Simple Summary
Cervical cancer persists as a significant global health burden, particularly in regions where screening and vaccination programmes are limited. Consequently, the identification of reliable, non-invasive biomarkers is paramount to enhance early detection and patient management. Extracellular vesicles (EVs) comprise lipid bilayer-enclosed particles released by cells into bodily fluids, carrying a molecular cargo that reflects the molecular signature of their cell of origin. This systematic review and meta-analysis assessed the diagnostic and prognostic utility of EVs in cervical cancer. Our findings indicate that EV-derived biomarkers, particularly non-coding RNAs, exhibit high diagnostic accuracy, yielding a pooled area under the receiver operating characteristic (ROC) curve of 0.87. However, evidence supporting their prognostic utility remains inconclusive due to methodological heterogeneity. In conclusion, EVs represent a promising liquid biopsy modality for cervical cancer detection; however, the validation of their clinical application necessitates further large-scale studies employing standardised protocols.
Abstract
Background/Objectives: Cervical cancer remains a significant global health burden, underscoring the imperative for refined diagnostic and prognostic methodologies. This study aimed to evaluate the potential of extracellular vesicles (EVs) as non-invasive biomarkers for cervical cancer, focusing on diagnosis and prognosis. Methods: We conducted a systematic review and meta-analysis in accordance with PRISMA guidelines to assess the diagnostic and prognostic accuracy of EV-based biomarkers. We searched PubMed, EMBASE, and Web of Science for relevant studies. Twelve articles met the inclusion criteria: eight related to diagnostic accuracy, three to prognosis, and one to both outcomes. Six studies met the criteria for meta-analysis. We used a random-effects model to synthesise diagnostic data, while prognostic data were synthesised narratively. Results: The meta-analysis yielded a pooled area under the receiver operating characteristic curve (AUC) of 0.87 (95% CI 0.80–0.92) for EVs in the diagnosis of cervical cancer, indicating high accuracy. The evaluated diagnostic biomarkers were primarily non-coding RNAs. For prognosis, data heterogeneity precluded quantitative synthesis; however, individual studies identified diverse EV-associated molecules correlated with recurrence and survival. GRADE assessment indicated a high risk of bias and heterogeneity across studies. Conclusions: Extracellular vesicles demonstrate robust promise as diagnostic biomarkers for cervical cancer; however, their prognostic utility remains inconclusive due to methodological and clinical heterogeneity. Future research must prioritise the standardisation of isolation protocols and the execution of large-scale, prospective studies to validate EV biomarkers for clinical application. Systematic Review Registration: PROSPERO, identifier: CRD420251014411.
1. Introduction
Cervical cancer persists as the fourth leading cause of cancer-related deaths among women worldwide, underscoring its status as a significant public health concern [1]. Although incidence is relatively low in Western Europe, it continues to be the leading cause of cancer mortality among middle-aged women in Eastern European countries [1]. These global disparities are intrinsically linked to varying rates of Human Papillomavirus (HPV) infection, vaccination coverage, and the implementation of robust screening programmes [2]. Despite advancements in screening methods, including cytology and HPV testing, mortality rates have largely remained stable in developed countries [1,3]. Timely diagnosis is paramount, as early-stage detection allows for curative, fertility-sparing interventions and is associated with high survival rates. In contrast, advanced stages necessitate aggressive multimodal therapies and carry a significantly poorer prognosis [4]. Given that early-stage cervical cancer is often asymptomatic, elucidating disease progression through non-invasive means is crucial for timely diagnosis and monitoring [4]. Although histological examination remains the definitive diagnostic standard [4], conventional tissue biopsies may not accurately reflect the entire genomic and molecular landscape of a tumour due to intrinsic intratumoral heterogeneity [5]. As a non-invasive alternative, liquid biopsies offer a dynamic approach to cancer monitoring, providing real-time information on disease status and treatment response [5]. Circulating blood contains various tumour-derived materials, including circulating tumour cells (CTCs), circulating tumour DNA (ctDNA), and extracellular vesicles (EVs). Among these, EVs offer significant numerical and biological advantages as non-invasive biomarkers [6]
Extracellular vesicles (EVs), as defined by the International Society for Extracellular Vesicles (ISEV), encompass a heterogeneous population of lipid bilayer-enclosed particles released from cells [7]. EVs are broadly categorised by size and biogenesis: exosomes (typically 30–150 nm) are derived from the endosomal system and released upon the fusion of multivesicular bodies (MVBs) with the plasma membrane. In contrast, microvesicles (or ectosomes, generally 50 nm–1 μm) are generated by the direct outward budding and fission of the plasma membrane, while apoptotic bodies (1–5 μm) are larger fragments released during programmed cell death [7]. EVs are ubiquitous in most bodily fluids, and their concentration (often exceeding 109 exosomes/mL) is significantly higher than that of circulating tumour cells (CTCs, <100 cells/mL) [6,8].
In the context of cancer diagnostics, exosomes have garnered particular attention due to their unique biogenesis and function as highly specific mediators of intercellular communication [9]. In contrast to microvesicles, which are often shed non-specifically via plasma membrane budding, or apoptotic bodies, which represent passive cellular remnants of programmed death, exosomes are actively secreted via the endosomal pathway [10]. This mechanism enables exosomes to carry a selective molecular cargo that accurately reflects the molecular status of the parental tumour cell [10]. Furthermore, the lipid bilayer structure of exosomes imparts inherent stability, actively protecting their enclosed cargo (including proteins and nucleic acids) from enzymatic degradation in circulation, a critical feature for effective biomarker analysis and validation [8].
Crucially, in cervical cancer, tumour-derived exosomes are not merely diagnostic markers but active mediators of oncogenesis [10]. They propagate the effects of HPV E6 and E7 oncoproteins by transferring specific viral transcripts, proteins, and non-coding RNAs to surrounding cells, thereby facilitating key tumour progression processes such as immune evasion, angiogenesis, and metastasis within the tumour microenvironment [11,12]. This capacity to encapsulate stable, functional, and disease-specific molecules directly involved in the pathogenesis of HPV-driven cancer distinguishes exosomes as the most relevant and studied EV subtype for liquid biopsy applications [11,12].
Extracellular vesicles can be isolated from various biofluids, most commonly plasma or serum, although urine, saliva, and cervicovaginal secretions have also been explored in gynaecological malignancies [7]. Their detection and analysis rely on complementary methodologies including ultracentrifugation, size-exclusion chromatography, nanoparticle tracking analysis, and transmission electron microscopy, ensuring both structural and molecular validation of vesicle integrity [7].
Consequently, this systematic review and meta-analysis aim to evaluate the potential of extracellular vesicles, with a specific emphasis on exosomes, as non-invasive biomarkers for the early diagnosis and prognosis of cervical cancer.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13], the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach for quality of evidence assessment [14], and the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool for the evaluation of risk of bias in individual studies [15]. The study protocol was registered prospectively in PROSPERO (registration number: CRD420251014411) prior to the commencement of the literature search.
2.2. Eligibility Criteria
Studies were eligible if they comprised original research or replication studies published in peer-reviewed journals, with full-text availability in English, Portuguese, or Spanish, without restriction regarding the year of publication.
In accordance with the PECO framework (Population, Exposure, Comparator, Outcome), specific inclusion and exclusion criteria are detailed in Table 1. Included observational studies were required to evaluate EVs as potential biomarkers and report specific quantitative data on EVs for cervical cancer detection or prognosis. Due to the limited number of studies anticipated in this emerging field, no restrictions regarding sample size were applied. Studies failing to report adequate details regarding EV isolation and characterisation methods were excluded, as were those focusing solely on therapeutic applications of extracellular vesicles without addressing their diagnostic or prognostic utility.

Table 1.
Inclusion and exclusion criteria based on PECO.
2.3. Information Sources
An initial scoping search was conducted in January 2025 to inform the development of the review protocol. This preliminary search facilitated the refinement of the research question and search strategy. Following the approval of the protocol by PROSPERO on 26 March 2025, a comprehensive systematic search was executed according to the pre-registered methodology. The search results were exported to EndNote 21.5 for Mac (Build 20846, Clarivate Analytics).
The following electronic databases were searched: PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science Core Collection, WHO International Clinical Trials Registry Platform, EU Clinical Trials Register, and ClinicalTrials.gov. Databases were searched from their inception until 30 April 2025 to ensure comprehensive coverage of this novel topic.
The search strategy utilised Boolean operators targeting title, abstract, and keywords fields with the following terms: (“cervical cancer” OR “cervical neoplasms” OR “uterine cervix cancer” OR “HPV-related cervical cancer”) AND (“Extracellular vesicl*” OR “plasma Extracellular vesicl*” OR “serum Extracellular vesicl*” OR “blood Extracellular vesicl*” OR “exosome” OR “microvesicles” OR “ectosomes” OR “oncosomes” OR “vesicular cargo” OR “liquid biopsy”) AND (“healthy” OR “cervical intraepithelial neoplasia” OR “CIN”) AND (“biomarker$” OR “biological biomarker” OR “diagnosis” OR “prognosis” OR “miRNA” OR “HPV” OR “ctDNA” OR “long non-coding RNAs (lncRNAs)”).
Additionally, annual conference abstracts from the International Papillomavirus Conference (IPVC) and the International Multidisciplinary HPV Congress (EUROGIN) from 2020 to 2025 were screened. Reference lists of included articles were manually scrutinised to retrieve potentially relevant studies that had not been identified in the initial search.
2.4. Study Selection
The screening process commenced on 4 January 2025. All duplicates were identified and removed automatically using EndNote 21.5. Two reviewers (F.S. and M.F.-D.) screened titles and abstracts independently and in a blinded manner, adhering to a predefined list of selection criteria. In instances where publications used overlapping patient cohorts, data were extracted from the study providing the most detailed information on EV biomarkers. A step-by-step comparison of the entire process was conducted, and any disagreements prompted a re-evaluation of the records in question. Eligibility was discussed among all authors involved in the selection process until consensus was achieved. The same procedure was applied to the scrutiny of reference lists of included studies to identify any additional relevant records.
From the selected articles, six were included for meta-analysis based on the availability of the Area Under the Curve (AUC). All studies assessed diagnostic outcomes. Both significant and non-significant results were considered for the analysis.
2.5. Data Extraction
All data variables to be extracted were defined a priori. A standardised data extraction spreadsheet was developed to minimize bias. The extracted variables were: (i) study, author, and publication year; (ii) country; (iii) study design; (iv) type of sample (e.g., plasma or serum); (v) EV subtype (e.g., exosome, microvesicle); (vi) EV isolation method; (vii) EV characterisation method; (viii) EV quantification method; (ix) specific biomarker identified (e.g., miRNA, lncRNA); (x) expression level (e.g., upregulated or downregulated); (xi) techniques used to identify and quantify the biomarkers; (xii) sample size (cervical cancer cases, healthy controls, and/or CIN controls); and (xiii) outcomes (e.g., diagnostic or prognostic) including descriptive statistics and association measures where available.
F.S. and M.F.-D. completed the initial data extraction independently. RN and MS subsequently reviewed the entire process, and any discrepancies were resolved by consensus. The data required for meta-analysis were collated by F.S. and M.F.-D. and subsequently reviewed by FC. JT and LPA provided a final verification of the quality of data entered into the database.
2.6. Risk of Bias in Individual Studies
The risk of bias in individual studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool [15], which assesses four key domains: patient selection, index test (in this case, extracellular vesicles as biomarkers), reference standard, and flow and timing.
Two independent reviewers (F.S. and M.F.-D.) performed the quality assessment for each included study. Each domain was assessed for risk of bias (high, low, or unclear) and applicability concerns. Following the initial assessment, results were compared, disagreements discussed, and a subsequent re-analysis performed. Finally, RN and MS reviewed the assessments to ensure the robustness of the evaluations.
The methodological quality of included studies was appraised considering key aspects of study design, conduct, analysis, and evaluation, informed by Simon et al.’s criteria [16] and the REMARK guidelines [17], whilst ensuring adherence to MISEV guidelines for EV research [7].
2.7. Summary Measures
Summary measures synthesised the diagnostic accuracy of exosomes as blood-based biomarkers.
For studies reporting multiple area under the ROC curve (AUC) values due to different experimental conditions, the lowest value corresponding to the worst-case scenario was selected, thus adopting a conservative approach. In one study, where the AUC was not directly available, it was estimated from the reported sensitivity and specificity values. For studies including an external validation group, AUC values obtained from these groups were prioritized.
Data were analysed using R (v 4.4.2) software [18]. A logit transformation was applied to AUC values to approximate a normal distribution, and the variance of the logit transformation was estimated for each study. A random-effects model was applied using the restricted maximum likelihood (REML) method through the ‘rma’ function of the ‘metafor’ package, accounting for the expected heterogeneity between studies. The summary measure and its 95% confidence intervals were calculated and subsequently back-transformed to the original AUC scale to facilitate interpretation.
Heterogeneity among studies was assessed using the Cochran Q test and the I2 statistic. The Q test evaluates whether observed differences in results are compatible with chance alone, with a p-value < 0.10 indicating significant heterogeneity. The I2 statistic quantifies the percentage of total variation across studies due to heterogeneity rather than chance, with values of 25%, 50%, and 75% suggesting low, moderate, and high heterogeneity, respectively [19,20]. The presence of publication bias was assessed visually through a funnel plot and quantitatively using Egger’s test. A p-value < 0.05 in Egger’s test indicated possible publication bias.
2.8. Confidence in Cumulative Evidence
Confidence in the cumulative evidence was assessed using the GRADE framework [14], adapted for observational studies. Observational studies start with a low-certainty rating, which can be upgraded or downgraded based on five dimensions: risk of bias (using tools such as QUADAS-2) [15], inconsistency (heterogeneity quantified by I2 and Cochran’s Q) [20], indirectness (relevance of study populations and outcomes), imprecision (evaluated by 95% confidence intervals), and publication bias (assessed through funnel plots and Egger’s test). Sensitivity analyses were performed to exclude poor-quality studies. These assessments were performed independently by F.S. and M.F.-D. and verified by FC.
3. Results
3.1. Study Selection
The electronic search across four databases yielded 260 records, with one additional record identified from clinical trial registry platforms. Following the removal of duplicates via EndNote (n = 27) and manual screening (n = 11), 223 unique records remained. After screening titles and abstracts, 23 studies were retained for full-text assessment. Subsequently, 12 studies met the eligibility criteria and were included in the qualitative analysis. (Figure 1).
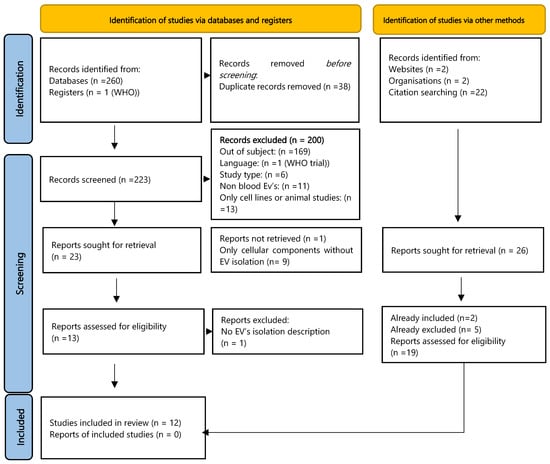
Figure 1.
Flowchart describing the study selection process [13].
Searches of websites and organisational resources identified four potential studies, and manual citation searching of included articles revealed 22 potentially relevant titles (including updated searches). Of these, two had already been included in our final sample, and five had been excluded during the process. Nineteen studies had not appeared in our initial searches; screening of their abstracts resulted in the exclusion of nine based on study type (e.g., abstract, review) and 10 based on failure to meet PECO criteria.
A meta-analysis was conducted on six articles related to cervical cancer diagnosis, focusing solely on extracellular vesicles as a collective biomarker entity, considering them as mediators or carriers of intercellular communication. Crucially, the specific biomarkers transported by isolated extracellular vesicles were not analysed individually due to their substantial heterogeneity across the included studies.
Among the included studies, the primary focus was on long non-coding RNAs (lncRNAs) [21,22], mRNA [23], plasma tRFs [24], and various miRNAs [23,25,26,27] or proteins [28,29]. Regarding prognosis, performing a meta-analysis was not feasible; although two studies reported AUC values [22,30], methodological disparities precluded the statistical pooling of results.
3.2. Study Characteristics
For the diagnostic evaluation, nine studies were selected, comprising a total of 659 cervical cancer patients (635 included in the meta-analysis), 482 healthy controls (458 included in the meta-analysis), and 139 CIN controls (all included in the meta-analysis) (Table 2).

Table 2.
Characteristics of studies included in the study—outcome: Diagnostic.
For the prognostic evaluation, four studies were included (one of which assessed both outcomes), comprising 234 cervical cancer patients and 77 healthy controls (Table 3).

Table 3.
Characteristics of studies included in the study: outcome: Prognostic.
3.3. Results of Syntheses for Diagnosis
The meta-analysis of the six studies meeting the eligibility criteria yielded a pooled AUC of 0.87 (95% CI: 0.80, 0.92), indicating robust diagnostic accuracy for exosomes as potential biomarkers for cervical cancer (Figure 2).
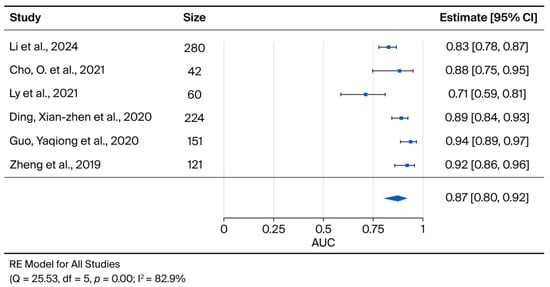
Figure 2.
The forest-plot displays the Area under the curve (AUC) for each selected paper, along with the summary measure and its 95% confidence intervals.
The age of participants varied considerably across the included studies, spanning a broad spectrum from <45 years [22] to >60 years [24,27] (details in Table 4). Reporting of Human Papillomavirus (HPV) status was inconsistent; while some studies documented HPV infection in a subset of cases (Positive: 97, Negative: 17) [21], others lacked this information [22,23]. Reported disease stages according to the FIGO classification ranged from early (I–II) to advanced (III–IV) disease.

Table 4.
Patient Characteristics: Outcome: Diagnosis.
Regarding exosome-specific biomarkers, differential expression patterns were observed in cervical cancer patients compared with healthy controls and/or individuals with CIN across various cargo types, including microRNAs (miRNAs) [23,25,26,27,30,31], lncRNAs [21,22], tRNA fragments (tRFs) [24], and proteins [28,29,32]. These findings suggest that these exosomal constituents mediate critical roles in tumorigenesis, immune modulation, and intercellular communication.
Several miRNAs, including miR-142-3p [23], miR-125a-5p [25], and a panel of eight miRNAs (let-7a-3p, let-7d-3p, miR-30d-5p, miR-144-5p, miR-182-5p, miR-183-5p, miR-215-5p, and miR-4443) [27], have been implicated in cervical carcinogenesis, potentially acting as oncogenes or tumour suppressors. Their exosomal enrichment and differential expression may influence signalling pathways governing proliferation, apoptosis, and metastasis.
Furthermore, the upregulation of lncRNA DLX6-AS1 [21] and lncRNA-EXOC7 [22] identified these transcripts as potential diagnostic biomarkers. The individual study AUCs for these specific biomarkers ranged from 0.71 for miR-125a-5p [25] to 0.94 for lncRNA-EXOC7 (analysed in serum and exosomes) [22]. Among the evaluated targets, lncRNA-EXOC7 appeared to possess the greatest potential for early diagnosis, demonstrating AUC values of 0.9388 and 0.8982 in serum and plasma, respectively [22] (Table 2).
Due to substantial variations in patient populations, subgroup analyses based on HPV status, histology, or disease stage were not feasible. Furthermore, statistical heterogeneity across the included studies was substantial (I2 = 82.9%), justifying the use of a random-effects model in the meta-analysis.
3.4. Results of Syntheses for Prognosis
Significant heterogeneity was observed regarding study populations and methodologies across the four studies included for prognostic analysis. Patient cohorts encompassed a broad age range, extending from early adulthood to the post-menopausal period. Histological subtypes were predominantly squamous cell carcinoma; however, several studies also included adenocarcinoma [30,31] and, less frequently, adenosquamous carcinoma [30,31]. Disease staging varied considerably, ranging from early (FIGO I–II) to advanced stages (FIGO III–IV) (Table 3 and Table 5).

Table 5.
Patient Characteristics: Outcome: Prognosis.
Regarding biomarkers, considerable methodological variability was evident, particularly concerning the isolation and quantification of exosomes. Furthermore, the lack of consensus regarding reference values for exosome concentrations in healthy versus diseased individuals limited the interpretability of findings.
Among the included studies, the analysis by Someya et al. [30] demonstrated that a nine-miRNA signature (miR-148a-5p, 1915-3p, 3960, 183-5p, 196b-5p, 200c-3p, 182-5p, 374a-5p, and 431-5p) held the most significant prognostic potential, exhibiting differential expression between patients with no evidence of recurrence and those experiencing relapse. Further analysis revealed an inverse correlation between miR-374a-5p/miR-431-5p and tumour-infiltrating T cells (CD8+ and FOXP3+), implying a possible role in immune suppression and resistance to concurrent chemoradiotherapy (CCRT), thus underscoring its potential in personalised cervical cancer management [30].
3.5. Reporting Biases
Potential publication bias related to diagnostic outcomes was assessed using Egger’s test, yielding a p-value of 0.436, which suggests no significant evidence of publication bias (Figure 3).
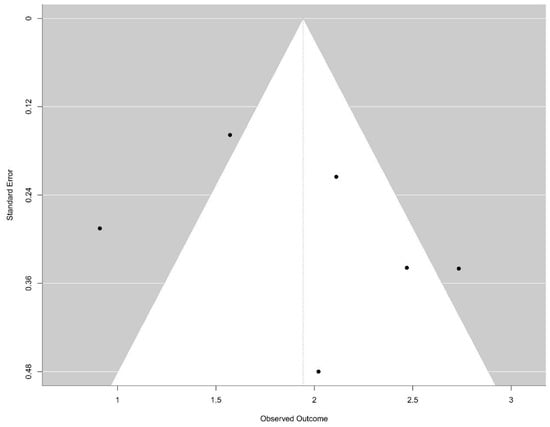
Figure 3.
Funnel-plot: There is no evidence of bias publication (Egger test, p = 0.436).
Visual inspection of the funnel plot revealed a relatively symmetrical distribution of studies around the pooled effect size; however, the limited number of included studies (n = 6) restricted the statistical power to detect true asymmetry. Regarding prognostic outcomes, the included studies exhibited several limitations and high heterogeneity in study design and reporting quality.
The GRADE assessment indicated that confidence in the pooled estimates from this review is significantly compromised, primarily due to a high risk of bias within individual studies, marked inconsistency across findings, potential indirectness stemming from sample and methodological heterogeneity, and evidence of imprecision. Collectively, these factors preclude firm conclusions regarding the investigated diagnostic modalities (Table 6). A summary of the risk of bias identified across studies is detailed in Table 7.

Table 6.
GRADE assessment for the certainty of evidence.

Table 7.
Risk assessment.
4. Discussion
4.1. Summary of Main Results
This systematic review and meta-analysis reinforce the emerging paradigm of extracellular vesicles, specifically exosomes, as promising non-invasive biomarkers, particularly in the context of cervical cancer. The high diagnostic accuracy identified, with a combined AUC of 0.87 (95% CI: 0.80, 0.92), underscores their potential for early detection. The study also highlighted that the prognostic utility of extracellular vesicles remains inconclusive, a finding constrained by significant methodological heterogeneity among the included studies.
4.2. Results in the Context of Published Literature
The high diagnostic accuracy found in our review is consistent with results from studies in other tumour types, such as gastric [33], colorectal [34], and breast cancer [35], where EV-associated biomarkers have demonstrated AUC values ranging from 0.81 to 0.91.
These findings underscore the potential biological advantages of liquid biopsies over conventional methods, particularly regarding the quantitative characteristics of these analytes. Mechanistically, this diagnostic sensitivity is underpinned by the high abundance of circulating extracellular vesicles. In contrast to rare circulating tumour cells (<10 cells/mL), exosomes are present in plasma at concentrations typically ranging from 109 to 1012 particles/mL, providing a vast reservoir of tumour-derived material for downstream analysis [36]. Furthermore, while absolute diagnostic cutoff values vary across studies due to differing normalisation strategies, the discriminatory power of these biomarkers is driven by the magnitude of their differential expression. For instance, our analysis highlighted markers such as lncRNA-EXOC7, which exhibited pronounced fold-changes, ranging from 2.93- to 3.18-fold elevations in cervical cancer patients relative to healthy controls. Such substantial signal-to-noise ratios are critical for early detection, enabling the identification of disease prior to the onset of symptoms [22].
These results emphasise the broad potential of extracellular vesicles as non-invasive diagnostic tools across various malignancies, which is crucial given the limitations of conventional diagnostic methods and the often advanced stage at diagnosis [37].
Consistent with other cancers, cervical cancer exhibits differential expression patterns of EV-associated molecules, including miRNAs, lncRNAs, and proteins. This suggests shared mechanisms in cancer progression and immune modulation across various malignancies. Moreover, the discovery of specific biomarkers, like lncRNA-EXOC7, with high diagnostic potential echoes findings for other tumours, where unique EV cargoes correlate with distinct cancer types [37].
However, a recurring theme in the broader EV literature, and a pivotal finding in our analysis, is the considerable heterogeneity among studies. This methodological variability is not unique to cervical cancer research and is a primary reason why our review was unable to perform a meta-analysis on the prognostic utility of extracellular vesicles. This limitation is also evident in the literature on endometrial cancer, where EV-associated proteins have shown high diagnostic accuracy and links to recurrence risk [38], yet studies on EV-derived miRNA signatures have revealed substantial inconsistencies, with only a minority of differentially expressed miRNAs overlapping between vesicular and whole serum analyses [39]. Similarly, while EV-associated miRNAs such as miR-21-5p and miR-214-3p have been implicated in predicting chemoresistance in ovarian cancer [40], clinical studies remain limited by small sample sizes and considerable variability in detection platforms [41,42]. In breast cancer, meta-analyses have identified EV-associated proteins like PD-L2 and sHLA-G as robust prognostic markers for overall and disease-free survival [43]. Furthermore, the presence of PD-L1-positive extracellular vesicles has shown potential in predicting response to immunotherapy, particularly in triple-negative breast cancer subtypes [44]. Nevertheless, conflicting associations with patient outcomes persist, mainly due to tumour heterogeneity and divergent EV detection and characterisation methodologies.
4.3. Strengths and Weaknesses
The primary strength of this study lies in its novelty as the first dedicated quantitative synthesis of the current evidence landscape. In contrast to previous reviews that have addressed the role of EVs in gynecological malignancies in a broad or qualitative manner [11], we provide a targeted meta-analysis specifically for cervical cancer, establishing a robust pooled AUC of 0.87. Furthermore, our strict adherence to PRISMA guidelines ensures a systematic and transparent appraisal of the literature. This methodological rigour not only minimizes selection bias but also confers a high degree of statistical power to our conclusions, distinguishing these findings from earlier descriptive summaries.
The primary weakness of the review is the significant heterogeneity and high risk of bias identified across the EV cargo analysis of the included studies. Although a meta-analysis was performed on the overall diagnostic accuracy of extracellular vesicles, the extreme variability in the types of EV-associated molecules (miRNAs, lncRNAs, and proteins) analysed and the lack of standardised protocols limited the capacity to draw definitive conclusions about the specific cargoes. This methodological inconsistency is compounded by inherent technical challenges in the field. As discussed by Thakur et al., the lack of standardized isolation and purification methods leads to variability in vesicle yield and purity, affecting the reproducibility of results. Furthermore, the co-isolation of non-vesicular contaminants, such as lipoproteins and protein aggregates, alongside difficulties in normalizing data from complex clinical biofluids, remains a critical hurdle for the clinical translation of EV biomarkers [45]. These factors ultimately affect the generalisability of results, requiring a cautious interpretation of the findings.
4.4. Implications for Practice and Future Research
The high diagnostic accuracy of extracellular vesicle biomarkers suggests their potential for future use in clinical practice as a non-invasive screening or diagnostic tool for cervical cancer, which could improve early detection rates and patient outcomes. However, the studies analyzed herein relied predominantly on standard characterisation techniques, such as Western blotting, transmission electron microscopy (TEM), and nanoparticle tracking analysis (NTA). While fundamental, these bulk methods often lack the sensitivity to capture stochastic heterogeneity or detect trace-level cargoes in complex clinical matrices. To bridge the gap towards clinical translation, future workflows must adopt a rigorous two-step paradigm: standardized isolation followed by high-resolution profiling. First, adherence to MISEV guidelines is imperative to ensure the reproducible enrichment of vesicles using established methods like ultracentrifugation or size exclusion chromatography [7].
Subsequently, the integration of advanced biophysical and nanoplasmonic platforms is required for detailed downstream characterisation. Atomic Force Microscopy (AFM) offers a distinct advantage in post-isolation assessment, enabling the nanomechanical analysis of vesicle size, morphology, and stiffness, thereby confirming the structural integrity of the isolates [46]. Complementarily, optical techniques such as Localized Surface Plasmon Resonance (LSPR) and Surface-Enhanced Raman Scattering (SERS) provide unparalleled sensitivity for molecular interrogation. Once vesicles are captured on sensor surfaces, LSPR facilitates label-free quantification, while SERS allows for the ‘molecular fingerprinting’ of specific cargoes, including HPV E6/E7 oncoproteins, at the single-vesicle level [47,48].
For future research, validating this integrated workflow of robust isolation and high-sensitivity detection in large-scale, prospective multicentric cohorts represents the requisite next step to confirm the diagnostic and prognostic utility of extracellular vesicles in routine oncological practice.
5. Conclusions
In conclusion, this systematic review and meta-analysis provide robust evidence for the diagnostic potential of extracellular vesicles as biomarkers for cervical cancer. While their promise as prognostic indicators is not yet fully established, the findings support a crucial shift towards more standardised methodologies to unlock the full clinical utility of these non-invasive biomarkers.
Author Contributions
F.S., Writing—original draft, Conceptualization, Investigation and Methodology. M.F.-D., Writing—review & editing, Validation, Supervision, Methodology, and Conceptualisation. R.J.N., Writing—review & editing. M.M.S., Writing—review & editing, Data curation and Methodology. J.M.P.T., Writing—review & editing and Validation; F.A.C., Formal analysis, Data curation, and Conceptualisation. L.P.A., Writing—review & editing and Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article as all data were obtained from previously published studies, which are cited within the manuscript.
Acknowledgments
During the preparation of this manuscript, the author used OpenEvidence, for the purposes of text suggestion. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CC | cervical cancer |
| EVs | Extracellular Vesicles |
| AUC | Area Under the Curve |
| miRNAs | microRNAs |
| lncRNAs | long non-coding RNAs |
| ctDNA | circulating tumour DNA |
References
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020, Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018, a worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Lindquist, S.; Kjaer, S.K.; Frederiksen, K.; Ornskov, D.; Munk, C.; Waldstrom, M. Comparative analysis of HPV testing versus cytology in Danish cervical cancer screening: Insights from a large-scale implementation study. Gynecol. Oncol. 2024, 191, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer-Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Jalali, M.; Lu, Y.; del Real Mata, C.; Rak, J.; Mahshid, S. Nanoscopic technologies toward molecular profiling of single extracellular vesicles for cancer liquid biopsy. Appl. Phys. Rev. 2025, 12, 011312. [Google Scholar] [CrossRef]
- Lawrence, S.R.; Shah, K.M. Prospects and Current Challenges of Extracellular Vesicle-Based Biomarkers in Cancer. Biology 2024, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The biology, function, and applications of exosomes in cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef]
- Leetanaporn, K.; Hanprasertpong, J.; Navakanitworakul, R. Molecular insights and clinical impacts of extracellular vesicles in cancer. Oncol. Rev. 2021, 15, 542. [Google Scholar] [CrossRef]
- Buglyó, B.S.G. The Role of Exosomes in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 8. [Google Scholar] [CrossRef]
- Wang, K.H.; Ding, D.C. The Role and Applications of Exosomes in Gynecological Cancer: A Review. Cell Transplant. 2023, 32, 9636897231195240. [Google Scholar] [CrossRef]
- Cakir, M.O.; Selek, M.; Yilmaz, B.; Ozdogan, M.; Ashrafi, G.H. Exosomes in HPV-Associated Cancers: From Biomarkers to Engineered Therapeutics. Cancers 2025, 17, 3386. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Mallett, S.; Takwoingi, Y.; Davenport, C.F.; Hyde, C.J.; Whiting, P.F.; Deeks, J.J.; Leeflang, M.M.; QUADAS-C Group. QUADAS-C: A Tool for Assessing Risk of Bias in Comparative Diagnostic Accuracy Studies. Ann. Intern. Med. 2021, 174, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.M.; Paik, S.; Hayes, D.F. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J. Natl. Cancer Inst. 2009, 101, 1446–1452. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumour MARKer prognostic studies (REMARK). Eur. J. Cancer 2005, 41, 1690–1696. [Google Scholar] [CrossRef]
- Hamza, T.; Schwarzer, G.; Salanti, G. crossnma: An R package to synthesize cross-design evidence and cross-format data using network meta-analysis and network meta-regression. BMC Med. Res. Methodol. 2024, 24, 169. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev 2019, 10, Ed000142. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Ding, X.Z.; Zhang, S.Q.; Deng, X.L.; Qiang, J.H. Serum Exosomal lncRNA DLX6-AS1 Is a Promising Biomarker for Prognosis Prediction of Cervical Cancer. Technol. Cancer Res. Treat. 2021, 20, 1533033821990060. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Wang, K.; He, Y. Appraising the Value of Serum and Serum-Derived Exosomal LncRNA-EXOC7 as a Promising Biomarker in Cervical Cancer. Clin. Lab. 2020, 66, 1357–1363. [Google Scholar] [CrossRef]
- Cho, O.; Kim, D.W.; Cheong, J.Y. Screening Plasma Exosomal RNAs as Diagnostic Markers for Cervical Cancer: An Analysis of Patients Who Underwent Primary Chemoradiotherapy. Biomolecules 2021, 11, 1691. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yang, R.; Jin, X.; Han, Q.; She, Z.; Ge, P. Plasma-Derived Exosomal tRF-Phe-GAA-001 and tRF-Gly-GCC-037 as Novel Diagnostic Biomarkers for Cervical Cancer. Indian J. Clin. Biochem. 2024, 40, 683–690. [Google Scholar] [CrossRef]
- Lv, A.; Tu, Z.; Huang, Y.; Lu, W.; Xie, B. Circulating exosomal miR-125a-5p as a novel biomarker for cervical cancer. Oncol. Lett. 2020, 21, 54. [Google Scholar] [CrossRef]
- Ma, G.; Song, G.; Zou, X.; Shan, X.; Liu, Q.; Xia, T.; Zhou, X.; Zhu, W. Circulating plasma microRNA signature for the diagnosis of cervical cancer. Cancer Biomark. 2019, 26, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Hou, L.; Ma, Y.; Zhou, L.; Wang, F.; Cheng, B.; Wang, W.; Lu, B.; Liu, P.; Lu, W.; et al. Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol. Cancer 2019, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Molika, P.; Leetanaporn, K.; Rungkamoltip, P.; Roytrakul, S.; Hanprasertpong, J.; Navakanitworakul, R. Proteomic analysis of small extracellular vesicles unique to cervical cancer. Transl. Cancer Res. 2023, 12, 3113–3128. [Google Scholar] [CrossRef] [PubMed]
- Ao, K.; Yin, M.; Lyu, X.; Xiao, Y.; Chen, X.; Zhong, S.; Wen, X.; Yuan, J.; Ye, M.; Zhang, J.; et al. METTL3-mediated HSPA9 m6A modification promotes malignant transformation and inhibits cellular senescence by regulating exosomal mortalin protein in cervical cancer. Cancer Lett. 2024, 587, 216658. [Google Scholar] [CrossRef]
- Someya, M.; Hasegawa, T.; Tsuchiya, T.; Kitagawa, M.; Fukushima, Y.; Gocho, T.; Mafune, S.; Ikeuchi, Y.; Kozuka, Y.; Idogawa, M.; et al. Predictive value of an exosomal microRNA-based signature for tumor immunity in cervical cancer patients treated with chemoradiotherapy. Med. Mol. Morphol. 2023, 56, 38–45. [Google Scholar] [CrossRef]
- Cho, O.; Kim, D.W.; Cheong, J.Y. Plasma Exosomal miRNA Levels after Radiotherapy Are Associated with Early Progression and Metastasis of Cervical Cancer: A Pilot Study. J. Clin. Med. 2021, 10, 2110. [Google Scholar] [CrossRef]
- Molika, P.; Bissanum, R.; Surachat, K.; Pattanapanyasat, K.; Hanprasertpong, J.; Chotigeat, W.; Navakanitworakul, R. Exploration of Extracellular Vesicle Long Non-Coding RNAs in Serum of Patients with Cervical Cancer. Oncology 2024, 102, 53–66. [Google Scholar] [CrossRef]
- Xue, J.; Qin, S.; Ren, N.; Guo, B.; Shi, X.; Jia, E. Extracellular vesicle biomarkers in circulation for the diagnosis of gastric cancer: A systematic review and meta-analysis. Oncol. Lett. 2023, 26, 423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, T.; Wu, Q.; Yang, X.; Hao, J.; Deng, X.; Yang, S.; Gu, C.; Wang, Z. Diagnostic performance of various liquid biopsy methods in detecting colorectal cancer: A meta-analysis. Cancer Med. 2020, 9, 5699–5707. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Tian, X.; Wang, Q.; Huang, H.; Lu, X.; Qi, M.; Cao, X.; Lei, J. Diagnostic and predictive value of liquid biopsy-derived exosome miR-21 for breast cancer: A systematic review and meta-analysis. Expert. Rev. Mol. Diagn. 2023, 23, 315–324. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Andresen, T.L.; Simonsen, J.B. What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 109–116. [Google Scholar] [CrossRef]
- Andre, M.; Caobi, A.; Miles, J.S.; Vashist, A.; Ruiz, M.A.; Raymond, A.D. Diagnostic potential of exosomal extracellular vesicles in oncology. BMC Cancer 2024, 24, 322. [Google Scholar] [CrossRef]
- Herrero, C.; de la Fuente, A.; Casas-Arozamena, C.; Sebastian, V.; Prieto, M.; Arruebo, M.; Abalo, A.; Colás, E.; Moreno-Bueno, G.; Gil-Moreno, A.; et al. Extracellular Vesicles-Based Biomarkers Represent a Promising Liquid Biopsy in Endometrial Cancer. Cancers 2019, 11, 2000. [Google Scholar] [CrossRef]
- Paterson, E.; Blenkiron, C.; Danielson, K.; Henry, C. Recommendations for extracellular vesicle miRNA biomarker research in the endometrial cancer context. Transl. Oncol. 2022, 23, 101478. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kim, H.S.; Park, S.J.; Lee, E.J.; Kim, S.I.; Song, G.; Lim, W. Inhibition of miR-214-3p Aids in Preventing Epithelial Ovarian Cancer Malignancy by Increasing the Expression of LHX6. Cancers 2019, 11, 1917. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Lei, N.; Zhou, J.; Chen, M.; Guo, R.; Qin, B.; Li, Y.; Chang, L. Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion. Cell Death Dis. 2022, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Su, X.; Li, S.; Liu, Z.; Wang, Q.; Zeng, H. Low serum exosomal miR-484 expression predicts unfavorable prognosis in ovarian cancer. Cancer Biomark. 2020, 27, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, O.; Wormland, S.; Bittner, A.K.; Collenburg, M.; Horn, P.A.; Kimmig, R.; Kasimir-Bauer, S.; Rebmann, V. Programmed death receptor ligand-2 (PD-L2) bearing extracellular vesicles as a new biomarker to identify early triple-negative breast cancer patients at high risk for relapse. J. Cancer Res. Clin. Oncol. 2023, 149, 1159–1174. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.; Li, X.; Guo, A. CCAAT enhancer binding protein delta activates vesicle associated membrane protein 3 transcription to enhance chemoresistance and extracellular PD-L1 expression in triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2024, 43, 115. [Google Scholar] [CrossRef]
- Thakur, A.; Parra, D.C.; Motallebnejad, P.; Brocchi, M.; Chen, H.J. Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 2022, 10, 281–294. [Google Scholar]
- Ridolfi, A.; Brucale, M.; Montis, C.; Caselli, L.; Paolini, L.; Borup, A.; Boysen, A.T.; Loria, F.; van Herwijnen, M.J.C.; Kleinjan, M.; et al. AFM-Based High-Throughput Nanomechanical Screening of Single Extracellular Vesicles. Anal. Chem. 2020, 92, 10274–10282. [Google Scholar] [CrossRef]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef]
- Beeram, R.; Vepa, K.R.; Soma, V.R. Recent Trends in SERS-Based Plasmonic Sensors for Disease Diagnostics, Biomolecules Detection, and Machine Learning Techniques. Biosensors 2023, 13, 328. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).