Current Evidence on the Relation Between Microbiota and Oral Cancer—The Role of Fusobacterium nucleatum—A Narrative Review
Simple Summary
Abstract
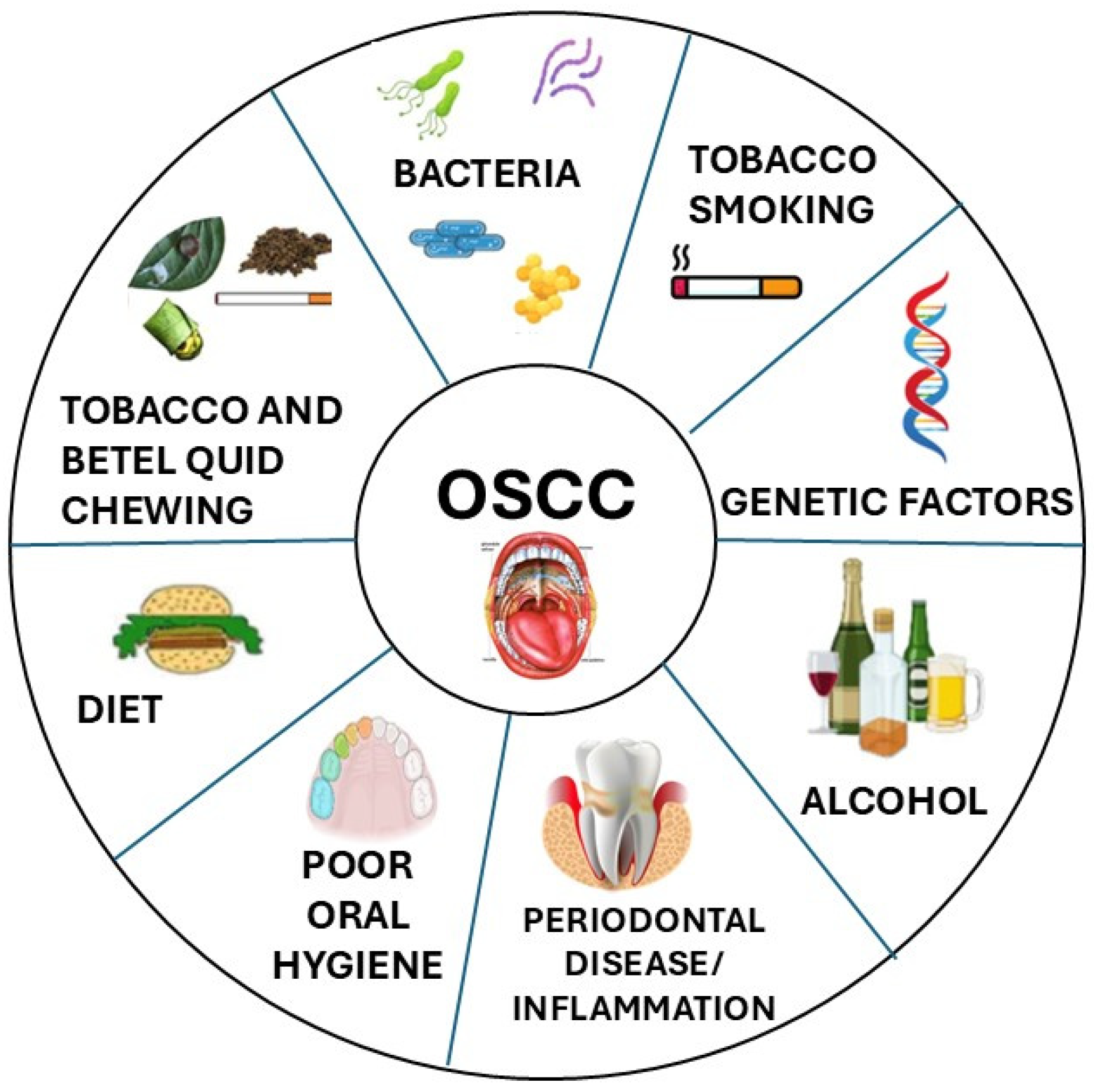
1. Introduction—Epidemiology and Clinical Aspects
2. The Oral Microbiota
2.1. Fusobacterium Nucleatum and Cancer
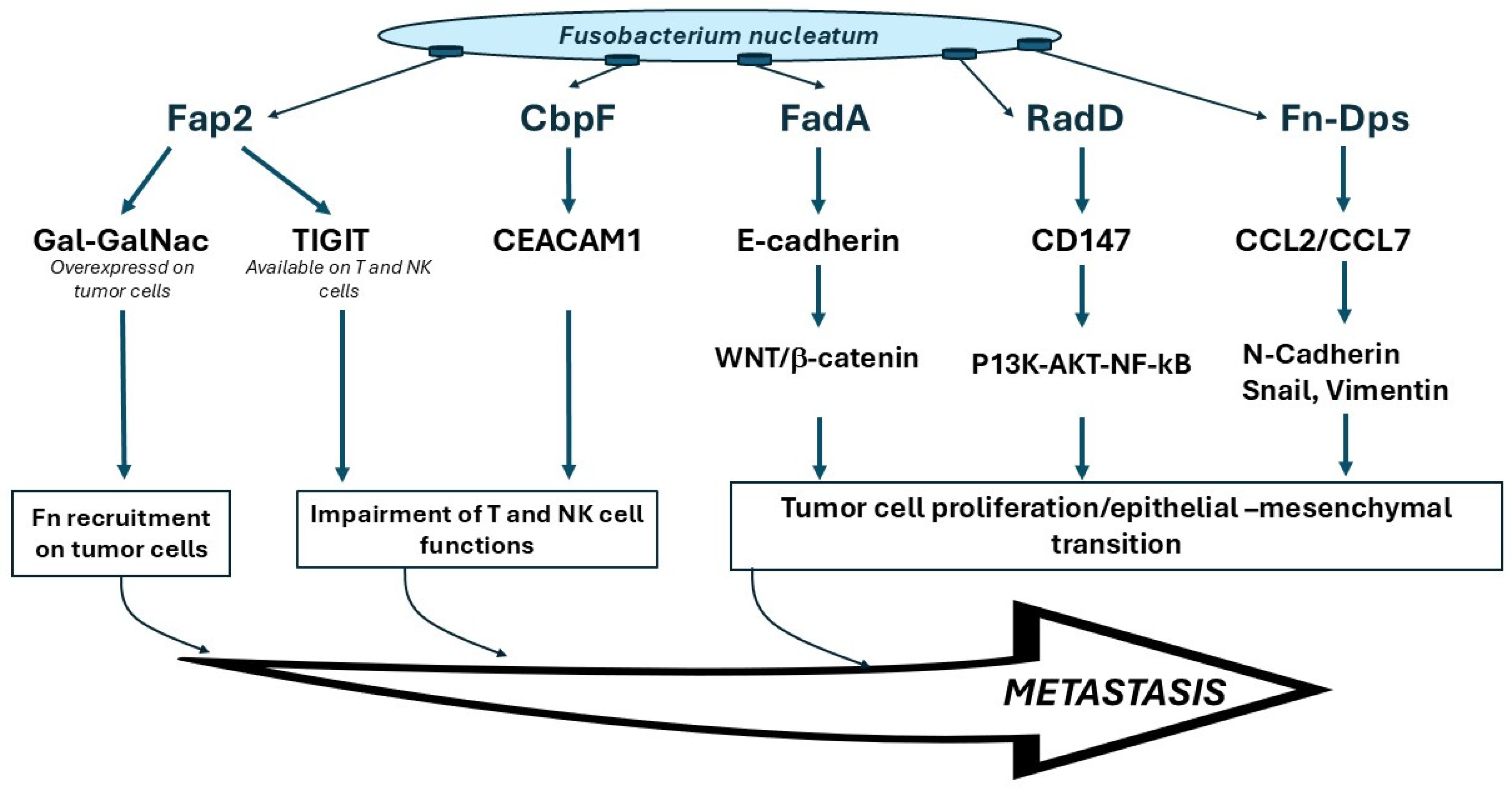
2.2. F. nucleatum and OSCC
3. Research Questions for Translational Research on Fn and OSCC
3.1. Where Does the Association Between Fn and OSCC Come from? Is Fn a Cause or a Consequence of Oral Carcinogenesis?
3.2. Are the Fn Subspecies or Clades in OSCC the Same as Those Promoting CRC?
3.3. What Is the Consequent Clinical Meaning of Fn in OSCC? Different Translational Perspectives Can Be Hypothesized
- Fn is a classical initiating or promoting agent involved in the early progression from dysplasia to cancer/transformation. In this case, the microbiota would become a target for prevention, and modifications of the microbiota in high-risk subjects could reduce the OSCC risk. In this case, Fn should already be abundant in preneoplastic lesions.
- The microbiota is modified by the presence of cancer, and Fn abundance is a consequence of a tumor’s impact on the oral environment and saliva (deriving from necrosis and cellular lysis, tumor metabolism, etc.). In this case, the Fn load would be proportional to the tumor bulk and increase in bulkier, more locally advanced OSCC.
- The microbiota is a direct disease modifier. Independently from its role as a potential risk factor, it could drive carcinogenic progression toward specific genotypes and therefore clinical phenotypes. In this case, characterization of the genetic alterations in different OSCCs and their association with Fn may help to dissect the role of Fn in tumor growth and metastasis [81].
- The microbiota influences the immune response, and consequently tumor–host interaction and balance, and this would explain the specific impact described on nodal involvement progression and recurrence, as an effective immune response against tumor could reduce the nodal spread.
3.4. What Is the Prognostic Value of Fn in OSCC?
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Patel, S.G.; Singh, B. Jatin Shah’s Head and Neck Surgery and Oncology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Miranda-Galvis, M.; Loveless, R.; Kowalski, L.P.; Teng, Y. Impacts of Environmental Factors on Head and Neck Cancer Pathogenesis and Progression. Cells 2021, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Licitra, L.; Bernier, J.; Cvitkovic, E.; Grandi, C.; Spinazze, S.; Bruzzi, P.; Gatta, G.; Molinari, R. Cancer of the nasopharynx. Crit. Rev. Oncol. Hematol. 2003, 45, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Human Papillomaviruses; World Health Organization: Lyon, France, 2007.
- AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2016.
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Bussu, F.; Muresu, N.; Crescio, C.; Gallus, R.; Rizzo, D.; Cossu, A.; Sechi, I.; Fedeli, M.; Cossu, A.; Delogu, G.; et al. Low Prevalence of HPV Related Oropharyngeal Carcinogenesis in Northern Sardinia. Cancers 2022, 14, 4205. [Google Scholar] [CrossRef]
- Gallus, R.; Nauta, I.H.; Marklund, L.; Rizzo, D.; Crescio, C.; Mureddu, L.; Tropiano, P.; Delogu, G.; Bussu, F. Accuracy of p16 IHC in Classifying HPV-Driven OPSCC in Different Populations. Cancers 2023, 15, 656. [Google Scholar] [CrossRef]
- Gallus, R.; Gheit, T.; Holzinger, D.; Petrillo, M.; Rizzo, D.; Petrone, G.; Micciche, F.; Mattiucci, G.C.; Arciuolo, D.; Capobianco, G.; et al. Prevalence of HPV Infection and p16(INK4a) Overexpression in Surgically Treated Laryngeal Squamous Cell Carcinoma. Vaccines 2022, 10, 204. [Google Scholar] [CrossRef]
- Nauta, I.H.; Heideman, D.A.M.; Brink, A.; van der Steen, B.; Bloemena, E.; Koljenovic, S.; de Jong, R.J.B.; Leemans, C.R.; Brakenhoff, R.H. The unveiled reality of human papillomavirus as risk factor for oral cavity squamous cell carcinoma. Int. J. Cancer 2021, 149, 420–430. [Google Scholar] [CrossRef]
- Bussu, F.; Sali, M.; Gallus, R.; Vellone, V.G.; Zannoni, G.F.; Autorino, R.; Dinapoli, N.; Santangelo, R.; Martucci, R.; Graziani, C.; et al. HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker? Br. J. Cancer 2013, 108, 1157–1162. [Google Scholar] [CrossRef]
- McIlvanna, E.; Linden, G.J.; Craig, S.G.; Lundy, F.T.; James, J.A. Fusobacterium nucleatum and oral cancer: A critical review. BMC Cancer 2021, 21, 1212. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Marchais, M.; Vacher, S.; Hilmi, M.; Schnitzler, A.; Meseure, D.; Leclere, R.; Lecerf, C.; Dubot, C.; Jeannot, E.; et al. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci. Rep. 2021, 11, 7870. [Google Scholar] [CrossRef] [PubMed]
- Gallimidi, A.B.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Li, Y.H.; Tian, X. Quorum sensing and bacterial social interactions in biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef]
- Lazarevic, V.; Whiteson, K.; Hernandez, D.; Francois, P.; Schrenzel, J. Study of inter- and intra-individual variations in the salivary microbiota. BMC Genom. 2010, 11, 523. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Ganly, I.; Yang, L.; Giese, R.A.; Hao, Y.; Nossa, C.W.; Morris, L.G.T.; Rosenthal, M.; Migliacci, J.; Kelly, D.; Tseng, W.; et al. Periodontal pathogens are a risk factor of oral cavity squamous cell carcinoma, independent of tobacco and alcohol and human papillomavirus. Int. J. Cancer 2019, 145, 775–784. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Seneviratne, C.J.; Jayasinghe, R.D.; Vo, P.T.; Bostanci, N.; Choi, Y. Bacteriome and mycobiome dysbiosis in oral mucosal dysplasia and oral cancer. Periodontology 2000 2024, 96, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Rangel, R.; Pickering, C.R.; Sikora, A.G.; Spiotto, M.T. Genetic Changes Driving Immunosuppressive Microenvironments in Oral Premalignancy. Front. Immunol. 2022, 13, 840923. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Udaltsova, N.; Engels, E.A.; Katzel, J.A.; Yanik, E.L.; Katki, H.A.; Lingen, M.W.; Silverberg, M.J. Oral Leukoplakia and Risk of Progression to Oral Cancer: A Population-Based Cohort Study. J. Natl. Cancer Inst. 2020, 112, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Frustino, J.; Villa, A.; Nguyen, B.C.; Woo, S.B.; Johnson, W.E.; Varelas, X.; Kukuruzinska, M.; Monti, S. Total RNA sequencing reveals gene expression and microbial alterations shared by oral pre-malignant lesions and cancer. Hum. Genom. 2023, 17, 72. [Google Scholar] [CrossRef]
- Unlu, O.; Demirci, M.; Paksoy, T.; Eden, A.B.; Tansuker, H.D.; Dalmizrak, A.; Aktan, C.; Senel, F.; Sunter, A.V.; Yigit, O.; et al. Oral microbial dysbiosis in patients with oral cavity cancers. Clin. Oral Investig. 2024, 28, 377. [Google Scholar] [CrossRef]
- Saikia, P.J.; Pathak, L.; Mitra, S.; Das, B. The emerging role of oral microbiota in oral cancer initiation, progression and stemness. Front. Immunol. 2023, 14, 1198269. [Google Scholar] [CrossRef]
- Morales-Sanchez, A.; Fuentes-Panana, E.M. Human viruses and cancer. Viruses 2014, 6, 4047–4079. [Google Scholar] [CrossRef]
- Lax, A.J. Opinion: Bacterial toxins and cancer—A case to answer? Nat. Rev. Microbiol. 2005, 3, 343–349. [Google Scholar] [CrossRef]
- Megraud, F.; Bessede, E.; Varon, C. Helicobacter pylori infection and gastric carcinoma. Clin. Microbiol. Infect. 2015, 21, 984–990. [Google Scholar] [CrossRef]
- Hatakeyama, M. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 196–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Zhang, R.G.; Duan, G.C. Pathogenic mechanisms of the oncoprotein CagA in H. pylori-induced gastric cancer (Review). Oncol. Rep. 2016, 36, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Jungas, T.; Verbeke, P.; Ojcius, D.M. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect. Immun. 2004, 72, 3743–3751. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Jermanus, C.; Barbetta, B.; Choi, C.; Verbeke, P.; Ojcius, D.M.; Yilmaz, O. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral Microbiol. 2010, 25, 89–101. [Google Scholar] [CrossRef]
- Laliani, G.; Sorboni, S.G.; Lari, R.; Yaghoubi, A.; Soleimanpour, S.; Khazaei, M.; Hasanian, S.M.; Avan, A. Bacteria and cancer: Different sides of the same coin. Life Sci. 2020, 246, 117398. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Vogelmann, R.; Amieva, M.R. The role of bacterial pathogens in cancer. Curr. Opin. Microbiol. 2007, 10, 76–81. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Tang, Z.; Huang, Y.; Huang, M.; Liu, H.; Ziebolz, D.; Schmalz, G.; Jia, B.; Zhao, J. More Than Just a Periodontal Pathogen—The Research Progress on Fusobacterium nucleatum. Front. Cell. Infect. Microbiol. 2022, 12, 815318. [Google Scholar] [CrossRef]
- McGuire, A.M.; Cochrane, K.; Griggs, A.D.; Haas, B.J.; Abeel, T.; Zeng, Q.; Nice, J.B.; MacDonald, H.; Birren, B.W.; Berger, B.W.; et al. Evolution of invasion in a diverse set of Fusobacterium species. mBio 2014, 5, e01864. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, T.; Zhou, J.; Zhi, M.; Shen, S.; Wang, Y.; Gu, X.; Li, Z.; Gao, H.; Wang, P.; et al. Pangenomic Study of Fusobacterium nucleatum Reveals the Distribution of Pathogenic Genes and Functional Clusters at the Subspecies and Strain Levels. Microbiol. Spectr. 2023, 11, e0518422. [Google Scholar] [CrossRef] [PubMed]
- Kook, J.K.; Park, S.N.; Lim, Y.K.; Cho, E.; Jo, E.; Roh, H.; Shin, Y.; Paek, J.; Kim, H.S.; Kim, H.; et al. Genome-Based Reclassification of Fusobacterium nucleatum Subspecies at the Species Level. Curr. Microbiol. 2017, 74, 1137–1147. [Google Scholar] [CrossRef]
- Krieger, M.; AbdelRahman, Y.M.; Choi, D.; Palmer, E.A.; Yoo, A.; McGuire, S.; Kreth, J.; Merritt, J. Stratification of Fusobacterium nucleatum by local health status in the oral cavity defines its subspecies disease association. Cell Host Microbe 2024, 32, 479–488.e4. [Google Scholar] [CrossRef]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Miguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Wu, N.; Yang, X.; Zhang, R.; Li, J.; Xiao, X.; Hu, Y.; Chen, Y.; Yang, F.; Lu, N.; Wang, Z.; et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb. Ecol. 2013, 66, 462–470. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Suehiro, Y.; Hashimoto, S.; Hoshida, T.; Fujimoto, M.; Watanabe, M.; Imanaga, D.; Sakai, K.; Matsumoto, T.; Nishioka, M.; et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J. Gastroenterol. 2018, 53, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Emgard, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Fardini, Y.; Wang, X.; Temoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Baik, J.E.; Lagana, S.M.; Han, R.P.; Raab, W.J.; Sahoo, D.; Dalerba, P.; Wang, T.C.; Han, Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/beta-catenin modulator Annexin A1. EMBO Rep. 2019, 20, e47638. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, S.; Chen, F.; Li, Y.; Huang, Y.; Liu, W.; Zhang, G. Fn-Dps, a novel virulence factor of Fusobacterium nucleatum, disrupts erythrocytes and promotes metastasis in colorectal cancer. PLoS Pathog. 2023, 19, e1011096. [Google Scholar] [CrossRef]
- Brewer, M.L.; Dymock, D.; Brady, R.L.; Singer, B.B.; Virji, M.; Hill, D.J. Fusobacterium spp. target human CEACAM1 via the trimeric autotransporter adhesin CbpF. J. Oral Microbiol. 2019, 11, 1565043. [Google Scholar] [CrossRef]
- Casasanta, M.A.; Yoo, C.C.; Udayasuryan, B.; Sanders, B.E.; Umana, A.; Zhang, Y.; Peng, H.; Duncan, A.J.; Wang, Y.; Li, L.; et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci. Signal. 2020, 13, eaba9157. [Google Scholar] [CrossRef]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef]
- Abed, J.; Maalouf, N.; Manson, A.L.; Earl, A.M.; Parhi, L.; Emgard, J.E.M.; Klutstein, M.; Tayeb, S.; Almogy, G.; Atlan, K.A.; et al. Colon Cancer-Associated Fusobacterium nucleatum May Originate from the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front. Cell. Infect. Microbiol. 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Ji, X.; Li, Y.; Estilo, C.; Yegnanarayana, R.; Singh, B.; Li, X.; Saxena, D. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Al-Hebshi, N.N.; Nasher, A.T.; Maryoud, M.Y.; Homeida, H.E.; Chen, T.; Idris, A.M.; Johnson, N.W. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017, 7, 11773. [Google Scholar] [CrossRef]
- Yost, S.; Stashenko, P.; Choi, Y.; Kukuruzinska, M.; Genco, C.A.; Salama, A.; Weinberg, E.O.; Kramer, C.D.; Frias-Lopez, J. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int. J. Oral Sci. 2018, 10, 32. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell. Infect. Microbiol. 2019, 9, 476. [Google Scholar] [CrossRef]
- Su, S.C.; Chang, L.C.; Huang, H.D.; Peng, C.Y.; Chuang, C.Y.; Chen, Y.T.; Lu, M.Y.; Chiu, Y.W.; Chen, P.Y.; Yang, S.F. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis 2021, 42, 127–135. [Google Scholar] [CrossRef]
- Nie, F.; Wang, L.; Huang, Y.; Yang, P.; Gong, P.; Feng, Q.; Yang, C. Characteristics of Microbial Distribution in Different Oral Niches of Oral Squamous Cell Carcinoma. Front. Cell. Infect. Microbiol. 2022, 12, 905653. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Huang, X.; Wang, Q.; Fu, R.; Wen, X.; Liu, J.; Zhang, L.F. Nucleatum enhances oral squamous cell carcinoma proliferation via E-cadherin/beta-Catenin pathway. BMC Oral Health 2024, 24, 518. [Google Scholar]
- Chen, Z.; Wong, P.Y.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.W.; Cai, L.; Lei, P.; Mou, Q.; Wong, S.H.; et al. The Intersection between Oral Microbiota, Host Gene Methylation and Patient Outcomes in Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 3425. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Dharavath, B.; Manavalan, S.; Rane, A.; Redhu, A.K.; Sunder, R.; Butle, A.; Mishra, R.; Joshi, A.; Togar, T.; et al. Fusobacterium nucleatum is associated with inflammation and poor survival in early-stage HPV-negative tongue cancer. NAR Cancer 2022, 4, zcac006. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fang, J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Borozan, I.; Zaidi, S.H.; Harrison, T.A.; Phipps, A.I.; Zheng, J.; Lee, S.; Trinh, Q.M.; Steinfelder, R.S.; Adams, J.; Banbury, B.L.; et al. Molecular and Pathology Features of Colorectal Tumors and Patient Outcomes Are Associated with Fusobacterium nucleatum and Its Subspecies animalis. Cancer Epidemiol. Biomark. Prev. 2022, 31, 210–220. [Google Scholar] [CrossRef]
- Crowley, C.; Selvaraj, A.; Hariharan, A.; Healy, C.M.; Moran, G.P. Fusobacterium nucleatum subsp. polymorphum recovered from malignant and potentially malignant oral disease exhibit heterogeneity in adhesion phenotypes and adhesin gene copy number, shaped by inter-subspecies horizontal gene transfer and recombination-derived mosaicism. Microb. Genom. 2024, 10, 001217. [Google Scholar]
- Bi, D.; Zhu, Y.; Gao, Y.; Li, H.; Zhu, X.; Wei, R.; Xie, R.; Wei, Q.; Qin, H. A newly developed PCR-based method revealed distinct Fusobacterium nucleatum subspecies infection patterns in colorectal cancer. Microb. Biotechnol. 2021, 14, 2176–2186. [Google Scholar] [CrossRef]
- Hara, Y.; Baba, Y.; Oda, E.; Harada, K.; Yamashita, K.; Toihata, T.; Kosumi, K.; Iwatsuki, M.; Miyamoto, Y.; Tsutsuki, H.; et al. Presence of Fusobacterium nucleatum in relation to patient survival and an acidic environment in oesophagogastric junction and gastric cancers. Br. J. Cancer 2024, 131, 797–807. [Google Scholar] [CrossRef]
- Hasegawa, T.; Ouchi, T.; Shibukawa, Y.; Asoda, S.; Nakagawa, T. Etiology of Oral Potentially Malignant Disorders and Squamous Cell Carcinoma Based on Cellular Stress Regulation and Matrix Stiffness. Front. Biosci. 2023, 28, 265. [Google Scholar] [CrossRef]
| Reference | Role | Major Findings |
|---|---|---|
| [66] | OSCC carcinogenesis | Fn was the most significantly abundant genus in the OSCC samples |
| [70,71] | OSCC carcinogenesis | A greater abundance of Fn in OSCC lesions compared to contralateral normal tissue |
| [16] | OSCC prognosis | The presence of Fn was associated with improved overall survival, relapse-free survival and metastasis-free survival in their merged OSCC cohort |
| [74] | OSCC prognosis | Fn enrichment in HNSCC tumor tissues correlated with better cancer-specific survival and a lower relapse rate |
| [75] | OSCC prognosis | The presence of Fn in OSCC correlated with enhanced spread of cancers cells, tissue invasion and metastatic potential that inevitably correlated with poorer survival in early-stage HPV-negative tongue cancers, clearly indicating a worsened prognosis in patients with Fn |
| [78] | OSCC carcinogenesis | Fn subsp. polymorphum is most abundant in malignant oral mucosa, and strains with high copy number of TVSS adhesin-encoding genes show highest adhesion to oral keratinocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiscuzzu, F.; Crescio, C.; Varrucciu, S.; Rizzo, D.; Sali, M.; Delogu, G.; Bussu, F. Current Evidence on the Relation Between Microbiota and Oral Cancer—The Role of Fusobacterium nucleatum—A Narrative Review. Cancers 2025, 17, 171. https://doi.org/10.3390/cancers17020171
Chiscuzzu F, Crescio C, Varrucciu S, Rizzo D, Sali M, Delogu G, Bussu F. Current Evidence on the Relation Between Microbiota and Oral Cancer—The Role of Fusobacterium nucleatum—A Narrative Review. Cancers. 2025; 17(2):171. https://doi.org/10.3390/cancers17020171
Chicago/Turabian StyleChiscuzzu, Federica, Claudia Crescio, Simona Varrucciu, Davide Rizzo, Michela Sali, Giovanni Delogu, and Francesco Bussu. 2025. "Current Evidence on the Relation Between Microbiota and Oral Cancer—The Role of Fusobacterium nucleatum—A Narrative Review" Cancers 17, no. 2: 171. https://doi.org/10.3390/cancers17020171
APA StyleChiscuzzu, F., Crescio, C., Varrucciu, S., Rizzo, D., Sali, M., Delogu, G., & Bussu, F. (2025). Current Evidence on the Relation Between Microbiota and Oral Cancer—The Role of Fusobacterium nucleatum—A Narrative Review. Cancers, 17(2), 171. https://doi.org/10.3390/cancers17020171







