Simple Summary
Non-restorative low anterior resection (NRLAR) may result in inferior oncological outcomes compared to restorative low anterior resection (RLAR) and abdominoperineal resection (APR) for the surgical treatment of rectal cancer. This study aimed to retrospectively evaluate the intermediate-term oncological outcomes of patients who underwent RLAR, NRLAR, or APR for primary rectal cancer. This analysis included all elective NRLAR, RLAR, and APR procedures for primary rectal carcinoma performed across 11 Dutch centers from 2013 to 2020. Multivariate Cox regression analyses confirmed NRLAR as an independent predictor for poorer DFS (HR 1.34; 95% CI: 1.01–1.80; p = 0.046), OS (HR 1.57; 95% CI: 1.04–2.36, p = 0.032), and higher LR risk (HR 2.66; 95% CI: 1.53–4.65; p <= 0.001). Therefore, NRLAR should be avoided when technically feasible alternatives exist.
Abstract
Background: Non-restorative low anterior resection (NRLAR) may result in inferior oncological outcomes compared to restorative low anterior resection (RLAR) and abdominoperineal resection (APR). While NRLAR is often performed when poor functional or technical challenges are anticipated, comprehensive data on its oncological outcomes remain scarce. This study aimed to retrospectively evaluate the intermediate-term oncological outcomes of patients—who underwent RLAR, NRLAR, or APR for primary rectal cancer. Methods: This analysis included all elective NRLAR, RLAR, and APR procedures for primary rectal carcinoma performed across 11 Dutch centers from 2013 to 2020. The primary outcome was 3-year disease-free survival (DFS). Secondary outcomes included 3-year overall survival (OS) and 3-year local recurrence (LR). KaplanMeier survival analysis with log-rank testing and multivariate Cox regression analysis were employed. Results: A total of 253 (12.5%) patients underwent NRLAR, 1109 (55.0%) RLAR, and 656 (32.5%) APR. NRLAR was associated with a lower 3-year DFS (71.4%) versus RLAR (82.0%) and APR (77.4%) (p = 0.003). The 3-year OS was lower for NRLAR (82.9%) versus RLAR (93.5%) and APR (90.2%) (p < 0.001), with a higher 3-year LR rate for NRLAR (8.1%) versus RLAR (3.3%) and APR (4.5%) (p = 0.003). Multivariate Cox regression analyses confirmed NRLAR as an independent predictor for poorer DFS (HR 1.34; 95% CI: 1.01–1.80; p = 0.046), OS (HR 1.57; 95% CI: 1.04–2.36, p = 0.032), and higher LR risk (HR 2.66; 95% CI: 1.53–4.65; p <= 0.001). Conclusions: NRLAR is associated with poorer intermediate-term oncological outcomes. When technically feasible, restorative options should be considered, and prospective studies are required to further investigate causal relationships.
1. Introduction
The cornerstone of surgical intervention in rectal cancer is radical surgery according to the total mesorectal excision (TME) principle [1,2]. While a restorative low anterior resection (RLAR) remains the most common procedure, sphincter preservation may be unattainable for either oncological or functional reasons. In these patients, an abdominoperineal resection (APR) is required [3]. A possible third approach constitutes a non-restorative low anterior resection; this involves cross-stapling of the rectal stump and constructing an end colostomy, commonly called an ultra-low Hartmann’s procedure [4].
In randomized controlled trials, NRLAR constitutes less than 5% of cases [5,6]. In the real world, in unselected patient populations—especially in northern Europe—NRLAR’s incidence can surge to as high as 25% of all low anterior resections [7,8,9,10]. Although sphincter preservation was feasible with acceptable oncological margins, the rationale for non-restorative surgery is usually unspecified and remains ill-defined. Unplanned NRLAR may unfold due to technical challenges encountered during pelvic dissection, such as a narrow pelvis, obesity, bulky tumors, or intricate anatomical configurations, especially in male patients [11,12].
While existing studies suggest an association between NRLAR and inferior oncological outcomes compared to APR and restorative surgery, the bulk of this evidence predates the advent of laparoscopic TME (L-TME), robot-assisted TME (R-TME), and transanal TME (TaTME) [13,14,15]. Moreover, studies that incorporate these techniques rely on relatively older data and often are hampered by limited sample size [15,16].
Therefore, the primary objective of this study is to elucidate the three-year oncological outcomes among a large cohort of rectal cancer patients undergoing NRLAR, RLAR, and APR within dedicated laparoscopic, robot-assisted, and transanal centers.
2. Materials and Methods
2.1. Study Design
This study was designed as a retrospective multicenter cohort analysis conducted by the Minimally Invasive Rectal Cancer Taskforce (MIRECA). The objective was to compare intermediate-term oncological outcomes after NRLAR, RLAR, and APR. The cohort has been previously described [17].
2.2. Study Population
All consecutive patients who underwent oncological rectal resection for MRI-defined primary rectal adenocarcinoma positioned at a distance of ≤12 cm of the anorectal junction (ARJ) between 1 January 2015, and 31 December 2020, were identified from eleven centers in the national Dutch ColoRectal Audit (DCRA) database. The DCRA is mandatory nationwide registry with prospective data collection and independent quality control. Each participating hospital was considered a dedicated high-volume unit, performing a minimum of 40 TME procedures annually [18,19].
Patients were excluded if they (1) underwent local excision only, (2) had prior transanal endoscopic microsurgery (TEM) before TME, (3) presented with metastatic disease (cM1), or (4) underwent surgery in a palliative or emergency context. The study protocol and reporting adhered to the STROBE guideline [20].
2.3. Outcomes and Definitions
The primary endpoint was 3-year disease-free survival (DFS). Secondary endpoints included 3-year overall survival (OS), 3-year local recurrence (LR) rate, and 3-year systemic recurrence (SR). Resection type was defined according to the procedure carried out during the index TME. Detailed definitions of all outcomes are provided in Supplementary File S1.
2.4. Treatment Approach and Follow-Up
All cases were evaluated by a multidisciplinary tumor board at the local institution. Treatment strategies, including neoadjuvant therapy, were decided in accordance with National guidelines [21]. Surgical resection consisted of a full TME, performed as either NRLAR, LAR, or APR depending on tumor location and patient-related factors. Postoperative follow-up was carried out according to national recommendations.
2.5. Statistical Analysis
Comparisons between groups were stratified by type of resection (NRLAR, RLAR, and APR). Categorical variables were tested with Fisher’s exact test. Continuous variables were compared with the Wilcoxon rank-sum test if not normally distributed and with Student’s t-test otherwise. Three-year oncological outcomes were estimated with Kaplan–Meier methods and compared using the log-rank test.
Multivariable Cox regression with backward selection (significance level 0.05) was applied to explore associations between resection type and oncological outcome (DFS, OS, LR, and SR). Variables were selected based on literature evidence and included age, sex, body mass index, ASA classification, distance of the inferior border of the tumor to the ARJ, neoadjuvant therapy, pathological TNM classification, CRM involvement, and the minimally invasive TME technique. Variance inflation factors (VIF) were calculated to check for multicollinearity. Multiple imputation was performed when data were missing at random. Statistical significance was defined as p < 0.05. All analyses were conducted in ‘R’ version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Baseline Characteristics
The MIRECA dataset documented 3049 TME procedures. Following the exclusion of all ineligible patients, 2018 patients were included in the analysis. Of these, 1109 (55.0%) underwent RLAR, 656 (32.5%) underwent APR, and 253 (12.5%) underwent NRLAR.
Patients in the NRLAR group were notably older (mean age 75.48 years (standard deviation (SD): 9.72), compared to 65.54 (SD: 9.74) in the RLAR group and 69.34 (SD: 11.03) in the APR group, respectively, p < 0.001). They also more frequently presented as ASA III classification (34.4%, compared to 14.0% in RLAR and 23.5% in APR, p < 0.001), had a higher incidence of previous abdominal surgery (37.2%, compared to 24.5% in RLAR and 29.7% in APR, p < 0.001), exhibited EMVI exceeding 5 mm on MRI more frequently (22.9%, compared to 13.9% in RLAR and 17.2% in APR, p < 0.001), and had a higher prevalence of cT3-stage (67.2%, compared to 57.2% in RLAR and 55.0% in APR, p < 0.001) (Table 1).

Table 1.
Patient and tumor characteristics.
In the APR group, patients had a shorter distance of the inferior border of the tumor to the ARJ on MRI (1.50 cm [0.0, 3.2], versus 7.00 [5.0, 9.0] and 6.00 [3.5, 8.0] in RLAR and NRLAR, respectively, p < 0.001). They also more frequently displayed MRF involvement (39.3%, versus 19.4%, and 30.4% in RLAR and NRLAR, respectively, p < 0.001), had a higher incidence of cT4-stage (13.3%, versus 5.0%, and 5.5% in RLAR and NRLAR, respectively, p < 0.001), and more often received chemoradiotherapy (37.2%, versus 24.3%, and 24.3% in RLAR and NRLAR, respectively, p < 0.001).
3.2. Surgical Characteristics and Postoperative Outcomes
NRLAR was predominantly performed laparoscopically (46.6%), while RLAR and APR were more frequently performed using the robot-assisted technique (53.0% and 47.1%, respectively). Conversion rates were higher in the NRLAR group than in the RLAR and APR groups (8.3% versus 3.5% and 4.0%, respectively, p = 0.011). Intraoperative complications occurred more frequently in the APR group than in the RLAR and NRLAR groups (10.7% versus 4.2% versus 6.3%, respectively, p < 0.001) (Table 2).

Table 2.
Surgical outcomes.
3.3. Pathological Outcomes
Overall, pT-stages were higher in the NRLAR group (p = 0.005). The quality of the TME specimen was reported lowest in the APR group (p < 0.001). Pathological CRM involvement was 2.8% for RLAR, 7.3% for APR, and 4.2% for NRLAR (p <0.001). Perforations were observed in 1.0% of patients undergoing RLAR, 4.4% patients undergoing APR, and 2.0% patients undergoing NRLAR (p < 0.001) (Table 2).
3.4. Oncological Outcomes
Table 2 compares oncological outcomes. NRLAR demonstrated significantly inferior rates of 3-year DFS, 3-year OS, and 3-year LR.
The 3-year DFS rate was 82.0% after RLAR, 77.4% after APR, and 71.4% after NRLAR (log-rank: p = 0.003; Figure 1A). Multivariable Cox regression analyses demonstrated that NRLAR was independently associated with worse 3-year DFS (HR 1.34; 95% CI: 1.01–1.80; p = 0.046) (Table 3). Other independent predictors for 3-year DFS included age >80, ASA class III/IV, chemoradiation, CRM involvement, pT4 stage, and pN1–2 stage.
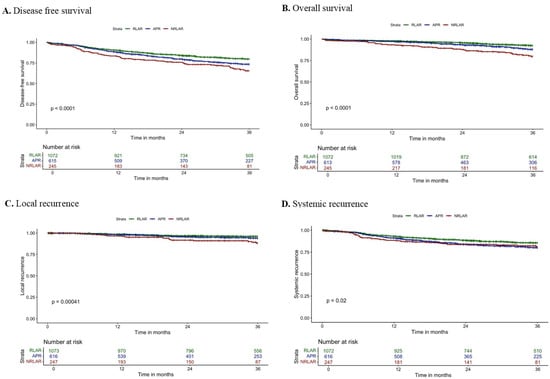
Figure 1.
Kaplan–Meier survival curves of (A) 3-year disease-free survival, (B) 3-year overall survival, (C) 3-year local recurrence, and (D) 3-year systemic recurrence.

Table 3.
Multivariable Cox regression analysis for disease-free survival and overall survival.
The 3-year OS rate was 93.5% after RLAR, 90.2% after APR, and 82.9% after NRLAR (log-rank: p < 0.001; Figure 1B). Multivariable Cox regression analyses demonstrated that NRLAR was independently associated with worse 3-year OS (HR 1.57; 95% CI: 1.04–2.36, p = 0.032) (Table 3). Other independent predictors for 3-year OS included age >70, ASA class III/IV, chemoradiation, pT4 stage, and pN1–2 stage.
The 3-year LR rate was 3.3% after RLAR, 4.5% after APR, and 8.2% after NRLAR (log-rank: p = 0.003; Figure 1C). Multivariable Cox regression analyses demonstrated that NRLAR was independently associated with a higher 3-year LR rate (HR 2.66; 95% CI: 1.53–4.65; p <= 0.001) (Table 4). Other independent predictors for 3-year LR included CRM involvement, pT4-stage, and pN1–2 stage.

Table 4.
Multivariable Cox regression analysis for local recurrence and systemic recurrence.
The 3-year SR rate was 12.5% after RLAR, 16.4% after APR, and 15.0% after NRLAR (log-rank: p = 0.077; Figure 1D). Multivariable Cox regression analyses demonstrated no independent association between resection type and 3-year LR rate (Table 4). Factors that were independently associated with 3-year SR included ASA class III/IV, radiotherapy, chemoradiation, pT4 stage, and pN1–2 stage.
4. Discussion
In this retrospective multicenter study encompassing 2018 patients diagnosed with rectal cancer across 11 dedicated centers in the Netherlands, NRLAR demonstrated inferior intermediate-term oncological outcomes compared to RLAR and APR. After correction for confounding, NRLAR emerged as an independent predictor for decreased 3-year DFS and OS, alongside higher rates of 3-year LR.
The observed 3-year DFS rates after RLAR (82.0%) and APR (77.4%) align with those reported in prominent randomized controlled trials (RCTs) comparing open TME with L-TME, such as the ALaCART, COLOR II, and ACOSOG Z6051 trials [22,23,24]. However, previous RCTs have frequently excluded NRLAR patients from comparative analyses, limiting the available literature. Two retrospective analyses conducted by Hol et al. and Roodbeen et al. did compare oncological outcomes following NRLAR [15,16]. In line with the observed 3-year DFS after NRLAR (71.4%) of the present study, Hol et al. reported a 3-year DFS rate (70.1%) and also found an independent association between NRLAR and inferior 3-year DFS [16]. Roodbeen et al. reported an even lower 3-year DFS rate for NRLAR (62.0%) but found no significant association between NRLAR and 3-year DFS in multivariate analysis [15]. This discrepancy may be attributed to factors beyond resection type, such as the relatively higher incidence of CRM involvement and elevated pT stages within their NRLAR cohort.
The 3-year OS rates observed following NRLAR (82.9%), RLAR (93.5%), and APR (90.2%) are consistent with previous studies [25,26,27]. Hol et al. and Roodbeen et al. reported similar 3-year OS rates and also demonstrated NRLAR’s independent association with inferior 3-year OS after adjusting for confounding factors [15,16]. Moreover, two older studies reporting data from 1995 to 2003 and 2006 to 2010 found lower overall OS rates across resection types. This disparity is likely due to older data and different treatment regimens at the time. However, both studies mirrored the current study’s trend, with the lowest OS rates for NRLAR [13,14].
The 3-year LR rates after RLAR (3.3%), APR (4.5%), and NRLAR (8.1%) align with previous studies reporting the long-term oncological outcomes of these resections [28,29]. Both Ortiz et al. and Hol et al. reported elevated 3-year LR rates after NRLAR and highlighted significantly higher rates of CRM positivity in the NRLAR group as a strong contributor to the higher 3-year LR rates [14,16]. In contrast to these findings, in the study by Roodbeen et al., positive CRM rates were comparable between resection types and could not explain the differences observed in LR rate and survival [15].
Regarding SR, multivariate analyses did not establish an independent association between NRLAR and higher SR rates compared to RLAR and APR. The observed 3-year SR rates (NRLAR: 15.0%, RLAR: 12.5%, APR: 16.4%) align with prior studies reporting on the long-term oncological outcomes of TME [28,30]. However, Hol et al. did find a significant association between NRLAR and SR, possibly due to a higher incidence of advanced cN status and variations in EMVI, a strong predictor for distant metastasis, which was not available in their dataset [16,31,32]. In the present multivariable analyses, neoadjuvant radiotherapy and chemoradiation were unexpectedly associated with worse SR rates. This finding likely reflects selection bias, where patients receiving neoadjuvant therapies often represent a cohort with more advanced or biologically aggressive disease, leading to higher recurrence rates despite intensive treatment. Interestingly, despite presenting with more advanced disease, patients in the NRLAR group received neoadjuvant chemoradiation at similar rates to RLAR patients and less frequently than APR patients. This likely reflects selection bias, as older and frailer patients were often considered unfit for multimodal therapy. While such undertreatment may have contributed to poorer outcomes, NRLAR remained independently associated with inferior DFS, OS, and LR after adjusting for neoadjuvant therapy, consistent with previous studies where treatment was equally administered across groups.
The independent relationship between NRLAR and DFS, OS, and LR is likely non-causal, as the mere construction of an anastomosis should not inherently influence oncological or survival outcomes, provided a proper oncological resection is performed. Given the multifactorial nature of cancer recurrence and survival, numerous factors must be considered when evaluating these outcomes after NRLAR.
NRLAR patients were inherently exposed to baseline risk factors such as advanced age, ASA III/IV classification, increased T stage, and higher EMVI prevalence on MRI, placing this group at a greater risk of recurrence or death. These factors were accounted for in the present multivariable Cox regression analysis, which demonstrated that NRLAR remained independently associated with poorer oncological outcomes after statistical correction for these confounders. While recognizing that not all potential confounding factors could be included due to the limited number of events, the results suggest that worse outcomes after NRLAR may not merely result from selective bias.
Although the dataset did not include specific information on pelvic sepsis, previous studies have suggested that this complication may contribute to the increased rates of LR observed in NRLAR patients. This phenomenon, often secondary to anastomotic leakage or rectal stump blow-out, has been associated with significant local inflammatory responses that may compromise oncological outcomes [33,34]. While anastomotic leakage in RLAR patients is often promptly detected and managed, delayed recognition and treatment of pelvic sepsis in NRLAR patients could exacerbate its impact on oncological and survival outcomes [35,36]. However, further research is needed to elucidate the specific role of pelvic sepsis in rectal cancer prognosis.
Disparities in neoadjuvant therapy administration may have influenced survival outcomes. NRLAR patients received neoadjuvant chemotherapy significantly less often than RLAR patients despite their unfavorable pathological profiles. This may be because the local multidisciplinary cancer board considered some NRLAR patients too frail for both a restorative procedure and neoadjuvant therapy, given their older age and higher ASA class. Studies in which neoadjuvant therapy was equally administered between groups observed similar associations with NRLAR, indicating that therapy differences alone do not fully explain these outcome variations [15,16]. Furthermore, neoadjuvant therapy guidelines changed drastically between the analyses performed by previous studies and the present study [2,37]. Adjuvant chemotherapy was administered in a limited number of patients (2.3% RLAR vs. 2.1% APR vs. 2.0% NRLAR), as it is not routinely provided in the Netherlands due to the uncertain oncological benefits in MRI-defined rectal tumors [38,39,40,41]. While adjuvant therapy is typically reserved for patients with unfavorable pathological risk factors, such as positive margins or high tumor grade, there was no significant difference in its administration between groups.
Other factors that warrant consideration include intraoperative technical challenges. The decision not to restore bowel continuity may have been made intraoperatively following difficult TME dissection, as indicated by the higher conversion rate for NRLAR patients. Along this line of thought, it should be considered that the ability to construct an anastomosis serves as a measure of surgical difficulty, which is potentially associated with poorer oncological and survival outcomes. Technical challenges during resection, as evidenced by the higher conversion rates in the NRLAR group, may reflect the complexity of pelvic dissection in this population. While conversions have been hypothesized to correlate with worse survival outcomes in previous literature, this association is largely attributed to incomplete resections or positive CRM involvement, both robust predictors for LR, DFS, and OS [42,43]. In this cohort, however, NRLAR demonstrated resection quality comparable to RLAR and superior to APR in terms of CRM positivity and TME specimen completeness. Therefore, while conversions in NRLAR likely reflect procedural difficulty, they are unlikely to have significantly influenced the oncological outcomes observed in this study. Strikingly, when examining all three resection types, APR reported the highest rates of incomplete and nearly complete TME specimens (8.8% and 23.0%, respectively), the highest rate of CRM involvement (7.3%) and perforation rate (4.4%). It must be acknowledged that APR is typically reserved for tumors located at or fixated to the anal sphincter or just above it. In such cases, the distal location of the tumor corresponds to a tapered mesorectum, which increases the technical complexity of achieving an R0 resection. Nevertheless, NRLAR still outperformed APR regarding oncological outcomes and survival, suggesting that the inferior outcomes after NRLAR likely stem from factors beyond TME specimen quality and CRM involvement alone.
This study is subject to several limitations that warrant acknowledgment. Importantly, the retrospective design introduces the risk of selection bias and limits the possibility of establishing causality. Despite the use of multivariable regression to account for key confounders, residual confounding cannot be ruled out. The unequal group sizes and the higher age and comorbidity burden in the NRLAR cohort reflect real-world surgical decision making, where non-restorative resection is more often performed in older and frailer patients. Although these baseline imbalances were adjusted for in the multivariable analyses, residual confounding may still be present. Although dedicated techniques were employed in expert centers specializing in L-TME, R-TME, or TaTME, variability in surgeon experience across hospitals could have influenced the decision between the types of resection. Furthermore, the present study included MRI-defined rectal tumors within 12 cm from the ARJ, as a recent study suggested that tumors between 12 and 15 cm from the ARJ might be classified as sigmoid tumors, should the novel sigmoidal take-off definition be employed [44]. Classifying tumors according to this definition was not feasible for the current study due to the inability to evaluate preoperative MRI scans in some centers. Another important limitation is that relevant confounders, such as the surgeon’s experience and intraoperative decision-making details, could not be fully accounted for. An additional key limitation of this study is the absence of functional and quality-of-life outcomes, restricting assessment of NRLAR’s benefit profile. As NRLAR aims to prevent severe functional impairments, focusing solely on oncological outcomes limits clinical applicability. Another limitation of this study is the absence of systematically documented reasons for selecting NRLAR over RLAR or APR. The choice for a non-restorative approach is often multifactorial, depending on patient frailty, intraoperative anatomy, and technical complexity, but these factors are rarely recorded in a standardized fashion. As a result, the clinical rationale for NRLAR could not be assessed in this cohort. Lastly, the decision to perform a non-restorative resection may reflect anticipation of procedural challenges, indicated by the higher conversion rate and intraoperative complications, introducing selection bias despite multivariable adjustments. The strengths of the present study lie in the large national cohort, the use of a prospective audited registry, and the consistency of high-volume expert centers, which help mitigate some of these inherent shortcomings. Nevertheless, these findings should be interpreted with caution, and prospective studies or randomized controlled trials are required to confirm the observed associations.
Despite these limitations, these findings carry significant clinical implications. This study underscores NRLAR’s independent association with worse oncological outcomes compared to RLAR and APR, corroborating previous reports. Given the absence of prospective studies or RCTs providing insight into NRLAR’s outcomes, avoidance of NRLAR for rectal cancer treatment is recommended.
Future Perspectives
In scenarios where non-restorative resection is deemed necessary, removal of the rectal stump through intersphincteric APR (iAPR) should be pursued. Compared with NRLAR, iAPR may decrease the risk of residual mesorectum and potentially lower the incidence of diversion proctitis and pelvic sepsis [45]. However, caution is warranted as iAPR is technically demanding and requires substantial experience and subspecialized training. Ongoing randomized trials comparing iAPR with NRLAR are expected to shed further light on their postoperative surgical morbidity [46].
Future research should not only focus on oncological safety but also systematically incorporate patient-reported outcomes and functional endpoints. The rationale for considering a non-restorative resection is often to avoid the severe functional sequelae of a very low anastomosis, such as low anterior resection syndrome, urgency, or incontinence. Therefore, a comprehensive evaluation of surgical strategies must balance oncological outcomes with quality of life and functional preservation.
Ongoing prospective studies, such as the nationwide VANTAGE trial, are specifically designed to address this gap by integrating oncological, functional, and quality-of-life outcomes into a single framework. These data will be essential to guide truly patient-centered decision making and to clarify the role of NRLAR compared to restorative and abdominoperineal approaches.
5. Conclusions
In this multicenter cohort of 2018 patients with MRI-defined rectal cancer, NRLAR was associated with significantly poorer oncological outcomes compared with RLAR and APR. Three-year disease-free survival was 71.4% after NRLAR versus 82.0% after RLAR and 77.4% after APR (p = 0.003), while overall survival was 82.9% for NRLAR compared to 93.5% and 90.2%, respectively (p < 0.001). Local recurrence was more than doubled after NRLAR (8.1%) compared to RLAR (3.3%) and APR (4.5%) (p = 0.003). After multivariable adjustment, NRLAR remained an independent predictor of inferior DFS (HR 1.34), OS (HR 1.57), and higher LR risk (HR 2.66). While this association cannot be interpreted as causal, it likely reflects a combination of patient frailty, technical challenges during low pelvic dissection, and the potential impact of pelvic sepsis. These findings highlight the need for careful surgical decision making and underscore the importance of prospective studies to further clarify causal mechanisms and optimize outcomes in this patient group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17183074/s1, File S1.
Author Contributions
Substantial contributions to the conception and design of the work: R.T.J.G., M.B., T.A.B., R.H., and E.C.J.C.; drafting the article: R.T.J.G., M.B., and T.A.B.; revising the article critically for important intellectual content: R.H. and E.C.J.C.; final approval of the version to be published: R.T.J.G., M.B., T.A.B., R.H., and E.C.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Medical Research Ethics Committees United (MEC-U) AW19- 347 023/W18.100 2022-03-31.
Informed Consent Statement
The Medical Research Ethics Committees United (MEC-U) (AW19-023/W18.100) waived the need for informed consent, which was approved by the local ethics boards of all participating institutions.
Data Availability Statement
Data will be made available upon reasonable request by the corresponding author.
Acknowledgments
We would like to thank all collaborators of the MIRECA Study Group: G.J.D. van Acker, T.S. Aukema, H.J. Belgers, F.H. Beverdam, J.G. Bloemen, K. Bosscha, S.O. Breukink, P.P.L.O. Coene, R.M.P.H. Crolla, P. van Duijvendijk, E.B. van Duyn, I.F. Faneyte, S.A.F. Fransen, A.A.W. van Geloven, M.F. Gerhards, W.M.U. van Grevenstein, K. Havenga, I.H.J.T. de Hingh, C. Hoff, G. Kats, J.W.A. Leijtens, M.F. Lutke Holzik, J. Melenhorst, M.M. Poelman, A. Pronk, A.H.W. Schiphorst, J.M.J. Schreinemakers, C. Sietses, A.B. Smits, I Somers, E.J. Spillenaar Bilgen, H.B.A.C. Stockmann, A.K. Talsma, P.J. Tanis, J. Tuynman, E.G.G. Verdaasdonk, F.A.R.M. Warmerdam, H.L. van Westreenen, and D.D.E. Zimmerman.
Conflicts of Interest
ECJC is proctor for Intuitive Surgical. No (financial) support from this organization has been received for the submitted manuscript. There have not been any other activities or relations that have influenced the submitted work. All other authors declare no support from any organization for the submitted work, no financial relationships with organizations that may have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
References
- Heald, R.J.; Husband, E.M.; Ryall, R.D.H. The mesorectum in rectal cancer surgery—The clue to pelvic recurrence? Br. J. Surg. 2005, 69, 613–616. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Corman, M.L.W. Ernest Miles. Dis. Colon Rectum 1980, 23, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H. Nouveau procédé d’ablation des cancers de la partie terminale du colon pelvien. In Trentieme Congres De Chi-Rurgie; Association Française de Chirurgie: Paris, France, 1921; Volume 28, p. 411. [Google Scholar]
- Bujko, K.; Nowacki, M.P.; Kępka, L.; Olędzki, J.; Bębenek, M.; Kryj, M.; the Polish Colorectal Study Group. Postoperative complications in patients irradiated pre-operatively for rectal cancer: Report of a randomised trial comparing short-term radiotherapy vs chemoradiation. Color. Dis. 2005, 7, 410–416. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef]
- Rutegård, M.; Haapamäki, M.; Matthiessen, P.; Rutegård, J. Early postoperative mortality after surgery for rectal cancer in Sweden, 2000–2011. Color. Dis. 2014, 16, 426–432. [Google Scholar] [CrossRef]
- Jørgensen, J.B.; Erichsen, R.; Pedersen, B.G.; Laurberg, S.; Iversen, L.H. Stoma reversal after intended restorative rectal cancer resection in Denmark: Nationwide population-based study. BJS Open 2020, 4, 1162–1171. [Google Scholar] [CrossRef]
- Santoro, R.; Del Corpo, G.; Chiappini, A.; Maria, F.M.S.; Di Cicco, M.; Callegaro, E.; Costanzo, F.; Sandri, G.B.L. Accreditation for colorectal cancer surgery in Italy. Preliminary results of a new program in a district hospital. Il G. Di Chir.-J. Ital. Surg. Assoc. 2019, 40, 504–512. [Google Scholar]
- Hurtado, H.O.; Cazador, A.C. The Rectal Cancer Project of the Spanish Association of Surgeons. Rev. Med. Hosp. Gen. Méx. 2017, 80, 106–110. [Google Scholar] [CrossRef]
- Cecil, T.D.; Taffinder, N.; Gudgeon, A.M. A personal view on laparoscopic rectal cancer surgery. Color. Dis. 2006, 8, 30–32. [Google Scholar] [CrossRef]
- Lawday, S.; Flamey, N.; E Fowler, G.; Leaning, M.; Dyar, N.; Daniels, I.R.; Smart, N.J.; Hyde, C. Quality of life in restorative versus non-restorative resections for rectal cancer: Systematic review. BJS Open 2022, 5, zrab101. [Google Scholar] [CrossRef]
- Anderin, C.; Martling, A.; Hellborg, H.; Holm, T. A Population-based Study on Outcome in Relation to the Type of Resection in Low Rectal Cancer. Dis. Colon Rectum 2010, 53, 753–760. [Google Scholar] [CrossRef]
- Ortiz, H.; Wibe, A.; Ciga, M.A.; Kreisler, E.; Garcia-Granero, E.; Roig, J.V.; Biondo, S. Multicenter Study of Outcome in Relation to the Type of Resection in Rectal Cancer. Dis. Colon Rectum 2014, 57, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Roodbeen, S.X.; Blok, R.D.; Borstlap, W.A.; Bemelman, W.A.; Hompes, R.; Tanis, P.J.; The Dutch Snapshot Research Group; Wevers, K. Does oncological outcome differ between restorative and nonrestorative low anterior resection in patients with primary rectal cancer? Color. Dis. 2020, 23, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Hol, J.C.; Burghgraef, T.A.; Rutgers, M.L.; Crolla, R.M.; van Geloven, N.A.; Leijtens, J.W.; Polat, F.; Pronk, A.; Smits, A.B.; Tuynman, J.B.; et al. Comparison of three-year oncological results after restorative low anterior resection, non-restorative low anterior resection and abdominoperineal resection for rectal cancer. Eur. J. Surg. Oncol. (EJSO) 2022, 49, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Hol, J.C.; A Burghgraef, T.; Rutgers, M.L.W.; Crolla, R.M.P.H.; Geloven, N.A.W.v.; Hompes, R.; A Leijtens, J.W.; Polat, F.; Pronk, A.; Smits, A.B.; et al. Comparison of laparoscopic versus robot-assisted versus transanal total mesorectal excision surgery for rectal cancer: A retrospective propensity score-matched cohort study of short-term outcomes. Br. J. Surg. 2021, 108, 1380–1387. [Google Scholar] [CrossRef]
- Burghgraef, T.A.; Sikkenk, D.J.; Verheijen, P.M.; El Moumni, M.; Hompes, R.; Consten, E.C.J. The learning curve of laparoscopic, robot-assisted and transanal total mesorectal excisions: A systematic review. Surg. Endosc. 2022, 36, 6337–6360. [Google Scholar] [CrossRef]
- Burghgraef, T.A.; Sikkenk, D.J.; Crolla, R.M.P.H.; Fahim, M.; Melenhorst, J.; El Moumni, M.; van der Schelling, G.; Smits, A.B.; Stassen, L.P.S.; Verheijen, P.M.; et al. Assessing the learning curve of robot-assisted total mesorectal excision: A multicenter study considering procedural safety, pathological safety, and efficiency. Int. J. Color. Dis. 2023, 38, 9. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Tanis, P.; Beets, G.; Verhoef, C.; Punt, C.J.A.; Moons, L.; Beets-Tan, R.G.H.; Intven, M.P.W.; Marijnen, C.A.M.; Meijerink, M.; Stoker, J.; et al. Dutch Colorectal Cancer Guideline. Oncoline.nl. 2019. Available online: https://www.mdl.nl/files/richlijnen/Richtlijn%20Colorectaal%20Carcinoom.pdf (accessed on 19 September 2023).
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Stevenson, A.R.L.; Solomon, M.J.; Lumley, J.W.; Hewett, P.; Clouston, A.D.; Gebski, V.J.; Davies, L.; Wilson, K.M.; Hague, W.; Simes, J. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015, 314, 1356–1363. [Google Scholar] [CrossRef]
- Fleshman, J.; Branda, M.E.; Sargent, D.J.; Boller, A.M.; George, V.V.; Abbas, M.A.; Peters, W.R.; Maun, D.C.; Chang, G.J.; Herline, A.; et al. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer. Ann. Surg. 2019, 269, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Bonjer, H.J.; Deijen, C.L.; Abis, G.A.; Cuesta, M.A.; Van Der Pas, M.H.G.M.; de Lange-de Klerk, E.S.M.; Lacy, A.M.; Bemelman, W.A.; Andersson, J.; Angenete, E.; et al. A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer. N. Engl. J. Med. 2015, 372, 1324–1332. [Google Scholar] [CrossRef]
- Stevenson, A.R.L.; Solomon, M.J.; Brown, C.S.B.; Lumley, J.W.; Hewett, P.; Clouston, A.D.; Gebski, V.J.; Wilson, K.; Hague, W.; Simes, J. Disease-free Survival and Local Recurrence After Laparoscopic-assisted Resection or Open Resection for Rectal Cancer. Ann. Surg. 2019, 269, 596–602. [Google Scholar] [CrossRef]
- Mari, G.; Santambrogio, G.; Crippa, J.; Cirocchi, R.; Origi, M.; Achilli, P.; Ferrari, G.; Megna, S.; Desio, M.; Cocozza, E.; et al. 5 year oncological outcomes of the HIGHLOW randomized clinical trial. Eur. J. Surg. Oncol. (EJSO) 2022, 49, 641–646. [Google Scholar] [CrossRef]
- Serra-Aracil, X.; Zarate, A.; Bargalló, J.; Gonzalez, A.; Serracant, A.; Roura, J.; Delgado, S.; Mora-López, L.; Ta-LaTME study Group; Pallisera-Lloveras, A.; et al. Transanal versus laparoscopic total mesorectal excision for mid and low rectal cancer (Ta-LaTME study): Multicentre, randomized, open-label trial. Br. J. Surg. 2022, 110, 150–158. [Google Scholar] [CrossRef]
- Feng, Q.; Tang, W.; Zhang, Z.; Wei, Y.; Ren, L.; Chang, W.; Zhu, D.; Liang, F.; He, G.; Xu, J. Robotic versus laparoscopic abdominoperineal resections for low rectal cancer: A single-center randomized controlled trial. J. Surg. Oncol. 2022, 126, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Segev, L.; Schtrechman, G.M.; Kalady, M.F.; Liska, D.; Gorgun, I.E.; Valente, M.A.D.; Nissan, A.; Steele, S.R.M. Long-term Outcomes of Minimally Invasive Versus Open Abdominoperineal Resection for Rectal Cancer: A Single Specialized Center Experience. Dis. Colon Rectum 2021, 65, 361–372. [Google Scholar] [CrossRef]
- Battersby, N.J.; How, P.; Moran, B.; Stelzner, S.; West, N.P.; Branagan, G.; Strassburg, J.; Quirke, P.; Tekkis, P.; Pedersen, B.G.; et al. Prospective Validation of a Low Rectal Cancer Magnetic Resonance Imaging Staging System and Development of a Local Recurrence Risk Stratification Model. Ann. Surg. 2016, 263, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.R.S.; Simillis, C.; Hunter, C.; Chand, M.; Bhoday, J.; Garant, A.; Vuong, T.; Artho, G.; Rasheed, S.; Tekkis, P.; et al. A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br. J. Cancer 2017, 116, 1513–1519. [Google Scholar] [CrossRef]
- Denost, Q.; Rouanet, P.; Faucheron, J.-L.; Panis, Y.; Meunier, B.; Cotte, E.; Meurette, G.; Portier, G.; Sabbagh, C.; Loriau, J.; et al. Impact of early biochemical diagnosis of anastomotic leakage after rectal cancer surgery: Long-term results from GRECCAR 5 trial. Br. J. Surg. 2021, 108, 605–608. [Google Scholar] [CrossRef]
- Koedam, T.W.A.; Bootsma, B.T.; Deijen, C.L.; van de Brug, T.; Kazemier, G.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Haglind, E.; Tuynman, J.B.; et al. Oncological Outcomes After Anastomotic Leakage After Surgery for Colon or Rectal Cancer. Ann. Surg. 2020, 275, e420–e427. [Google Scholar] [CrossRef]
- Tøttrup, A.; Frost, L. Pelvic Sepsis After Extended Hartmann’s Procedure. Dis. Colon Rectum 2005, 48, 251–255. [Google Scholar] [CrossRef]
- Artioukh, D.Y.; Smith, R.A.; Gokul, K. Risk factors for impaired healing of the perineal wound after abdominoperineal resection of rectum for carcinoma. Color. Dis. 2006, 9, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B.; Oliveira, J. Rectal cancer: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2008, 19, ii31–ii32. [Google Scholar] [CrossRef] [PubMed]
- Breugom, A.J.; van Gijn, W.; Muller, E.W.; Berglund, Å.; van den Broek, C.B.M.; Fokstuen, T.; Gelderblom, H.; Kapiteijn, E.; Leer, J.W.H.; Marijnen, C.A.M.; et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann. Oncol. 2015, 26, 696–701. [Google Scholar] [CrossRef]
- Kulaylat, A.S.; Hollenbeak, C.S.; Stewart, D.B. Adjuvant Chemotherapy Improves Overall Survival of Rectal Cancer Patients Treated with Neoadjuvant Chemoradiotherapy Regardless of Pathologic Nodal Status. Ann. Surg. Oncol. 2016, 24, 1281–1288. [Google Scholar] [CrossRef]
- Sainato, A.; Nunzia, V.C.L.; Valentini, V.; De Paoli, A.; Maurizi, E.R.; Lupattelli, M.; Aristei, C.; Vidali, C.; Conti, M.; Galardi, A.; et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother. Oncol. 2014, 113, 223–229. [Google Scholar] [CrossRef]
- Denost, Q.; Fleming, C.A.; Burghgraef, T.; Celerier, B.; Geitenbeek, R.; Rullier, E.; Tuynman, J.; Consten, E.; Hompes, R. Dutch MIRECA Collaborative Group (Pubmed Citable) An International Multicenter Prospective Study Evaluating the Long-term Oncological Impact of Adjuvant Chemotherapy in ypN+ Rectal Cancer. Ann. Surg. 2022, 277, 299–304. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; van de Velde, C.J.; van der Worp, E.; Kapiteijn, E.; Quirke, P.; van Krieken, J.H.M. Pathology Review Committee for the Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group Macroscopic Evaluation of Rectal Cancer Resection Specimen: Clinical Significance of the Pathologist in Quality Control. J. Clin. Oncol. 2002, 20, 1729–1734. [Google Scholar] [CrossRef]
- Quirke, P.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Couture, J.; O’CAllaghan, C.; Myint, A.S.; Bessell, E.; Thompson, L.C.; et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009, 373, 821–828. [Google Scholar] [CrossRef]
- A Burghgraef, T.; Hol, J.C.; Rutgers, M.L.; Brown, G.; Hompes, R.; Sietses, C.; Consten, E.C.J.; the MIRECA Study Group; Crolla, R.M.P.H.; Geitenbeek, R.T.J.; et al. Implications of the new MRI-based rectum definition according to the sigmoid take-off: Multicentre cohort study. BJS Open 2023, 7, zrad018. [Google Scholar] [CrossRef] [PubMed]
- Westerduin, E.; Aukema, T.S.; van Geloven, A.A.W.; Bemelman, W.A.; Tanis, P.J.; the Dutch Snapshot Research Group. What to do with the rectal stump during sphincter preserving rectal cancer resection with end colostomy: A collaborative snapshot study. Color. Dis. 2018, 20, 696–703. [Google Scholar] [CrossRef] [PubMed]
- HAPIrect Collaborative Study Group; Smedh, K.; Sverrisson, I.; Chabok, A.; Nikberg, M. Hartmann’s procedure vs abdominoperineal resection with intersphincteric dissection in patients with rectal cancer: A randomized multicentre trial (HAPIrect). BMC Surg. 2016, 16, 43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).