Gene Therapy Strategies for Hepatocellular Carcinoma (HCC): Current Landscape and Future Directions
Simple Summary
Abstract
1. Introduction
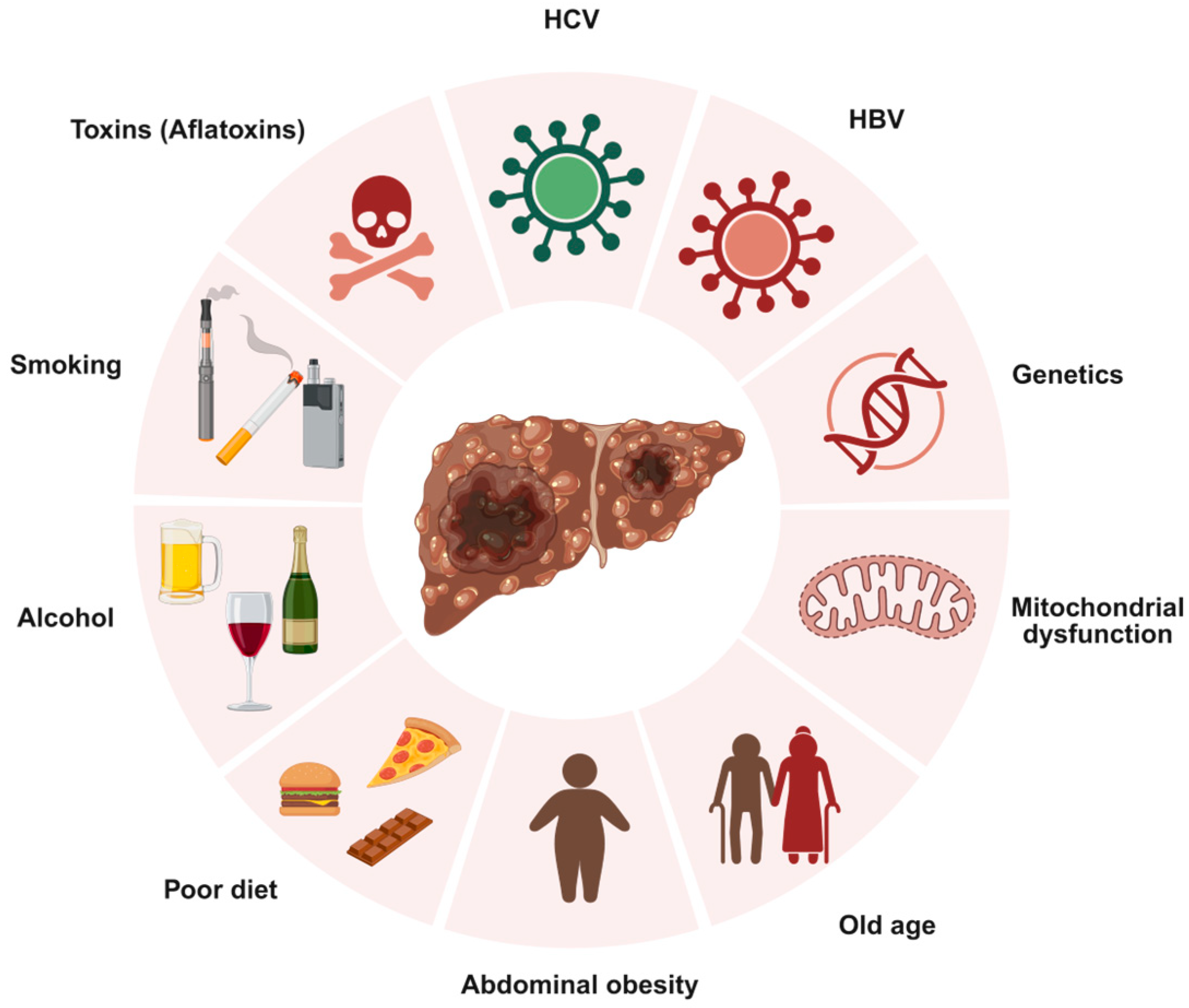
1.1. Hepatocellular Carcinoma (HCC): Epidemiology, Etiology, and Clinical Challenges
1.2. Rationale for Gene Therapy in HCC
1.3. Scope and Objectives of the Review
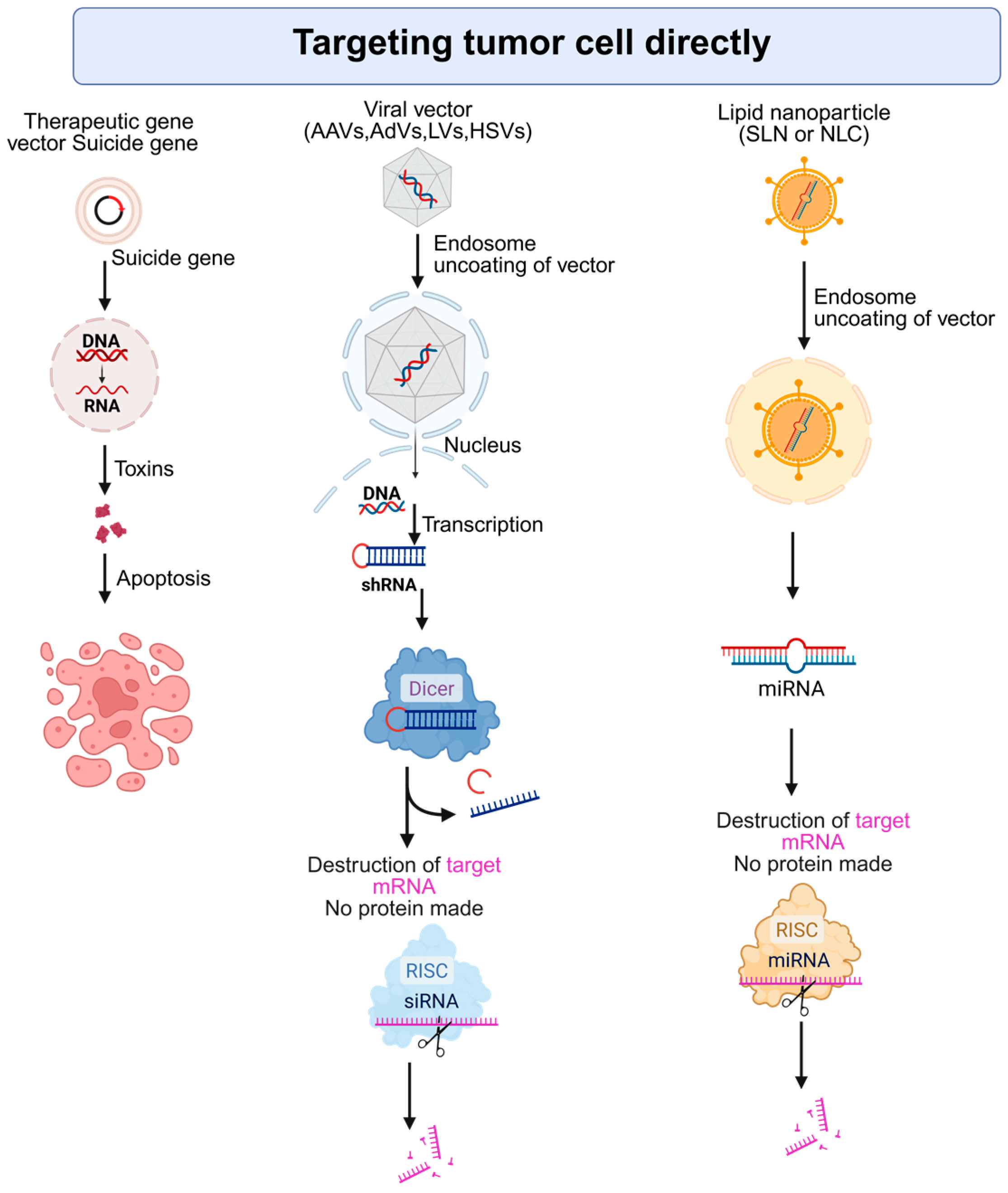
2. Gene Therapy Strategies Targeting Tumor Cells Directly
2.1. Suicide Gene Therapy Triggering Selective Tumor Cell Death
2.1.1. Clinical Application and Trial Outcomes of Suicide Gene Therapy
2.1.2. Enhancing Efficacy of Suicide Gene Therapy with Combination Therapies
2.2. Restoring Tumor Suppressor Function: Focus on p53
2.3. Oncolytic Virotherapy (OV): Harnessing Viruses for Targeted Tumor Destruction
2.3.1. Genetically Engineered Viruses for Tumor Selectivity
2.3.2. Mechanisms of Oncolysis and Immune Activation
2.3.3. Clinical Evidence and Safety Considerations
2.4. Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-Associated Protein 9 (CRISPR/Cas9) Gene Editing in HCC: Precision Medicine at the Genomic Level
2.4.1. Gene Knockout, Knock-In, and Functional Modulation
2.4.2. Delivery and Specificity: Major Barriers to Clinical Translation
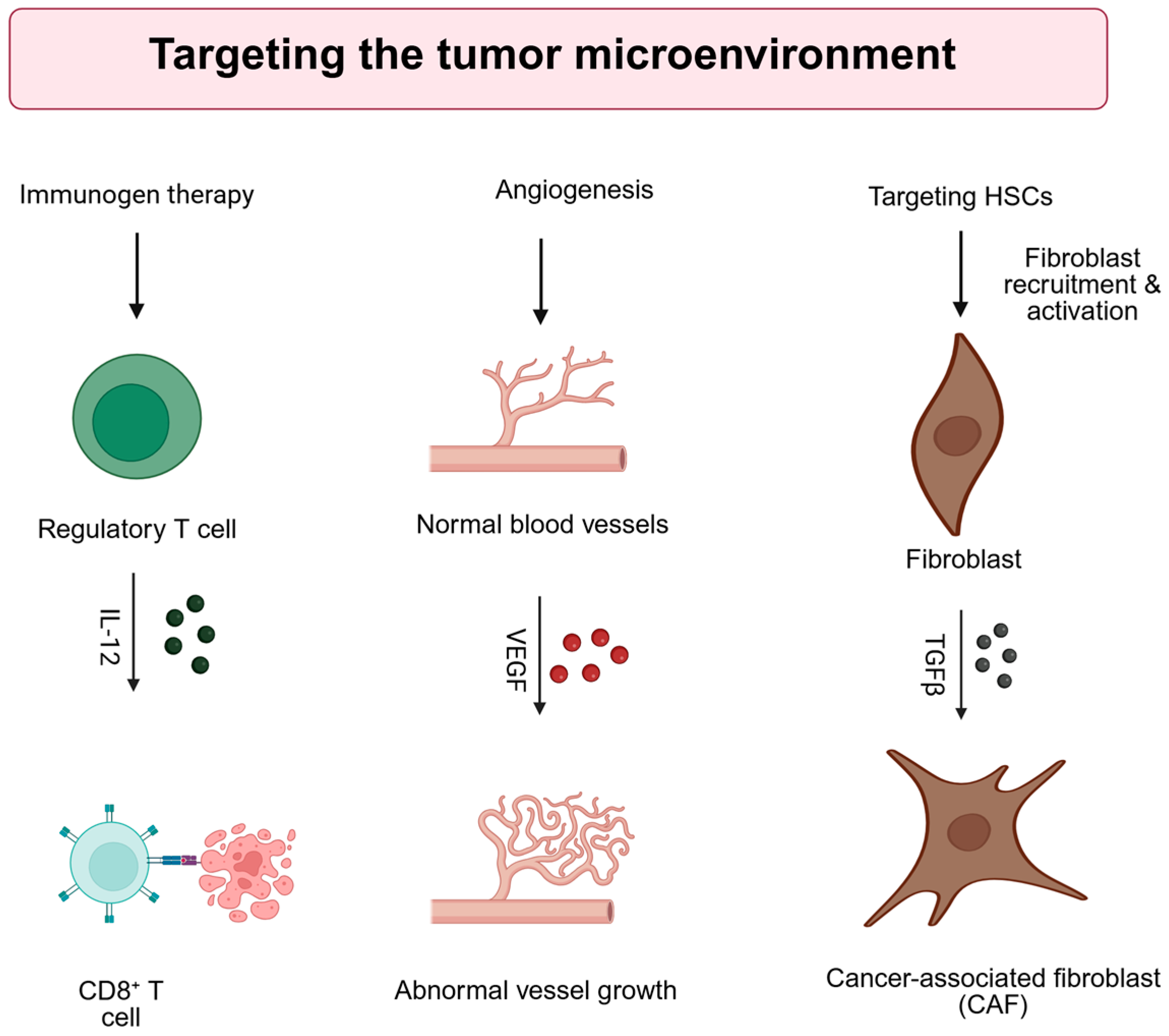
3. Gene Therapy Strategies Targeting the Tumor Microenvironment (TME)
3.1. Immunogen Therapy
3.2. Anti-Angiogenic Gene Therapy
3.3. Gene Therapy Targeting Hepatic Stellate Cells (HSCs)
4. Chimeric Antigen Receptor (CAR)-T Cells
5. Vector Design and Delivery Systems
5.1. Viral Vectors
5.1.1. Adeno-Associated Viruses (AAVs)
5.1.2. Adenoviruses (AdVs)
5.1.3. Lentiviruses (LVs)
5.1.4. Herpes Simplex Viruses (HSVs)
5.2. Non-Viral Vectors
5.2.1. Inorganic Nanoparticles (INs)
5.2.2. Lipid Nanoparticles (LNPs)
5.2.3. Polymeric Nanoparticles (PNPs)
5.3. Safety and Immunogenicity Profiles
5.4. Targeted Delivery Strategies
5.4.1. Transcriptional Targeting: Tumor-Specific Promoters (TSPs)
5.4.2. Ligand-Mediated Targeting
6. Clinical Trials for Gene Therapy and Outcomes of HCC
7. Challenges, Future Directions, and Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef]
- Yarchoan, M.; Agarwal, P.; Villanueva, A.; Rao, S.; Dawson, L.A.; Llovet, J.M.; Finn, R.S.; Groopman, J.D.; El-Serag, H.B.; Monga, S.P.; et al. Recent Developments and Therapeutic Strategies against Hepatocellular Carcinoma. Cancer Res. 2019, 79, 4326–4330. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Toniutto, P.; Zanetto, A.; Ferrarese, A.; Burra, P. Current challenges and future directions for liver transplantation. Liver Int. 2017, 37, 317–327. [Google Scholar] [CrossRef]
- Kim, H.C. Chemoembolization combined with radiofrequency ablation is the best option for the local treatment of early hepatocellular carcinoma? Clin. Mol. Hepatol. 2023, 29, 984–986. [Google Scholar] [CrossRef]
- Lewis, S.; Dawson, L.; Barry, A.; Stanescu, T.; Mohamad, I.; Hosni, A. Stereotactic body radiation therapy for hepatocellular carcinoma: From infancy to ongoing maturity. JHEP Rep. 2022, 4, 100498. [Google Scholar] [CrossRef]
- Chakraborty, E.; Sarkar, D. Emerging Therapies for Hepatocellular Carcinoma (HCC). Cancers 2022, 14, 2798. [Google Scholar] [CrossRef]
- Blaese, R.M.; Culver, K.W.; Miller, A.D.; Carter, C.S.; Fleisher, T.; Clerici, M.; Shearer, G.; Chang, L.; Chiang, Y.; Tolstoshev, P.; et al. T lymphocyte-directed gene therapy for ADA- SCID: Initial trial results after 4 years. Science 1995, 270, 475–480. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.C.; Lu, X.; Huang, Y.; Lin, X.; Mahmood, I.; Przepiorka, D.; Gavin, D.; Lee, S.; Liu, K.; George, B.; et al. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2019, 25, 1142–1146. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.A.; Kersten, M.J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, F.; Kay, M.A. The deLIVERed promises of gene therapy: Past, present, and future of liver-directed gene therapy. Mol. Ther. 2025, 33, 1966–1987. [Google Scholar] [CrossRef]
- Kattenhorn, L.M.; Tipper, C.H.; Stoica, L.; Geraghty, D.S.; Wright, T.L.; Clark, K.R.; Wadsworth, S.C. Adeno-Associated Virus Gene Therapy for Liver Disease. Hum. Gene Ther. 2016, 27, 947–961. [Google Scholar] [CrossRef]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef]
- Shu, S.A.; Wang, J.; Tao, M.H.; Leung, P.S. Gene Therapy for Autoimmune Disease. Clin. Rev. Allergy Immunol. 2015, 49, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Wheeler, D.A.; Roberts, L.R. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.e23. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.; Imbeaud, S.; Letouze, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015, 47, 505–511. [Google Scholar] [CrossRef] [PubMed]
- von Fritschen, M.; Janosz, E.; Blume, C.; Jagle, U.; Keating, K.; Schneider, C.K. What’s in a word? Defining “gene therapy medicines”. Mol. Ther. Methods Clin. Dev. 2024, 32, 101348. [Google Scholar] [CrossRef]
- Duzgunes, N. Origins of Suicide Gene Therapy. Methods Mol. Biol. 2019, 1895, 1–9. [Google Scholar] [CrossRef]
- Beck, C.; Cayeux, S.; Lupton, S.D.; Dorken, B.; Blankenstein, T. The thymidine kinase/ganciclovir-mediated “suicide” effect is variable in different tumor cells. Hum. Gene Ther. 1995, 6, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Moolten, F.L. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: Paradigm for a prospective cancer control strategy. Cancer Res. 1986, 46, 5276–5281. [Google Scholar] [PubMed]
- Senter, P.D.; Su, P.C.; Katsuragi, T.; Sakai, T.; Cosand, W.L.; Hellstrom, I.; Hellstrom, K.E. Generation of 5-fluorouracil from 5-fluorocytosine by monoclonal antibody-cytosine deaminase conjugates. Bioconjugate Chem. 1991, 2, 447–451. [Google Scholar] [CrossRef]
- Qu, L.; Wang, Y.; Gong, L.; Zhu, J.; Gong, R.; Si, J. Suicide gene therapy for hepatocellular carcinoma cells by survivin promoter-driven expression of the herpes simplex virus thymidine kinase gene. Oncol. Rep. 2013, 29, 1435–1440. [Google Scholar] [CrossRef]
- Liu, J.; Zou, W.G.; Lang, M.F.; Luo, J.; Sun, L.Y.; Wang, X.N.; Qian, Q.J.; Liu, X.Y. Cancer-specific killing by the CD suicide gene using the human telomerase reverse transcriptase promoter. Int. J. Oncol. 2002, 21, 661–666. [Google Scholar] [CrossRef]
- Michaelsen, S.R.; Christensen, C.L.; Sehested, M.; Cramer, F.; Poulsen, T.T.; Patterson, A.V.; Poulsen, H.S. Single agent- and combination treatment with two targeted suicide gene therapy systems is effective in chemoresistant small cell lung cancer cells. J. Gene Med. 2012, 14, 445–458. [Google Scholar] [CrossRef]
- Sun, X.; Xing, L.; Deng, X.; Hsiao, H.T.; Manami, A.; Koutcher, J.A.; Clifton Ling, C.; Li, G.C. Hypoxia targeted bifunctional suicide gene expression enhances radiotherapy in vitro and in vivo. Radiother. Oncol. 2012, 105, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kawashita, Y.; Ohtsuru, A.; Kaneda, Y.; Nagayama, Y.; Kawazoe, Y.; Eguchi, S.; Kuroda, H.; Fujioka, H.; Ito, M.; Kanematsu, T.; et al. Regression of hepatocellular carcinoma in vitro and in vivo by radiosensitizing suicide gene therapy under the inducible and spatial control of radiation. Hum. Gene Ther. 1999, 10, 1509–1519. [Google Scholar] [CrossRef]
- Sangro, B.; Mazzolini, G.; Ruiz, M.; Ruiz, J.; Quiroga, J.; Herrero, I.; Qian, C.; Benito, A.; Larrache, J.; Olague, C.; et al. A phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther. 2010, 17, 837–843. [Google Scholar] [CrossRef]
- Li, N.; Zhou, J.; Weng, D.; Zhang, C.; Li, L.; Wang, B.; Song, Y.; He, Q.; Lin, D.; Chen, D.; et al. Adjuvant adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of liver transplantation in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2007, 13, 5847–5854. [Google Scholar] [CrossRef]
- Naing, C.; Ni, H.; Aung, H.H.; Htet, N.H.; Nikolova, D. Gene therapy for people with hepatocellular carcinoma. Cochrane Database Syst. Rev. 2024, 6, CD013731. [Google Scholar] [CrossRef]
- Marukawa, Y.; Nakamoto, Y.; Kakinoki, K.; Tsuchiyama, T.; Iida, N.; Kagaya, T.; Sakai, Y.; Naito, M.; Mukaida, N.; Kaneko, S. Membrane-bound form of monocyte chemoattractant protein-1 enhances antitumor effects of suicide gene therapy in a model of hepatocellular carcinoma. Cancer Gene Ther. 2012, 19, 312–319. [Google Scholar] [CrossRef]
- Han, S.R.; Lee, C.H.; Im, J.Y.; Kim, J.H.; Kim, J.H.; Kim, S.J.; Cho, Y.W.; Kim, E.; Kim, Y.; Ryu, J.H.; et al. Targeted suicide gene therapy for liver cancer based on ribozyme-mediated RNA replacement through post-transcriptional regulation. Mol. Ther. Nucleic Acids 2021, 23, 154–168. [Google Scholar] [CrossRef]
- Li, J.; Zhou, P.; Li, L.; Zhang, Y.; Shao, Y.; Tang, L.; Tian, S. Effects of Cationic Microbubble Carrying CD/TK Double Suicide Gene and alphaVbeta3 Integrin Antibody in Human Hepatocellular Carcinoma HepG2 Cells. PLoS ONE 2016, 11, e0158592. [Google Scholar] [CrossRef][Green Version]
- Hughes, B.W.; Wells, A.H.; Bebok, Z.; Gadi, V.K.; Garver, R.I., Jr.; Parker, W.B.; Sorscher, E.J. Bystander killing of melanoma cells using the human tyrosinase promoter to express the Escherichia coli purine nucleoside phosphorylase gene. Cancer Res. 1995, 55, 3339–3345. [Google Scholar][Green Version]
- Krohne, T.U.; Shankara, S.; Geissler, M.; Roberts, B.L.; Wands, J.R.; Blum, H.E.; Mohr, L. Mechanisms of cell death induced by suicide genes encoding purine nucleoside phosphorylase and thymidine kinase in human hepatocellular carcinoma cells in vitro. Hepatology 2001, 34, 511–518. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, M.; Zhang, Y.; Chen, W.; Zhang, L.; Chen, L. An ultrasonic nanobubble-mediated PNP/fludarabine suicide gene system: A new approach for the treatment of hepatocellular carcinoma. PLoS ONE 2018, 13, e0196686. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Chung, T.K.; Parker, W.B.; Allan, P.W.; Clemons, L.; Lowman, D.; Hong, J.; Hunt, F.R.; Richman, J.; Conry, R.M.; et al. Phase I dose-escalating trial of Escherichia coli purine nucleoside phosphorylase and fludarabine gene therapy for advanced solid tumors. Ann. Oncol. 2015, 26, 1481–1487. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Xue, R.; Liu, M.; Bai, J.; Bao, J.; Wang, Y.; Jiang, N.; Li, Z.; Wang, W.; et al. Deep whole-genome analysis of 494 hepatocellular carcinomas. Nature 2024, 627, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Zilfou, J.T.; Lowe, S.W. Tumor suppressive functions of p53. Cold Spring Harb. Perspect. Biol. 2009, 1, a001883. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Li, G.; Li, P.; Wang, H.; Fang, X.; He, T.; Li, J. Twenty years of Gendicine(R) rAd-p53 cancer gene therapy: The first-in-class human cancer gene therapy in the era of personalized oncology. Genes Dis. 2024, 11, 101155. [Google Scholar] [CrossRef]
- Yang, Z.X.; Wang, D.; Wang, G.; Zhang, Q.H.; Liu, J.M.; Peng, P.; Liu, X.H. Clinical study of recombinant adenovirus-p53 combined with fractionated stereotactic radiotherapy for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2010, 136, 625–630. [Google Scholar] [CrossRef]
- Guan, Y.S.; Liu, Y.; He, Q.; Li, X.; Yang, L.; Hu, Y.; La, Z. p53 gene therapy in combination with transcatheter arterial chemoembolization for HCC: One-year follow-up. World J. Gastroenterol. 2011, 17, 2143–2149. [Google Scholar] [CrossRef]
- Hutcheson, J.; Bourgo, R.J.; Balaji, U.; Ertel, A.; Witkiewicz, A.K.; Knudsen, E.S. Retinoblastoma protein potentiates the innate immune response in hepatocytes: Significance for hepatocellular carcinoma. Hepatology 2014, 60, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, H.; Zhang, J.; Liu, S.; Liu, Y.; Zheng, D. AAV-mediated TRAIL gene expression driven by hTERT promoter suppressed human hepatocellular carcinoma growth in mice. Life Sci. 2008, 82, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Li, C.; Hao, Q.; Miao, H.; Zhang, L.; Li, L.; Zhou, Z. VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat. Commun. 2017, 8, 14058. [Google Scholar] [CrossRef]
- Xie, W.; Hao, J.; Zhang, K.; Fang, X.; Liu, X. Adenovirus armed with VGLL4 selectively kills hepatocellular carcinoma with G2/M phase arrest and apoptosis promotion. Biochem. Biophys. Res. Commun. 2018, 503, 2758–2763. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Wei, X.; Cui, Q.; Lou, W.; Wang, G.; Hu, X.; Qian, C. Potent antitumor activity of oncolytic adenovirus-mediated SOCS1 for hepatocellular carcinoma. Gene Ther. 2013, 20, 84–92. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Liu, W. Hypermethylated KCNQ1 acts as a tumor suppressor in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018, 503, 3100–3107. [Google Scholar] [CrossRef]
- Wei, J.X.; Lv, L.H.; Wan, Y.L.; Cao, Y.; Li, G.L.; Lin, H.M.; Zhou, R.; Shang, C.Z.; Cao, J.; He, H.; et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology 2015, 61, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Li, T.; Guan, L.; Liu, F.E.; Chen, X.M.; Zhao, J.; Lin, S.; Liu, Z.Z.; Zhang, H.Q. CTNNA3 is a tumor suppressor in hepatocellular carcinomas and is inhibited by miR-425. Oncotarget 2016, 7, 8078–8089. [Google Scholar] [CrossRef]
- Huan, H.; Wen, X.; Chen, X.; Wu, L.; Liu, W.; Habib, N.A.; Bie, P.; Xia, F. C/EBPalpha Short-Activating RNA Suppresses Metastasis of Hepatocellular Carcinoma through Inhibiting EGFR/beta-Catenin Signaling Mediated EMT. PLoS ONE 2016, 11, e0153117. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhu, P.; Zhang, X.; Zhang, L.; Chen, X.; Lu, F.; Yu, Z.; Liu, S. PCDH9 acts as a tumor suppressor inducing tumor cell arrest at G0/G1 phase and is frequently methylated in hepatocellular carcinoma. Mol. Med. Rep. 2017, 16, 4475–4482. [Google Scholar] [CrossRef]
- Lawler, S.E.; Speranza, M.C.; Cho, C.F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef]
- Twumasi-Boateng, K.; Pettigrew, J.L.; Kwok, Y.Y.E.; Bell, J.C.; Nelson, B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer 2018, 18, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019, 18, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef]
- Stojdl, D.F.; Lichty, B.D.; tenOever, B.R.; Paterson, J.M.; Power, A.T.; Knowles, S.; Marius, R.; Reynard, J.; Poliquin, L.; Atkins, H.; et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 2003, 4, 263–275. [Google Scholar] [CrossRef]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- Eager, R.M.; Nemunaitis, J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011, 18, 305–317. [Google Scholar] [CrossRef]
- Dhungel, B.; Andrzejewski, S.; Jayachandran, A.; Shrestha, R.; Ramlogan-Steel, C.A.; Layton, C.J.; Steel, J.C. Evaluation of the Glypican 3 promoter for transcriptional targeting of hepatocellular carcinoma. Gene Ther. 2018, 25, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xu, M.; Cao, Z.; Li, W.; Chen, L.; Xie, X.; Wang, W.; Liu, J. Ultrasound-Assisted miR-122-Loaded Polymeric Nanodroplets for Hepatocellular Carcinoma Gene Therapy. Mol. Pharm. 2020, 17, 541–553. [Google Scholar] [CrossRef]
- Park, B.H.; Hwang, T.; Liu, T.C.; Sze, D.Y.; Kim, J.S.; Kwon, H.C.; Oh, S.Y.; Han, S.Y.; Yoon, J.H.; Hong, S.H.; et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: A phase I trial. Lancet Oncol. 2008, 9, 533–542. [Google Scholar] [CrossRef]
- Liu, T.C.; Hwang, T.; Park, B.H.; Bell, J.; Kirn, D.H. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol. Ther. 2008, 16, 1637–1642. [Google Scholar] [CrossRef]
- Lemay, C.G.; Rintoul, J.L.; Kus, A.; Paterson, J.M.; Garcia, V.; Falls, T.J.; Ferreira, L.; Bridle, B.W.; Conrad, D.P.; Tang, V.A.; et al. Harnessing oncolytic virus-mediated antitumor immunity in an infected cell vaccine. Mol. Ther. 2012, 20, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Woller, N.; Gurlevik, E.; Fleischmann-Mundt, B.; Schumacher, A.; Knocke, S.; Kloos, A.M.; Saborowski, M.; Geffers, R.; Manns, M.P.; Wirth, T.C.; et al. Viral Infection of Tumors Overcomes Resistance to PD-1-immunotherapy by Broadening Neoantigenome-directed T-cell Responses. Mol. Ther. 2015, 23, 1630–1640. [Google Scholar] [CrossRef]
- Koks, C.A.; Garg, A.D.; Ehrhardt, M.; Riva, M.; Vandenberk, L.; Boon, L.; De Vleeschouwer, S.; Agostinis, P.; Graf, N.; Van Gool, S.W. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int. J. Cancer 2015, 136, E313–E325. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Prestwich, R.J.; Harrington, K.J.; Pandha, H.S.; Vile, R.G.; Melcher, A.A.; Errington, F. Oncolytic viruses: A novel form of immunotherapy. Expert Rev. Anticancer. Ther. 2008, 8, 1581–1588. [Google Scholar] [CrossRef]
- Guo, Z.S.; Liu, Z.; Kowalsky, S.; Feist, M.; Kalinski, P.; Lu, B.; Storkus, W.J.; Bartlett, D.L. Oncolytic Immunotherapy: Conceptual Evolution, Current Strategies, and Future Perspectives. Front. Immunol. 2017, 8, 555. [Google Scholar] [CrossRef] [PubMed]
- Breitbach, C.J.; Burke, J.; Jonker, D.; Stephenson, J.; Haas, A.R.; Chow, L.Q.; Nieva, J.; Hwang, T.H.; Moon, A.; Patt, R.; et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 2011, 477, 99–102. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Galle, P.R.; Chao, Y.; Erinjeri, J.; Heo, J.; Borad, M.J.; Luca, A.; Burke, J.; Pelusio, A.; Agathon, D.; et al. PHOCUS: A Phase 3, Randomized, Open-Label Study of Sequential Treatment with Pexa-Vec (JX-594) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2024, 13, 248–264. [Google Scholar] [CrossRef]
- Shen, Y.; Bai, X.; Zhang, Q.; Liang, X.; Jin, X.; Zhao, Z.; Song, W.; Tan, Q.; Zhao, R.; Jia, W.; et al. Oncolytic virus VG161 in refractory hepatocellular carcinoma. Nature 2025, 641, 503–511. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, C.C.; Johnson, L.; Bagus, B.; Choi, S.; Nicholas, C.; Shen, A.; Boyle, L.; Pandey, K.; Soria, C.; Kunich, J.; et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell 2004, 6, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.J.; Li, Q.J.; Lao, X.M.; Yang, H.; Li, S.P. Transarterial injection of recombinant human type-5 adenovirus H101 in combination with transarterial chemoembolization (TACE) improves overall and progressive-free survival in unresectable hepatocellular carcinoma (HCC). BMC Cancer 2015, 15, 707. [Google Scholar] [CrossRef]
- Yi, L.; Ning, Z.; Xu, L.; Shen, Y.; Zhu, X.; Yu, W.; Xie, J.; Meng, Z. The combination treatment of oncolytic adenovirus H101 with nivolumab for refractory advanced hepatocellular carcinoma: An open-label, single-arm, pilot study. ESMO Open 2024, 9, 102239. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, R.; Zhang, B.; Lai, C.; Li, L.; Shen, J.; Tan, X.; Shao, J. Research progress and application of the CRISPR/Cas9 gene-editing technology based on hepatocellular carcinoma. Asian J. Pharm. Sci. 2023, 18, 100828. [Google Scholar] [CrossRef]
- Amjad, E.; Pezzani, R.; Sokouti, B. A review of the literature on the use of CRISPR/Cas9 gene therapy to treat hepatocellular carcinoma. Oncol. Res. 2024, 32, 439–461. [Google Scholar] [CrossRef] [PubMed]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Wiedenheft, B.; Sternberg, S.H.; Doudna, J.A. RNA-guided genetic silencing systems in bacteria and archaea. Nature 2012, 482, 331–338. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Geisinger, J.M.; Turan, S.; Hernandez, S.; Spector, L.P.; Calos, M.P. In vivo blunt-end cloning through CRISPR/Cas9-facilitated non-homologous end-joining. Nucleic Acids Res. 2016, 44, e76. [Google Scholar] [CrossRef]
- Yao, X.; Wang, X.; Liu, J.; Shi, L.; Huang, P.; Yang, H. CRISPR/Cas9-mediated Targeted Integration In Vivo Using a Homology-mediated End Joining-based Strategy. J. Vis. Exp. 2018, 133, e56844. [Google Scholar] [CrossRef]
- Huang, L.; Yang, C.; Chen, Y.; Deng, H.; Liao, Z.; Xiao, H. CRISPR-Mediated Base Editing: Promises and Challenges for a Viable Oncotherapy Strategy. Hum. Gene Ther. 2023, 34, 669–681. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, D.; Wang, D.; Zhou, J.; Chen, Q.; Lai, J. Prime editing: A gene precision editing tool from inception to present. FASEB J. 2024, 38, e70148. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, Y.; Yu, B.; Hu, Y.; Zhang, N.; Zheng, Y.; Yang, M.; Xu, F.J. A Lactose-Derived CRISPR/Cas9 Delivery System for Efficient Genome Editing In Vivo to Treat Orthotopic Hepatocellular Carcinoma. Adv. Sci. 2020, 7, 2001424. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Sun, L.; Pu, Y.; Yu, J.; Feng, W.; Dong, C.; Zhou, B.; Du, D.; Zhang, Y.; Chen, Y.; et al. Ultrasound-Controlled CRISPR/Cas9 System Augments Sonodynamic Therapy of Hepatocellular Carcinoma. ACS Cent. Sci. 2021, 7, 2049–2062. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Liu, H.; Wu, D.; Hu, Y.; Liang, Q.; Liu, M.; Liu, H.; Wu, J.; Zhang, J.; Deng, Y.; et al. CRISPR/Cas9-mediated PD-1 attenuation enhances tumor infiltrating lymphocyte-based adoptive cellular therapy in humanized-PDX model of hepatocellular carcinoma. Transl. Oncol. 2025, 60, 102484. [Google Scholar] [CrossRef]
- Liu, H.; Ge, W.; Yu, X.; Luo, J.; Zhang, J.; Yang, M.; Cao, L.; Zhang, Y.; Wang, R.; Yang, C.; et al. CRISPR/Cas9-mediated SHP-1-knockout T cells combined with simvastatin enhances anti-tumor activity in humanized-PDX HCC model. iScience 2025, 28, 112266. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, X.; Lei, Y.; Wang, G.; Liu, M. Glypican-3: A Novel and Promising Target for the Treatment of Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 824208. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S.; Lee, H.; Kim, S.; Kim, D.H. Therapeutic Glypican-3 CRISPR Genome-Editing Using UltraLarge Porous Silica Nano-Depot for the Treatment of Hepatocellular Carcinoma. Small Sci. 2025, 5, 2400447. [Google Scholar] [CrossRef]
- Zhang, B.C.; Luo, B.Y.; Zou, J.J.; Wu, P.Y.; Jiang, J.L.; Le, J.Q.; Zhao, R.R.; Chen, L.; Shao, J.W. Co-delivery of Sorafenib and CRISPR/Cas9 Based on Targeted Core-Shell Hollow Mesoporous Organosilica Nanoparticles for Synergistic HCC Therapy. ACS Appl. Mater. Interfaces 2020, 12, 57362–57372. [Google Scholar] [CrossRef]
- Nie, J.J.; Liu, Y.; Qi, Y.; Zhang, N.; Yu, B.; Chen, D.F.; Yang, M.; Xu, F.J. Charge-reversal nanocomolexes-based CRISPR/Cas9 delivery system for loss-of-function oncogene editing in hepatocellular carcinoma. J. Control. Release 2021, 333, 362–373. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Feng, K.K.; Lu, J.Y.; Le, J.Q.; Li, W.L.; Zhang, B.C.; Li, C.L.; Song, X.H.; Tong, L.W.; Shao, J.W. CRISPR/Cas9-based application for cancer therapy: Challenges and solutions for non-viral delivery. J. Control. Release 2023, 361, 727–749. [Google Scholar] [CrossRef]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013, 31, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Alsahli, M.A.; Almatroudi, A.; Alrumaihi, F.; Al Abdulmonem, W.; Moawad, A.A.; Alwanian, W.M.; Almansour, N.M.; Rahmani, A.H.; Khan, A.A. Innovative Strategies of Reprogramming Immune System Cells by Targeting CRISPR/Cas9-Based Genome-Editing Tools: A New Era of Cancer Management. Int. J. Nanomed. 2023, 18, 5531–5559. [Google Scholar] [CrossRef] [PubMed]
- Sas, Z.; Cendrowicz, E.; Weinhauser, I.; Rygiel, T.P. Tumor Microenvironment of Hepatocellular Carcinoma: Challenges and Opportunities for New Treatment Options. Int. J. Mol. Sci. 2022, 23, 3778. [Google Scholar] [CrossRef]
- Duchs, M.J.; Kratzer, R.F.; Vieyra-Garcia, P.; Strobel, B.; Schonberger, T.; Gross, P.; Aljayyoussi, G.; Gupta, A.; Lang, I.; Klein, H.; et al. Riboswitch-controlled IL-12 gene therapy reduces hepatocellular cancer in mice. Front. Immunol. 2024, 15, 1360063. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chiang, T.; Liu, C.H.; Chern, G.G.; Lin, T.T.; Gao, D.Y.; Chen, Y. Delivery of siRNA Using CXCR4-targeted Nanoparticles Modulates Tumor Microenvironment and Achieves a Potent Antitumor Response in Liver Cancer. Mol. Ther. 2015, 23, 1772–1782. [Google Scholar] [CrossRef]
- Li, B.; Niu, H.; Zhao, X.; Huang, X.; Ding, Y.; Dang, K.; Yang, T.; Chen, Y.; Ma, J.; Liu, X.; et al. Targeted anti-cancer therapy: Co-delivery of VEGF siRNA and Phenethyl isothiocyanate (PEITC) via cRGD-modified lipid nanoparticles for enhanced anti-angiogenic efficacy. Asian J. Pharm. Sci. 2024, 19, 100891. [Google Scholar] [CrossRef]
- Lai, Q.; Li, W.; Hu, D.; Huang, Z.; Wu, M.; Feng, S.; Wan, Y. Hepatic stellate cell-targeted chemo-gene therapy for liver fibrosis using fluorinated peptide-lipid hybrid nanoparticles. J. Control. Release 2024, 376, 601–617. [Google Scholar] [CrossRef]
- Wang, K.; Chen, H.; Qin, S.; Chen, S.; Zhang, Q.; Chen, J.; Di, D.; Su, G.; Yuan, Y. Co-delivery of pirfenidone and siRNA in ZIF-based nanoparticles for dual inhibition of hepatic stellate cell activation in liver fibrotic therapy. Colloids Surf. B Biointerfaces 2023, 231, 113567. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Chern, G.J.; Hsu, F.F.; Huang, K.W.; Sung, Y.C.; Huang, H.C.; Qiu, J.T.; Wang, S.K.; Lin, C.C.; Wu, C.H.; et al. A multifunctional nanocarrier for efficient TRAIL-based gene therapy against hepatocellular carcinoma with desmoplasia in mice. Hepatology 2018, 67, 899–913. [Google Scholar] [CrossRef] [PubMed]
- Altomonte, J.; Marozin, S.; De Toni, E.N.; Rizzani, A.; Esposito, I.; Steiger, K.; Feuchtinger, A.; Hellerbrand, C.; Schmid, R.M.; Ebert, O. Antifibrotic properties of transarterial oncolytic VSV therapy for hepatocellular carcinoma in rats with thioacetamide-induced liver fibrosis. Mol. Ther. 2013, 21, 2032–2042. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, S.; Xu, M.; Wu, X.; Dou, W.; Li, H.; Zhang, Z.; Zhang, S. CAR-T cell therapy for hepatocellular carcinoma: Current trends and challenges. Front. Immunol. 2024, 15, 1489649. [Google Scholar] [CrossRef]
- Cheadle, E.J.; Gornall, H.; Baldan, V.; Hanson, V.; Hawkins, R.E.; Gilham, D.E. CAR T cells: Driving the road from the laboratory to the clinic. Immunol. Rev. 2014, 257, 91–106. [Google Scholar] [CrossRef]
- Sadelain, M.; Brentjens, R.; Riviere, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Shi, Y.; Kaseb, A.O.; Qi, X.; Zhang, Y.; Chi, J.; Lu, Q.; Gao, H.; Jiang, H.; Wang, H.; et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin. Cancer Res. 2020, 26, 3979–3989. [Google Scholar] [CrossRef]
- Steffin, D.; Ghatwai, N.; Montalbano, A.; Rathi, P.; Courtney, A.N.; Arnett, A.B.; Fleurence, J.; Sweidan, R.; Wang, T.; Zhang, H.; et al. Interleukin-15-armoured GPC3 CAR T cells for patients with solid cancers. Nature 2025, 637, 940–946. [Google Scholar] [CrossRef]
- Fu, Q.; Zheng, Y.; Fang, W.; Zhao, Q.; Zhao, P.; Liu, L.; Zhai, Y.; Tong, Z.; Zhang, H.; Lin, M.; et al. RUNX-3-expressing CAR T cells targeting glypican-3 in patients with heavily pretreated advanced hepatocellular carcinoma: A phase I trial. eClinicalMedicine 2023, 63, 102175. [Google Scholar] [CrossRef]
- Tamaki, N.; Kuno, A.; Matsuda, A.; Tsujikawa, H.; Yamazaki, K.; Yasui, Y.; Tsuchiya, K.; Nakanishi, H.; Itakura, J.; Korenaga, M.; et al. Serum Wisteria Floribunda Agglutinin-Positive Sialylated Mucin 1 as a Marker of Progenitor/Biliary Features in Hepatocellular Carcinoma. Sci. Rep. 2017, 7, 244. [Google Scholar] [CrossRef]
- Terris, B.; Cavard, C.; Perret, C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J. Hepatol. 2010, 52, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, J.; Xing, J.; Li, W.; Li, H.; Ke, X.; Zhang, J.; Ren, T.; Shang, Y.; Yang, H.; et al. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J. Hepatol. 2014, 61, 859–866. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Xiang, J.; Long, L.; Green, S.; Yang, Z.; Zimdahl, B.; Lu, J.; Cheng, N.; Horan, L.H.; et al. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin. Cancer Res. 2017, 23, 478–488. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Y.; Han, W. Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment. Protein Cell 2017, 8, 896–925. [Google Scholar] [CrossRef]
- Adkins, S. CAR T-Cell Therapy: Adverse Events and Management. J. Adv. Pract. Oncol. 2019, 10, 21–28. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef]
- Ghosh, S.; Brown, A.M.; Jenkins, C.; Campbell, K. Viral Vector Systems for Gene Therapy: A Comprehensive Literature Review of Progress and Biosafety Challenges. Appl. Biosaf. 2020, 25, 7–18. [Google Scholar] [CrossRef]
- Minskaia, E.; Galieva, A.; Egorov, A.D.; Ivanov, R.; Karabelsky, A. Viral Vectors in Gene Replacement Therapy. Biochemistry 2023, 88, 2157–2178. [Google Scholar] [CrossRef] [PubMed]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct. Target. Ther. 2021, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.I.; Sheppard, N.C.; Riley, J.L. Genetic engineering of T cells for immunotherapy. Nat. Rev. Genet. 2021, 22, 427–447. [Google Scholar] [CrossRef]
- Wang, S.W.; Gao, C.; Zheng, Y.M.; Yi, L.; Lu, J.C.; Huang, X.Y.; Cai, J.B.; Zhang, P.F.; Cui, Y.H.; Ke, A.W. Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol. Cancer 2022, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Dhungel, B.; Jayachandran, A.; Layton, C.J.; Steel, J.C. Seek and destroy: Targeted adeno-associated viruses for gene delivery to hepatocellular carcinoma. Drug Deliv. 2017, 24, 289–299. [Google Scholar] [CrossRef]
- Donsante, A.; Miller, D.G.; Li, Y.; Vogler, C.; Brunt, E.M.; Russell, D.W.; Sands, M.S. AAV vector integration sites in mouse hepatocellular carcinoma. Science 2007, 317, 477. [Google Scholar] [CrossRef]
- Chandler, R.J.; LaFave, M.C.; Varshney, G.K.; Trivedi, N.S.; Carrillo-Carrasco, N.; Senac, J.S.; Wu, W.; Hoffmann, V.; Elkahloun, A.G.; Burgess, S.M.; et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J. Clin. Investig. 2015, 125, 870–880. [Google Scholar] [CrossRef]
- Meumann, N.; Schmithals, C.; Elenschneider, L.; Hansen, T.; Balakrishnan, A.; Hu, Q.; Hook, S.; Schmitz, J.; Brasen, J.H.; Franke, A.C.; et al. Hepatocellular Carcinoma Is a Natural Target for Adeno-Associated Virus (AAV) 2 Vectors. Cancers 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Huang, P.P.; Zhang, R.; Ma, B.Y.; Zhou, X.M.; Sun, Y.F. Targeting adeno-associated virus and adenoviral gene therapy for hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J.; Benko, M.; Harrach, B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 2003, 84, 2895–2908. [Google Scholar] [CrossRef]
- Shahryari, A.; Saghaeian Jazi, M.; Mohammadi, S.; Razavi Nikoo, H.; Nazari, Z.; Hosseini, E.S.; Burtscher, I.; Mowla, S.J.; Lickert, H. Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders. Front. Genet. 2019, 10, 868. [Google Scholar] [CrossRef]
- Chang, J. Adenovirus Vectors: Excellent Tools for Vaccine Development. Immune. Netw. 2021, 21, e6. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.D.; Byrne, B.J.; Corti, M. Evolving Horizons: Adenovirus Vectors’ Timeless Influence on Cancer, Gene Therapy and Vaccines. Viruses 2023, 15, 2378. [Google Scholar] [CrossRef]
- He, T.C.; Zhou, S.; da Costa, L.T.; Yu, J.; Kinzler, K.W.; Vogelstein, B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 1998, 95, 2509–2514. [Google Scholar] [CrossRef]
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Kumari, R.; Mittal, S.K. Current Use of Adenovirus Vectors and Their Production Methods. Methods Mol. Biol. 2019, 1937, 155–175. [Google Scholar] [CrossRef]
- Poletti, V.; Mavilio, F. Designing Lentiviral Vectors for Gene Therapy of Genetic Diseases. Viruses 2021, 13, 1526. [Google Scholar] [CrossRef]
- Manjunath, N.; Wu, H.; Subramanya, S.; Shankar, P. Lentiviral delivery of short hairpin RNAs. Adv. Drug Deliv. Rev. 2009, 61, 732–745. [Google Scholar] [CrossRef]
- Jadlowsky, J.K.; Hexner, E.O.; Marshall, A.; Grupp, S.A.; Frey, N.V.; Riley, J.L.; Veloso, E.; McConville, H.; Rogal, W.; Czuczman, C.; et al. Long-term safety of lentiviral or gammaretroviral gene-modified T cell therapies. Nat. Med. 2025, 31, 1134–1144. [Google Scholar] [CrossRef]
- Liu, K.C.; Lin, B.S.; Gao, A.D.; Ma, H.Y.; Zhao, M.; Zhang, R.; Yan, H.H.; Yi, X.F.; Lin, S.J.; Que, J.W.; et al. Integrase-deficient lentivirus: Opportunities and challenges for human gene therapy. Curr. Gene Ther. 2014, 14, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, H.; Xue, M.; Zheng, C.; Chen, Q. HSV-1 as a gene delivery platform for cancer gene therapy. Trends Pharmacol. Sci. 2025, 46, 324–336. [Google Scholar] [CrossRef]
- Le Hars, M.; Joussain, C.; Jegu, T.; Epstein, A.L. Non-replicative herpes simplex virus genomic and amplicon vectors for gene therapy-an update. Gene Ther. 2025, 32, 173–183. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging non-viral vectors for gene delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, M.; Yang, Z.; Liu, X.; Xu, Z.; Liu, S.; Feng, G.; Tang, S.; Li, Z.; Zhang, Y.; et al. Recent advances in nanotechnology approaches for non-viral gene therapy. Biomater. Sci. 2022, 10, 6862–6892. [Google Scholar] [CrossRef]
- Abtahi, M.S.; Fotouhi, A.; Rezaei, N.; Akalin, H.; Ozkul, Y.; Hossein-Khannazer, N.; Vosough, M. Nano-based drug delivery systems in hepatocellular carcinoma. J. Drug Target. 2024, 32, 977–995. [Google Scholar] [CrossRef] [PubMed]
- Graur, F.; Puia, A.; Mois, E.I.; Moldovan, S.; Pusta, A.; Cristea, C.; Cavalu, S.; Puia, C.; Al Hajjar, N. Nanotechnology in the Diagnostic and Therapy of Hepatocellular Carcinoma. Materials 2022, 15, 3893. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic nanoparticles in cancer therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef]
- Lin, G.; Revia, R.A.; Zhang, M. Inorganic Nanomaterial-Mediated Gene Therapy in Combination with Other Antitumor Treatment Modalities. Adv. Funct. Mater. 2021, 31, 2007096. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Rizwan, M.; Zubair, M.; Inam, A.; Ali, S. Biological synthesis, characterization of three metal-based nanoparticles and their anticancer activities against hepatocellular carcinoma HepG2 cells. Ecotoxicol. Environ. Saf. 2021, 223, 112575. [Google Scholar] [CrossRef]
- Salah, Z.; Abd El Azeem, E.M.; Youssef, H.F.; Gamal-Eldeen, A.M.; Farrag, A.R.; El-Meliegy, E.; Soliman, B.; Elhefnawi, M. Effect of Tumor Suppressor MiR-34a Loaded on ZSM-5 Nanozeolite in Hepatocellular Carcinoma: In Vitro and In Vivo Approach. Curr. Gene Ther. 2019, 19, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Stavljenic Milasin, I.; Batu Eken, Z.; Mravak-Stipetic, M.; Pavelic, K.; Ozer, F. Effects of Zeolite as a Drug Delivery System on Cancer Therapy: A Systematic Review. Molecules 2021, 26, 6196. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Younis, M.A.; Khalil, I.A.; Elewa, Y.H.A.; Kon, Y.; Harashima, H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. J. Control. Release 2021, 331, 335–349. [Google Scholar] [CrossRef]
- Rong, J.; Liu, T.; Yin, X.; Shao, M.; Zhu, K.; Li, B.; Wang, S.; Zhu, Y.; Zhang, S.; Yin, L.; et al. Co-delivery of camptothecin and MiR-145 by lipid nanoparticles for MRI-visible targeted therapy of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2024, 43, 247. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Shapiro, G.I.; LoRusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Plummer, R.; Meyer, T.; Sodergren, M.H.; Basu, B.; Chee, C.E.; Huang, K.W.; Palmer, D.H.; Ma, Y.T.; Evans, T.R.J.; et al. MTL-CEBPA, a Small Activating RNA Therapeutic Upregulating C/EBP-alpha, in Patients with Advanced Liver Cancer: A First-in-Human, Multicenter, Open-Label, Phase I Trial. Clin. Cancer Res. 2020, 26, 3936–3946. [Google Scholar] [CrossRef]
- Deng, Z.; Yang, H.; Tian, Y.; Liu, Z.; Sun, F.; Yang, P. An OX40L mRNA vaccine inhibits the growth of hepatocellular carcinoma. Front. Oncol. 2022, 12, 975408. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, H.; Huang, L. Smart polymeric nanoparticles for cancer gene delivery. Mol. Pharm. 2015, 12, 314–321. [Google Scholar] [CrossRef]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, C.G.; Kozielski, K.L.; Vaughan, H.J.; Nakata, M.M.; Kim, J.; Higgins, L.J.; Pomper, M.G.; Green, J.J. Polymeric nanoparticles as cancer-specific DNA delivery vectors to human hepatocellular carcinoma. J. Control. Release 2017, 263, 18–28. [Google Scholar] [CrossRef]
- Devulapally, R.; Foygel, K.; Sekar, T.V.; Willmann, J.K.; Paulmurugan, R. Gemcitabine and Antisense-microRNA Co-encapsulated PLGA-PEG Polymer Nanoparticles for Hepatocellular Carcinoma Therapy. ACS Appl. Mater. Interfaces 2016, 8, 33412–33422. [Google Scholar] [CrossRef]
- Vaughan, H.J.; Zamboni, C.G.; Hassan, L.F.; Radant, N.P.; Jacob, D.; Mease, R.C.; Minn, I.; Tzeng, S.Y.; Gabrielson, K.L.; Bhardwaj, P.; et al. Polymeric nanoparticles for dual-targeted theranostic gene delivery to hepatocellular carcinoma. Sci. Adv. 2022, 8, eabo6406. [Google Scholar] [CrossRef]
- Punuch, K.; Wongwan, C.; Jantana, S.; Somboonyosdech, C.; Rodponthukwaji, K.; Kunwong, N.; Nguyen, K.T.; Sirivatanauksorn, V.; Sirivatanauksorn, Y.; Srisawat, C.; et al. Study of siRNA Delivery via Polymeric Nanoparticles in Combination with Angiogenesis Inhibitor for The Treatment of AFP-Related Liver Cancer. Int. J. Mol. Sci. 2022, 23, 12666. [Google Scholar] [CrossRef]
- Elfiky, A.M.; Mohamed, R.H.; Abd El-Hakam, F.E.; Yassin, M.A.; ElHefnawi, M. Targeted delivery of miR-218 via decorated hyperbranched polyamidoamine for liver cancer regression. Int. J. Pharm. 2021, 610, 121256. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, D.; Srivastava, J.; Ebeid, K.; Gredler, R.; Akiel, M.; Jariwala, N.; Robertson, C.L.; Shen, X.N.; Siddiq, A.; Fisher, P.B.; et al. Combination of Nanoparticle-Delivered siRNA for Astrocyte Elevated Gene-1 (AEG-1) and All-trans Retinoic Acid (ATRA): An Effective Therapeutic Strategy for Hepatocellular Carcinoma (HCC). Bioconjugate Chem. 2015, 26, 1651–1661. [Google Scholar] [CrossRef]
- Duan, D. Lethal immunotoxicity in high-dose systemic AAV therapy. Mol. Ther. 2023, 31, 3123–3126. [Google Scholar] [CrossRef]
- Bradbury, A.; Markusic, D.; Muhuri, M.; Ou, L. Editorial: Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 2023, 14, 1227231. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Wang, H.; Chen, W.; Mou, X.; Wang, S. Expression of coxsackie and adenovirus receptor is correlated with inferior prognosis in liver cancer patients. Oncol. Lett. 2019, 17, 2485–2490. [Google Scholar] [CrossRef]
- Dai, Z.; Si, Y.; Xiong, S.; Li, Y.; Ye, J.; Gao, Q.; Ma, D.; Jin, X.; Li, F. Chimeric Ad5/35 oncolytic adenovirus overcome preexisting neutralizing antibodies and enhance tumor targeting efficiency. Cancer Gene Ther. 2025, 32, 418–436. [Google Scholar] [CrossRef]
- Zafar, S.; Quixabeira, D.C.A.; Kudling, T.V.; Cervera-Carrascon, V.; Santos, J.M.; Gronberg-Vaha-Koskela, S.; Zhao, F.; Aronen, P.; Heinio, C.; Havunen, R.; et al. Ad5/3 is able to avoid neutralization by binding to erythrocytes and lymphocytes. Cancer Gene Ther. 2021, 28, 442–454. [Google Scholar] [CrossRef]
- Vaughan, H.J.; Zamboni, C.G.; Luly, K.M.; Li, L.; Gabrielson, K.L.; Hassan, L.F.; Radant, N.P.; Bhardwaj, P.; Selaru, F.M.; Pomper, M.G.; et al. Non-Viral Gene Delivery to Hepatocellular Carcinoma via Intra-Arterial Injection. Int. J. Nanomed. 2023, 18, 2525–2537. [Google Scholar] [CrossRef]
- Taghdiri, M.; Mussolino, C. Viral and Non-Viral Systems to Deliver Gene Therapeutics to Clinical Targets. Int. J. Mol. Sci. 2024, 25, 7333. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Eygeris, Y.; Ryals, R.C.; Jozic, A.; Sahay, G. Strategies for non-viral vectors targeting organs beyond the liver. Nat. Nanotechnol. 2024, 19, 428–447. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, Q.; Wei, L. The Emerging Influences of Alpha-Fetoprotein in the Tumorigenesis and Progression of Hepatocellular Carcinoma. Cancers 2021, 13, 5096. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Pathak, S.; Kumar, N.; Jayandharan, G.R. Development of an Optimized Promoter System for Exosomal and Naked AAV Vector-Based Suicide Gene Therapy in Hepatocellular Carcinoma. ACS Omega 2024, 9, 30945–30953. [Google Scholar] [CrossRef]
- Huang, K.W.; Lai, Y.T.; Chern, G.J.; Huang, S.F.; Tsai, C.L.; Sung, Y.C.; Chiang, C.C.; Hwang, P.B.; Ho, T.L.; Huang, R.L.; et al. Galactose Derivative-Modified Nanoparticles for Efficient siRNA Delivery to Hepatocellular Carcinoma. Biomacromolecules 2018, 19, 2330–2339. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, M.; Chen, Y.; Hua, L.; Xu, T.; Wang, C.; Li, Y.; Zhu, B. Folate-targeted selenium nanoparticles deliver therapeutic siRNA to improve hepatocellular carcinoma therapy. RSC Adv. 2018, 8, 25932–25940. [Google Scholar] [CrossRef]
- Singh, D.; Singh, M. Hepatocellular-Targeted mRNA Delivery Using Functionalized Selenium Nanoparticles In Vitro. Pharmaceutics 2021, 13, 298. [Google Scholar] [CrossRef]
- Zafar, A.; Khan, M.J.; Abu, J.; Naeem, A. Revolutionizing cancer care strategies: Immunotherapy, gene therapy, and molecular targeted therapy. Mol. Biol. Rep. 2024, 51, 219. [Google Scholar] [CrossRef] [PubMed]
| NCT Number | Study Status | Disease Conditions | Interventions | Phases | Enrollment | Locations |
|---|---|---|---|---|---|---|
| NCT00844623 | Completed | HCC | Adenoviral vector containing thymidine kinase (TK99UN) | 1 | 10 | Spain |
| Recombinant human adenovirus | ||||||
| NCT05872841 | Unknown | Primary HCC | rhAdV type 5 + TACE | 2 | 38 | China |
| NCT01869088 | Unknown | HCC | rhAdV type 5 | 3 | 266 | China |
| NCT05113290 | Unknown | HCC | rhAdV type 5 + Sorafenib | 4 | 66 | China |
| NCT03790059 | Unknown | HCC | H101 oncolytic virus | NA | 160 | China |
| NCT06685354 | Not yet recruiting | HCC | rhAdV type 5 | 2 | 11 | China |
| NCT00669136 | Terminated | HCC, Hepatoma, Liver Cancer | AFP + GM-CSF Plasmid Prime and AFP Adenoviral Vector Boost | 1 | 2 | United States (USA) |
| NCT03780049 | Unknown | HCC | H101|HAIC of FOLFOX| DRUG: DRUG: Placebos | 3 | 304 | China |
| NCT05675462 | Recruiting | HCC | Human recombinant type 5 adenovirus (Oncorine) Tislelizumab+Lenvatinib | 1 | 25 | China |
| NCT02202564 | Completed | Liver Cancer|HCC| Liver Transplantation | AdV-TK|ganciclovir | 2 | 81 | China |
| NCT00300521 | Completed | HCC|Liver Transplantation | AdV-TK | 2 | 40 | China |
| NCT03313596 | Unknown | HCC | AdV-Tk | 3 | 180 | China |
| NCT04612504 | Unknown | HCC | Recombinant oncolytic adenovirus SynOV1.1 | 1 | 15 | China |
| NCT02509169 | Unknown | Advanced HCC | Transcatheter arterial embolization (TAE) plus P53 gene | 2 | 60 | China |
| NCT00003147 | Terminated | Liver Cancer | Ad5CMV-p53 gene | 1 | 30 | USA |
| NCT02561546 | Unknown | HCC|Diabetes | Recombinant adenovirus p53 injection (rAdp53) Trans-catheter embolization | 2 | 40 | China |
| NCT00093548 | Withdrawn | Liver Cancer | Alpha fetoprotein adenoviral vector vaccine | 1, 2 | 0 | USA |
| NCT Number | Study Status | Disease Conditions | Interventions/Drugs | Phases | Enrollment | Locations |
|---|---|---|---|---|---|---|
| NCT05620706 | Recruiting | Advanced HCC | GPC3 CAR-T cells | NA | 20 | China |
| NCT03884751 | Completed | HCC | CAR-GPC3 T Cells | 1 | 9 | China |
| NCT03146234 | Completed | HCC | CAR-GPC3 T cells | NA | 7 | China |
| NCT03980288 | Completed | Advanced HCC | CAR-GPC3 T Cells | 1 | 6 | China |
| NCT04951141 | Unknown | HCC | anti-GPC3 CAR-T cells | 1 | 10 | China |
| NCT02715362 | Unknown | HCC | TAI-GPC3-CART cells | 1, 2 | 30 | China |
| NCT05103631 | Recruiting | Liver Cancer | CATCH T cells | 1 | 27 | USA |
| NCT06198296 | Not yet recruiting | HCC | 21.15.GPC3-CAR T cells | 1 | 21 | USA |
| NCT03198546 | Recruiting | HCC | GPC3 and/or TGFβ targeting CAR-T cells | 1 | 30 | China |
| NCT02905188 | Completed | HCC | GLYCAR T cells|DRUG: Cytoxan|DRUG: Fludarabine | 1 | 9 | USA |
| NCT05926726 | Recruiting | HCC | CAR-GPC3 T cells | NA | 12 | China |
| NCT03130712 | Unknown | HCC | GPC3-CART cells | 1, 2 | 10 | China |
| NCT06144385 | Recruiting | Liver Cancer|HCC | CAR-GPC3 T cells | 1 | 20 | China |
| NCT06641453 | Not yet recruiting | HCC | GPC3-CART cells|DRUG: Fludarabine Phosphate for Injection|DRUG: Cyclophosphamide for Injection | 1, 2 | 30 | China |
| NCT05003895 | Recruiting | HCC | Cyclophosphamide|CAR-T cell|Fludarabine | 1 | 38 | USA |
| NCT06461624 | Recruiting | Advanced HCC | anti-GPC3 CAR-T | 1 | 15 | China |
| NCT03302403 | Unknown | HCC | CAR-CD19 T cell|CAR-BCMA T cell|CAR-GPC3 T cell|CAR-CLD18 T cell|Fludarabine|Cyclophosphamide | NA | 18 | China |
| NCT02395250 | Completed | Advanced HCC | anti-GPC3 CAR T | 1 | 13 | China |
| NCT02959151 | Unknown | HCC | CAR-T cell | 1, 2 | 20 | China |
| NCT06968195 | Not yet recruiting | Advanced HCC | Anti-GPC3-CAR Autologous TL | 1 | 24 | USA |
| NCT05120271 | Recruiting | HCC | CAR-GPC3 T Cells | 1, 2 | 110 | USA, United Kingdom (UK) |
| NCT06560827 | Recruiting | HCC | CT011 CAR-GPC3 T Cells | 1 | 30 | China |
| NCT05783570 | Recruiting | Advanced HCC | EU307 CAR-T Cell | 1 | 12 | Korea Republic |
| NCT05652920 | Recruiting | HCC | Ori-C101 (GPC3-directed chimeric antigen receptor modified T cells) | 1, 2 | 105 | China |
| NCT05155189 | Recruiting | HCC | C-CAR031|DRUG: Lenvatinib|PD-1(L1) monoclonal antibody | 1 | 44 | China |
| NCT06891742 | Recruiting | HCC | GPC3-targeted chimeric antigen receptor autologous T cell injection (OriC902) | 1 | 44 | China |
| NCT Number | Study Status | Disease Conditions | Interventions/Drugs | Phases | Enrollment | Locations |
|---|---|---|---|---|---|---|
| NCT01171651 | Completed | HCC | JX-594 followed by sorafenib | 2 | 25 | Korea Republic |
| NCT01636284 | Completed | HCC | JX-594 recombinant vaccina GM-CSF | 2 | 16 | USA, Korea, Spain |
| NCT00554372 | Completed | HCC | JX-594: Recombinant vaccinia virus (TK-deletion plus GM-CSF) | 2 | 30 | USA, Canada, Korea Republic, |
| NCT02562755 | Completed | HCC | Pexastimogene Devacirepvec (Pexa Vec)|DRUG: Sorafenib | 3 | 459 | USA, UK |
| NCT01387555 | Completed | HCC | JX-594 recombinant vaccina GM-CSF|OTHER: Best Supportive Care | 2 | 129 | USA, Canada, France, Germany, Korea, Taiwan |
| NCT00629759 | Completed | Neoplasms, Liver | JX-594: Recombinant vaccinia virus (TK-deletion plus GM-CSF) | 1 | 14 | Korea Republic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermi, A.G.; Younis, R.M.; Rodriguez, K.; Sarkar, D. Gene Therapy Strategies for Hepatocellular Carcinoma (HCC): Current Landscape and Future Directions. Cancers 2025, 17, 3608. https://doi.org/10.3390/cancers17223608
Ermi AG, Younis RM, Rodriguez K, Sarkar D. Gene Therapy Strategies for Hepatocellular Carcinoma (HCC): Current Landscape and Future Directions. Cancers. 2025; 17(22):3608. https://doi.org/10.3390/cancers17223608
Chicago/Turabian StyleErmi, Ali Gawi, Rabha M. Younis, Kayla Rodriguez, and Devanand Sarkar. 2025. "Gene Therapy Strategies for Hepatocellular Carcinoma (HCC): Current Landscape and Future Directions" Cancers 17, no. 22: 3608. https://doi.org/10.3390/cancers17223608
APA StyleErmi, A. G., Younis, R. M., Rodriguez, K., & Sarkar, D. (2025). Gene Therapy Strategies for Hepatocellular Carcinoma (HCC): Current Landscape and Future Directions. Cancers, 17(22), 3608. https://doi.org/10.3390/cancers17223608






