1. Introduction
Cancer exerts tremendous financial strain on communities and health systems. As the second leading cause of death worldwide, it accounts for approximately 10 million deaths a year. Cancer incidence increases with age, and it is expected that these numbers will only rise as a consequence of the growing population aging [
1]. Colorectal cancer (CRC) is the third most common cancer type worldwide, accounting for approximately 10% of all cancer-related deaths [
2,
3]. At early stages, CRC is often symptomless, and it is usually diagnosed in an advanced and/or metastatic stage when treatment options are limited. Treatment success is influenced by the cancer genotype and the corresponding tumor microenvironment [
4]. There are different CRC subtypes which are classified based on unique morphological and molecular traits. Adenocarcinomas account for about 95% of colorectal cancer cases, while neuroendocrine tumors and small-cell carcinomas represent the remaining 5% [
5]. The stage of the disease detected at the time of diagnosis or after surgery generally has a significant impact on the patient’s prognosis [
6,
7].
The stage and the metastatic potential of CRC are classified based on the TNM or a Dukes classification [
8]. Genetic instability is a major characteristic of CRC and arises through two basic processes. The most common mechanism, accounting for around 84% of instances, is chromosomal instability, defined by changes in the number and shape of chromosomes, including deletions and translocations [
9]. The second is related to hypermutation and microsatellite instability (MSI) as a result of poor DNA mismatch repair (MMR), and it is observed in around 15% of sporadic CRC cases [
10]. However, these traditional classifications based on tumor size, location, and the stage do not fully capture the complexity of CRC. Therefore, precise analysis of specific genetic and molecular alterations within the tumor might provide important and patient-specific information for future personalized therapy options.
The molecular status of proteins associated with cellular death and growth belonging to apoptotic, Wnt/β catenin, and Hippo YAP pathways might be associated with different stages of CRC and represent important prognostic markers and possible therapeutic targets.
Apoptotic inducing factor M3 (AIFM3), a mitochondrial protein involved in the execution phase of apoptosis [
11], is widely expressed across multiple tissues and shows abnormal expression in various cancers [
12]. Although AIFM3 has been found in colon tissue, until recently, clinical implications of AIFM3 were not reported for CRC.
Vestigial-like family member 4 (VGLL4) is a member of the VGLL family of transcriptional cofactors and is involved in negative regulation of the Wnt signaling pathway, negative regulation of cell growth, and negative regulation of Hippo signaling. VGLL4 levels in cancer cells are typically lower compared to normal tissues, and reduced expression of VGLL4 is often associated with poor survival outcomes in numerous cancers, including lung, gastric, breast, colorectal, bladder, pancreatic adenocarcinoma, and esophageal squamous cancer. On the other hand, higher VGLL4 expression is positively correlated with better survival rates [
13]. Its interaction with TEAD transcription factors affects the Wnt/β-catenin and Hippo–YAP1 signaling pathways, which are critical for cellular development and differentiation. Due to its negative regulation of pathways involved in tumor proliferation, the VGLL4 acts as a tumor suppressor.
WNT4 is a member of the WNT protein family, important for key cellular activities such as cell growth and development [
14]. Upregulation of Wnt signaling pathways is one of the main drivers of CRC development and progression. In CRC, WNT4 is significantly elevated in patient serum and originates largely from cancerous tissues, suggesting that it may serve as a potential biomarker. The WNT4 promotes CRC progression by inducing epithelial–mesenchymal transition (EMT), activating fibroblasts, and enhancing angiogenesis through the Wnt/β-catenin signaling pathway. These effects contribute to the aggressive nature of CRC and elevate its metastatic potential [
15]. WNT4 has also been observed to play a role in other cancer types, particularly breast cancer, where it may contribute to aberrant cell proliferation.
In this study, it was hypothesized that AIFM3, VGLL4, and WNT4 proteins play an important role in molecular events leading to development of CRC and might represent an important new prognostic marker. Molecular classification of these proteins in different stages of CRC could contribute to the development of personalized treatments, particularly for individuals at higher risk
Scheme 1.
2. Materials and Methods
2.1. Ethics
This study was performed in accordance with the Helsinki Declaration and has been approved by the Ethics Committee of the School of Medicine, University of Mostar.
2.2. Tissue Procurement and Immunohistochemistry
Patient recruitment was carried out in collaboration with healthcare professionals to identify appropriate candidates. Participants were approached directly during medical visits, followed by subsequent communication and informational sessions to guarantee informed consent. No medications were administered to participants prior to the study, as it focused on patients receiving their first CRC diagnosis. The youngest patient was 45, and the oldest was 89 years of age.
A total of 43 tumor tissue samples and control tissues were taken at laparoscopic CRC surgery in paraffin blocks (12 samples from Dukes A, 11 samples from Dukes B, 10 samples from Dukes C, and 10 samples from Dukes D) were received from the Department of Pathology, Cytology, and Forensic Medicine at University Hospital Mostar (
Table 1). The study included CRC tumor samples across all cancer stages from patients of both sexes and all age ranges, as described above. Stratified randomization ensured an even distribution of cancer stages as the key characteristics across treatment groups.
Samples were prescreened, selecting only those with adequate paraffin block material for further immunohistochemistry (IHC) analysis and complete clinical data. Exclusion criteria included incomplete laboratory results, insufficient IHC material, and lack of control tissue. The control tissue was normal tissue adjacent to tumor tissue.
Tumor samples were examined and measured, with each sample containing both tumor and healthy control tissue. The tissue blocks were processed following the method outlined by Vukojevic et al. [
16]. In summary, the tissues were dehydrated using an increasing series of ethanol solutions (70% to 100%) and then embedded in paraffin. Sections (4 μm) were cut from the paraffin tissue blocks by microtome (Leica RM2155, Pittsburgh, PA, USA). The sections were deparaffinized in xylene, rehydrated in decreasing ethanol solutions, and rinsed in distilled water. Histological evaluation of the collected tissue samples was performed using hematoxylin and eosin (HE) staining. The HE staining was used for assessing tissue morphology and preservation, as well as to identify tumor regions, differentiate between cancerous and normal tissues, and evaluate the histopathological features of the samples to ensure accurate classification and selection of regions for subsequent analyses.
The next step in the immunofluorescence protocol involved heating the tissue in citrate buffer (pH 6) at 95 °C in a steam bath for 15 min, then allowing it to cool to room temperature. Afterwards, the tissue sections were incubated in blocking buffer (ab64226, Abcam, Cambridge, UK) for 20 min, followed by a one-hour incubation with the appropriate primary antibody combinations (
Table 2). After being rinsed in PBS, the tissue sections were incubated with the secondary antibody for one hour. After final rinsing in the PBS, sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and cover-slipped.
To minimize non-specific background signals, isotype-matched controls and secondary-only samples were used. Isotype-matched controls replaced the primary antibody with one of the same isotype but without specificity for the target, helping to identify non-specific binding. Secondary-only samples, where the primary antibody was omitted, allowed for the detection of any non-specific binding of the secondary antibody. These controls ensured that observed signals were specific to the target protein and not due to background noise, such as residual paraffin (
Figure S1).
2.3. Data Collection and Processing
Hematoxylin- and eosin-stained tissue sections were analyzed by microscope (BX40, Olympus, Tokyo, Japan), and immunofluorescence images were captured by an immunofluorescence microscope (BX51, Olympus, Tokyo, Japan) mounted with a digital camera (Nikon Ri-D2, Nikon, Tokyo, Japan).
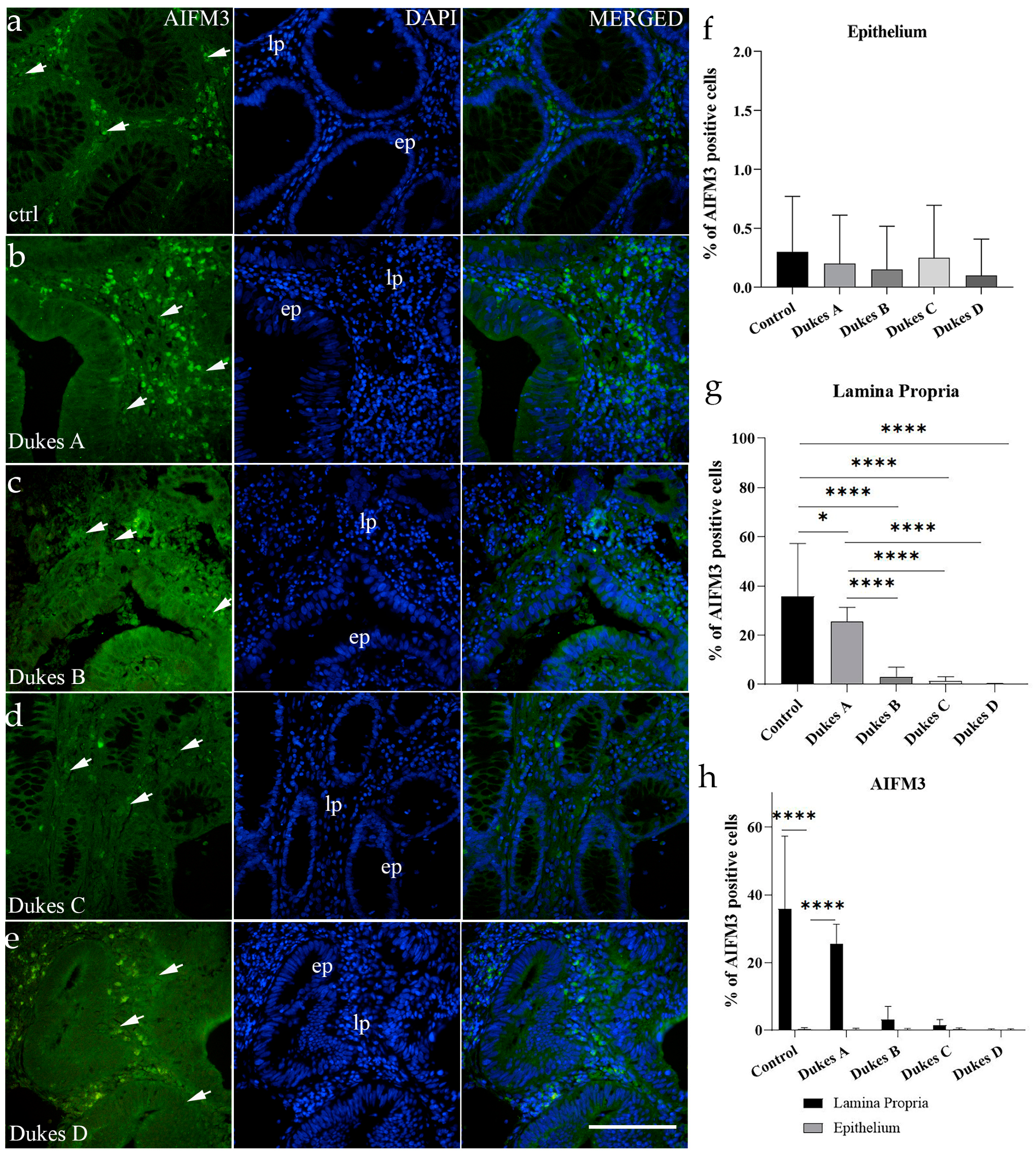
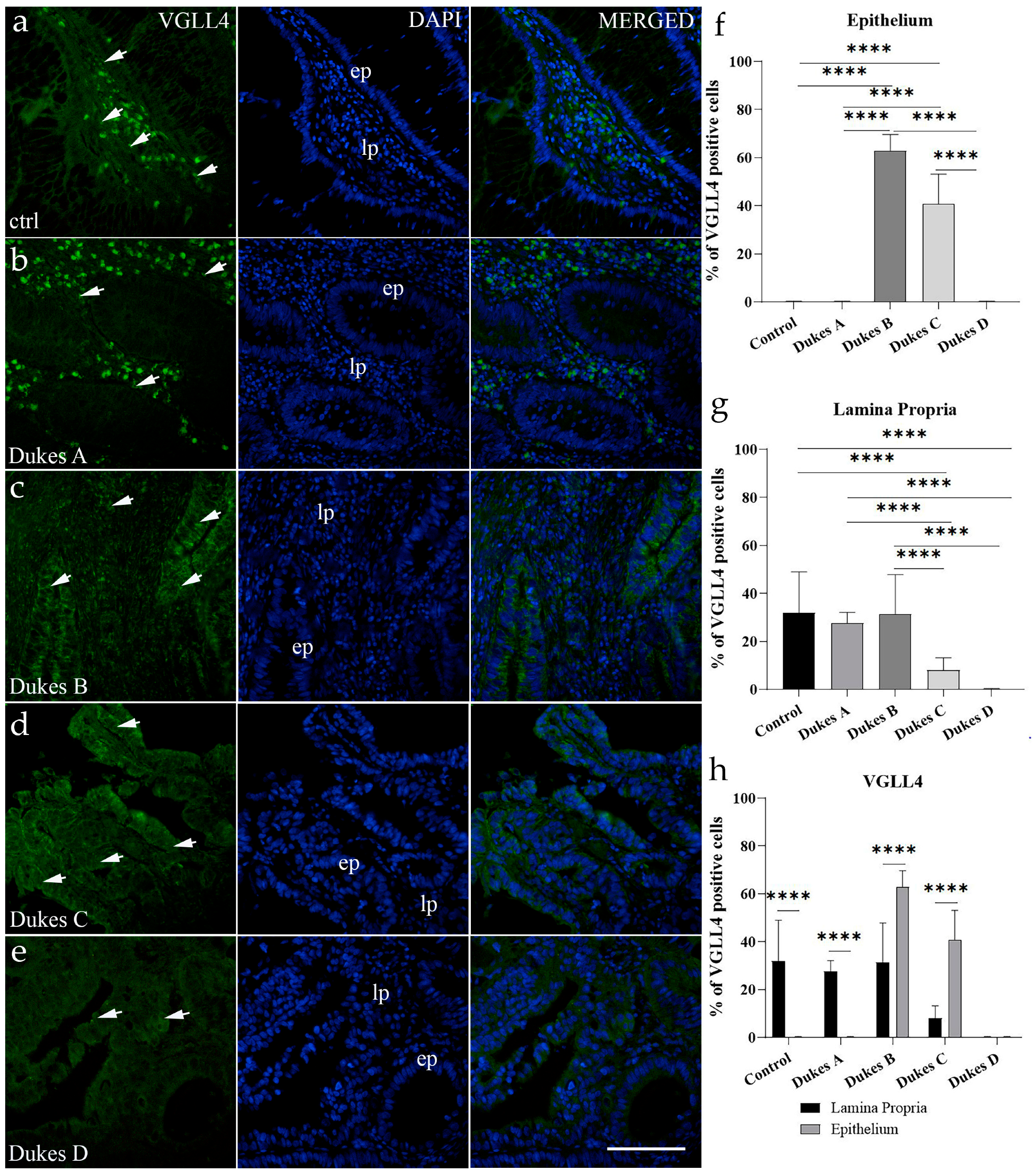
For AIFM3, VGLL4, and WNT4, positive cell counts were performed on captured immunofluorescence images for all groups, in the epithelium and lamina propria. Regarding the classification of positive cells, any cells showing nucleic, cytoplasmic, or membranous staining, regardless of intensity, were considered positive, and cells with no apparent immunofluorescent signal were considered unstained (negative), when merged with DAPI. Image processing was conducted by ImageJ (National Institutes of Health, Bethesda, MD, USA). For quantitative analysis, images were set to be divided into squares (20 μm × 20 μm at 40× magnification).
Positive and negative cell counts followed a standardized method: only squares fully encompassing the region of interest were evaluated, avoiding duplicate counts by analyzing alternate sections. Cells along the right and lower boundaries of each square were included, while those on the left and upper boundaries were excluded. The percentage of positive cells was determined by analyzing ten representative images within the regions of interest for each sample. The results were then averaged per sample and across the examined groups. Data were presented as mean ± standard deviation (SD). For image assembly, Adobe Photoshop (Adobe Photoshop CS, 2004, Berkeley, CA, USA: Peachpit Press) was applied.
2.4. The Examination of RNA Transcripts
The TCGA colon and rectal adenocarcinoma (COADREAD) gene expression dataset, obtained through RNA sequencing (polyA + IlluminaHiSeq), was retrieved from the Xena database at the University of California Santa Cruz (
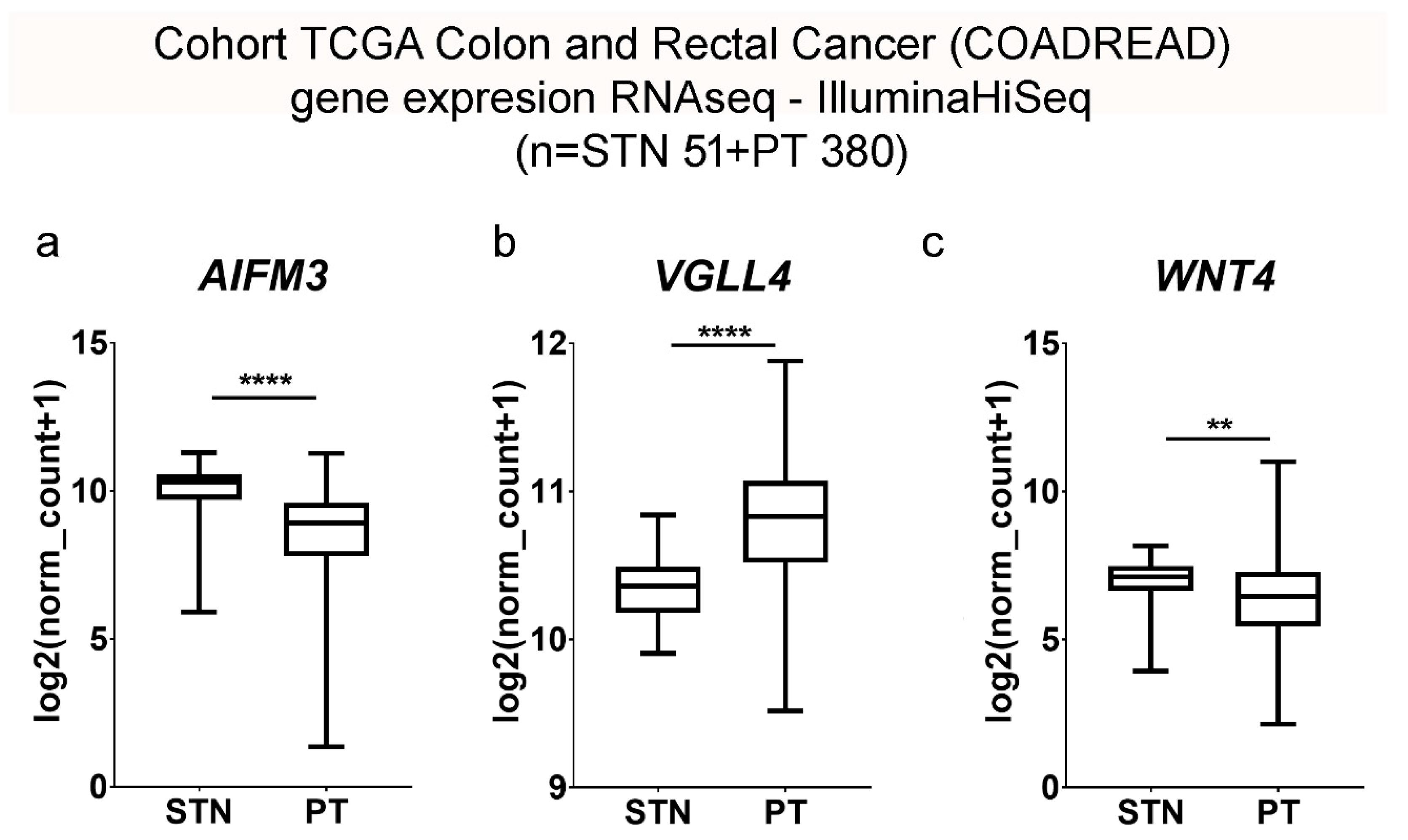
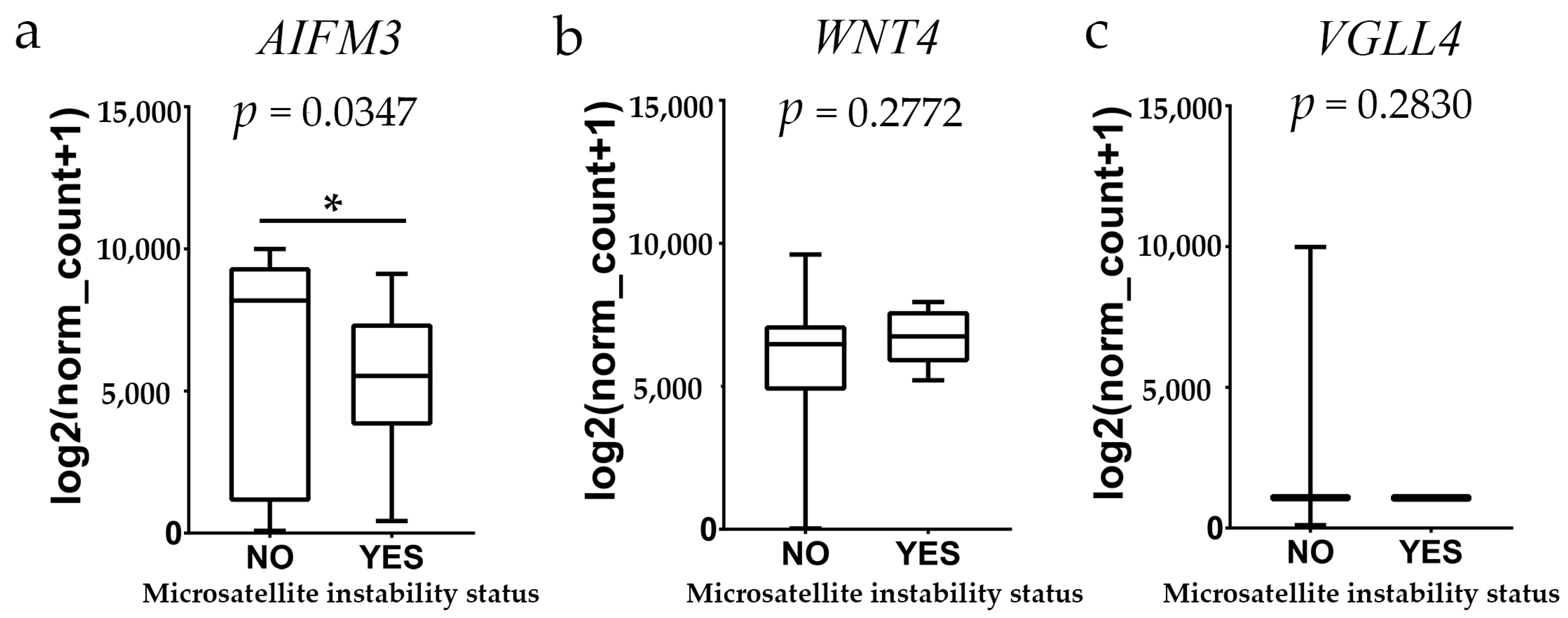
https://xenabrowser.net/datapages/; accessed on 4 May 2024) and utilized for the current studies. This dataset combined findings from TCGA colon adenocarcinoma and rectal adenocarcinoma cases, specifically those with no prior history of neoadjuvant treatment, and was measured using the Illumina HiSeq 2000 RNA Sequencing platform at the University of North Carolina’s TCGA genome characterization center. The dataset provided gene-level transcription estimates represented as log2(x + 1)-transformed RSEM normalized counts. Data regarding overall survival, gene expression of samples, and information on microsatellite instability were exported as text files and processed in Microsoft Excel 2019 MSO version 2305 (Microsoft Corp., Redmond, WA, USA). After curation of the data to remove duplicates and samples lacking expression data for the factors studied, 431 patient samples were preserved for analysis, which included 380 primary tumors (PT) and 51 normal solid tissues from healthy resection margins (STN). The expression of AIFM3, WNT4, and VGLL4 in relation to microsatellite instability (MSI) status in colorectal cancer was analyzed using a cohort of 108 patients. Among the cohort, 98 patients were classified as MSI-stable, while 10 were MSI-unstable.
2.5. Statistical Data Analysis
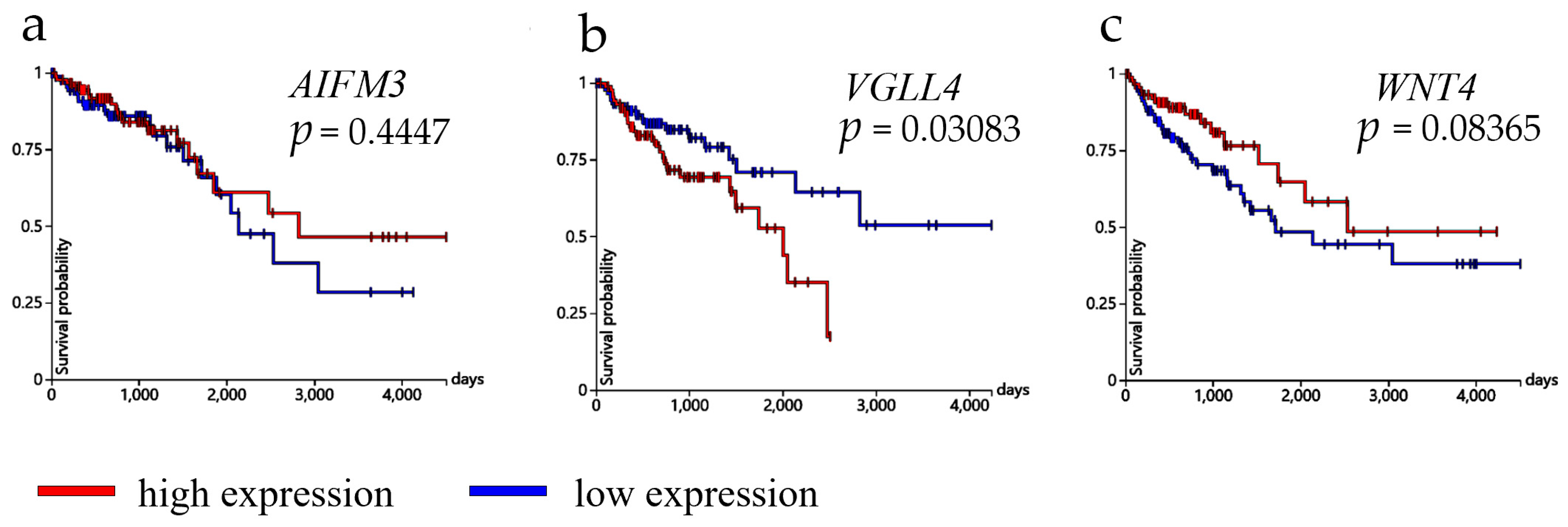
The GraphPad was used for statistical alalysis (GraphPad Software, San Diego, CA, USA). Ordinary one-way ANOVA was used, and afterwards Tukey’s multiple comparison tests was performed for protein expression comparison between control and CRC tissues (at all tumor stages) in the lamina propria and epithelium. A two-way ANOVA test was used for control tissue and each examined CRC stage within each group. Šídák’s multiple comparisons test was then used to compare the protein expression (lamina propria vs. epithelium). To compare the expression of AIFM3, VGLL4, and WNT4 markers between tumor and normal tissue specimens, an unpaired t-test was used. Survival analysis was conducted using expression quartiles for each gene. The statistical evaluation of survival duration was performed using the Kaplan–Meier method along with the log-rank test. The Mann–Whitney U test was used to test differences in gene expression levels between MSI-stable and MSI-unstable groups. A difference was considered statistically significant if the p-value was less than 0.05.
4. Discussion
Cancer development can be a consequence of hyperactivation of cellular proliferation and inadequate activation of apoptosis, both contributing to the uncontrolled survival of malignant cells [
17]. Therefore, disruptions in the expression and function of signaling molecules involved in the regulation of apoptosis and cellular growth represent important molecular events involved in cancer initiation and progression. Stimulation of cancer cell death by activation of cellular apoptotic pathways and inhibition of uncontrolled proliferation are key targets in cancer therapies. The usual apoptotic markers, such as Fas receptor, Fas ligand, and circulating DNA, are often used to monitor therapy progress [
18]. These are reasons for a continued search for novel—and investigation of known—molecules involved in the control of apoptosis and cellular proliferation, especially those that could serve as relevant prognostic markers for better survival and therapy targets. In this study, using tissue samples of patients with different stages of CRC, the expression levels of three important signaling molecular markers AIFM3, VGLL4, and WNT4 were evaluated.
Recently, apoptosis-related protein AIFM3 was found to be increased in breast cancer and cholangiocarcinoma [
19]. Chua-on et al. reported elevated AIFM3 levels in the sera of cholangiocarcinoma patients, originating from the tumor tissue [
20]. Unlike the data from breast cancer and cholangiocarcinoma patients, in our study, CRC patients’ samples showed a decrease in AIFM-positive cells in the lamina propria. Analysis revealed a significant reduction in AIFM-positive cells within the lamina propria, and this reduction was mostly pronounced in advanced CRC stages (Dukes B–D), where AIFM-positive cells were very low or undetectable. Furthermore, the epithelial regions of CRC samples exhibited a markedly low percentage of AIFM-positive cells. These findings suggest that the loss of AIFM-positive cells may be associated with CRC progression and highlight a potential divergence in the tumor microenvironment between CRC and other cancer types, indicating possible tissue specificity. To elucidate the functional consequences of reduced AIFM-positive cells’ presence in CRCs, further studies are warranted.
Another important hallmark of tumors is uncontrollable cellular growth. This usually occurs through mutations in cellular checkpoint proteins, dysregulation of tumor suppressor genes such as p53, and activation of oncogenes or mutations in components of growth signaling receptors and downstream pathways, leading to their activation and increased cellular proliferation [
21]. Two very important pathways for cellular growth are the Hippo–YAP1 and Wnt/β-catenin pathways. The activity of these pathways can be used as a diagnostic marker in cancer prognosis, and high activation correlates with worse survival rates [
22]. One of the described prognostic markers is VGLL4 [
22]. In breast cancer, it binds TEAD1, and this interaction antagonizes TEAD1–YAP1-induced cell proliferation in Hippo–YAP1 signaling. In CRCs, it was shown that the binding of VGLL4 to the TEAD4–TCF4 complex inhibits Wnt/β-catenin signaling [
23]. Our findings support the growing evidence that low expression of VGLL4 is associated with poorer survival outcomes in various cancers, particularly breast and colorectal cancer [
23,
24]. In breast cancer, diminished VGLL4 levels correlate with shorter relapse-free and disease-specific survival, highlighting its role as an independent prognostic factor [
24]. This aligns with studies demonstrating that VGLL4 overexpression inhibits cancer cell proliferation and migration, suggesting its function as a tumor suppressor [
23,
24].
Similarly, in colorectal cancer, reduced VGLL4 expression is linked to worse survival and inversely associated with Wnt/β-catenin target gene expression. Lower levels of VGLL4 also correlate with adverse clinical parameters such as tumor size and lymph node metastasis. Our results here using CRC samples partially confirmed these data. A decrease in VGLL4-positive cells was detected in the lamina propria in later stages of CRC at Dukes C and D. However, the results differed in the epithelium—no VGLL4-positive cells could be detected in healthy and early-stage CRC Dukes A. One can argue that in early tumor stages under normal conditions, VGLL4 activity is not needed or is limited; however, with the tumor progression beyond mucosa, as in cancers of the Dukes B stage, it is upregulated and serves as a tumor suppressor. Our findings reinforce VGLL4’s importance in CRC progression and indicate a need for further investigation into its mechanistic role as a tumor suppressor, especially in CRC cancers.
The third protein investigated in this study was WNT4, a protein likely to be involved in the progression of CRC, and its expression might serve as an important progression indicator. Activators of the Wnt pathway, such as WNT4, can be classified as tumor promoters [
25]. Canonical Wnt signaling, also known as Wnt/β-catenin, relies on nuclear translocation of β-catenin and activation of downstream genes regulating cell proliferation. Generally, turnover of β-catenin is regulated by phosphorylation-dependent ubiquitination by a complex formed by glycogen synthase kinase 3β (GSK3β), casein kinase I (CK I), Axin, and adenomatous polyposis (APC). Once the Wnt ligand binds to its core receptor (LRP5/6 and frizzled proteins), it disrupts the GSK3β/CK I/Axin/APC complex by recruiting activated cytosolic Dvl protein. This leads to β-catenin cytosolic accumulation and, finally, translocation to the nucleus. There, β-catenin works with other transcription factors to activate Wnt target genes [
26]. The WNT4 protein activates canonical Wnt signaling and epithelial–mesenchymal transition (EMT) in CRC. It is found in elevated levels in the serum of CRC patients [
15]. Results in the study here showed the upregulated expression of WNT4 in the epithelium of CRC samples at Dukes A–D. However, the percentage of WNT-positive cells in the lamina propria was seen to increase from the early-stage to late-stage Dukes A to Dukes D. This could be a manifestation of epithelial–mesenchymal transition (EMT) initiation, which happens in more advanced stages of CRC, Dukes C and D. This represents excellent pilot data that could be considered in future studies focused on the evaluation of WNT4’s role in CRC.
Microsatellite instability (MSI) represents a significant form of genetic hypermutability stemming from deficiencies in the DNA mismatch repair (MMR) system. This phenomenon is particularly relevant in colorectal cancer (CRC), where MSI is detected in approximately 15–20% of cases, predominantly as a result of sporadic hypermethylation of the MLH1 promoter or associated with Lynch syndrome due to germline mutations in MMR genes such as MLH1, MSH2, MSH6, and PMS [
27,
28]. The presence of MSI in tumors is characterized by distinct molecular features, including a higher tumor mutational burden and an increased neoantigen load, which correlate with enhanced immune responses [
29,
30]. Clinically, MSI-unstable tumors are often associated with better prognoses and improved responses to immune checkpoint inhibitors compared to their microsatellite-stable (MSS) counterparts [
31]. As such, MSI status serves as a critical biomarker for diagnosis, prognosis, and therapeutic decision-making in CRC management, highlighting its essential role in tailoring patient-specific treatment strategies [
28,
32].
In this study, we conducted a comprehensive analysis of microsatellite instability (MSI) in colorectal cancer (COADREAD), utilizing data sourced from the XENA database. Our analysis included a cohort of 108 patients, among which 98 were classified as MSI-stable and 10 as MSI-unstable. Gene expression profiling was performed to identify potential biomarkers associated with MSI status. Notably, AIFM3 exhibited a statistically significant difference in expression levels between the MSI-stable and MSI-unstable groups, suggesting its potential utility as a biomarker for MSI in colorectal cancer. In contrast, VGLL4 and WNT4 did not show significant differences in expression between the two groups, indicating that these genes may not serve as reliable indicators of MSI status in this context. These findings contribute to the understanding of the molecular underpinnings of colorectal cancer and highlight AIFM3 as a candidate biomarker warranting further investigation.
The primary limitation of our study is the relatively small sample size (N = 43), which may impact the generalizability of the findings. A larger cohort would provide more robust statistical power and strengthen the validity of the conclusions. Additionally, while this study focused on specific proteins, further exploring other proteins and genes within the Wnt signaling pathway could offer a more comprehensive understanding of their roles in CRC. These limitations highlight areas for future research, which we aim to pursue as part of a broader investigation.
5. Conclusions
This study provides novel findings highlighting the expression levels of three important apoptotic and growth signaling proteins, AIFM3, VGLL4, and WNT4, involved in different clinically relevant stages of progression in patients with colorectal cancer (CRC). Data also provide an excellent basis for future, more in-depth studies on molecular mechanisms in CRC associated with the expression and function of these proteins and related signaling pathways.
The key findings involve a significant reduction in AIFM3-positive cells in the lamina propria, especially in advanced CRC stages (Dukes B–D), contrasting with increased AIFM3 levels in breast cancer and cholangiocarcinoma. Furthermore, AIFM3 showed a statistically significant difference in expression between MSI-stable and MSI-unstable colorectal cancer, highlighting its potential as a biomarker for MSI status. VGLL4, a known prognostic marker and tumor suppressor, showed decreased expression in the lamina propria during late CRC stages, suggesting that its downregulation correlates with the tumor progression beyond mucosa. Conversely, WNT4, a Wnt pathway activator, exhibited increasing expression in both the epithelium and lamina propria across CRC stages, consistent with its role in epithelial–mesenchymal transition (ETM) in advanced disease.
Therefore, this study highlights the clinical potential of AIFM3, VGLL4, and WNT4 in colorectal cancer (CRC) progression. VGLL4 emerges as a promising prognostic biomarker, with its decreased expression correlating with advanced disease stages. Similarly, the upregulation of WNT4, a key mediator of epithelial–mesenchymal transition, suggests its role in promoting metastasis and its potential as a therapeutic target, while the reduction of AIFM3-positive cells in CRC compared to other cancers highlights its potential role in restoring apoptotic pathways. The distinct expression patterns of these proteins in the tumor epithelium and the surrounding microenvironment underscore their importance in shaping tumor progression and providing a foundation for personalized diagnostic and therapeutic strategies. Future studies will focus on elucidating the mechanisms underlying these findings and validating their clinical utility in CRC management.