Abstract
Under the acyclic diene metathesis (ADMET) reaction condition, the C3-vinyl groups of cinchona alkaloids readily react with each other to form a C-C bond. A novel type of cinchona alkaloid polymers was synthesized from dimeric cinchona squaramides using the Hoveyda-Grubbs’ second-generation catalysts (HG2) by means of ADMET reaction. The chiral polymers, containing cinchona squaramide moieties in their main chains, were subsequently employed as catalysts for the enantioselective Michael reaction to give the corresponding chiral adducts in high yields with excellent enantioselectivity and diastereoselectivity. Both enantiomers from the asymmetric Michael reaction were distinctively prepared while using the polymeric catalysts, possessing pseudoenantiomeric structures. The catalysts were readily recovered from the reaction mixture and recycled several times due to the insolubility of the cinchona-based squaramide polymers.
1. Introduction
Polymeric catalysts have been widely used in organic syntheses. Their easy recoverability, applicability to continuous flow systems, and simple modification and control of their microenvironment have attracted significant attention [1,2]. Chiral catalysts, for asymmetric syntheses, require the precise control of the reaction microenvironment to optimize the catalyst performance. Cinchona alkaloid and its derivatives have demonstrated their splendid catalytic activity in many kinds of enantioselective reactions. The polymeric version of the cinchona alkaloid compounds, as catalysts, has been highly desired. We prepared various chiral polymeric catalysts, possessing cinchona alkaloid moieties in their main chains, by various kinds of polymerization techniques, such as etherification [3], ion-exchange [4,5], and neutralization [6,7]. These optically active polymeric catalysts have been employed in enantioselective reactions, including C-C bond forming reactions. The utilization of the chiral main-chain polymeric catalysts confirmed their splendid catalytic activity in enantioselective reactions. In several cases, superior ability in stereoselections was achieved with the optically active main-chain polymeric catalysts. From these results, the catalytic moiety should have reasonable conformation in the optically active polymer main-chain. These results also facilitated the development of novel optically active polymeric catalyst. We recently initiated the application of Mizoroki–Heck (MH) coupling reaction to polymer synthesis of cinchona alkaloid derivatives [8,9,10]. The preparation of optically active polymeric catalysts requires the chiral functionalities to act as a catalytic active site, which must be maintained during and after the polymer synthesis. A chiral monomer requires extra functionalities for a polymerization that is not associated with catalytically active functionalities. The polymerization reaction conditions must be tolerant to these active functional groups. Moreover, the MH reaction fulfills these requirements. The copolymerization of the dimers [11] of cinchona alkaloid derivatives and aromatic diiodide in the presence of a Pd catalyst produced chiral polymers in good yield. This polymerization effectively utilized the C3-vinyl group of the cinchona alkaloid compounds.
The acyclic diene metathesis (ADMET) polymerization is another possible polymerization that utilizes the C3-vinyl group of cinchona alkaloid. Dimers of cinchona squaraminde derivatives [8,10] possess vinyl groups in both cinchona moieties and they are considered to be optically active diene. ADMET polymerization does not require other monomers, such as 1,4-diiodobenzene, which are required in MH polymerization. Since the dimers of cinchona alkaloid always contain two vinyl groups, they can be polymerized directly under the condition of ADMET reaction. The concept of ADMET has been discussed in scientific literature since the 1970s [12,13,14]. The first successful ADMET polymerization was reported by Wagner’s group in 1987. ADMET polymerization is highly tolerant to various functionalities and it proceeds under mild polymerization conditions [3]. In 1991, Wagner et al. reported the reaction mechanism of the ADMET polymerization [15]. This polymerization method can be used to give a different kinds of polymers and polymer architectures that cannot be accomplished with other polymerization methods. Different kinds of polymers, such as unsaturated poly[carbo(dimethyl)silanes] [16], unsaturated polyethers [17,18,19], hydrocarbons [20], unsaturated polycarbonates [21], unsaturated polyamines [22], unsaturated polyacetals [23], organoboronates [24], carbosilane-/carbosiloxane-based homopolymers/copolymers [25], and unsaturated polyesters [26] were prepared by the Wagener group. Most of the polymers, which were synthesized using ADMET polymerization, were optically inactive polymers. One exception is the chiral polymers synthesized from -amino acid derivatives [27]. The ADMET polymerization technique has not been adopted in the synthesis of optically active polymers derived from cinchona alkaloid until our recent publication of preliminary results of the polymerization [28]. Cinchona alkaloids and their derivatives are widely employed for the preparation of different types of catalysts, and their most important application is as chiral organocatalysts in asymmetric reactions [29,30,31,32,33]. Since cinchona alkaloids exhibit various functionalities, such as a quinuclidine tertiary N2, secondary alcohol, a quinoline ring, and a vinylic unit, it was feasible to modify their functionalities for the design of novel polymeric catalysts [34,35,36]. Rawal et al. successfully introduced cinchona squaramide derivatives as important organocatalysts by their pioneering work [37], and exhibited outstanding catalytic performance for enantioselective C-C bond forming reaction, such as the Michael-type reactions [38,39]. The cinchona-based squaramides contain an acidic NH that can be a hydrogen bond donor, and the quinuclidine tertiary nitrogen could also be a hydrogen bond acceptor and a base in enantioselective Michael reactions. A number of modifications of the catalyst have been reported after Rawal’s group initiated the excellent work on cinchona alkaloid squaramide organocatalysts. Various catalysts containing cinchona alkaloid squaramide have been developed and used for different types of asymmetric reactions [40,41,42,43,44,45,46, 47, 48].
In this paper, we describe the ADMET polymerization of cinchona squaramide dimers using the Hoveyda–Grubbs second-generation catalysts to prepare novel optically active polymeric catalysts. We applied these optically active polymeric catalysts for the enantioselective Michael type reactions to examine the catalytic performance and stereoselectivity. The enantioselective Michael reaction is one of the most important C-C bond forming reactions for the construction of chiral building blocks. Because the resulting ADMET polymeric organocatalysts were insoluble in some generally utilized organic solvents, we also examined their recyclability.
2. Results and Discussion
Synthesis of Dimeric Cinchona Squaramides and Their Corresponding Polymers by ADMET Polymerization
Cinchona-based squaramide dimers (1–3) (Figure 1) were prepared from dimethylsquarate and the C9-amino substituted cinchona alkaloids according to the literature procedures [8]. The C9-amino derivatives were synthesized by the Mitsunobu-type azide formation, followed by the Staudinger reaction according to the previously reported procedure [37,45]. Dimers 1 (a cinchonine-derived catalyst), 2C (a cinchonidine-derived catalyst), and 2Q (a quinine-derived catalyst) were successfully prepared. Another dimeric cinchona squaramide (3), which contained a chiral diamine moiety between two squaramides, was also synthesized [10]. The treatment of the two equivalents of dimethylsquarate with (1R,2R)-1,2-diamino-1,2-diphenylethane afforded the bissquaramide. Subsequently, the C9-aminated quinine could react with the chiral bissquaramide to afford 3 in good yield.
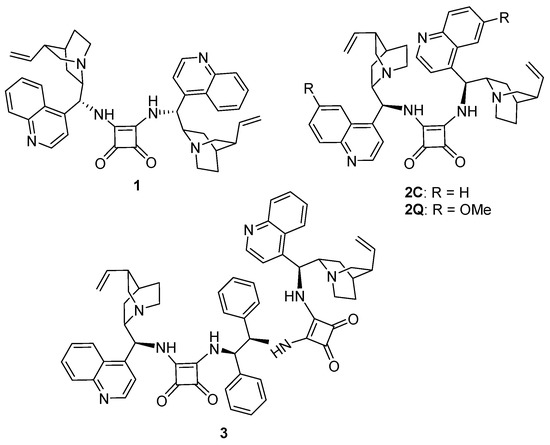
Figure 1.
Dimeric cinchona squaramides 1–3.
The dimeric cinchona squaramides that are shown in Figure 1 possess two olefinic double bonds in their structures. These squaramides are chiral dienes and they amenable to ADMET polymerization. A variety of diene compounds has been polymerized under the ADMET reaction condition to afford the polymers [15,16,17,18,19,20,21,22]. We synthesized the chiral polymers by repetitive ADMET reactions of 1–3. The ADMET polymerization of 1–3 with catalyst HG2A readily proceeded in dry DMF, affording chiral polymers P1–3, respectively, which contained cinchona squaramides in repeated units, in their main chains (Scheme 1). Further, the copolymerization of 2C and achiral triallyl ether (4) was also performed to prepare a hyperbranched polymer (P4). After the copolymerization, the reaction mixture was poured into ether to precipitate the product polymer. P1–3 were soluble in dichloromethane (DCM), methanol (MeOH), CDCl3, as well as in highly polar solvents, such as DMF and DMSO. P4 was slightly soluble in these solvents. All of the polymers were insoluble in generally employed organic solvents, including ether, tetrahydrofuran (THF), hexane, toluene, acetonitrile, and EtOAc.
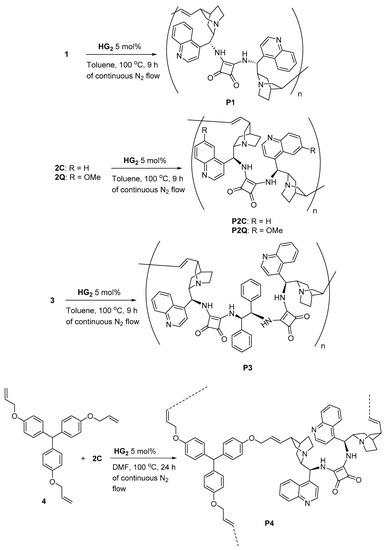
Scheme 1.
Synthesis of P1–P4 from 1–4, using HG2.
Table 1 summarizes the results of the ADMET polymerizations of different cinchona squaramides with HG2. First, we examined the condition for the polymerization of the dimeric cinchona squaramides. Different reaction conditions were examined while using 2Q, as shown in Table 1. In DMF, 2Q was polymerized using catalyst HG2A (5 mol%) to give the quinine-derived polymer (P2Q) with 81% yield (entry 1). It was also observed that a low concentration of 2Q afforded a low yield of P2Q (entry 2). Moreover, HG2A achieved better results than HG2B (Figure 2). We observed that the ADMET polymerization of 2Q proceeded readily in toluene at 100 °C. After 9 h stirring at the same temperature, P2Q was generated with a 92% isolated yield (entry 6). Polymerization at 85 °C caused a lowering of the yield in prolonged reaction time (entry 7). Other dimeric cinchona squaramides were also polymerized to afford their corresponding chiral ADMET polymers under the optimized condition for the polymerization of 2Q (entries 8–11). All of the polymers obtained by ADMET polymerization possessed Mn > 40,000, except for P3.

Table 1.
Synthesis of P1–P4 by acyclic diene metathesis (ADMET) polymerization a.
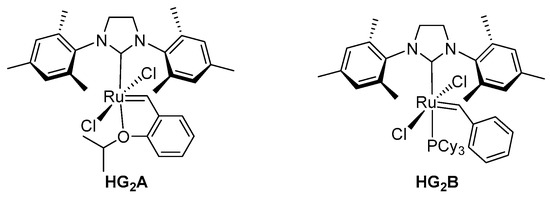
Figure 2.
Hoveyda-Grubbs 2nd generation catalysts (HG2).
The enantioselective Michael addition reactions between 5 and 6 (Scheme 2) were investigated to evaluate the catalytic activity of the resulting cinchona squaramide polymers. Table 2 illustrates the results of the enantioselective reaction. The reaction between 5 and 6 smoothly occurred with the low-molecular-weight catalyst (1) in MeOH to afford the product (7) with 91% yield and 93% enantiomeric excess (ee) of the major diastereomers (7a and c) (Table 2, entry 1). The corresponding P1 successfully catalyzed the reaction in the same solvent to yield 6 in good yield and high stereoselectivities (entry 2). The solvent effect on the catalytic performance of P1 was also investigated. MeOH, acetonitrile, THF, EtOAc, hexane, dichloromethane, ether, hexane, and toluene were employed, because they are generally used organic solvents. Among these solvents, P1 was partially soluble in MeOH, DCM, and CDCl3. P1 was insoluble in the other solvents utilized and the reaction proceeded in heterogeneous condition. However, P1 was swollen well in these solvents. Highly catalytic activity was obtained, even in heterogeneous condition. In DCM, its diastereoselectivity improved and higher enantioselectivity (97% ee) for the major diastereomer was achieved using (entry 3). Under a heterogeneous condition, in THF, the asymmetric reaction readily proceeded to afford the chiral product with high enantioselectivity (entry 4). Relatively prolonged reaction times were required for the reaction in EtOAc, ether, toluene, and hexane. However, high ees were obtained in all cases (entries 5–8). Toluene afforded poor isolated yield and decreased in diastereoselectivity with P1 (entry 7). When CH3CN was used as a solvent for the enantioselective reaction, the reaction was completed in 9 h at room temperature (entry 9). Very high diastereoselectivity (1:>100) was achieved with P1 in acetonitrile. In this case, the ee of the major diastereomers was 95%. The temperature effect on the catalyst performance in acetonitrile was also investigated. The reaction was performed in the range of −10 to 80 °C (entries 9–12) to see the temperature effect on the stereoselectivity. The diastereoselectivity decreased with increasing temperature. Interestingly, the high ee of 95% was still achieved at 80 °C.
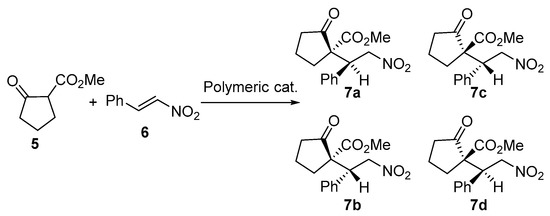
Scheme 2.
Asymmetric Michael addition of 5 and 6 to yield 7a–d.

Table 2.
Asymmetric Michael addition of 5 and 6 using the ADMET polymeric catalyst a.
Cinchonine and cinchonidine are pseudoenantiomers. When 2C was employed, the major products of the asymmetric Michael addition were 7b and 7d (entry 13). The corresponding polymeric catalyst P2C also afforded the same isomers (entry 14). Quinine possesses the same configuration as cinchonidine. 2Q and its corresponding polymeric catalyst (P2Q) catalyzed the reaction in the same direction of stereo selection (entries 15 and 16). We introduced a chiral diamine moiety (C9-aminated quinine) between cinchonidine squaramide (chiral bissquaramide) to afford 3, which was thereafter polymerized under an ADMET condition to produce P3. The same asymmetric reaction was observed with P3 as a catalyst. When compared to P2C, somewhat higher diastereoselectivity was achieved with P3 (entry 17). Another chiral polymer (P4) was prepared by the copolymerization of 3 and 4. Although the diastereoselectivity decreased, an excellent ee was achieved with P4 (entry 18).
The asymmetric reactions proceeded in a heterogeneous system since the chiral ADMET polymers were insoluble in most of the generally utilized organic solvents. The utilization of P1 facilitated the easy separation of the catalyst from the reaction mixture by filtration. The recyclability test was, therefore, performed with P1 in acetonitrile at room temperature. At the end of the reaction, the polymer was readily separated from the reaction mixture and washed with ether three times. The recovered polymer was employed in subsequent reactions. Although the third and fourth reuse cycles exhibited lower ee (Table 3, entries 3 and 4) than the first and second, the catalytic performance of P1 was maintained for several cycles.

Table 3.
Recycling of P1 a.
Finally, we applied P1 as a polymeric organocatalyst in the asymmetric Michael addition of various kinds of substrates. The reaction between ethyl 2-oxocyclopentanecarboxylate and 6 proceeded readily in acetonitrile to afford the corresponding asymmetric product 8 in good yield and excellent diastereoselectivity of >100:1, dr, and high ee (Scheme 3). Further, 6 was allowed to react with two other Michael donors, including pentane-2,4-dione and anthrone. In the case of anthrone, product 10 was obtained with low enantiopurity. The Michael donor (5) readily reacted with 4-fluoro-trans-β-nitrostyrene, 4-methyl-trans-β-nitrostyrene, and (2-nitrovinyl)thiophene in the presence of P1 to afford the Michael adducts (11, 12, and 13) with good yields and high ees. The stereoselectivity of the chiral product that was obtained from methyl-trans-β-nitrostyrene was not determined due to the incomplete separation of the products by chiral HPLC analysis.
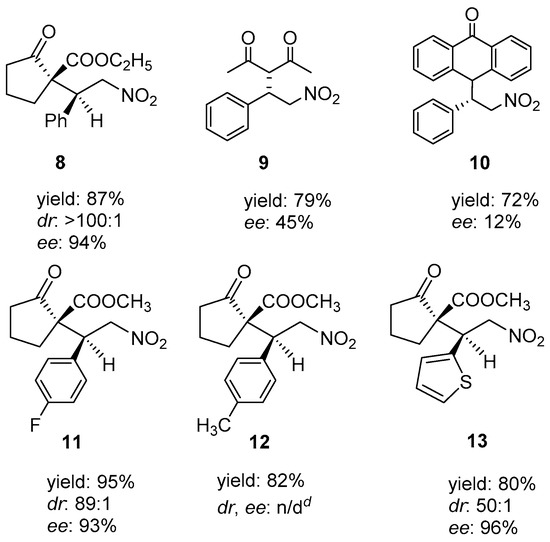
Scheme 3.
Asymmetric Michael addition reaction involving different combinations of the Michael donors and acceptors, using P1. Reactions were conducted with the Michael donor (0.50 mmol), Michael acceptor (0.55 mmol), and P1 (5 mol%) in acetonitrile (2.5 mL) at 25 °C. Isolated yield of the product after purification by column chromatography. dr and ee were determined by chiral HPLC (Chiralcel OD-H) for compounds 8, 11–13; Chiralcel AD-H for compound 9 and Chiralcel AS-H for compound 10. d dr and ee were not determined due to the incomplete separation of the products by chiral HPLC.
3. Materials and Methods
3.1. Materials and General Considerations
All of the solvents and reagents were purchased from Sigma Aldrich (St. Luis, MO, USA), Wako Pure Chemical Industries, Ltd. (Tokyo, Japan), or Tokyo Chemical Industry (TCI) Co., Ltd. (Tokyo, Japan). in the highest available purity and they were utilized as received. The reactions were monitored by thin-layer chromatography (TLC) using precoated silica gel plates (Merck 5554, 60F254). Column chromatography was performed using a silica gel column (Wakogel C-200, 100–200 mesh). The NMR spectra were recorded on JEOL JNM-ECS400 spectrometers in deuterated chloroform (CDCl3) or deuterated dimethyl sulfoxide (DMSO-d6) solvent at room temperature operating at 400 (1H) and 100 MHz (13C{1H}). Tetramethylsilane (TMS) and CDCl3 were used as the internal standards for 1H and 13C NMR, respectively. The chemical shifts are reported in parts per million (ppm) while using TMS as a reference, and the J values are reported in hertz (Hz). The infrared (IR) spectra were recorded on a JEOL JIR-7000 Fourier-transform (FT)–IR (FT–IR) spectrometer and they were reported in reciprocal centimeters (cm−1). High-performance liquid chromatography (HPLC) was performed on a Jasco HPLC system consisting of a DG-980-50 three-line degasser, PU-980 HPLC pump, and CO-965 column oven and equipped with a chiral column (Chiralpak OD-H, AD-H Daicel), using hexane/2-propanol as the eluent. A Jasco UV-975 UV detector was employed for peak detection. Size-exclusion chromatography (SEC) was performed using a Tosoh HLC 8020 instrument with UV (254 nm) for refractive index detection. Dimethylformamide (DMF) with 0.5 wt% LiCl/LiBr was used as the carrier solvent at a flow rate of 1.0 mL min−1 at 40 °C. Two polystyrene gel columns with 10 µm beads were utilized. A calibration curve was generated for determining the number average molecular weight (Mn) and molecular weight distribution (Mw/Mn), using polystyrene standards. The optical rotation was recorded on a JASCO DIP-149 digital polarimeter using a 10 cm thermostated microcell.
3.2. Synthesis of Cinchona-Based Squaramide Containing the Chiral ADMET Polymer
3.2.1. Polymer P1
Squaramide 1 (133.0 mg, 0.200 mmol), HG2A (6.26 mg, 0.010 mmol), and toluene (0.5 mL) were collected in a dried Schlenk tube, after which they were set in an oil bath with a condenser. The Schlenk tube was connected to continuous N2 gas flow. The reaction mixture was stirred for 9 h after setting the desired reaction temperature (100 °C). Thereafter, the reaction mixture was cooled to room temperature and then poured into diethyl ether (50 mL). Next, the solid polymer product was purified by reprecipitation in diethyl ether (70–80 mL) three times. The precipitate was filtered out and vacuum-dried at 40 °C for 3 h to afford the desired polymer (P1 with 77% yield as a brownish solid), which is an ADMET polymeric organocatalyst. [α]25D = +90.99 (c 0.175 g/dL in DMF at 26.0 °C). 1H NMR (400 MHz, DMSO-d6, 25 °C); δ 8.37, 8.43, 8.84, 8.98 (1H), 7.48–8.26 (aromatic H), 5.71–6.08 (vinylic H), 5.43 (2H), 5.02–5.15 (m, 2H), 3.05, 2.89, 2.72, 2.67, 2.34, 2.10, 1.55, 1.45, 1.26, 1.09, 0.93, 0.76, and 0.65 (quinuclidine H) ppm. IR (KBr): ν = 3380, 3208, 2936, 2863, 1793, 1672, 1360, 1258, 1239, 1085, 1030, 975, 848, 692, and 624 cm−1. Mn (SEC) = 4.7 × 104, Mw/Mn = 1.04.
Other optically active polymers were prepared from different cinchona-based squaramides (1–3) with HG2A while using the same process. Table 1 summarizes the relevant results.
3.2.2. Representative Procedure for the Asymmetric Michael Reaction between β-Ketoesters and Trans-β-Nitrostyrene to Yield Nitroolefins Using P1
Methyl 2-oxocyclopentanecarboxylate (5) (63 µL, 0.50 mmol) and trans-β-nitrostyrene (6) (82.05 mg, 0.55 mmol) were placed in a vessel with 2.5 mL of a solvent. Subsequently, P1 (5 mol%) was added to the mixture. The reaction mixture was then stirred at 25 °C for a specified time. The solvent was evaporated with a rotary evaporator after the complete consumption of 5, which was monitored by TLC. The residue was washed with ether, which was subsequently separated by decantation. Unused P1 was recovered for reuse. The collected ether solution was concentrated in vacuo, and the crude product was purified by silica gel column chromatography (100–200 mesh) with hexane/ethyl acetate (EtOAc), 6.0/1.0, as the eluent to afford 7 as a colorless oil. 1H NMR (400 MHz, 25 °C, CDCl3); δ = 7.29–7.23 (m, 5H), 5.14 (dd, J = 13.8 Hz, 3.8 Hz, 1H), 5.00 (dd, J = 13.8 Hz, 10.7 Hz, 1H), 4.08 (dd, J = 10.8 Hz, 3.8 Hz, 1H), 3.74 (s, 3H), 2.38–2.33 (m, 2H), and 2.04–1.84.
4. Conclusions
In summary, we successfully synthesized chiral cinchona squaramide polymers P1–P3 by ADMET polymerization. The vinyl group in cinchona alkaloid exhibited good reactivity in ADMET polymerization when HG2 was employed to afford the optically active polymers in high yields. The copolymerization with triallyl ether also readily proceeded to afford P4. Asymmetric Michael reaction of 5 with 6 was efficiently catalyzed by the cinchona squaramide polymers to afford the Michael adducts with high stereoselectivity and isolated yields. P1a afforded 7a and c as major diastereomers, while P2C, P2Q, P3, and P4 afforded 7b and d. Both pseudoenantiomeric polymeric catalysts exhibited excellent performance in the same asymmetric reaction with high stereoselectivity. Different stereoisomers can be obtained by the proper selection of the catalytic structure of the cinchonidine and cinchonine derivatives. The recovery of the catalysts from the reaction mixture was dramatically improved by using the insoluble polymeric organocatalysts. These catalysts can be reused several times in the same asymmetric reaction without any significant loss in their catalytic activity.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/5/591/s1, details and data supplemental to the main text is shown here.
Author Contributions
Conceptualization, methodology, S.I.; supervision, S.I., formal analysis and data curation, M.S.U.; S.A.C. writing—original draft preparation, M.S.U.; writing—review and editing, S.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Naoki Haraguchi of the Toyohashi University of Technology, for useful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Itsuno, S.; Haraguchi, N. Catalyst Immobilization, Methods and Applications, Chapter 2; Benaglia, M., Puglisi, A., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 23–75. [Google Scholar]
- Itsuno, S.; Ullah, M.S. Flow Chemistry in Organic Synthesis, Science of Synthesis; Jamison, T.F., Koch, G., Eds.; Thieme: New York, NY, USA, 2018; pp. 347–380. [Google Scholar]
- Itsuno, S.; Paul, D.K.; Ishimoto, M.; Haraguchi, N. Designing Chiral Quaternary Ammonium Polymers: Novel Type of Polymeric Catalyst for Asymmetric Alkylation Reaction. Chem. Lett. 2010, 39, 86–87. [Google Scholar] [CrossRef]
- Itsuno, S.; Paul, D.K.; Salam, M.A.; Haraguchi, N. Main-Chain Ionic Chiral Polymers: Synthesis of Optically Active Quaternary Ammonium Sulfonate Polymers and Their Application in Asymmetric Catalysis. J. Am. Chem. Soc. 2010, 132, 2864–2865. [Google Scholar] [CrossRef]
- Parvez, M.M.; Haraguchi, N.; Itsuno, S. Molecular design of chiral quaternary ammonium polymers for asymmetric catalysis applications. Org. Biomol. Chem. 2012, 10, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, N.; Kiyono, H.; Takemura, Y.; Itsuno, S. Design of main-chain polymers of chiral imidazolidinone for asymmetric organocatalysis application. Chem. Commun. 2012, 48, 4011–4013. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, N.; Takenaka, N.; Najwa, A.; Takahara, Y.; Mun, M.K.; Itsuno, S. Synthesis of Main-Chain Ionic Polymers of Chiral Imidazolidinone Organocatalysts and Their Application to Asymmetric Diels–Alder Reactions. Adv. Synth. Catal. 2018, 360, 112–123. [Google Scholar] [CrossRef]
- Ullah, M.S.; Itsuno, S. Synthesis of cinchona alkaloid squaramide polymers as bifunctional chiral organocatalysts for the enantioselective Michael addition of β-ketoesters to nitroolefins. Mol. Catal. 2017, 438, 239–244. [Google Scholar] [CrossRef]
- Parvez, M.M.; Haraguchi, N.; Itsuno, S. Synthesis of Cinchona Alkaloid-Derived Chiral Polymers by Mizoroki–Heck Polymerization and Their Application to Asymmetric Catalysis. Macromolecules 2014, 47, 1922–1928. [Google Scholar] [CrossRef]
- Ullah, M.S.; Itsuno, S. Cinchona Squaramide-Based Chiral Polymers as Highly Efficient Catalysts in Asymmetric Michael Addition Reaction. ACS Omega 2018, 4573–4582. [Google Scholar] [CrossRef]
- Boratynski, P.J. Dimeric Cinchona alkaloids. Mol. Divers. 2015, 19, 385–422. [Google Scholar] [CrossRef]
- Lindmarkhamberg, M.; Wagener, K.B. Acyclic metathesis polymerization: The olefin metathesis reaction of 1,5-hexadiene and 1,9-decadiene. Macromolecules 1987, 20, 2949–2951. [Google Scholar] [CrossRef]
- Wagener, K.B.; Boncella, J.M.; Nel, J.G.; Duttweiler, R.P.; Hillmyer, M.A. The key to successful acyclic diene metathesis polymerization chemistry. Macromol. Chem. 1990, 191, 365–374. [Google Scholar] [CrossRef]
- Wagener, K.B.; Nel, J.G.; Konzelman, J.; Boncella, J.M. Acyclic diene metathesis copolymerization of 1,5-hexadiene and 1, 9-decadiene. Macromolecules 1990, 23, 5155–5157. [Google Scholar] [CrossRef]
- Wagener, K.B.; Boncella, J.M.; Nel, J.G. Acyclic diene metathesis copolymerization of 1,5-hexadiene and 1,9-decadiene. Macromolecules 1991, 24, 2649–2657. [Google Scholar] [CrossRef]
- Wagener, K.B.; Smith, D.W. Acyclic diene metathesis polymerization: Synthesis and characterization of unsaturated poly[carbo(dimethyl)silanes]. Macromolecules 1991, 24, 6073–6078. [Google Scholar] [CrossRef]
- Wagener, K.B.; Brzezinska, K. Acyclic diene metathesis (ADMET) polymerization: Synthesis of unsaturated polyethers. Macromolecules 1991, 24, 5273–5277. [Google Scholar] [CrossRef]
- Wagener, K.B.; Patton, J.T. Acyclic diene metathesis (ADMET) polymerization. Synthesis of unsaturated polycarbonates. Macromolecules 1993, 26, 249–253. [Google Scholar] [CrossRef]
- Portmess, J.D.; Wagener, K.B. Acyclic diene metathesis (ADMET) polymerization: The synthesis of unsaturated polyamines. J. Polym. Sci. Pol. Chem. 1996, 34, 1353–1357. [Google Scholar] [CrossRef]
- Wolfe, P.S.; Wagener, K.B. An ADMET route to unsaturated polyacetals. Macromol. Rapid Commun. 1998, 19, 305–308. [Google Scholar] [CrossRef]
- Wolfe, P.S.; Wagener, K.B. Investigation of Organoboronates in Metathesis Polymerization. Macromolecules 1999, 32, 7961–7967. [Google Scholar] [CrossRef]
- Brzezinska, K.R.; Schitter, R.; Wagener, K.B. Carbosilane/carbosiloxane-based ADMET homopolymers and copolymers possessing latent reactivity. J. Polym. Sci. Pol. Chem 2000, 38, 1544–1550. [Google Scholar] [CrossRef]
- Cummings, S.K.; Smith, D.W.; Wagener, K.B. Environmental degradation of biodegradable polyesters 1. Poly(ε-caprolactone), poly[(R)-3-hydroxybutyrate], and poly(L-lactide) films in controlled static seawater. Macromol. Rapid Commun. 1995, 16, 347–355. [Google Scholar] [CrossRef]
- Patton, J.T.; Boncella, J.M.; Wagener, K.B. Acyclic diene metathesis (ADMET) polymerization: The synthesis of unsaturated polyesters. Macromolecules 1992, 25, 3862–3867. [Google Scholar] [CrossRef]
- Hopkins, T.E.; Pawlow, J.H.; Koren, D.L.; Deters, K.S.; Solivan, S.M.; Davis, J.A.; Gomez, F.J.; Wagener, K.B. Chiral Polyolefins Bearing Amino Acids. Macromology 2001, 34, 7920–7922. [Google Scholar] [CrossRef]
- Ullah, M.S.; Itsuno, S. Synthesis of Cinchona Alkaloid Derived Chiral Squaramide Polymers by ADMET Polymerization and Their Application to Asymmetric Catalysis. Chem. Lett. 2018, 47, 1220–1223. [Google Scholar] [CrossRef]
- Song, C.E. Cinchona Alkaloids in Synthesis and Catalysis; Wiley: Weinheim, Germany, 2009. [Google Scholar]
- Jianga, L.; Chen, Y.C. Recent advances in asymmetric catalysis with cinchona alkaloid-based primary amines. Catal. Sci. Technol. 2011, 1, 354–365. [Google Scholar] [CrossRef]
- Yeboah, E.M.O.; Yeboah, S.O.; Singh, G.S. Recent applications of Cinchona alkaloids and their derivatives as catalysts in metal-free asymmetric synthesis. Tetrahedron 2011, 67, 1725–1762. [Google Scholar] [CrossRef]
- Marcelli, T. Organocatalysis: Cinchona catalysts. WIREs Comput. Mol. Sci. 2011, 1, 142–152. [Google Scholar] [CrossRef]
- Ingemann, S.; Hiemstra, H. Comprehensive Enantioselective Organocatalysis; Dalko, P.I., Ed.; Wiley: Weinheim, Germany, 2013; pp. 119–160. [Google Scholar]
- Itsuno, S.; Parvez, M.M.; Haraguchi, N. Polymeric chiral organocatalysts. Polym. Chem. 2011, 2, 1942–1949. [Google Scholar] [CrossRef]
- Itsuno, S.; Hassan, M.M. Polymer-immobilized chiral catalysts. RSC Adv. 2014, 4, 52023–52043. [Google Scholar] [CrossRef]
- Haraguchi, N.; Itsuno, S. Polymeric Chiral Catalyst Design and Chiral Polymer Synthesis; Itsuno, S., Ed.; Wiley: Hoboken, NJ, USA, 2011; Volume 2, pp. 17–61. [Google Scholar]
- Malerich, J.P.; Hagihara, K.; Rawal, V.H. Chiral Squaramide Derivatives are Excellent Hydrogen Bond Donor Catalysts. J. Am. Chem. Soc. 2008, 130, 14416–14417. [Google Scholar] [CrossRef]
- Tsakos, M.; Kokotos, C.G. Primary and secondary amine-(thio)ureas and squaramides and their applications in asymmetric organocatalysis. Tetrahedron 2013, 69, 10199–10222. [Google Scholar] [CrossRef]
- Zhao, B.L.; Du, D.M. Chiral Squaramide-Catalyzed Michael/Alkylation Cascade Reaction for the Asymmetric Synthesis of Nitro-Spirocyclopropanes. Eur. J. Org. Chem. 2015, 2015, 5350–5359. [Google Scholar] [CrossRef]
- Lee, J.W.; Ryu, T.H.; Oh, J.S.; Bae, H.Y.; Jang, H.B.; Song, C.E. Self-association-free dimeric cinchona alkaloid organocatalysts: Unprecedented catalytic activity, enantioselectivity and catalyst recyclability in dynamic kinetic resolution of racemic azlactones. Chem. Commun. 2009, 46, 7224–7226. [Google Scholar] [CrossRef]
- Rao, K.S.; Ramesh, P.; Chowhan, L.R.; Trivedi, R. Asymmetric Mannich reaction: Highly enantioselective synthesis of 3-amino-oxindoles via chiral squaramide based H-bond donor catalysis. RSC Adv. 2016, 6, 84242–84247. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Chiral Squaramide-Catalyzed Highly Enantioselective Michael Addition of 2-Hydroxy-1,4-naphthoquinones to Nitroalkene. Adv. Synth. Catal. 2011, 353, 1241–1246. [Google Scholar] [CrossRef]
- Konishi, H.; Lam, T.Y.; Malerich, J.P.; Rawal, V.H. Enantioselective α-Amination of 1,3-Dicarbonyl Compounds Using Squaramide Derivatives as Hydrogen Bonding Catalysts. Org. Lett. 2012, 12, 2028–2031. [Google Scholar] [CrossRef]
- Yang, W.; Jia, Y.; Du, D.M. Squaramide-catalyzed enantioselective Michael addition of malononitrile to chalcones. Org. Biomol. Chem. 2012, 10, 332–338. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Highly Enantioselective Michael Addition of Nitroalkanes to Chalcones Using Chiral Squaramides as Hydrogen Bonding Organocatalysts. Org. Lett. 2010, 12, 5450–5453. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Cinchona-based squaramide-catalysed cascade aza-Michael–Michael addition: Enantioselective construction of functionalized spirooxindole tetrahydroquinolines. Chem. Commun. 2013, 49, 8842–8844. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Chiral squaramide-catalyzed highly diastereo- and enantioselective direct Michael addition of nitroalkanes to nitroalkenes. Chem. Commun. 2011, 47, 12706–12708. [Google Scholar] [CrossRef]
- Vakulya, B.; Varga, S.; Csampai, A.; Soos, T. Highly Enantioselective Conjugate Addition of Nitromethane to Chalcones Using Bifunctional Cinchona Organocatalysts. Org. Lett. 2005, 7, 1967–1969. [Google Scholar] [CrossRef]
- Tripathi, C.B.; Kayal, S.; Mukherjee, S. Catalytic Asymmetric Synthesis of α,β-Disubstituted α,γ-Diaminophosphonic Acid Precursors by Michael Addition of α-Substituted Nitrophosphonates to Nitroolefins. Org. Lett. 2012, 14, 3296–3299. [Google Scholar] [CrossRef]
- Chhanda, S.A.; Itsuno, S. Design and synthesis of chiral hyperbranched polymers containing cinchona squaramide moieties and their catalytic activity in the asymmetric Michael addition reaction. J. Catal. 2019, 377, 543–549. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).