of Indium(III) Bis(arylimino)acenaphthene Complexes. Inorg. Chem. 2017, 56, 7811–7820. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Santos, C.I.M.; Welter, R.; Aullón, G.; Lodeiro, C.; Avilés, T. Comparison of the Structure and Stability of New α-Diimine Complexes of Copper(I) and Silver(I): Density Functional Theory versus Experimental. Inorg. Chem. 2010, 49, 8699–8708. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.Y.; Tan, L.J.; Ng, S.M.; Chai, Y.T.; Ganguly, R.; Du, Y.; Yeow, E.K.L.; Soo, H.S. Spectroscopic Characterization and Mechanistic Studies on Visible Light Photoredox Carbon–Carbon Bond Formation by Bis(arylimino)acenaphthene Copper Photosensitizers. ACS Catal. 2018, 8, 11277–11286. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Kiskin, M.A.; Kholin, K.V.; Khrizanforov, M.N.; Budnikova, Y.G.; Babeshkin, K.A.; Efimov, N.N.; Goloveshkin, A.S.; Imshennik, V.K.; et al. Generation of a Hetero Spin Complex from Iron(II) Iodide with Redox Active Acenaphthene-1,2-Diimine. Molecules 2021, 26, 2998. [Google Scholar] [CrossRef] [PubMed]
- Fedushkin, I.L.; Makarov, V.M.; Sokolov, V.G.; Fukin, G.K.; Maslov, M.O.; Ketkov, S.Y. Compounds of chromium, titanium, and zirconium with different reduced forms of acenaphthene-1,2-diimine. Russ. Chem. Bull. 2014, 63, 870–882. [Google Scholar] [CrossRef]
- Villa, M.; Miesel, D.; Hildebrandt, A.; Ragaini, F.; Schaarschmidt, D.; Jacobi von Wangelin, A. Synthesis and Catalysis of Redox-Active Bis(imino)acenaphthene (BIAN) Iron Complexes. ChemCatChem 2017, 9, 3203–3209. [Google Scholar] [CrossRef]
- Tanahashi, H.; Ikeda, H.; Tsurugi, H.; Mashima, K. Synthesis and Characterization of Paramagnetic Tungsten Imido Complexes Bearing α-Diimine Ligands. Inorg. Chem. 2016, 55, 1446–1452. [Google Scholar] [CrossRef]
- Romashev, N.F.; Gushchin, A.L.; Fomenko, I.S.; Abramov, P.A.; Mirzaeva, I.V.; Kompan’kov, N.B.; Kal’nyi, D.B.; Sokolov, M.N. A new organometallic rhodium(I) complex with dpp-bian ligand: Synthesis, structure and redox behaviour. Polyhedron 2019, 173, 114110. [Google Scholar] [CrossRef]
- Gushchin, A.L.; Romashev, N.F.; Shmakova, A.A.; Abramov, P.A.; Ryzhikov, M.R.; Fomenko, I.S.; Sokolov, M.N. Novel redox active rhodium(III) complex with bis(arylimino)acenaphthene ligand: Synthesis, structure and electrochemical studies. Mendeleev Commun. 2020, 30, 81–83. [Google Scholar] [CrossRef]
- Romashev, N.F.; Abramov, P.A.; Bakaev, I.V.; Fomenko, I.S.; Samsonenko, D.G.; Novikov, A.S.; Tong, K.K.H.; Ahn, D.; Dorovatovskii, P.V.; Zubavichus, Y.V.; et al. Heteroleptic Pd(II) and Pt(II) Complexes with Redox-Active Ligands: Synthesis, Structure, and Multimodal Anticancer Mechanism. Inorg. Chem. 2022, 61, 2105–2118. [Google Scholar] [CrossRef]
- Romashev, N.F.; Mirzaeva, I.V.; Bakaev, I.V.; Komlyagina, V.I.; Komarov, V.Y.; Fomenko, I.S.; Gushchin, A.L. Structure of a Binuclear Rhodium(I) Complex with the Acenaphthene-1,2-diimine Ligand. J. Struct. Chem. 2022, 63, 242–251. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and .alpha.-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, C. Accessing Multiple Catalytically Active States in Redox-Controlled Olefin Polymerization. ACS Catal. 2017, 7, 7490–7494. [Google Scholar] [CrossRef]
- Anderson, W.C.; Rhinehart, J.L.; Tennyson, A.G.; Long, B.K. Redox-Active Ligands: An Advanced Tool To Modulate Polyethylene Microstructure. J. Am. Chem. Soc. 2016, 138, 774–777. [Google Scholar] [CrossRef]
- Kaiser, J.M.; Anderson, W.C.; Long, B.K. Photochemical regulation of a redox-active olefin polymerization catalyst: Controlling polyethylene microstructure with visible light. Polym. Chem. 2018, 9, 1567–1570. [Google Scholar] [CrossRef]
- Saini, A.; Smith, C.R.; Wekesa, F.S.; Helms, A.K.; Findlater, M. Conversion of aldimines to secondary amines using iron-catalysed hydrosilylation. Org. Biomol. Chem. 2018, 16, 9368–9372. [Google Scholar] [CrossRef]
- Maier, T.M.; Gawron, M.; Coburger, P.; Bodensteiner, M.; Wolf, R.; van Leest, N.P.; de Bruin, B.; Demeshko, S.; Meyer, F. Low-Valence Anionic α-Diimine Iron Complexes: Synthesis, Characterization, and Catalytic Hydroboration Studies. Inorg. Chem. 2020, 59, 16035–16052. [Google Scholar] [CrossRef]
- Kluwer, A.M.; Koblenz, T.S.; Jonischkeit, T.; Woelk, K.; Elsevier, C.J. Kinetic and Spectroscopic Studies of the [Palladium(Ar-bian)]-Catalyzed Semi-Hydrogenation of 4-Octyne. J. Am. Chem. Soc. 2005, 127, 15470–15480. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, Z.; Yu, F.; Ma, S.; Holuigue, A.; Tromp, D.S.; Elsevier, C.J.; Yu, Y. [Pd(Ar-BIAN)(alkene)]-Catalyzed Highly Chemo-, Regio-, and Stereoselective Semihydrogenation of 1,2-Allenyl Phosphonates and Related Compounds. Angew. Chem. 2006, 118, 5119–5122. [Google Scholar] [CrossRef]
- Sandl, S.; Maier, T.M.; van Leest, N.P.; Kröncke, S.; Chakraborty, U.; Demeshko, S.; Koszinowski, K.; de Bruin, B.; Meyer, F.; Bodensteiner, M.; et al. Cobalt-Catalyzed Hydrogenations via Olefin Cobaltate and Hydride Intermediates. ACS Catal. 2019, 9, 7596–7606. [Google Scholar] [CrossRef]
- Maier, T.M.; Sandl, S.; Shenderovich, I.G.; Jacobi von Wangelin, A.; Weigand, J.J.; Wolf, R. Amine-Borane Dehydrogenation and Transfer Hydrogenation Catalyzed by α-Diimine Cobaltates. Chem.–Eur. J. 2019, 25, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Ramadan, D.R.; Ragaini, F. Transition Metal Catalyzed Reductive Cyclization Reactions of Nitroarenes and Nitroalkenes. ChemCatChem 2019, 11, 4450–4488. [Google Scholar] [CrossRef]
- Viganò, M.; Ragaini, F.; Buonomenna, M.G.; Lariccia, R.; Caselli, A.; Gallo, E.; Cenini, S.; Jansen, J.C.; Drioli, E. Catalytic Polymer Membranes under Forcing Conditions: Reduction of Nitrobenzene by CO/H2O Catalyzed by Ruthenium Bis(arylimino)acenaphthene Complexes. ChemCatChem 2010, 2, 1150–1164. [Google Scholar] [CrossRef]
- Ragaini, F.; Cenini, S.; Borsani, E.; Dompé, M.; Gallo, E.; Moret, M. Synthesis of N-Arylpyrroles, Hetero-Diels−Alder Adducts, and Allylic Amines by Reaction of Unfunctionalized Dienes with Nitroarenes and Carbon Monoxide, Catalyzed by Ru(CO)3(Ar-BIAN). Organometallics 2001, 20, 3390–3398. [Google Scholar] [CrossRef]
- Yakub, A.M.; Moskalev, M.V.; Bazyakina, N.L.; Fedushkin, I.L. Carbon—Carbon and Carbon—Nitrogen bond formation reactions catalyzed by the magnesium and calcium acenaphthene-1,2-diimine complexes. Russ. Chem. Bull. 2018, 67, 473–478. [Google Scholar] [CrossRef]
- Li, L.; Lopes, P.S.; Figueira, C.A.; Gomes, C.S.B.; Duarte, M.T.; Rosa, V.; Fliedel, C.; Avilés, T.; Gomes, P.T. Cationic and Neutral (Ar-BIAN)Copper(I) Complexes Containing Phosphane and Arsane Ancillary Ligands: Synthesis, Molecular Structure and Catalytic Behaviour in Cycloaddition Reactions of Azides and Alkynes. Eur. J. Inorg. Chem. 2013, 2013, 1404–1417. [Google Scholar] [CrossRef]
- Li, L.; Lopes, P.S.; Rosa, V.; Figueira, C.A.; Lemos, M.A.N.D.A.; Duarte, M.T.; Avilés, T.; Gomes, P.T. Synthesis and structural characterisation of (aryl-BIAN)copper(I) complexes and their application as catalysts for the cycloaddition of azides and alkynes. Dalton Trans. 2012, 41, 5144. [Google Scholar] [CrossRef]
- Fomenko, I.S.; Gushchin, A.L.; Shul’pina, L.S.; Ikonnikov, N.S.; Abramov, P.A.; Romashev, N.F.; Poryvaev, A.S.; Sheveleva, A.M.; Bogomyakov, A.S.; Shmelev, N.Y.; et al. New oxidovanadium(IV) complex with a BIAN ligand: Synthesis, structure, redox properties and catalytic activity. New J. Chem. 2018, 42, 16200–16210. [Google Scholar] [CrossRef]
- Fomenko, I.S.; Gushchin, A.L. Mono- and binuclear complexes of group 5 metals with diimine ligands: Synthesis, reactivity and prospects for application. Russ. Chem. Rev. 2020, 89, 966–998. [Google Scholar] [CrossRef]
- Lukoyanov, A.N.; Fomenko, I.S.; Gongola, M.I.; Shul’pina, L.S.; Ikonnikov, N.S.; Shul’pin, G.B.; Ketkov, S.Y.; Fukin, G.K.; Rumyantcev, R.V.; Novikov, A.S.; et al. Novel Oxidovanadium Complexes with Redox-Active R-Mian and R-Bian Ligands: Synthesis, Structure, Redox and Catalytic Properties. Molecules 2021, 26, 5706. [Google Scholar] [CrossRef] [PubMed]
- Gryca, I.; Czerwińska, K.; Machura, B.; Chrobok, A.; Shul’pina, L.S.; Kuznetsov, M.L.; Nesterov, D.S.; Kozlov, Y.N.; Pombeiro, A.J.L.; Varyan, I.A.; et al. High Catalytic Activity of Vanadium Complexes in Alkane Oxidations with Hydrogen Peroxide: An Effect of 8-Hydroxyquinoline Derivatives as Noninnocent Ligands. Inorg. Chem. 2018, 57, 1824–1839. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Shvydkiy, N.V.; Guedes da Silva, M.F.C.; Kirillova, M.V.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’pin, G.B. A new binuclear oxovanadium(V) complex as a catalyst in combination with pyrazinecarboxylic acid (PCA) for efficient alkane oxygenation by H2O2. Dalton Trans. 2013, 42, 11791. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, I.; Gushchin, A.; Abramov, P.; Sokolov, M.; Shul’pina, L.; Ikonnikov, N.; Kuznetsov, M.; Pombeiro, A.; Kozlov, Y.; Shul’pin, G. New Oxidovanadium(IV) Complexes with 2,2′-bipyridine and 1,10-phenathroline Ligands: Synthesis, Structure and High Catalytic Activity in Oxidations of Alkanes and Alcohols with Peroxides. Catalysts 2019, 9, 217. [Google Scholar] [CrossRef]
- Nunes, C.D.; Vaz, P.D.; Félix, V.; Veiros, L.F.; Moniz, T.; Rangel, M.; Realista, S.; Mourato, A.C.; Calhorda, M.J. Vanadyl cationic complexes as catalysts in olefin oxidation. Dalton Trans. 2015, 44, 5125–5138. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, I.S.; Vincendeau, S.; Manoury, E.; Poli, R.; Abramov, P.A.; Nadolinny, V.A.; Sokolov, M.N.; Gushchin, A.L. An oxidovanadium(IV) complex with 4,4′-di-tert-butyl-2,2′-bipyridine ligand: Synthesis, structure and catalyzed cyclooctene epoxidation. Polyhedron 2020, 177, 114305. [Google Scholar] [CrossRef]
- Fomenko, I.S.; Mikhailov, A.A.; Vorobyev, V.; Kuratieva, N.V.; Kostin, G.A.; Schaniel, D.; Nadolinny, V.A.; Gushchin, A.L. Solution and solid-state light-induced transformations in heterometallic vanadium-ruthenium nitrosyl complex. J. Photochem. Photobiol. A Chem. 2021, 407, 113044. [Google Scholar] [CrossRef]
- Fomenko, I.S.; Nadolinnyi, V.A.; Efimov, N.N.; Kokovkin, V.V.; Gushchin, A.L. Binuclear Oxidovanadium(IV) Complex with the Bridging Chloranilate Ligand: Synthesis and Magnetic Properties. Russ. J. Coord. Chem. 2019, 45, 776–781. [Google Scholar] [CrossRef]
- Apostolopoulou, A.; Vlasiou, M.; Tziouris, P.A.; Tsiafoulis, C.; Tsipis, A.C.; Rehder, D.; Kabanos, T.A.; Keramidas, A.D.; Stathatos, E. Oxidovanadium(IV/V) Complexes as New Redox Mediators in Dye-Sensitized Solar Cells: A Combined Experimental and Theoretical Study. Inorg. Chem. 2015, 54, 3979–3988. [Google Scholar] [CrossRef]
- Gusevskaya, E.V.; Menini, L.; Parreira, L.A.; Mesquita, R.A.; Kozlov, Y.N.; Shul’pin, G.B. Oxidation of isoeugenol to vanillin by the “H2O2–vanadate–pyrazine-2-carboxylic acid” reagent. J. Mol. Catal. A Chem. 2012, 363–364, 140–147. [Google Scholar] [CrossRef]
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: New York, NY, USA, 2002. [Google Scholar]
- Shul’pin, G.B.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.L. A hydroperoxo-rebound mechanism of alkane oxidation with hydrogen peroxide catalyzed by binuclear manganese(IV) complex in the presence of an acid with involvement of atmospheric dioxygen. Inorg. Chim. Acta 2017, 455, 666–676. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiy, A.; Kulikova, V.S. Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid’. Part 12. Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2 2001, 1351–1371. [Google Scholar] [CrossRef]
- De la Cruz, M.H.C.; Kozlov, Y.N.; Lachter, E.R.; Shul’pin, G.B. Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 13. Kinetics and mechanism of the benzene hydroxylation. New J. Chem. 2003, 27, 634–638. [Google Scholar] [CrossRef]
- Ferretti, F.; Rota, L.; Ragaini, F. Unexpected coordination behavior of ruthenium to a polymeric α-diimine containing the poly[bis(arylimino)acenaphthene] fragment. Inorg. Chim. Acta 2021, 518, 120257. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- ADF; SCM. Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824, Erratum in Phys. Rev. B 1986, 34, 7406–7406. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-type basis sets for the elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Autschbach, J.; Pritchard, B. Calculation of molecular g-tensors using the zeroth-order regular approximation and density functional theory: Expectation value versus linear response approaches. Theor. Chem. Acc. 2011, 129, 453–466. [Google Scholar] [CrossRef]
- Autschbach, J.; Patchkovskii, S.; Pritchard, B. Calculation of Hyperfine Tensors and Paramagnetic NMR Shifts Using the Relativistic Zeroth-Order Regular Approximation and Density Functional Theory. J. Chem. Theory Comput. 2011, 7, 2175–2188. [Google Scholar] [CrossRef] [PubMed]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic regular two-component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]
- Pye, C.C.; Ziegler, T. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package. Theor. Chem. Acc. 1999, 101, 396–408. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Ehlers, A.; Baerends, E.-J. Geometry optimizations in the zero order regular approximation for relativistic effects. J. Chem. Phys. 1999, 110, 8943–8953. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(µ-H)2. Appl. Organomet. Chem. 2010, 24, 464–472. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).