Simple Summary
Extramammary Paget’s disease (EMPD) is a rare skin cancer that primarily affects the genital and perianal regions. Surgery remains the primary treatment option, but it often involves invasive procedures that can cause discomfort and functional impairment, and still carry a risk of recurrence. These limitations have stimulated interest in less invasive options. This review focuses on two main aspects. First, the role of imaging in detecting EMPD, defining lesion margins, and monitoring patients over time without repeated biopsies. Second, microscopic evidence is presented for non-surgical treatments such as topical agents, radiotherapy, or photodynamic therapy. By critically analyzing the literature, we explore how these approaches may help individuals who cannot undergo surgery or wish to avoid it. Our findings provide insights for future research and support more tailored management of patients with EMPD.
Abstract
Background/Objectives: Extramammary Paget’s disease (EMPD) is a rare cutaneous malignancy arising in areas rich in apocrine glands that poses diagnostic and therapeutic difficulties. Although surgery remains the standard of care, achieving clear margins is challenging and recurrence rates are high. This review explores the contribution of non-invasive imaging for diagnosis and monitoring, and evaluates conservative, non-surgical therapies as alternatives to radical surgery. Methods: Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), a systematic review was conducted: eligible studies included interventional and observational research, as well as case series and reports, assessing non-invasive diagnostic methods or non-surgical treatments for EMPD. Data extraction and risk-of-bias evaluation were performed independently by multiple reviewers, and a narrative synthesis summarized therapeutic outcomes and diagnostic performance. Results: Of 808 identified records, 82 met the inclusion criteria: 66 focused on non-surgical therapies, 15 on diagnostic techniques, and one on both. Reflectance confocal microscopy (RCM) and photodynamic diagnosis (PDD) showed high concordance with histopathology, aiding both diagnosis and margin delineation. Among therapies, topical imiquimod and photodynamic therapy (PDT) demonstrated encouraging response rates, while radiotherapy, laser ablation, and systemic chemotherapy were less consistently reported. Evidence quality was limited by small cohorts, heterogeneous regimens, and variable follow-up. Conclusions: Non-invasive imaging enhances diagnostic accuracy and surgical planning, while non-surgical treatments—particularly imiquimod and PDT—offer viable alternatives in selected cases. Larger prospective studies are needed to establish standardized protocols and clarify long-term outcomes.
1. Introduction
Extramammary Paget’s disease (EMPD) represents an uncommon form of cutaneous malignancy that arises in areas rich in apocrine glands [1]. Its occurrence is exceptionally rare, with incidence estimates ranging from 0.1 to 2.4 cases per million individuals per year [2,3]. The disease predominantly affects the genital region, with the vulva being the most frequent site in women, followed by the penoscrotal area in men; other sites include the perianal region and, less commonly, the axilla. Among reported cases, EMPD is most often diagnosed in Caucasian women, particularly those aged between 50 and 80 years [1,2,3,4].
EMPD is a rare cutaneous malignancy that may arise through two distinct pathways, each carrying different prognostic significance. Primary EMPD originates within the epidermis, most often presenting as an intraepithelial (in situ) neoplasm, though it may progress to an invasive form capable of regional or distant metastasis. In some cases, it is associated with an underlying adenocarcinoma of skin appendages or subcutaneous glands. Secondary EMPD, by contrast, reflects epidermal involvement by malignant cells derived from non-cutaneous adenocarcinomas—most commonly anorectal, urothelial, or other visceral primaries—via metastasis or epidermotropic colonization [4,5]. These associated tumors may occur simultaneously or develop at a different time point [6,7,8,9].
The definitive diagnosis of EMPD relies on biopsy and anatomopathological assessment. Microscopically, the epidermis shows the presence of Paget cells, which are large with abundant pale, finely granular cytoplasm and pleomorphic round nuclei. These cells are usually scattered individually but may also appear in small clusters [4]. Immunohistochemical staining typically reveals positivity for cytokeratins, epithelial membrane antigen, and carcinoembryonic antigen [5].
Surgery remains the mainstay of care, most commonly performed as wide local excision (WLE) or Mohs micrographic surgery (MMS) [10]. The real challenge, however, lies in defining the true extent of the disease. EMPD may spread microscopically beyond what is visible, arise in multiple foci, and show irregular borders. As a result, obtaining histologically negative margins without resorting to extensive or even mutilating procedures is difficult. Recurrence is therefore common, emphasizing the importance of accurate margin assessment in shaping both prognosis and long-term outcomes [6,7,8,11,12].
Non-invasive imaging technologies have opened new possibilities for diagnosing and managing cutaneous malignancies. Techniques such as dermoscopy, reflectance confocal microscopy (RCM), photodynamic diagnosis (PDD), optical coherence tomography (OCT), positron emission tomography/computed tomography (PET/TC), and magnetic resonance imaging (MRI) enable visualization of skin architecture at various depths without the need for biopsy. Among these, RCM provides immediate feedback during surgery, making it especially valuable for delineating tumor margins and identifying residual disease. This real-time information can significantly improve the precision, speed, and overall outcomes of MMS and other operative strategies in EMPD treatment [5].
Over the years, several non-surgical approaches have been tried in EMPD, but their use is still quite limited. This is partly because the disease is rare, and partly because we do not yet have large, controlled studies to guide practice [13,14,15,16]. Reported alternatives include radiotherapy, photodynamic therapy (PDT), laser treatment, and different topical agents. Among these agents, imiquimod has attracted particular interest, showing favorable outcomes in certain clinical contexts [17,18,19,20,21,22]. This drug, a toll-like receptor 7 agonist, works by stimulating the innate immune system and triggering cytokines such as interferon-α, interleukin-6, and Tumor Necrosis Factor α (TNF-α), which together contribute to an antitumor effect. Although approved for actinic keratosis, superficial basal cell carcinoma, and genital warts, clinicians have extended its use to EMPD [4]. Other topical creams, like 5-fluorouracil, have also been tested, while systemic chemotherapy is generally considered only when the disease is invasive or metastatic [5].
When limited to the epidermis and managed appropriately, primary EMPD is usually associated with a good prognosis. In contrast, once the disease becomes invasive and involves regional lymph nodes or distant organs, systemic therapy is often required and the outlook is considerably poorer [22].
This systematic review focuses on non-invasive and conservative strategies for the management of EMPD, integrating diagnostic and therapeutic perspectives within a unified framework. Non-invasive imaging techniques are central, as they improve the accuracy of disease detection, margin assessment, and postoperative surveillance, thereby informing and optimizing treatment decisions. In parallel, conservative non-surgical therapies are discussed as complementary options that align with the same minimally invasive philosophy—offering meaningful clinical benefit for patients in whom radical surgery is contraindicated or undesirable. Together, these approaches represent, for the first time, a comprehensive continuum of care that unites diagnosis and treatment within a single patient-centered management strategy, emphasizing the evolving paradigm toward less invasive yet effective disease control.
2. Materials and Methods
2.1. Study Design
This systematic review was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1) [23].
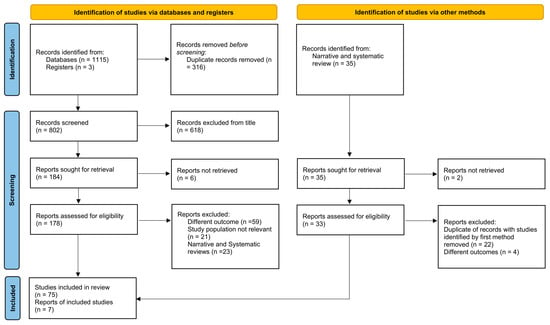
Figure 1.
PRISMA flow chart illustrating the study selection process of studies. The chart shows records identified through databases, registries, and other sources, followed by screening, eligibility assessment, and final inclusion in the review. A total of 82 studies (75 from articles and 7 articles retrieved from narrative and systematic review) were included after removing duplicates and excluding records due to irrelevance or ineligible outcomes.
2.2. Inclusion and Exclusion Criteria
Given the scarcity of research on this subject, we adopted broad eligibility criteria. We considered all interventional studies, whether randomized or not, carried out in male or female patients of any age and from any geographical area. To be eligible, studies had to provide data on healing rates at different time points, outcomes of non-surgical therapies, local recurrence rates, and management or follow-up using non-invasive imaging methods. Studies focusing on patients with EMPD secondary to an underlying malignancy or in advanced stages of disease were excluded.
We also accepted conference abstracts and proceedings, as well as single case reports, since these often represent valuable individual experiences that would otherwise remain underrepresented in the literature. Conversely, narrative reviews, systematic reviews, and meta-analyses were initially set aside. During the data extraction phase, articles referenced within these reviews were assessed for eligibility, and those meeting the inclusion criteria, without duplication of previously included studies, were extracted and included in the analysis (See Section 2.4). Finally, only articles written in English, Spanish, French, German, or Italian were included in the review.
2.3. Search Strategy
A comprehensive literature search was conducted to identify studies evaluating non-surgical therapies and non-invasive diagnostic techniques for EMPD. Searches were performed up to July 2024 across five major databases: PubMed, Ovid Embase, Cochrane CENTRAL, Web of Science Core Collection, and ClinicalTrials.gov. Two independent reviewers (F.P. and M.V.) performed the search and screening processes, with a third reviewer (M.A.) resolving disagreements. No restrictions were applied regarding publication date, language, or study design. The search strategy was developed around four core concepts following Patient/Population, Interventions, Comparison, and Outcome strategy. Patient/Population: EMPD; Interventions: non-surgical and topical treatments like imiquimod, fluorouracil, PDT, radiotherapy and non-invasive diagnostic tools like RCM and PDD; Comparison: not applicable; Outcome terms like recurrence, diagnosis, follow-up, retreatment. All retrieved records were exported to Endnote for automatic de-duplication and then imported into an excel file for independent screening of titles/abstracts and full texts. When abstracts were later published as full articles, the full articles were given priority over the abstracts.
2.4. Data Extraction
During the initial screening phase, all records retrieved from the database were reviewed by two independent investigators (M.V. and F.P.) and a third reviewer (M.A.) in case of disagreement. From an initial set of 802 references, 618 were excluded after evaluation of titles only. The remaining studies underwent a second-level screening, which required careful examination of abstracts, and, when necessary, consultation of the results section, aimed at verifying whether the outcomes of interest were reported. Specifically, we considered: (i) healing rates at 6 months, 1 year, and 3 years for non-surgical treatments (including imiquimod, radiotherapy, photodynamic therapy, 5-fluorouracil, or other therapies); (ii) local recurrence rates at different time points (to be defined in the synthesis stage); and (iii) the use and role of confocal microscopy in the follow-up of EMPD.
The intermediate screening of abstracts was conducted independently by two authors (F.D’O. and M.L.). Each study was coded in a predesigned Excel file: articles judged relevant by both reviewers were assigned a score of “1,” while non-eligible studies were assigned “0.” In cases of disagreement between the two reviewers, a third author (F.P.) acted as an adjudicator. After this step, 75 studies were retained for study inclusion. A total of 35 articles were retrieved from 23 narrative and systematic reviews with titles consistent with the outcomes of interest by two authors (F.D’O. and M.L.). In cases of disagreement between the two reviewers, a third author (F.P.) acted as an adjudicator. Of these, only 7 were ultimately included in the final analysis, as most were duplicates of studies already identified through the primary search strategy, while a smaller proportion were excluded due to reports not being retrievable or because they addressed different outcomes. In total, 82 studies were included in the final review.
2.5. Risk of Bias
The methodological quality of the selected studies was independently assessed by two reviewers (L.L. and K.M.C.) in accordance with the Risk Of Bias In Non-randomized Studies-of Interventions (ROBINS-I) tool, applying the most appropriate domains for each study. The evaluation considered potential confounding, selection of participants, classification and deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results. Two reviewers independently assessed each study (L.L. and K.M.C.), and disagreements were resolved through discussion. When necessary, consensus was reached with the involvement of a third author (F.P.). The assessment of methodological rigor and potential sources of bias was incorporated into the overall interpretation of the findings.
2.6. Statistical Analysis
A narrative synthesis was carried out, supported by summary tables that combined therapeutic and diagnostic information from the included studies. The analysis encompassed treatment characteristics (such as prior management, schedules, outcomes, recurrence and adverse effects) together with data on non-invasive diagnostic strategies, their aims and main findings. Continuous variables were summarized using means or medians. Categorical variables were analyzed and presented as frequencies and percentages. Due to the heterogeneity and limited availability of data, no additional analyses were conducted. When information was incomplete, calculations were based on the number of cases or studies for which data were available.
3. Results
3.1. Study Selection
The PRISMA flow chart (Figure 1) illustrates the process of study selection. Ultimately, 82 studies were retained for qualitative synthesis: 66 addressing non-surgical therapeutic strategies for EMPD, 15 focusing on non-invasive diagnostic approaches, and one study contributing to both categories.
3.2. Study Quality, Bias Results and Limitations
Overall study quality was heterogeneous. Most publications were case reports or small case series, while observational designs were less frequent. In the therapeutic literature, the absence of control arms was common, and outcome assessment was usually descriptive, with limited use of standardized measures or formal statistical analysis. Reporting of recurrence, retreatment, and adverse events was inconsistent, and follow-up durations varied widely across studies. Diagnostic investigations were similarly limited, often consisting of feasibility assessments in small cohorts without systematic comparisons to reference standards. Methodological details such as patient selection, timing of assessment, and interpretation criteria were frequently incomplete. Taken together, the evidence base was characterized by variability in study design, reporting quality, and data completeness.
As most of the included studies were small case series rather than randomized controlled trials, this may have introduced a certain degree of bias and limited the robustness of the conclusions. A publication bias cannot be entirely excluded, as smaller reports are more likely to present positive findings. Furthermore, because conference abstracts and proceedings were included to ensure comprehensive coverage of the literature, some overlap with subsequently published full articles may have occurred, potentially leading to partial duplication of cases. Obvious duplicates were excluded whenever identified, and this limitation was considered in the interpretation of the findings.
The included studies were highly heterogeneous in terms of design, patient characteristics, disease stage, treatment modality, and outcome measures. Therefore, a statistical pooling of results was not methodologically appropriate, and findings were summarized descriptively. This heterogeneity, combined with the lack of controlled or comparative trials, restricts the generalizability of the conclusions and prevents firm recommendations regarding optimal diagnostic or therapeutic strategies.
In addition, the literature search was completed in July 2024, corresponding to the period of data extraction and analysis. Although no significant new studies were identified in a supplementary check performed before submission, the potential time lag between the last search and publication represents a limitation.
3.3. Study and Population Characteristics
A total of 82 studies met the inclusion criteria: 66 investigating therapeutic strategies, 15 focusing on diagnostic approaches, and one article contributing to both categories. Therapeutic reports described a wide range of non-surgical options, including topical agents such as imiquimod [24,25], PDT in different settings [24], radiotherapy either as a primary or adjuvant treatment [25], laser procedures, and less conventional strategies such as boron neutron capture therapy [26] or systemic chemotherapy [27]. Sample sizes varied substantially, from single-patient case reports to larger cohorts exceeding 90 individuals [28]. Diagnostic studies mainly assessed RCM and PDD, ranging from individual case descriptions [29] to broader series such as Tan et al., which included 73 patients [30], generally reporting concordance with biopsy findings, margin definition, and characteristic imaging features of Paget cells. Across all reports, details on lesion size, disease duration, and number of lesions were often missing, and follow-up periods ranged from a few months to more than five years [31].
3.4. Diagnostic Characteristics
Sixteen studies evaluated non-invasive diagnostic techniques for EMPD (Table 1). The majority of these studies investigated the use of RCM, either alone or combined with PDD. Sample sizes ranged from single-patient case reports [29] to larger cohorts, such as that by Tan et al. with 73 patients [30] and Huang et al. with 36 patients and 130 sections [32].
RCM was evaluated in a single study for the primary diagnosis, detection of residual disease, and assessment of surgical margins in patients with recurrent EMPD. Of the 22 clinically suspicious sites examined in 5 patients (4 men, 1 woman; median age 70 years, range 56–77), 9 (40.9%) were positive for recurrent disease on high-resolution confocal microscopy and histopathologically confirmed, while 13 (59.1%) were negative on RCM, with 3 of these 13 positive for EMPD on histopathology. Overall, RCM demonstrated a sensitivity of 75% and a specificity of 100% in detecting recurrent or persistent EMPD. False-negative results occurred in two patients, mainly at lesion margins near previous biopsy sites. The use of video mosaicking appeared to improve lesion detection [33,34].
Commonly described features included large hyper-reflective Paget cells within a disrupted honeycomb pattern, glandular nests at the dermoepidermal junction, and “target cells” with a bright nucleus and a peripheral dark halo [32,35].
PDD, alone or in association with RCM, was mainly applied for margin delineation. Cheng et al. [35] reported that surgical margins required for clearance ranged between 0.5 and 2.0 cm beyond the visible lesion. Huang et al. [32] documented that 63.8% of tumor margins extended beyond the macroscopic boundary, while the combination of PDD with RCM reduced this proportion to 20.8%.
Follow-up data were reported inconsistently. Cheng et al. observed local recurrence in 6 of 36 patients (15.4%) within 12 months, and one case of nodal metastasis at 36 months [35]. Zhou et al. [36] described recurrence rates of 26.7% and 28.6% in control groups, compared with 4% in the experimental group using Wood lamp plus 5-aminolevulinic acid-mediated photodynamic therapy (5-ALA-PDT). Several single-patient reports documented absence of relapse during follow-up periods of up to two years [35,37].

Table 1.
Summary of studies on non-invasive diagnostic techniques in EMPD, including sample size, type and purpose of the method (diagnosis, residual disease, or margin detection), timing, concordance with histopathology, key RCM features, treatment context, recurrence, and follow-up.
Table 1.
Summary of studies on non-invasive diagnostic techniques in EMPD, including sample size, type and purpose of the method (diagnosis, residual disease, or margin detection), timing, concordance with histopathology, key RCM features, treatment context, recurrence, and follow-up.
| Authors | No. of Patients and Sections | Type of Non-Invasive Diagnosis | Purpose (Diagnosis, Monitoring, Margin Detection) | Time (Before or After Treatment) | Concordance to Different Approaches | RCM Features of Lesions | Type of Treatment | Recurrence | Follow-Up Period (Years) |
|---|---|---|---|---|---|---|---|---|---|
| Zhou et al. [36] | 15 21 | (A) Wide local excision (control group, 15 patients) (B) Wood lamp examination + 5-ALA-PDT (experimental group, 21 patients) | Margin detections | Pre-treatment | - | - | Surgery | Recurrence rate (A) 26.7% (B) 28.6% | 4.0 |
| Zhan-Yan et al. [38] | 14 23 | Reflectance confocal microscopy | Diagnosis and margin detections | - | Biopsy confirmed | Typical Paget cells were characterized by a mild bright nucleus and dark cytoplasm, frequently twice the size of keratinocytes or larger. At the dermoepidermal junction, tumor nests were seen as dark glandular structures | Surgery | - | - |
| Guitera et al. [39] | 10 - | Reflectance confocal microscopy | Diagnosis | - | Biopsy confirmed | Presence of large atypical Pagetoid cells | Surgery | - | - |
| Debarbieux et al. [40] | 1 - | Reflectance confocal microscopy | Diagnosis and margin detection | Pre-treatment and intraoperative | Biopsy confirmed | Intra-epidermal large dark isolated or nested cells, with few of them exhibiting a target appearance. | Surgery | - | - |
| Suppa et al. [31] | 1 - | Reflectance confocal microscopy | Diagnosis | - | Biopsy confirmed | Large round cells with abundant and hypo-reflective cytoplasm with peripheral dark halo at epidermis. | Previously treated with topical 5% imiquimod cream | Recurrence after 13 years since first treatment | - |
| Terrier et al. [37] | 1 - | Reflectance confocal microscopy combined with the “Spaghetti” Technique | Margin detection | Pre-treatment | Histological examination identified minimum clear margins of 3 mm | Dark and roundish cavities in the epidermis, corresponding to Paget cells. | Surgery | - | 2.0 |
| Yélamos et al. [41] | 5 22 | Reflectance confocal microscopy | Margin detection and residual disease | Pre-treatment | Three false-negative RCM at the margins of EMPD, close to previous biopsy sites. | Target cells with bright center and peripheral dark halo forming nests of Paget cells at DEJ. Focal dark holes in the stratum spinosum. | Surgery, topical 5% imiquimod cream, radiotherapy plus oral ERBB2-TKI | Recurrent disease on handheld RCM and histopathologically confirmed: 9/22 (40.9%); Negative on HRCM: 13/22 (59.1%), of which 3 were positive for EMPD on histopathological examination. | - |
| Chuchvara et al. [29] | 1 - | Reflectance confocal microscopy | Diagnosis | - | Similarities to melanoma on dermoscopy, histopathology, and RCM versus immuno-histochemistry staining revealing pigmented EMPD | Atypical hyperreflective dendritic cells and hyporeflective round nucleated cells within a disarranged honeycomb pattern at the level of epidermis and DEJ | - | - | - |
| Wu et al. [32] | 36 130 | Photodynamic diagnosis plus reflectance confocal microscopy | Margin detection | Pre- and post-treatment | Tumor margins beyond macroscopic line: 83/130 (63.8%) Tumor margins beyond photodynamic diagnosis marker line: 46/130 (35.4%) Tumor margins beyond photodynamic diagnosis plus reflectance confocal microscopy marker line: 27/130 (20.8%) | Highly refractive, large-nucleated cells on the epidermis | Surgery | - | - |
| Kibbi et al. [42] | 33 41 | Reflectance confocal microscopy | Margin detection | Post-treatment | RCM correlation with scouting punch biopsies (kappa, 0.93) | Dark holes in the epidermis (Paget’s cells) and glandular nests of cells | Surgery: 21/33 Radiotherapy: 5/33 Imiquimod 6/33 Photodynamic therapy: 1/33 | - | - |
| Huang et al. [34] | 1 - | Photodynamic diagnosis plus reflectance confocal microscopy | Diagnosis and margin detection | Pre-treatment | - | Highly refractive, large-nucleated cells on the epidermis | Surgery | - | - |
| Ganhewa et al. [43] | 1 - | Reflectance confocal microscopy | Residual disease | Pre-treatment | - | Paget cells with distinctive holes in the honeycomb’ pattern of keratinocytes | 5-FU | - | - |
| Navarrete-Dechent et al. [44] | 33 36 | Reflectance confocal microscopy | Margin detection | Post-treatment (mean margin needed to clear 1.8 cm) | RCM correlation with scouting punch biopsies (kappa, 0.93; p < 0.001) | - | Surgery | - | 1.5 |
| Tan et al. [30] | 73 - | Reflectance confocal microscopy | Diagnosis | - | RCM: 54/73 (74.0%) Biopsy: 52/67 (77.6%) | Disarranged honeycomb pattern in the upper epidermis and basal lamina; Paget cells in stratum spinosum; inflammatory cells in dermis; dilated vessels in tortuous morphology in the superficial dermis. | - | - | - |
| Filonenko et al. [45] | 1 - | Photodynamic diagnosis | Margin detection | Before first and second cycle of treatment | - | - | Photodynamic therapy | Relapse-free follow-up at 2 years and 3 months after treatment | 2.3 |
| Cheng et al. [35] | 36 166 | Photodynamic diagnosis plus reflectance confocal microscopy | Margin detection | Post-treatment (mean margin needed to clear 0.5–2.0 cm) | - | - | Surgery | Local recurrence: 6/36, 15.4% (2–12 months postoperatively) Lymph node metastasis without local recurrence: 1/36, 2.8% (36 months postoperatively) | 3.0 |
3.5. Treatment Characteristics
A total of 67 studies reported non-surgical therapies for EMPD (Table 2). Among them, topical imiquimod 5% was the most frequently investigated. When considering only monotherapy cohorts with clearly reported outcomes, data extracted from Hata et al., Clément et al., and Rioli et al. indicated a complete response (CR) in 57.9% of patients (133/230). Partial responses (PR) were seen in 31.0% (38/124), and stable disease (SD) in 9.5% (2/21) of cases. Disease progression was rarely documented [25,46,47].
Photodynamic therapy was the second most commonly evaluated modality. Across the available studies evaluating PDT as monotherapy, the CR rate was 83.1% (83/100) [26,48]. PR was reported in 24.8% (6/24), SD in 42.4% (6/14), and PD in 7.0% (1/13) of patients [49].
Radiotherapy was described in multiple case series, often after surgery, but also as primary treatment, with generally favorable outcomes [27,43].
Other approaches were less frequently reported, including laser ablation [49], topical 5-fluorouracil [43], systemic chemotherapy [27], and boron neutron capture therapy [26].
Treatment regimens were highly heterogeneous. Imiquimod was applied between 2 and 7 times weekly, while PDT protocols varied in photosensitizer, light source, and number of cycles. Follow-up ranged from a few months in case reports to over five years in larger series [50].

Table 2.
Summary of studies on non-surgical therapeutic approaches for EMPD, including patient numbers, type and line of treatment, previous therapies, treatment regimens, clinical outcomes (CR, PR, SD, PD), recurrence and retreatment data, reported adverse effects, and follow-up duration.
Table 2.
Summary of studies on non-surgical therapeutic approaches for EMPD, including patient numbers, type and line of treatment, previous therapies, treatment regimens, clinical outcomes (CR, PR, SD, PD), recurrence and retreatment data, reported adverse effects, and follow-up duration.
| Authors | No. of Patients | Type of Non-Surgical Treatment | Line of Treatment | Previous Treatments | Dosage and Time | Therapeutic Outcome CR, PR, SD, PD (Months) | Recurrence: Yes/No (Months) | Retreatments | Side Effects | Follow-Up Period (Years) |
|---|---|---|---|---|---|---|---|---|---|---|
| Escolà et al. [51] | 76 | Topical 5% imiquimod cream Photodynamic therapy (ALA in 20.5% and mALA in 79.5%) Topical 5-Fluorouracil Radiotherapy (82.4% external beam radiotherapy with photons or electrons, 11.8% brachytherapy and 5.9% orthovoltage radiotherapy) Carbon dioxide laser | First line | - | Dose schedules ranged from once daily to twice weekly and duration from 1 to 104 weeks Total of 1–10 treatments at intervals ranging from 1 to 5 weeks Once or twice daily for 2–10 weeks Doses ranged between 8 and 64 Gy delivered in 1–33 fractions - | CR 52.2%; PR 30.6%; SD 13.4%; PD 3.7% (<3 months) CR 14.6%; PR 45.8%; SD 33.3%; PD 6.3% (<3 months) CR 18.8%; PR 37.5%; SD 37.5%; PD 6.3% (<3 months) CR 65.2%; PR 26.1%; SD 4.4%; PD 4.4% (<3 months) CR 0.0%; PR 50.0%; SD 25.0%; PD 25.0% (<3 months) | - | - | - | 5.0 |
| Xiang et al. [52] | 1 | Hematoporphyrin injection photodynamic therapy | First line | - | Intravenous dose of Hematoporphyrin Injection (HiPorfin; 5 mL: 25 mg) at 5 mg/kg in 250 mL saline. Laser wavelength 630 nm, power density 100 mW/cm2, irradiation duration 30 min per light spot, energy density 180 J/cm2 | CR 100% (41 months) | No (41 months) | No | Redness, necrosis, scab, ulceration, granulation, scarring | 3.4 |
| Filonenko et al. [45] | 1 | Chlorin e6 photodynamic therapy | First line | - | Chlorin e6 intravenous 1.0 mg/kg. Irradiation (λ 662 nm, power density 130–150 mW/cm2, 194 min, light dose 300 J/cm2) | CR 100% (6 months) | Yes (21 months) | Photodynamic therapy (second cycle) | Redness, pain, bleeding, ulceration, scarring | 4.0 |
| Xiang et al. [53] | 1 | Hematoporphyrin photodynamic therapy | First line | - | Hematoporphyrin injection (HpD) 5 mg/kg intravenously in 250 mL saline solution. Laser wavelength 630 nm, power density 100 mW/cm2, irradiation time 30 min per spot, energy density 180 J/cm2 | CR 100% (3 months) | Yes (18 months) | No | Pain, swelling, redness, scarring | 3.5 |
| Do et al. [54] | 11 | Radiation therapy | Adjuvant | Surgical treatment | - | - | - | - | - | 2.9 |
| Borella et al. [50] | 51 | Topical 5% imiquimod cream | First line | - | 2 or 3 applications weekly, from a minimum of 24 weeks to a maximum of 72 weeks | CR 43.1% (66 months) | - | - | Erosions, burning, fever | 5.5 |
| van der Linden et al. [55] | 23 | Topical 5% imiquimod cream | First line | - | 3 times a week for 16 weeks | CR 52.2%; PR 30.4% (12 months) | Yes (31 months) | Topical 5% imiquimod cream Surgery | Fatigue, headache | 2.6 |
| Wang et al. [56] | 11 | Hematoporphyrin derivatives photodynamic therapy | First line | - | HpD injection 3 mg/kg or 5 mg/kg plus 250 mL normal saline in 60 min. Fluorescence at 48 and 72 h. Laser 630 nm red light, dosage level 150–200 J/cm2 | CR 90.1%; PR 9.1% (1 month) CR 72.7% (17.4 months) | No | No | Pain, infection, photosensitivity and uroschesis | 1.5 |
| Sadko et al. [57] | 1 | Topical 5% imiquimod cream | Adjuvant | Surgical treatment (vulvectomy) | 3 times a week for 1 month, suspended for 2 weeks and resumed for 2 months | CR 100% (10 months) | No | No | Erythema | 0.8 |
| Ferrara et al. [49] | 10 | Fractional carbon dioxide laser abrasion, followed by photodynamic therapy | First line | - | Fractional carbon dioxide laser abrasion. 3 h occlusive application of ALA, 100 J/cm2 irradiation, 630 nm lamp. Combination repeated every 2 weeks for a total of 5 times | CR 20%; PR 80% (12 months) | Yes (12 months) | No | Swelling, pain, residual hyperpigmentation | 1.0 |
| Ganhewa et al. [43] | 1 | Topical 5-fluorouracil | First line | - | - | PR 100% (6 months) | - | - | Erythema | 0.5 |
| Liu et al. [58] | 119 (including surgical patients) | Topical therapy (imiquimod, 75%), radiation, chemotherapy, photodynamic therapy | First line and adjuvant | Surgical treatment | - | - | Yes | Surgical treatment (80%) | - | 4.7 |
| Preti et al. [21] | 17 | Topical 5% imiquimod cream (13) Laser vaporization (4) | First line | - | 3–4 applications weekly - | - | - | - | - | 7.9 |
| Tanimura et al. [59] | 6 | Radiotherapy | First line | - | 51 Gy | CR 100% (6 months) | Yes (13 months) | Surgical treatment | Erosions and candidiasis | 2.7 |
| Rioli et al. [47] | 13 | Photodynamic therapy | Second line | Laser, surgery, imiquimod, PDT, fluorouracil, radiotherapy, brachytherapy, cryotherapy, ingenol mebutate | Topical 16% methyl aminolevulinate (MAL); red light (630 nm) fluency 37 J/cm2 for 7 to 10 minutes | CR 15%; PR 38%; SD 38%; PD 7% (3 months) | Yes (5 months) | Topical 5% imiquimod cream, surgery, carbon dioxide laser, cryotherapy, ingenol mebutate, fluorouracil, radiotherapy | Pain | 3.2 |
| Bauman et al. [60] | 1 | Topical 5% imiquimod cream Photodynamic therapy | First line | - | Once for 5 days per week for 1 month, followed by 2 months for 3 nights a week. Monthly 5-ALA photodynamic therapy for 6 months. After 6 months, imiquimod discontinued and quarterly photodynamic therapy performed | CR 100% (12 months) | No | - | - | 5.0 |
| Nitecki et al. [61] | 20 | Topical 5% imiquimod cream | Adjuvant (15) First line (5) | Surgical treatment | - | - | Yes (12.5 months) | Topical 5% imiquimod cream | Local irritation | 3.8 |
| Sawada et al. [62] | 9 | Topical 5% imiquimod cream | First line | - | 3 times per week for 16 weeks; one case for 6 weeks | CR 56%; PR 44% (4 months) | Yes (34 months) | Topical 5% imiquimod cream | Local irritation | 3.4 |
| van der Linden et al. [7] | 24 | Topical 5% imiquimod cream | First line | - | 3 times a week for 16 weeks | CR 52.2%; PR 30.4% (7 months) | Yes (12 months) | Topical 5% imiquimod cream Surgery | Fatigue and headache | 2.6 |
| Vicentini et al. [63] | 1 | Unconventional PDT | Next line | Imiquimod applications, LASER treatments and conventional photodynamic therapy | 3 PDT sessions with 16% methyl-aminolevulinate cream with the light emitting fabric at irradiance 6 mW/cm2, fluence. 37 J/cm2 | SD 100% (2 and 5 months) | - | - | - | 0.4 |
| Dogan et al. [64] | 1 | Topical 5% imiquimod cream | Adjuvant | Surgical resection and re-resection | Twice weekly was applied for 3 months | CR 100% (3 months) | No | - | Erythema | 0.5 |
| Knight et al. [65] | 1 | Topical 5% imiquimod cream | First line | - | Once for 5 days and 2 rest days per week for 4 months | CR 100% (4 months) | Yes (18 months) | - | Viral infection | 1.5 |
| Cowan et al. [66] | 8 | Topical 5% imiquimod cream | Second line | Surgical treatment | 3 times per week for 3 months | CR 75%; PR 13% (3 months) | Yes (35 months) | Topical 5% imiquimod cream | Erythema | 2.9 |
| Higgins et al. [67] | 1 | Topical 5% imiquimod cream | Adjuvant | Surgical treatment | 3-times weekly for 19 months | PR 100% (3 months); CR 100% (9 months) | Yes (9 months) | Topical 5% imiquimod cream | - | 4.0 |
| Al Youssef et al. [68] | 1 | Photodynamic therapy | First line | - | Topical methyl 5-aminolevulinate (5-MAL) and exposure to 37 J/ cm2 of visible red light (630 nm). Three PDT sessions at 4-week intervals | CR 100% (3 months) | No | - | Pain | 1.0 |
| Gao et al. [24] | 38 | Photodynamic therapy | Adjuvant (31) First line (7) | Surgical treatment | Aminolevulinic acid (5-ALA) and exposure to 120 J/cm2 with 635 nm laser for 15 min, for 3 times (adjuvant) or 4–6 times (first line) | CR 100% (<6 months) | Yes (6 and 12 months) | - | Pain and swelling | 1.0 |
| Marchitelli et al. [69] | 10 | Topical 5% imiquimod cream | Adjuvant (3) First line (7) | Surgical treatment | Every other day until the lesions were no longer clinically detected | CR 90%; PR 10% (5 months) | No | - | Irritation | 1.5 |
| Jing et al. [70] | 2 | Photodynamic therapy and topical 5% imiquimod cream combination | Next line | CO2 laser therapy Cryosurgery | 6 cycles of 20% 5-aminolevulinic acid (ALA) photodynamic therapy and topical imiquimod | CR 100% (6 and 12 months) | No | - | - | 3.0 |
| Luyten et al. [71] | 21 | Topical 5% imiquimod cream | Adjuvant (6) First line (15) | Surgical treatment | 2 or 3 times per week for a mean duration of 15.4 weeks | CR 52.4%; PR 28.6%; SD 9.5% (4 months) | No | - | Local reaction | - |
| Hata et al. [72] | 41 | Radiotherapy | Adjuvant (17) First line (24) | Surgical treatment | Total doses of 45–80.2 Gy (median, 60 Gy) were delivered in 23–43 fractions (median, 33 fractions). Irradiation took place 5 days per week and fraction sizes were 1.8–2.2 Gy (median, 1.8 Gy) | CR:100% (2–9 months) | Yes (3–44 months) | - | All patients had ≤grade 2 dermatitis, 13 ≤grade 2 colitis, 12 cystitis, 16 ≤grade 2 hematologic toxicities | 3.4 |
| Itonaga et al. [73] | 14 | Radiotherapy | Definitive (3) Definitive after relapse (6) Adjuvant (5) | Surgical treatment | Median total irradiation dose was 50 Gy, delivered in 20–33 fractions | CR 100% (71, 4 months) | Yes (13–83 months) | - | Local reaction | 6.0 |
| Fontanelli et al. [48] | 32 | Photodynamic therapy (M-ALA) | First line (32) | - | M-ALA PDT treatment was repeated every 3 weeks | CR 3.9%, PR 78.1%, NC 12.5% (11 months) | Yes (6, 10, 18 months) | Surgical treatment | - | 1.5 |
| Mann et al. [74] | 1 | Radiotherapy | First line | - | cumulative dose was 6400 cGy delivered in 200 cGy fractions over 6.5 weeks | CR | no | - | - | 5.0 |
| Clément et al. [46] | 8 | Photodynamic therapy | First line | - | 3 h after topical application of methyl aminolevulinic acid emulsion, they underwent illumination with red light (570–670 nm) at a dose of 37 J/cm2 for 10 min. In the event of relapse, a further cycle was given at week 6. | CR 87.5% (3 months), PR 12.5% | Yes (4–14 months) | - | - | 1.2 |
| Hata et al. [25] | 22 | Radiotherapy | First line | - | A total dose of 45–70.2 Gy was delivered in 25–39 fractions (median, 33) | CR 86.3%, NC 13.7% (8–133 months) | Yes (3–43 months) | Surgical treatment | Local reactions | 5 |
| Green et al. [75] | 27 | Topical 5% imiquimod cream | First line | Surgical treatment | Once daily for 14 weeks | CR 78% (3 months) | Yes (11 months) | - | Lesional tenderness and erythema | 1.0 |
| Qiang et al. [76] | 17 | Photodynamic therapy | First line | - | Topical 20% 5-aminolevulinic acid was applied for 6 h. Each lesion was irradiated with 633 nm red light three times, 1 week apart, at a total dose of 339 J/cm2 | CR 52.4% (6 months) | Yes (3 months) | - | Local reactions | 2.0 |
| Housel et al. [77] | 8 | Photodynamic therapy | First line | - | Four patients received topical ALA only as a photosensitizer, three received intravenous porfimer sodium only, and one received both. 632.8 nm argon-pumped dye laser, and some were also treated using a red lamp (590–729 nm) | -PDT using intravenous porfimer sodium CR 78% -PDT using topical ALA showed a CR 50% (9–88 MONTHS) | - | - | Local reactions | 8.0 |
| Fukui et al. [78] | 5 | Photodynamic therapy following carbon dioxide laser | -First line (2 patients) -neoadjuvant (3) | Surgical treatment | Carbon dioxide (CO2) laser abrasion, followed by 3 h of occlusive application of aminolaevulinic acid (ALA) and then 100 J/cm2 irradiation with a 630 nm excimer dye laser. This combination treatment regime was repeated every 2 weeks for a total of 3 times | CR 100 % (6 weeks) | Yes (12 months) | - | Local reactions | 1.0 |
| Tae Heung et al. [79] | 1 | Radiotherapy | Third line | Failure of local excision. Imiquimod 5% cream for two months | A total of 5040 cGy in 28 fractions was given | CR 100% | No | - | - | 1.0 |
| Geisler et Manahan [80] | 1 | Topical imiquimod 5% cream | Second line | Surgical treatment (multiple resections) | Once daily for 3 months | CR 100% | No | - | - | 1.0 |
| Vereecken et al. [81] | 1 | Topical imiquimod 5% cream | First line | - | Once daily for 3 months | CR 100% | No | - | Local erythema | 1.0 |
| Raspagliesi et al. [82] | 7 | Photodynamic therapy | 5 MAL-PDT was applied for 3 h and than irradiated with red-light (620 nm) using a total light dose of 37 J/cm2 for a period of 10 min. Patients were treated once every 3 weeks, for a total of three treatments | CR 57% (4 months) | - | - | local edema and mild-moderate local pain | 0.5 | ||
| Holt et Stanley [83] | 1 | Radiotherapy | First line | - | 40 Gray in 10 fractions | CR 100% | - | - | Mild local reactions | 3.0 |
| Madan et al. [84] | 1 | Photodynamic therapy | First line | - | ALA was applied followed 6 h later by irradiation using a filtered xenon-arc lamp | CR 100% | Yes (9 months) | intravenously administered porfimer sodium followed by one topical PDT treatment | - | 1.5 |
| Seok-Hyun et al. [85] | 3 | Radiotherapy | First line | - | 54–78 Gy delivered in 6–8 weeks | CR 100% | Yes (2 years) | None | Local desquamation, mild late atrophic skin changes | 0.5–8-11 |
| Mikasa et al. [86] | 2 | Photodynamic therapy | First line | - | ALA-PDT treatments were applied to parts of the lesions at a total dose of 200–300 J/cm2 | CR 100% | Yes (2 months) | two more PDT treatments | - | 0.6 |
| Luk et al. [87] | 6 | Radiotherapy | First line (2), postexcisional relapse (3) and adjuvant treatment (1) | Surgical excision | 60 Gy delivered at 2 Gy/fraction, 5 fraction/week | CR 83.3%, PR 16.7% | Yes (18 months) | Surgical treatment | Acute confluent wet desquamation and mild late skin atrophy | 1.2–14.8 |
| Berman et al. [88] | 1 | Topical imiquimod 5% cream | First line | - | Once daily for 6 weeks | CR 100% | no | - | Moderate erythema | 0.5 |
| Guerrieri et Back [89] | 1 | Radiotherapy | First line | - | 60 Gy in 30 fractions | CR 100% | no | - | Moist desquamation | 1.0 |
| Moreno-Arias et al. [90] | 2 | Radiotherapy | First line | - | 100 kV, 440 cGy/day, 3 days a week over 3 weeks until a total dose of 3960 cGy was completed | CR 100% | no | - | Hypopigmentation and local atrophy | 2.0–3.0 |
| Burrows et al. [91] | 5 | Radiotherapy | First line | - | 4050 cGy rays in nine fractions over 3 weeks | CR 100% | No | - | Moist desquamation | 0.5–8.0 |
| Kwan et al. [92] | 1 | Brachytherapy | First line | - | A total dose of 42 Gy in 14 fractions was delivered at the 5 mm depth from the skin surface over 18 days. | CR 100% | No | - | Wet desquamation, hypopigmentation | 0.5 |
| Voigt et al. [27] | 1 | Systemic chemotherapy | First line | - | Carboplatin administered on day 1 (400 mg/m2 intravenously), and calcium folinate on days 1–5 (170 mg/m2 intravenously) 1 h before 5-fluorouracil (350 mg/m2 intravenously). Six cycles were performed. Treatment cycles were repeated every 4 weeks. | CR 100% | No | - | Thrombocytopenia, necessitating dosage modification | 1.0 |
| Marchitelli et al. [93] | 10 | 5% Imiquimod cream | First line | - | cream was applied every other day until clinical lesions disappeared (3 to 6 months) | CR 80% | No | - | Local reaction | 1.5 |
| Karam et Dorigo [28] | 92 | Radiotherapy | First line (41) Adjuvant (51) | Surgical treatment | evaluated patients diagnosed with invasive EMPD using data collected from the SEER program. radiation fields, doses, fractionation schedules and radiation sources vary widely making these reports difficult to compare | - | - | - | - | - |
| Machado et al. [94] | 1 | 5% Imiquimod cream | First line | - | Three times/week for three months | CR 100% | No | - | - | 0.5 |
| Makino et al. [26] | 2 | Boron neutron capture therapy (BNCT) (experimental form of RT) | First line | - | The tumors were irradiated at the Kyoto University Research Reactor with thermal neutrons of 5 MW for 65 min and 78 min, respectively | CR 100% | No | - | - | 1.0 |
| Schmid et al. [95] | 1 | 5% Imiquimod Cream | First line | - | Once daily for 3 months | CR 100% | No | - | - | 1.0 |
| Tae Heung et al. [79] | 1 | Radiotherapy | Adjuvant | Surgical treatment (positive margin) Imiquimod | Imiquimod ointment was applied for 2 months, followed by multiple skin biopsies, which revealed Paget’s disease in all specimens. A total dose of 5040 cGy in 28 fractions was given within a 3-month period | CR 100% | No | - | desquamation of the radiation-exposed skin | 1.0 |
| Besa et al. [96] | 9 | Radiotherapy | First line (4) Adjuvant (5) | Surgical treatment | Doses ranged from 40 to 60 Gy | CR 100% | No | - | Local erythema, swelling, pain, and rectal urgency | 1.0 |
| Choi et al. [1] | 10 | 5% Imiquimod cream | Adjuvant | Surgical treatment | Postoperatively, topical imiquimod was applied every other night, 3 times a week, over the margin and adjacent normal skin for a period of 6 months | CR 100% (no recurrence observed) | No | - | - | 6.0 |
| van der Linden et al. [97] | 10 | 5% Imiquimod cream | First line | - | Once daily for 10–24 weeks (median 13) | CR 40%, PR 40%, NR 20% | - | Surgical treatment | - | 3.3 |
| van der Linden et al. [98] | 18 | 5% Imiquimod Cream | First line | - | - | CR 20%, PR 40% NR 20% | - | - | - | 3.0 |
| Wang et al. [99] | 13 | Surgery (5) ALA-PDT + surgery (8) | Neoadjuvant | - | Four sessions of topical PDT mediated with 20% 5-aminolevulinic acid (ALA-PDT) were applied prior to surgery | CR (40%) CR 63% | Yes 25% 9% | - | Pain during light irradiation | 1.0 |
| Chen et al. [100] | 16 | Photodynamic therapy | Adjuvant | Surgical treatment | Postoperatively, all patients underwent three courses of ALA PDT to the operative site and nearby areas | CR 100% | Recurrence rate 12.5% | Surgical treatment | Infection, lower limb movement disorder | 3.0 |
| Zhou et al. [36] | 36 | A)Wide local excision (control group, 15 patients) B) Wood lamp examination + 5-ALA-PDT (experimental group, 21 patients) | Neoadjuvant | - | Wood’s lamp with 5-ALA PDT defined tumor margins. 5-ALA emulsion was applied beyond the lesion, fluorescence traced, then biopsies done before resection. | CR 100% | Recurrence rate 26.7% 28.6% | - | Local pain during light irradiation | 4.0 |
4. Discussion
Biopsy remains the definitive diagnostic procedure for EMPD, yet several non-invasive imaging modalities have emerged as valuable adjunctive tools. These techniques, while not definitive, may facilitate earlier detection, refine treatment planning, and reduce the need for repeated biopsies.
RCM is the most extensively investigated. Multiple studies report strong correlation with histopathology in detecting Paget’s cells and tumor nests in the epidermis [11,28,41]. Characteristic findings consist of large hyporeflective cells with well-defined hyperreflective borders, observed either individually or grouped in small nests within the epidermis. Beyond diagnosis, RCM enables intraoperative margin mapping and postoperative assessment to improve surgical precision. However, false negatives remain an issue [41], emphasizing the need for standardized criteria and validation in larger cohorts.
PDD, although not specific for EMPD, has proven useful for margin delineation. Fluorescence often reveals subclinical spread beyond visible margins, guiding both surgery and PDT [37]. When combined with RCM, PDD appears to improve margin accuracy, reducing overtreatment and preserving tissue while maintaining oncological safety [32].
Dermoscopy may highlight suspicious features, aiding lesion triage for biopsy, but lacks specificity for EMPD [5].
Overall, current evidence supports RCM and PDD as the most reliable non-invasive tools in EMPD, particularly for diagnosis and margin assessment. Dermoscopy and OCT remain investigational, while MRI and PET/CT appear promising for staging and monitoring. These methods should be regarded as complementary to histopathology. Further, large-scale, multicentric prospective studies are needed to validate diagnostic accuracy, standardize imaging markers, and clarify their impact on recurrence and long-term outcomes [5].
The evidence supporting the use of topical imiquimod for EMPD remains fragmentary and largely based on small series or isolated case reports, with only a few larger cohort studies available. This inevitably introduces publication bias and limits the strength of current recommendations. In several studies, imiquimod was used either as a first-line approach or as an adjuvant following surgery, often reflecting the lack of standardized guidelines across different centers [24,25,64].
Despite these limitations, the available data indicate that imiquimod can induce meaningful therapeutic responses. Larger cohorts such as Borella et al. (n = 51) reported a CR in 43.1% of patients (median follow-up of 5.5 years) [50], while van der Linden et al. observed CR in 52.2% of 23 cases (median follow-up of 2.6 years) [55]. Similar results were described by Luyten et al. (52.4% CR, 28.6% PR) [71], whereas Green et al. reported higher rates (78% CR, daily application for 14 weeks) [75]. Smaller series and case reports frequently described complete clearance (100% CR) with varied regimens [51,71], but these outcomes must be interpreted cautiously given the sample size. Interestingly, Escolà et al. documented more heterogeneous results, with CR ranging from 0% to 65% across different treatment arms [51], further underlining the variability of real-world data.
Several factors may influence treatment success. Perianal and multifocal disease have been associated with lower likelihood of CR [50], and studies rarely reported lesion size, although larger and more extensive lesions may correlate with worse outcomes. Treatment regimens were inconsistent, ranging from daily application to two or three times weekly, with durations spanning weeks to more than a year. In the largest series, three to four weekly applications over several months were the most frequent schedules and seemed to achieve balanced efficacy and tolerability [7,75]. Extending treatment beyond six months has been suggested to maximize response [71], but higher frequency increased local toxicity without clearly improving clearance [51].
Adverse events were generally mild to moderate in severity and included erythema, burning, erosions, fatigue, and local tenderness [25,75]. Notably, even patients who interrupted treatment early due to intolerance sometimes achieved CR, raising the hypothesis that the local inflammatory reaction, rather than cumulative dosing, may be the critical determinant of efficacy. Recurrence was reported inconsistently across studies, with some series describing relapses occurring within 12–35 months.
Taken together, current findings suggest that imiquimod is a promising non-surgical alternative for selected EMPD patients, particularly when surgery is contraindicated or refused. However, response rates vary widely across studies, follow-up remains limited, and recurrence is not uncommon. Larger prospective trials with standardized dosing schedules are needed to define its real therapeutic potential, optimize treatment duration, and identify patients most likely to benefit.
PDT has been investigated across different clinical contexts, with efficacy ranging from excellent outcomes to more modest responses. Several reports documented CR in all treated patients. Xiang et al. described durable remission lasting up to 41 months with hematoporphyrin-based PDT [53], while Filonenko et al. reported CR at 6 months following chlorin e6 PDT, although relapse occurred at 21 months [45]. Gao et al. documented CR in all 38 patients treated with ALA-PDT in either the adjuvant or first-line setting, but recurrences were noted at 6 and 12 months [24]. Other smaller series confirmed high short-term CR rates, such as Al Youssef et al. with MAL-PDT [68] and Fukui et al. with CO2 laser plus PDT [78]. By contrast, more modest efficacy was observed in other cohorts. Rioli et al. reported CR in only 15% of patients, with partial or stable disease in the majority [47], while Fontanelli et al. documented a CR rate of just 3.9%, with most patients achieving only partial responses and multiple relapses during follow-up [48].
Adverse events were common but generally manageable. The most frequent were local pain, erythema, edema, and swelling [24,52]. More severe local reactions such as necrosis, ulceration, and scarring were reported occasionally [45,52]. Additional side effects included photosensitivity, infection, and hyperpigmentation [32,49].
Recurrence rates varied considerably. While some single-case reports described durable remission without relapse [52,68], larger series showed higher recurrence: Gao et al. noted relapses within 12 months despite initial CR [24], Fontanelli et al. reported multiple recurrences during follow-up [48], and Chen et al. and Zhou et al. documented recurrence rates of 12.5% and 26.7%, respectively [36,100].
These findings indicate that PDT can induce high initial response rates, including complete remissions in a proportion of patients. Although high response rates are achievable, maintaining long-term remission remains challenging, as relapse may occur even after initial complete clearance.
Although current evidence supports the diagnostic and therapeutic potential of non-invasive imaging and topical or photodynamic approaches in EMPD, several methodological and practical limitations restrict their clinical applicability. Most available studies are retrospective, single-center, and include small or heterogeneous patient populations, often combining primary and recurrent cases or different anatomic sites. Such variability complicates cross-comparison and may account for the wide range of reported complete response rates. Furthermore, inconsistent endpoints—clinical versus histological clearance—and heterogeneous follow-up durations make it difficult to assess true long-term efficacy and recurrence risk.
From a clinical standpoint, these findings highlight the need for a tailored, multimodal strategy rather than a one-size-fits-all approach. RCM and PDD may be best positioned as adjuncts for preoperative mapping and postoperative surveillance, but they cannot yet replace histopathology as the gold standard. Similarly, imiquimod and PDT should be considered options for carefully selected patients—particularly those unfit for or unwilling to undergo surgery—rather than routine alternatives. Treatment selection should be guided by disease extent, anatomical site, and patient comorbidities, while acknowledging the absence of standardized protocols.
While non-surgical modalities can achieve meaningful responses in selected patients, surgical management remains the cornerstone of EMPD treatment, providing superior local control and lower recurrence rates. Margin-controlled techniques, including MMS, show recurrence rates of about 11%, compared with roughly 37% for WLE [13]. Conservative or limited resections may be appropriate for frail or elderly patients, though recurrence risk is higher. By contrast, topical imiquimod achieves complete responses in 30–54% of cases, but recurrence occurs in up to 35% and local irritation is common [18]. PDT can induce high initial clearance, yet long-term remission is less consistent. Overall, surgery—particularly margin-controlled approaches—should be considered the preferred curative option whenever feasible, while conservative or nonsurgical treatments are best reserved for patients with contraindications or high surgical morbidity.
Future investigations should prioritize prospective, multicentric trials with standardized imaging protocols and uniform response criteria. For imaging, establishing validated diagnostic markers and reproducible interpretation guidelines is crucial to minimizing observer variability and false negatives. For topical and photodynamic therapies, randomized controlled studies comparing regimens, durations, and combination strategies are needed to clarify optimal dosing and maintenance approaches. Long-term follow-up data are also essential to determine whether initial complete responses translate into durable remission.
Finally, translational studies exploring biomarkers of treatment response and predictors of recurrence could refine patient selection and move the field toward personalized therapy. Integration of molecular profiling with advanced imaging may eventually allow more accurate staging, dynamic monitoring, and early detection of relapse—key steps toward improving outcomes in EMPD.
5. Single-Center Case Series
As a complement to the findings of our systematic review, we present our single-center experience and case series of EMPD treated with topical imiquimod and monitored longitudinally with RCM.
In our clinical experience, we have treated three patients with penile or scrotal EMPD using topical imiquimod. All patients underwent regular follow-up visits every six weeks and were monitored longitudinally with RCM, during which treatment continuation was decided based on the presence or absence of microscopic disease persistence.
The first case involved a 74-year-old man with recurrent penile EMPD after first-line surgery. He was treated with imiquimod five times per week for six-week cycles, with complete remission achieved after several courses and stable disease-free follow-up at one year.
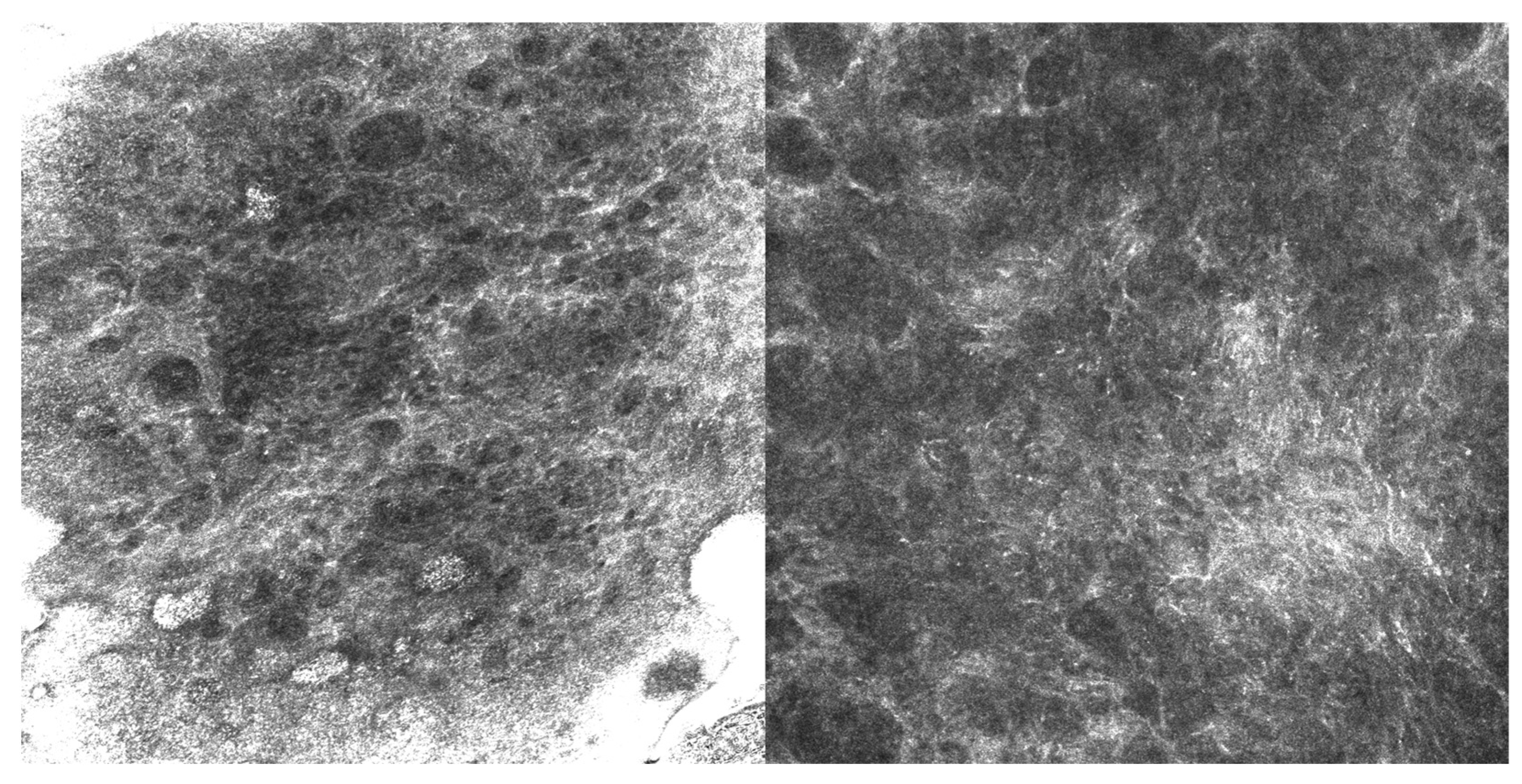
The second case, a 63-year-old man with EMPD, also received imiquimod following surgical excision with positive margins. He experienced a microscopic recurrence, which was documented by RCM (Figure 2) and required retreatment; he subsequently remained stable without evidence of disease at one-year follow-up.
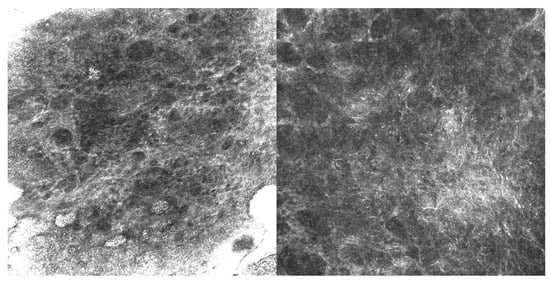
Figure 2.
RCM images (500 µm × 500 µm) showing Paget’s cells with clearly defined hyperreflective borders and hyporeflective cytoplasm, larger than the surrounding keratinocytes and scattered within the spinous layer.
The third case concerned a 52-year-old man with EMPD and positive surgical margins. After initiation of imiquimod, complete clinical and microscopic remission was obtained within six months.
6. Conclusions
Current knowledge about EMPD management remains limited and not straightforward: the disease is rare, presents differently across patients, and there are no universally accepted therapeutic rules to follow. The literature, mostly made of small case series and retrospective reports, suggests that non-invasive imaging can help improve diagnostic accuracy. Still, this remains within limits, since such methods are not standardized for this condition, and their role is more supportive than definitive in planning treatment or follow-up. On the therapeutic side, topical immunotherapy and PDT have shown encouraging results, especially when surgery is either not possible or would be excessively harmful.
All of this underlines the need for prospective studies able to clarify diagnostic criteria, refine treatment choices, and offer reliable outcome measures. Combining new diagnostic tools with conservative options could open the way to a more individualized and less invasive management of EMPD.
Author Contributions
Conceptualization, F.D., F.P., E.D., M.V., S.G. and M.A.; methodology, F.D., F.P., L.L. and K.M.C.; software, F.P., L.L. and K.M.C.; validation, F.D., M.L. and F.P.; formal analysis, F.D., F.P., L.L. and K.M.C.; investigation, F.D., M.L. and F.P.; resources, F.D., M.L. and F.P.; data curation, F.D., M.L. and F.P.; writing—original draft preparation, F.D., G.F. and F.P.; writing—review and editing, F.D., G.F. and F.P.; visualization, F.D. and F.P.; supervision, F.P., M.V. and M.A.; project administration, F.P., E.D., M.V., S.G. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EMPD | Extramammary Paget’s Disease |
| RCM | Reflectance Confocal Microscopy |
| PDT | Photodynamic Therapy |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| WLE | Wide Local Excision |
| MMS | Mohs Micrographic Surgery |
| OCT | Optical Coherence Tomography |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| ROBINS-I | Risk Of Bias In Non-randomized Studies-of Interventions |
| CR | Complete Response |
| PR | Partial Response |
| SD | Stable Disease |
| PD | Progressive Disease |
| ALA | Aminolevulinic Acid |
| MAL | Methyl Aminolevulinate |
| HpD | Hematoporphyrin Derivative |
| BNCT | Boron Neutron Capture Therapy |
| RT | Radiotherapy |
| Gy | Gray |
| MeSH | Medical Subject Headings |
| DEJ | Dermo-Epidermal Junction |
| ERBB2-TKI | ERBB2 Tyrosine Kinase Inhibitor |
| NC | No Change |
| SEER | Surveillance, Epidemiology, and End Results |
References
- Choi, J.-H.; Jue, M.-S.; Kim, E.-J.; Joh, O.-J.; Song, K.-Y.; Park, H.-J. Extramammary Paget Disease: Minimal Surgical Therapy. Ann. Dermatol. 2013, 25, 213. [Google Scholar] [CrossRef]
- Morris, C.R.; Hurst, E.A. Extramammary Paget Disease: A Review of the Literature—Part I: History, Epidemiology, Pathogenesis, Presentation, Histopathology, and Diagnostic Work-up. Dermatol. Surg. 2020, 46, 151–158. [Google Scholar] [CrossRef]
- Dukharan, V.; Della Porta, A.L.; Gregory, S.; Erdag, G. Extramammary Paget Disease of Peristomal Skin Secondary to Bladder Urothelial Carcinoma. J. Dermatol. Res. Ther. 2021, 7, 106. [Google Scholar] [CrossRef]
- Mayo-Martínez, F.; Moro, R.; Millán-Esteban, D.; Ríos-Viñuela, E.; Bautista, I.J.; Nagore, E.; Sanmartín, O.; Llombart, B. Topical Imiquimod in Primary Cutaneous Extramammary Paget’s Disease: A Systematic Review. Cancers 2023, 15, 5665. [Google Scholar] [CrossRef]
- Bayan, C.-A.Y.; Khanna, T.; Rotemberg, V.; Samie, F.H.; Zeitouni, N.C. A review of non-invasive imaging in extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1862–1873. [Google Scholar] [CrossRef]
- Ishizuki, S.; Nakamura, Y. Extramammary Paget’s Disease: Diagnosis, Pathogenesis, and Treatment with Focus on Recent Developments. Curr. Oncol. 2021, 28, 2969–2986. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, M.; Meeuwis, K.; Van Hees, C.; Van Dorst, E.; Bulten, J.; Bosse, T.; IntHout, J.; Boll, D.; Slangen, B.; Van Seters, M.; et al. The Paget Trial: A Multicenter, Observational Cohort Intervention Study for the Clinical Efficacy, Safety, and Immunological Response of Topical 5% Imiquimod Cream for Vulvar Paget Disease. JMIR Res. Protoc. 2017, 6, e178. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Barcellini, A.; Mazzeo, R.; Gallo, R.; Vitale, M.G.; Passarelli, A.; Mangili, G.; Pignata, S.; Palaia, I. Vulvar Paget’s Disease: A Systematic Review of the MITO Rare Cancer Group. Cancers 2023, 15, 1803. [Google Scholar] [CrossRef]
- Wilkinson, E.J.; Brown, H.M. Vulvar Paget disease of urothelial origin: A report of three cases and a proposed classification of vulvar Paget disease. Hum. Pathol. 2002, 33, 549–554. [Google Scholar] [CrossRef]
- Kim, S.J.; Thompson, A.K.; Zubair, A.S.; Otley, C.C.; Arpey, C.J.; Baum, C.L.; Roenigk, R.K.; Lohse, C.M.; Brewer, J.D. Surgical Treatment and Outcomes of Patients with Extramammary Paget Disease: A Cohort Study. Dermatol. Surg. 2017, 43, 708–714. [Google Scholar] [CrossRef]
- Sanderson, P.; Innamaa, A.; Palmer, J.; Tidy, J. Imiquimod therapy for extramammary Paget’s disease of the vulva: A viable non-surgical alternative. J. Obstet. Gynaecol. 2013, 33, 479–483. [Google Scholar] [CrossRef]
- Choi, S.; Oh, Y.; Roh, M.R.; Chung, K.Y.; Oh, B.H. Initial topical monotherapy may increase the risk of recurrence in patients with extramammary Paget’s disease. J. Dermatol. 2021, 48, 585–591. [Google Scholar] [CrossRef]
- Preti, M.; Joura, E.; Vieira-Baptista, P.; Van Beurden, M.; Bevilacqua, F.; Bleeker, M.C.G.; Bornstein, J.; Carcopino, X.; Chargari, C.; Cruickshank, M.E.; et al. The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD) and the European Federation for Colposcopy (EFC) consensus statements on pre-invasive vulvar lesions. Int. J. Gynecol. Cancer 2022, 32, 830–845. [Google Scholar] [CrossRef]
- Edey, K.A.; Allan, E.; Murdoch, J.B.; Cooper, S.; Bryant, A. Interventions for the treatment of Paget’s disease of the vulva. Cochrane Database Syst. Rev. 2019, 2019, CD009245. [Google Scholar] [CrossRef] [PubMed]
- Snast, I.; Sharon, E.; Kaftory, R.; Noyman, Y.; Oren-Shabtai, M.; Lapidoth, M.; Hodak, E.; Mimouni, D.; Mazor, S.; Levi, A. Nonsurgical Treatments for Extramammary Paget Disease: A Systematic Review and Meta-Analysis. Dermatology 2020, 236, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, L.; Cafasso, V.; Conte, C.; Cuomo, L.; Giampaolino, P.; Lavitola, G.; Bifulco, G. Medical and Surgical Strategies in Vulvar Paget Disease: Let’s Throw Some Light! J. Pers. Med. 2023, 13, 100. [Google Scholar] [CrossRef]
- Zampogna, J.C.; Flowers, F.P.; Roth, W.I.; Hassenein, A.M. Treatment of primary limited cutaneous extramammary Paget’s disease with topical imiquimod monotherapy: Two case reports. J. Am. Acad. Dermatol. 2002, 47, S229–S235. [Google Scholar] [CrossRef]
- Mirer, E.; El Sayed, F.; Ammoury, A.; Lamant, L.; Messer, L.; Bazex, J. Treatment of mammary and extramammary Paget’s skin disease with topical imiquimod. J. Dermatol. Treat. 2006, 17, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Borella, F.; Gallio, N.; Mangherini, L.; Cassoni, P.; Bertero, L.; Benedetto, C.; Preti, M. Recent advances in treating female genital human papillomavirus related neoplasms with topical imiquimod. J. Med. Virol. 2023, 95, e29238. [Google Scholar] [CrossRef]
- Nasioudis, D.; Bhadra, M.; Ko, E.M. Extramammary Paget disease of the vulva: Management and prognosis. Gynecol. Oncol. 2020, 157, 146–150. [Google Scholar] [CrossRef]
- Preti, M.; Micheletti, L.; Borella, F.; Cosma, S.; Marrazzu, A.; Gallio, N.; Privitera, S.; Tancredi, A.; Bevilacqua, F.; Benedetto, C. Vulvar Paget’s disease and stromal invasion: Clinico-pathological features and survival outcomes. Surg. Oncol. 2021, 38, 101581. [Google Scholar] [CrossRef]
- Hatta, N.; Yamada, M.; Hirano, T.; Fujimoto, A.; Morita, R. Extramammary Paget’s disease: Treatment, prognostic factors and outcome in 76 patients. Br. J. Dermatol. 2007, 158, 313–318. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Wang, W.; Yang, Y.; Wang, H.; Lu, Y.; Fan, D. Efficacy and safety of topical ALA-PDT in the treatment of EMPD. Photodiagnosis Photodyn. Ther. 2015, 12, 92–97. [Google Scholar] [CrossRef]
- Hata, M.; Omura, M.; Koike, I.; Wada, H.; Miyagi, E.; Tayama, Y.; Odagiri, K.; Minagawa, Y.; Ogino, I.; Inoue, T. Role of Radiotherapy as Curative Treatment of Extramammary Paget’s Disease. Int. J. Radiat. Oncol. 2011, 80, 47–54. [Google Scholar] [CrossRef]
- Makino, E.; Sasaoka, S.; Aihara, T.; Sakurai, Y.; Maruhashi, A.; Ono, K.; Fujimoto, W.; Hiratsuka, J. 1013 The First Clinical Trial of Boron Neutron Capture Therapy Using 10B-para-boronophenylalanine for Treating Extramammary Paget’s Disease. Eur. J. Cancer 2012, 48, S244–S245. [Google Scholar] [CrossRef]
- Voigt, H.; Basserrmann, R.; Nafhrath, W. Cytoreductive combination chemotherapy for regionally advanced unresectable extramammary Paget carcinoma. Cancer 1992, 70, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Karam, A.; Dorigo, O. Treatment outcomes in a large cohort of patients with invasive Extramammary Paget’s disease. Gynecol. Oncol. 2012, 125, 346–351. [Google Scholar] [CrossRef]
- Chuchvara, N.; Reilly, C.; Haroon, A.; Wassef, C.; Maghari, A.; Rao, B. Atypical cells on reflectance confocal microscopy may not represent melanoma: A case of axillary pigmented extramammary Paget disease. J. Cutan. Pathol. 2020, 47, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Huang, J.; Zhang, Y.; Zeng, L.; Tong, X.; Gao, L.; Zeng, J. Evaluation of in vivo reflectance confocal microscopy in the diagnosis of extramammary Paget’s disease. Microsc. Res. Tech. 2022, 85, 283–289. [Google Scholar] [CrossRef]
- Suppa, M.; Marneffe, A.; Miyamoto, M.; Rorive, S.; Boone, M.; Del Marmol, V. Apport de la microscopie confocale par réflectance dans le diagnostic de la maladie de Paget extra-mammaire. Ann. Dermatol. Vénéréologie 2015, 142, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, L.; Lu, X.; Li, J.; Wang, Y.; Zang, J.; Mo, X.; Shao, X.; Wang, L.; Cheng, W.; et al. Utility of photodynamic diagnosis plus reflectance confocal microscopy in detecting the margins of extramammary Paget disease. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kibbi, N.; Owen, J.L.; Worley, B.; Wang, J.X.; Harikumar, V.; Downing, M.B.; Aasi, S.Z.; Aung, P.P.; Barker, C.A.; Bolotin, D.; et al. Evidence-Based Clinical Practice Guidelines for Extramammary Paget Disease. JAMA Oncol. 2022, 8, 618. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Wu, M.; Zhao, J.; Zhang, W.; Zhao, L.; Mo, X.; Fang, F. Diagnosis of subclinical extramammary Paget’s disease with a combination of noninvasive photodynamic diagnosis and reflectance confocal microscopy. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 371–374. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Q.; Zhang, W.; Huang, L.; Sun, J.; Zhao, L.; Zhao, Y.; Tian, C.; Cheng, W.; Shao, X.; et al. Impact of combining photodynamic diagnosis with reflectance confocal microscopy, on tumor margin detection and surgical outcomes in patients with extramammary Paget disease. Indian J. Dermatol. Venereol. Leprol. 2023, 90, 447–452. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Song, C.; Lou, Y.; Fu, B. The application of Wood’s lamp combined with 5-aminolevulinic acid for defining tumor margins in patients with extramammary Paget’s disease. Photodiagnosis Photodyn. Ther. 2021, 35, 102490. [Google Scholar] [CrossRef]
- Terrier, J.-E.; Tiffet, O.; Raynaud, N.; Cinotti, E. In Vivo Reflectance Confocal Microscopy Combined with the “Spaghetti” Technique: A New Procedure for Defining Surgical Margins of Genital Paget Disease. Dermatol. Surg. 2015, 41, 862–864. [Google Scholar] [CrossRef]
- Pan, Z.-Y.; Liang, J.; Zhang, Q.-A.; Lin, J.-R.; Zheng, Z.-Z. In vivo reflectance confocal microscopy of extramammary Paget disease: Diagnostic evaluation and surgical management. J. Am. Acad. Dermatol. 2012, 66, e47–e53. [Google Scholar] [CrossRef]
- Guitera, P.; Scolyer, R.A.; Gill, M.; Akita, H.; Arima, M.; Yokoyama, Y.; Matsunaga, K.; Longo, C.; Bassoli, S.; Bencini, P.L.; et al. Reflectance confocal microscopy for diagnosis of mammary and extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e24–e29. [Google Scholar] [CrossRef] [PubMed]
- Debarbieux, S.; Dalle, S.; Depaepe, L.; Jeanniot, P.Y.; Poulalhon, N.; Thomas, L. Extramammary Paget’s disease of the scalp: Examination by in vivo and ex vivo reflectance confocal microscopy. Ski. Res. Technol. 2014, 20, 124–126. [Google Scholar] [CrossRef]
- Yélamos, O.; Hibler, B.P.; Cordova, M.; Hollmann, T.J.; Kose, K.; Marchetti, M.A.; Myskowski, P.L.; Pulitzer, M.P.; Rajadhyaksha, M.; Rossi, A.M.; et al. Handheld Reflectance Confocal Microscopy for the Detection of Recurrent Extramammary Paget Disease. JAMA Dermatol. 2017, 153, 689. [Google Scholar] [CrossRef]
- Kibbi, N. Reflectance Confocal Microscopy as a Promising Adjunctive Tool for Treatment Planning in Extramammary Paget’s Disease. Dermatol. Surg. 2021, 47, 688. [Google Scholar] [CrossRef]
- Ganhewa, A.D.; Shumack, S.; Harvey, R.A.; Guitera, P. Extramammary Paget’s disease of the scrotum: Case report and assessment with in vivo reflectance confocal microscopy. Australas. J. Dermatol. 2021, 62 (Suppl. S1), 97. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Aleissa, S.; Cordova, M.; Hibler, B.P.; Erlendsson, A.M.; Polansky, M.; Cordova, F.; Lee, E.H.; Busam, K.J.; Hollmann, T.; et al. Treatment of Extramammary Paget Disease and the Role of Reflectance Confocal Microscopy: A Prospective Study. Dermatol. Surg. 2021, 47, 473–479. [Google Scholar] [CrossRef]
- Filonenko, E.; Kaprin, A.; Volchenko, N.; Grigorievykh, N.; Ivanova-Radkevich, V. Photodynamic therapy in a patient with perianal extramammary Paget’s disease. Photodiagnosis Photodyn. Ther. 2023, 42, 103603. [Google Scholar] [CrossRef]
- Clément, E.; Sparsa, A.; Doffoel-Hantz, V.; Durox, H.; Prey, S.; Bonnetblanc, J.-M.; Caly, H.; Aubard, Y.; Bedane, C. Traitement de la maladie de Paget extramammaire par photothérapie dynamique topique. Ann. Dermatol. Vénéréologie 2012, 139, 103–108. [Google Scholar] [CrossRef]
- Rioli, D.-I.; Samimi, M.; Beneton, N.; Hainaut, E.; Martin, L.; Misery, L.; Quereux, G. Efficacy and tolerance of photodynamic therapy for vulvar Paget’s disease: A multicentric retrospective study. Eur. J. Dermatol. 2018, 28, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Fontanelli, R.; Papadia, A.; Martinelli, F.; Lorusso, D.; Grijuela, B.; Merola, M.; Solima, E.; Ditto, A.; Raspagliesi, F. Photodynamic therapy with M-ALA as non surgical treatment option in patients with primary extramammary Paget’s disease. Gynecol. Oncol. 2013, 130, 90–94. [Google Scholar] [CrossRef]
- Ferrara, F.; Bardazzi, F.; Messori, S.; Abbenante, D.; Barisani, A.; Vaccari, S. Photodynamic therapy following fractional CO2 laser for treatment of primary vulvar Paget’s disease: Does it really work? J. Dermatol. Treat. 2021, 32, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Borella, F.; Preti, M.; Vieira-Baptista, P.; Pérez-López, F.R.; Bertero, L.; Gallio, N.; Micheletti, L.; Benedetto, C. Vulvar Paget’s disease: Outcomes of 51 patients treated with imiquimod cream. Maturitas 2022, 163, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Escolà, H.; Llombart, B.; Escolà-Rodríguez, A.; Barchino-Ortiz, L.; Marcoval, J.; Alcaraz, I.; Beà-Ardébol, S.; Toll, A.; Miñano-Medrano, R.; Rodríguez-Jiménez, P.; et al. Therapeutic outcomes and survival analysis of Extramammary Paget’s disease: A multicentre retrospective study of 249 patients. J. Am. Acad. Dermatol. 2024, 90, 66–73. [Google Scholar] [CrossRef]
- Xiang, X.; Li, Y.; Liu, X.; Ma, G. Case report: Hematoporphyrin injection (HpD) photodynamic therapy for the treatment of groin extramammary Paget’s disease in an elderly male patient. Photodiagnosis Photodyn. Ther. 2023, 44, 103727. [Google Scholar] [CrossRef]
- Xiang, X.; Li, Y.; Tang, Y.; Liu, R.; Ma, T.; Liu, X.; Ma, G. Efficacy of hematoporphyrin injection (HpD) photodynamic therapy in the treatment of widespread extramammary Paget’s disease. Photodiagnosis Photodyn. Ther. 2023, 42, 103649. [Google Scholar] [CrossRef]
- Do, J.; Do, S.-I.; Kim, H.-S. Identification of Predictive Factors for Post-operative Recurrence and Clinical Outcomes of Primary Vulvar Extramammary Paget Disease. Vivo 2023, 37, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, M.; Van Hees, C.L.; Van Beurden, M.; Bulten, J.; Van Dorst, E.B.; Esajas, M.D.; Meeuwis, K.A.; Boll, D.; Van Poelgeest, M.I.; De Hullu, J.A. The Paget Trial: Topical 5% imiquimod cream for noninvasive vulvar Paget disease. Am. J. Obstet. Gynecol. 2022, 227, 250.e1–250.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, P.; Li, C.; Zhou, Z.; Zhang, L.; Zhang, G.; Wang, X. Efficacy and safety of HpD-PDT for Extramammary Paget’s Disease refractory to conventional therapy: A prospective, open-label and single arm pilot study. Photodiagnosis Photodyn. Ther. 2022, 37, 102670. [Google Scholar] [CrossRef] [PubMed]
- Sadko, K.; Płaszczyńska, A.; Czarny, J. Extramammary Paget disease of the vulva treated with imiquimod. Dermatol. Rev. 2022, 109, 368–376. [Google Scholar] [CrossRef]
- Liu, K.X.; Huang, V.; Chen, C.A.; Elco, C.P.; Chen, S.T.; Stern, R.S.; Wu, P.A. Longitudinal multicentre retrospective cohort study of treatment outcomes in extramammary Paget disease. Br. J. Dermatol. 2021, 185, 219–221. [Google Scholar] [CrossRef]
- Tanimura, H.; Tsuda, M.; Shijimaya, T.; Nagano, N.; Nakamaru, S.; Makimura, K.; Kiyohara, T. Six Cases of Extramammary Paget’s Disease Treated with Radiotherapy. Ski. Res. 2021, 20, 11–17. [Google Scholar] [CrossRef]
- Bauman, T.M.; Rosman, I.S.; Sheinbein, D.M. Extramammary Paget’s disease of the scrotum with complete response to imiquimod and photodynamic therapy. BMJ Case Rep. 2018, 2018, bcr2017221696. [Google Scholar] [CrossRef]
- Nitecki, R.; Davis, M.; Watkins, J.C.; Wu, Y.E.; Vitonis, A.F.; Muto, M.G.; Berkowitz, R.S.; Horowitz, N.S.; Feltmate, C.M. Extramammary Paget Disease of the Vulva. Int. J. Gynecol. Cancer 2018, 28, 632–638. [Google Scholar] [CrossRef]
- Sawada, M.; Kato, J.; Yamashita, T.; Yoneta, A.; Hida, T.; Horimoto, K.; Sato, S.; Uhara, H. Imiquimod 5% cream as a therapeutic option for extramammary Paget’s disease. J. Dermatol. 2018, 45, 216–219. [Google Scholar] [CrossRef]
- Vicentini, C.; Carpentier, O.; Lecomte, F.; Thecua, E.; Mortier, L.; Mordon, S.R. Treatment of a vulvar Paget’s disease by photodynamic therapy with a new light emitting fabric based device. Lasers Surg. Med. 2017, 49, 177–180. [Google Scholar] [CrossRef]
- Dogan, A.; Hilal, Z.; Krentel, H.; Cetin, C.; Hefler, L.A.; Grimm, C.; Tempfer, C.B. Paget’s Disease of the Vulva Treated with Imiquimod: Case Report and Systematic Review of the Literature. Gynecol. Obstet. Investig. 2017, 82, 1–7. [Google Scholar] [CrossRef]
- Knight, S.R.; Proby, C.; Ziyaie, D.; Carey, F.; Koch, S. Extramammary Paget disease of the perianal region: The potential role of imiquimod in achieving disease control. J. Surg. Case Rep. 2016, 2016, rjw110. [Google Scholar] [CrossRef]
- Cowan, R.A.; Black, D.R.; Hoang, L.N.; Park, K.J.; Soslow, R.A.; Backes, F.J.; Gardner, G.J.; Abu-Rustum, N.R.; Leitao, M.M.; Eisenhauer, E.L.; et al. A pilot study of topical imiquimod therapy for the treatment of recurrent extramammary Paget’s disease. Gynecol. Oncol. 2016, 142, 139–143. [Google Scholar] [CrossRef]
- Higgins, C.; Pagano, R.; Veysey, E.; Howard, A. Primary Paget’s disease of the vulva successfully treated with imiquimod. Australas. J. Dermatol. 2016, 57, 3–85. [Google Scholar] [CrossRef]
- Youssef, A.A.; Reygagne, P. Successful treatment of extramammary Paget’s disease of the scalp with photodynamic therapy. Int. J. Dermatol. 2016, 55, 580–582. [Google Scholar] [CrossRef]
- Marchitelli, C.; Peremateu, M.S.; Sluga, M.C.; Berasategui, M.T.; Lopez, D.G.; Wernicke, A.; Velazco, A.; Gogorza, S. Treatment of Primary Vulvar Paget Disease with 5% Imiquimod Cream. J. Low. Genit. Tract Dis. 2014, 18, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Juan, X.; Li, X.; Jiayuan, C.; Qin, H.; Qing, L.; Shengmei, X. Complete remission of two patients with recurrent and wide spread extramammary Paget disease obtained from 5-aminolevulinic acid-based photodynamic therapy and imiquimod combination treatment. Photodiagnosis Photodyn. Ther. 2014, 11, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Luyten, A.; Sörgel, P.; Clad, A.; Gieseking, F.; Maass-Poppenhusen, K.; Lellé, R.J.; Harter, P.; Buttmann, N.; Petry, K.U. Treatment of extramammary Paget disease of the vulva with imiquimod: A retrospective, multicenter study by the German Colposcopy Network. J. Am. Acad. Dermatol. 2014, 70, 644–650. [Google Scholar] [CrossRef]
- Hata, M.; Koike, I.; Wada, H.; Miyagi, E.; Kasuya, T.; Kaizu, H.; Matsui, T.; Mukai, Y.; Ito, E.; Inoue, T. Radiation therapy for extramammary Paget’s disease: Treatment outcomes and prognostic factors. Ann. Oncol. 2014, 25, 291–297. [Google Scholar] [CrossRef]
- Itonaga, T.; Nakayama, H.; Okubo, M.; Mikami, R.; Nogi, S.; Tajima, Y.; Sugahara, S.; Tokuuye, K. Radiotherapy in Patients with Extramammary Paget’s Disease—Our Own Experience and Review of the Literature. Oncol. Res. Treat. 2014, 37, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Lavaf, A.; Tejwani, A.; Ross, P.; Ashamalla, H. Perianal Paget disease treated definitively with radiotherapy. Curr. Oncol. 2012, 19, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Green, J.S.; Burkemper, N.M.; Fosko, S.W. Failure of Extensive Extramammary Paget Disease of the Inguinal Area to Clear with Imiquimod Cream, 5%: Possible Progression to Invasive Disease During Therapy. Arch. Dermatol. 2011, 147, 704. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, C.; Gao, T.; Jiao, B.; Qi, X.; Long, H.; Qiao, H.; Wang, L.; Lv, Y.; Hu, X.; et al. Long-term Follow-up of In situ Extramammary Paget’s Disease in Asian Skin Types IV/V Treated with Photodynamic Therapy. Acta Derm. Venereol. 2010, 90, 159–164. [Google Scholar] [CrossRef]
- Housel, J.P.; Izikson, L.; Zeitouni, N.C. Noninvasive Extramammary Paget’s Disease Treated with Photodynamic Therapy: Case Series from the Roswell Park Cancer Institute. Dermatol. Surg. 2010, 36, 1718–1724. [Google Scholar] [CrossRef]
- Fukui, T.; Watanabe, D.; Tamada, Y.; Matsumoto, Y. Photodynamic Therapy Following Carbon Dioxide Laser Enhances Efficacy in the Treatment of Extramammary Paget’s Disease. Acta Derm. Venereol. 2009, 89, 150–154. [Google Scholar] [CrossRef]
- Kim, T.H.; Chang, I.H.; Kim, T.-H.; Lee, S.Y.; Myung, S.C. Extramammary Paget’s Disease of Scrotum Treated with Radiotherapy. Urology 2009, 74, 474.e1–474.e3. [Google Scholar] [CrossRef]
- Geisler, J.P.; Manahan, K.J. Imiquimod in vulvar Paget’s disease: A case report. J. Reprod. Med. 2008, 53, 811–812. [Google Scholar]
- Vereecken, P.; Awada, A.; Ghanem, G.; Marques da Costa, C.; Larsimont, D.; Simoens, C.; Mendes da Costa, P.; Hendlisz, A. A therapeutic approach to perianal extramammary Paget’s disease: Topical imiquimod can be useful to prevent or defer surgery. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2007, 13, CS75-7. [Google Scholar]
- Raspagliesi, F.; Fontanelli, R.; Rossi, G.; Ditto, A.; Solima, E.; Hanozet, F.; Kusamura, S. Photodynamic therapy using a methyl ester of 5-aminolevulinic acid in recurrent Paget’s disease of the vulva: A pilot study. Gynecol. Oncol. 2006, 103, 581–586. [Google Scholar] [CrossRef]
- Holt, K.; Stanley, L. Radiotherapy and Perianal Paget’s Disease. J. Radiother. Pract. 2006, 5, 55–60. [Google Scholar] [CrossRef]
- Madan, V.; Loncaster, J.; Allan, D.; Lear, J.; Sheridan, L.; Leach, C.; Allan, E. Extramammary Paget’s disease treated with topical and systemic photodynamic therapy. Photodiagnosis Photodyn. Ther. 2005, 2, 309–311. [Google Scholar] [CrossRef]
- Son, S.-H.; Lee, J.-S.; Kim, Y.-S.; Ryu, M.-R.; Chung, S.-M.; Namkoong, S.-E.; Han, G.-T.; Lee, H.-J.; Yoon, S.-C. The Role of Radiation Therapy for the Extramammary Paget’s Disease of the Vulva; Experience of 3 Cases. Cancer Res. Treat. 2005, 37, 365. [Google Scholar] [CrossRef]
- Mikasa, K.; Watanabe, D.; Kondo, C.; Kobayashi, M.; Nakaseko, H.; Yokoo, K.; Tamada, Y.; Matsumoto, Y. 5-Aminolevulinic Acid-Based Photodynamic Therapy for the Treatment of Two Patients with Extramammary Paget’s Disease. J. Dermatol. 2005, 32, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Luk, N.M.; Yu, K.H.; Yeung, W.K.; Choi, C.L.; Teo, M.L. Extramammary Paget’s disease: Outcome of radiotherapy with curative intent: Extramammary Paget’s disease. Clin. Exp. Dermatol. 2003, 28, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Spencer, J.; Villa, A.; Poochareon, V.; Elgart, G. Successful treatment of extramammary Paget’s disease of the scrotum with imiquimod 5% cream: Treatment of Paget’s disease of the scrotum with imiquimod 5% cream. Clin. Exp. Dermatol. 2003, 28, 36–38. [Google Scholar] [CrossRef]
- Guerrieri, M.; Back, M.F. Extramammary Paget’s disease: Role of radiation therapy. Australas. Radiol. 2002, 46, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arias, G.A.; Conill, C.; Castells-Mas, A.; Arenas, M.; Grimalt, R. Radiotherapy for Genital Extramammary Paget’s Disease in Situ. Dermatol. Surg. 2001, 27, 587–590. [Google Scholar] [CrossRef]
- Burrows, N.P.; Jones, D.H.; Hudson, P.M.; Pye, R.J. Treatment of extramammary Paget’s disease by radiotherapy. Br. J. Dermatol. 2010, 132, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Kwan, W.H.; Teo, P.M.L.; Ngar, Y.K.; Yu, K.H.; Choi, P.H.K. Perineal Paget’s disease: Effective treatment with fractionated high dose rate brachytherapy. Clin. Oncol. 1995, 7, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Marchitelli, C.; Sluga, C.; Berasategui, M.T.; Secco, G.; Peremateu, S.; Wernicke, A.; Velazco, A.; Gogorza, S. Treatment of recurrent primary vulvar Paget’s disease with 5% imiquimod cream. J. Low. Genit. Tract Dis. 2013, 17, e85–e115. [Google Scholar] [CrossRef]
- Machado, L.O.; Amaral, A.; Vieira-Baptista, P.; Costa, A.; Costa, A.R.; Beires, J. O413 Treatment of Vulvar Paget’s Disease with Imiquimod: Clinical Report and Revision of the Literature. Int. J. Gynecol. Obstet. 2012, 119, S407. [Google Scholar] [CrossRef]
- Schmid, M.; Zierhofer-Tonar, U.; Seeber, A.; Volc-Platzer, B. P79 Successful treatment of extramammary Paget’s disease with topical imiquimod. Melanoma Res. 2010, 20, e78. [Google Scholar] [CrossRef]
- Besa, P.; Rich, T.A.; Delclos, L.; Edwards, C.L.; Ota, D.M.; Wharton, J.T. Extramammary paget’s disease of the perineal skin: Role of radiotherapy. Int. J. Radiat. Oncol. 1992, 24, 73–78. [Google Scholar] [CrossRef]
- Van Der Linden, M.; Van Esch, E.; Bulten, J.; Dreef, E.; Massuger, L.; Van Der Steen, S.; Bosse, T.; De Hullu, J.; Van Poelgeest, M. The immune cell infiltrate in the microenvironment of vulvar Paget disease. Gynecol. Oncol. 2018, 151, 453–459. [Google Scholar] [CrossRef]
- Van Der Linden, M.; Oonk, M.H.M.; Van Doorn, H.C.; Bulten, J.; Van Dorst, E.B.L.; Fons, G.; Lok, C.A.R.; Van Poelgeest, M.I.E.; Slangen, B.M.F.; Massuger, L.F.A.G.; et al. Vulvar Paget disease: A national retrospective cohort study. J. Am. Acad. Dermatol. 2019, 81, 956–962. [Google Scholar] [CrossRef]
- Wang, H.; Lv, T.; Zhang, L.; Lai, Y.; Tang, L.; Tang, Y.; Huang, Z.; Wang, X. A prospective pilot study to evaluate combined topical photodynamic therapy and surgery for extramammary paget’s disease. Lasers Surg. Med. 2013, 45, 296–301. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Dai, Y.; Yang, Z.; Zhang, X.; Li, D. Excision combined with photodynamic therapy for scrotal Paget’s disease in patients aged over 60 years. Aging Male 2020, 23, 854–859. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).