Abstract
Developing a novel ammonia synthesis process from N2 and H2 is of interest to the catalysis and hydrogen research communities. γ-Alumina-supported nickel was determined capable of serving as an efficient catalyst for ammonia synthesis using nonthermal plasma under atmospheric pressure without heating. The catalytic activity was almost unrelated to the crystal structure and the surface area of the alumina carrier. The activity of Ni/Al2O3 was quantitatively compared with that of Fe/Al2O3 and Ru/Al2O3, which contained active metals for the conventional Haber–Bosch process. The activity sequence was Ni/Al2O3 > Al2O3 > Fe/Al2O3 > no additive > Ru/Al2O3, surprisingly indicating that the loading of Fe and Ru decreased the activity of Al2O3. The catalytic activity of Ni/Al2O3 was dependent on the amount of loaded Ni, the calcination temperature, and the reaction time. XRD, visual, and XPS observations of the catalysts before the plasma reaction indicated the generation of NiO and NiAl2O4 on Al2O3, the latter of which was generated upon high-temperature calcination. The NiO species was readily reduced to Ni metal in the plasma reaction, whereas the NiAl2O4 species was difficult to reduce. The catalytic behavior could be attributed to the production of fine Ni metal particles that served as active sites. The PN2/PH2 ratio dependence and rate constants of formation and decomposition of ammonia were finally determined for 5.0 wt% Ni/Al2O3 calcined at 773 K. The ammonia yield was 6.3% at an applied voltage of 6.0 kV, a residence time of reactant gases of 0.12 min, and PH2/PN2 = 1.
1. Introduction
New methods for the synthesis of ammonia have been widely investigated in many catalysis fields to improve or displace the current Haber–Bosch process due to its extreme reaction conditions and high consumption of energy supply. In the field of heterogeneous catalysis, new catalytic systems, including mainly Ru as an active site, were reported [1,2,3,4,5,6,7,8,9,10,11]. However, relatively high yields were usually accompanied by low reaction rates, while high reaction rates were accompanied by low yields. At present, such a trade-off correlation is very difficult to overcome. In the field of homogeneous catalysis [12,13,14,15,16], various metal complexes were reported to be active for ammonia synthesis. The turnover numbers of the recently reported catalysts were 100–200, indicating the need for extensive research efforts to make a practical system. Electrocatalysis was also reported using Ag-Pd alloys and Ru/supports as electrode catalysts [17,18,19,20,21,22,23], but their reaction rates were far lower than those of the Haber–Bosch process. In catalysis using nonthermal atmospheric-pressure plasma for the activation of a nitrogen molecule [24,25,26,27,28,29,30,31,32,33,34], Ru- or Fe-loaded ceramic membranes or metal oxides such as BaTiO3 were used, and the yields reached a few percent. However, the production rates were slow, due to the slow-flowing velocity of the reactant gases via the ceramic layers [34,35,36,37].
In contrast, we have recently reported that a wool-like metal electrode, used to produce nonthermal plasma, functioned as an efficient catalyst for ammonia production from N2 and H2 under atmospheric pressure without heating [38,39]. The order of activity at each initial experiment was Au > Pt > Pd > Ag > Cu > Fe > Mo > Ni > W > Ti > Al [39]. Most of the metals were not reported as active for ammonia synthesis in the Haber–Bosch process. After the activity was stabilized by repeated experiments, the ammonia yield was 3.5% at H2/N2 = 3 on copper [38], which was the highest among the values reported at atmospheric pressure. In a typical Haber–Bosch process, the triple bond between the two nitrogen atoms is activated by electron donation from the catalyst. However, in the plasma process, the nitrogen molecule is activated by the plasma, and the catalyst provides the reaction site for the activated species. Although a detailed ammonia synthesis mechanism for the plasma catalysis remained unclear, these new findings made us consider that some metal oxides might also be active for this reaction.
Various nickel-containing materials have been reported to be active for the catalytic decomposition of ammonia [40,41,42,43,44,45,46]. Since the ammonia decomposition reaction is an equilibrium reaction at a middle temperature range, these earlier works might suggest the possibility of the nickel-containing materials working as active catalysts for ammonia synthesis. In fact, several nickel-containing nitride compounds have been reported as active for ammonia synthesis [47,48,49,50,51], although very severe reaction conditions, such as high reaction temperature and high pressure, were often required. In the current work, the catalytic activity of nickel-supported alumina was studied in a nonthermal atmospheric pressure plasma without any heating and compared with those of iron- or ruthenium-supported alumina that are well-known to be active for the conventional Haber–Bosch process. We found that the activity of Ni/Al2O3 was much higher than that of Fe/Al2O3 and Ru/Al2O3, and surprisingly, the activities of the latter two compounds were lower than that of alumina alone. The dependencies of the ammonia synthesis on the PH2/PN2 ratio and the residence time of the reactant gases were also studied on Al2O3 and 5.0 wt% Ni/Al2O3 calcined at 773 K. The new reaction system effectively produced ammonia with yields of 3.0% and 6.3% on the catalysts at a residence time of reactant gases of 0.12 min and PH2/PN2 = 1. This method provides new insights into ammonia synthesis that will be significant for the future hydrogen economy.
2. Results and Discussion
2.1. Comparison of the Activities of Various Oxide Catalysts
It has been reported that alumina, used as a carrier in this study, shows catalytic activity for the plasma synthesis of ammonia. In this study, we first compared the catalytic activity of alumina as a function of surface area and crystal structure. The results are summarized in Figure 1, in which the supplier of alumina, sample name, surface area, and amount used in plasma experiments are listed in Table S1 (in Supporting Information). In the reaction conditions, H2/N2 means the ratio of partial pressure of supplied H2 and that of supplied N2. The horizontal axis of the figure is the total surface area of alumina actually used in the catalyst experiment. The colors of the plots indicate that the structure of alumina is alpha-type (yellow), theta (white), gamma (red), or a mixture of theta and gamma (blue). The red broken line indicates the average activity of all Al2O3. It is clear that the catalytic activity did not change significantly depending on the crystal structure or surface area, although it did change somewhat. In this study, we employed gamma-type alumina as the support because it has a medium-high surface area and the crystal structure is stable.
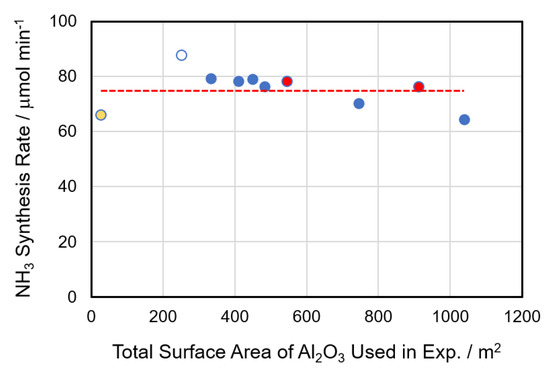
Figure 1.
Comparison of activities of various Al2O3 as a function of total surface area and crystal structure. The crystal structure of α, θ, γ, and θ + γ is shown by yellow, white, red, and blue plots. The red broken line is the average NH3 synthesis rate. Reaction conditions: applied voltage, 6 kV; frequency, 50 kHz; electrode length, 150 mm; total flow rate, 100 mL min−1; and H2/N2 = 1.
The catalytic activities of Ni/Al2O3 were quantitatively compared with the activities of Fe/Al2O3 and Ru/Al2O3 in Figure 2, where the components listed in the figure were determined by XRD measurements after the reactions. The amounts of loaded metals were all 5.0 wt% as metal, which are indicated by the first numerical values of the sample names. KC and SC mean γ-Al2O3 purchased from Kanto Chemical Co., Japan, and Strem Chemicals Inc., USA, and the numerical values such as 773 are the calcination temperatures of catalysts in Kelvin. At first, it should be noted that the activities of Al2O3(SC) and Al2O3(KC) were similar and that the activities of 5Ni/Al2O3(SC, 773) and 5Ni/Al2O3(KC, 773) were also similar, although the former comparison was already shown in Figure 1. The results ensured again that there was no problem in using either the SC or KC alumina as the carrier without distinction. The activities of the catalysts were in the following order 5Ni/Al2O3 > Al2O3 > 5Fe/Al2O3 > no additive (a blank experiment) > 5Ru/Al2O3, surprisingly indicating that the loading of iron and ruthenium decreased the activity of Al2O3. As indicated in the introduction, the plasma atmosphere works as a preactivation port of nitrogen and hydrogen molecules, and the catalysts should work as a reaction port of preactivated molecules. Fe and Ru would not be useful for this purpose, although they are active for activation of the triple bond between two nitrogen atoms. It is widely accepted in the Haber–Bosch process that Fe and Ru can gather hydrogen atoms adsorbed on supports and exhibit high activity for their reaction with a nitrogen atom activated on the metal site. The loading of Fe or Ru might decrease the number of hydrogen atoms on alumina, which resulted in smaller ammonia yields than that of alumina alone. The results also suggested that there will be many opportunities in the field to develop an active catalyst for ammonia synthesis from preactivated nitrogen with hydrogen, as well as revealing the reaction mechanism and active species.
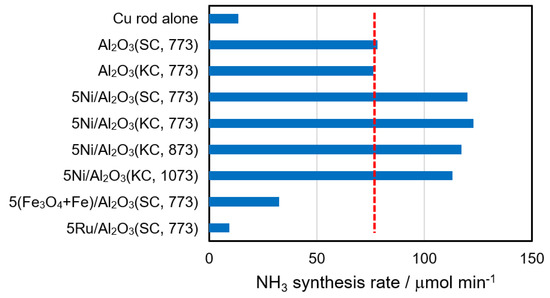
Figure 2.
Comparison of activities of Ni-, Fe-, and Ru-supported γ-Al2O3 catalysts. The red broken line indicates the average activity of Al2O3(SC) and Al2O3(KC). Reaction conditions were the same as those in Figure 1.
Recently a spillover rate of a hydrogen atom on titania was reported to be much faster than that on alumina by DFT calculations [52] and experimental measurements [53]. The surface diffusion of a hydrogen atom might be important for the catalytic ammonia synthesis using the plasma atmosphere. TiO2 particles (diameter of 0.3–0.6 mm, a rutile type) purchased from Kanto Chemical Co., Japan was used as a parent catalyst and 5.0 wt% of Ni was loaded on TiO2 in a similar manner to that in the preparation of 5Ni/Al2O3. TiO2 and 5Ni/TiO2 were employed as catalysts for the plasma ammonia synthesis after calcination at 773 K for 4 h in air. The formation rates of ammonia on TiO2 and 5Ni/TiO2 were 32.7 and 45.0 µmol min−1, respectively, at H2/N2 = 1, total flow rate = 100 mL min−1, 6 kV, and 50 kHz (a typical reaction condition same as those applied for 5Ni/Al2O3). No deactivation was observed on the catalysts. Unfortunately, the ammonia formation rates were lower than those of Al2O3 and 5Ni/Al2O3 catalysts. The catalytic activity was compared with various parameters of oxides such as a relative dielectric constant [34] and a lattice constant, but no clear correlation could be found for explanation of the catalysis.
2.2. Activity and Active Sites of Ni/Al2O3 for Ammonia Synthesis
The catalytic activities of Al2O3(KC) and Ni/Al2O3(KC) were studied as a function of reaction time and amount of loaded nickel, and typical ammonia synthesis rates are summarized in Figure 3 and Figure 4 together with the activity of a Cu inner electrode alone (the experiment is called “a blank test” hereafter). As shown in Figure 3, the catalytic activity of the blank test slightly decreased with reaction time, although the reason for the change remained unknown. Additionally, the activity of the ammonia synthesis increased by using Al2O3 as the catalyst and further improved when Ni was supported on Al2O3. The activity of the Ni/Al2O3 catalyst changed with the amount of Ni loaded, the calcination temperature, and the reaction time. The activities of Ni/Al2O3(KC, 773) catalysts containing 0.50–20.0 wt% Ni showed characteristic changes with reaction time (Figure 4). When the amount of Ni loaded was small (0.50–1.00 wt%), the activity increased in the early stage of the reaction, reached the maximum activity, and then decreased. In contrast, the activities of 5.0–20.0 wt% Ni/Al2O3(KC, 773) were high in the initial stages and gradually decreased with the reaction time. Only 2.5 wt% Ni/Al2O3(KC, 773) showed almost constant activity. As will be shown in the following section, we proposed that these activity enhancements and reductions were caused by changes in the Ni state on the support by the plasma irradiation. The second important observation in Figure 4 is that the catalytic activity is almost independent of the amount of loaded Ni. We will suggest that a very small amount of Ni functions as a catalytically active site, and that the amount does not change with increasing Ni loading.
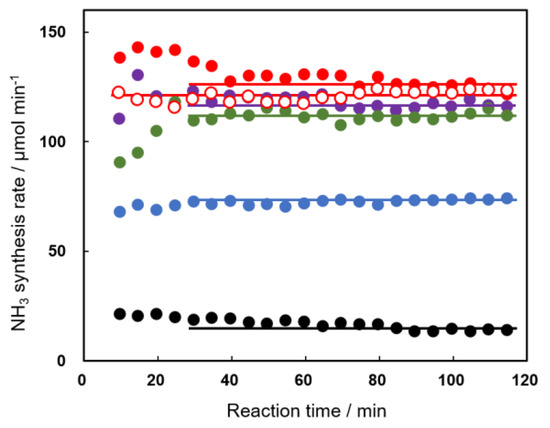
Figure 3.
Time courses for the catalytic activities of the copper rod (black), Al2O3(KC) (blue), and 5Ni/Al2O3(KC, 773) (closed red, the 1st run; open red, the 2nd run on the same catalyst), 5Ni/Al2O3(KC, 873) (purple), and 5Ni/Al2O3(KC, 1073) (green) for the plasma synthesis of ammonia. Reaction conditions were the same as those in Figure 1.
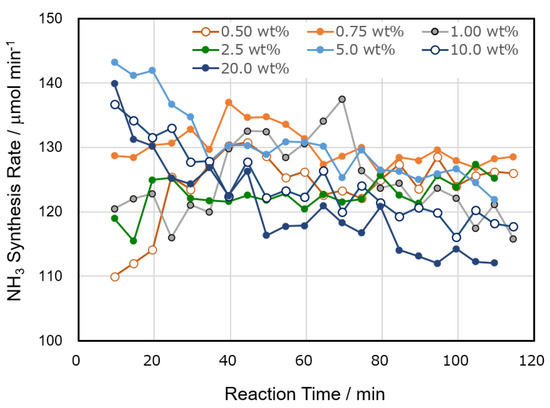
Figure 4.
Time courses for the catalytic activities of Al2O3-loaded Ni catalysts (KC, 773) for the plasma synthesis of ammonia. The amounts of loaded Ni are shown in the figure. Reaction conditions were the same as those in Figure 1.
Figure 3 summarizes the effect of calcination temperature, where a 5.0 wt% sample was employed for this study, since the characterization of Ni would become difficult when the amount of Ni supported was too small. Although the activity of 5Ni/Al2O3(KC, 773) decreased with the reaction time, as also shown in Figure 4, it was confirmed, in the second experiment (open red circles in Figure 3) on the catalyst once used, that the activity stabilized at approximately 120 µmol min−1 after a 2 h reaction. On 5Ni/Al2O3(KC, 873), the activity gradually decreased, although the degree of decrease was smaller than that on 5Ni/Al2O3(KC, 773). On the other hand, the behavior of 5Ni/Al2O3(KC, 1073) was essentially different from those of the above two catalysts. The activity increased at the initial stage, then stabilized after a reaction time of 30 min. It should be noted that the activities on the three catalysts nearly agreed at approximately 120 µmol min−1 at 120 min, which was similar to those observed in Figure 4.
These changes were attributed to changes in the nickel species, resulting from the calcination temperature and the reductive atmosphere of the plasma experiment. The X-ray diffraction patterns of Ni/Al2O3 [51,52,53,54,55,56] were measured before and after the reaction shown in Figure 3, and the results are summarized in Figure 5. XRD peaks of the γ-alumina carrier appeared at 2θ = 38, 45, and 67 degrees, assignable to the diffractions from (311), (400), and (440) faces, and the pattern was almost the same as that of 5Ni/Al2O3(KC, 773) before the plasma reaction (Figure 5a), although not shown in the figure for simplicity. Before the catalysts were used in the plasma reaction, diffraction peaks attributable to the NiO phase (2θ = 43 and 63 degrees) could hardly be observed as clear peaks on the three samples. The peaks of NiAl2O4 (2θ = 19.1, 31.4, 37.0, 45.0, 55.3, 59.7, and 65.5 degrees) were observed on the 873 K and 1073 K calcined samples. The findings were in good agreement with reports that the NiAl2O4 formation from NiO and Al2O3 begins upon calcination at 723 K [57] and becomes vigorous at 873 K and above. The respective colors of 5Ni/Al2O3 calcined at 773, 873, and 1073 K were pale greenish-gray, light sky blue, and pale blue; these corresponded well to the pale green-pale gray of NiO (depending on the oxygen content) and the pale blue of 5NiAl2O4 [58], again indicating the production of these oxides on alumina. Upon the plasma experiments, the XRD patterns and the sample colors were changed. On the used 5Ni/Al2O3(KC, 773), the formation of Ni metal particles was confirmed with the XRD peaks of 2θ = 44.5, 52, and 76 degrees [49,50], although their intensities were very small. The diffraction peaks originating from NiO disappeared at the same time. On the used 5Ni/Al2O3(KC, 873) and 5Ni/Al2O3(KC, 1073), the XRD peaks of metallic Ni did not appear irrespective of the disappearance of the NiO peaks, which could be due to the formation of small Ni particles. The sample colors were greatly changed. Black, gray, and grayish pale blue were the colors for the used 5Ni/Al2O3(KC, 773), 5Ni/Al2O3(KC, 873), and 5Ni/Al2O3(KC, 1073), respectively. The color changes supported the formation of metallic Ni (black), which was shown in the XRD experiments.
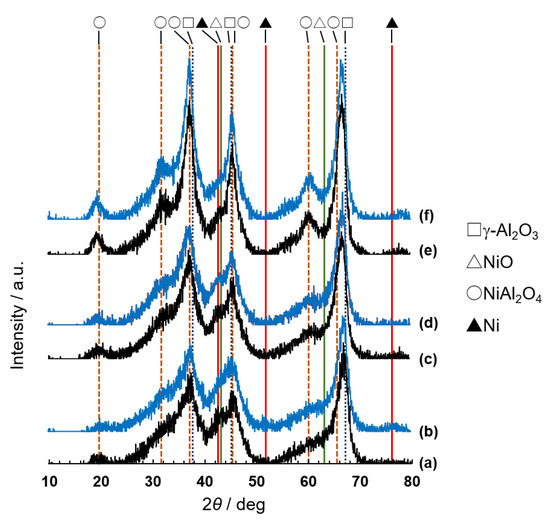
Figure 5.
XRD patterns of 5Ni/Al2O3(KC, 773) (a,b), 5Ni/Al2O3(KC, 873) (c,d), and 5Ni/Al2O3(KC, 1073) (e,f) before (black) and after (blue) the plasma experiments shown in Figure 3. The diffraction lines could be assigned to γ-Al2O3 (open square), NiO (open triangle), NiAl2O4 (open circle), and metallic Ni particles (closed triangle).
For the ammonia synthesis in the plasma atmosphere, the following active sites were suggested based on the above observations. The reduction of NiO to Ni occurred very easily with activated hydrogen molecules to form atomically dispersed Ni metal particles, the resultant very small Ni particles were converted to fine particles of Ni metal, and finally, the fine particles gathered slowly to form large particles. The fine particles were active for the ammonia synthesis, while large Ni particles were not. The low catalytic activity of large Ni particles was supported by the previous finding that wool-like Ni metal wires showed very low catalytic activity for the reaction [39]. With 5Ni/Al2O3(KC, 773), the formation of fine Ni fine particles [55] finished immediately after the plasma reaction started because of the facile reducibility of NiO. The prolonged reaction time, however, caused agglomeration of the Ni particles, which induced a decrease in the catalytic activity, as shown in Figure 3 and Figure 4. With 5Ni/Al2O3(KC, 1073), the NiO on the surface was reduced to Ni particles, but the number of the atomically dispersed particles was smaller than that on 5Ni/Al2O3(KC, 773) due to the limited amount of supported Ni, which was loaded as both NiO and NiAl2O4. Since the reduction of NiAl2O4 was difficult under the present reaction conditions [55,59], the number of fine Ni particles gradually increased during the reaction by moving of the atomically dispersed particles, which resulted in the increase in the catalytic activity for the first 30 min, as shown in Figure 3. After the 120 min reaction, the changes in catalytic activity on the three catalysts nearly disappeared, and they reached nearly the same conversion level. The similar changes in activities can be observed on the Ni/Al2O3(KC, 773) catalysts with low amounts of loaded Ni. As shown in Figure 4, the activities of catalysts with Ni contents of 0.50–1.00 wt% increased in the early stage of the reaction, reached the respective maximum values, and then decreased. The phenomena could be explained with the changes of Ni particles from very small ones to fine ones and then to large ones. In the present discussion, the active phase was assumed to be fine particles, but it might be particles with an amorphous structure.
The above discussion was supported by the XPS characterization of the catalysts. The XPS spectra of 5Ni/Al2O3(KC, 1073) are summarized in Figure 6 as typical examples. The Ni 2p3/2 peaks were reported to appear at 852, 854–855, and 856–857 eV together with satellite peaks [60,61,62,63,64], which could be attributed to Ni0 (metal), Ni2+ of NiO, and Ni2+ of NiAl2O4 (a spinel compound), respectively. With the present 5Ni/Al2O3(KC, 1073), three peaks were observed at 852.5, 855.0, and 856.1 eV before and after the reaction, as shown in Figure 6, indicating the formation of Ni metal, NiO, and NiAl2O4 species. The intensity ratios of the three peaks were approximately 0:37:63 before the reaction and 38:0:62 after the reaction. The findings indicated the easy and quantitative reduction of NiO to Ni0 and the difficult reduction of NiAl2O4, which were in good agreement with behaviors observed in reforming reactions [55,56]. With 5Ni/Al2O3(KC, 773), only NiO was observed before the plasma reaction, with nearly all of the species being reduced to Ni metal after the reaction. The XPS spectra of 5Ni/Al2O3(KC, 873) revealed a change in the ratio of Ni metal, NiO, and NiAl2O4 from 0:81:19 to 83:0:17 before and after the reaction. All findings supported the discussion in the previous section that Ni metal on the alumina support, produced by the reduction of NiO in the plasma reaction, would be active for the ammonia synthesis, although the XRD peaks attributable to Ni metal were not clearly recognized.
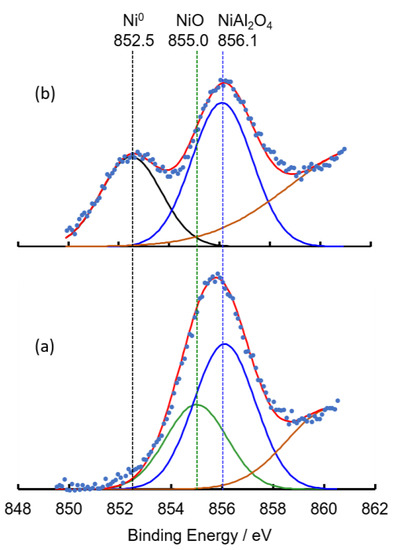
Figure 6.
XPS spectra of Ni 2p3/2 for 5Ni/Al2O3(KC, 1073) before (a) and after (b) the plasma experiments shown in Figure 3. The blue points are the measured values. The deconvolution showed the presence of NiO (green), NiAl2O4 (blue), and metallic Ni species (black) with a base line (brown) including satellite peaks. The red lines are the sums of the respective calculated lines.
2.3. Kinetic Analysis of Ammonia Synthesis on Ni/Al2O3
The ammonia synthesis rates and yields on Al2O3 and 5Ni/Al2O3(KC, 773) were examined as a function of the molar ratio of H2 and N2 (Figure 7). The synthesis rates were maximized at H2/N2 = 2 and 1 on Al2O3 and 5Ni/Al2O3, respectively, and reached 93 and 121 µmol min−1. The respective yields increased monotonously as the H2/N2 ratio increased due to a decrease in the concentration of the introduced N2. The yields reached 4.1% and 4.6% at H2/N2 = 3 (the stoichiometric ratio for ammonia synthesis) on Al2O3 and 5Ni/Al2O3, respectively. The synthesis rates and yields were greater than those observed in previous metal wire systems [38,39]. To investigate the dependencies on the individual concentrations in a gas-phase reaction, we usually used inert gases such as helium or argon to adjust the respective partial pressures. However, it has been widely reported for plasma reactions that these gases show the remarkable Penning effect, which largely changes the experimental results. Thus, we had to perform the experiments using a mixture of only N2 and H2 without any diluent. The correlation in Figure 7 was analyzed using the following kinetic equation, where the reaction rate was expressed using a power equation.
rNH3 = kPH2αPN2β
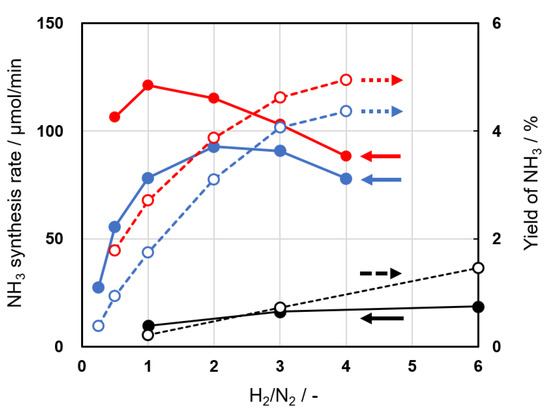
Figure 7.
Change in the synthesis rate (closed) and yield (open) of ammonia as a function of the N2/H2 ratio using Al2O3(SC) (blue) and 5Ni/Al2O3(KC, 773) (red) catalysts. The results of the blank experiment (black) are also shown for comparison. Reaction conditions: applied voltage, 6 kV; frequency, 50 kHz; electrode length, 150 mm; total flow rate, and 100 mL min−1.
A least-squares method was applied to determine the α and β values. α(Al2O3(SC)) = 1.59, β(Al2O3(SC)) = 0.82 and α(5Ni/Al2O3(KC, 773)) = 1.12, β(5Ni/Al2O3(KC, 773)) = 0.99 were obtained from the calculations. These values differed from those reported on well-known solid catalysts (α = 1.5–2.2 and β = 0.9–1.2 on Fe, and α = −0.43 and β = 1.0 on Ru-Cs/MgO) [65], and previously reported values of α = 0.77 and β = 1.16 on Cu-wool-like electrodes stabilized by the repetition of the plasma experiments [35]. The changes in the partial pressure dependencies with the catalysts were recognized, but a detailed discussion would require an indepth understanding of the reaction mechanisms. The positive values of the partial pressure of hydrogen on the Al2O3 and Ni/Al2O3 catalysts are significant for the practical application of the current method because the increase in the hydrogen partial pressure would not result in a decrease in the ammonia formation rate.
The production rate of ammonia was studied as a function of the residence time of the reactant gases. The residence time of the mixture in the reaction port was determined by V/F (min), where V is the volume of void space in the reaction port (mL), and F the total flow rate of the reactants (mL min−1). The results are summarized in Figure 8. The yield of NH3 increased as the residence time increased, and the rate of NH3 synthesis became slower for longer times. It should be noted that the degree of decrease of NH3 synthesis rate on Al2O3(SC) (Figure 8, closed blue) was much higher than that on 5Ni/Al2O3(KC, 773) (closed red), although the reason for the difference is not clear yet.
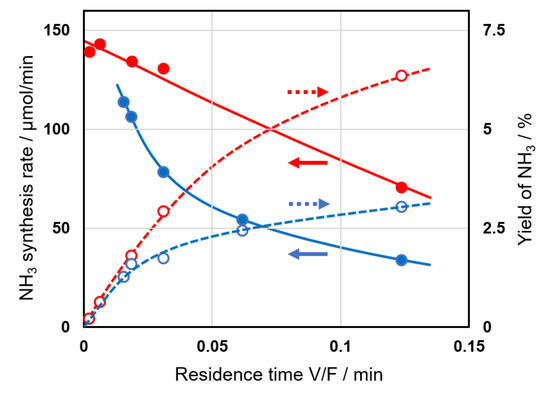
Figure 8.
Dependence of the synthesis rate (closed) and yield (open) of ammonia on the residence time (V/F) of the reactants gases using Al2O3(SC) (blue) and 5Ni/Al2O3(KC, 773) (red) catalysts. Reaction conditions: applied voltage, 6 kV; frequency, 50 kHz; electrode length, 25–150 mm; volume of reaction port (V), 0.515–3.09 mL (the volume was calculated based on the degree of void space (0.52) and the volume of catalyst bed); total flow rate (F), 25–200 mL min−1; H2/N2 = 1.
The correlation was analyzed using the following equations,
dx/dt = r0 − kdx
x = (r0/kd)(1 − exp(−kd t))
Here, we assumed that the formation rate on the catalyst was constant (r0 (min−1)) because the changes in the partial pressures of N2 and H2 were small before and after the reaction, and both pressures could be treated as being approximately constant. Additionally, the decomposition rate of ammonia was proportional to the rate constant (kd (min−1)) and the partial pressure of produced ammonia (x (-)). Equation (2) was thus formulated and converted to Equation (3) by integration. We applied a least-squares method to determine the parameters of Al2O3(SC) and 5Ni/Al2O3(KC, 773). r0 and kd were 0.73 and 8.4 min−1 for the former and 1.10 and 8.4 min−1 for the latter. We have already reported that the r0 and kd values of the stabilized Cu-wool catalyst were 0.268 and 8.9 min−1, respectively [38], which showed a significant increase in the r0 value and a nearly unchanged kd value from the values of 0.097 and 8.4 min−1 of the fresh Cu-wool catalyst [39]. The current values determined for Al2O3(SC) and 5Ni/Al2O3(KC, 773) indicated further increases in the r0 values and again unchanged kd values. As shown in Figure 8, the maximum ammonia yields were achieved at a residence time of 0.12 min within the current experimental conditions and were 3.0% and 6.3% on Al2O3(SC) and 5Ni/Al2O3(KC, 773).
3. Materials and Methods
3.1. Experimental Methods
A copper rod with an 8 mm diameter was employed as the inner electrode in the plasma experiments. The quartz tubular reactor and electrodes are shown in Figure S1 (in Supporting Information). The outer diameter and the thickness of the quartz tube were 12.7 mm and 1.0 mm, respectively. The outer side of the quartz reactor was surrounded by the outer electrode, which consisted of a copper net. The internal electrode was connected to a high-voltage power supply, and the outer electrode acted as a grounded electrode. Oxide catalysts were mounted between the quartz tube and the inner electrode, as shown in Figure S1B. The weight of the catalyst used in the experiment varied depending on the bulk density, and was approximately 2.64–3.85 g. All experiments were performed at atmospheric pressure without heating. A mixture of N2 and H2 was flowed into the reactor from the top of the reactor, and the exit gas was delivered to a dilute H2SO4 aqueous solution to gather the produced ammonia. Typical reaction conditions included an applied voltage of 6 kV and a frequency of 50 kHz for the reaction port (Figures S2 and S3), which was 150 mm in length. A mixture of H2/N2 = 1 was flowed into the reactor at a flow rate of 100 mL min−1, unless otherwise stated. It is important to note that the level of ammonia produced in the current reaction system somewhat varied with the reaction time. The average production rates and yields of ammonia at 60–120 min of reaction time were used to compare the catalytic activity of the respective catalysts. The ammonia yield and the production rate were calculated based on the supplied nitrogen molecules as follows:
Yield of ammonia (%) = 100 × conc. of NH3 produced (mol min−1)/2 × conc. of N2 supplied (mol min−1)
Production rate of ammonia (µmol min−1) = amount of ammonia produced in the predetermined time (µmol)/sampling interval (min)
3.2. Materials
Alumina samples, Al2O3, were commercially obtained from Strem Chemicals Inc. (Newburyport, MA., USA) (abbreviated as SC) or Kanto Chemical Co. (Tokyo, Japan) (KC), both of which were of γ-type. The former was in powder form and possessed a BET surface area of 200.1 m2g−1, and the latter was granular and exhibited 238.3 m2g−1. Although the surface areas of these substances were slightly different, they were used for the present experiments without distinction (SC and KC are shown in the sample names). Ni-, Fe-, and Ru-supported alumina samples were prepared by a conventional impregnation method using Ni(NO3)2·6H2O, Fe2(NO3)3·9H2O, and Ru3(CO)12, respectively. The metal loadings were 0.5–20.0 wt% metal. The granular Al2O3(KC) was ground into 0.3–0.6 mm particles before loading the active component. The alumina carriers, SC and KC, were dropped into the solutions and the metal species were supported by an impregnation method at 353 K. All catalysts were dried at 393 K overnight and calcined at 773–1073 K for 4 h in air. The calcination temperature is shown in the respective sample names (in Kelvin). After the calcination, the grain sizes of metal/Al2O3(SC) were adjusted to 0.3–0.6 mm for use in catalytic runs by compression molding. The compression-molded catalyst was occasionally powdered during the plasma experiment. The pulverization and uneven distribution of the catalyst in the reaction tube made the plasma experiment difficult. To avoid such annoying conditions, most of the experiments in this study were carried out using ground Al2O3(KC) as a carrier.
When the sample was filled in a reaction tube, the volume of voids between the catalyst particles was actually measured. The ratio of the void volume to the catalyst bed volume was 0.52, which was used for the calculation of the residence time of reactant gases.
3.3. Characterization of Catalysts
The structure of the prepared catalyst was confirmed by X-ray diffraction (XRD) analysis before and after the plasma experiment, in which a Rigaku Ultima IV X-ray diffractometer (Akishima, Tokyo, Japan) with Cu Kα (40 kV, 40 mA) radiation and a Ni filter was used. X-ray photoelectron spectroscopy (XPS) spectra of nickel were also measured to determine the chemical binding energies and valence states of nickel using a JEOL JPS-9010TR spectrometer (Akishima, Tokyo, Japan) (Mg Kα) before and after the plasma experiments. In the latter, the samples were transferred from the reaction port in the plasma reactor to the measurement chamber using a nitrogen box.
4. Conclusions
Alumina-supported nickel is a novel and very effective catalyst for ammonia synthesis using nonthermal atmospheric-pressure plasma. The yield of ammonia from H2 and N2 reached 6.3% without heating, which is the highest among the values examined at ambient temperature. A computational study indicated that the highest barrier for the ammonia decomposition on Ni was the combination of activated nitrogen atoms to form a dinitrogen molecule [38]. The current reaction is the reverse of the decomposition reaction, and the nitrogen molecule was activated by a plasma system and not by the catalyst surface, which would result in the good activity of Ni/Al2O3. The reaction mechanism, including the active species in the gas phase and on the surface, should be investigated in the near future. However, the current results pave the way for a new approach to ammonia synthesis that may be significant for future hydrogen applications.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/5/590/s1, Figure S1: Overall view and sectional view of the reactor, Figure S2: Waveforms of voltage and current, Figure S3: The Lissajous curves of V(t) and I(t) for the plasma reactions, Table S1: detailed data of Figure 1.
Author Contributions
Conceptualization, M.I.; reaction on Ni/Al2O3, M.H., T.S. and K.S.; reaction on metal oxides, R.H.; reaction on Ni/TiO2, H.T.; writing—original draft preparation, review and editing, M.I.; supervision, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS, METI, 17H03460) and the New Energy and Industrial Technology Development Organization (NEDO, MITI, 17100665-0).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hara, M.; Kitano, M.; Hosono, H. Ru-Loaded C12A7:e– Electride as a Catalyst for Ammonia Synthesis. ACS Catal. 2017, 7, 2313–2324. [Google Scholar] [CrossRef]
- Abe, H.; Niwa, Y.; Kitano, M.; Inoue, Y.; Sasase, M.; Nakao, T.; Tada, T.; Yokoyama, T.; Hara, M.; Hosono, H. Anchoring Bond Between Ru and N Atoms of Ru/Ca2NH Catalyst: Crucial for the High Ammonia Synthesis Activity. J. Phys. Chem. C 2017, 121, 20900–20904. [Google Scholar] [CrossRef]
- Kobayashi, V.; Kitano, M.; Kawamura, S.; Yokoyama, T.; Hosono, H. Kinetic evidence: The rate-determining step for ammonia synthesis over electride-supported Ru catalysts is no longer the nitrogen dissociation step. Catal. Sci. Technol. 2017, 7, 47–50. [Google Scholar] [CrossRef]
- Li, J.; Kitano, M.; Ye, T.; Sasase, M.; Yokoyama, T.; Hosono, H. Chlorine-Tolerant Ruthenium Catalyst Derived Using the Unique Anion-Exchange Properties of 12CaO·7Al2O3 for Ammonia Synthesis. ChemCatChem 2017, 9, 3078–3083. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Sasase, M.; Kishida, K.; Kobayashi, Y.; Nishiyama, K.; Tada, T.; Kawamura, S.; Yokoyama, T.; Hara, M.; et al. Self-Organized Ruthenium-Barium Core-Shell Nanoparticles on a Mesoporous Calcium Amide Matrix for Efficient Low-Temperature Ammonia Synthesis. Angew. Chem. Int. Ed. 2018, 57, 2648–2652. [Google Scholar] [CrossRef]
- Ogo, S.; Sekine, Y. Catalytic Reaction Assisted by Plasma or Electric Field. Chem. Rec. 2017, 17, 726–738. [Google Scholar] [CrossRef]
- Murakami, K.; Manabe, R.; Nakatsubo, H.; Yabe, T.; Ogo, S.; Sekine, Y. Elucidation of the role of electric field on low temperature ammonia synthesis using isotopes. Catal. Today 2018, 303, 271–275. [Google Scholar] [CrossRef]
- Manabe, R.; Nakatsubo, H.; Gondo, A.; Murakami, K.; Ogo, S.; Tsuneki, H.; Ikeda, M.; Ishikawa, A.; Nakai, H.; Sekine, Y. Electrocatalytic synthesis of ammonia by surface proton hopping. Chem. Sci. 2017, 8, 5434–5439. [Google Scholar] [CrossRef]
- Ni, J.; Jing, B.; Lin, J.; Lin, B.; Zhao, Z.; Jiang, L. Effect of rare earth on the performance of Ru/MgAl-LDO catalysts for ammonia synthesis. J. Rare Earths 2018, 36, 135–141. [Google Scholar] [CrossRef]
- Ogura, Y.; Sato, K.; Miyahara, S.I.; Kawano, Y.; Toriyama, T.; Yamamoto, T.; Matsumura, S.; Hosokawa, S.; Nagaoka, K. Efficient ammonia synthesis over a Ru/La0.5Ce0.5O1.75 catalyst pre-reduced at high temperature. Chem. Sci. 2018, 9, 2230–2237. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, S.; Xiong, X.; Hu, B.; Song, C. Effect of Graphitic Carbon Nitride on the Electronic and Catalytic Properties of Ru Nanoparticles for Ammonia Synthesis. Catal. Lett. 2016, 146, 2324–2329. [Google Scholar] [CrossRef]
- Yandulov, D.V.; Schrock, R.R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 2003, 301, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Hu, S.; Luo, G.; Kang, X.; Luo, Y.; Hou, Z. Dinitrogen cleavage and hydrogenation by a trinuclear titanium polyhydride complex. Science 2013, 340, 1549–1952. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Nishibayashi, Y. Developing more sustainable processes for ammonia synthesis. Coord. Chem. Rev. 2013, 257, 2551–2564. [Google Scholar] [CrossRef]
- Tanaka, H.; Nishibayashi, Y.; Yoshizawa, K. Interplay between Theory and Experiment for Ammonia Synthesis Catalyzed by Transition Metal Complexes. Acc. Chem. Res. 2016, 49, 987–995. [Google Scholar] [CrossRef]
- Kuriyama, S.; Arashiba, K.; Nakajima, K.; Matsuo, Y.; Tanaka, H.; Ishii, K.; Yoshizawa, K.; Nishibayashi, Y. Catalytic transformation of dinitrogen into ammonia and hydrazine by iron-dinitrogen complexes bearing pincer ligand. Nat. Commun. 2016, 7, 12181. [Google Scholar] [CrossRef]
- Amar, I.A.; Lan, R.; Petit, C.T.G.; Tao, S. Solid-state electrochemical synthesis of ammonia: A review. J. Solid State Electrochem. 2011, 15, 1845–1860. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; Fitzpatrick, P. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process. 2015, 90, 24–33. [Google Scholar] [CrossRef]
- Khoenkhoen, N.; de Bruin, B.; Reek, J.N.H.; Dzik, W.I. Reactivity of Dinitrogen Bound to Mid- and Late-Transition-Metal Centers. Eur. J. Inorg. Chem. 2015, 2015, 567–598. [Google Scholar] [CrossRef]
- Saadatjou, N.; Jafari, A.; Sahebdelfar, S. Ruthenium Nanocatalysts for Ammonia Synthesis: A Review. Chem. Eng. Commun. 2015, 202, 420–448. [Google Scholar] [CrossRef]
- Klinsrisuk, S.; Irvine, J.T.S. Electrocatalytic ammonia synthesis via a proton conducting oxide cell with BaCe0.5Zr0.3Y0.16Zn0.04O3-δ electrolyte membrane. Catal. Today 2017, 286, 41–50. [Google Scholar] [CrossRef]
- Kosaka, F.; Noda, N.; Nakamura, T.; Otomo, J. In situ formation of Ru nanoparticles on La1-xSrxTiO3-based mixed conducting electrodes and their application in electrochemical synthesis of ammonia using a proton-conducting solid electrolyte. J. Mater. Sci. 2017, 52, 2825–2835. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Z.; Bai, X.; Bai, M.; Ning, W. Plasma synthesis of ammonia with a microgap dielectric barrier discharge at ambient pressure. IEEE Trans. Plasma Sci. 2003, 31, 1285–1291. [Google Scholar] [CrossRef]
- Neyts, E.C. Plasma-Surface Interactions in Plasma Catalysis. Plasma Chem. Plasma Process. 2016, 36, 185–212. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.A. Bogaerts, Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef]
- Whitehead, J.C. Plasma-catalysis: The known knowns, the known unknowns and the unknown unknowns. J. Phys. D Appl. Phys. 2016, 49, 243001. [Google Scholar] [CrossRef]
- Nakajima, J.; Sekiguchi, H. Synthesis of ammonia using microwave discharge at atmospheric pressure. Thin Solid Films 2008, 516, 4446–4451. [Google Scholar] [CrossRef]
- Uyama, H.; Matsumoto, O. Synthesis of ammonia in high-frequency discharges. Plasma Chem. Plasma Process. 1989, 9, 13–24. [Google Scholar] [CrossRef]
- Uyama, H.; Matsumoto, O. Synthesis of ammonia in high-frequency discharges. II. Synthesis of ammonia in a microwave discharge under various conditions. Plasma Chem. Plasma Process. 1989, 9, 421–432. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Z.; Bai, M.; Bai, X.; Gao, H. Synthesis of ammonia using CH4/N2 plasmas based on micro-gap discharge under environmentally friendly condition. Plasma Chem. Plasma Process. 2008, 28, 405–414. [Google Scholar] [CrossRef]
- Peng, P.; Cheng, Y.; Hatzenbeller, R.; Addy, M.; Zhou, N.; Schiappacasse, C.; Chen, D.; Zhang, Y.; Anderson, E.; Liu, Y.; et al. Ru-based multifunctional mesoporous catalyst for low-pressure and non-thermal plasma synthesis of ammonia. Int. J. Hydrogen Energy 2017, 42, 19056–19066. [Google Scholar] [CrossRef]
- van Helden, J.H.; Wagemans, W.; Yagci, G.; Zijlmans, R.A.B.; Schram, D.C.; Engeln, R.A.H.; Lombardi, G.; Stancu, D.; Ropcke, J. Detailed study of the plasma-activated catalytic generation of ammonia in N2-H2 plasmas. J. Appl. Phys. 2007, 101, 043305. [Google Scholar] [CrossRef]
- Mizushima, T.; Mastumoto, K.; Ohkita, H.; Kakuta, N. Catalytic effects of metal-loaded membrane-like alumina tubes on ammonia synthesis in atmospheric pressure plasma by dielectric barrier discharge. Plasma Chem. Plasma Process. 2007, 27, 1–11. [Google Scholar] [CrossRef]
- Gomez-Ramirez, A.; Cotrino, J.; Lambert, R.M.; Conzalez-Elipe, A.R. Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor. Plasma Sources Sci. Technol. 2015, 24, 06501. [Google Scholar] [CrossRef]
- Gomez-Ramirez, A.; Montoro-Damas, A.; Cotrino, J.; Lambert, R.M.; Conzalez-Elipe, A.R. About the enhancement of chemical yield during the atmospheric plasma synthesis of ammonia in a ferroelectric packed bet reactor. Plasma Process. Polym. 2017, 14, e1600081. [Google Scholar] [CrossRef]
- Kim, H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Plasma Catalysis for Environmental Treatment and Energy Applications. Plasma Chem Plasma Process 2016, 36, 45–72. [Google Scholar] [CrossRef]
- Aihara, K.; Akiyama, M.; Deguchi, T.; Tanaka, M.; Hagiwara, R.; Iwamoto, M. Remarkable catalysis of a wool-like copper electrode for NH3 synthesis from N2 and H2 in non-thermal atmospheric plasma. Chem. Commun. 2016, 52, 13560–13563. [Google Scholar] [CrossRef]
- Iwamoto, M.; Akiyama, M.; Aihara, K.; Deguchi, T. Ammonia Synthesis on Wool-Like Au, Pt, Pd, Ag, or Cu Electrode Catalysts in Nonthermal Atmospheric-Pressure Plasma of N2 and H2. ACS Catal. 2017, 7, 6924–6929. [Google Scholar] [CrossRef]
- Muroyama, H.; Saburi, C.; Matsui, T.; Eguchi, K. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Appl. Catal. A Gen. 2012, 443–444, 119–124. [Google Scholar] [CrossRef]
- Duan, X.; Qian, G.; Liu, Y.; Ji, J.; Zhou, X.; Chen, D.; Yuan, W. Structure sensitivity of ammonia decomposition over Ni catalysts: A computational and experimental study. Fuel Process. Technol. 2013, 108, 112–117. [Google Scholar] [CrossRef]
- Sato, K.; Abe, N.; Kawagoe, T.; Miyahara, S.; Honda, K.; Nagaoka, K. Supported Ni catalysts prepared from hydrotalcite-like compounds for the production of hydrogen by ammonia decomposition. Int. J. Hydrogen Energy 2017, 42, 6610–6617. [Google Scholar] [CrossRef]
- Polanski, J.; Bartczak1, P.; Ambrozkiewicz1, W.; Sitko1, R.; Siudyga, T.; Mianowski, A.; Szade, J.; Balin, K.; Leltko, J. Ni-Supported Pd Nanoparticles with Ca Promoter: A New Catalyst for Low-Temperature Ammonia Cracking. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, F.; Shao, J.; Dai, Y.; Ding, J.; Tang, Z. Attapulgite clay supported Ni nanoparticles encapsulated by porous silica: Thermally stable catalysts for ammonia decomposition to COx free hydrogen. Int. J. Hydrogen Energy 2016, 41, 21157–21165. [Google Scholar] [CrossRef]
- Atsumi, R.; Noda, R.; Takagi, H.; Vecchione, L.; Carlo, A.D.; Del Prete, Z.; Kuramoto, K. Effects of Steam on Ni/Al2O3 Catalysts for Ammonia Decomposition. Ind. Eng. Chem. Res. 2014, 53, 17849–17853. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, J.; Ge, Q.; Xu, H.; Li, W. Effects of CeO2 addition on Ni/Al2O3 catalysts for the reaction of ammonia decomposition to hydrogen. Appl. Catal. B Environ. 2008, 80, 98–105. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J.; Mckay, D. A comparison of the reactivity of lattice nitrogen in Co3Mo3N and Ni2Mo3N catalysts. J. Mol. Catal. A Chem. 2009, 305, 125–129. [Google Scholar] [CrossRef]
- Taylor, D.W.; Smith, P.J.; Dowdenc, D.A.; Kemball, C.; Whan, D.A. Ammonia synthesis and related reactions over iron-cobalt and iron-nickel alloy catalysts. Part I. Catalysts reduced at 853 K. Appl. Catal. 1982, 3, 161–176. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H. Novel class of ammonia synthesis catalysts. Chem. Commun. 2000, 1057–1058. [Google Scholar] [CrossRef]
- Abghoui, Y.; Skulason, E. Computational Predictions of Catalytic Activity of Zincblende (110) Surfaces of Metal Nitrides for Electrochemical Ammonia Synthesis. J. Phys. Chem. C 2017, 121, 6141–6151. [Google Scholar] [CrossRef]
- Ouyang, B.; Zhang, Y.; Zhang, Z.; Fan, H.J.; Rawat, R.S. Nitrogen-Plasma-Activated Hierarchical Nickel Nitride Nanocorals for Energy Applications. Small 2017, 13, 1604265. [Google Scholar] [CrossRef] [PubMed]
- Spreafico, C.; Karim, W.; Ekinci, Y.; van Bokhoven, J.A.; VandeVondele, J. Hydrogen Adsorption on Nanosized Platinum and Dynamics of Spillover onto Alumina and Titania. J. Phys. Chem. C 2017, 121, 17862–17872. [Google Scholar] [CrossRef]
- Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; VandeVondele, J.; Ekinci, Y.; van Bokhoven, J.A. Catalyst support effects on hydrogen spillover. Nature 2017, 541, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, L.; Hill, J.M. Comparison of reducibility and stability of alumina-supported Ni catalysts prepared by impregnation and co-precipitation. Appl. Catal. A Gen. 2006, 301, 16–24. [Google Scholar] [CrossRef]
- Akande, A.J.; Idem, R.O.; Dalai, A.K. Synthesis, characterization and performance evaluation of Ni/Al2O3 catalysts for reforming of crude ethanol for hydrogen production. Appl. Catal. A Gen. 2005, 287, 159–175. [Google Scholar] [CrossRef]
- Ragupathi, C.; Bijaya, J.J.; Surendhar, P.; Kennedy, L.J. Comparative investigation of nickel aluminate (NiAl2O4) nano and microstructures for the structural, optical and catalytic properties. Polyhedron 2014, 72, 1–7. [Google Scholar] [CrossRef]
- El-Shobaky, G.A.; Al-Noaimi, A.N.; Saber, T.M.H. Structure and catalytic activity of nickel oxide/alumina (NiO/Al2O3) prepared by impregnation or coprecipitation. Bull. Soc. Chim. France 1987, 930–934, CODEN BSCFAS. [Google Scholar]
- Jeevanandam, P.; Koltypin, Y.; Gedanken, A. Preparation of nanosized nickel aluminate spinel by a sonochemical method. Mater. Sci. Eng. B 2002, 90, 125–132. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, T.; Zhang, P.; Qi, R.; Huang, R.; Song, X.; Gao, L. Facile synthesis of hollow hierarchical Ni/γ-Al2O3 nanocomposites for methane dry reforming catalysis. RSC Adv. 2014, 4, 51184–51193. [Google Scholar] [CrossRef]
- Inoue, H.; Hatanaka, N.; Kidena, K.; Murata, S.; Nomura, M. Reforming of Methane with Carbon Dioxide over Nickel-loaded Zeolite Catalysts. J. Jpn. Petroleum Inst. 2002, 45, 314–320. [Google Scholar] [CrossRef][Green Version]
- Xiang, L.; Gong, Y.L.; Li, J.C.; Wang, Z.W. Influence of hydrothermal modification on the properties of Ni/Al2O3 catalyst. Appl. Surf. Sci. 2004, 239, 94–100. [Google Scholar] [CrossRef]
- Al-Ubaid, A.; Wolf, E.E. Steam reforming of methane on reduced non-stoichiometric nickel aluminate catalysts. Appl. Catal. 1988, 40, 73–85. [Google Scholar] [CrossRef]
- Salagre, P.; Fierro, J.L.G.; Medina, F.; Sueiras, J.E. Characterization of nickel species on several γ-alumina supported nickel samples. J. Mol. Catal. A Chem. 1996, 106, 125–134. [Google Scholar] [CrossRef]
- Zhang, L.; Shu, X.; Zhang, L. Influence of Ni Loading on Catalytic Activity of NiO/γ-Al2O3 for Hydrogenation of Coal Pyrolysis. Asian J. Chem. 2013, 25, 5071–5075. [Google Scholar] [CrossRef]
- Hagen, S.; Barfod, R.; Fehrmann, R.; Jacobsen, C.J.H.; Teunis-sen, H.T.; Chorkendorff, I. Ammonia synthesis with barium-promoted iron–cobalt alloys supported on carbon. J. Catal. 2003, 214, 327–335. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).