Intraoperative Ultrasound in the Management of Rare Lesions Involving the Intradural Extramedullary Spinal Compartment: A Quick, but Effective Helping Hand to Define the Optimal Surgical Strategy
Simple Summary
Abstract
1. Introduction
2. Disembryogenetic Cysts
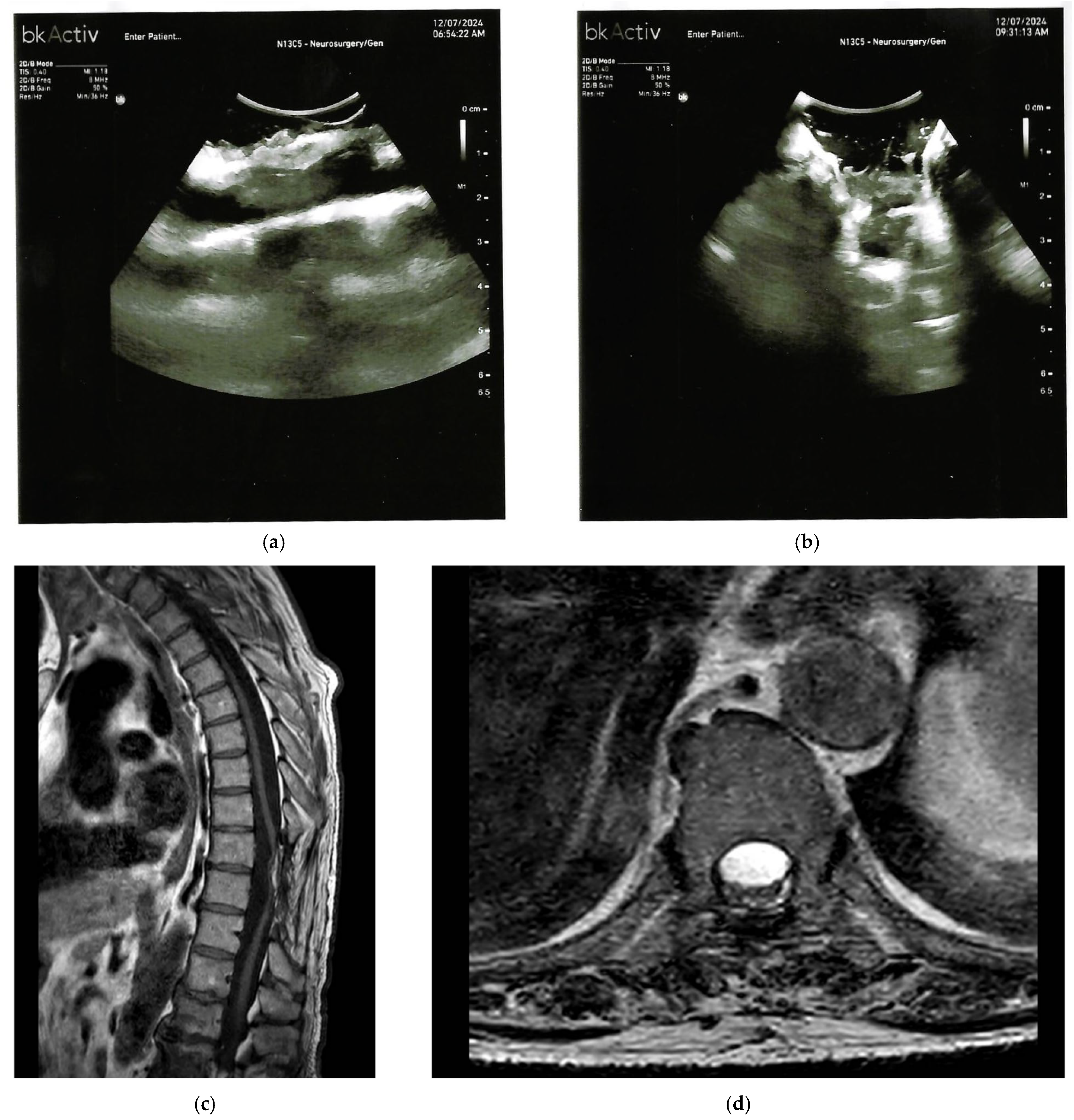
3. Spinal Chronic Subdural Hematoma (CSSDH)
4. Spinal Cord Tethering
5. Hemangioblastomas
6. Intradural Spinal Arachnoid Cysts
| Lesion Type | MRI Characteristics | IOUS Characteristics |
|---|---|---|
| Neuroenteric or Respiratory Cysts | Well circumscribed, cerebrospinal fluid-like signal on T2; no enhancing solid component; focal cord compression. | Anechoic or hypoechoic cavity with well-defined margins, echogenic lining, occasional septations; eccentric cord displacement. |
| Spinal Chronic Subdural Hematoma (CSSDH) | Hyperintense on T1 or T2 (late subacute); isointense or hypointense (chronic); elongated dorsal collection with cord displacement. | Hypoechoic or anechoic subdural collection beneath echogenic dura; internal echoes or septations; bounded by echogenic membrane. |
| Spinal Cord Tethering or Scarring | Focal dorsal cerebrospinal fluid loculation; anterior cord displacement (“scalpel sign”); adhesions or thickened filum. | Hyperechoic adhesions bridging dura and cord; effaced subarachnoid space; reduced cord excursion; stiffness reduction post detethering (for example, 93.84 kilopascals to 9.35 kilopascals via shear wave elastography). |
| Intradural Extramedullary Hemangioblastomas | Well circumscribed nodule with cystic components or syrinx; enhancing on contrast; associated with von Hippel Lindau disease. | Well defined, homogeneous, isoechoic nodule; cystic components; vascular profiling via Doppler or micro-Doppler for feeders or drainers. |
| Intradural Spinal Arachnoid Cysts or Webs | Cerebrospinal fluid filled; subtle subarachnoid communications; cord compression if symptomatic. | Anechoic cavities with definable walls; subtle communications; boundaries and cerebrospinal fluid flow assessment. |
7. Summary and Key Message
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| CSF | cerebrospinal fluid |
| CSSDH | chronic spinal subdural hematoma |
| CT | computed tomography |
| GTR | gross total resection |
| iCT | intraoperative computed tomography |
| iMRI | intraoperative magnetic resonance imaging |
| IOUS | intraoperative ultrasound |
| MRI | magnetic resonance imaging |
| SWE | shear wave elastography |
| TCS | tethered cord syndrome |
| VHL | Von Hippel–Lindau (disease) |
| µDoppler | micro-Doppler ultrasound |
References
- Prada, F.; Vetrano, I.G.; Filippini, A.; Del Bene, M.; Perin, A.; Casali, C.; Legnani, F.; Saini, M.; DiMeco, F. Intraoperative ultrasound in spinal tumor surgery. J. Ultrasound 2014, 17, 195–202. [Google Scholar] [CrossRef]
- Haciyakupoglu, E.; Yuvruk, E.; Onen, M.R.; Naderi, S. The Use of Intraoperative Ultrasonography in Intradural Spinal Tumor Surgery. Turk. Neurosurg. 2019, 29, 237–241. [Google Scholar] [CrossRef]
- Coburger, J.; König, R.W.; Scheuerle, A.; Engelke, J.; Hlavac, M.; Thal, D.R.; Wirtz, C.R. Navigated High Frequency Ultrasound: Description of Technique and Clinical Comparison with Conventional Intracranial Ultrasound. World Neurosurg. 2014, 82, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Budu, A.; Sims-Williams, H.; Poeata, I. Using Intraoperative Ultrasonography for Spinal Cord Tumor Surgery. World Neurosurg. 2017, 97, 104–111. [Google Scholar] [CrossRef]
- Unsgaard, G.; Ommedal, S.; Muller, T.; Gronningsaeter, A.; Nagelhus Hernes, T.A. Neuronavigation by intraoperative three-dimensional ultrasound: Initial experience during brain tumor resection. Neurosurgery 2002, 50, 804–812; discussion 812. [Google Scholar] [CrossRef]
- Fredrickson, V.L.; Russin, J.J.; Strickland, B.A.; Bakhsheshian, J.; Amar, A.P. Intraoperative Imaging for Vascular Lesions. Neurosurg. Clin. N. Am. 2017, 28, 603–613. [Google Scholar] [CrossRef]
- Hall, W.A.; Truwit, C.L. Intraoperative magnetic resonance imaging. Acta Neurochir. Suppl. 2011, 109, 119–129. [Google Scholar] [CrossRef]
- Coburger, J.; Scheuerle, A.; Thal, D.R.; Engelke, J.; Hlavac, M.; Wirtz, C.R.; König, R. Linear array ultrasound in low-grade glioma surgery: Histology-based assessment of accuracy in comparison to conventional intraoperative ultrasound and intraoperative MRI. Acta Neurochir. 2015, 157, 195–206. [Google Scholar] [CrossRef]
- Konovalov, N.A.; Onoprienko, R.A.; Kaprovoy, S.V.; Brinyuk, E.S.; Zakirov, B.A.; Poluektov, Y.M.; Ivanov, S.V.; Kudymets, M.O.; Ilyinsky, N.O. Surgical treatment of intramedullary spinal cord tumors: A systematic review. Zh. Vopr. Neirokhir. Im. N. N. Burdenko 2025, 89, 103–108. [Google Scholar] [CrossRef]
- Smith, H.; Taplin, A.; Syed, S.; Adamo, M.A. Correlation between intraoperative ultrasound and postoperative MRI in pediatric tumor surgery. J. Neurosurg. Pediatr. 2016, 18, 578–584. [Google Scholar] [CrossRef]
- Gopakumar, S.; Gadgil, N.; McDonald, M.F.; Gadot, R.; Ropper, A.E. Neurenteric Cyst: Case Report and Operative Video. Cureus 2020, 12, e8714. [Google Scholar] [CrossRef]
- Gauden, A.J.; Khurana, V.G.; Tsui, A.E.; Kaye, A.H. Intracranial neuroenteric cysts: A concise review including an illustrative patient. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2012, 19, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Breshears, J.D.; Rutkowski, M.J.; McDermott, M.W.; Cha, S.; Tihan, T.; Theodosopoulos, P.V. Surgical Management of Intracranial Neuroenteric Cysts: The UCSF Experience. J. Neurol. Surg. Part B Skull Base 2015, 76, 475–479. [Google Scholar] [CrossRef][Green Version]
- Elmesallamy, W.A.E.A.; Yakout, H.; Hassanen, S.; Elshekh, M. The role of intraoperative ultrasound in management of spinal intradural mass lesions and outcome. Egypt. J. Neurosurg. 2023, 38, 38. [Google Scholar] [CrossRef]
- Regelsberger, J.; Fritzsche, E.; Langer, N.; Westphal, M. Intraoperative sonography of intra- and extramedullary tumors. Ultrasound Med. Biol. 2005, 31, 593–598. [Google Scholar] [CrossRef]
- Dauleac, C.; Messerer, R.; Obadia-Andre, N.; Afathi, M.; Barrey, C.Y. Cysts associated with intramedullary ependymomas of the spinal cord: Clinical, MRI and oncological features. J. Neurooncol. 2019, 144, 385–391. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.-H.; Lee, C.-H.; Kim, S.; Kim, Y.-R.; Kim, K.-T.; Kim, J.; Rhee, J.M.; Jo, W.-Y.; Oh, H.; et al. The utility of intraoperative ultrasonography for spinal cord surgery. PLoS ONE 2024, 19, e0305694. [Google Scholar] [CrossRef]
- Abla, A.A.; Oh, M.Y. Spinal chronic subdural hematoma. Neurosurg. Clin. N. Am. 2000, 11, 465–471. [Google Scholar] [CrossRef]
- Diyora, B.; Sharma, A.; Mamidanna, R.; Kamat, L. Chronic cervicothoracic spinal subdural hematoma. Neurol. Med. Chir. 2009, 49, 310–312. [Google Scholar] [CrossRef]
- Fujikawa, Y.; Fujishiro, T.; Tanabe, H.; Takami, T.; Wanibuchi, M. Pathogenesis of Spinal Subdural Hematoma Based on Histopathological Findings: A Case Report. NMC Case Rep. J. 2025, 12, 41–46. [Google Scholar] [CrossRef]
- Braun, P.; Kazmi, K.; Nogués-Meléndez, P.; Mas-Estellés, F.; Aparici-Robles, F. MRI findings in spinal subdural and epidural hematomas. Eur. J. Radiol. 2007, 64, 119–125. [Google Scholar] [CrossRef]
- Vasudeva, V.S.; Abd-El-Barr, M.; Pompeu, Y.A.; Karhade, A.; Groff, M.W.; Lu, Y. Use of Intraoperative Ultrasound During Spinal Surgery. Glob. Spine J. 2017, 7, 648–656. [Google Scholar] [CrossRef]
- Akimoto, J.; Fukuhara, H.; Nakamura, Y.; Yoshioka, Y.; Onodera, S. Spinal Chronic Subdural Hematoma Cured by Lumbar Drainage: A Case Report and Literature Review. NMC Case Rep. J. 2025, 12, 121–125. [Google Scholar] [CrossRef]
- Oichi, Y.; Toda, H.; Yamagishi, K.; Tsujimoto, Y. Multiple Spinal Chronic Subdural Hematomas Associated with Thoracic Hematomyelia: A Case Report and Literature Review. World Neurosurg. 2019, 131, 95–103. [Google Scholar] [CrossRef]
- National institute of Neurological Disorders and Stroke. Tethered Spinal Cord Syndrome. Available online: https://www.ninds.nih.gov/health-information/disorders/tethered-spinal-cord-syndrome (accessed on 25 September 2025).
- Bratelj, D.; Stalder, S.; Capone, C.; Jaszczuk, P.; Dragalina, C.; Pötzel, T.; Gebhard, C.; Fiechter, M. Spinal cord tethering and syringomyelia after trauma: Impact of age and surgical outcome. Sci. Rep. 2023, 13, 11442. [Google Scholar] [CrossRef] [PubMed]
- Keykhosravi, E.; Faravani, E.; Dehghani Dashtabi, S.; Emadzadeh, M.; Alamdaran, S.A.; Aminzadeh, B. Comparison of Ultrasonographic Findings Between Patients with Tethered Cord Syndrome and Healthy Children. Iran. J. Med. Sci. 2023, 48, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Almotairi, F.S.; Basalamah, A.A.; Amir, A.; Al-Habib, A.F. Intraoperative demonstration of reduced distal spinal cord stiffness following untethering of the spinal cord using ultrasound shear wave elastography (SWE). World Neurosurg. X 2023, 20, 100225. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, I.; Valentini, L. The role of intraoperative ultrasound in surgery of occult spinal dysraphisms. Brain Spine 2022, 2, 101569. [Google Scholar] [CrossRef]
- Squintani, G.; Basaldella, F.; Badari, A.; Rasera, A.; Tramontano, V.; Pinna, G.; Moscolo, F.; Soda, C.; Ricci, U.; Ravenna, G.; et al. Intraoperative Neurophysiological Monitoring in Tethered Cord Syndrome Surgery: Predictive Values and Clinical Outcome. J. Clin. Neurophysiol. 2025, 42, 257–263. [Google Scholar] [CrossRef]
- Li, D.; Choe, S.; Borys, E.; Serrone, J.C.; Germanwala, A.V. Primary Intradural Extramedullary Sporadic Spinal Hemangioblastomas: Case Report and Systematic Review. World Neurosurg. 2021, 152, 84–94. [Google Scholar] [CrossRef]
- Kawanishi, M.; Tanaka, H.; Itoh, Y.; Yokoyama, K.; Yamada, M.; Sugie, A.; Miyake, A. Intradural extramedullary hemangioblastoma of the thoracic cord: A case report. Surg. Neurol. Int. 2021, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Soloukey, S.; Verhoef, L.; Generowicz, B.S.; De Zeeuw, C.I.; Koekkoek, S.K.E.; Vincent, A.J.P.E.; Dirven, C.M.F.; Harhangi, B.S.; Kruizinga, P. Case report: High-resolution, intra-operative µDoppler-imaging of spinal cord hemangioblastoma. Front. Surg. 2023, 10, 1153605. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.P.; Zhang, J.; Ghinda, D.; Glikstein, R.; Agid, R.; Rodesch, G.; Tampieri, D.; terBrugge, K.G. Imaging diagnosis and the role of endovascular embolization treatment for vascular intraspinal tumors. Neurosurg. Focus 2015, 39, E16. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, P.; Hejrati, N.; Charalampidis, A.; Wu, P.H.; Schneider, M.; Wilson, J.R.; Gao, A.F.; Massicotte, E.M.; Fehlings, M.G. Spinal arachnoid cysts: A case series & systematic review of the literature. Brain Spine 2022, 2, 100904. [Google Scholar] [CrossRef]
- Klekamp, J. A New Classification for Pathologies of Spinal Meninges-Part 2: Primary and Secondary Intradural Arachnoid Cysts. Neurosurgery 2017, 81, 217–229. [Google Scholar] [CrossRef]
- Zhang, D.; Papavassiliou, E. Spinal Intradural Arachnoid Webs Causing Spinal Cord Compression with Inconclusive Preoperative Imaging: A Report of 3 Cases and a Review of the Literature. World Neurosurg. 2017, 99, 251–258. [Google Scholar] [CrossRef]
- Naggar, A.; El Ouali, I.; Aidi, S.; Melhaoui, A.; Ech-cherif El Kettani, N.; Fikri, M.; Jiddane, M.; Touarsa, F. Spinal arachnoid web: A systematic review of a rare entity, with two illustrative case reports. Egypt. J. Radiol. Nucl. Med. 2024, 55, 178. [Google Scholar] [CrossRef]
- Ramazanoglu, A.F.; Sarikaya, C.; Varol, E.; Aydin, S.O.; Etli, M.U.; Avci, F.; Naderi, S. Surgical treatment of spinal arachnoid cysts: Cyst excision or fenestration? Turk. Neurosurg. 2022, 32, 1002–1006. [Google Scholar] [CrossRef]


| Parameter | Intraoperative Ultrasound (IOUS) | Intraoperative MRI (iMRI) | Intraoperative CT (iCT) |
|---|---|---|---|
| Cost | Low (cost-effective, no specialized room required) | High (requires dedicated operating room and equipment) | Moderate (less expensive than iMRI but requires scanner) |
| Examination Time | Minimal (real time, less than 5 min per scan) | Prolonged (10 to 30 min per scan, interrupts surgery) | Short (5 to 10 min, but may require repositioning) |
| Effectiveness for Lesion Localization | High (real-time visualization of cystic or hypoechoic structures, margins, and cord displacement) | Excellent (superior soft tissue resolution for rare lesions) | Good (bone and gross anatomy, limited for soft tissue) |
| Effectiveness for Resection Control | High (immediate assessment of residuals, Doppler for vascularity, elastography for stiffness) | Excellent (gold standard for residual tumor detection) | Moderate (limited for intradural details without contrast) |
| Radiation Exposure | None | None | Present (ionizing radiation) |
| Real Time Capabilities | Excellent (dynamic imaging during surgery) | Limited (static scans) | Limited (static scans) |
| Advantages for Rare Lesions | Versatile for underreported echo patterns (for example, anechoic cysts, hypoechoic collections); minimizes exposure | Detailed anatomy, but less practical for quick decisions | Useful for bony landmarks but insufficient for cystic or vascular rarities |
| Disadvantages | Operator dependent; lower resolution for deep structures | High cost and time; not real time | Radiation risk; poor soft tissue contrast |
| References | Based on [1,7,8] from the manuscript | Extrapolated from [7,8] | Extrapolated from [7,8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesce, A.; Di Carlo, L.; Palmieri, M.; Novegno, F.; Iaquinandi, A.; Denaro, L.; Armocida, D.; Santoro, A.; Salvati, M.; Ius, T.; et al. Intraoperative Ultrasound in the Management of Rare Lesions Involving the Intradural Extramedullary Spinal Compartment: A Quick, but Effective Helping Hand to Define the Optimal Surgical Strategy. Cancers 2025, 17, 3607. https://doi.org/10.3390/cancers17223607
Pesce A, Di Carlo L, Palmieri M, Novegno F, Iaquinandi A, Denaro L, Armocida D, Santoro A, Salvati M, Ius T, et al. Intraoperative Ultrasound in the Management of Rare Lesions Involving the Intradural Extramedullary Spinal Compartment: A Quick, but Effective Helping Hand to Define the Optimal Surgical Strategy. Cancers. 2025; 17(22):3607. https://doi.org/10.3390/cancers17223607
Chicago/Turabian StylePesce, Alessandro, Luca Di Carlo, Mauro Palmieri, Federica Novegno, Andrea Iaquinandi, Luca Denaro, Daniele Armocida, Antonio Santoro, Maurizio Salvati, Tamara Ius, and et al. 2025. "Intraoperative Ultrasound in the Management of Rare Lesions Involving the Intradural Extramedullary Spinal Compartment: A Quick, but Effective Helping Hand to Define the Optimal Surgical Strategy" Cancers 17, no. 22: 3607. https://doi.org/10.3390/cancers17223607
APA StylePesce, A., Di Carlo, L., Palmieri, M., Novegno, F., Iaquinandi, A., Denaro, L., Armocida, D., Santoro, A., Salvati, M., Ius, T., & Frati, A. (2025). Intraoperative Ultrasound in the Management of Rare Lesions Involving the Intradural Extramedullary Spinal Compartment: A Quick, but Effective Helping Hand to Define the Optimal Surgical Strategy. Cancers, 17(22), 3607. https://doi.org/10.3390/cancers17223607






