Simple Summary
Clinical applications of unmanipulated haploidentical hematopoietic cell transplantation with GvHD (graft versus host disease) prophylaxis using post-transplant cyclophosphamide (haplo-HCT with PTCy) have become widespread in therapy of various types of hematological malignancies. The aim of this prospective non-interventional multicenter study was to document frequency of potential non-infectious and infection-related complications and main transplant outcomes after the first haplo-HCT with PT-Cy between 2017 and 2019 in 129 adult patients. The median follow-up was 37.3 months [95% CI: 34.3–39.7]. We have identified mucositis (37.5%), renal insufficiency (18%), and cardiovascular complications (10.9%) as the three main non-infectious complications. Infections were common after haplo-HCT with PTCy—bacterial in 65.1%, viral in 51.6% and fungal in 18.6% recipients. Two-year OS was 58.1% [95% CI: 50.2–67.3]; NRM—27.1% [95% CI: 19.7–35]; PFS—50.4% [95% CI: 42.5–59.8]; and GRFS—38.8% [95% CI: 31.2–48.1]. We found that disease remission status at transplant had an impact on transplant outcomes—PFS, chronic GvHD, and GRFS.
Abstract
Background: Haploidentical hematopoietic cell transplantations (haplo-HCTs) with post-transplant cyclophosphamide (PT-Cy) are standard practice, but complications causing morbidity and mortality are not well described. Methods: The aim of this prospective non-interventional multicenter study was to document frequency of potential non-infectious and infection-related complications and main transplant outcomes after the first unmanipulated haplo-HCT with PT-Cy between 2017 and 2019 in 129 adult patients with hematological malignancies. The median follow-up was 37.3 months [95% CI: 34.3–39.7]. Results: The cumulative incidence (CI) of acute graft versus host disease (aGvHD) at day +100 was 22.4% grade II-IV [95% CI: 15.5–30.1] and 8.8% grade III-IV [95% CI: 4.6–14.6], respectively. The cumulative incidence of chronic GvHD (cGvHD) at 24 months was 25.8% [95% CI: 18.5–33.6]; extensive cGvHD was 10.9% [95% CI: 6.3–17.1], respectively. The most frequent non-infectious complications for the whole study population were mucositis—37.5% (n = 48); renal insufficiency—18% (n = 23); and cardiovascular complications—10.9% (n = 14). The following infection-related complications were diagnosed: bacterial in 84 (65.1%), viral in 66 (51.6%), and fungal in 24 (18.6%) recipients. Two-year OS was 58.1% [95% CI: 50.2–67.3]; NRM—27.1% [95% CI: 19.7–35]; PFS—50.4% [95% CI: 42.5–59.8]; and GRFS—38.8% [95% CI: 31.2–48.1]. About 50% of all deaths were directly caused by infection or infection-related conditions. Conclusions: Disease remission status at transplant significantly affected PFS, chronic GvHD, and GRFS. Although clinical applications of haplo-HCT with PTCy are widespread, the study confirms the need to reduce infection-related mortality after this type of GvHD prophylaxis.
1. Introduction
The use of haploidentical T-cell replete hematopoietic cell transplantations (haplo-HCT) with post-transplant cyclophosphamide (PT-Cy) is commonly applied among transplant centers, especially in patients lacking an HLA-compatible stem cell donor [1,2,3,4]. The main advantages of this procedure are the rapid access to a donor, an over 95% chance of identifying a donor, and cost-effectiveness [2,3,5]. Historically, the main complications after haplo-HCT were graft versus host disease (GvHD) and graft failure [5]. The introduction of haplo-HCT with the PT-Cy strategy has changed the landscape of post-transplant outcomes [5,6]. The cyclophosphamide is an alkylating agent metabolized by the hepatic CYP450 enzyme into an active metabolite—aldophosphamide, which is oxidized by aldehyde dehydrogenase (ALDH) [5]. In the context of haploidentical stem cell transplantation, post-transplant cyclophosphamide as a GvHD prophylaxis functionally impairs proliferating recipient and alloreactive donor T cells, which have decreased levels of ALDH, while regulatory T cells and stem cells having increased ALDH levels are spared [5,6,7]. This is the key proposed mechanism of action of PTCy in haploHCT procedures.
Despite widespread use of PT-Cy in transplantation from haploidentical donors, multicenter real-world data regarding the types and frequency of complications after these procedures are relatively small [5,6]. There are numerous retrospective reports of different non-infectious and infectious post-transplant complications occurring after haplo-HCT: cytokine release syndrome (CRS); hemorrhagic cystitis (HC); transplant-associated thrombotic microangiopathy (TA-TMA); hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS); diffuse alveolar hemorrhage (DAH); idiopathic pneumonia syndrome (IPS); cardiac toxicity; and infections, especially of viral origin [7,8,9,10,11,12,13,14,15,16,17,18,19]. Some of them were considered probably or definitely related to the toxicity profile of cyclophosphamide [20]. The pattern of immune reconstitution, infectious complications, and immune-based complications is different after haplo transplants [5,6]. In recent retrospective reports focused on this issue, infectious complications and infection-related mortality are the major factors influencing the outcomes after haplo-HCT with PT-Cy [17,18,19,21,22,23].
In the present study, we aimed to prospectively investigate frequency of early and late non-infectious and infectious complications after PT-Cy-based haplo-HCT and determine essential survival outcomes.
2. Materials and Methods
2.1. Study Design and Inclusion Criteria
This was a prospective observational multicenter study performed on behalf of the Transplant Complications Working Party (TCWP) of the European Society for Blood and Marrow Transplantation (EBMT). The EBMT centers willing to participate were registered, and data collection followed the usual EBMT reporting guidelines (https://www.ebmt.org)—participating centers have been asked to record a MED-A D0 and MED-A D100 form (which required the minimal patient data in reporting the EBMT system ProMISE) and then to complete the Study Follow-up Form at 100 days, 12 months, and 24 months after transplantation. Data privacy was ensured according to ethical standards and the EBMT reporting guidelines. The inclusion criteria were the following: first alloHCT (previous autologous HCT was allowed), recipient age 18 years at transplantation, hematological malignancy, haploidentical donor, PT-Cy-based GvHD prophylaxis, bone marrow (BM) or peripheral blood (PB) as stem cell source, and year of transplantation between 2017 and 2019. The only exclusion criterion was ex vivo T-cell depletion.
In the present study, we aimed to prospectively investigate frequency of main early and late non-infectious and infectious complications after PT-Cy-based haplo-HCT and determine essential survival outcomes. We have performed analyses in the whole study population and according to disease remission status at transplant. The rationale for analyses in the CR (complete remission) versus the no-CR group was expected unfavorable results and outcomes in patients transplanted in an active disease. According to the study protocol, the following non-infectious complications were collected: mucositis, renal insufficiency, cardiovascular complications, VOD/SOS, TA-TMA, DAH, ELS (endothelial leakage syndrome), ES (engraftment syndrome), non-infective HC (haemorrhagic cystitis), PRES (posterior reversible encephalopathy syndrome), IPS (idiopathic pneumonia syndrome), BOS (bronchiolitis obliterans syndrome), COP (cryptogenic organizing pneumonia), osteoporosis, endocrine effects, PTLD (post-transplant lymphoproliferative disease) and other secondary malignancies, early graft loss, and graft failure and graft versus host disease (GvHD). Among infectious complications, data on bacterial, viral, and fungal infections and their main clinical presentation were collected. Data for all non-infectious and infectious complications were not collected, so only frequencies are presented and not incidences. The main recipient, donor, and transplant procedure characteristics, such as age and gender, cytomegalovirus (CMV) serostatus, disease diagnosis and its status at transplantation, HCT-CI (hematopoietic cell transplantation—comorbidity index), Karnofsky performance status, intensity of conditioning regimen, stem cell source, and GvHD prophylaxis regimen, were included. The definition of conditioning intensity, grading of acute GvHD and chronic GvHD, early graft loss, and graft failure were performed using published criteria [7,24,25,26,27].
The list of transplant centers contributing data to this study and the list of collected parameters are presented in the Supplementary Materials.
2.2. Statistical Methods
The primary endpoint was the frequency of each non-infectious and infectious complication after transplant. Secondary endpoints comprised survival outcomes: overall survival (OS), progression-free survival (PFS), relapse incidence (RI), non-relapse mortality (NRM), GvHD-relapse-free survival (GRFS), acute GvHD (aGvHD) grades II–IV and III–IV, and overall and extensive chronic GvHD (cGvHD).
The median values and ranges were used for continuous variables and percentages for categorical variables. Patient-, disease-, and transplant-related variables were compared between the two groups (CR at transplant vs. no CR) using the Mann–Whitney U test for numerical variables, and the Chi-squared or Fisher’s exact test for categorical variables.
OS was defined as the time from alloHCT to death, regardless of the cause. PFS was defined as the time from alloHCT to relapse or death from any cause. RI was defined as disease recurrence after alloHCT. NRM was defined as death without previous relapse. GRFS was defined as the first occurrence of grade III-IV acute GvHD or extensive chronic GvHD or relapse/progression or death from any cause. Acute GvHD was graded according to the modified Seattle-Glucksberg criteria and chronic GvHD according to the revised Seattle criteria. Neutrophil engraftment was defined as an achievement of an absolute neutrophil count (ANC) ≥ 0.5 G/L for 3 days without growth factor support, and platelet engraftment as a platelet count (PLT) > 50 G/L without transfusion. All the outcomes were measured from the time of stem cell infusion. The probabilities of OS, PFS, and GRFS were calculated with the Kaplan–Meier test, and those of RI, NRM, and acute and chronic GvHD with the cumulative incidence estimator to accommodate for competing risks. For NRM, relapse was the competing risk, and for relapse, the competing risk was NRM. For acute and chronic GvHD, death without the event and relapse were the competing risks. Median follow-up time was estimated using the reverse Kaplan–Meier method. Univariate analyses were performed using the log-rank test for OS and PFS, while Gray’s test was used for competing risk outcomes.
Statistical analyses were performed with R 4.1.2 (R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
3. Results
3.1. Patients, Disease, and Transplant Characteristics
A total of 129 adult patients undergoing haplo-HCT using PT-Cy between 2017 and 2019 were included. The analysis was performed for the whole study population and compared two groups of recipients: those in CR (complete remission) (n = 68) at transplant versus no-CR disease status (n = 58).
The patient, donor, and procedure characteristics for the whole study population and according to remission status at haplo-HCT are shown in Table 1.

Table 1.
Patient, donor, and procedure characteristics for the whole study population and according to remission status at haplo-HCT.
Patients in the no-CR group were older (median age 58.9 vs. 45.1; p < 0.001), and 75.8% of them were diagnosed with lymphoma and myelodysplastic/myeloproliferative disorders, while 86.8% of the CR group patients were diagnosed with acute leukemias (p < 0.001). Bone marrow was the main stem cell source in the no-CR group (69% vs. 48.5% in the CR group; p = 0.021).
The median follow-up was 37.3 months [95% CI: 34.3–39.7]. The two main causes of death were the original disease and infection. The original disease was diagnosed as the main cause of death in 34.6% of patients in the CR group and in 36.7% in the no-CR group, respectively. Infection-related deaths were reported in 26.9% of the CR patients and in 46.7% of the no-CR recipients, respectively.
3.2. Non-Infectious Complications
As mentioned above, we analyzed occurrence of all significant non-infectious post-transplant complications, including endothelial syndromes, organ toxicities, GvHD, and graft failure.
The most frequent non-infectious complications for the whole study population were mucositis—37.5% (n = 48); renal insufficiency—18% (n = 23); and cardiovascular —10.9% (n = 14). The rest of the analyzed non-infectious complications were diagnosed in less than 5% of the recipients: HC—3.9% (n = 5), DAH—3.9% (n = 5), VOD/SOS—3.1% (n = 4), engraftment syndrome—3.1% (n = 4), thrombotic microangiopathy—1.6% (n = 2), capillary leak syndrome (CLS)—1.6% (n = 2), idiopathic pneumonia syndrome—2.3% (n = 3), posterior reversible encephalopathy syndrome—0.8% (n = 1) and osteoporosis—3.1% (n = 4). There were no reported events of endocrine complications, post-transplant lymphoproliferative disease (PTLD), or other secondary malignancies. Some of the complications mentioned above were observed more often in the CR vs. no-CR group: mucositis 44.8% vs. 29.3%, cardiovascular complications 11.9% vs. 6.9%, HC 6% vs. 1.7%, VOD/SOS 4.5% vs. 1.7%, ES 6% vs. 0%, and CLS 3% vs. 0%, respectively. Renal complications were diagnosed most frequently in the no-CR vs. CR group: 19% vs. 14.9%, respectively. Frequency of the main non-infectious complications in the whole study population and according to remission status is shown in Figure 1.
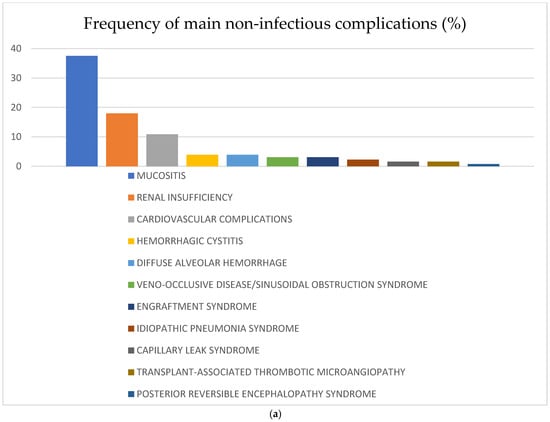
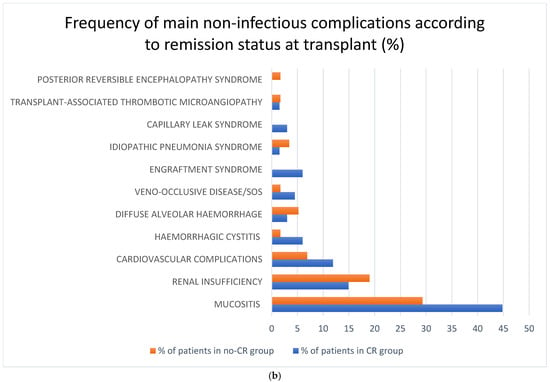
Figure 1.
(a) Frequency of non-infectious complications after haplo-HCT with PT-Cy in the whole population; (b) frequency of non-infectious complications after haplo-HCT with PT-Cy according to remission status at transplant. Abbreviations: SOS—sinusoidal obstruction syndrome.
Cytokine release syndrome (CRS) was diagnosed in eight (6.2%) patients, mostly in grade 1 according to Lee criteria (only one patient developed CRS of grade 2), and six in the CR group [27]. It should be mentioned that according to certain transplant center protocols, steroids were used in 14 (10.9%) and calcineurin inhibitors in 16 (12.4%) recipients before PT-Cy administration. There was no report of tocilizumab administration in the analyzed population.
Neutrophil engraftment (ANC—absolute neutrophil count ≥ 0.5 G/L) occurred in 109 (94.8%) patients, and platelets (>50 G/L) in 79 (63.2%). Growth factors were used in 97 (75.8%) recipients, mostly G-CSF (in 94 patients). Full donor chimerism was achieved in 100 (90.1%) patients.
Early graft loss was diagnosed in five patients: two in the CR group and three in the no-CR group, respectively. Graft failure was diagnosed in six (4.7%) patients: one in the CR group and five in the no-CR group.
3.3. GvHD
The cumulative incidence of acute GvHD at day +30 and at day +100 for grade II-IV acute GvHD was 12% [95% CI: 7–18.4] and 22.4% [95% CI: 15.5–30.1], and for grade III-IV aGvHD 5.6% [95% CI: 2.5–10.6] and 8.8% [95% CI: 4.6–14.6], respectively. There was no significant difference between the CR vs. no-CR group in aGvHD incidence—for grade II-IV at day +30 10.4% [95% CI: 4.6–19.2] vs. 14.8% [95% CI: 6.9–25.6] and at day +100 23.9% [95% CI: 14.4–34.7] vs. 22.2% [95% CI: 12.2–34.1], respectively (Figure 2a). Complete resolution of aGvHD was achieved in 75.7% of patients; most of them (83.3%) were treated with corticosteroids.
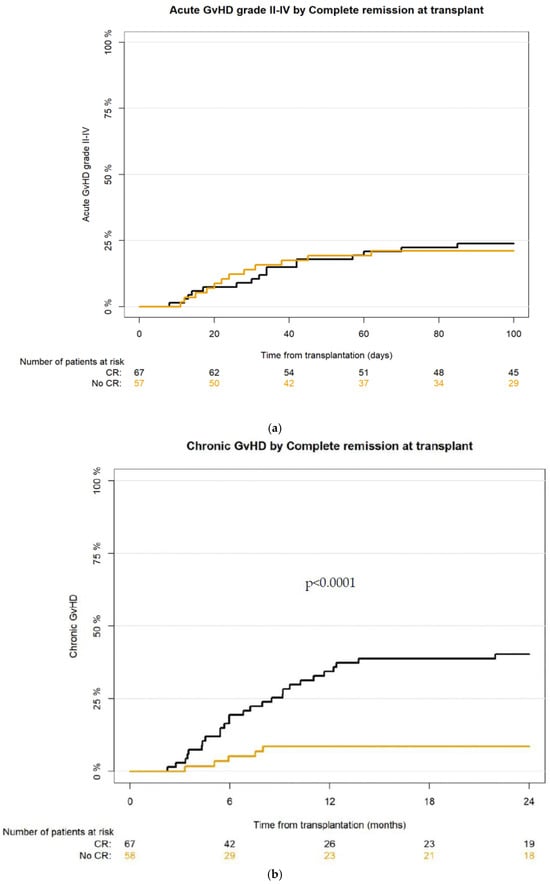
Figure 2.
(a) Cumulative incidence of aGvHD grade II-IV after haplo-HCT with PT-Cy in CR vs. no-CR group; (b) cumulative incidence of cGvHD after haplo-HCT with PT-Cy in CR vs. no-CR group.
The cumulative incidence for cGvHD at 12 months and at 24 months was 21.9% [95% CI: 15.1–29.4] and 25.8% [95% CI: 18.5–33.6], respectively; for extensive cGvHD—9.4% [95% CI: 5.1–15.2] and 10.9% [95% CI: 6.3–17.1], respectively. According to the National Institute of Health (NIH) scoring severity system [26], mild cGvHD was recognized in 13 (39.4%), moderate in 15 (45.5%), and severe in 5 (15.2%) recipients who developed chronic GvHD.
The incidence of cGvHD and extensive cGvHD was significantly higher in the CR group of patients, CI for cGvHD at 12 months for CR group was 34.3% [95%CI: 23.2–45.8] vs. 8.6% [95% CI: 3.1–17.7] in the no-CR group, and at 24 months 40.3% [95% CI: 28.4–51.9] vs. 8.6% [95% CI: 3.1–7.7], respectively (p < 0.001) (Figure 2b). The incidence of extensive cGvHD at 12 months for CR group was 13.4% [95% CI: 6.6–22.8] vs. 5.2% [95% CI: 1.4–13.8] in the no-CR group, and at 24 months 16.4% [95% CI: 8.7–26.3] vs. 5.2% [95% CI: 1.3–13.1], respectively.
3.4. Infectious Complications
Infection-related complications were frequently diagnosed in the whole study population: bacterial in 84 (65.1%), viral in 66 (51.6%), and fungal in 24 (18.6%) (Figure 3). There was only one case of infection (0.8%) of parasitic origin. There were no clinically relevant differences according to frequency of bacterial and fungal infectious complications between the CR vs. the no-CR group: bacterial infection was diagnosed in 66.2% in the CR and 63.8% in the no-CR group, and fungal—in 17.6% and 17.2%, respectively. A disparity was observed in viral infections—61.8% in the CR vs. 42.1% in the no-CR recipients, respectively. The most frequent clinical presentations of infection were pneumonia in 42 (32.6%), septic shock in 20 (15.5%), haemorrhagic cystitis in 20 (15.5%), gut infection in 19 (14.7%), skin infection in 9 (7%), and central nervous system involvement in 4 (3.1%) patients, respectively.
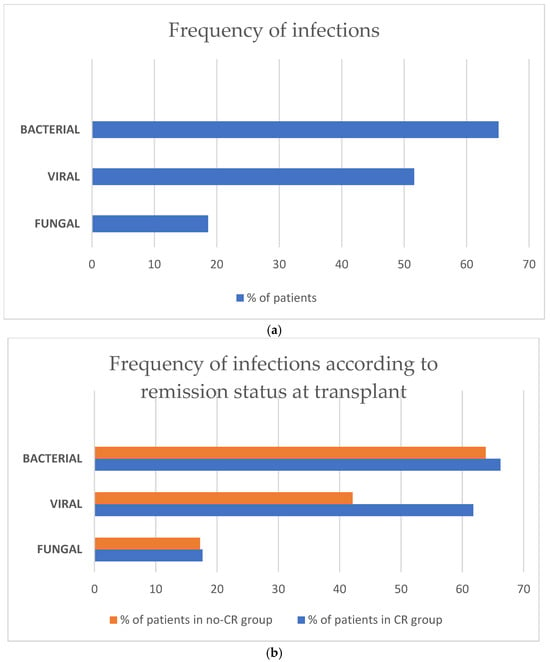
Figure 3.
(a) Frequency of infectious complications after haplo-HCT with PT-Cy in the whole population. (b) Frequency of infectious complications after haplo-HCT with PT-Cy according to remission status at transplant.
Acute respiratory distress syndrome (ARDS) due to infection was diagnosed in 14 (10.9%) patients, and multi-organ failure (MOF) in 12 (9.3%) of them. Neither infectious-related hepatitis nor nephritis was present in the whole population.
3.5. Transplantation Outcomes
The analyzed transplantation outcomes are presented in Table 2.

Table 2.
Univariate analysis of transplantation outcomes for the whole study population and according to remission status at haplo-HCT.
Main survival outcomes according to remission status at transplant are presented in Figure 4.
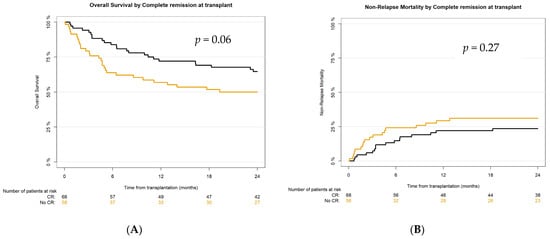
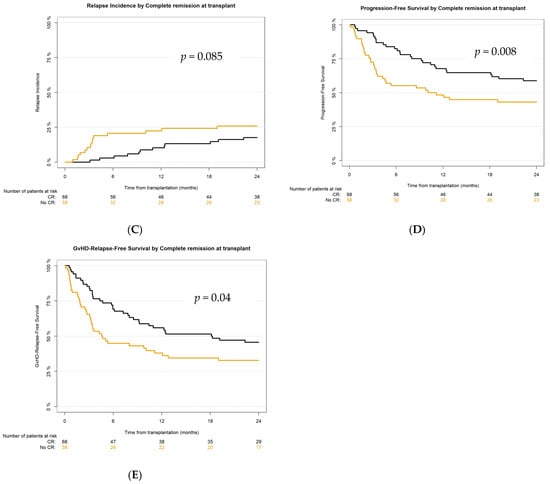
Figure 4.
Survival outcomes after haplo-HCT with PT-Cy according to remission status at transplant: (A) overall survival (OS), (B) non-relapse mortality (NRM), (C) relapse incidence (RI), (D) progression-free survival (PFS), (E) GvHD-relapse-free survival (GRFS).
4. Discussion
The present study prospectively and comprehensively analyzed real-world multicenter data on non-infectious and infection-related complications and main transplant outcomes after unmanipulated haplo-HCT with PT-Cy in 129 adult patients with hematological malignancies.
For non-infectious complications, we focused mainly on organ toxicities, complications of endothelial origin, and graft versus host disease. Among organ toxicities, besides mucositis, the most frequent complication was renal insufficiency, diagnosed in 18% (n = 23) of patients. Renal insufficiency was lower in our cohort than published data in the post-allogeneic stem cell transplant setting, which reports a frequency of 50% [28,29]. The main limitation of our analysis is lack of data on severity grading of adverse events.
Cardiovascular complications were the next most frequently occurring organ toxicity, recognized in 10.9% (n = 14) of recipients. Cardiac complications after haplo-HCT with PT-Cy have been investigated recently in retrospective studies showing the cumulative incidence as high as 19% and it was settled that they are dose-dependent [15,30]. Our prospective and real-life data show lower frequency of cardiac toxicity, and it is worth highlighting that over 99% of patients received cyclophosphamide in a standard total dose of 100 mg/kg, 55.8% of them in +3 and +4 days, and 42.6% according to the Genova protocol, i.e., +3 and +5 days after transplant [31]. Recently, data was published on cardiac complications after haplo-HCT with PT-Cy in 58 elderly patients (aged ≥ 65 years) and those with cardiac comorbidities [32]. Authors compared the standard cyclophosphamide dose of 100 mg/kg in 25 recipients with a reduced total dose to 70 mg/kg combined with a low dose of antithymocyte globulin in 33 [32]. Cardiac complications were reported in 44% of patients with standard dose versus 12% in the reduced dose group, but it was still a higher proportion than in our cohort of 129 recipients. For older patients and those with cardiovascular diseases, this reduced dose strategy could be a valid option in mitigating cardiac toxicity after haplo-HCT with PT-Cy [32].
According to our findings, frequency of the main complications of endothelial origin was less than 4% each. These results are lower than published data [7]. For example, SOS/VOD in our study was diagnosed in only four recipients (3.9%), while incidence of this complication in adults is diagnosed in about 5–10% depending on the risk group [7,14,33,34]. One of the possible explanations is a wider time gap between conditioning and administration of calcineurin inhibitors compared to conventional protocols. The other non-infectious complication that is expected, due to high doses of cyclophosphamide, is haemorrhagic cystitis (HC) [7,8,9,10,11]. An early HC after haploPTCy was reported in almost 30% of patients [10], but the main cause of HC in haplo transplants is viral infections—HC in general was diagnosed in 55–62% of recipients with BK PyV (BK Polyomavirus) as a main factor in 91% of them [9,10,11]. In our cohort, non-infectious HC was a rare complication recorded in 5 (3.9%) patients, and HC of infectious origin in 20 (15.5%) of them, which constitutes a lower frequency than in published data [7,8,9,10,11].
Cytokine release syndrome (CRS) was reported as a common complication in haploPTCy transplant recipients, especially with onset after day 0 and before cyclophosphamide infusion, in more than 70% of patients [16,35,36,37]. In our study, we confirmed CRS only in 8 (6.2%) patients, and with severity mostly of grade 1. An important information to note is that steroids were used in 14 (10.9%) and calcineurin inhibitors in 16 (12.4%) recipients before PT-Cy administration, according to each center’s protocol. It is probable that this complication was underdiagnosed in the analyzed population due to the absence of clear criteria, which were refined after the initiation of the study [38].
In addition, almost 95% of the patients in this study engrafted, and graft failure was rare—we identified this complication in 6 (4.7%) patients. In published data, graft failure or poor graft function after haplo transplants, even with the use of PT-Cy, is still reported as an important issue with frequency reaching 27.5% [7,39].
Regarding aGvHD, the incidence of this complication in our study was similar to that reported recently after haplo transplants with PT-Cy and significantly lower than in historically published data reaching 40% [1,7,35,40]. Cumulative incidence of acute GvHD grade II-IV at day +100 was 22.4% and grade III-IV 8.8% compared with 18–33% and 9.4–13%, respectively, reported by others [41,42,43]. The rate of any grade chronic GvHD was 25.8% and is definitely lower than 46.9% as previously published, and in our cohort, extensive GvHD was 10.9% at 2 years, which is close to results reported by others with a frequency of 15.6% and 3.1%, respectively [7,41,42,43].
Infections are considered the main complication of haplo-HCT with PT-Cy, affecting NRM, and in our cohort, they were the main or contributing cause of death, as previously described in other studies [17,18,21,35]. The frequency of bacterial infections was the highest in the analyzed population, followed by viral and, less commonly, fungal infections, but in the CR group, we noticed the predominance of viral infections. In published reports, there are differences in cumulative incidence of infection by pathogen type—in some of them, viral, especially due to CMV, are the most often diagnosed infections, but bacterial in others [17,18,21,22,23,31,44,45,46,47].
The main transplant outcomes in our study were not inferior to what was previously reported [7,41,42,48,49,50]. According to NRM, the 2-year CI in the analyzed population was 27.1% in comparison with the published 31% [39], and there was no significant difference between the CR vs. the no-CR group. OS in our cohort at 2 years was 58.1% (better in the CR group—64.6%)—in previously reported studies it was 54–57% [7,41,42,48,49,50]. PFS at 24 months for the whole study population was 50.4% (for the CR group 58.8%)—in published reports, 49–54% [7,41,42,50]. And finally, 2-year GvHD-relapse-free survival in the analyzed cohort was 38.8%, in the CR group—45.6%, as compared to about 36% in previous reports [41,51]. However, active disease was diagnosed in almost 45% (n = 58) of recipients at transplant, and even in this very poor prognosis cohort, haplo-HCT with PT-Cy was feasible and effective, resulting in 2-year OS, PFS, and GRFS at 50%, 43.1%, and 32.8%, respectively.
5. Conclusions
In conclusion, our prospective study provided multicenter real-world evidence on non-infectious and infection-related complications of T cell-replete haploidentical stem cell transplantation with post-transplant cyclophosphamide and emphasizes the impact of infections on transplant outcomes. For 28 patients of all 56 reported deaths in the study, the mortality was directly caused by infections or was infection-related. Non-infectious complications were, in general, less common in our cohort than what has been previously described in haplo-HCT with PT-Cy. Disease remission status at transplant significantly affected PFS, chronic GvHD, and GRFS. Refinement of the transplantation procedure with PT-Cy should be the subject of further studies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers17244029/s1. Table S1: The list of transplant centers contributing data to this study. Table S2: The list of main collected parameters. Infection-related complications. Table S3: The list of main collected parameters. Non-infectious complications.
Author Contributions
Conceptualization, A.T. and G.W.B.; methodology, G.W.B., C.P., E.P., P.A. and W.B.; data reporting, S.S., M.A., J.P., J.L.L.L., U.S., P.J., A.K., R.M.B., M.E. and M.-A.B.; formal analysis, all; statistical analysis, C.P.; writing—original draft preparation, A.T.; writing—review and editing, all; visualization, A.T.; construction of manuscript and critical revision, H.S., O.P., I.M. and Z.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This was a prospective observational multicenter study performed on behalf of the Transplant Complications Working Party (TCWP) of the European Society for Blood and Marrow Transplantation (EBMT). The EBMT centers willing to participate were registered, and data collection followed the usual EBMT reporting guidelines.
Informed Consent Statement
Due to this being a retrospective study, the informed consent is waived.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bashey, A.; Zhang, X.; Sizemore, C.A.; Manion, K.; Brown, S.; Holland, H.K.; Morris, L.E.; Solomon, S.R. T-Cell–Replete HLA-Haploidentical Hematopoietic Transplantation for Hematologic Malignancies Using Post-Transplantation Cyclophosphamide Results in Outcomes Equivalent to Those of Contemporaneous HLA-Matched Related and Unrelated Donor Transplantation. J. Clin. Oncol. 2013, 31, 1310–1316. [Google Scholar] [CrossRef]
- Bashey, A.; Solomon, S.R. T-cell replete haploidentical donor transplantation using post-transplant CY: An emerging standard-of-care option for patients who lack an HLA-identical sibling donor. Bone Marrow Transplant. 2014, 49, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.M.; Dominietto, A.; di Grazia, C.; Lamparelli, T.; Gualandi, F.; Ibatici, A.; Bregante, S.; Van Lint, M.T.; Varaldo, R.; Ghiso, A.; et al. Unmanipulated Haploidentical Transplants Compared with Other Alternative Donors and Matched Sibling Grafts. Biol. Blood Marrow Transplant. 2014, 20, 1573–1579. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Ciceri, F.; de la Cámara, R.; Glass, B.; Greco, R.; Hazenberg, M.D.; Kalwak, K.; McLornan, D.P.; Neven, B.; et al. Hematopoietic cell transplantation and cellular therapies in Europe 2022. CAR-T activity continues to grow; transplant activity has slowed: A report from the EBMT. Bone Marrow Transplant. 2024, 59, 803–812. [Google Scholar] [CrossRef]
- Cytryn, S.; Abdul-Hay, M. Haploidentical Hematopoietic Stem Cell Transplantation Followed by ‘Post-Cyclophosphamide’: The Future of Allogeneic Stem Cell Transplant. Clin. Hematol. Int. 2020, 2, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, S.H.C.; Rambaldi, B.; Shapiro, R.M.; Romee, R. Key Aspects of the Immunobiology of Haploidentical Hematopoietic Cell Transplantation. Front. Immunol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Sureda, A.; Corbacioglu, S.; Greco, R.; Kroger, N.; Carreras, E. The EBMT Handbook: Hematopoietic Cell Transplantation and Cellular Therapies; Springer Nature: Dordrecht, The Netherlands, 2024; pp. 303–501. [Google Scholar]
- Ruggeri, A.; Roth-Guepin, G.; Battipaglia, G.; Mamez, A.; Malard, F.; Gomez, A.; Brissot, E.; Belhocine, R.; Vekhoff, A.; Lapusan, S.; et al. Incidence and risk factors for hemorrhagic cystitis in unmanipulated haploidentical transplant recipients. Transpl. Infect. Dis. 2015, 17, 822–830. [Google Scholar] [CrossRef]
- Copelan, O.R.; Sanikommu, S.R.; Trivedi, J.S.; Butler, C.; Ai, J.; Ragon, B.K.; Jacobs, R.; Knight, T.G.; Usmani, S.Z.; Grunwald, M.R.; et al. Higher Incidence of Hemorrhagic Cystitis Following Haploidentical Related Donor Transplantation Compared with Matched Related Donor Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 785–790. [Google Scholar] [CrossRef]
- Arango, M.; Cardona, D. Hemorrhagic Cystitis after Haploidentical Transplantation with Post-Transplantation Cyclophosphamide: Protective Effect of MESNA Continuous Infusion. Biol. Blood Marrow Transplant. 2020, 26, 1492–1496. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, G.; Xu, J.; Yang, R.; Muhashi, M.; Aizezi, G.; Jiang, M. Incidence of late-onset hemorrhagic cystitis and its effect on PFS in acute leukemia patients after haplo-PBSCT: The 5-year single-center data. Front. Oncol. 2022, 12, 913802. [Google Scholar] [CrossRef]
- Matsumoto, K.; Fujishita, K.; Matsuda, M.; Oka, S.; Fujisawa, Y.; Imai, T.; Machida, T. Idiopathic pneumonia syndrome re-fractory to ruxolitynib after post-transplant cyclophosphamide-based haploidentical hematopoietic stem cell transplantation: Lung pathological findings from an autopsy case. Acta Medica Okayama 2022, 76, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, P.; Andrés-Zayas, C.; Carbonell, D.; Chicano, M.; Bailén, R.; Oarbeascoa, G.; Suárez-González, J.; Centurión, I.G.; Dorado, N.; Gallardo, D.; et al. Association between gene polymorphisms in the cyclophosphamide metabolism pathway with complications after haploidentical hematopoietic stem cell transplantation. Front. Immunol. 2022, 13, 1002959. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Centurión, I.; Bailén, R.; Oarbeascoa, G.; Muñoz, C.; Luque, A.Á.; Boyra, M.E.; Calleja, E.; Rincón, D.; Dorado, N.; Barzallo, P.; et al. Transjugular Intrahepatic Portosystemic Shunt for Very Severe Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome (VOD/SOS) after Unmanipulated Haploidentical Hematopoietic Stem Cell Transplantation with Post-transplantation Cyclophosphamide. Biol. Blood Marrow Transplant. 2020, 26, 2089–2097. [Google Scholar] [CrossRef]
- Duléry, R.; Mohty, R.; Labopin, M.; Sestili, S.; Malard, F.; Brissot, E.; Battipaglia, G.; Médiavilla, C.; Banet, A.; Van de Wyngaert, Z.; et al. Early Cardiac Toxicity Associated With Post-Transplant Cyclophosphamide in Allogeneic Stem Cell Transplantation. JACC CardioOncol. 2021, 3, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Abboud, R.; Wan, F.; Mariotti, J.; Arango, M.; Castagna, L.; Romee, R.; Hamadani, M.; Chhabra, S. Cytokine release syndrome after haploidentical hematopoietic cell transplantation: An international multicenter analysis. Bone Marrow Transplant. 2021, 56, 2763–2770. [Google Scholar] [CrossRef]
- Slade, M.; Goldsmith, S.; Romee, R.; DiPersio, J.F.; Dubberke, E.R.; Westervelt, P.; Uy, G.L.; Lawrence, S.J. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transpl. Infect. Dis. 2016, 19. [Google Scholar] [CrossRef]
- Esquirol, A.; Pascual, M.J.; Kwon, M.; Pérez, A.; Parody, R.; Ferra, C.; Cadenas, I.G.; Herruzo, B.; Dorado, N.; Hernani, R.; et al. Severe infections and infection-related mortality in a large series of haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide. Bone Marrow Transplant. 2021, 56, 2432–2444. [Google Scholar] [CrossRef]
- Little, J.S.; Shapiro, R.M.; Aleissa, M.M.; Kim, A.; Chang, J.B.P.; Kubiak, D.W.; Zhou, G.; Antin, J.H.; Koreth, J.; Nikiforow, S.; et al. Invasive Yeast Infection after Haploidentical Donor Hematopoietic Cell Transplantation Associated with Cytokine Release Syndrome. Biol. Blood Marrow Transplant. 2022, 28, 508.e1–508.e8. [Google Scholar] [CrossRef]
- Kanakry, C.G.; Fuchs, E.J.; Luznik, L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat. Rev. Clin. Oncol. 2015, 13, 10–24. [Google Scholar] [CrossRef]
- Crocchiolo, R.; Bramanti, S.; Vai, A.; Sarina, B.; Mineri, R.; Casari, E.; Tordato, F.; Mauro, E.; Timofeeva, I.; Lugli, E.; et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl. Infect. Dis. 2015, 17, 242–249. [Google Scholar] [CrossRef]
- Atilla, E.; Atilla, P.A.; Bozdağ, S.C.; Demirer, T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection 2017, 45, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Mohty, R.; Brissot, E.; Battipaglia, G.; Ruggeri, A.; Dulery, R.; Bonnin, A.; Médiavilla, C.; Sestili, S.; Belhocine, R.; Vekhoff, A.; et al. Infectious complications after post-transplantation cyclophosphamide and anti-thymocyte globulin-based haploidentical stem cell transplantation. Br. J. Haematol. 2019, 187, E64–E68. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Ballen, K.; Rizzo, D.; Giralt, S.; Lazarus, H.; Ho, V.; Apperley, J.; Slavin, S.; Pasquini, M.; Sandmaier, B.M.; et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biol. Blood Marrow Transplant. 2009, 15, 1628–1633. [Google Scholar] [CrossRef]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4–10. [Google Scholar] [CrossRef]
- Lee, S.J. Classification systems for chronic graft-versus-host disease. Blood 2017, 129, 30–37. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Renaghan, A.D.; Jaimes, E.A.; Malyszko, J.; Perazella, M.A.; Sprangers, B.; Rosner, M.H. Acute Kidney Injury and CKD Associated with Hematopoietic Stem Cell Transplantation. Clin. J. Am. Soc. Nephrol. 2019, 15, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kanduri, S.R.; Cheungpasitporn, W.; Thongprayoon, C.; Bathini, T.; Kovvuru, K.; Garla, V.; Medaura, J.; Vaitla, P.; Kashani, K.B. Incidence and mortality of acute kidney injury in patients undergoing hematopoietic stem cell transplantation: A systematic review and meta-analysis. Qjm: Int. J. Med. 2020, 113, 621–632. [Google Scholar] [CrossRef]
- Ohmoto, A.; Fuji, S. Cardiac complications associated with hematopoietic stem-cell transplantation. Bone Marrow Transplant. 2021, 56, 2637–2643. [Google Scholar] [CrossRef]
- Raiola, A.M.; Angelucci, E.; Sica, S.; Bacigalupo, A. Haploidentical bone marrow transplants with post transplant cyclophosphamide on day + 3 + 5: The Genova protocol. Blood Rev. 2022, 62, 101031. [Google Scholar] [CrossRef]
- Duléry, R.; Malard, F.; Brissot, E.; Banet, A.; Sestili, S.; Belhocine, R.; Calabro, M.; Van de Wyngaert, Z.; Bonnin, A.; Ledraa, T.; et al. Reduced post-transplant cyclophosphamide dose with antithymocyte globulin in peripheral blood stem cell haploidentical transplantation. Bone Marrow Transplant. 2023, 58, 1215–1222. [Google Scholar] [CrossRef]
- Cairo, M.S.; Cooke, K.R.; Lazarus, H.M.; Chao, N. Modified diagnostic criteria, grading classification and newly elucidated pathophysiology of hepatic SOS/VOD after haematopoietic cell transplantation. Br. J. Haematol. 2020, 190, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Malard, F.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; Blaise, D.; Brissot, E.; et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A refined classification from the European society for blood and marrow transplantation (EBMT). Bone Marrow Transplant. 2023, 58, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Bejanyan, N.; Pidala, J.A.; Wang, X.; Thapa, R.; Nishihori, T.; Elmariah, H.; Lazaryan, A.; Khimani, F.; Davila, M.L.; Mishra, A.; et al. A phase 2 trial of GVHD prophylaxis with PTCy, sirolimus, and MMF after peripheral blood haploidentical transplantation. Blood Adv. 2021, 5, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.V.; Hamadani, M.; Szabo, A.; Pasquini, M.C.; Shah, N.N.; Drobyski, W.R.; Shaw, B.E.; Saber, W.; Rizzo, J.D.; Jerkins, J.; et al. Peripheral Blood Grafts for T Cell–Replete Haploidentical Transplantation Increase the Incidence and Severity of Cytokine Release Syndrome. Biol. Blood Marrow Transplant. 2018, 24, 1664–1670. [Google Scholar] [CrossRef]
- Yao, J.M.; Otoukesh, S.; Kim, H.; Yang, D.; Mokhtari, S.; Samara, Y.; Blackmon, A.; Arslan, S.; Agrawal, V.; Pourhassan, H.; et al. Tocilizumab for Cytokine Release Syndrome Management After Haploidentical Hematopoietic Cell Transplantation With Post-Transplantation Cyclophosphamide-Based Graft-Versus-Host Disease Prophylaxis. Biol. Blood Marrow Transplant. 2023, 29, 515.e1–515.e7. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Gómez-Centurión, I.; Rojas, R.M.M.; Bailén, R.; Muñoz, C.; Sabell, S.; Oarbeascoa, G.; Fernández-Caldas, P.; Carbonell, D.; Gayoso, J.; Martínez-Laperche, C.; et al. Poor graft function after haploidentical stem cell transplantation with post-transplant cyclophosphamide. Ann. Hematol. 2023, 102, 1561–1567. [Google Scholar] [CrossRef]
- Montoro, J.; Piñana, J.L.; Hernández-Boluda, J.C.; Hernani, R.; Lorenzo, I.; Pérez, A.; Guerreiro, M.; Balaguer-Rosello, A.; Sanz, G.F.; Carretero, C.; et al. Uniform graft-versus-host disease prophylaxis with posttransplant cyclophosphamide, sirolimus, and mycophenolate mofetil following hematopoietic stem cell transplantation from haploidentical, matched sibling and unrelated donors. Bone Marrow Transplant. 2020, 55, 2147–2159. [Google Scholar] [CrossRef]
- Duléry, R.; Goudet, C.; Mannina, D.; Bianchessi, A.; Granata, A.; Harbi, S.; Maisano, V.; Chabannon, C.; Malard, F.; Brissot, E.; et al. Reduced post-transplant cyclophosphamide doses in haploidentical hematopoietic cell transplantation for elderly patients with hematological malignancies. Bone Marrow Transplant. 2022, 58, 386–392. [Google Scholar] [CrossRef]
- Ruggeri, A.; Labopin, M.; Bacigalupo, A.; Gülbas, Z.; Koc, Y.; Blaise, D.; Bruno, B.; Irrera, G.; Tischer, J.; Diez-Martin, J.L.; et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer 2018, 124, 1428–1437. [Google Scholar] [CrossRef]
- Pruitt, A.; Gao, F.; De Togni, E.; Cochran, H.; Godbole, S.; Slade, M.; Abboud, R. Impact of donor age and relationship on outcomes of peripheral blood haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2023, 58, 855–862. [Google Scholar] [CrossRef]
- Mikulska, M.; Bartalucci, C.; Raiola, A.M.; Oltolini, C. Does PTCY increase the risk of infections? Blood Rev. 2023, 62, 101092. [Google Scholar] [CrossRef] [PubMed]
- Fayard, A.; Daguenet, E.; Blaise, D.; Chevallier, P.; Labussière, H.; Berceanu, A.; Yakoub-Agha, I.; Socié, G.; Charbonnier, A.; Suarez, F.; et al. Evaluation of infectious complications after haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide following reduced-intensity and myeloablative conditioning: A study on behalf of the Francophone Society of Stem Cell Transplantation and Cellular Therapy (SFGM-TC). Bone Marrow Transplant. 2019, 54, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.R.; Abid, M.B.; Auletta, J.J.; Bashey, A.; Beitinjaneh, A.; Castillo, P.; Chemaly, R.F.; Chen, M.; Ciurea, S.; Dandoy, C.E.; et al. Posttransplant cyclophosphamide is associated with increased cytomegalovirus infection: A CIBMTR analysis. Blood 2021, 137, 3291–3305. [Google Scholar] [CrossRef]
- Chorão, P.; Henriques, M.; Villalba, M.; Montoro, J.; Balaguer-Roselló, A.; González, E.M.; Gómez, M.D.; Gómez, I.; Solves, P.; Santiago, M.; et al. Cytomegalovirus Reactivations in Allogeneic Hematopoietic Stem Cell Transplantation from HLA-Matched and Haploidentical Donors with Post-Transplantation Cyclophosphamide. Biol. Blood Marrow Transplant. 2024, 30, 538.e1–538.e10. [Google Scholar] [CrossRef]
- Baron, F.; Labopin, M.; Tischer, J.; Ciceri, F.; Raiola, A.M.; Blaise, D.; Sica, S.; Vydra, J.; Fanin, R.; Diez-Martin, J.L.; et al. Comparison of HLA-mismatched unrelated donor transplantation with post-transplant cyclophosphamide versus HLA-haploidentical transplantation in patients with active acute myeloid leukemia. Bone Marrow Transplant. 2022, 57, 1657–1663. [Google Scholar] [CrossRef]
- Sugita, J.; Kamimura, T.; Ishikawa, T.; Ota, S.; Eto, T.; Kuroha, T.; Miyazaki, Y.; Kumagai, H.; Matsuo, K.; Akashi, K.; et al. Reduced dose of posttransplant cyclophosphamide in HLA-haploidentical peripheral blood stem cell transplantation. Bone Marrow Transplant. 2020, 56, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Bashey, A.; Zhang, M.-J.; McCurdy, S.R.; Martin, A.S.; Argall, T.; Anasetti, C.; Ciurea, S.O.; Fasan, O.; Gaballa, S.; Hamadani, M.; et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell–Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J. Clin. Oncol. 2017, 35, 3002–3009. [Google Scholar] [CrossRef]
- Im, A.; Rashidi, A.; Wang, T.; Hemmer, M.; MacMillan, M.L.; Pidala, J.; Jagasia, M.; Pavletic, S.; Majhail, N.S.; Weisdorf, D.; et al. Risk Factors for Graft-versus-Host Disease in Haploidentical Hematopoietic Cell Transplantation Using Post-Transplant Cyclophosphamide. Biol. Blood Marrow Transplant. 2020, 26, 1459–1468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).