Simple Summary
Pancreatic cancer is a common cause of cancer-related death and carries an exceedingly poor prognosis. Increasingly, patients with localized disease are receiving chemotherapy and/or radiation therapy prior to surgical resection, a treatment plan known as neoadjuvant therapy (NT). Unfortunately, a significant number of patients who initiate NT will be unable to undergo surgery, and it remains unclear which patients are at risk of not completing NT. In this retrospective intention-to-treat cohort study, patient frailty was independently associated with a decreased likelihood of surgical resection and worse overall survival. These findings suggest that frail patients may benefit from different treatment sequencing or from receiving personalized care aimed at strengthening their overall health early in treatment.
Abstract
Background: Neoadjuvant therapy (NT) is increasingly utilized for patients with localized pancreatic ductal adenocarcinoma (PDAC). Toxicities during NT are common, often leading to the inability to undergo surgical resection, yet risk factors for attrition are poorly understood. Therefore, we sought to evaluate the impact of baseline frailty on outcomes of patients with PDAC undergoing NT. Methods: All patients with potentially resectable (PR) or borderline resectable (BR) PDAC who initiated neoadjuvant chemotherapy and/or chemoradiation between 2019 and 2025 at a single institution were assessed retrospectively in an intention-to-treat fashion. The association between frailty as defined by the modified 11-item frailty index (mFI-11) and receipt of surgical resection as well as other secondary endpoints was assessed. Comprehensive functional frailty assessments were prospectively obtained in a subset of patients. Results: Among 252 eligible patients, the median age was 67 years, 56.7% were male, 90.9% were White, 49.6% had PR disease, and 5.2% were frail according to mFI-11. After a median 3.6 months of NT, 62.7% underwent surgical resection. Frail individuals had worse performance status and increased comorbidities compared with non-frail patients. On multivariable analysis, male sex, BR anatomic staging, initial use of Gemcitabine + nab-paclitaxel, and frailty (OR 0.09; 95%CI 0.02–0.44) were associated with reduced odds of undergoing resection. Along with increased baseline CA 19-9 levels, frailty was independently associated with worse overall survival (HR 3.00; 95%CI 1.46–6.20). Among 39 patients who underwent formal functional frailty assessment, only abnormal posture was associated with lower odds of surgical resection following NT (OR, 0.22; 95% CI, 0.05–0.92), and no aspects of functional frailty were associated with overall survival. Conclusions: Among patients with localized PDAC initiating NT, frailty as assessed by mFI-11 was associated with reduced odds of undergoing surgical resection and worse overall survival. Future research should focus on efforts to improve functional status during NT.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the third most common cause of cancer-related death in the United States and has a poor 5-year overall survival (OS) rate of approximately 13% [1]. Traditionally, patients with localized PDAC have undergone surgical resection followed by adjuvant chemotherapy. However, over the past several decades, there has been a steady shift toward the use of neoadjuvant therapy (NT) prior to surgery [2]. Advantages of NT include potential tumor downstaging, improved likelihood of margin-negative resection, the opportunity to treat micrometastatic disease that initial surgery would fail to address, and ensuring the receipt of systemic therapy, which can be difficult to deliver postoperatively due to surgical complications, reduced performance status, or early recurrence [3,4].
Despite these advantages, there are several downsides to administering NT. Treatment-associated adverse events are common, and severe toxicities can occur which can lead to delays or interruptions to treatment as well as functional decline [5,6]. At the same time, challenges associated with interdisciplinary patient care coordination persist [7], and the optimal treatment regimens and duration have yet to be determined [8]. All of these factors complicate the delivery of NT and increase the potential for patients to not ultimately undergo surgical resection, which is associated with worse OS outcomes [9,10]. Nevertheless, risk factors for surgical attrition are poorly understood [10].
Frailty can be understood as a geriatric syndrome characterized by an increased vulnerability to adverse health outcomes, arising from the cumulative effect of multiple health deficits and a diminished capacity to respond to physiological stressors [11]. Frailty is common among patients with PDAC and is associated with worse long-term outcomes [12,13]. Cancer patients who are frail experience higher rates of postoperative complications, worse responses to chemotherapy, and reduced OS [14,15]. Despite these realities, the impact of frailty on patient outcomes during NT is unknown. A better understanding of the outcomes of frail patients could identify opportunities to develop patient-centered interventions to support physiological needs during NT and/or clarify alternative treatment sequencing.
2. Materials and Methods
2.1. Overall Study Design
The study was designed to evaluate the impact of baseline frailty on outcomes of patients with localized PDAC undergoing NT. In this retrospective intention-to-treat cohort study, all patients with potentially resectable (PR) or borderline resectable (BR) PDAC who received at least one cycle of NT with the intent of undergoing surgical resection were included. Patients were seen at either the Ohio State University Comprehensive Cancer Center’s Pancreatic Cancer Multidisciplinary Clinic (PMDC) or in a physician’s own clinic. The study was approved by the Ohio State University Institutional Review Board. Patients diagnosed between November 2018 and January 2025 were included, with a data cut-off date of 31 August 2025. Patients were excluded from this study if they were diagnosed with locally advanced (LA) or metastatic cancer at presentation, if comorbidities were deemed to be prohibitive of subsequent surgical resection, or if upfront surgery was selected.
The modified 11-item frailty index (mFI-11) was used to assess the baseline frailty of all patients included in the study [16]. The specific conditions included in the mFI-11 are a history of diabetes mellitus, non-independent functional status, chronic obstructive pulmonary disease, congestive heart failure, myocardial infarction, past percutaneous coronary intervention/cardiac surgery/angina, hypertension requiring the use of medication, peripheral vascular disease, impaired sensorium, transient ischemic attack/cerebrovascular accident without deficit, and cerebrovascular accident with deficit. Each comorbidity or deficit was coded as 1 if present and 0 if absent. These values were summed, then divided by the total number of variables considered, 11, yielding a score between 0 and 1 for each patient, with higher scores reflecting increased frailty. Frailty was defined as a score of 0.55 or greater [17].
2.2. Comprehensive Frailty Assessments
A subset of patients evaluated in the PMDC underwent comprehensive frailty evaluation by trained physical therapists (PT). The indications for a patient to receive a frailty assessment included the presence of baseline neuropathy, Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≥ 1, self-reported sedentary lifestyle, or verbalized concerns regarding their ability to tolerate NT or surgery. Patients were retrospectively categorized as either frail or not frail based on the Fried frailty phenotype [18]. This widely used classification system proposes the five domains of exhaustion, grip strength, gait speed, weight loss, and physical activity as necessary to assess functional frailty. Abnormalities in three or more of these areas are sufficient for a frailty designation. Exhaustion was verbally assessed using the scale from the Common Terminology Criteria for Adverse Events (CTCAE v5.0). A patient was considered to be experiencing exhaustion if they admitted “fatigue that is not relieved by rest” (fatigue ≥ Grade 2 on CTCAE). Grip strength was assessed with a Jamar hydraulic hand dynamometer and was identified as abnormal if the patient’s stronger (left vs. right) measure fell below established BMI and sex-matched cutoffs [19]. Gait speed was assessed via a timed 3-m walk, with any time-to-complete greater than 3.62 s considered to be abnormal. Physical activity status was assessed retrospectively based on the PT’s subjective assessment describing level of functioning, ability to complete activities of daily living, participation in recreational activities, and mobility limitations. Weight loss was defined as a loss of 10 lbs. or more in the prior 12 months.
2.3. Statistical Analysis
The primary aim of the study was to evaluate baseline patient frailty as a potential predictor of the ability to undergo surgical resection following NT completion. Continuous variables were first evaluated for normality using the Shapiro–Wilk test. Normally distributed variables were analyzed with Independent Samples t-tests and reported as means, while non-normally distributed variables were assessed using the non-parametric Mann–Whitney test and presented as medians. Categorical variables were compared using chi-square tests, with Fisher’s Exact tests applied when assumptions for chi-square were not met. To address missing data points prior to regression analysis, we performed multiple imputation, generating five imputed datasets. Results from these datasets were pooled to obtain overall estimates and assess statistical significance. To determine the association of variables with surgical resection status, logistic regression analysis was performed. First, univariate analyses were conducted to assess associations between baseline frailty, as well as clinical, demographic, and oncologic determinants of health, and performance of surgical resection. All variables with p < 0.10 on univariate analysis were entered into a multivariable logistic regression to identify factors independently associated with surgical resection status. Similarly, to assess the relationship of factors with OS, univariate Cox regression analyses were performed, with p < 0.10 on univariate analysis prompting factors to be considered for Cox multivariable regression analysis. For both multivariable logistic regression and multivariable Cox regression analyses, age, sex, and anatomic staging were included in the analysis, regardless of their univariate significance. All statistical analyses were performed using IBM SPSS (version 29.0) with statistical significance set at p < 0.05 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Patient Characteristics
Among the 252 patients who met the inclusion criteria, the median age was 67 years, 56.7% were male, and 90.9% were White. A similar proportion had PR (49.6%) and BR (50.4%) disease, and most patients (65.9%) received FOLFIRINOX as their initial chemotherapy, with 24.6% also receiving chemoradiation. Based on mFI-11, 13 patients (5.2%) were considered frail, while 239 (94.8%) were not. Table 1 compares the clinical and demographic characteristics of frail and non-frail patients. Frail patients had a higher Charlson Comorbidity Index score than non-frail patients (median (IQR), 9 (3) vs. 5 (2); p < 0.001) and were more likely to have an ECOG PS greater than 1 (46.2% vs. 5.0%; p < 0.001). Overall, frail patients were less likely to undergo surgical resection (15.4% vs. 65.3%; p < 0.001).

Table 1.
Patient Clinical and Demographic Characteristics Based on Baseline mFI-11 Frailty Status.
3.2. Predictors of Resection Status and OS
Median follow-up for the cohort was 46.5 months. Table 2 displays the outcomes of univariate and multivariable logistic regression analysis of factors associated with surgical resection. On multivariable analysis, male sex (OR, 0.50; 95% CI, 0.29–0.89), frailty (OR, 0.09; 95% CI, 0.02–0.44), BR stage (OR, 0.42; 95% CI, 0.24–0.73), and initial use of Gemcitabine + nab-paclitaxel during NT (OR, 0.43; 95% CI, 0.22–0.84) were independently associated with decreased odds of resection.

Table 2.
Univariate and Multivariable Logistic Regression of Factors Associated with Surgical Resection.
Compared to frail patients, non-frail patients experienced superior median OS (22.3 [95% CI 17.60–27.06] vs. 12.1 [95% CI 5.42–18.72] months) and improved 2-year OS (48.4% vs. 10.3%) (Figure 1). On multivariable Cox regression, frailty (HR, 3.00; 95% CI, 1.46–6.20), increased baseline CA 19-9 levels (HR, 1.00; 95% CI, 1.00–1.01), ECOG PS (HR, 1.37; 95% CI, 0.98–1.91), and use of Gemcitabine + nab-paclitaxel (HR, 2.19; 95% CI, 1.43–3.33) were associated with worse OS (Table 3).
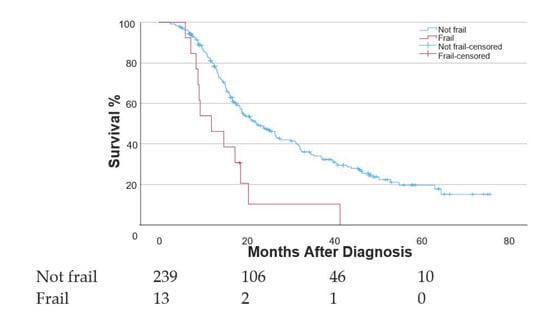
Figure 1.
Kaplan–Meier curve displaying overall survival measured from the time of diagnosis among patients who had frail and non-frail mFI-11 scores.

Table 3.
Cox Regression Analysis of Factors Associated with Overall Survival.
3.3. Functional Frailty Analysis
Of the 252 patients initially included in this study, 39 underwent comprehensive frailty assessment before beginning NT. Among this subset, 23 (59.0%) were functionally frail according to Fried criteria. No association was observed between functional frailty and subsequent surgical resection; however, functional frailty was associated with significant differences in albumin levels, ECOG PS scores, and initial neoadjuvant chemotherapy regimen compared to non-frail patients (Table S1). When each aspect of the functional frailty assessment was considered in a univariate logistic regression analysis, only the finding of abnormal patient posture (OR, 0.22; 95% CI, 0.05–0.92) was associated with surgical resection. This association persisted even after controlling for age, sex, anatomic stage, and CCI score (OR, 0.08; 95% CI, 0.01–0.68) (Table S2). No aspects of functional frailty were associated with OS on Cox regression analysis.
4. Discussion
Contemporary management of localized PDAC consists of multimodality therapy. Although the preferred sequencing remains controversial, NT is increasingly being recommended for most patients with localized PDAC [20,21]. While achieving surgical resection remains prognostically important and a patient-centered endpoint, risk factors for surgical attrition remain poorly understood [10]. In this single-institution retrospective but intention-to-treat study, we found that frail patients, as assessed by the mFI-11, are less likely than their non-frail counterparts to eventually undergo surgical resection. Beyond this association, patient frailty also proved to be an independent predictor of OS. These findings carry important implications for treatment decision-making and utilization of patient-centered resources during cancer therapy.
While frailty has been the center of extensive research in the oncology literature, relatively little research has been conducted on the impact of frailty during NT for PDAC. In addition, most prospective clinical trials limit inclusion to those with good PS [22,23,24]. For example, Fong et al. investigated ECOG PS, which captures some elements of functional frailty, and noted that a drop in PS during NT is independently associated with decreased odds of eventual surgical exploration in patients with PDAC [25]. On the other hand, Dickey et al. reported that baseline ECOG PS alone was not associated with attrition following NT [26].
Furthermore, Funamizu et al. reported that frail patients are less likely to complete adjuvant therapy following pancreatic cancer resection and demonstrate shorter OS [27]. On the other hand, frailty has been assessed in patients undergoing NT for other cancers, such as muscle-invasive bladder cancer, in which frailty was associated with worse OS [28]. Additionally, metrics that capture elements of frailty, such as total number of comorbidities and skeletal muscle wasting during NT, are associated with decreased odds of resection and decreased survival in other cancers, respectively [29,30].
The impact of frailty on the outcomes of patients undergoing NT is important, but whether this represents a modifiable risk factor that can be intervened upon remains uncertain. Given the retrospective nature of our study and the fragmented care that many patients at our institution receive, we were unable to assess the frequency with which frail patients received formal physical therapy or participated in formal prehabilitation programs and whether this improved their functional status during NT. While prehabilitation is a promising strategy for improving patient condition before major surgery, only limited evidence exists for its incorporation into the neoadjuvant setting [31,32,33]. Therefore, additional research on targeted interventions in this patient population is warranted. Alternate treatment sequencing might be considered for those patients with borderline (i.e., upfront surgical resection) or prohibitive (i.e., definitive chemotherapy and/or chemoradiation) frailty status. Finally, prioritizing less invasive surgical approaches may be of particular benefit to frail patients as well [34].
Frailty is a clinical syndrome with a wide variety of features often present in patients with PDAC. One lens through which frailty can be viewed is a cumulative deficit model. This model, as represented by the mFI-11, suggests that the impact of individual comorbidities, past medical events, and other physical deficits can be summed together to accurately depict one’s state of frailty [16]. Alternatively, the phenotype model aims to capture the functional manifestations of frailty, including weakness, slowness, weight loss, exhaustion, and decreased physical activity, as exemplified by the Fried Frailty Index [18].
A strength of our study is the use of both a widely available calculated frailty index as well as comprehensive formal frailty assessments in a subset of patients. On the other hand, the selection of mFI-11 and the use of a relatively high threshold to define frailty may have influenced our study findings. Previous studies have reported higher rates of frailty among patients with PDAC using other frailty indices such as the 5-item mFI, the Charlson Comorbidity Index, and the Fried Frailty Index [12,35,36]. It is also important to recognize that only patients who were deemed potentially candidates for eventual surgical resection were included in our study. Thus, other studies with less stringent inclusion criteria may report higher rates of frailty. Nevertheless, future research on the optimal frailty index for predicting outcomes of NT and informing patient-centered decision making is warranted.
Most patients in the more recent era of our PMDC underwent a comprehensive functional frailty assessment encompassing measures of exhaustion, pain, balance, posture, gait, sit-to-stand performance, grip strength, independence in activities of daily living, and fall history, among other domains. Interestingly, normal posture was the only metric found to be significantly associated with receipt of surgical resection. While these results could be related to the smaller sample size of this subset analysis, one possible interpretation of this finding is that abnormal sitting posture at rest is a potentially valuable indicator of poor functional health and is able to successfully predict a patient’s ability to tolerate NT. On the other hand, these results question whether formal frailty assessments, which are resource-intensive, provide additional value beyond standard clinical assessment. Future investigation into the prognostic value of these metrics with larger groups of patients is necessary.
Whereas strengths of our study include its intention-to-treat nature, where all patients who initiated NT were included, and the use of both a widely available mFI-11 and comprehensive functional frailty assessments, the primary limitation is its retrospective single-institution design. Importantly, both measured and unmeasured differences could exist between frail and non-frail patients (e.g., clinical judgments regarding patient fitness that influence treatment decision making) that confound our results. In addition, several important measures during NT that could influence surgical attrition were not available in our study data, such as chemotherapy dose intensity and toxicity profiles. Functional frailty assessments were also only obtained at baseline. Conducting multiple functional frailty assessments over the course of NT could characterize the importance of an individual’s trend in frailty and potentially identify critical time points when targeted interventions could be most beneficial. Finally, functional assessments were performed in only a small subset, and these patients are probably not representative of the entire cohort, as they were often referred due to concerns regarding their ability to tolerate NT. Indeed, of those classified as non-frail by Fried criteria, most still had deficiencies in at least one assessment (i.e., pre-frail).
5. Conclusions
Among patients with PR or BR PDAC undergoing NT, frailty as measured by the mFI-11 was independently associated with a decreased likelihood of surgical resection and worse OS. Frail patients may benefit from alternative treatment sequencing or individualized patient-centered interventions designed to improve physiologic resilience in the early stages of treatment. Future research with larger cohorts is warranted to clarify our findings on the relationship between functional frailty during NT and surgical outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17244030/s1. Table S1: Patient Clinical and Demographic Characteristics Based on Baseline Fried Frailty Classification; Table S2: Univariate and Multivariable Logistic Regression of Factors Associated with Surgical Resection.
Author Contributions
Conceptualization, J.M.C.; methodology, N.R.W. and J.M.C.; formal analysis, N.R.W. and A.K.W.; investigation, N.R.W., T.L., K.G., A.I., S.T. and M.E.D.; data curation, N.R.W. and T.L.; writing—original draft preparation, N.R.W. and J.M.C.; writing—review and editing, N.R.W., T.M.P., A.B.B. and J.M.C.; visualization, N.R.W.; supervision, J.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Ohio State University College of Medicine Bennett Medical Student Research Scholarship (N.R.W.).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ohio State University Cancer Institutional Review Board on 18 January 2024 (protocol number 2022C0015).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Aquina, C.T.; Ejaz, A.; Tsung, A.; Pawlik, T.M.; Cloyd, J.M. National Trends in the Use of Neoadjuvant Therapy Before Cancer Surgery in the US from 2004 to 2016. JAMA Netw. Open 2021, 4, e211031. [Google Scholar] [CrossRef] [PubMed]
- Springfeld, C.; Ferrone, C.R.; Katz, M.H.G.; Philip, P.A.; Hong, T.S.; Hackert, T.; Büchler, M.W.; Neoptolemos, J. Neoadjuvant Therapy for Pancreatic Cancer. Nat. Rev. Clin. Oncol. 2023, 20, 318–337. [Google Scholar] [CrossRef]
- Stoop, T.F.; Theijse, R.T.; Seelen, L.W.F.; Groot Koerkamp, B.; van Eijck, C.H.J.; Wolfgang, C.L.; van Tienhoven, G.; van Santvoort, H.C.; Molenaar, I.Q.; Wilmink, J.W.; et al. Preoperative Chemotherapy, Radiotherapy and Surgical Decision-Making in Patients with Borderline Resectable and Locally Advanced Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Vecchia, S.; Anselmi, E.; Toscani, I.; Guasconi, M.; Perrone, G.; Citterio, C.; Banchini, F.; Giuffrida, M. Neoadjuvant Treatment in Localized Pancreatic Cancer of the Elderly: A Systematic Review of the Current Literature. Cancers 2025, 17, 747. [Google Scholar] [CrossRef]
- Van Dam, J.L.; Janssen, Q.P.; Besselink, M.G.; Homs, M.Y.V.; Van Santvoort, H.C.; Van Tienhoven, G.; De Wilde, R.F.; Wilmink, J.W.; Van Eijck, C.H.J.; Groot Koerkamp, B. Neoadjuvant Therapy or Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomised Controlled Trials. Eur. J. Cancer 2022, 160, 140–149. [Google Scholar] [CrossRef]
- Schreiber, L.; Zeh, R.; Monsour, C.; Ejaz, A.; Tsung, A.; Pawlik, T.M.; Miller, E.; Noonan, A.; Krishna, S.G.; Santry, H.; et al. Multi-Specialty Physician Perspectives on Barriers and Facilitators to the Use of Neoadjuvant Therapy for Pancreatic Ductal Adenocarcinoma. HPB 2022, 24, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Sarna, A.; Arango, M.J.; Bates, S.E.; Bhutani, M.S.; Bloomston, M.; Chung, V.; Dotan, E.; Ferrone, C.R.; Gambino, P.F.; et al. Best Practices for Delivering Neoadjuvant Therapy in Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2025, 160, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.J.; Maxwell, J.E.; Katz, M.H.G.; Snyder, R.A. Surgical Considerations for Neoadjuvant Therapy for Pancreatic Adenocarcinoma. Cancers 2023, 15, 4174. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Colby, S.; Guthrie, K.A.; Lowy, A.M.; Chiorean, E.G.; Philip, P.; Sohal, D.; Ahmad, S. Failure to Undergo Resection Following Neoadjuvant Therapy for Resectable Pancreatic Cancer: A Secondary Analysis of SWOG S1505. J. Natl. Compr. Canc. Netw. 2024, 22, e237099. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A. Frailty Defined by Deficit Accumulation and Geriatric Medicine Defined by Frailty. Clin. Geriatr. Med. 2011, 27, 17–26. [Google Scholar] [CrossRef]
- Ngo-Huang, A.; Holmes, H.M.; Des Bordes, J.K.A.; Parker, N.H.; Fogelman, D.; Petzel, M.Q.B.; Song, J.; Bruera, E.; Katz, M.H.G. Association between Frailty Syndrome and Survival in Patients with Pancreatic Adenocarcinoma. Cancer Med. 2019, 8, 2867–2876. [Google Scholar] [CrossRef]
- Giri, S.; Al-Obaidi, M.; Harmon, C.; Clark, D.; Ubersax, C.; Dai, C.; Young-Smith, C.; Outlaw, D.; Gbolahan, O.; Khushman, M.; et al. Patient-Reported Geriatric Assessment-Based Frailty Index among Older Adults with Gastrointestinal Malignancies. J. Am. Geriatr. Soc. 2023, 71, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The Prevalence and Outcomes of Frailty in Older Cancer Patients: A Systematic Review. Ann. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and Cancer: Implications for Oncology Surgery, Medical Oncology, and Radiation Oncology. CA Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef]
- Velanovich, V.; Antoine, H.; Swartz, A.; Peters, D.; Rubinfeld, I. Accumulating Deficits Model of Frailty and Postoperative Mortality and Morbidity: Its Application to a National Database. J. Surg. Res. 2013, 183, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Cotton, J.; Cabot, J.; Buckner, J.; Field, A.; Pounds, L.; Quint, C. Increased Frailty Associated with Higher Long-Term Mortality after Major Lower Extremity Amputation. Ann. Vasc. Surg. 2022, 86, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A. Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Fried, L.P.; Borhani, N.O.; Enright, P.; Furberg, C.D.; Gardin, J.M.; Kronmal, R.A.; Kuller, L.H.; Manolio, T.A.; Mittelmark, M.B.; Newman, A.; et al. The Cardiovascular Health Study: Design and Rationale. Ann. Epidemiol. 1991, 1, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Stoop, T.F.; Javed, A.A.; Oba, A.; Koerkamp, B.G.; Seufferlein, T.; Wilmink, J.W.; Besselink, M.G. Pancreatic Cancer. Lancet 2025, 405, 1182–1202. [Google Scholar] [CrossRef]
- Davis, C.H.; Augustinus, S.; De Graaf, N.; Wellner, U.F.; Johansen, K.; Andersson, B.; Beane, J.D.; Björnsson, B.; Busch, O.R.; Gleeson, E.M.; et al. Impact of Neoadjuvant Therapy for Pancreatic Cancer: Transatlantic Trend and Postoperative Outcomes Analysis. J. Am. Coll. Surg. 2024, 238, 613–621. [Google Scholar] [CrossRef]
- Reni, M.; Macchini, M.; Orsi, G.; Procaccio, L.; Malleo, G.; Carconi, C.; Rapposelli, I.G.; Bencardino, K.; Scartozzi, M.; Balzano, G.; et al. Preoperative mFOLFIRINOX versus PAXG for Stage I–III Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma (PACT-21 CASSANDRA): Results of the First Randomisation Analysis of a Randomised, Open-Label, 2 × 2 Factorial Phase 3 Trial. Lancet 2025. Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; Van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Duong, M.; Sohal, D.P.S.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M.; et al. Surgical Outcome Results From SWOG S1505: A Randomized Clinical Trial of mFOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Perioperative Treatment of Resectable Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2020, 272, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Fong, Z.V.; Verdugo, F.L.; Fernandez-del Castillo, C.; Ferrone, C.R.; Allen, J.N.; Blaszkowsky, L.S.; Clark, J.W.; Parikh, A.R.; Ryan, D.P.; Weekes, C.D.; et al. Tolerability, Attrition Rates, and Survival Outcomes of Neoadjuvant FOLFIRINOX for Nonmetastatic Pancreatic Adenocarcinoma: Intent-to-Treat Analysis. J. Am. Coll. Surg. 2023, 236, 1126–1136. [Google Scholar] [CrossRef]
- Dickey, E.M.; Min, L.; Dhawan, A.; Martos, M.; Franceschi, D.; Livingstone, A.S.; Hosein, P.; Terrero, G.; Datta, J.; Merchant, N.; et al. Factors Driving Attrition from Neoadjuvant Therapy to Pancreatectomy in Localized Pancreatic Cancer. Ann. Surg. Oncol. 2025. Epub ahead of printing. [Google Scholar] [CrossRef]
- Funamizu, N.; Mori, S.; Sakamoto, A.; Honjo, M.; Tamura, K.; Sakamoto, K.; Ogawa, K.; Umeda, Y.; Aoki, T.; Takada, Y. Novel Modified Frailty Index Predicts Completion of Adjuvant Chemotherapy in Resectable Pancreatic Cancer in a Dual Center Study. Sci. Rep. 2025, 15, 17000. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kohada, Y.; Kobatake, K.; Tasaka, R.; Iwamoto, H.; Ueno, T.; Fujita, A.; Furutani, T.; Hashimoto, K.; Kurimura, Y.; et al. Impact of the Preoperative Modified 5-Item Frailty Index on the Efficacy of Neoadjuvant Chemotherapy in Patients with Muscle Invasive Bladder Cancer. Urol. Oncol. Semin. Orig. Investig. 2025, 43, 695.e11–695.e19. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Li, X.; Lin, G.; Ma, Y.; Xiao, R.; Li, H.; Qiu, M.; Yang, F. Skeletal Muscle Wasting during Neoadjuvant Therapy as a Prognosticator in Patients with Esophageal and Esophagogastric Junction Cancer: A Systematic Review and Meta-Analysis. Int. J. Surg. 2022, 97, 106206. [Google Scholar] [CrossRef]
- Bruce, K.H.; Xu, C.; Shah, R.P.; McGree, M.E.; Fought, A.J.; Yadav, S.; Langstraat, C.L.; Cliby, W.A.; Kumar, A. Real-World Outcomes of Neoadjuvant Chemotherapy for Advanced Ovarian Cancer: What Happens to Patients Who Never Undergo Surgery? Gynecol. Oncol. 2025, 201, 137–143. [Google Scholar] [CrossRef]
- Ngo-Huang, A.T.; Parker, N.H.; Xiao, L.; Schadler, K.L.; Petzel, M.Q.B.; Prakash, L.R.; Kim, M.P.; Tzeng, C.-W.D.; Lee, J.E.; Ikoma, N.; et al. Effects of a Pragmatic Home-Based Exercise Program Concurrent with Neoadjuvant Therapy on Physical Function of Patients with Pancreatic Cancer: The PancFit Randomized Clinical Trial. Ann. Surg. 2023, 278, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.H.; Ngo-Huang, A.; Lee, R.E.; O’Connor, D.P.; Basen-Engquist, K.M.; Petzel, M.Q.B.; Wang, X.; Xiao, L.; Fogelman, D.R.; Schadler, K.L.; et al. Physical Activity and Exercise during Preoperative Pancreatic Cancer Treatment. Support. Care Cancer 2019, 27, 2275–2284. [Google Scholar] [CrossRef]
- Nemkov, T.; Cendali, F.; Dzieciatkowska, M.; Stephenson, D.; Hansen, K.C.; Jankowski, C.M.; D’Alessandro, A.; Marker, R.J. A Multiomics Assessment of Preoperative Exercise in Pancreatic Cancer Survivors Receiving Neoadjuvant Therapy: A Case Series. Pathophysiology 2024, 31, 166–182. [Google Scholar] [CrossRef]
- Farah, E.; Al Abbas, A.; Abreu, A.A.; Cheng, M.; Yopp, A.; Wang, S.; Mansour, J.; Porembka, M.; Zeh, H.J.; Polanco, P.M. Minimally Invasive Pancreaticoduodenectomy: A Favorable Approach for Frail Patients with Pancreatic Cancer. Surgery 2024, 175, 1168–1175. [Google Scholar] [CrossRef]
- Khalid, A.; Pasha, S.A.; Demyan, L.; Standring, O.; Newman, E.; King, D.A.; DePeralta, D.; Gholami, S.; Weiss, M.J.; Melis, M. Modified 5-Item Frailty Index (mFI-5) May Predict Postoperative Outcomes after Pancreatoduodenectomy for Pancreatic Cancer. Langenbecks Arch. Surg. 2024, 409, 286. [Google Scholar] [CrossRef]
- Dias-Santos, D.; Ferrone, C.R.; Zheng, H.; Lillemoe, K.D.; Fernández-del Castillo, C. The Charlson Age Comorbidity Index Predicts Early Mortality after Surgery for Pancreatic Cancer. Surgery 2015, 157, 881–887. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).