Improving the Potential for Predicting Prostate Cancer Progression in Patients on Active Surveillance Using Explainable Artificial Intelligence
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Targeted Biopsy Protocol
2.3. MRI Acquisition Parameters
2.4. Image Segmentation and Radiomic Feature Extraction
2.5. Data Preprocessing
- •
- Baseline features derived from the initial observation;
- •
- Delta features, calculated as the arithmetic difference between the final and baseline observations;
- •
- Time series of features, incorporating all available examinations.
2.6. Predictive Modeling
2.7. Statistical Analysis
2.8. Workflow of the Study
- ROI segmentation on T2WI and ADC maps (Section 2.4);
- Extraction of radiomic features (first-order, shape, and texture) and further analysis of their robustness (Section 2.4);
- Formation of datasets depending on the scans and features considered (Section 2.5);
- Predictive modeling for various datasets, including the consideration of different ML algorithms, tuning of hyperparameters via LOOCV, selection of the best models, and their interpretation and improvement using SHAP (Section 2.6).
3. Results
3.1. Patient Characteristics
3.2. Progression Prediction Models Based on Baseline Features, Delta Features, and Time Series of Features
- Temporal Context. Features were calculated from:
- (I)
- The baseline (initial) observation (Baseline features);
- (II)
- The difference between the final (last) and baseline observations (Delta features);
- (III)
- All available observations (Time series of features).
- Feature Subset. For each temporal context, we created three feature subsets:
- (A)
- Radiomic features from T2WI and ADC maps;
- (B)
- Radiomic features and PSA value;
- (C)
- Radiomic features and PSAd value.
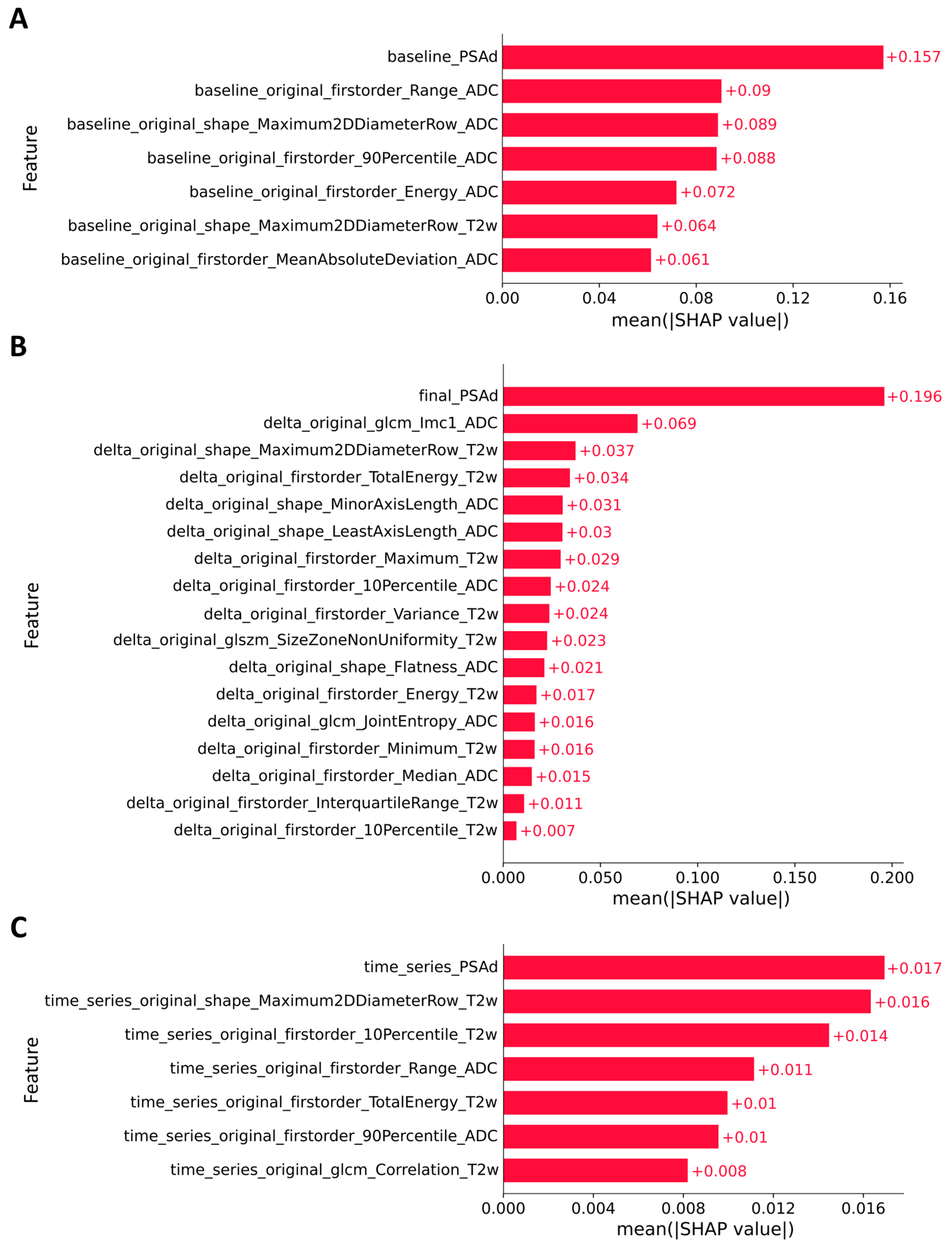
3.3. Explainable Artificial Intelligence
3.4. Combination of Predictions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Broering, J.M.; Kantoff, P.W.; Carroll, P.R. Contemporary Trends in Low Risk Prostate Cancer: Risk Assessment and Treatment. J. Urol. 2007, 178, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Shill, D.K.; Roobol, M.J.; Ehdaie, B.; Vickers, A.J.; Carlsson, S.V. Active Surveillance for Prostate Cancer. Transl. Androl. Urol. 2021, 10, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, L.F.; Schenk, J.M.; Zheng, Y.; Liu, M.; Zhu, K.; Brooks, J.D.; Carroll, P.R.; Dash, A.; De La Calle, C.M.; Ellis, W.J.; et al. Long-Term Outcomes in Patients Using Protocol-Directed Active Surveillance for Prostate Cancer. JAMA 2024, 331, 2084. [Google Scholar] [CrossRef]
- Wilt, T.J.; Jones, K.M.; Barry, M.J.; Andriole, G.L.; Culkin, D.; Wheeler, T.; Aronson, W.J.; Brawer, M.K. Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N. Engl. J. Med. 2017, 377, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.H.; Ollendorf, D.A.; Pearson, S.D.; Barry, M.J.; Kantoff, P.W.; Stewart, S.T.; Bhatnagar, V.; Sweeney, C.J.; Stahl, J.E.; McMahon, P.M. Active Surveillance Compared With Initial Treatment for Men With Low-Risk Prostate Cancer: A Decision Analysis. JAMA 2010, 304, 2373. [Google Scholar] [CrossRef]
- Bellardita, L.; Valdagni, R.; Van Den Bergh, R.; Randsdorp, H.; Repetto, C.; Venderbos, L.D.F.; Lane, J.A.; Korfage, I.J. How Does Active Surveillance for Prostate Cancer Affect Quality of Life? A Systematic Review. Eur. Urol. 2015, 67, 637–645. [Google Scholar] [CrossRef]
- Venkitaraman, R.; Norman, A.; Woode-Amissah, R.; Fisher, C.; Dearnaley, D.; Horwich, A.; Huddart, R.; Khoo, V.; Thompson, A.; Parker, C. Predictors of Histological Disease Progression in Untreated, Localized Prostate Cancer. J. Urol. 2007, 178, 833–837. [Google Scholar] [CrossRef]
- Dall’Era, M.A.; Konety, B.R.; Cowan, J.E.; Shinohara, K.; Stauf, F.; Cooperberg, M.R.; Meng, M.V.; Kane, C.J.; Perez, N.; Master, V.A.; et al. Active Surveillance for the Management of Prostate Cancer in a Contemporary Cohort. Cancer 2008, 112, 2664–2670. [Google Scholar] [CrossRef]
- Klotz, L.; Zhang, L.; Lam, A.; Nam, R.; Mamedov, A.; Loblaw, A. Clinical Results of Long-Term Follow-Up of a Large, Active Surveillance Cohort With Localized Prostate Cancer. J. Clin. Oncol. 2010, 28, 126–131. [Google Scholar] [CrossRef]
- San Francisco, I.F.; Werner, L.; Regan, M.M.; Garnick, M.B.; Bubley, G.; DeWolf, W.C. Risk Stratification and Validation of Prostate Specific Antigen Density as Independent Predictor of Progression in Men With Low Risk Prostate Cancer During Active Surveillance. J. Urol. 2011, 185, 471–476. [Google Scholar] [CrossRef]
- Bul, M.; Zhu, X.; Valdagni, R.; Pickles, T.; Kakehi, Y.; Rannikko, A.; Bjartell, A.; Van Der Schoot, D.K.; Cornel, E.B.; Conti, G.N.; et al. Active Surveillance for Low-Risk Prostate Cancer Worldwide: The PRIAS Study. Eur. Urol. 2013, 63, 597–603. [Google Scholar] [CrossRef]
- Bokhorst, L.P.; Valdagni, R.; Rannikko, A.; Kakehi, Y.; Pickles, T.; Bangma, C.H.; Roobol, M.J. A Decade of Active Surveillance in the PRIAS Study: An Update and Evaluation of the Criteria Used to Recommend a Switch to Active Treatment. Eur. Urol. 2016, 70, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Moschini, M.; Carroll, P.R.; Eggener, S.E.; Epstein, J.I.; Graefen, M.; Montironi, R.; Parker, C. Low-Risk Prostate Cancer: Identification, Management, and Outcomes. Eur. Urol. 2017, 72, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelrijck, M.; Ji, X.; Helleman, J.; Roobol, M.J.; Van Der Linden, W.; Nieboer, D.; Bangma, C.H.; Frydenberg, M.; Rannikko, A.; Lee, L.S.; et al. Reasons for Discontinuing Active Surveillance: Assessment of 21 Centres in 12 Countries in the Movember GAP3 Consortium. Eur. Urol. 2019, 75, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.H.; Marchetti, K.A.; Singhal, U.; Morgan, T.M. Active Surveillance for Prostate Cancer: Selection Criteria, Guidelines, and Outcomes. World J. Urol. 2022, 40, 35–42. [Google Scholar] [CrossRef]
- Loeb, S.; Vellekoop, A.; Ahmed, H.U.; Catto, J.; Emberton, M.; Nam, R.; Rosario, D.J.; Scattoni, V.; Lotan, Y. Systematic Review of Complications of Prostate Biopsy. Eur. Urol. 2013, 64, 876–892. [Google Scholar] [CrossRef]
- Evans, R.; Loeb, A.; Kaye, K.S.; Cher, M.L.; Martin, E.T. Infection-Related Hospital Admissions After Prostate Biopsy in United States Men. Open Forum Infect. Dis. 2017, 4, ofw265. [Google Scholar] [CrossRef]
- Moore, C.M.; Giganti, F.; Albertsen, P.; Allen, C.; Bangma, C.; Briganti, A.; Carroll, P.; Haider, M.; Kasivisvanathan, V.; Kirkham, A.; et al. Reporting Magnetic Resonance Imaging in Men on Active Surveillance for Prostate Cancer: The PRECISE Recommendations—A Report of a European School of Oncology Task Force. Eur. Urol. 2017, 71, 648–655. [Google Scholar] [CrossRef]
- Barrett, T.; Haider, M.A. The Emerging Role of MRI in Prostate Cancer Active Surveillance and Ongoing Challenges. Am. J. Roentgenol. 2017, 208, 131–139. [Google Scholar] [CrossRef]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J. Urol. 2018, 199, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Thurtle, D.; Barrett, T.; Thankappan-Nair, V.; Koo, B.; Warren, A.; Kastner, C.; Saeb-Parsy, K.; Kimberley-Duffell, J.; Gnanapragasam, V.J. Progression and Treatment Rates Using an Active Surveillance Protocol Incorporating Image-guided Baseline Biopsies and Multiparametric Magnetic Resonance Imaging Monitoring for Men with Favourable-risk Prostate Cancer. BJU Int. 2018, 122, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Doan, P.; Scheltema, M.J.; Amin, A.; Shnier, R.; Geboers, B.; Gondoputro, W.; Moses, D.; Van Leeuwen, P.J.; Haynes, A.M.; Matthews, J.; et al. Final Analysis of the Magnetic Resonance Imaging in Active Surveillance Trial. J. Urol. 2022, 208, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Rajwa, P.; Pradere, B.; Quhal, F.; Mori, K.; Laukhtina, E.; Huebner, N.A.; D’Andrea, D.; Krzywon, A.; Shim, S.R.; Baltzer, P.A.; et al. Reliability of Serial Prostate Magnetic Resonance Imaging to Detect Prostate Cancer Progression During Active Surveillance: A Systematic Review and Meta-Analysis. Eur. Urol. 2021, 80, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Sushentsev, N.; Rundo, L.; Blyuss, O.; Gnanapragasam, V.J.; Sala, E.; Barrett, T. MRI-Derived Radiomics Model for Baseline Prediction of Prostate Cancer Progression on Active Surveillance. Sci. Rep. 2021, 11, 12917. [Google Scholar] [CrossRef]
- Totaro, A.; Di Paola, V.; Campetella, M.; Scarciglia, E.; Boldrini, L.; Manfredi, R. Radiomic Features on Prostatic Multiparametric Magnetic Resonance Imaging Enable Progression Risk in Patients on Active Surveillance: A Pilot Study. J. Radiol. Clin. Imaging 2022, 5, 78–86. [Google Scholar] [CrossRef]
- Sushentsev, N.; Rundo, L.; Blyuss, O.; Nazarenko, T.; Suvorov, A.; Gnanapragasam, V.J.; Sala, E.; Barrett, T. Comparative Performance of MRI-Derived PRECISE Scores and Delta-Radiomics Models for the Prediction of Prostate Cancer Progression in Patients on Active Surveillance. Eur. Radiol. 2022, 32, 680–689. [Google Scholar] [CrossRef]
- Midya, A.; Hiremath, A.; Huber, J.; Sankar Viswanathan, V.; Omil-Lima, D.; Mahran, A.; Bittencourt, L.K.; Harsha Tirumani, S.; Ponsky, L.; Shiradkar, R.; et al. Delta Radiomic Patterns on Serial Bi-Parametric MRI Are Associated with Pathologic Upgrading in Prostate Cancer Patients on Active Surveillance: Preliminary Findings. Front. Oncol. 2023, 13, 1166047. [Google Scholar] [CrossRef]
- Sushentsev, N.; Rundo, L.; Abrego, L.; Li, Z.; Nazarenko, T.; Warren, A.Y.; Gnanapragasam, V.J.; Sala, E.; Zaikin, A.; Barrett, T.; et al. Time Series Radiomics for the Prediction of Prostate Cancer Progression in Patients on Active Surveillance. Eur. Radiol. 2023, 33, 3792–3800. [Google Scholar] [CrossRef]
- Kuru, T.H.; Wadhwa, K.; Chang, R.T.M.; Echeverria, L.M.C.; Roethke, M.; Polson, A.; Rottenberg, G.; Koo, B.; Lawrence, E.M.; Seidenader, J.; et al. Definitions of Terms, Processes and a Minimum Dataset for Transperineal Prostate Biopsies: A Standardization Approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU Int. 2013, 112, 568–577. [Google Scholar] [CrossRef]
- Barrett, T.; Slough, R.; Sushentsev, N.; Shaida, N.; Koo, B.C.; Caglic, I.; Kozlov, V.; Warren, A.Y.; Thankappannair, V.; Pinnock, C.; et al. Three-Year Experience of a Dedicated Prostate mpMRI Pre-Biopsy Programme and Effect on Timed Cancer Diagnostic Pathways. Clin. Radiol. 2019, 74, 894.e1–894.e9. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Leger, S.; Agolli, L.; Pilz, K.; Troost, E.G.C.; Richter, C.; Löck, S. Assessing Robustness of Radiomic Features by Image Perturbation. Sci. Rep. 2019, 9, 614. [Google Scholar] [CrossRef]
- Sfoungaristos, S.; Perimenis, P. PSA Density Is Superior than PSA and Gleason Score for Adverse Pathologic Features Prediction in Patients with Clinically Localized Prostate Cancer. Can. Urol. Assoc. J. 2012, 6, 46–50. [Google Scholar] [CrossRef]
- Vellekoop, A.; Loeb, S.; Folkvaljon, Y.; Stattin, P. Population Based Study of Predictors of Adverse Pathology among Candidates for Active Surveillance with Gleason 6 Prostate Cancer. J. Urol. 2014, 191, 350–357. [Google Scholar] [CrossRef]
- Alberts, A.R.; Roobol, M.J.; Drost, F.H.; Van Leenders, G.J.; Bokhorst, L.P.; Bangma, C.H.; Schoots, I.G. Risk-stratification Based on Magnetic Resonance Imaging and Prostate-specific Antigen Density May Reduce Unnecessary Follow-up Biopsy Procedures in Men on Active Surveillance for Low-risk Prostate Cancer. BJU Int. 2017, 120, 511–519. [Google Scholar] [CrossRef]
- Caglic, I.; Sushentsev, N.; Gnanapragasam, V.J.; Sala, E.; Shaida, N.; Koo, B.C.; Kozlov, V.; Warren, A.Y.; Kastner, C.; Barrett, T. MRI-Derived PRECISE Scores for Predicting Pathologically-Confirmed Radiological Progression in Prostate Cancer Patients on Active Surveillance. Eur. Radiol. 2021, 31, 2696–2705. [Google Scholar] [CrossRef]
- Sammut, C.; Webb, G.I. Encyclopedia of Machine Learning; Springer Science & Business Media: New York, NY, USA, 2011; pp. 1–1058. [Google Scholar]
- Peterson, L. K-Nearest Neighbor. Scholarpedia 2009, 4, 1883. [Google Scholar] [CrossRef]
- Sperandei, S. Understanding Logistic Regression Analysis. Biochem. Med. 2014, 24, 12–18. [Google Scholar] [CrossRef]
- Vapnik, V.N. Statistical Learning Theory; Wiley: New York, NY, USA, 1998; pp. 1–768. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Chapman & Hall/CRC: New York, NY, USA, 1984; pp. 1–368. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Friedman, J.H. Stochastic Gradient Boosting. Comput. Stat. Data Anal. 2002, 38, 367–378. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.-Y. LightGBM: A Highly Efficient Gradient Boosting Decision Tree. In Proceedings of the 31st International Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017; p. 30. [Google Scholar]
- Prokhorenkova, L.; Gusev, G.; Vorobev, A.; Dorogush, A.V.; Gulin, A. CatBoost: Unbiased Boosting with Categorical Features. arXiv 2017, arXiv:1706.09516. [Google Scholar]
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Bergstra, J.S.; Bardenet, R.; Bengio, Y.; Kégl, B. Algorithms for Hyper-Parameter Optimization. In Proceedings of the 25th International Conference on Neural Information Processing Systems (NIPS 2011), Granada, Spain, 12–14 December 2011; p. 24. [Google Scholar]
- Watanabe, S. Tree-Structured Parzen Estimator: Understanding Its Algorithm Components and Their Roles for Better Empirical Performance. arXiv 2023, arXiv:2304.11127. [Google Scholar] [CrossRef]
- Akiba, T.; Sano, S.; Yanase, T.; Ohta, T.; Koyama, M. Optuna: A Next-Generation Hyperparameter Optimization Framework. arXiv 2019, arXiv:1907.10902. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017; pp. 4768–4777. [Google Scholar]
- Chandramouli, S.; Leo, P.; Lee, G.; Elliott, R.; Davis, C.; Zhu, G.; Fu, P.; Epstein, J.I.; Veltri, R.; Madabhushi, A. Computer Extracted Features from Initial H&E Tissue Biopsies Predict Disease Progression for Prostate Cancer Patients on Active Surveillance. Cancers 2020, 12, 2708. [Google Scholar] [CrossRef]
- Nayan, M.; Salari, K.; Bozzo, A.; Ganglberger, W.; Lu, G.; Carvalho, F.; Gusev, A.; Schneider, A.; Westover, B.M.; Feldman, A.S. A Machine Learning Approach to Predict Progression on Active Surveillance for Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 161.e1–161.e7. [Google Scholar] [CrossRef]
- Zhou, H.; Xie, M.; Shi, H.; Shou, C.; Tang, M.; Zhang, Y.; Hu, Y.; Liu, X. Integrating Multimodal Imaging and Peritumoral Features for Enhanced Prostate Cancer Diagnosis: A Machine Learning Approach. PLoS ONE 2025, 20, e0323752. [Google Scholar] [CrossRef]
- Zhao, W.; Hou, M.; Wang, J.; Song, D.; Niu, Y. Interpretable Machine Learning Model for Predicting Clinically Significant Prostate Cancer: Integrating Intratumoral and Peritumoral Radiomics with Clinical and Metabolic Features. BMC Med. Imaging 2024, 24, 353. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; He, H.; Yan, Y.; Zhang, L.; Zhang, Q. Multi-Regional Multiparametric Deep Learning Radiomics for Diagnosis of Clinically Significant Prostate Cancer. J. Digit. Imaging. Inform. Med. 2025. [Google Scholar] [CrossRef]
- Nicoletti, G.; Mazzetti, S.; Maimone, G.; Cignini, V.; Cuocolo, R.; Faletti, R.; Gatti, M.; Imbriaco, M.; Longo, N.; Ponsiglione, A.; et al. Development and Validation of an Explainable Radiomics Model to Predict High-Aggressive Prostate Cancer: A Multicenter Radiomics Study Based on Biparametric MRI. Cancers 2024, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- Vraka, A.; Marfil-Trujillo, M.; Ribas-Despuig, G.; Flor-Arnal, S.; Cerdá-Alberich, L.; Jiménez-Gómez, P.; Jimenez-Pastor, A.; Martí-Bonmatí, L. Radiomics-Based Early Triage of Prostate Cancer: A Multicenter Study from the CHAIMELEON Project 2025. medRxiv 2025. [Google Scholar] [CrossRef]
- Li, Z.; Yang, L.; Wang, X.; Xu, H.; Chen, W.; Kang, S.; Huang, Y.; Shu, C.; Cui, F.; Zhang, Y. Intralesional and Perilesional Radiomics Strategy Based on Different Machine Learning for the Prediction of International Society of Urological Pathology Grade Group in Prostate Cancer. BMC Med. Imaging 2025, 25, 266. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Fan, Z.; Jiang, S.; Chen, X.; Guo, H.; Dong, S.; Jiang, T. Interpretable Multimodal Deep Learning Model for Predicting Post-Surgical International Society of Urological Pathology Grade in Primary Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 3730–3743. [Google Scholar] [CrossRef]
- Liu, B.; Ding, X.; Xu, H.; Bai, X.; Zhang, X.; Cui, M.; Guo, A.; Mu, X.; Xie, L.; Kang, H.; et al. Preoperative Assessment of Extraprostatic Extension in Prostate Cancer Using an Interpretable Tabular Prior-Data Fitted Network-Based Radiomics Model From MRI. Magn. Reson. Imaging 2025. [Google Scholar] [CrossRef]
- Kotb, A.F.; Tanguay, S.; Luz, M.A.; Kassouf, W.; Aprikian, A.G. Relationship between Initial PSA Density with Future PSA Kinetics and Repeat Biopsies in Men with Prostate Cancer on Active Surveillance. Prostate Cancer Prostatic Dis. 2011, 14, 53–57. [Google Scholar] [CrossRef][Green Version]
- Sushentsev, N.; Caglic, I.; Rundo, L.; Kozlov, V.; Sala, E.; Gnanapragasam, V.J.; Barrett, T. Serial Changes in Tumour Measurements and Apparent Diffusion Coefficients in Prostate Cancer Patients on Active Surveillance with and without Histopathological Progression. Br. J. Radiol. 2022, 95, 20210842. [Google Scholar] [CrossRef]
- Solari, E.L.; Gafita, A.; Schachoff, S.; Bogdanović, B.; Villagrán Asiares, A.; Amiel, T.; Hui, W.; Rauscher, I.; Visvikis, D.; Maurer, T.; et al. The Added Value of PSMA PET/MR Radiomics for Prostate Cancer Staging. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 527–538. [Google Scholar] [CrossRef]
- Faiella, E.; Pileri, M.; Ragone, R.; De Nicola, A.M.; Beomonte Zobel, B.; Grasso, R.F.; Santucci, D. Promising Results About the Possibility to Identify Prostate Cancer Patients Employing a Random Forest Classifier: A Preliminary Study Preoperative Patients Selection. Diagnostics 2025, 15, 421. [Google Scholar] [CrossRef]
- Wibmer, A.; Hricak, H.; Gondo, T.; Matsumoto, K.; Veeraraghavan, H.; Fehr, D.; Zheng, J.; Goldman, D.; Moskowitz, C.; Fine, S.W.; et al. Haralick Texture Analysis of Prostate MRI: Utility for Differentiating Non-Cancerous Prostate from Prostate Cancer and Differentiating Prostate Cancers with Different Gleason Scores. Eur. Radiol. 2015, 25, 2840–2850. [Google Scholar] [CrossRef]
- Sushentsev, N.; Comune, R.; Sinci, K.A.; Blyuss, O.; Caglič, I.; Kastner, C.; Barrett, T. A 10-year Analysis of MRI-driven Prostate Cancer Diagnosis and Active Surveillance: Trends and Implications. BJU Int. 2025, 136, 224–226. [Google Scholar] [CrossRef]
- Cattell, R.; Chen, S.; Huang, C. Robustness of radiomic features in magnetic resonance imaging: Review and a phantom study. Vis. Comput. Ind. Biomed. Art 2019, 2, 19. [Google Scholar] [CrossRef]
- Scalco, E.; Belfatto, A.; Mastropietro, A.; Rancati, T.; Avuzzi, B.; Messina, A.; Valdagni, R.; Rizzo, G. T2w-MRI signal normalization affects radiomics features reproducibility. Med. Phys. 2020, 47, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Přibil, J.; Přibilová, A.; Frollo, I. Analysis of the influence of different settings of scan sequence parameters on vibration and noise generated in the open-air MRI scanning area. Sensors 2019, 19, 4198. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total Cohort (n = 76) | Progressors (n = 29) | Non-Progressors (n = 47) | p (Progressors vs. Non-Progressors) |
|---|---|---|---|---|
| Age, years | 66 (61–69) | 66 (60–69) | 66 (61.5–69) | 1.0 |
| Gland volume, mL | 44.75 (36.0–70.0) | 41.0 (29.0–47.0) | 55.0 (39.5–80.5) | 0.005 |
| PSA, ng/mL | 5.04 (3.62–7.42) | 5.63 (4.02–7.7) | 4.51 (3.35–7.15) | 0.255 |
| PSAd | 0.10 (0.07–0.16) | 0.12 (0.08–0.27) | 0.09 (0.06–0.12) | 0.007 |
| Biopsy ISUP grade group | 0.726 | |||
| 1 | 58 (76.3%) | 21 (72.4%) | 37 (78.7%) | |
| 2 | 18 (23.7%) | 8 (27.6%) | 10 (21.3%) | |
| PI-RADS | 0.021 | |||
| 3 | 16 (21.1%) | 2 (6.9%) | 14 (29.8%) | |
| 4 | 26 (34.2%) | 9 (31%) | 17 (36.2%) | |
| 5 | 34 (44.7%) | 18 (62.1%) | 16 (34%) | |
| AS follow-up time, months | 42 (32.5–63.25) | 40 (33–49) | 43 (30.5–67.5) | 0.233 |
| Dataset | Optimal Model | Balanced Accuracy | F1-Score | AUC |
|---|---|---|---|---|
| I.A | XGBoost | 0.695 | 0.6 | 0.623 |
| I.B | CatBoost | 0.659 | 0.571 | 0.662 |
| I.C | GB | 0.719 | 0.642 | 0.704 |
| II.A | LightGBM | 0.764 | 0.704 | 0.817 |
| II.B | CatBoost | 0.792 | 0.741 | 0.8 |
| II.C | XGBoost | 0.753 | 0.691 | 0.740 |
| II.D | CatBoost | 0.778 | 0.72 | 0.8 |
| II.E | GB | 0.85 | 0.814 | 0.844 |
| III.A | LSTM | 0.744 | 0.696 | 0.726 |
| III.B | LSTM | 0.742 | 0.679 | 0.787 |
| III.C | LSTM | 0.805 | 0.759 | 0.843 |
| Dataset, Model | Number of Optimal Features | Balanced Accuracy | F1-Score | AUC |
|---|---|---|---|---|
| I.C, GB | 7 | 0.76 | 0.702 | 0.793 |
| II.E, GB | 17 | 0.865 | 0.836 | 0.913 |
| III.C, LSTM | 7 | 0.861 | 0.828 | 0.917 |
| Dataset, Model, # Features | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| I.C, GB, 7 | 0.690 | 0.830 | 0.714 | 0.813 |
| II.E, GB, 17 | 0.793 | 0.936 | 0.885 | 0.88 |
| III.C, LSTM, 7 | 0.828 | 0.894 | 0.828 | 0.894 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vershinina, O.; Sushentsev, N.; Zaikin, A.; Blyuss, O.; Barrett, T.; Ivanchenko, M. Improving the Potential for Predicting Prostate Cancer Progression in Patients on Active Surveillance Using Explainable Artificial Intelligence. Cancers 2025, 17, 3598. https://doi.org/10.3390/cancers17223598
Vershinina O, Sushentsev N, Zaikin A, Blyuss O, Barrett T, Ivanchenko M. Improving the Potential for Predicting Prostate Cancer Progression in Patients on Active Surveillance Using Explainable Artificial Intelligence. Cancers. 2025; 17(22):3598. https://doi.org/10.3390/cancers17223598
Chicago/Turabian StyleVershinina, Olga, Nikita Sushentsev, Alexey Zaikin, Oleg Blyuss, Tristan Barrett, and Mikhail Ivanchenko. 2025. "Improving the Potential for Predicting Prostate Cancer Progression in Patients on Active Surveillance Using Explainable Artificial Intelligence" Cancers 17, no. 22: 3598. https://doi.org/10.3390/cancers17223598
APA StyleVershinina, O., Sushentsev, N., Zaikin, A., Blyuss, O., Barrett, T., & Ivanchenko, M. (2025). Improving the Potential for Predicting Prostate Cancer Progression in Patients on Active Surveillance Using Explainable Artificial Intelligence. Cancers, 17(22), 3598. https://doi.org/10.3390/cancers17223598






