Abstract
The coexistence of pollutants presents a great challenge to the implementation of photocatalysts. In this work, a novel MIL-101(Fe)/TiO2 composite prepared by in situ growth of MIL-101(Fe) on TiO2 was developed for the synergetic oxidation of MC-LR and Cr(VI) reduction. The heterojunction material shows elevated photocatalytic behavior under ultraviolet compared with the unary pollutant system. Furthermore, quenching experiments and electron spin resonance confirm that the enhanced photodegradation behavior is related to the synergistic effect between the photocatalytic reduction and oxidation process, in which MC-LR consumes the holes and Cr(VI) captures electrons, followed by efficient charge separation through the conventional double-transfer mechanism between MIL-101(Fe) and TiO2. This investigation provides a deeper understanding of the construction of MOFs/semiconductor heterojunctions for the pollutants removal in multi-component contaminants system.
1. Introduction
Heavy metal and organic pollutions pose a major threat to the environmental issues and human health [1,2,3]. Among them, hexavalent chromium (Cr(VI)) and microcystin-LR (MC-LR) are the most popular, mainly originating from industrial load and algal blooms respectively [4,5,6]. They can simultaneously exist in eutrophic lakes [7,8]. For example, heavy metal pollution exists in Taihu Lake, Jiangsu and Lake Dian, Yunnan China, where cyanobacterial blooms occur frequently [9,10]. This makes their removal more challenging due to the competition of coexisting substances. Therefore, it is urgent that develop a promising method to remove them more efficiently be developed. In general, methods for their removal include separation, adsorption, and photodegradation [11,12,13], in which photocatalytic detoxification is commonly identified as a sustainable strategy.
To date, many efforts have been devoted to the treatment of Cr(VI) or MC-LR. For example, MIL-53(Fe), MIL-125(Ti) and NH2-MIL-88B (Fe) exhibit high photocatalytic efficiency in terms of Cr(VI) reduction [14,15,16]. Regarding to the removal of MC-LR, doping of metal in TiO2, such as Bi-, N- or F-doping catalysts is popular and more efficient with a removal efficiency of 75% within several hours, in which the photocatalytic activities are significantly enhanced compared with those of undoped TiO2 [17,18]. Some researchers have also studied the simultaneous reduction of Cr(VI) and the oxidation of other pollutants using photocatalyst with any competitive reaction [19,20]; however, these photocatalysts are far from expected levels primarily due to the low active sites and rapid combination of electron−hole pairs [18,21,22]. Thus, it is desirable to assemble a photocatalyst to achieve synergetic oxidation of MC-LR and reduction of Cr(VI) with the characteristic of high active sites and a low combination of electron−hole pairs.
Metal-organic frameworks (MOFs), especially Fe-based MOF with aromatic dicarboxylate ligands [23], have received considerable attention for environmental remediation purposes owing to their unique large specific surface area, excellent chemical stability and hierarchical porosity [24]. In addition, their molecular structure (e.g., organic linkers separated by metal nodes) and the band between the highest occupied molecular orbital and the lowest unoccupied molecular orbital (HOMO−LUMO) are beneficial to harvest light and make it easily be photoexcited [25,26,27,28,29]. Therefore, the MOFs is very suitable for use as photocatalysts. Nevertheless, their photocatalytic performance is still limited because of their low conductivity and narrow band [29]. Recently, it has been found that a rational complex of MOFs with various semiconductor nanoparticles is able to not only optimize the generation of active species but also boost catalytic performance. For instance, g-C3N4/MIL-101(Fe), g-C3N4/PDI@NH2-MIL-53(Fe), TiO2/MIL-100(Fe) and Ag/AgCl/MIL-101(Fe) have been designed and used as photocatalysts for the removal of Cr (VI) and organic pollutants, such as bisphenol A, tetracycline and carbamazepine [30,31,32,33]. However, to the best of our knowledge, there are still no reports on the synergetic removal of Cr (VI) and MC-LR. This is probably because the MC-LR degradation, especially the destruction of the toxic foundational groups of MC-LR, is usually involves hole oxidation [34].
As is known, the semiconductor NPs loaded into their pores with position control can favor charges separation and regulate the generation of kind of reactive species [24,35,36]. In the present work, MIL-101(Fe)/TiO2 hybrids were constructed through rationally position control assembly which can allow the excited electrons (e−) to transfer between TiO2 and the adjacent metal with an appropriate redox pair, facilitating the electron generation for the Cr(VI) reduction and greatly inhibiting the charge recombination. Thus, more holes can be remained in the ligand of the MOF for the destruction of MC-LR. The simultaneous reduction of Cr(VI) and oxidation of MC-LR led to increased activity in both processes in comparison to separate reactions. This enhanced mechanism was explored through an energy diagram where the position of conduction and the valance bands for both MIL-101(Fe) and TiO2 were determined and the preferential role of holes in the MIL-101(Fe) matrix and photo generated e− in the TiO2 matrix were experimentally verified by radical scavenger experiments, electron spin resonance (ESR) and electrochemical test, etc. This work provides a new strategy employing a semiconductor/MOF material as a catalyst for the synergetic removals of pollutants in hybrids system.
2. Results and Discussion
2.1. Characterization of Catalysts
Installation of TiO2 within the structure of MIL-101(Fe) followed a previous reported methods [37] with minor modification. In detail, the precursor of MIL-101(Fe) was decorated on TiO2 based on the conversion of Fe3+ to γ-FeOOH at a constant temperature of 95 °C [37]. Then, γ-FeOOH could be converted to MIL-101(Fe) in the presence of terephthalic acid through a hydrothermal reaction. As a final result, MIL-101(Fe)/TiO2 was obtained (showing a deep orange color like MIL-101(Fe)) (Figure 1a). Fourier transform infrared (FTIR) spectra were further recordedto confirm the successful synthesis of MIL-101(Fe)/TiO2. As shown in Figure 1b, no vibrational peak is foundfor the bare TiO2 from 1100 to 2000 cm−1 and the broad peak from 850 cm−1 to 530 cm−1 is typical for the bare TiO2. While several characteristic peaks are found in MIL-101(Fe)/TiO2, such as 1706, 1583, and 1383 cm−1, which are the typical peaks for MIL-101(Fe). Among them, the peak at 1706 cm−1 conforms to the C=O bond in carboxyl groups [38,39]. The vibration peak at 1383 and 1583 cm−1 are assigned to the symmetric and asymmetric vibrations of O−C=O respectively, exhibiting blue shift (1583 cm−1) and are slightly weak compared with those of MIL-101(Fe) [40]. These changes may attribute to that the introduction of TiO2 limiting the formation of MIL-101(Fe). In addition, blow the 1200 cm−1, 744 and 545 cm−1 assigned to C-H and Fe-O respectively [41], are diminished in the nanocomposites compared with MIL-101(Fe). We speculate that a strong interaction between the TiO2 and MIL-101(Fe) exists and an alteration of the organic moiety ligation to the metal cluster takes place [42].
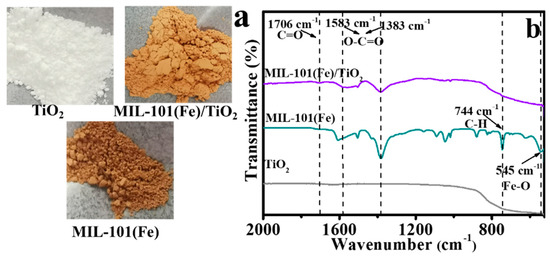
Figure 1.
Different colors (a), and FTIR spectra (b) of MIL-101(Fe), TiO2, and MIL-101(Fe)/TiO2 samples.
Scanning electron microscopy (SEM) and the high-resolution transmission electron microscopy (TEM) images were also used to study the formation of MIL-101(Fe)/TiO2. As shown in Figure 2, MIL-101(Fe) is a regular polyhedron (Figure 2a). The surface of bare TiO2 is smooth (Figure 2b); however, after stirring together with FeCl3•6H2O for 2 h at 95 °C, the surface of TiO2 becomes rough (Figure 2c,e) which is attributed to the formation of FeOOH originating from the transformation of Fe3+ [43]. Notably, after a hydrothermal reaction, the small FeOOH nanosticks are disappeared and were subsequently converted into MIL-101(Fe) (Figure 2d,f). The final morphology features of the as-prepared MIL-101(Fe)/TiO2 is completely different with those of MIL-101(Fe), and possessed a diameter of ~25 nm agreeing with that of pristine P25. High resolution TEM further indicated that core-shell nanoparticles of MIL-101(Fe)/TiO2 formed and the thickness of the outer MIL-101(Fe) wasabout 3.3 nm (Figure 2g). Therefore, it can be concluded that the growth of MOF (Fe) is not prevented in the presence of TiO2, and its morphology features change greatly.
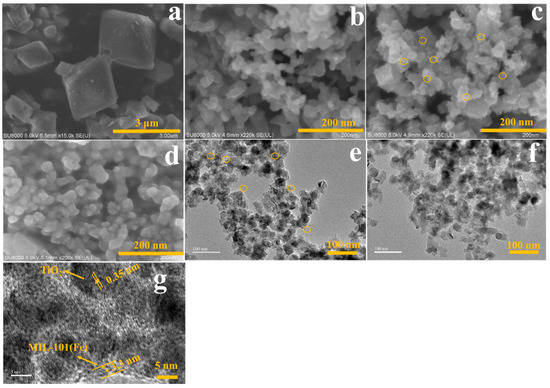
Figure 2.
SEM images of MIL-101(Fe) (a) and TiO2 (b); SEM images of the MOF precursor/TiO2 (c) and MIL-101(Fe)/TiO2 (d); (e) TEM images of MOF precursor/TiO2 and MIL-101(Fe)/TiO2 (f); (g) High-resolution TEM image of MIL-101(Fe)/TiO2.
Deeper insight into the interactions between TiO2 and MIL-101(Fe) was investigated using X-ray photoelectron spectroscopy (XPS) spectra (Figure 3). In Figure 3a, despite weak the signal of Ti 2p in MIL-101(Fe)/TiO2, two common peaks at 464.8 eV and 459.1 eV are still observed which show slight positive shift compared with that of bare TiO2 [44]. For the O 1s spectra of MIL-101(Fe)/TiO2, a lattice O in TiO2 (O-Ti) still exists with a binding energy of 530.1 eV (Figure 3b). Two splitting peaks at around 531.3 and 532.2 eV were also obtained after deconvolution of O 1s spectrum of MIL-101(Fe)/TiO2 (Figure 3b), which were assigned to the oxygen components of the terephthalate linkers and Fe-O bonds of MIL-101(Fe), respectively [41]. The spectra of Fe 2p of TiO2/MIL-101(Fe) showed two main peaks at 711.5 and 725.4 eV (Figure 3c), which are the characteristic of Fe 2p3/2 and Fe 2p1/2 in MIL-101 (Fe), respectively [30]. The peaks at 714.0 and 728.3 eV are assigned to Fe(III) oxidation states in MIL-101(Fe) [45].Two satellite peaks at 719.0 and 731.9 eV were also observed. The results indicate that the iron is principally Fe3+ in MIL-101(Fe)/TiO2 [46,47]. Moreover, all of the Fe 2p peaks from MIL-101(Fe)/TiO2 display negative shifts compared with those in MIL-101(Fe) (Figure 3c), indicating an increase in electron density around the Fe atoms. This result can be attributed to the interaction between two components in MIL-101(Fe)/TiO2, where Fe can attract electron from Ti, and subsequently low and increase the binding energy of Fe and Ti respectively. These discussions collectively indicate that the strong interaction between MIL-101(Fe) and TiO2, which might facilitate the charge transfer between the two components [48].
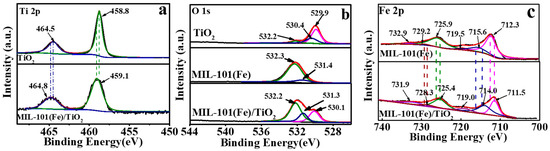
Figure 3.
(a) High-resolution of Ti 2p spectra of TiO2 and MIL-101(Fe)/TiO2; (b) High-resolution of O 1s of various photocatalysts; (c) High-resolution of Fe 2p of MIL-101(Fe) and MIL-101(Fe)/TiO2.
UV−Vis spectra shows that the pristine P25 only absorbed UV light (<400 nm) (Figure 4a). After the incorporation of MIL-101(Fe), the light absorption expands to the visible light range, suggesting the improved light absorption of the MIL-101(Fe)/TiO2 composites. Tauc-plots (Figure 4b) were deduced from the UV−Vis spectra to obtain the band gaps. And the band gaps (Eg) of P25 and MIL-101(Fe) were 3.20 and 2.5 eV, respectively. Notably, the band gap of the MIL-101(Fe)/TiO2 heterojunction is between that of the two individual materials.
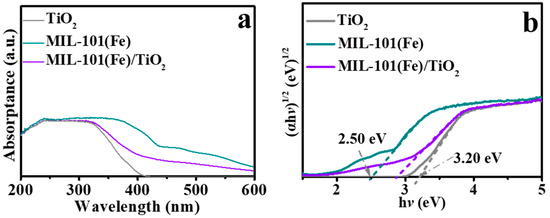
Figure 4.
(a) UV−Vis spectra of the as-prepared samples; (b) plots obtained using the Kubelka−Munk function transformation for the calculated absorbance versus photon energy.
2.2. Photocatalytic Synergetic Oxidation of MC-LR and Reduction of Cr(VI)
The photocatalytic performance of MIL-101(Fe)/TiO2 over Cr(VI) reduction was investigated systematically. Figure 5a shows that the removal curve of Cr(VI) using over various catalysts under UV irradiation. The adsorption of Cr(VI) in the dark can be neglected using different photocatalysts. Bare MIL-101(Fe) exhibited the lowest removal efficiency of Cr(VI) (0.011 min−1). It was also obvious that the introduction of TiO2 into MOF(Fe) resulted in higher Cr(VI) reduction rates (Figure 5b) which indicated that the photocatalytic behavior of bare MIL-101(Fe) could be enhanced by coupling it with the semiconductor particle P25 due to the high charge transfer via heterojunction. To investigate the influence of the MOF (Fe) proportion on the photocatalytic degradation efficiencies of Cr(VI) and MC-LR, three MIL-101(Fe)/TiO2 composites with low, medium and high content MIL-101(Fe) were synthesized by regulating the amounts of Fe3+. Accordingly, we name the as-prepared MIL-101(Fe)/TiO2 as L-, M- or H-MIL-101(Fe)/TiO2, respectively. Compared with the rate constant of the bare TiO2 (0.129 min−1), L-MIL-101 (Fe)/TiO2 exhibits the highest removal content and rate. The removal efficiency reaches to 100% within 20 min irradiation. Results also showed that the removal efficiency of Cr(VI) was inversely proportional to MOF (Fe) content in MIL-101(Fe)/TiO2. The corresponding rate constants were 0.306 min−1, 0.031 min−1 and 0.027 min−1 for L-, M-, and H-MIL-101(Fe)/TiO2, respectively (Figure 5b). The reason for this might be attributed to the excess of MIL-101(Fe) within composites will block TiO2, restricting the availability of light and the electron migration.
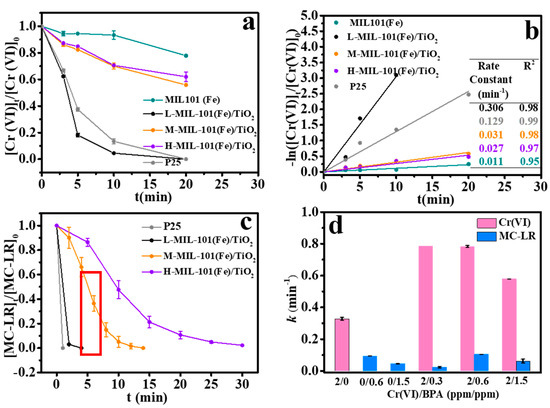
Figure 5.
(a) Photocatalytic reduction of Cr(VI) by various photocatalysts; (b) the kinetics of Cr(VI) reduction by various photocatalysts; (c) photocatalytic oxidation of MC-LR by various photocatalysts; (d) synergetic photocatalytic reduction of Cr(VI) and degradation of MC-LR over L-MIL-101(Fe)/TiO2 at different initial concentration ratios.
The decomposition of MC-LR by various photocatalysts was also tested. As shown in Figure 5c, 100% of MC-LR can be removed within 30 min by all catalysts, and the degradation efficiency of MC-LR over L-, and M-MIL-101(Fe)/TiO2 was close to that of P25. Previous study showed that MC-LR can be directly oxidized by holes [34]. Therefore, it is proposed that the removal of Cr(VI) can be promoted with the co-existence of MC-LR attributing a synergy of oxidation and reduction processes. Accordingly, the photocatalytic effect of L-MIL-101(Fe)/TiO2 was conducted in the mixture system with an the initial concentration of 2 ppm Cr(VI) and a series of concentrations (0.3, 0.6 and 1.5 ppm) of MC-LR. As shown in Figure 5d, in the case of the sole Cr(VI) system, the constant rate of Cr(VI) is 0.33 min−1. While, with the addition of MC-LR, the removal rate of Cr(VI) is enhanced, with the constant rates of 0.79, 0.78 and 0.53 min−1 in the hybrid system of Cr(VI)/MC-LR of 2/0.3, 2/0.6, 2/1.5, respectively. This might be attributed to the simultaneous occurrence of oxidation and reduction reaction [20,31]. In the mixture of the two targeted pollutants, MC-LR might consume holes reducing the hole-electron pairs recombination and Cr(VI) is reduced by electron. However, the presence of Cr(VI) slightly facilitates the degradation of MC-LR. The single constant rate of MC-LR was 0.05 min−1, however, 0.06 min−1 was observed with the coexistence of Cr(VI). This might attributed to the fact that hole is not the only reactive species to degrade MC-LR, and quenching experiments and ESR technology were further studied to prove our speculation.
The usability of L-MIL-101(Fe)/TiO2 was also tested. Figure 6 shows that MC-LR removal efficiency reached 95% within 20 min in the three consecutive cycles. In addition, the removal efficiency of Cr(VI) was still higher than 95% within 30 min after three times use. The above results shows that the as prepared composite is an efficient photocatalyst for synergetic Cr(VI) and MC-LR removal in aqueous solution. MIL-101(Fe)/TiO2 exhibited superiority compared to the partial catalysts in Table S5.
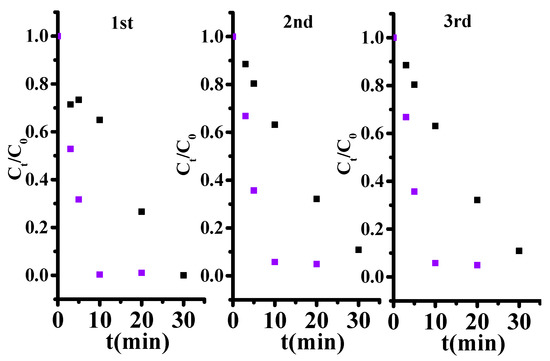
Figure 6.
The usability of L-MIL-101(Fe)/TiO2 within three recycles. Black dot and purple.dot represent the dynamic of Cr(VI) reduction and MC-LR degradation respectively. [Cr(VI)]0 = 2 ppm, [MC-LR]0 = 1.5 ppm, Catalysts dose = 0.05 g/L.
2.3. Possible Synergetic Mechanisms of Photocatalysis
Usually, the catalytic activity of MOF/TiO2 is mainly related to the production of free radicals, such as superoxide radical (•O2−), hydroxyl radical (•OH) and singlet oxygen (1O2) etc [22,49]. In order to further understand the formation of the free radicals of the materials during UV irradiation, the photocatalytic performance of MIL-101 (Fe)/TiO2 on MC-LR degradation was also evaluated through quenching experiments. Potassium iodide(KI), superoxide dismutase(SOD), iso-propylalcohol(IPA) and histidine(His) were added to the system as the scavenger of holes (h+), •O2−, •OH and 1O2, respectively. As displayed in Figure 7a, with the addition of IPA, there is less suppression of MC-LR degradation, which indicates that •OH is not the predominant free radical. However, the addition of KI, His and SOD markedly reduce MC-LR removal by 100%, 71.1% and 56.8%, respectively, indicating that h+, 1O2 and •O2− were involved and h+ dominates the degradation of MC-LR.
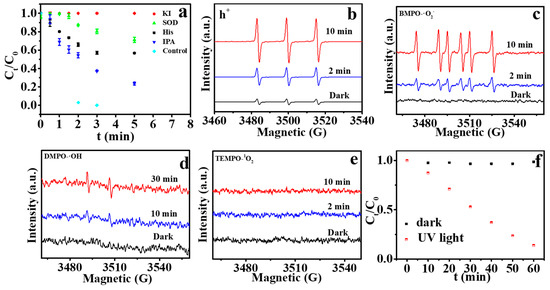
Figure 7.
(a) Removal efficiency of MC-LR in the presence of different scavengers by L-MIL-101(Fe)/TiO2; (b–e) ESR spectrum of the reaction system obtained by various probes (CPH, BMPO, DMPO and TEMPO for h+, •O2−, •OH, and 1O2 respectively) under UV irradiation; (f) FFA decay with irradiation time over MIL-101(Fe)/TiO2.
Using the ESR spin-trap technique with various probes [50,51,52], reactive species involved with the photocatalytic degradation were further confirmed in MIL-101 (Fe)/TiO2 catalyst suspensions. As shown in Figure 7b,c, both the h+ and •O2− signals were discerned using a probe of 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine (CPH) and 5-tert-butoxycar-bonyl 5-methyl-1-pyrroline N-oxide (BMPO) respectively, and their intensities increase obviously after 2 min of irradiation. 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) was used as an •OH probe was using to verify the direct generation of •OH [50]. The signal strength of •OH was relatively weak even after 30 min irradiation (Figure 7d). 2,2,6,6-tetramethylpiperidine (TEMP) as a probe of 1O2, has a characteristic peak signal of 1:1:1 [53]. However, there was no TEMP-1O2 signal (Figure 7e), which may be attributed to the small amounts 1O2. Furfuryl alcohol (FFA) is also a specific probe for 1O2 [54]. Thus, the degradation of 0.2 mM FFA was also carried out to confirm the existence of 1O2. Figure 7f shows that the content of FFA decreases diagonally within 60 min, with 16% left at the end, suggesting that the reaction of 1O2 with FFA occurred. As a final result, h+, •O2− and 1O2 were the main involved reactive species in the MIL-101 (Fe)/TiO2 suspensions, which is consistent with the MC-LR degradation quenching experiments.
Regarding to the reduction of Cr(VI), as we know, the photogenerated e− is the main species. Herein, a solid proof is supplied that the photocurrent is observed over the MIL-101(Fe)/TiO2 photocatalysts under UV irradiation (Figure 8a). The current density of MIL-101(Fe)/TiO2 is higher than that of bare MIL-101(Fe). That is why MIL-101(Fe)/TiO2 is more effective in Cr(VI) reduction than that of MIL-101(Fe). To further clarify the intrinsic electronic properties of MIL-101(Fe)/TiO2 to favor the reduction of Cr(VI), Mott−Schottky (M–S) measurements were conducted to estimate the flat band potentials (Vfb) of the prepared material. Based on the theory of M–S, the space charge capacitances (C) at the interface of semiconductor/electrolyte can be used to determine Vfb position. In the typical Mott–Schottky plots, 1/C2 and Ag/AgCl potential are Y-axis and X-axis, respectively and the flat band potential is the intersects of the tangent and the X axis. As shown in Figure 8b, the band gaps of MIL-101(Fe) is confirmed as 2.5 eV based on the Kubelka−Munk functions (Figure 4b). According to the M–S plots, the flat-band potential (Vfb) of MIL-101(Fe) is −0.70 V vs. Ag/AgCl at pH 6.8. MIL-101(Fe) is considered as an n-type semiconductor due to the positive slope of M–S plots [55]. It is well known that the conduction band (CB) of n-type semiconductor is very close to its Vfb [56]. Therefore, the redox potential of CB (ECB) of MIL-101(Fe) is deduced with the value of −0.50 V vs. NHE. The valence band (EVB) is the difference between Eg and ECB. Thus, the EVB value of MIL-101(Fe) is 2.0 V vs. NHE. It has been reported that the CB and VB of TiO2 are -0.4 and 2.8 V vs. NHE respectively [1]. The energy position of the CB edge of the MIL-101(Fe) is negative compared to that of TiO2. Therefore, in the MIL-101(Fe)/TiO2 photocatalyst, the photogenerated e− will be migrated on the CB of TiO2, and the photogenerated holes are injected into the VB of MIL-101 (Fe), resulting in the effective separation of photogenerated electron-hole pairs (Figure 8a).
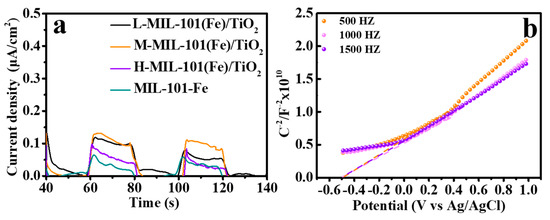
Figure 8.
(a) Photocurrent of MIL-101(Fe) and MIL-101(Fe)/TiO2 catalysts under UV irradiation; (b) Typical Mott–Schottky plots of MIL-101(Fe).
As is demonstrated in the above discussion aabove, the flow pathway of photogenerated e− and the ROS generation mechanism can be clearly verified in MIL-101(Fe)/TiO2. The schematic mechanism of simultaneous Cr(VI) reduction and MC-LR oxidation are shown in Figure 9, the more negative CB edge potential (-0.40 V vs. NHE) compared with that of O2/•O2− (−0.33 V vs. NHE) is provided by TiO2 over MIL-101(Fe)/TiO2, in which is enriched in photogenerated e− under UV irradiation. Accordingly, the adsorbed O2 by MIL-101(Fe)/TiO2 can be converted to •O2− by photogenerated e−, therefore •O2− plays important role in MC-LR degradation in a single MC-LR system (Figure 7a). In addition, it is also thermodynamically feasible that the bulk of aggregated e− (−0.4 V vs. NHE) can facilitate Cr(VI) (+0.51 V vs. NHE) reduction in the mixture system. However, h+ (2.0 V vs. NHE) can barely oxidize H2O to generation •OH in the VB of MIL-101(Fe) over MIL-101(Fe)/TiO2, which is consistent with the weak ESR weak signal generation and its minor role of •OH in degrading MC-LR. Therefore, the photogenerated holes are enhanced and play the main roles in the synergetic oxidation of MC-LR. Additionally, due to the structure of metal-organic chromophore linkers as a catalytic center in MIL101(Fe) [45], the generation of 1O2 might originated from the photosensitization of MIL101(Fe)/TiO2 under UV irradiation [57], and is also involved the synergetic degradation reaction.
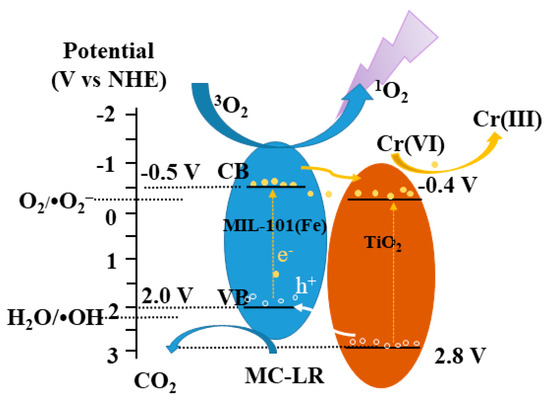
Figure 9.
Schematic mechanism of simultaneous Cr(VI) reduction and MC-LR oxidation over 0.1 M/P under UV light irradiation.
3. Materials, Experiment and Analysis Methods
3.1. Materials
Ferric chloride hexahydrate (FeCl3·6H2O), potassium dichromate (K2Cr2O7) and sodium hydroxide (NaOH) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Beijing, China). N, N-dimethylformamide (DMF) was purchased from Aladdin Co. (Shanghai, China). Terephthalic acid (BDC), TEMP and TiO2 nanoparticles (Aeroxide P25) were purchased from Sigma-Aldrich (St. Louis, MO, USA). BMPO and DMPO was purchased from DOJINDO Molecular Technologies, Inc. (Kumamoto, Japan). Microcystin-LR (MC-LR) was of high-performance liquid chromatography grade with purity ≥95% (Algal Science Inc., Taiwan, China). CPH was purchased from Enzo Biochem (New York, NY, USA) All reagents were of analytical grade.
3.2. Catalyst Preparation
The synthesis method of MIL-101(Fe) was as follows. 206 mg BDC and 675 mg FeCl3•6H2O were suspended in 15 mL DMF. The mixture was poured into a 100 mL high-pressure autoclave and sealed to be heated at 110 °C for 24 h, followed by cooling to room temperature naturally to obtaining orange crystals. The as-prepared sample was further purified using hot DMF and ethanol for several days. Finally, MIL-101(Fe) was centrifuged and collected, and then dried using an oven.
MIL-101(Fe)/TiO2 heterojunction was prepared using the following method. Exactly, 0.025 g commercial P25 was dispersed in 100 mL deionized water and ultrasonicated for 30 min, followed by the addition of FeCl3·6H2O and stirring for 2 min. The mixture was then transferred to a hot plate and stirred for 2 h at 95 °C. Then, the reaction kettle was cooled to room temperature and the residual Fe3+ was washed several times with deionized water and discarded, leaving the γ-FeOOH/TiO2 [37].Finally, 0.2 g BDC was added to γ-FeOOH/TiO2 and dissolved in DMF (15 mL). The resulting solution was finally transferred to a Teflon autoclave, and sealed to be heated at 110 °C for 24 h. The as-synthesized MIL-101(Fe)/TiO2 was centrifuged and purified with DMF and acetone for several days. Then, the sample was dried in an oven at 70 °C for 10 h. The mass ratio of TiO2 and MIL-101(Fe) was controlled by the amounts of Fe3+. Finally, the as-prepared catalysts were named as L-MIL-101(Fe)/TiO2, M-MIL-101(Fe)/TiO2 and H-MIL-101(Fe)/TiO2 with the mass ratio of FeCl3·6H2O: TiO2 of 1:9.3, 1:1.9, and 1:0.9 respectively.
3.3. Characterization
The as-synthesis catalysts were characterized via XRD (Bruker D8 Advance, Karlsruhe, Germany) with Cu Kα radiation (λ = 1.54178 Å). Morphology analysis of catalysts was obtained by SEM (Hitachi SU-8020, Hitachi, Japan) and the high-resolution TEM (JEOL-2100F, Tokyo, Japan). Surface chemical states was analyzed by XPS (ESCALAB 250Xi, Thermo Scientific, Waltham, MA, USA). FTIR spectra was investigated using a spectrometer (Nicolet iN10MX, Thermo Scientific, Waltham, MA, USA). UV−vis absorption was characterized with a UV 3600 (Shimadzu, Kyoto, Japan). ESR measurement were performed on a Bruker EMX spectrometer (Bruker, Karlsruhe, Germany).
3.4. Electrochemical Measurements
The photocurrent measurements were carried out on a Shanghai Chenhua CHI-660b electrochemical system. The working electrode were prepared on ITO glass plates. A volume of 300 μL 20 mg/L photocatalysts were mixed with 100 μL Nafion. Then the working electrode was prepared by dropping the mixture solution, followed by natural drying. Three-electrode method was used in all electrochemical tests with Pt plate as the counter electrode, and Ag/AgCl as the reference electrode (in 0.1 M Na2C2O4). The light source was similar to that of photocatalytic degradation experiments.
Further, to assess the flat band potentials of the prepared material, Mott-Schottky (M-S) measurements were performed on a Shanghai Chenhua CHI-660E electrochemical system. The M–S measurements were undertaken at frequencies of 500, 1000, and 1500 Hz, respectively. The electrolyte was 0.1 M Na2SO4 (pH = 6.8).
3.5. Photocatalytic Reaction
MC-LR and Cr(VI) were chosen as the model pollutants to test the photocatalytic properties of the as-prepared materials. Experiments of Cr(VI) reduction and MC-LR oxidation degradation were carried out in 100 mL quartz beaker separately. Reaction systems contained 50 mL 0.05 g/L catalysts, Cr(VI) and MC-LR were added to the above solution with the initial concentration of 2 ppm and 1.5 ppm, respectively. The resulting solution underwent 30 min of stirring in the dark to reach adsorption equilibrium. Then ultraviolet irradiation started using a 500 W Xenon lamp with a 200–400 nm filter (25 mW/cm2), and 10 cm interval away from the reaction cell. 1 mL solution was sampled at a certain interval time and filtered with 0.45 μm filter for further analysis. The synergetic oxidation of MC-LR and reduction of Cr(VI) in aqueous media was also conducted under the same condition mentioned above.
3.6. Chemical Analysis
MC-LR concentration was determined using a Aligent 1260 high performance liquid chromatography (Algal Science Inc., Taiwan) equipped with a UV detector and a ZORBAX Eclipse Plus C18 column (150 mm × 4.6 mm × 5 μm). The detection wavelength was 238 nm. The Cr (VI) concentration was detected using a typically colorimetric method at 540 nm, as proposed by previous study [32].
4. Conclusions
In summary, a MIL-101(Fe)/TiO2 composite was constructed by in situ growth of MIL-101(Fe) on TiO2. It can be used to achieve the synergetic photoreduction of Cr(VI) and photooxidation of MC-LR. During the synergistic photocatalytic degradation process, Cr(VI) reduction activity is enhanced compared with that of the bare TiO2 and MIL-101(Fe), while the MC-LR oxidation rate was also slightly enhanced. Here, MC-LR acts as “hole scavengers” to improve the separation of photo-induced charge carriers. Moreover, the electrochemical investigation also demonstrates that the introduction of TiO2 to MIL-101(Fe) can accept photoexcited electrons and effectively avoid the recombination of charge carriers. We anticipate that this assembly strategy will provide new insight into a broad range of catalytic applications, particularly to those that need the simultaneously treatment of mixed contaminants simultaneously.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11101186/s1, Table S1. The precise position, FWHM and %area of Ti 2p fitted for different TiO2 and MIL-101(Fe)/TiO2, Table S2. The precise position, FWHM and %area of O 1s fitted for different TiO2 and MIL-101(Fe)/TiO2, Table S3. The precise position, FWHM and %area of Fe 2p fitted for MIL-101(Fe), Table S4. The precise position, FWHM and %area of Fe 2p fitted for MIL-101(Fe)/TiO2, Table S5. Rate constant kCr and k MC-LR of photocatalytic reduction of Cr (VI) and MC-LR.
Author Contributions
Conceptualization, Y.W. (Yarui Wang) and L.Z.; methodology, Y.W. (Yarui Wang), W.Y. and L.Z.; software, Y.W. (Yarui Wang), W.Y. and L.Z.; validation, Y.W. (Yarui Wang), Y.W and L.Z.; formal analysis, Y.W. (Yarui Wang), F.G. and L.Z.; investigation, Y.W. (Yarui Wang) and L.Z.; resources, L.Z.; data curation, Y.W. (Yarui Wang) and F.G.; writing—original draft preparation, Y.W. (Yarui Wang); writing—review and editing, L.Z. and Y.W. (Yawei Wang); supervision, L.Z. and Y.W. (Yawei Wang); All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 21876184, 21625702), National Key Research and Development Program of China (2020YFA0907504).
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.; Chen, F.; Yu, W.; Zhao, L. An efficient floating adsorption-photocatalyst to decarboxylate D-Glu and D-MeAsp of Microcystin-LR via holes direct oxidation. Chem. Eng. J. 2021, 413, 127543. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Shi, Z.; Lu, T.-T.; Chen, D.; Wang, Q.; Zhan, Z. A direct Z-scheme BiOBr/TzDa COF heterojunction photocatalyst with enhanced performance on visible-light driven removal of organic dye and Cr (VI). Sep. Purif. Technol. 2021, 275, 119216. [Google Scholar] [CrossRef]
- Su, Y.-Q.; Yuan, S.; Guo, Y.-C.; Tan, Y.-Y.; Mao, H.-T.; Cao, Y.; Chen, Y.-E. Highly efficient and sustainable removal of Cr (VI) in aqueous solutions by photosynthetic bacteria supplemented with phosphor salts. Chemosphere 2021, 283, 131031. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.-H.; Xu, X.-R.; Jiang, D.; Kong, L.-J.; Sun, Y.; Hu, Y.-X.; Hao, Q.-W.; Chen, H. Bentonite-supported nanoscale zero-valent iron/persulfate system for the simultaneous removal of Cr(VI) and phenol from aqueous solutions. Chem. Eng. J. 2016, 302, 213–222. [Google Scholar] [CrossRef]
- Lu, D.; Chai, W.; Yang, M.; Fang, P.; Wu, W.; Zhao, B.; Xiong, R.; Wang, H. Visible light induced photocatalytic removal of Cr (VI) over TiO2-based nanosheets loaded with surface-enriched CoOx nanoparticles and its synergism with phenol oxidation. Appl. Catal. B Environ. 2016, 190, 44–65. [Google Scholar] [CrossRef]
- Gong, M.; Xiao, S.; Yu, X.; Dong, C.; Ji, J.; Zhang, D.; Xing, M. Research progress of photocatalytic sterilization over semiconductors. RSC Adv. 2019, 9, 19278–19284. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Diao, X.; He, L. Effects of algal bloom formation, outbreak, and extinction on heavy metal fractionation in the surficial sediments of Chaohu Lake, Environ. Environ. Sci. Pollut. Res. 2015, 22, 14269–14279. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, E.; Shen, J. The accumulation and potential ecological risk of heavy metals in microalgae from a eutrophic lake (Taihu Lake, China). Environ. Sci. Pollut. Res. 2015, 22, 17123–17134. [Google Scholar] [CrossRef]
- Fan, X.; Ding, S.; Chen, M.; Gao, S.; Fu, Z.; Gong, M.; Tsang, D.; Wang, Y.; Zhang, C. Peak Chromium pollution in summer and winter caused by high mobility of chromium in sediment of a Eutrophic Lake: In situ evidence from high spatiotemporal sampling. Environ. Sci. Technol. 2019, 53, 4755–4764. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, X.; Yu, C.; Zhou, W.; Wei, L. Construct interesting CuS/TiO2 architectures for effective removal of Cr(VI) in simulated wastewater via the strong synergistic adsorption and photocatalytic process. Sci. Total Environ. 2021, 796, 148941. [Google Scholar] [CrossRef]
- Chen, M.; Long, Z.; Dong, R.; Wang, L.; Zhang, J.; Li, S.; Zhao, X.; Hou, X.; Shao, H.; Jiang, X. Titanium incorporation into Zr-Porphyrinic Metal–Organic Frameworks with enhanced antibacterial activity against multidrug-resistant pathogens. Small 2020, 16, e1906240. [Google Scholar] [CrossRef] [PubMed]
- Hasija, V.; Nguyen, V.-H.; Kumar, A.; Raizada, P.; Krishnan, V.; Khan, A.A.P.; Singh, P.; Lichtfouse, E.; Wang, C.; Huong, P.T. Advanced activation of persulfate by polymeric g-C3N4 based photocatalysts for environmental remediation: A review. J. Hazard. Mater. 2021, 413, 125324. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Jafari, N.; Ebrahimpour, K.; Karimi, M.; Rostamnia, S.; Behnami, A.; Ghanbari, R.; Mohammadi, A.; Rahimi, B.; Abdolahnejad, A. A novel ternary heterogeneous TiO2/BiVO4/NaY-Zeolite nanocomposite for photocatalytic degradation of microcystin-leucine arginine (MC-LR) under visible light. Ecotoxicol. Environ. Saf. 2021, 210, 111862. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Shen, L.; Jing, F.; Qin, N.; Wu, L. Preparation of MIL-53(Fe)-reduced graphene oxide nanocomposites by a simple self-assembly strategy for increasing interfacial contact: Efficient visible-light photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 9507–9515. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Wu, Z.; Jiang, L.; Li, H. Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr(VI) reduction. J. Hazard. Mater. 2015, 286, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Meng, X.; Liu, H.; Ye, J. An Amine-Functionalized Iron(III) Metal-Organic Framework as Efficient Visible-Light Photocatalyst for Cr(VI) Reduction. Adv. Sci. 2015, 2, 1500006. [Google Scholar] [CrossRef]
- Yang, J.; Chen, D.-X.; Deng, A.-P.; Huang, Y.-P.; Chen, C.-C. Visible-light-driven photocatalytic degradation of microcystin-LR by Bi-doped TiO2. Res. Chem. Intermed. 2011, 37, 47–60. [Google Scholar] [CrossRef]
- Pelaez, M.; de la Cruz, A.A.; Stathatos, E.; Falaras, P.; Dionysiou, D.D. Visible light-activated N-F-codoped TiO2 nanoparticles for the photocatalytic degradation of microcystin-LR in water. Catal. Today 2009, 144, 19–25. [Google Scholar] [CrossRef]
- Fu, H.; Lu, G.; Li, S. Adsorption and photo-induced reduction of Cr(VI) ion in Cr(VI)-4CP (4-chlorophenol) aqueous system in the presence of TiO2 as photocatalyst. J. Photochem. Photobiol. A Chem. 1998, 114, 81–88. [Google Scholar] [CrossRef]
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J. Hazard. Mater. 2015, 287, 364–372. [Google Scholar] [CrossRef]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuki, P. Photocatalysis with reduced TiO2: From black TiO2 to cocatalyst-free hydrogen production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Chen, T.; Pan, X. Metal–organic-framework-based materials for antimicrobial applications. ACS Nano 2021, 15, 3808–3848. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, M.; Li, Z. Fe-Based metal–organic frameworks for highly selective photocatalytic benzene hydroxylation to phenol. ACS Catal. 2015, 5, 6852–6857. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Yeung, K.; Pan, H.; Wu, S. Porous Iron-carboxylate metal–organic framework: A novel bioplatform with sustained antibacterial efficacy and nontoxicity. ACS Appl. Mater. Interfaces 2017, 9, 19248–19257. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, J.; Feng, X.; Li, J.; Hao, Y.; Zhang, J.; Wang, H.; Yin, A.; Zhou, J.; Ma, X.; et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 2019, 10, 2177. [Google Scholar] [CrossRef]
- Ma, D.; Li, P.; Duan, X.; Li, J.; Shao, P.; Lang, Z.; Bao, L.; Zhang, Y.; Lin, Z.; Wang, B. A hydrolytically stable Vanadium(IV) metal–organic framework with photocatalytic bacteriostatic activity for autonomous indoor humidity control. Angew. Chem. 2020, 59, 3905–3909. [Google Scholar] [CrossRef]
- Kataoka, Y.; Sato, K.; Miyazaki, Y.; Masuda, K.; Tanaka, H.; Naito, S.; Mori, W. Photocatalytic hydrogen production from water using porous material [Ru2(p-BDC)2] n. Energy Environ. Sci. 2009, 2, 397–400. [Google Scholar] [CrossRef]
- de Miguel, M.; Ragon, F.; Devic, T.; Serre, C.; Horcajada, P.; García, H. Evidence of Photoinduced Charge Separation in the Metal-Organic Framework MIL-125(Ti)-NH. ChemPhysChem 2012, 13, 3651–3654. [Google Scholar] [CrossRef]
- Wang, C.-C.; Du, X.-D.; Li, J.; Guo, X.-X.; Wang, P.; Zhang, J. Photocatalytic Cr (VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B Environ. 2016, 193, 198–216. [Google Scholar] [CrossRef]
- He, L.; Dong, Y.; Zheng, Y.; Jia, Q.; Shan, S.; Zhang, Y. A novel magnetic MIL-101(Fe)/TiO2 composite for photo degradation of tetracycline under solar light. J. Hazard. Mater. 2019, 361, 85–94. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Ben Hammouda, S.; Doshi, B.; Guijarro, N.; Min, X.; Tang, C.-J.; Sillanpää, M.; Sivula, K.; Wang, S. MIL-101(Fe)/g-C3N4 for enhanced visible-light-driven photocatalysis toward simultaneous reduction of Cr(VI) and oxidation of bisphenol A in aqueous media. Appl. Catal. B Environ. 2020, 272, 119033. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, W.; Sen, T.; Yu, Y.; Liu, Y.; Zhang, J.; Wang, L. Metal–Organic Framework MIL-101(Fe) nanoparticles decorated with Ag nanoparticles for regulating the photocatalytic phenol oxidation pathway for Cr(VI) reduction. ACS Appl. Nano Mater. 2021, 4, 4513–4521. [Google Scholar] [CrossRef]
- Li, Y.; Fang, Y.; Cao, Z.; Li, N.; Chen, D.; Xu, Q.; Lu, J. Construction of g-C3N4/PDI@ MOF heterojunctions for the highly efficient visible light-driven degradation of pharmaceutical and phenolic micropollutants. Appl. Catal. B Environ. 2019, 250, 150–162. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, Y.; Yang, J.; Wang, P.; Cheng, G. Unique ability of BiOBr to decarboxylate D-Glu and D-MeAsp in the photocatalytic degradation of Microcystin-LR in water. Environ. Sci. Technol. 2011, 45, 1593–1600. [Google Scholar]
- Qiu, H.; Pu, F.; Liu, Z.; Deng, Q.; Sun, P.; Ren, J.; Qu, X. Depriving bacterial adhesion-related molecule to inhibit biofilm formation using CeO 2-decorated metal-organic frameworks. Small 2019, 15, 1902522. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xu, X.; Ma, Y.; Cho, H.S.; Ding, D.; Wang, C.; Wu, J.; Oleynikov, P.; Jia, M.; Cheng, J.; et al. Filling metal–organic framework mesopores with TiO2 for CO2 photoreduction. Nat. Cell Biol. 2020, 586, 549–554. [Google Scholar] [CrossRef]
- He, X.; Fang, H.; Gosztola, D.J.; Jiang, Z.; Jena, P.; Wang, W.-N. Mechanistic Insight into photocatalytic pathways of MIL-100(Fe)/TiO2 composites. ACS Appl. Mater. Interfaces 2019, 11, 12516–12524. [Google Scholar] [CrossRef]
- Zhou, R.-Y.; Yu, J.-X.; Chi, R.-A. Selective removal of phosphate from aqueous solution by MIL-101(Fe)/bagasse composite prepared through bagasse size control. Environ. Res. 2020, 188, 109817. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Jin, W.; Hu, Q.; Zhao, Y. Adsorption behavior of arsenicals on MIL-101(Fe): The role of arsenic chemical structures. J. Colloid Interface Sci. 2019, 554, 692–704. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Umar, A.; Khan, M.A.; Algarni, H.; Kansal, S.K. Removal of fluoroquinolone drug, levofloxacin, from aqueous phase over iron based MOFs, MIL-100(Fe). J. Solid State Chem. 2020, 281, 121029. [Google Scholar] [CrossRef]
- Liu, Z.; He, W.; Zhang, Q.; Shapour, H.; Bakhtari, M.F. Preparation of a GO/MIL-101(Fe) Composite for the removal of methyl orange from aqueous solution. ACS Omega 2021, 6, 4597–4608. [Google Scholar] [CrossRef]
- Fazlali, F.; Hajian, A.; Afkhami, A.; Bagheri, H. A superficial approach for fabricating unique ternary AgI@TiO2/Zr-MOF composites: An excellent interfacial with improved photocatalytic light-responsive under visible light. J. Photochem. Photobiol. A Chem. 2020, 400, 112717. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, C.; Qian, J.; Lu, Z.; Pan, S.; Pan, B. Highly efficient water decontamination by using sub-10 nm FeOOH confined within millimeter-sized mesoporous polystyrene beads. Environ. Sci. Technol. 2017, 51, 9210–9218. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Lu, L.; Liang, C.; Liu, Q.; Zhu, G. Heterojunction of facet coupled g-C3N4/surface-fluorinated TiO2 nanosheets for organic pollutants degradation under visible LED light irradiation. Appl. Catal. B Environ. 2014, 156, 331–340. [Google Scholar] [CrossRef]
- Huo, Q.; Liu, G.; Sun, H.; Fu, Y.; Ning, Y.; Zhang, B.; Zhang, X.; Gao, J.; Miao, J.; Zhang, X.; et al. CeO2-modified MIL-101(Fe) for photocatalysis extraction oxidation desulfurization of model oil under visible light irradiation. Chem. Eng. J. 2021, 422, 130036. [Google Scholar] [CrossRef]
- Zheng, X.-X.; Fang, Z.-P.; Dai, Z.-J.; Cai, J.-M.; Shen, L.-J.; Zhang, Y.-F.; Au, C.-T.; Jiang, L.-L. Iron-based metal–organic frameworks as platform for H2S selective conversion: Structure-dependent desulfurization activity. Inorg. Chem. 2020, 59, 4483–4492. [Google Scholar] [CrossRef]
- Fu, J.; Wang, X.; Wang, T.; Zhang, J.; Guo, S.; Wu, S.; Zhu, F. Covalent Functionalization of graphene oxide with a presynthesized metal–organic framework enables a highly stable electrochemical sensing. ACS Appl. Mater. Interfaces 2019, 11, 33238–33244. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Duan, S.; She, H.; Huang, J.; Wang, Q. In-situ incorporation of Copper(II) porphyrin functionalized zirconium MOF and TiO2 for efficient photocatalytic CO2 reduction. Sci. Bull. 2019, 64, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhuo, Y.; Ezugwu, C.I.; Wang, C.-C.; Li, C.; Liu, S. Synergetic Molecular oxygen activation and catalytic oxidation of formaldehyde over defective MIL-88B(Fe) nanorods at room temperature. Environ. Sci. Technol. 2021, 55, 8341–8350. [Google Scholar] [CrossRef]
- Gu, X.; Qin, N.; Zhang, P.; Hu, Y.; Zhang, Y.-N.; Zhao, G. In-situ synthesis of {111} TiO2/Ti photoelectrode to boost efficient removal of dimethyl phthalate based on a bi-functional interface. Chem. Eng. J. 2021, 422, 129980. [Google Scholar] [CrossRef]
- Bing, J.; Hu, C.; Nie, Y.; Yang, M.; Qu, J. Mechanism of catalytic ozonation in Fe2O3/Al2O3@SBA-15 aqueous suspension for destruction of ibuprofen. Environ. Sci. Technol. 2015, 49, 1690–1697. [Google Scholar] [CrossRef]
- Hailili, R.; Wang, Z.-Q.; Xu, M.; Wang, Y.; Gong, X.-Q.; Xu, T.; Wang, C. Layered nanostructured ferroelectric perovskite Bi5FeTi3O15 for visible light photodegradation of antibiotics. J. Mater. Chem. A 2017, 5, 21275–21290. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Wang, Z.U.; Wang, H.; Lu, J.; Yu, S.-H.; Jiang, H.-L. Singlet oxygen-engaged selective photo-oxidation over pt nanocrystals/porphyrinic MOF: The roles of photothermal effect and pt electronic state. J. Am. Chem. Soc. 2017, 139, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.; Joens, J.A.; O’Shea, K.E. Fundamental Studies of the singlet oxygen reactions with the potent marine toxin domoic acid. Environ. Sci. Technol. 2020, 54, 6073–6081. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, X.; Liu, W. The synthesis strategies and photocatalytic performances of TiO2/MOFs composites: A state-of-the-art review. Chem. Eng. J. 2020, 391, 123601. [Google Scholar] [CrossRef]
- Huang, H.; Li, D.; Lin, Q.; Zhang, W.; Shao, Y.; Chen, Y.; Sun, M.; Fu, X. Efficient degradation of benzene over LaVO4/TiO2 nanocrystalline heterojunction photocatalyst under visible light irradiation. Environ. Sci. Technol. 2009, 43, 4164–4168. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, X.; Zhang, W.; Zhang, S.; Tian, Y.; Chen, Z.; Fang, W.; Ma, J. Intraligand charge transfer boosts visible-light-driven generation of singlet oxygen by metal-organic frameworks. Appl. Catal. B Environ. 2020, 273, 119087. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).